Abstract
Purpose:
Patients with esophageal cancer treated with chemoradiation and surgery can develop pulmonary complications. Four-dimensional computed tomography–ventilation (4DCT-ventilation) is a developing imaging modality that uses 4DCT data to calculate lung ventilation. 4DCT-ventilation has been studied in the lung-cancer population but has yet to be extended to patients with esophageal cancer. The purpose of this study was to characterize 4DCT-ventilation–based spatial lung function for patients with esophageal cancer.
Methods and Materials:
Thirty-five patients with esophageal cancer who underwent 4DCT scans participated in the study. A 4DCT-ventilation map was calculated using the patient’s 4DCT imaging and a density change–based algorithm. To assess each patient’s ventilation profile, radiologist interpretations and quantitative metrics were used. A radiologist interpreted the 4DCT-ventilation images for lobar-based defects and gravity-dependent atelectasis. The 4DCT-ventilation maps were reduced to single metrics intended to reflect the degree of ventilation heterogeneity. The quantitative metrics included the coefficient of variation and metrics based on the ventilation in each lung and each lung third (superior-inferior ventilation [Vent-SI] and anteroposterior ventilation). The functional profile of patients with esophageal cancer was characterized and compared (using the Mann-Whitney test) for cohorts based on thoracic comorbidities and radiologist-identified defects.
Results:
Radiologist observations revealed that 26% of patients with esophageal cancer had lobar-based defects and 46% had gravity-dependent atelectasis. The baseline values were 0.52 ± 0.20 (mean ± SD), 11.2 ± 12.5, and 72.5 ± 14.6 for the coefficient of variation, the ventilation ratio of right to left lung, and Vent-SI metrics, respectively. The Vent-SI values were significantly different between patients with and without thoracic comorbidities (P = .05), and the anteroposterior ventilation metric was able to delineate patients with and without gravity-dependent atelectasis (P < .01).
Conclusions:
Our data demonstrate that approximately 30% of patients with esophageal cancer have significant ventilation heterogeneities. The current work uses radiologist observations and quantitative metrics to characterize 4DCT ventilation–based lung function for patients with esophageal cancer and presents data that can be used for future applications of 4DCT-ventilation to reduce thoracic toxicity for patients with esophageal cancer. © 2018 Elsevier Inc. All rights reserved.
Summary
Four-dimensional computed tomography (4DCT)–ventilation has been studied in the lung cancer population but has yet to be extended to patients with esophageal cancer. The purpose of this study was to characterize 4DCT-ventilation–based spatial lung function for patients with esophageal cancer. The study used radiologist observations and developed quantitative metrics to assess pretreatment spatial lung function profiles for patients with esophageal cancer. The study presents data that can be used for 4DCT-ventilation application aimed at reducing thoracic toxicity for patients with esophageal cancer.
Introduction
Four-dimensional computed tomography (4DCT)–based lung ventilation imaging is an imaging modality that uses 4DCT data to calculate a surrogate for lung ventilation. 4DCT imaging is generally acquired for patients with lung cancer during the simulation process and is used to aid with breathing-motion management during radiation treatment.1,2 Because 4DCT scans are acquired as part of standard of care for many patients with lung cancer, calculating 4DCT-ventilation images has the advantage of providing lung function information with no extra procedure required for the patient. 4DCT-ventilation research has progressed from work investigating calculation methodologies3-5 to studies validating the generated images against other forms of lung function imaging,6-10 including positron emission tomography,10 single-photon emission computed tomography,6,8 hyperpolarized He3 magnetic resonance imaging, and xenon-based computed tomography.11 The validation studies10 have reported correlation coefficients between 4DCT-ventilation and other forms of lung function imaging in the range of 0.2 to 0.8.
The 2 proposed clinical applications for 4DCT-ventilation in thoracic radiation therapy (RT) have been for functional avoidance and for imaging-based dose-response assessment. Functional-avoidance RT aims to design RT plans that avoid functional portions of the lung (as measured by 4DCT-ventilation), with the hypothesis that reducing the dose to functional regions of the lung can reduce treatment-related thoracic side effects.12-15 Dose-response assessment proposes to measure normal lung radiation changes due to treatment using 4DCT-ventilation functional imaging.16-18 Both functional avoidance and imaging-based dose-response assessment are currently being evaluated in ongoing prospective clinical trials for patients with lung cancer ( NCT02528942, NCT02308709, NCT02843568).
4DCT-ventilation has been researched and applied in the lung cancer population but has yet to be extended to patients with esophageal cancer. The purpose of this work was to explore a novel application of 4DCT-ventilation assessment in patients with esophageal cancer. Although clinical practice varies across centers, a portion of patients with esophageal cancer undergo 4DCT simulations to help contour the target and aid with breathing-motion management during treatment.19,20 In situations in which 4DCTs are acquired for patients with esophageal cancer, 4DCT-ventilation images can be generated for patients with esophageal cancer without requiring an extra imaging procedure.
There are several potential applications for 4DCT-ventilation in esophageal cancer patients. As in patients with lung cancer, thoracic toxicity is a significant limitation in the treatment of patients with esophageal cancer. The typical treatment regimen for advanced esophageal cancer is chemoradiation, followed by surgery. Studies have reported that for standard esophageal cancer treatment regimens of chemoradiation followed by surgery, thoracic complications can occur for 15% to 27% of patients.21-24 4DCT-ventilation can potentially be used for both functional avoidance and imaging-based lung assessment for patients with esophageal cancer to reduce and predict thoracic complications after treatment.
Before evaluating 4DCT-ventilation functional avoidance and dose-response applications for patients with esophageal cancer, studies are needed that characterize baseline spatial lung function. Although some studies have assessed baseline lung function for patients with lung cancer,25,26 few studies have assessed pretreatment imaging-based lung function for patients with esophageal cancer. The purpose of this study was, therefore, to quantitatively characterize pretreatment 4DCT ventilation–based spatial lung function for patients with esophageal cancer.
Methods and Materials
Study population
Data from 70 randomly selected patients with esophageal or lung cancer who were treated at our institution from 2010 to 2017 and had undergone 4DCT simulations were used for this study (study institutional review board approval number of BLINDED FOR REVIEW). Of the 70 patients used for the study, 35 were patients with esophageal cancer, and 35 had lung cancer. Because there is no precedent for evaluating spatial lung function for patients with esophageal cancer, our intention was to compare the 4DCT-ventilation data to a population whose lung function has been well studied. Therefore, in addition to the cohort of patients with esophageal cancer, the study used a cohort of 35 patients with lung cancer.
All 4DCT scans were acquired before radiation treatment as part of the treatment-planning process. The 4DCTs were performed on either a Siemens Somatom (Siemens Healthcare, Tarrytown, NY) or a Philips Brilliance Big Bore CT. A similar gated lung protocol was used for patients with both lung and esophageal cancer with 120 kVp, 3-mm slices, and variable mAs. No audio-visual coaching was used during the 4DCT, and no effort was made to use similar tidal volumes for the 4DCTs across different patients. However, the 4DCT breathing traces were reviewed for all patients to ensure consistent, nonerratic breathing.
The patient and clinical data broken down into esophageal and lung cancer cohorts are presented in Table 1. As expected, the lung cancer cohort had a larger percentage of patients (63%) with thoracic comorbidities (chronic obstructive pulmonary disease [COPD] and emphysema) than the esophageal patient cohort (23%). Using Fisher’s exact test, the rates of emphysema and COPD were significantly different (P < .01) between the esophageal and lung cancer cohorts. Although the rates of smokers and former smokers were higher for patients with lung cancer (86%) than for patients with esophageal cancer (69%), the differences were not significantly different (P = .15 using Fisher’s exact test). Most patients in both cohorts had advanced disease (≥stage 3). All patients were treated with standard doses per fraction (1.5-2 Gy per fraction), and no patients who were selected for the study were treated with stereotactic body radiation therapy.
Table 1.
Patient and clinical parameters for the patient population with esophageal and lung cancer used for this study
| Parameter | No. (%) |
|---|---|
| Lung cancer population | |
| No. of patients | 35 |
| Sex | |
| Female | 20 (57) |
| Male | 15 (43) |
| COPD/emphysema | |
| Yes | 22 (63) |
| No | 13 (37) |
| Smoking status | |
| Nonsmoker | 5 (14) |
| Current smoker | 6 (17) |
| Former smoker | 24 (69) |
| Histology | |
| NSCLC | 33 (94) |
| SCLC | 2 (6) |
| Stage | |
| IIB | 1 (3) |
| IIIA | 17 (49) |
| IIIB | 14 (40) |
| IV | 3 (8) |
| Treatment regimens | |
| 60 Gy (30 fr of 2 Gy) | 18 (51) |
| 50 Gy (25 fr of 2 Gy) | 7 (20) |
| 50.04 Gy (28 fr of 1.8 Gy) | 8 (23) |
| 45 Gy (30 fr of 1.5 Gy) | 2 (6) |
| Esophageal cancer population | |
| No. of patients | 35 |
| Sex | |
| Female | 9 (26) |
| Male | 26 (74) |
| COPD/emphysema | |
| Yes | 8 (23) |
| No | 27 (77) |
| Smoking status | |
| Nonsmoker | 11 (31) |
| Current smoker | 4 (12) |
| Former smoker | 20 (57) |
| Histology | |
| Adenocarcinoma | 29 (83) |
| Squamous cell carcinoma | 6 (17) |
| Stage | |
| IB | 3 (8.5) |
| IIA | 8 (23) |
| IIB | 2 (5.5) |
| IIIA | 8 (23) |
| IIIB | 8 (23) |
| IIIC | 3 (8.5) |
| IV | 3 (8.5) |
| Treatment regimens | |
| 60 Gy (30 fr of 2 Gy) | 1 (3) |
| 50 Gy (25 fr of 2 Gy) | 12 (34) |
| 50.4 Gy (28 fr of 1.8 Gy) | 15 (43) |
| 45 Gy (25 fr of 1.8 Gy) | 7 (20) |
Abbreviations: COPD = chronic obstructive pulmonary disease; fr = fraction; NSCLC = non-small cell lung cancer; SCLC = small cell lung cancer.
4DCT-ventilation image calculations
The Hounsfield unit (HU) calculation technique3,4 was applied to calculate 4DCT-ventilation images for each patient using the 4DCT data. We will briefly describe the algorithm used for the study. The first step was to perform deformable image registration to link lung voxel elements from the inhale-to-exhale phases of the 4DCT. The deformable registration algorithm used for the study had been previously validated for thoracic registrations with a spatial accuracy of 1.25 mm.27 Once the lung voxel elements were linked, a lung segmentation was performed to determine the ventilation calculation space by contouring the lungs and delineating out the main-stem bronchi and pulmonary vasculature. A density change–based algorithm3,4 was applied to calculate ventilation using the following equation:
| (1) |
where Vin and Vex are the inhale and exhale volumes, and HUin and HUex are the inhale and exhale phases of HU of the individual lung voxels, respectively.28 Equation 1 is applied on a voxel to voxel level to produce a 3-dimensional map of ventilation. 4DCT-ventilation images are presented as values calculated from Equation 1 multiplied by 100 (for display purposes).
4DCT-ventilation image heterogeneity assessments
To assess each patient’s spatial ventilation profile, radiologist observations and quantitative metrics were used. A radiologist interpreted the 4DCT-ventilation images and provided a binary yes–no score for 2 physiological phenomena: (1) lobar-based defects (resulting from the tumor or accompanying thoracic comorbidities) and (2) gravity-dependent atelectasis (a phenomenon where patients have reduced lung function in the posterior portions of the lungs because of being imaged in the supine position).29 The radiologist interpreted the 4DCT-ventilation images using MIM version 6.7 (Cleveland, OH).
For quantitative analysis, the 4DCT-ventilation maps were reduced to single metrics intended to reflect the degree of ventilation obstruction and heterogeneity. Four ventilation-based heterogeneity metrics were developed: coefficient of variation (CoV), superior–inferior ventilation (Vent-SI), right-to-left lung ventilation ratio (Vent-RL), and anterior–posterior ventilation (Vent-AP). The CoV is defined as the ratio of the SD over the mean and has been previously used to describe the heterogeneity of a ventilation image.10,30,25 The Vent-RL metric intends to calculate the average ventilation of each lung relative to the other lung. For patients with lung cancer, the Vent-RL metric has been previously calculated as the average ventilation in the ipsilateral lung over the average ventilation in the contralateral lung,30,25 with the idea that patients with lobar-based defects will present with an ipsilateral-to-contralateral ventilation ratio of <1 (presuming both the tumor and subsequent ventilation defect is in the ipsilateral lung). In contrast, patients with a homogeneous ventilation distribution will have an ipsilateral-to-contralateral ventilation ratio closer to unity. In the case of patients with esophageal cancer, it was not logical to use ipsilateral and contralateral lung designations; therefore, we used the following equation to calculate Vent-RL:
| (2) |
Equation 2, used to calculate the Vent-RL metric, presented a value that represented deviation from a uniform right-left ventilation distribution, with increasing values from zero representing an increasingly heterogeneous ventilation distribution.
The remaining 2 metrics (Vent-SI and Vent-AP) are based on the idea of dividing the lung into geometric thirds, which is currently a standard clinical assessment used to interpret nuclear medicine ventilation-perfusion scans.31 To calculate the Vent-SI metric, each lung was first divided into geometric superior-inferior thirds, and the average ventilation was calculated in each third. The Vent-SI metric was then calculated as the ratio of the minimum in any lung third over the average ventilation value of all lung thirds. The idea of the Vent-SI metric is that lobar-based defects are often represented as decreased ventilation values in superior–inferior thirds of the lung.31,32 The Vent-AP metric was similar in concept to the Vent-SI metric. The lungs were first divided into anterior–posterior thirds, and the Vent-AP metric was taken as the ratio of the minimum ventilation in any anterior–posterior third over the average of all thirds. The Vent-AP metric was created to characterize the gravity-dependent atelectasis phenomena, which presents as reduced ventilation values in the posterior portions of the lung.
Pretreatment spatial lung function of patients with esophageal cancer is presented as rates of the radiologist’s noted lobar-based defects and gravity-dependent atelectasis as well as baseline quantitative 4DCT-ventilation metrics (shown as the mean ± standard deviation [SD]). Three different comparisons were made using the radiologist interpretations and quantitative metrics. First, the esophageal cancer cohort was compared with the lung cancer cohort. To assess spatial lung function for patients with and without thoracic comorbidities, patients were divided into cohorts with and without clinically diagnosed COPD or emphysema. In addition, comparisons in quantitative 4DCT-ventilation metrics were made for patients with and without radiologist-identified lobar-based defects. Data comparing 4DCT-ventilation metrics relative to thoracic comorbidities and radiologist-identified lobar-based defects are presented for the esophageal patient cohort (N = 35) and the total patient cohort (patients with esophageal or lung cancer, N = 70). To assess the efficacy of the developed Vent-AP metric, patients with esophageal cancer with and without radiologist-defined gravity-dependent atelectasis were compared. All comparisons were tested for significance using the Mann-Whitney test.
Results
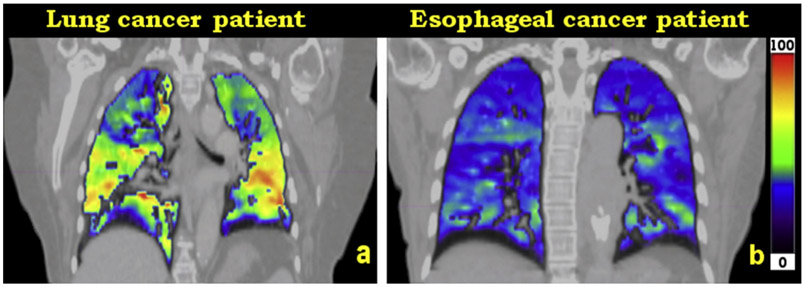
Radiologist observation revealed that 74% of patients with lung cancer had lobar-based defects, whereas 26% of patients with esophageal cancer had lobar-based ventilation defects. Figure 1 presents examples of image heterogeneity cases of 4DCT-ventilation images for a patient with esophageal cancer and a patient with lung cancer. Figure 1a presents a representative 4DCT-ventilation image of a patient with lung cancer who has an upper lobe defect in the right lung, and Figure 1b illustrates a representative 4DCT-ventilation image of a patient with esophageal cancer who had homogeneous lung function. The esophageal case presents with lower ventilation values when compared to the lung cancer patient (observed as an overall darker colormap for the patient with esophageal cancer). The decrease in the magnitude of the ventilation values is due to the breathing effort33 captured by the 4DCT scan for the representative patient with esophageal cancer compared with the patient with lung cancer in Figure 1.
Fig. 1.
Representative patient examples for a patient with lung cancer with a lobar-based defect (a) and a patient with esophageal cancer with homogeneous lung function (b). The 2 images use the same window-level ranges. The patient with esophageal cancer has decreased magnitude of ventilation values (because of a smaller magnitude of breath [lower breathing effort] captured during the 4-dimensional computed tomography scan) but presents with more homogeneous spatial lung function compared with the patient with lung cancer.
The baseline quantitative 4DCT-ventilation values for patients with esophageal cancer were 0.52 ± 0.20, 11.2 ± 12.5, and 72.5 ± 14.6 for the CoV, Vent-RL, and Vent-SI metrics, respectively (Table 2). By comparison, the CoV, Vent-RL, and Vent-SI metrics for the esophageal cohort all displayed values that indicated a more homogeneous lung function compared with patients with lung cancer; however, none of the differences between the 2 groups were statistically significant (P > .08 for all metrics). Table 3 provides a further breakdown of 4DCT-ventilation derived quantitative metrics for patients with and without clinically diagnosed COPD or emphysema. The data in Table 3 are provided for both the esophageal cohort alone (35 patients) and the entire 70-patient esophageal and lung cohort. The 4DCT ventilation–derived metrics present mixed results. For the esophageal cancer cohort, only the Vent-SI metric produced significant differences between the COPD and no-COPD group (P = .05), whereas for the total 70-patient cohort, only the CoV values were different between the 2 groups.
Table 2.
Four-dimensional computed tomography–ventilation heterogeneity metrics for the esophageal and lung cancer patient cohorts
| Ventilation metrics |
Esophageal cancer cohort Mean ± SD |
Lung cancer cohort Mean ± SD |
P value |
|---|---|---|---|
| CoV | 0.52 ± 0.20 | 0.55 ± 0.21 | .54 |
| Vent-RL (%) | 11.2 ± 12.5 | 17.5 ± 20.5 | .08 |
| Vent-SI (%) | 72.5 ± 14.6 | 69.1 ± 12.9 | .18 |
Abbreviations: CoV = coefficient of variation; Vent-RL = right-to-left lung ventilation ratio; Vent-SI = superior-inferior ventilation.
Table 3.
Four-dimensional computed tomography–ventilation heterogeneity metrics compared between patients with and without clinically diagnosed emphysema or COPD
| No emphysema/ COPD Mean ± SD |
Emphysema/ COPD Mean ± SD |
P value |
|
|---|---|---|---|
| Cohort of patients with esophageal cancer (N = 35) | |||
| CoV | 0.52 ± 0.22 | 0.53 ± 0.13 | .32 |
| Vent-RL (%) | 11.1 ± 13.6 | 11.3 ± 8.6 | .47 |
| Vent-SI (%) | 70.1 ± 15.3 | 80.6 ± 8.1 | .05 |
| Cohort of patients with esophageal + lung cancer (N = 70) | |||
| CoV | 0.52 ± 0.22 | 0.57 ± 0.17 | .05 |
| Vent-RL (%) | 15.2 ± 20.0 | 13.2 ± 12.6 | .43 |
| Vent-SI (%) | 68.8 ± 15.1 | 73.7 ± 11.4 | .17 |
Abbreviations: COPD = chronic obstructive pulmonary disease; CoV = coefficient of variation; Vent-RL = right-to-left lung ventilation ratio; Vent-SI = superior-inferior ventilation.
Table 4 presents 4DCT-ventilation quantitative metrics categorized according to whether patients had radiologist-defined lobar-based defects. For the esophageal cohort, the CoV metric was able to separate patients with and without lobar-based defects, but for the total 70-patient cohort, both the CoV and Vent-SI values were significantly different between the 2 groups.
Table 4.
Four-dimensional computed tomography–ventilation heterogeneity metrics compared between patients with and without radiologist-identified lobar-based defects
| No lobar- based defect |
Lobar-based defect present |
P value | |
|---|---|---|---|
| Cohort of patients with esophageal cancer (N = 35) | |||
| CoV | 0.45 ± 0.10 | 0.72 ± 0.30 | <.01 |
| Vent-RL (%) | 11.0 ± 11.2 | 12.0 ± 16.4 | .52 |
| Vent-SI (%) | 75.4 ± 12.0 | 64.2 ± 18.8 | .10 |
| Cohort of patients with esophageal + lung cancer (N = 70) | |||
| CoV | 0.46 ± 0.09 | 0.61 ± 0.25 | .04 |
| Vent-RL (%) | 13.0 ± 16.2 | 15.8 ± 18.3 | .65 |
| Vent-SI (%) | 74.2 ± 13.2 | 67.4 ± 13.7 | .02 |
Abbreviations: CoV = coefficient of variation; Vent-RL = right-to-left lung ventilation ratio; Vent-SI = superior-inferior ventilation.
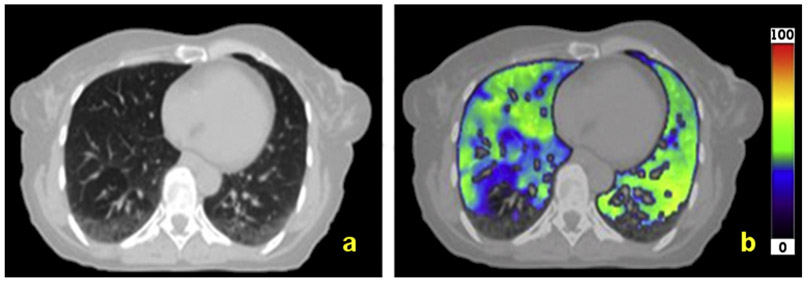
The esophageal cancer cohort had 46% of patients who were identified to have gravity-dependent atelectasis by the radiologist. Figure 2 presents an example of a patient with gravity-dependent atelectasis, as characterized by the decreased ventilation in the posterior portion of each lung. The Vent-AP values were significantly smaller (P < .01) for patients with gravity-dependent atelectasis (Vent-AP = 82.3 ± 20.7) compared with patients who were not identified to have gravity-dependent atelectasis (Vent-AP = 104.6 ± 24.1).
Fig. 2.
Representative computed tomography scan (a) and 4-dimensional computed tomography ventilation image (b) for a patient with gravity-dependent atelectasis.
Discussion
Before evaluating 4DCT-ventilation functional avoidance or dose-response applications for patients with esophageal cancer, studies are needed that characterize baseline spatial lung functional. Our data provide a first step in baseline characterization for assessment of spatial lung function. Clinically, both our data (Table 1) and data from previous studies22 demonstrate that patients with esophageal cancer generally have lower rates of thoracic comorbidities than patients with lung cancer.
Our data showed that 26% of patients with esophageal cancer had lobar-based defects and that the baseline values were 0.52, 11.2, and 72.5 for the CoV, Vent-RL, and Vent-SI metrics, respectively. The baseline lung function imaging data for patients with esophageal cancer echo the clinical findings: while patients with esophageal cancer tend to have more homogeneous lung function, the results can vary based on individual characteristics. For example, the radiologist observations noted that patients with esophageal cancer had a lower rate of lobar-based defects than patients with lung cancer. However, the quantitative 4DCT-ventilation metrics were not significantly different between the esophageal and lung cancer cohorts (Table 2). When the data were divided based on thoracic comorbidities and lobar-based defects, the quantitative 4DCT-ventilation metrics were better able to delineate the different groups: the Vent-SI metric was able to delineate patients with and without COPD (Table 3), and the CoV metrics was able to separate patients with and without lobar-based defects (Table 4). These results suggest that although patients with esophageal cancer have more homogeneous spatial lung function than patients with lung cancer, the data can be individualized according to whether patients have thoracic comorbidities or lobar-based defects. A reasonable comparison for the functional imaging profile of the esophageal cohort is patients with early-stage lung cancer25 who presented with 28% of patients having radiologist-noted lobar-based defects and average CoV values of 0.59.
Functional avoidance has been presented34 and is being investigated ( NCT02843568) for patients with early-stage lung cancer. Our results, which demonstrate that the spatial profiles of patients with esophageal cancer are similar to those of patients with early-stage lung cancer, provide a rationale for exploring functional avoidance for patients getting RT for esophageal tumors. The progression of the current work will incorporate functional planning for patients with esophageal cancer and evaluate how well the pretreatment 4DCT-ventilation metrics predict for the ability to spare functional portions of the lung.
One potential application of 4DCT-ventilation spatial data in patients with esophageal cancer is to monitor the response of normal lung tissue to treatment. In general, studies have used pulmonary function tests to assess and predict for thoracic toxicity in patients with esophageal cancer.35,36 Few studies have used functional imaging to assess lung function changes for patients with esophageal cancer. Two publications have evaluated the concept of using positron emission tomography–based functional imaging to assess for thoracic toxicity for patients with esophageal cancer.37,38 Hart et al37 demonstrated how pretreatment to posttreatment changes in standardized uptake value were predictive of clinical radiation pneumonitis in patients with esophageal cancer. Castillo et al38 showed that pretreatment standardized uptake value features predict for posttreatment symptomatic pneumonitis in patients with esophageal cancer. Of the 35 patients with esophageal cancer used for our study, 14 patients were considered evaluable for posttreatment toxicity (4 of whom had posttreatment thoracic complications). The 14-patient cohort was not considered sufficient to evaluate whether pretreatment 4DCT-ventilation metrics could predict for posttreatment toxicity. Future work will gather pretreatment and posttreatment functional imaging to evaluate whether pretreatment or serial-imaging features can predict for subsequent toxicity for patients with esophageal cancer.
Although the focus of this work was to assess a novel application of 4DCT-ventilation for patients with esophageal cancer, the data presented in our study can be useful in gleaning information for functional avoidance for patients with either esophageal or lung cancer. One important result from the current work is the characterization of patients with normal lung function. In functional avoidance, an important clinical consideration is whether a patient has a heterogeneous lung function profile conducive to designing radiation-treatment plans that avoid functional portions of the lung in favor of irradiating through less-functioning tissues.32 The idea is that for patients with homogenous lung function, there are no regions to preferentially spare, whereas heterogeneous ventilation distributions may be suitable for functional avoidance.
A particular challenge has been to identify clinical or quantitative measures of what constitutes homogeneous or “normal” lung function to help decide which patients would be good candidates for functional avoidance. Because 4DCT-ventilation was developed for radiation oncology applications, limited studies26 have evaluated 4DCT-ventilation images of patients with normal, nondiseased lung, and no studies have attempted to differentiate normal and abnormal 4DCT-ventilation-based lung function. The data presented in Table 2 for patients with esophageal cancer with no noted COPD or emphysema can theoretically be used to characterize quantitative metrics for patients with normal lung function because these patients do not have compromised lung function resulting from thoracic tumors or accompanying thoracic comorbidities. The CoV and Vent-SI values of 0.52 and 70.1%, respectively, for patients with esophageal cancer with no thoracic comorbidities can be used as a baseline to assess which patients may have normal lung function and therefore may not be good candidates for functional avoidance.
The other finding that has pertinence to functional avoidance (for patients with either esophageal or lung cancer) is the presence of gravity-dependent atelectasis. Gravity-dependent atelectasis is a known clinical phenomenon where a patient’s lung function is reduced in the posterior lower lobes of the lung as the patient undergoes imaging in the supine position. Gravity-dependent atelectasis can occur during the simulation and treatment phases of RT because patients are typically simulated and treated in the supine position. Functional avoidance aims to reduce the dose in functional portions of the lung in favor of irradiating through less-functional tissue. The potential significance of gravity-dependent atelectasis is that it results in regions of reduced lung function (that functional avoidance would increase the dose to), which may not be clinically significant but rather a transient effect29 resulting from the patient being imaged or treated in the supine position. Gravity-dependent atelectasis has been studied in other imaging modalities29,39 but has limited description using 4DCT-ventilation.
Nuclear medicine ventilation-perfusion studies have provided mixed results describing gravity-dependent atelectasis effects for ventilation and perfusion. Nyrén et al40 noted in anesthetized and mechanically ventilated healthy volunteers that regional lung ventilation did not differ with position, but perfusion was more uniform in the prone position compared with the supine position. Henderson et al41 noted that both ventilation and perfusion gravitational gradients were greater when patients were imaged in the supine position compared with the prone position. The mixed results suggest that an individualized approach is necessary to assess for gravitation effects. Our study developed a quantitative metric, Vent-AP, that was able to statistically differentiate (P < .01) between patients with and without gravity-dependent atelectasis. In the context of functional-avoidance planning, the Vent-AP metric can potentially provide quantitative data on whether a ventilation defect is due to gravity-dependent atelectasis or airway occlusion from the tumor.
Our study had several limitations. Our esophageal cancer cohort of 35 patients provided limited statistical power. Tables 3 and 4 demonstrate that as the cohort is expanded to include a combined 70 patients with esophageal cancer or lung cancer, the P values generally improve in separating patients with and without COPD and lobar-based defects. As with any imaging modality, 4DCT-ventilation is subject to uncertainties and image artifacts. The artifacts and uncertainties can be due to low-quality 4DCTs, spatial errors in deformable image registration,42,43 or nonlinear ventilation changes as a function of breathing effort.33,44 Methods for calculating and normalizing 4DCT-ventilation are still being further optimized.10,16,33,45,46 To reduce the potential uncertainties of the 4DCT-ventilation images, all 4DCTs and deformable registrations results were manually reviewed.
A limitation of the current work was that no functional imaging data were available that could be used to validate the findings from the 4DCT-ventilation imaging. Nuclear medicine planar ventilation-perfusion scans, which are the current clinical standard, are not typically acquired for patients with esophageal cancer and were therefore not available in the current retrospective cohort. Future prospective studies can collect multiple forms of lung-function imaging for patients with esophageal cancer to provide a validated assessment of baseline lung function. The quantitative metrics used in the current work were based on geometric methods intended to approximate lung anatomy. Defining lung division according to lung lobes rather than geometric delineations may improve the accuracy of the 4DCT-ventilation heterogeneity metrics. The choice to use metrics based on geometric thirds was made based on precedent from nuclear medicine31 and because the metrics could be calculated in an automated manner (which may be more suitable in busy clinical environments).
Another limitation of our study is that no follow-up 4DCT-ventilation imaging information was available. A prospective clinical trial collecting pre-RT and post-RT 4DCTs, and clinical toxicity data for patients with esophageal cancer, can provide valuable data to evaluate imaging-based changes and determine whether the imaging-based changes can be early predictors for clinical thoracic toxicity.
Conclusions
4DCT-ventilation imaging has been well studied for lung cancer applications but has yet to be extended to patients with esophageal cancer. Our study used radiologist observations and quantitative metrics to characterize pretreatment spatial lung function for patients with esophageal cancer. Our data showed lobar-based defects in 26% and gravity-dependent atelectasis in 46% of patients with esophageal cancer. The baseline values for patients with esophageal cancer were 0.52, 11.2, and 72.5 for the CoV, Vent-RL, and Vent-SI metrics, respectively. The CoV values were significantly different between patients with and without lobar-based defects, and the Vent-AP metric was able to delineate patients with and without gravity-dependent atelectasis. The current work is the first to characterize 4DCT-ventilation-based lung function for patients with esophageal cancer and presents data to aid with developing 4DCT-ventilation applications for patients with esophageal cancer.
Acknowledgments
Conflict of interest: This work was partially funded by grant R01CA200817 (Y.V., M.M., E.C., R.C., and T.G.).
References
- 1.Vedam SS, Keall PJ, Kini VR, et al. Acquiring a four-dimensional computed tomography dataset using an external respiratory signal. Phys Med Biol 2003;48:45–62. [DOI] [PubMed] [Google Scholar]
- 2.Keall PJ, Mageras GS, Balter JM, et al. The management of respiratory motion in radiation oncology report of AAPM task group 76a. Med Phys 2006;33:3874–3900. [DOI] [PubMed] [Google Scholar]
- 3.Simon BA. Non-invasive imaging of regional lung function using x-ray computed tomography. J Clin Monit Comput 2000;16:433–442. [DOI] [PubMed] [Google Scholar]
- 4.Guerrero T, Sanders K, Castillo E, et al. Dynamic ventilation imaging from four-dimensional computed tomography. Phys Med Biol 2006; 51:777–791. [DOI] [PubMed] [Google Scholar]
- 5.Du KF, Bayouth JE, Cao KL, et al. Reproducibility of registration-based measures of lung tissue expansion. Med Phys 2012;39:1595–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castillo R, Castillo E, McCurdy M, et al. Spatial correspondence of 4d ct ventilation and spect pulmonary perfusion defects in patients with malignant airway stenosis.Physics in Medicine and Biology 2012;57:1855–1871. [DOI] [PubMed] [Google Scholar]
- 7.Vinogradskiy Y, Koo PJ, Castillo R, et al. Comparison of 4-dimensional computed tomography ventilation with nuclear medicine ventilation-perfusion imaging: A clinical validation study. International Journal of Radiation Oncology Biology Physics 2014;89:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto T, Kabus S, Lorenz C, et al. Pulmonary ventilation imaging based on 4-dimensional computed tomography: Comparison with pulmonary function tests and SPECT ventilation images. Int J Radiat Oncol Biol Phys 2014;90:414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathew L, Wheatley A, Castillo R, et al. Hyperpolarized (3)He magnetic resonance imaging: Comparison with four-dimensional x-ray computed tomography imaging in lung cancer. Acad Radiol 2012; 19:1546–1553. [DOI] [PubMed] [Google Scholar]
- 10.Kipritidis J, Siva S, Hofman MS, et al. Validating and improving CT ventilation imaging by correlating with ventilation 4D-PET/CT using 68Ga-labeled nanoparticles. Med Phys 2014;41:011910. [DOI] [PubMed] [Google Scholar]
- 11.Ding K, Cao KL, Fuld MK, et al. Comparison of image registration based measures of regional lung ventilation from dynamic spiral CT with XE-CT. Med Phys 2012;39:5084–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vinogradskiy Y, Castillo R, Castillo E, et al. Use of 4-dimensional computed tomography-based ventilation imaging to correlate lung dose and function with clinical outcomes. International Journal of Radiation Oncology Biology Physics 2013;86:366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yaremko BP, Guerrero TM, Noyola-Martinez J, et al. Reduction of normal lung irradiation in locally advanced non–small-cell lung cancer patients, using ventilation images for functional avoidance. Int J Radiat Oncol Biol Phys 2007;68:562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto T, Kabus S, von Berg J, et al. Impact of four-dimensional computed tomography pulmonary ventilation imaging-based functional avoidance for lung cancer radiotherapy. Int J Radiat Oncol Biol Phys 2011;79:279–288. [DOI] [PubMed] [Google Scholar]
- 15.Ireland RH, Bragg CM, McJury M, et al. Feasibility of image registration and intensity-modulated radiotherapy planning with hyperpolarized helium-3 magnetic resonance imaging for non–small-cell lung cancer. Int J Radiat Oncol Biol Phys 2007;68: 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vinogradskiy YY, Castillo R, Castillo E, et al. Use of weekly 4dct-based ventilation maps to quantify changes in lung function for patients undergoing radiation therapy. Med Phys 2012;39:289–298. [DOI] [PubMed] [Google Scholar]
- 17.King MT, Maxim PG, Diehn M, et al. Analysis of long-term 4-dimensional computed tomography regional ventilation after radiation therapy. Int J Radiat Oncol Biol Phys 2015;92:683–690. [DOI] [PubMed] [Google Scholar]
- 18.Bayouth J, Du K, Christensen G, et al. Establishing a relationship between radiosensitivity of lung tissue and ventilation. Int J Radiat Oncol Biol Phys 2012;84:S31–S32. [Google Scholar]
- 19.Matzinger O, Gerber E, Bernstein Z, et al. EORTC-ROG expert opinion: Radiotherapy volume and treatment guidelines for neoadjuvant radiation of adenocarcinomas of the gastroesophageal junction and the stomach. Radiother Oncol 2009;92:164–175. [DOI] [PubMed] [Google Scholar]
- 20.Dieleman EMT, Senan S, Vincent A, et al. Four-dimensional computed tomographic analysis of esophageal mobility during normal respiration. Int J Radiat Oncol Biol Phys 2007;67:775–780. [DOI] [PubMed] [Google Scholar]
- 21.Asakura H, Hashimoto T, Zenda S, et al. Analysis of dose-volume histogram parameters for radiation pneumonitis after definitive concurrent chemoradiotherapy for esophageal cancer. Radiother Oncol 2010;95:240–244. [DOI] [PubMed] [Google Scholar]
- 22.Wang S-L, Liao Z, Vaporciyan AA, et al. Investigation of clinical and dosimetric factors associated with postoperative pulmonary complications in esophageal cancer patients treated with concurrent chemoradiotherapy followed by surgery. Int J Radiat Oncol Biol Phys 2006; 64:692–699. [DOI] [PubMed] [Google Scholar]
- 23.Lee HK, Vaporciyan AA, Cox JD, et al. Postoperative pulmonary complications after preoperative chemoradiation for esophageal carcinoma: Correlation with pulmonary dose-volume histogram parameters. Int J Radiat Oncol Biol Phys 2003;57:1317–1322. [DOI] [PubMed] [Google Scholar]
- 24.Tucker SL, Liu HH, Wang S, et al. Dose-volume modeling of the risk of postoperative pulmonary complications among esophageal cancer patients treated with concurrent chemoradiotherapy followed by surgery. Int J Radiat Oncol Biol Phys 2006;66:754–761. [DOI] [PubMed] [Google Scholar]
- 25.Vinogradskiy Y, Schubert L, Diot Q, et al. Regional lung function profiles of stage i and iii lung cancer patients: An evaluation for functional avoidance radiation therapy. Int J Radiat Oncol Biol Phys 2016;95:1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rankine LJ, Wang Z, Driehuys B, et al. The correlation of regional lung ventilation and gas transfer to red blood cells: Implications for functional-avoidance radiation therapy planning. Int J Radiat Oncol Biol Phys 2018;101:1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castillo E, Castillo R, White B, et al. Least median of squares filtering of locally optimal point matches for compressible flow image registration. Phys Med Biol 2012;57:4827–4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faught AM, Yamamoto T, Castillo R, et al. Evaluating which dose-function metrics are most critical for functional-guided radiation therapy. Int J Radiat Oncol Biol Phys 2017;99:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mata J, Altes T, Knake J, et al. Hyperpolarized 3He MR imaging of the lung: Effect of subject immobilization on the occurrence of ventilation defects. Acad Radiol 2008;15:260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brennan D, Schubert L, Diot Q, et al. Clinical validation of 4-dimensional computed tomography ventilation with pulmonary function test data. Int J Radiat Oncol Biol Phys 2015;92:423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parker JA, Coleman RE, Grady E, et al. SNM practice guideline for lung scintigraphy 4.0. J Nucl Med Technol 2012;40:57–65. [DOI] [PubMed] [Google Scholar]
- 32.Waxweiler T, Schubert L, Diot Q, et al. A complete 4DCT-ventilation functional avoidance virtual trial: Developing strategies for prospective clinical trials. J Appl Clin Med Phys 2017;18: 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du K, Reinhardt JM, Christensen GE, et al. Respiratory effort correction strategies to improve the reproducibility of lung expansion measurements. Med Phys 2013;40:123504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kadoya N, Cho SY, Kanai T, et al. Dosimetric impact of 4-dimensional computed tomography ventilation imaging-based functional treatment planning for stereotactic body radiation therapy with 3-dimensional conformal radiation therapy. Pract Radiat Oncol 2015; 5:e505–e512. [DOI] [PubMed] [Google Scholar]
- 35.Gergel TJ, Leichman L, Nava HR, et al. Effect of concurrent radiation therapy and chemotherapy on pulmonary function in patients with esophageal cancer: Dose-volume histogram analysis. Cancer J 2002;8:451–460. [DOI] [PubMed] [Google Scholar]
- 36.Kelsen DP, Minsky B, Smith M, et al. Preoperative therapy for esophageal cancer: A randomized comparison of chemotherapy versus radiation therapy. J Clin Oncol 1990;8:1352–1361. [DOI] [PubMed] [Google Scholar]
- 37.Hart JP, McCurdy MR, Ezhil M, et al. Radiation pneumonitis: Correlation of toxicity with pulmonary metabolic radiation response. Int J Radiat Oncol Biol Phys 2008;71:967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castillo R, Pham N, Castillo E, et al. Pre-radiation therapy fluorine 18 fluorodeoxyglucose pet helps identify patients with esophageal cancer at high risk for radiation pneumonitis. Radiology 2015;275:822–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morimoto S, Takeuchi N, Imanaka H, et al. Gravity-dependent atelectasis. Radiologic, physiologic and pathologic correlation in rabbits on high-frequency oscillation ventilation. Invest Radiol 1989;24:522–530. [PubMed] [Google Scholar]
- 40.Nyrén S, Radell P, Lindahl SGE, et al. Lung ventilation and perfusion in prone and supine postures with reference to anesthetized and mechanically ventilated healthy volunteers. Anesthesiology 2010;112: 682–687. [DOI] [PubMed] [Google Scholar]
- 41.Henderson AC, Sá RC, Theilmann RJ, et al. The gravitational distribution of ventilation-perfusion ratio is more uniform in prone than supine posture in the normal human lung. J Appl Physiol 2013;115: 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto T, Kabus S, von Berg J, et al. 4D-CT pulmonary ventilation image-guided radiotherapy planning is significantly influenced by deformable image registration algorithms and metrics. Int J Radiat Oncol Biol Phys 2010;78:S185. [Google Scholar]
- 43.Yamamoto T, Kabus S, Lorenz C, et al. 4D CT lung ventilation images are affected by the 4D CT sorting method. Med Phys 2013;40:10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mistry NN, Diwanji T, Shi X, et al. Evaluation of fractional regional ventilation using 4D-CT and effects of breathing maneuvers on ventilation. Int J Radiat Oncol Biol Phys 2013;87:825–831. [DOI] [PubMed] [Google Scholar]
- 45.Ding K, Bayouth JE, Buatti JM, et al. 4DCT-based measurement of changes in pulmonary function following a course of radiation therapy. Med Phys 2010;37:1261–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castillo E, Castillo R, Vinogradskiy Y, et al. The numerical stability of transformation-based CT ventilation. Int J Comput Assist Radiol Surg 2017;12:569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]