ABSTRACT
Macrophages are tissue-resident immune cells that are crucial for the initiation and maintenance of immune responses. Purinergic signaling modulates macrophage activity and impacts cellular plasticity. The ATP-activated purinergic receptor P2X7 (also known as P2RX7) has pro-inflammatory properties, which contribute to macrophage activation. P2X7 receptor signaling is, in turn, modulated by ectonucleotidases, such as CD39 (also known as ENTPD1), expressed in caveolae and lipid rafts. Here, we examined P2X7 receptor activity and determined impacts on ectonucleotidase localization and function in macrophages primed with lipopolysaccharide (LPS). First, we verified that ATP boosts CD39 activity and caveolin-1 protein expression in LPS-primed macrophages. Drugs that disrupt cholesterol-enriched domains – such as nystatin and methyl-β-cyclodextrin – decreased CD39 enzymatic activity in all circumstances. We noted that CD39 colocalized with lipid raft markers (flotillin-2 and caveolin-1) in macrophages that had been primed with LPS followed by treatment with ATP. P2X7 receptor inhibition blocked these ATP-mediated increases in caveolin-1 expression and inhibited the colocalization with CD39. Further, we found that STAT3 activation is significantly attenuated caveolin-1-deficient macrophages treated with LPS or LPS+BzATP. Taken together, our data suggest that P2X7 receptor triggers the initiation of lipid raft-dependent mechanisms that upregulates CD39 activity and could contribute to limit macrophage responses restoring homeostasis.
KEY WORDS: Extracellular ATP, Ectonucleotidases, Purinergic signaling, Lipid rafts, Macrophages, P2RX7
Summary: P2X7 receptor activation triggers the initiation of lipid raft-dependent regulatory pathways that upregulate CD39 activity, contributing to limit macrophage responses and inflammation.
INTRODUCTION
Macrophages are monocyte-derived or tissue-resident immune cells crucial for the initiation and maintenance of immune responses. These cells recognize pathogen-associated molecular pattern molecules (PAMPs) and damage-associated molecular pattern molecules (DAMPs) through pathogen recognition receptors (PRRs), such as Toll-like receptors (TLRs), thereby promoting inflammatory responses (reviewed in Gong et al., 2019; Gordon and Plüddemann, 2019).
Extracellular adenosine triphosphate (eATP) is a well-characterized DAMP that modulates macrophage function and plasticity (Barberá-Cremades et al., 2016; Savio and Coutinho-Silva, 2019). This nucleotide can be released from stressed, injured and dying cells or in response to TLR activation, reaching high concentrations within the extracellular milieu (Cohen et al., 2013). Once outside the cells, eATP can activate type 2 purinergic (P2) receptors. The P2 receptor family comprises the P2Y G-protein-coupled receptors (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14) and the P2X ligand-gated ion channels (P2X1, P2X2, P2X3, P2X4, P2X5, P2X6 and P2X17) (Ralevic and Burnstock, 1998; Abbracchio et al., 2006).
Of the latter group, the P2X7 receptor (also known as P2RX7) is the subtype that has been most-extensively studied during inflammation and was found to provide host defenses against parasites by inducing the activation of several microbicidal mechanisms (reviewed in Savio et al., 2018, Savio and Coutinho-Silva, 2019). The P2X7 receptor induces the production of reactive oxygen and nitrogen species, and the release of inflammatory cytokines, such as IL-1β and IL-18, by acting as the second signal to activate the NLRP3 inflammasome (Ferrari et al., 2006; Cruz et al., 2007). Furthermore, the P2X7 receptor is involved in the activation of signaling pathways, such as MyD88/NFκB, PI3K/Akt/mTOR and STAT3, and activation of mitogen-activated protein kinase (MAPK) pathway proteins, such as MEKs and ERK1/2 (MAPK3/MAPK1) (Bradford and Soltoff, 2002; Skaper et al., 2010; Liu et al., 2011; Bian et al., 2013; Savio et al., 2017a; de Andrade Mello et al., 2017b). It is thought that eATP-mediated P2X7 receptor signaling is a key component of macrophage inflammatory machinery and associated intercellular signaling responses (Savio et al., 2018; Zumerle et al., 2019).
eATP signaling is precisely regulated by nucleotide-metabolizing cell-surface enzymes, the so-called ectonucleotidases. The family of ectonucleoside triphosphate diphosphohydrolases (E-NTPDases) and that of 5′-nucleotidase (CD73, also known as NT5E) are the most important ectonucleotidases expressed in immune cells. E-NTPDases hydrolyze extracellular tri- and diphosphonucleosides to monophosphonucleosides. Ectonucleoside triphosphate diphosphohydrolase 1, 2 and 3 (ENTPD1, ENTPD2 and ENTPD3, respectively; hereafter referred to as CD39, CD39L1 and CD39L3, respectively) are ectoenzymes that are tightly bound to the plasma membrane through two transmembrane domains; CD39 is the dominant ecto-enzyme expressed in macrophages (Lévesque et al., 2010; Savio et al., 2017a). CD73 is a glycosylphosphatidylinositol-anchored enzyme that catalyzes the hydrolysis of AMP to adenosine (Zimmermann, 1996). These enzymes are important to regulate macrophage activation by degrading eATP to yield adenosine that generally has anti-inflammatory effects, acting mainly via A2A or A2B adenosine receptors (Lévesque et al., 2010; Cohen et al., 2013; Savio et al., 2017a). The expression of CD39 and CD73 is regulated by the transcription factors STAT3 and GFI-1, amongst others (Chalmin et al., 2012; Savio et al., 2017a). Both enzymes play relevant roles in the pathophysiology of several inflammatory diseases, including cancer, atherosclerosis, sepsis, autoimmune and neurological diseases (Cognato et al., 2011; Savio et al., 2017a,b; de Andrade Mello et al., 2017a; De Giorgi et al., 2017; Allard et al., 2017; Longhi et al., 2017; Vuerich et al., 2019; Takenaka et al., 2019). Nevertheless, cellular mechanisms that regulate the functionality of these enzymes in innate immune cells, such as macrophages, remain poorly defined.
Lipid rafts are membrane microdomains enriched in sphingolipids and cholesterol, which serve as a platform for the dynamic assembly of signaling complexes, including TLR-dependent signaling pathways, during the inflammatory process (Płóciennikowska et al., 2015). TLR2 has been described to be associated with lipid domains (Vieira et al., 2010; Fessler and Parks, 2011). In addition, TLR4 migrates to lipid rafts after stimulation with lipopolysaccharide (LPS) and forms complexes with other molecules, including CD14 (Triantafilou et al., 2002, 2007; Vieira et al., 2010; Fessler and Parks, 2011; Płóciennikowska et al., 2015). Furthermore, bacterial infections induce the formation of stable plasma membrane domains increasing the generation of ceramide from sphingomyelin in a reaction catalyzed by acid sphingomyelinase (Lu et al., 2012). The increase in ceramide levels is accompanied by an increased expression of TLR4 in lipid raft domains (Lu et al., 2012; Tawadros et al., 2015). Interestingly, P2X7 receptor activation can also stimulate sphingomyelinase activity, which promotes the formation of ceramide-enriched membrane domains (Garcia-Marcos et al., 2006; Lepine et al., 2006). Furthermore, both P2X7 receptor and CD39 have been described to be palmitoylated at conserved clusters of cysteine residues, and this post-translational modification targets these proteins to lipid rafts (Koziak et al., 2000; Gonnord et al., 2009; Murrell-Lagnado, 2017). Gangadharan et al. (2015) showed that caveolin-1 attenuates P2X7 receptor-dependent signaling by inducing endocytosis following activation of this receptor in osteoblasts. This phenomenon controls the duration and magnitude of P2X7 receptor activation, and can also remove other signaling proteins from the membrane. Therefore, we hypothesized that TLR activation and the eATP/P2X7 receptor proinflammatory signaling response in macrophages modifies lipid and protein dynamics in the plasma membrane by modulating CD39 activity in a lipid raft-dependent manner.
RESULTS
ATP-induced CD39 activity in LPS-primed macrophages is dependent on organizational integrity of membrane rafts
Initially, we evaluated whether treatment of peritoneal macrophages with drugs that disrupt cholesterol-enriched domains – such as nystatin and methyl-β-cyclodextrin (MβCD) – can impact CD39 or CD73 GPI-linked activity. Indeed, CD39 activity was decreased in peritoneal macrophages after treatment with nystatin or MβCD for 10 min (P<0.05), irrespectively of stimulation with LPS and ATP (Fig. 1A). In addition, treatment with imipramine – which inhibits acidic sphingomyelinase activity – attenuated the increase in CD39 enzyme activity induced by treatment with LPS+ATP (P<0.05; Fig. 1A).
Fig. 1.
P2X7-dependent increase in CD39 activity depends on lipid raft integrity in LPS-primed macrophages. (A,B) ATP hydrolysis (A) and AMP hydrolysis (B) in LPS-primed macrophages (1 µg/ml LPS for 4 h), stimulated with 500 µM ATP for 3 h that were then treated with 10 mM methyl-β-cyclodextrin (MβCD) or 50 mg/ml nystatin, for 10 min or benzyl alcohol (20 µM) for 30 min. One set of data is from cells treated with imipramine (30 µM) for 30 min before priming. Data are expressed as mean±s.e.m. of three independent experiments performed in triplicates. *P<0.05 when compared to unstimulated and untreated groups. #P<0.05 when non-treated groups were compared to drug-treated groups.
Next, to confirm that the increase in CD39 activity induced by LPS+ATP depends on raft formation during which the enzyme becomes more stable and active, we treated the cells with benzyl alcohol to increase membrane fluidity. After treatment with benzyl alcohol, there was no increase in ATP hydrolysis in cells stimulated with LPS+ATP (P>0.05; Fig. 1A), confirming that changes in plasma membrane fluidity and composition can interfere with the activity of CD39 enzyme. When evaluating CD73 functionality, we observed increased activity after priming cells with LPS (Fig. 1B). Nevertheless, CD73 activity was not affected by lipid raft disruption (Fig. 1B).
ATP triggers movement of CD39 to lipid rafts in LPS-primed macrophages
To confirm that CD39 is located within these membrane domains in macrophages, we double-labeled LPS- and ATP-treated peritoneal macrophages with antibodies recognizing CD39 and flotillin-2 (used as a general marker of lipid rafts) (Zhao et al., 2011). In control cells and cells treated with ATP only, CD39 and flotillin-2 colocalized in a less-structured pattern, with some (but not all) flotillin-2-positive spots observed in cells stained for CD39 (Fig. 2A). In contrast, cells treated with both LPS and ATP displayed numerous and more-conspicuous spots of fluorescence that were consistently double-stained for CD39 and flotillin-2 (Fig. 2A); this suggests a high CD39 concentration within membrane domains and is likely to represent lipid rafts. Treatment with MβCD diminished the double-labeled spots of fluorescence (Fig. 2A). These observations were confirmed by plug-in Coloc2 analysis performed with software Fiji/ImageJ (Fig. 2B-F), generating the Pearson correlation coefficient of the pixel-intensity correlation for double-labeled points that indicates colocalization.
Fig. 2.
ATP treatment increases colocalization of CD39 and flotillin-2 in LPS-primed peritoneal macrophages. Mouse peritoneal macrophages from wild-type animals were labeled with antibodies recognizing CD39 (red) and the lipid raft marker protein flotillin-2 (green), as well as with DAPI (blue) to stain the nucleus. (A) Cells were left untreated (Control; first row) or were treated with 1 µg/ml LPS for 4 h (second row), 500 µM ATP for 1 h (third row), with LPS and ATP (fourth row), or with ATP, LPS and 10 mM methyl-β-cyclodextrin (MβCD) (fifth row). CD39 and flotillin-2 colocalized in ‘spots’ when cells were treated with ATP or with ATP and LPS. Scale bars: 2 µm. (B-F) Representative intensity histogram outputs of Coloc2 analysis performed with Fiji/ImageJ software, and (G) Pearson correlation coefficient of the pixel-intensity correlation indicating colocalization of CD39 and flotillin-2. Data are expressed as mean±s.e.m. of three independent experiments. *,#P<0.05 compared with unstimulated control or with the LPS+ATP group, respectively.
As depicted in Fig. 2G, ATP+LPS treatment significantly increased the R value for colocalization, whereas MβCD treatment dissipated this effect, i.e. reducing the R value significantly when compared to both control and LPS+ATP groups (Fig. 2G).
P2X7 receptor activation triggers CD39 to caveolae of macrophages
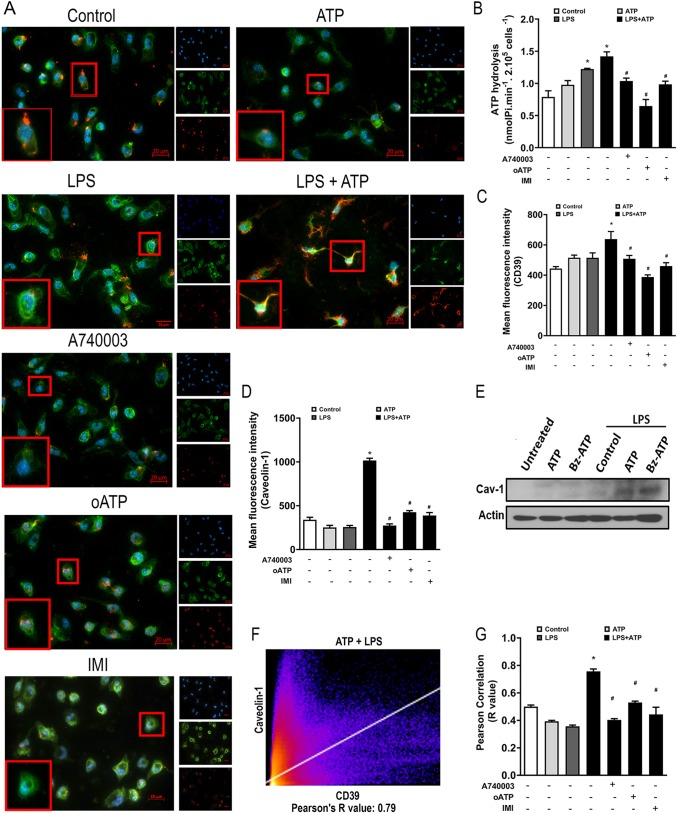
Given that CD39 has palmitoylation sites and has previously been reported to be located in caveolae of certain cell types (Koziak et al., 2000), we evaluated whether this enzyme can also be located in caveolae within the membrane of peritoneal macrophages treated with LPS+ATP. For this, we double-labeled LPS- and ATP-treated peritoneal macrophages (pre-treated or not with the P2X7 receptor inhibitor A740003 or imipramine) with antibodies recognizing CD39 and caveolin-1 (a marker of caveolar domains).
We verified that treatment with LPS+ATP induced a clear increase in caveolin-1 expression, showing some points consistently double labeled, suggesting colocalization of caveolin-1 and CD39 (Fig. 3A). Furthermore, this effect was prevented by P2X7 receptor blockade or the treatment with imipramine.
Fig. 3.
P2X7 receptor activation increases CD39 expression in caveolae of macrophages. LPS-primed peritoneal macrophages (1 µg/ml for 4 h) stimulated with 500 µM ATP for 1 h in the absence or presence of pre-treatment with the P2X7 receptor inhibitors A740003 (0.1 µM 30 min before priming), oxidized-ATP (oATP; 200 µM for 2 h before priming) or the sphingomyelinase inhibitor imipramine (IMI; 30 µM for 30 min before priming) were labeled with antibodies recognizing CD39 (green) and the lipid raft marker caveolin-1 (red), as well as with Hoechst 33258 (blue). (A) Representative immunofluorescence images of cells stained for CD39 and caveolin-1. (B-D) Enzymatic assay for ATP hydrolysis (B) and quantification of fluorescence intensity for CD39 (C) and caveolin-1 (D). (E) Representative western blot showing caveolin-1 expression in untreated and LPS-primed macrophages stimulated with P2X7 receptor agonists (500 µM ATP or 100 µM Bz-ATP). (F,G) Representative intensity histogram output (F) of Coloc2 analysis performed with Fiji/ImageJ and Pearson correlation coefficient of the pixel-intensity correlation (G) indicating colocalization. Data are expressed as mean±s.e.m. of three independent experiments. *,#P<0.05 compared with the unstimulated control or compared to the LPS+ATP group, respectively.
As we have previously shown, LPS and LPS+ATP treatment boost CD39 activity (Savio et al., 2017a), and these inhibitory effects are blocked by P2X7 receptor inhibitors (A740003 and oxidized-ATP) or imipramine (Fig. 3B). In addition, MFI analysis for CD39 and caveolin-1 showed that treatment with ATP+LPS significantly increases the cell surface levels of these proteins, whereas inhibition of P2X7 receptor blocks these effects (Fig. 3C and D). The increased expression of caveolin-1 in peritoneal macrophages after treatment with LPS+ATP or LPS+BzATP was confirmed by western blot experiments (Fig. 3E). Immunofluorescence images were also analyzed by Coloc2 plugin in Fiji/ImageJ software (Fig. 3F), and Pearson correlation coefficient values were calculated for double-labeled points (Fig. 3F and G). ATP+LPS treatment significantly increased the R value for colocalization, whereas pretreatment with P2X7 receptor antagonists or imipramine inhibited this effect (Fig. 3G).
Caveolin-1 is important for P2X7 receptor-mediated increase in STAT3 activation
Finally, we evaluated the relevance of caveolin-rich membrane domains in macrophages regarding the activation of the transcription factor STAT3, which is involved in the induction of CD39 expression in immune cells (Chalmin et al., 2012). LPS increases STAT-3 phosphorylation in wild type (WT) macrophages, and P2X7 receptor activation in response to Bz-ATP significantly potentiates the activation of this transcription factor, whereas treatment with 500 µM ATP did not increase levels of phosphorylated STAT3 (p-STAT3) (Fig. 4A-B). This is possibly due to the fact that Bz-ATP is 10× more potent than ATP in activating the P2X7 receptor. Nevertheless, we do not exclude participation of other mechanisms and, potentially, other P2 receptors in the regulation of STAT3 activation in these settings. However, when we used macrophages from caveolin-1-deficient mice, levels of p-STAT3 were significantly diminished in both LPS- and LPS+BzATP-treated groups when compared with WT groups (Fig. 4A,B). To test for the putative contribution of P2X7 receptor to STAT3 activation, we further pre-treated WT cells with P2X7 receptor inhibitors, i.e. A740003 and oxidized-ATP (oATP), and then exposed these cells to LPS or LPS+BzATP. We found that either inhibitor significantly decreased p-STAT3 levels in cells treated with LPS or LPS+Bz-ATP (data not shown). These data suggest that P2X7 receptor-dependent STAT3 phosphorylation in LPS-primed macrophages depends on caveolin-rich membrane domains.
Fig. 4.

Caveolin-1 is important for STAT3 activation in LPS-primed macrophages. (A,B) Representative western blot (A) and its densitometric analysis (B) of levels of active, i.e. phosphorylated STAT3 (p-STAT3) in untreated and LPS-primed macrophages derived from wild-type (WT) or caveolin-1−/− mice stimulated with P2X7 receptor agonists (500 µM ATP or 100 µM Bz-ATP). Data are expressed as mean±s.e.m. of three independent experiments. *P<0.05 compared with the unstimulated control group. #P<0.05 when comparing WT with caveolin-1−/− animals. ND, not detectable.
DISCUSSION
Purinergic signaling modulates macrophage activity and immune responses. Here, we show that the P2X7 receptor activates a lipid raft-dependent regulatory mechanism that modulates macrophage CD39 activity. P2X7 receptor activation increases activity of CD39 and targets this protein to lipid rafts, whereas drugs that disrupt cholesterol-enriched domains decreased its activity. P2X7 receptor inhibition blocked increases in caveolin-1 expression and inhibited colocalization with CD39. Interestingly, the formation of membrane rafts is linked to the functionality of various components of the purinergic signaling, such as the P2X7 receptor and CD39. The latter is anchored to the plasma membrane by two transmembrane domains. Drug- or detergent-induced disruption of these domains significantly decreases the activity of the CD39, further indicating that its activity requires the integrity of both transmembrane domains in the plasma membrane (Papanikolaou et al., 2005).
Grinthal and Guidotti (2006) have proposed that changes in the mechanical properties (dependent on lipid composition) of the plasma membrane domain where CD39 molecules are inserted modulate CD39 activity, by modifying the stability of its transmembrane domains. This is in agreement with our data, showing that drugs that disrupt lipid raft membrane domains (i.e. nystatin or MβCD) abolish the ATP-induced increase in CD39 activity in LPS-primed macrophages and decreases the CD39 colocalization with flotillin-2, suggesting that CD39 activity is dependent on its localization in specific lipid microdomains. Moreover, treating cells with benzyl alcohol, which acts by increasing membrane fluidity (Nagy et al., 2007), reverses increase in ATP hydrolysis after treatment with LPS+ATP, thereby demonstrating that changes in the composition and fluidity of the plasma membrane can modulate CD39 activity in macrophages.
Others and we infer that P2X7 receptor participates in lipid metabolism and formation of lipid rafts. In fact, the activation of this receptor stimulates the sphingomyelinase activity that convert sphingomyelin into ceramide promoting the formation of ceramide-enriched membrane domains (Garcia-Marcos et al., 2006; Lepine et al., 2006). In this context, it is possible that the P2X7 receptor stimulates the formation of stable lipid membrane domains in which CD39 becomes more stable and active.
The results obtained in this study are in keeping with this hypothesis, since treatment with ATP induced an increase in the activity of CD39. In this regard, we have already shown that P2X7 receptor activation boosts CD39 expression and activity in peritoneal macrophages (Savio et al., 2017a). In addition, we have reported that P2X7 receptor activation is important for activation of STAT3 (Savio et al., 2017a), a transcription factor that promotes CD39 expression in immune cells (Chalmin et al., 2012). Moreover, De Marchi et al. (2019) have recently shown that P2X7 receptor blockade reduces CD39 and CD73 expression in T effector and dendritic cells. Here, we found that caveolin-1 expression is key to P2X7 receptor-mediated phosphorylation of STAT3 in LPS-primed macrophages. Thus, the P2X7 receptor might modulate the enzymatic activity of CD39 at transcriptional level through a mechanism that, at least in part, is dependent upon formation of lipid membrane domains.
Our immunofluorescence experiments suggested that CD39 is located within lipid rafts in peritoneal murine macrophages, and that treatment with ATP induces aggregation of this protein and its association with other lipid raft markers, such as flotillin-2 or caveolin-1, although no coimmunoprecipitation experiments have been carried out to show physical interactions between these proteins. These membrane structures probably represent larger ceramide-enriched membrane domains, whose formation might be directly mediated through P2X7 receptor activation in response to treatment with ATP. Accordingly, this effect was prevented by either blockage of P2X7 receptor or treatment with the sphingomyelinase inhibitor imipramine. In the same line of evidence, the presence of palmitoylation sites in CD39 which colocalize with caveolin-1 from canine kidney cells has been reported (Koziak et al., 2000). Therefore, we hypothesize that P2X7 receptor activation promotes the formation of CD39-expressing caveolae.
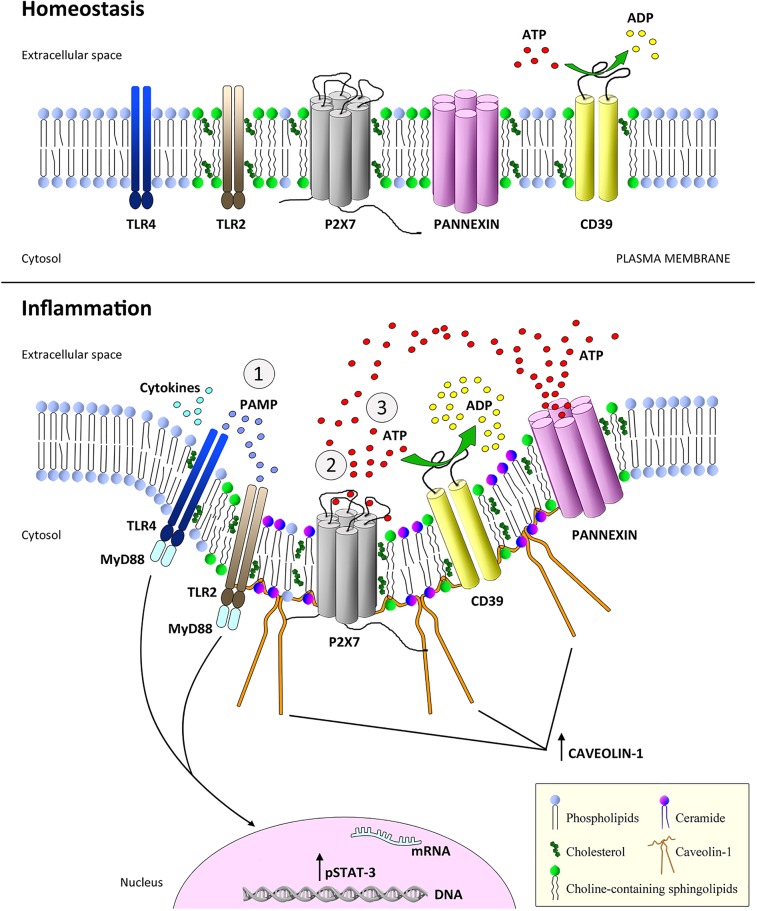
In summary, our study here demonstrated an increase in CD39 activity in response to stimulation of TLR4 (4 h) and P2X7 receptor. This increase was dependent on the integrity of membrane rafts, in which the CD39 is located after cells had been stimulated with LPS and ATP. The P2X7 receptor seems to modulate the functionality of CD39 by facilitating the formation of membrane domains, in which it becomes more stable and active (Fig. 5).
Fig. 5.
Schematic representation of the modulation of CD39 activity through P2X7 receptor signaling in activated macrophages. Under homeostatic conditions (upper panel) TLR2, the P2X7 receptor and CD39 are present in several lipid raft microdomains on the cell surface, while TLR4 is located outside the rafts. In contrast, during inflammation (lower panel) (1) the recognition of PAMPs – such as bacterial lipopolysaccharide (LPS) – by PRRs can induces ATP release, thereby activating the P2X7 receptor. This, in turn, induces (2) an extensive release of ATP that occurs via the P2X7 receptor itself or via pannexin hemichannels, thereby increasing caveolin-1 expression and the formation of caveolar domains on the plasma membrane. (3) In these domains, CD39 might become more stable and active, favoring the process of ATP hydrolysis to ADP and its metabolites, which, in a purinergic regulatory mechanism, could contribute to limit macrophage activation and inflammation.
To understand the mechanisms that modulate CD39 activity is crucial, given the immunoregulatory roles of this enzyme, as well as its impact on macrophage activation and proinflammatory responses mediated upon activation of the P2X7 receptor (Savio et al., 2017a). Our results support the involvement of purinergic signaling and lipid raft formation in cellular responses during inflammation and infection. They also suggest therapeutic approaches, including the administration of drugs that modulate the formation of lipid raft or of soluble apyrases that mimic the action of CD39.
MATERIALS AND METHODS
Animals and general reagents
We used male wild-type C57BL/6 mice (8–10 weeks old) in this study. In some experiments caveolin-1 null (caveolin-1−/−) mice (B6/129SJ) mice purchased from The Jackson Laboratory (Bar Harbor, ME) were used. Animals were housed at a ratio of five mice per cage, with water and food ad libitum, on a 12 h light/dark cycle (lights on at 7:00 am), and at a temperature of 22±1°C. The procedures for the care and use of animals were according to the guidelines of the Brazilian College of Animal Experimentation (COBEA) and to the Guide for the Care and Use of Laboratory Animals (National Research Council, USA). All experiments were approved by the Commission for the Ethical Use of Research Animals (CEUA) from the Federal University of Rio de Janeiro (UFRJ) (approved protocol number: IBCCF138) and by the Institutional Animal Care and Use Committees (IACUC) of Beth Israel Deaconess Medical Center (approved protocol number: 019-2015). ATP, ADP, AMP, oxidized-ATP, benzyl alcohol and imipramine were obtained from Sigma-Aldrich, MO. A740003 were purchased from Tocris Inc, Ellsville, MO.
Peritoneal macrophages
Murine macrophages were harvested from the peritoneal cavity of adult mice. Cells obtained from the peritoneal cavity were plated in suspension into 24- or 96-well tissue culture plates (TPP AG, Switzerland) at a density of 2×105 cells per well and incubated in non-supplemented Gibco® DMEM (Thermo Fisher Scientific, Rockford, IL) for 1 h, at 37°C, 5% CO2 atmosphere. Non-adherent cells were removed by washing three times with PBS, and adherent cells were cultured overnight in Gibco® DMEM (Thermo Fisher Scientific) complete medium before use in experiments.
Isolation, characterization and differentiation of bone marrow-derived macrophages
Medullar cells obtained from mouse tibias and femurs were resuspended in non-supplemented DMEM Gibco® (Thermo Fisher Scientific), passed through a filter for cell culture (Cell Strainer; 40 µm) and centrifuged for 10 min at 300 g at room temperature. Pellets were resuspended in red blood cells lysis buffer (cat. no. A10492-01, Thermo Fisher Scientific). Subsequently, cells were centrifuged for 10 min at 300 g and washed twice with PBS. Mononuclear bone marrow-derived macrophages (BMDMs) were then plated at a density of 1×108 cells in polystyrene flasks and cultured in DMEM Gibco® supplemented with 20% fetal bovine serum (Sigma-Aldrich), antibiotics (100 IU penicillin/ml and 100 mg streptomycin/ml; Gibco®) and 10 ng/ml macrophage colony-stimulating factor (M-CSF) (cat. no. 315-02, Peprotech, NJ). Cells were maintained at 37°C in an atmosphere of 5% CO2. After 4 days, culture medium was replaced, and non-adherent cells removed and discarded. After reaching 80% confluence, cells were detached from polystyrene plates upon addition of 0.25% trypsin-EDTA (Sigma-Aldrich) and plated again for subsequent experiments. The phenotype of BMDMs was determined by flow cytometry using the following antibodies: APC labeled anti-CD11b APC (cat. no. 101226, BioLegend, San Diego, CA) at 1:100 dilution and FITC labeled anti-F4/80 (cat. no. MCA497FT, AbD Serotec®, Bio-Rad, Hercules, CA) used at 1:100 dilution. Isotypes were used as negative control.
LPS priming and pharmacological treatments
For in vitro experiments, macrophages were left untreated or were primed with 1 µg/ml bacterial lipopolysaccharide (LPS) for 4 h to induce an inflammatory response, and then stimulated with 500 µM ATP or 100 µM BzATP to activate P2X7 receptors for 1 h or 3 h. Alternatively, cells were primed with 1 µg/ml LPS for 4 h, stimulated with 500 µM ATP for 3 h, and then treated with 50 mg/ml nystatin or 10 mM methyl-β-cyclodextrin (MβCD) for 10 min before, or with benzyl alcohol (to increase membrane fluidity; 20 mM) 30 min before ectonucleotidase assays (Nagy et al., 2007). In some wells, cells were pretreated with P2X7 receptor antagonists (300 µM oATP for 2 h or 100 nM A740003 for 30 min) or the sphingomyelinase inhibitor imipramine (30 µM for 30 min) before priming with LPS (Bai et al., 2014). All reagents were purchased from Sigma-Aldrich.
Ectonucleotidase activity assays
Activities of ectonucleoside triphosphate diphosphohydrolase 1 (CD39) and 5′-nucleotidase (CD73) were estimated in a reaction medium consisting of 20 mM HEPES buffer (pH 7.5) containing 1 mM CaCl2 (for ATP) or MgCl2 (for AMP), 120 mM NaCl, 5 mM KCl, 60 mM glucose, 1 mM sodium azide and 0.1% mM albumin (all reagents from Sigma-Aldrich). Peritoneal resident macrophages (2×105) were used in a final volume of 200 µl reaction medium, and enzymatic reactions were started by addition of ATP or AMP to a final concentration of 2 mM, followed by incubation for 30 min at 37°C. Reactions were stopped by addition of 200 µl of 10% trichloroacetic acid (TCA) (Sigma-Aldrich). Incubation times, protein concentrations, reaction mixtures and substrate concentrations were chosen according to a previous study (Vuaden et al., 2011). The amount of inorganic phosphate (Pi) released was measured using the colorimetric method described by Chan et al. (1986). Controls to correct for non-enzymatic Pi in samples were performed adding the nucleotides (ATP or AMP) after the reactions had been stopped with TCA. All reactions were performed in triplicates, and enzyme activities were expressed in nmol Pi released per minute per number of cells.
Immunocytochemistry
After LPS priming and pharmacological treatments described above, samples were fixed with 4% paraformaldehyde and 4% sucrose for 15 min at room temperature, and blocked with 10% horse serum and 1% BSA in PBS for 30 min at room temperature. Samples were then incubated (for 3 h at room temperature) with the following primary antibodies (in 0.1% BSA in PBS): C42A3 rabbit anti-flotillin-2 mAb (cat. no. 3436, Cell Signaling Technology, Danvers, MA) diluted 1:50 and goat anti-CD39 (cat. no. AF4398 (R&D Systems, Minneapolis, MN) diluted 1:200; or mice anti-caveolin-1 (7C8) (cat. no. NB100-615, Novus Biologicals, Littleton, CO) diluted 1:400 and goat anti-CD39 (cat. no. AF4398, R&D Systems) diluted 1:200. Cells were then washed and incubated at room temperature for 1 h with the following secondary antibodies (diluted 1:300, in 0.1% BSA in PBS): anti-rabbit IgG (H+L)-Alexa Fluor® 488 (Cell Signaling Technology) and anti-sheep IgG (H+L) Cy™5 (Jackson ImmunoResearch Laboratories, West Grove, PA); or anti-goat IgG (H+L)-Alexa Fluor® 488 and anti-mice IgG (H+L)-Alexa Fluor® 597 (Life Technologies, Eugene, OR). Finally, samples were stained with DAPI or Hoechst 33258 nuclear dye (1:10,000, cat. no. H3569, Life Technologies, Eugene, OR), and then mounted and examined in a fluorescence microscope Zeiss AxioVert 200M and the three-dimensional images (z-stack) in a Spinning Disk Confocal Microscope ZEISS Cell Observer SD (Peabody, MA).
Cell microscopy analysis
Mean fluorescence intensity (MFI) was measured in Zen Lite Blue software (Carl Zeiss). For this quantification, the background was initially subtracted and the region of interest (ROI) was selected in individual cells by using the freehand selection tool of the software to calculate the ratios of caveolin-1 and CD39 MFI in response to different treatments. This is provided by the software based on the intensity of the related pixels.
Colocalization analyses of the ratios of CD39 to flotillin-2 and of CD39 to caveolin-1 were performed using Coloc-2 Fiji-ImageJ plugin version 3.0. For specific analysis, different image channels (green and red) were overlaid in the same z-plane, generating yellow spots where the two molecules studied were present at the same pixel locations. Ten fields per condition per experiment were randomly chosen and measured by an investigator, who was blind to the experimental conditions. The point spread function (PSF) was calculated and set to 3.0, whereas randomizations were set to 10.0. The Pearson correlation coefficient was calculated as indicative of colocalization (Bolte and Cordelières, 2006; Adler and Parmryd, 2010; McCuaig et al., 2015; Schindelin et al., 2012).
Western blotting
Macrophages were lysed in ice-cold modified RIPA buffer (50 mM Tris-HCl pH 7.4; 1% NP-40; 0.25% sodium deoxycholate; 150 mM NaCl) supplemented with Complete Proteinase Inhibitor Cocktails (Roche Diagnostics) and Phosphatase Inhibitor Cocktails (Sigma-Aldrich, MO). The lysates were sonicated briefly on ice and centrifuged at 18,000 g for 10 min at 4°C. Protein concentrations were determined by Bio-Rad DC protein assay reagent (Bio-Rad Laboratories), using bovine serum albumin as the standard. Proteins (10 µg per lane) were boiled in XT Sample Buffer (cat. no. 161-0791, Bio-Rad Laboratories), separated using 4–12% Criterion XT Bis-Tris SDS-PAGE (Bio-Rad Laboratories) and transferred to PVDF membranes (cat. no. IPVH00010, Millipore) by semi-dry electroblotting. The latter were then probed with specific antibodies against proteins of interest. Bands were visualized using HRP-conjugated goat anti-mouse, donkey anti-rabbit or donkey anti-sheep IgG and the SuperSignal West Femto Maximum Sensitivity Substrate reagents applied (cat. no. PI-34096, Thermo Scientific) according to the manufacturer's instructions (Savio et al., 2017a).
Statistical analysis
Results are expressed as mean±standard error of mean (±s.e.m.). Statistical analysis was performed by one-way analysis of variance (ANOVA), followed by Tukey’s multiple range tests. Differences between groups were considered statistically significant when P<0.05.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: L.E.B.S., M.S.L., S.C.R., R.C.; Methodology: L.E.B.S., Y.W., R.C.S.; Software: L.E.B.S.; Validation: L.E.B.S.; Formal analysis: L.E.B.S., P.d.A.M., S.A.C.S.S., J.C.d.S., S.D.S.O., R.D.M., E.K., M.S.L.; Investigation: L.E.B.S., Y.W., M.S.L., S.C.R., R.C.S.; Resources: S.C.R., R.C.S.; Data curation: L.E.B.S., P.d.A.M., S.A.C.S.S., J.C.d.S., S.D.S.O.; Writing - original draft: L.E.B.S.; Writing - review & editing: L.E.B.S., P.d.A.M., S.D.S.O., R.D.M., E.K., M.S.L., S.C.R., R.C.S.; Supervision: S.C.R., R.C.S.; Project administration: R.C.; Funding acquisition: S.C.R., R.C.S.
Funding
This work was supported by funds from the Conselho Nacional de Desenvolvimento Científico e Tecnológico do Brasil (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), National Institutes of Health (NIH) (grant no. 5R01DK108894 to M.S.L. and grant no. R21CA221702 to S.C.R.) and U.S. Department of Defense (DOD) (grant no. W81XWH-16-0464 to S.C.R.). Deposited in PMC for release after 12 months.
References
- Abbracchio M. P., Burnstock G., Boeynaems J.-M., Barnard E. A., Boyer J. L., Kennedy C., Knight G. E., Fumagalli M., Gachet C., Jacobson K. A. et al. (2006). International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 58, 281-341. 10.1124/pr.58.3.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler J. and Parmryd I. (2010). Quantifying colocalization by correlation: the Pearson correlation coefficient is superior to the Mander's overlap coefficient. Cytometry A 77, 733-742. 10.1002/cyto.a.20896 [DOI] [PubMed] [Google Scholar]
- Allard B., Longhi M. S., Robson S. C. and Stagg J. (2017). The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol. Rev. 276, 121-144. 10.1111/imr.12528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai A., Moss A., Kokkotou E., Usheva A., Sun X., Cheifetz A., Zheng Y., Longhi M. S., Gao W., Wu Y. et al. (2014). CD39 and CD161 modulate Th17 responses in Crohn's disease. J. Immunol. 193, 3366-3377. 10.4049/jimmunol.1400346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberà-Cremades M., Baroja-Mazo A. and Pelegrín P. (2016). Purinergic signaling during macrophage differentiation results in M2 alternative activated macrophages. J. Leukoc. Biol. 99, 289-299. 10.1189/jlb.1A0514-267RR. [DOI] [PubMed] [Google Scholar]
- Bian S., Sun X., Bai A., Zhang C., Li L., Enjyoji K., Junger W. G., Robson S. C. and Wu Y. (2013). P2X7 integrates PI3K/AKT and AMPK-PRAS40-mTOR signaling pathways to mediate tumor cell death. PLoS One 8, 60184 10.1371/journal.pone.0060184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte S. and Cordelières F. P. (2006). A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213-232. 10.1111/j.1365-2818.2006.01706.x [DOI] [PubMed] [Google Scholar]
- Bradford M. D. and Soltoff S. P. (2002). P2X7 receptors activate protein kinase D and p42/p44 mitogen-activated protein kinase (MAPK) downstream of protein kinase C. Biochem. J. 366, 745-755. 10.1042/bj20020358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmin F., Mignot G., Bruchard M., Chevriaux A., Végran F., Hichami A., Ladoire S., Derangère V., Vincent J., Masson D. et al. (2012). Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity 36, 362-373. 10.1016/j.immuni.2011.12.019 [DOI] [PubMed] [Google Scholar]
- Chan K.-M., Delfert D. and Junger K. D. (1986). A direct colorimetric assay for Ca2+-stimulated ATPase activity. Anal. Biochem. 157, 375-380. 10.1016/0003-2697(86)90640-8 [DOI] [PubMed] [Google Scholar]
- Cognato G. P., Vuaden F. C., Savio L. E. B., Bellaver B., Casali E., Bogo M. R., Souza D. O. G., Sévigny J. and Bonan C. D. (2011). Nucleoside triphosphate diphosphohydrolases role in the pathophysiology of cognitive impairment induced by seizure in early age. Neuroscience 180, 191-200. 10.1016/j.neuroscience.2011.01.065 [DOI] [PubMed] [Google Scholar]
- Cohen H. B., Briggs K. T., Marino J. P., Ravid K., Robson S. C. and Mosser D. M. (2013). TLR stimulation initiates a CD39-based autoregulatory mechanism that limits macrophage inflammatory responses. Blood 122, 1935-1945. 10.1182/blood-2013-04-496216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz C. M., Rinna A., Forman H. J., Ventura A. L. M., Persechini P. M. and Ojcius D. M. (2007). ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J. Biol. Chem. 282, 2871-2879. 10.1074/jbc.M608083200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Andrade Mello P., Coutinho-Silva R. and Savio L. E. B. (2017a). Multifaceted effects of extracellular adenosine triphosphate and adenosine in the tumor-host interaction and therapeutic perspectives. Front. Immunol. 8, 1526 10.3389/fimmu.2017.01526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Andrade Mello P., Bian S., Savio L. E. B., Zhang H., Zhang J., Junger W., Wink M. R., Lenz G., Buffon A., Wu Y. et al. (2017b). Hyperthermia and associated changes in membrane fluidity potentiate P2X7 activation to promote tumor cell death. Oncotarget 8, 67254-67268. 10.18632/oncotarget.18595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Giorgi M., Enjyoji K., Jiang G., Csizmadia E., Mitsuhashi S., Gumina R. J., Smolenski R. T. and Robson S. C. (2017). Complete deletion of CD39 is atheroprotective in apolipoprotein E-deficient mice. J. Lipid Res. 58, 1292-1305. 10.1194/jlr.M072132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marchi E., Orioli E., Pegoraro A., Sangaletti S., Portararo P., Curti A., Colombo M. P., Di Virgilio F. and Adinolfi E. (2019). The P2X7 receptor modulates immune cells infiltration, ectonucleotidases expression and extracellular ATP levels in the tumor microenvironment. Oncogene 38, 3636-3650. 10.1038/s41388-019-0684-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D., Pizzirani C., Adinolfi E., Lemoli R. M., Curti A., Idzko M., Panther E. and Di Virgilio F. (2006). The P2X7 receptor: a key player in IL-1 processing and release. J. Immunol. 176, 3877-3883. 10.4049/jimmunol.176.7.3877 [DOI] [PubMed] [Google Scholar]
- Fessler M. B. and Parks J. S. (2011). Intracellular lipid flux and membrane microdomains as organizing principles in inflammatory cell signaling. J. Immunol. 187, 1529-1535. 10.4049/jimmunol.1100253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadharan V., Nohe A., Caplan J., Czymmek K. and Duncan R. L. (2015). Caveolin-1 regulates P2X7 receptor signaling in osteoblasts. Am. J. Physiol. Cell Physiol. 308, C41-C50. 10.1152/ajpcell.00037.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Marcos M., Pérez-Andrés E., Tandel S., Fontanils U., Kumps A., Kabré E., Gómez-Muñoz A., Marino A., Dehaye J.-P. and Pochet S. (2006). Coupling of two pools of P2X7 receptors to distinct intracellular signaling pathways in rat submandibular gland. J. Lipid Res. 47, 705-714. 10.1194/jlr.M500408-JLR200 [DOI] [PubMed] [Google Scholar]
- Gong T., Liu L., Jiang W. and Zhou R. (2019). DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 20, 95-112. 10.1038/s41577-019-0215-7 [DOI] [PubMed] [Google Scholar]
- Gonnord P., Delarasse C., Auger R., Benihoud K., Prigent M., Cuif M. H., Lamaze C. and Kanellopoulos J. M. (2009). Palmitoylation of the P2X7 receptor, an ATP-gated channel, controls its expression and association with lipid rafts. FASEB J. 23, 795-805. 10.1096/fj.08-114637 [DOI] [PubMed] [Google Scholar]
- Gordon S. and Plüddemann A. (2019). The mononuclear phagocytic system. generation of diversity. Front. Immunol. 10, 1893 10.3389/fimmu.2019.01893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinthal A. and Guidotti G. (2006). CD39, NTPDase 1, is attached to the plasma membrane by two transmembrane domains. Why? Purinergic Signal. 2, 391-398. 10.1007/s11302-005-5907-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziak K., Kaczmarek E., Kittel A., Sévigny J., Blusztajn J. K., Schulte Am Esch J. II, Imai M., Guckelberger O., Goepfert C., Qawi I. et al. (2000). Palmitoylation targets CD39/endothelial ATP diphosphohydrolase to caveolae. J. Biol. Chem. 275, 2057-2062. 10.1074/jbc.275.3.2057 [DOI] [PubMed] [Google Scholar]
- Lépine S., Le Stunff H., Lakatos B., Sulpice J. C. and Giraud F. (2006). ATP-induced apoptosis of thymocytes is mediated by activation of P2X7 receptor and involves de novo ceramide synthesis and mitochondria. Biochim. Biophys. Acta 1761, 73-82. 10.1016/j.bbalip.2005.10.001 [DOI] [PubMed] [Google Scholar]
- Lévesque S. A., Kukulski F., Enjyoji K., Robson S. C. and Sévigny J. (2010). NTPDase1 governs P2X7-dependent functions in murine macrophages. Eur. J. Immunol. 40, 1473-1485. 10.1002/eji.200939741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longhi M. S., Moss A., Jiang Z. G. and Robson S. C. (2017). Purinergic signaling during intestinal inflammation. J. Mol. Med. (Berl.) 95, 915-925. 10.1007/s00109-017-1545-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Xiao Y. and Li Z. (2011). P2X7 receptor positively regulates MyD88-dependent NF-κB activation. Cytokine 55, 229-236. 10.1016/j.cyto.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Lu D.-Y., Chen H.-C., Yang M.-S., Hsu Y.-M., Lin H.-J., Tang C.-H., Lee C.-H., Lai C.-K., Lin C.-J., Shyu W.-C. et al. (2012). Ceramide and Toll-like receptor 4 are mobilized into membrane rafts in response to Helicobacter pylori infection in gastric epithelial cells. Infect. Immun. 80, 1823-1833. 10.1128/IAI.05856-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCuaig R. D., Dunn J., Li J., Masch A., Knaute T., Schutkowski M., Zerweck J. and Rao S. (2015). PKC-Theta is a novel SC35 splicing factor regulator in response to T cell activation. Front. Immunol. 6, 562 10.3389/fimmu.2015.00562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell-Lagnado R. D. (2017). Regulation of P2X purinergic receptor signaling by cholesterol. Curr. Top. Membr. 80, 211-232. 10.1016/bs.ctm.2017.05.004 [DOI] [PubMed] [Google Scholar]
- Nagy E., Balogi Z., Gombos I., Akerfelt M., Björkbom A., Balogh G., Török Z., Maslyanko A., Fiszer-Kierzkowska A., Lisowska K. et al. (2007). Hyperfluidization-coupled membrane microdomain reorganization is linked to activation of the heat shock response in a murine melanoma cell line. Proc. Natl. Acad. Sci. USA 104, 7945-7950. 10.1073/pnas.0702557104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanikolaou A., Papafotika A., Murphy C., Papamarcaki T., Tsolas O., Drab M., Kurzchalia T. V., Kasper M. and Christoforidis S. (2005). Cholesterol-dependent lipid assemblies regulate the activity of the ecto-nucleotidase CD39. J. Biol. Chem. 280, 26406-26414. 10.1074/jbc.m413927200 [DOI] [PubMed] [Google Scholar]
- Płóciennikowska A., Hromada-Judycka A., Borzęcka K. and Kwiatkowska K. (2015). Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 72, 557-581. 10.1007/s00018-014-1762-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V. and Burnstock G. (1998). Receptors for purines and pyrimidines. Pharmacol. Rev. 50, 413-492. [PubMed] [Google Scholar]
- Savio L. E. B. and Coutinho-Silva R. (2019). Immunomodulatory effects of P2X7 receptor in intracellular parasite infections. Curr. Opin. Pharmacol. 47, 53-58. 10.1016/j.coph.2019.02.005 [DOI] [PubMed] [Google Scholar]
- Savio L. E. B. and Coutinho-Silva R. (2019). Immunomodulatory effects of P2X7 receptor in intracellular parasite infections. Curr. Opin Pharmacol. 7, 53-58. 10.1016/j.coph.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Savio L. E. B., de Andrade Mello P., Figliuolo V. R., de Avelar Almeida T. F., Santana P. T., Oliveira S. D. S., Silva C. L. M., Feldbrügge L., Csizmadia E., Minshall R. D. et al. (2017a). CD39 limits P2X7 receptor inflammatory signaling and attenuates sepsis-induced liver injury. J. Hepatol. 67, 716-726. 10.1016/j.jhep.2017.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savio L. E. B., Andrade M. G. J., de Andrade Mello P., Santana P. T., Moreira-Souza A. C. A., Kolling J., Longoni A., Feldbrügge L., Wu Y., Wyse A. T. S. et al. (2017b). P2X7 receptor signaling contributes to sepsis-associated brain dysfunction. Mol. Neurobiol. 54, 6459-6470. 10.1007/s12035-016-0168-9 [DOI] [PubMed] [Google Scholar]
- Savio L. E. B., de Andrade Mello P., da Silva C. G. and Coutinho-Silva R. (2018). The P2X7 receptor in inflammatory diseases: angel or demon? Front. Pharmacol. 9, 52 10.3389/fphar.2018.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676-682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper S. D., Debetto P. and Giusti P. (2010). The P2X7 purinergic receptor: from physiology to neurological disorders. FASEB J. 24, 337-345. 10.1096/fj.09-138883 [DOI] [PubMed] [Google Scholar]
- Takenaka M. C., Gabriely G., Rothhammer V., Mascanfroni I. D., Wheeler M. A., Chao C.-C., Gutiérrez-Vázquez C., Kenison J., Tjon E. C., Barroso A. et al. (2019). Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat. Neurosci. 22, 729-740. 10.1038/s41593-019-0370-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawadros P. S., Powers K. A., Ailenberg M., Birch S. E., Marshall J. C., Szaszi K., Kapus A. and Rotstein O. D. (2015). Oxidative stress increases surface toll-like receptor 4 expression in murine macrophages via ceramide generation. Shock 44, 157-165. 10.1097/SHK.0000000000000392 [DOI] [PubMed] [Google Scholar]
- Triantafilou M., Miyake K., Golenbock D. T. and Triantafilou K. (2002). Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J. Cell Sci. 115, 2603-2611. [DOI] [PubMed] [Google Scholar]
- Triantafilou M., Gamper F. G. J., Lepper P. M., Mouratis M. A., Schumann C., Harokopakis E., Schifferle R. E., Hajishengallis G. and Triantafilou K., (2007). Lipopolysaccharides from atherosclerosis-associated bacteria antagonize TLR4, induce formation of TLR2/1/CD36 complexes in lipid rafts and trigger TLR2-induced inflammatory responses in human vascular endothelial cells. Cell. Microbiol. 9, 2030-2039. 10.1111/j.1462-5822.2007.00935.x [DOI] [PubMed] [Google Scholar]
- Vieira F. S., Corrêa G., Einicker-Lamas M. and Coutinho-Silva R. (2010). Host-cell lipid rafts: a safe door for micro-organisms? Biol. Cell 102, 391-407. 10.1042/BC20090138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuaden F. C., Savio L. E. B., Bastos C. M. A., Bogo M. R. and Bonan C. D. (2011). Adenosine A2A receptor agonist (CGS-21680) prevents endotoxin-induced effects on nucleotidase activities in mouse lymphocytes. Eur. J. Pharmacol. 651, 212-217. 10.1016/j.ejphar.2010.11.003 [DOI] [PubMed] [Google Scholar]
- Vuerich M., Robson S. C. and Longhi M. S. (2019). Ectonucleotidases in intestinal and hepatic inflammation. Front. Immunol. 10, 507 10.3389/fimmu.2019.00507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F., Zhang J., Liu Y.-S., Li L. and He Y.-L. (2011). Research advances on flotillins. Virol. J. 8, 479 10.1186/1743-422X-8-479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H. (1996). Biochemistry, localization and functional roles of ecto-nucleotidases in the nervous system. Prog. Neurobiol. 49, 589-618. 10.1016/0301-0082(96)00026-3 [DOI] [PubMed] [Google Scholar]
- Zumerle S., Calì B., Munari F., Angioni R., Di Virgilio F., Molon B. and Viola A. (2019). Intercellular calcium signaling induced by ATP potentiates macrophage phagocytosis. Cell Rep. 27, 1-10.e4. 10.1016/j.celrep.2019.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]