Abstract
JWH-015, a cannabinoid receptor 2 (CB2) agonist has tumor regressive property in various cancer types. However, the underlying mechanism by which it acts in lung cancer is still unknown. Tumor associated macrophage (TAM) intensity has positive correlation with tumor progression. Also, macrophages recruited at the tumor site promote tumor growth by enhancing epithelial to mesenchymal (EMT) progression. In this study, we analyzed the role of JWH-015 on EMT and macrophage infiltration by regulation of EGFR signaling. JWH-015 inhibited EMT in NSCLC cells A549 and also reversed the mesenchymal nature of CALU-1 cells by downregulation of EGFR signaling targets like ERK and STAT3. Also, in vitro co-culture experiments of A549 with M2 polarized macrophages provided evidence that JWH-015 decreased migratory and invasive abilities which was proved by reduced expression of FAK, VCAM1 and MMP2. Furthermore, it decreased macrophage induced EMT in A549 by attenuating the mesenchymal character by downregulating EGFR and its targets. These results were confirmed in an in vivo subcutaneous syngenic mouse model where JWH-015 blocks tumor growth and also inhibits macrophage recruitment and EMT at the tumor site which was regulated by EGFR pathway. Finally, JWH-015 reduced metastatic lesions in a tail vein syngenic mouse model. These data confer the impact of this cannabinoid on anti-proliferative and anti-tumorigenic effects, thus enhancing our understanding of its therapeutic efficacy in NSCLC. Our findings open new avenues for cannabinoid receptor CB2 agonist- JWH-015 as a novel and potential therapeutic target based on EGFR downregulation mechanisms in NSCLC.
Keywords: NSCLC, JWH-015, EGFR, EMT, TAM
INTRODUCTION
Lung cancer is one of the major causes of cancer related deaths worldwide. Non-small cell lung cancer (NSCLC), the most common form of lung cancer, contributes to about 85% of cases with possibility of advanced stages in two-thirds of NSCLC patients. Adenocarcinoma and squamous cell carcinoma, the two most common histological subtypes of lung cancer are categorized as NSCLC. Tumor heterogeneity in NSCLC leads to poor patient outcome and drug resistance complications [1-2].
The genetic abnormalities associated with lung cancer are attributed to alterations in the signaling pathways which are targets for drug therapies. Most of these stimulatory signaling pathways are driven to malignant phenotype characterized by uncontrolled proliferation and apoptosis escape mechanism. Epidermal growth factor receptor (EGFR) is a member of family of four Receptor tyrosine kinases (RTKs) EGFR (ERBB1, HER1), ERBB2 (HER2, Neu), ERBB3 (HER3) and ERBB4 (HER4) [3]. EGFR is frequently overexpressed or mutated in NSCLC and was the first mutation identified to be more aberrated in non-smoking lung cancer patients. Hence, EGFR has been considered as a predicted biomarker in NSCLC patients which led to rise of several TKI (Tyrosine Kinase Inhibitors) [4]. Targeting EGFR would lead to better treatment of lung cancer in future.
Epithelial to Mesenchymal Transition (EMT) is a dynamic, significant event in cancer progression. EMT is correlated with malignant properties like migration, invasion, evasion of apoptosis, stemness, metastasis, etc. in vitro and in vivo. E-cadherin dysfunction contributes to poor patient prognosis in lung cancer. Also, EMT markers have been clinically associated with pathology of NSCLC [5]. EGFR is frequently attributed to dysfunction in tumors of epithelial origin, leading to EMT like features. In NSCLC cell line A549, EGF stimulates EMT by inducing a morphological change, downregulation of epithelial marker E-cadherin and upregulation of mesenchymal markers N-cadherin, fibronectin and vimentin. Loss of E-cadherin leads to disruption of cell-cell junctions, thus making the cells more motile and migratory. MMPs (matrix metalloproteinases) are secreted which degrade the ECM (extra-cellular matrix), thus allowing the cells to become more invasive, ultimately leading to metastasis to distant organs [6-7]. Reports suggest that EMT can lead to acquired resistance to conventional EGFR-TKI chemotherapeutic drugs, thus increasing the difficulty in lung cancer treatment.
The tumor microenvironment (TME) comprises of variety of cell types like cancer associated fibroblasts (CAF), natural killer (NK) cells, tumor associated macrophages (TAM), myeloid derived suppressor cells (MDSC), endothelial cells, etc. These infiltrated cells secrete various factors which play crucial role in cancer progression, EMT and metastasis. Emerging targets focus on the interplay between cancer cells and their microenvironment [8-9]. Macrophages are involved in regulation of tissue homeostasis, inflammation and are associated with several pathological diseases. TAM density is inversely correlated with patient prognosis in various cancer types. Also, TAMs have been related to angiogenesis, invasion, metastasis and immune modulation in different carcinomas [10]. Reports suggest that M2 macrophages induce EMT by regulating TLR4/IL-10 signaling in pancreatic cancer cells [11]. In hepatoma cells, activated macrophages enhance the migratory and invasive properties, leading to decreased E-cadherin levels [12]. Also, macrophages secreted activators for EGFR and STAT3 which enhanced the invasiveness in breast tumors [13]. These confirm a strong crosstalk between macrophages and tumor progression, mainly through stimulation of EMT.
The cannabinoid family is categorized into endogenous, synthetic and phytocannabinoids which activate specific G-protein coupled receptors- CB1 and CB2. CB1 is mainly expressed in the brain and CNS whereas CB2 is expressed in immune system. The use of cannabinoid agonists as anti-cancer agents has proven successful in various in vitro and in vivo cancer models such as glioma, breast, prostate, colon, leukemia and lymphoid tumors. They have been shown to modulate various cell survival pathways such as ERK, PI3K, p38 MAPK, AKT and ceramide pathways [14]. JWH-015 is a synthetic CB2 agonist which possesses anti-proliferative and anti-invasive effects in various cancer types [15-17]. Although JWH-015 is involved in modulating various signaling pathways, not much is known about how it regulates EGFR signaling in NSCLC.
In our present study, we show that JWH-015 has anti-migratory and anti-invasive effects in NSCLC cells. Also, we hypothesize that JWH-015 inhibits recruitment of macrophages to the tumor site by inhibiting EMT. This may be possible through downregulation of EGFR signaling by JWH-015 at the tumor site which might be activated by EGF secreted by the macrophages. We prove our hypothesis by performing in vitro co-culture experiments as well as in vivo. This study shall pave the way for novel target affecting EGFR pathway, which might be useful in NSCLC, considering the resistance of this cancer to various EGFR targeting drugs.
MATERIALS AND METHODS
Cell culture
Human NSCLC- A549 cells (ATCC) were cultured in DMEM (Corning Cellgro). Murine lung cancer ED1 cells (kindly provided by Dr. Ethan Dmitrovsky), CALU1 and THP1 cells (ATCC) were cultured in RPMI-1640 (Corning Cellgro). The media were supplemented with 10% FBS (Corning Cellgro) and 1% penicillin/ streptomycin (Corning Cellgro).
Reagents and antibodies
JWH-015 and SR144528 were purchased from Tocris Bioscience. Antibodies used were P-AKT, E-cadherin (Cell Signaling), P-ERK, ERK, AKT, GAPDH, P-EGFR, EGFR, VCAM-1, STAT3 (Santa Cruz), N-cadherin, P-FAK (Abcam), P-STAT3 (BD Biosciences).
Real Time Reverse Transcription PCR
RNA was isolated from tumors using TriZol (Invitrogen). RT-PCR was performed as described earlier [18].
Immunochemical (IHC) analyses
MTT assay
Cells were seeded at a density of 5000 cells per well in 96 well plates and allowed to grow for 24h. Briefly, cells were treated for 48h and cell viability was measured using the MTT assay (Roche) as described in the supplier’s protocols, based on the absorbance reading at 570nm with respect to the control.
Chemotaxis assays
For migration assay, 8μm transwell plates (Corning-Costar) were used. Briefly, cells were seeded in upper chamber and EGF (100ng/ml) or conditioned media from M2 macrophages were added to lower chambers [21,23]. 12 hours later, cells that migrated to lower chamber were fixed, stained using Hema stain and counted. For invasion assay, pre-coated Matrigel invasion chambers (BD Falcon) were used. After 24 hours of stimulation similar to migration assay, invaded cells were stained and counted. [21,24].
Immunofluorescence
Cells were seeded in 8 well chamber slides, treated, fixed and incubated with primary antibodies overnight at 4°C. After washing, cells were stained with Alexa Fluor- 488 or 594 conjugated secondary IgG antibodies and visualized under Olympus FV1000 Filter confocal microscope.
Western blotting
Cells were washed, lysed and protein estimation was performed using Bradford assay. Aliquots of cellular lysates (50μg) were electrophoresed on Novex SDS-PAGE, transferred to nitrocellulose membrane and blocked with 5% non-fat dry milk for an hour at room temperature. The membranes were probed overnight with specific primary antibody (1:1000) overnight at 4°C. After washing thrice with 1X TBST, blots were exposed to secondary antibody (1:2000) for an hour, washed thrice and detected using ECL chemiluminescence (Thermo Scientific).
Mouse xenograft model
EDI cells (3×106) in 100μl PBS were injected subcutaneously into the left flank of each syngenic 7 week old male FVB mouse. Once the tumors reached palpable size, they were treated with JWH-015 (7.5mg/kg) (n=5), for 3 weeks and sacrificed.
in vivo tumorigenicity assay
ED1 cells (1×106) in 100μl PBS were injected by tail vein in syngenic 7 week old male FVB mice. After a week of injection, mice were treated with ethanol control or JWH-015 (7.5mg/kg) (n=5), intraperitoneally for 3 weeks. After treatment, lungs were isolated and surface metastatic lesions were counted.
Flow cytometry
For FACS, single cell suspensions from tumor infiltrating cells were blocked with 1% FBS in PBS and incubated with anti-F4/80 PE, anti-CD11b APC, and anti-CD206 Alexa Fluor 488 for 1h. Then, the cells were washed and analyzed with FACS Caliber using CellQuest software (BD Biosciences).
Statistical analysis
Results were represented as mean ± SD which were analyzed using Student’s two-tailed t test. A value of P<0.05 was considered to be statistically significant.
RESULTS
JWH-015 inhibirs EGF induced proliferation in NSCLC cells
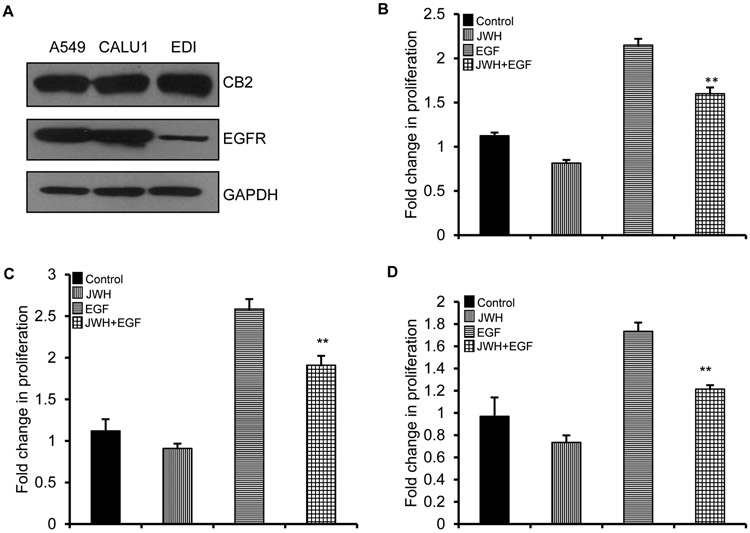
Previously, we have shown that JWH-015 exerts its effects by activating CB2 receptor in breast and lung cancer cells [15,17]. To understand the function of this ligand and how activation of CB2 affects EGFR pathway, we initially checked for the expression of CB2 and EGFR in lung cancer cell lines- A549 (epithelial cells), CALU-1 (mesenchymal cells) and ED1 (murine cells). Both the receptors were expressed in these cells (Fig. 1A). Also, JWH-015 significantly inhibited EGF induced proliferation in these cell lines (Fig. 1B, C, D).
Figure 1. JWH-015 inhibits EGF induced proliferation in NSCLC cells.
(A) NSCLC cell lines- A549, CALU1 and ED1 were subjected to immunoblot to determine expression of CB2 and EGFR. GAPDH is loading control. A549 (B), CALU1 (C) and EDI (D) cells were serum starved and treated with control, JWH-015 (5μM) in absence or presence of EGF (100ng/ml) for 48h and analyzed for viability by MTT assay. P<0.01 (**) as calculated by Student’s t test. Data represent mean ± SD for each experiment repeated three times.
JWH-015 inhibits EGF induced EMT in A549 cells
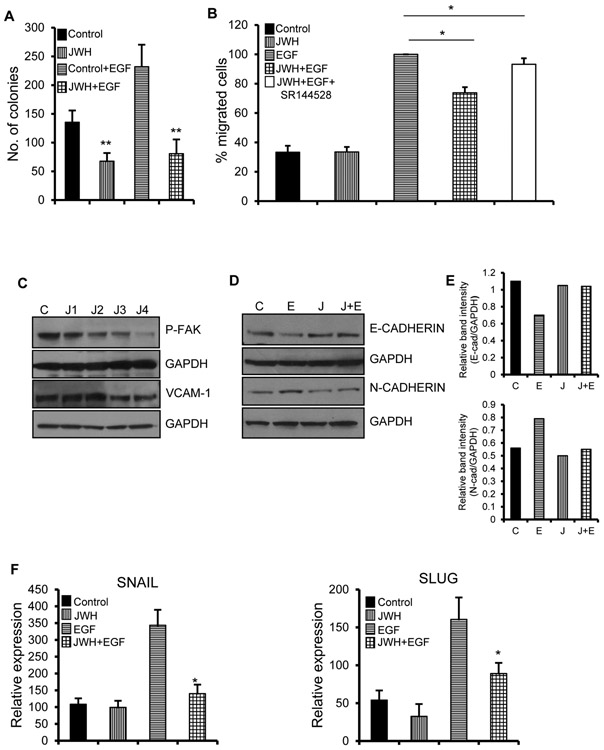
To study the effect of JWH-015 on EGF mediated proliferation, we performed colony formation assay, the ability of single cell to form clones. JWH-015 potently decreased the number of colonies in absence as well as presence of EGF in A549 cells (Fig. 2A). Cell migration is initiated by protrusion into the dense ECM and moving through this membrane with the help of actin rich structures in cell membranes. This movement is highly enhanced by secretion of chemoattractants like EGF which activate cancer cells [25]. JWH-015 inhibited EGF directed cell migration in A549 as shown by migration assay (Fig. 2B). This activity of JWH-015 was reverted when CB2 antagonist- SR144528 was used, proving the direct implication of CB2 receptor in this growth inhibitory effect.
Figure 2. JWH-015 inhibits EGF induced EMT in A549 cells.
(A) 1000 individual A549 cells were subjected to colony formation assay by treating with control or JWH-015 (5μM) for six days. Colonies were stained and counted. (B) A549 cells were treated with control or JWH-015 (5μM) for 48h and subjected to EGF (100ng/ml)-induced migration in combination with CB2 antagonist- SR144528. Number of cells migrated were stained and counted. (C) A549 cells were pre-treated with control (C) or JWH-015 at 1, 2, 3 and 4μM (J1, J2, J3, J4) for 48h and subjected to Immunoblot to determine expression of invasive markers like P-FAK and VCAM-1. A549 cells were pre-treated with control or JWH-015 (2.5μM) for 24h, stimulated with EGF (100ng/ml) for 48h and subjected to Western blot (D) with quantification (E) or Real time PCR (F) to determine expression of EMT markers. Control (C), JWH-015 (J), EGF (E), JWH-015+EGF (J+E). P<0.05 (*) and P<0.01 (**) as calculated by Student’s t test. Data represent mean ± SD for each experiment repeated three times.
Cancer progression begins with dissemination of tumor cells from the extra-cellular matrix (ECM) into other distant sites. The initial event involves detachment of cells due to regulation of cell adhesion molecules (CAM) like cadherins, integrins, selectins and immunoglobulin superfamily [26]. To test how JWH-015 affects the ability of cancer cells to detach from the tumor site and become more motile, we treated A549 cells with JWH-015 for 48h and checked for markers involved in ECM regulation like FAK, VCAM1 and MMP2. Focal adhesion kinase (FAK) is a tyrosine kinase that is activated by ECM-integrin binding. Activated FAK is involved in various cellular functions involved in tumorigenesis like cell adhesion, migration, invasion and evasion of apoptosis [26-27]. Vascular cell adhesion molecule-1 (VCAM-1), an adhesion molecule that belongs to the immunoglobulin superfamily promotes adhesion of tumor cells to vascular endothelial cells which ultimately leads to tumor angiogenesis and metastasis [28]. JWH-015 significantly inhibited p-FAK and VCAM-1 (Fig. 2C).
A549 is a human lung adenocarcinoma cell line with epithelial characteristics. It has been shown that the ligand EGF induced EMT in A549, thus converting it from epithelial to fibroblastic, mesenchymal phenotype [29]. EMT is marked by downregulation of epithelial markers like E-cadherin and upregulation of mesenchymal markers like N-cadherin, Snail, Slug and Vimentin. Also, loss of E-cadherin leads to loss of adherent junctions that exist between cells. The mesenchymal property helps the cells disperse and invade the basement membrane, thus becoming more migratory [8].
To study the effect of JWH-015 on EMT progression, we induced A549 cells with EGF to stimulate EMT in the presence or absence of JWH-015 and analyzed various EMT markers. As expected, EGF induced EMT by upregulation and downregulation of mesenchymal and epithelial markers respectively. Also, JWH-015 inhibited EGF induced EMT by reversing the EMT process. We observed decrease in mesenchymal markers like N-cadherin, Snail, Slug and increase in epithelial markers like E-cadherin (Fig. 2D, E, F). These results show that JWH-015 inhibits EMT by downregulation of EGFR pathway in A549 cells.
JWH-015 promotes mesenchymal to epithelial transition in CALU-1 cells
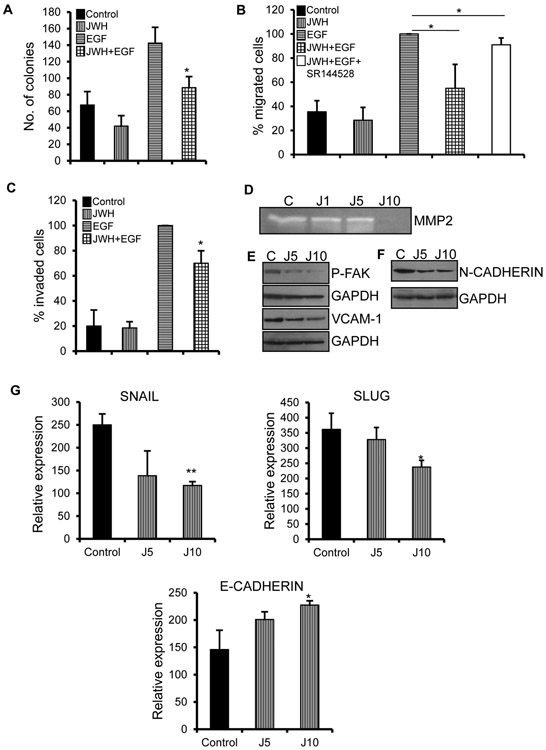
CALU-1 is a human lung adenocarcinoma cell line with fibroblastic, spindle shaped morphology, characteristic of mesenchymal property [30]. We further investigated the role of JWH-015 on EMT by assessing whether it can revert the mesenchymal phenotype to epithelial morphology. We initially checked for the effect of JWH-015 on proliferation of cells. JWH-015 inhibited EGF induced proliferation as shown by colony formation assay (Fig. 3A).
Figure 3. JWH-015 promotes mesenchymal to epithelial transition in CALU-1 cells.
(A) 1000 individual CALU1 cells were subjected to colony formation assay by treating with control or JWH-015 (5μM) for six days. Colonies were stained and counted. CALU1 cells were treated with control or JWH-015 (5μM) for 48h and subjected to EGF (100ng/ml)-induced migration in combination with CB2 antagonist- SR144528 (B) and invasion (C). Number of cells migrated or invaded were stained and counted. (D) CALU1 cells were treated with control or JWH-015 at 1, 5 and 10μM (J1, J5, J10) for 48h and supernatants were concentrated and run on zymogram gels. (E) CALU1 cells were pre-treated with control or JWH-015 at concentrations of 5 and 10μM (J5, J10) for 48h and subjected to Immunoblot to determine expression of P-FAK and VCAM-1. GAPDH is loading control. CALU1 cells were pre-treated with control or JWH-015 at 5 and 10μM (J5, J10) for 48h and subjected to Real time PCR (F) or Immunoblot (G) to determine expression of EMT markers. P<0.05 (*) and P<0.01 (**) as calculated by Student’s t test. Data represent mean ± SD for each experiment repeated three times.
A crucial step in EMT progression is the ability of cells to elongate, migrate and invade the ECM barrier by degradation of various enzymes secreted by the membrane. This migratory-invasive feature is the hallmark for metastasis. JWH-015 potently inhibited EGF induced migration (Fig. 3B) and invasion (Fig. 3C) in CALU-1 cells. The effects of JWH-015 in EGF induced migration were abrogated in the presence of CB2 antagonist- SR144528, thus showing CB2 dependent inhibitory effects. Also, we observed reduced secretion of MMP-2 in the presence of JWH-015 as shown by zymogram (Fig. 3D). MMPs are matrix metalloproteinases involved in ECM degradation. In early stage lung adenocarcinoma, higher expression of MMP-2 is involved in worse prognosis [31]. These results were strengthened by inhibition of other invasive markers like FAK and VCAM-1 (Fig. 3E).
Since we are interested in regulation of EGF induced EMT by JWH-015, we studied the signaling molecules involved in EGFR pathway. Activation of this pathway leads to onset of various events like cancer proliferation, migration, invasion and survival. Extra-cellular regulated kinase (ERK) is important downstream target of EGFR [32]. We treated CALU-1 cells with JWH-015 for 24h and then stimulated the cells with EGF at various time points to check the activation of EGFR targets. We found that JWH-015 decreased the phosphorylation of EGFR and ERK (Fig. S1).
To understand the molecular mechanism related to MET reversion, we treated CALU-1 cells with JWH-015 and checked for various EMT markers. We observed that JWH-015 inhibits and reverses the mesenchymal property of this cell line by increasing expression of epithelial markers like E-cadherin and decreasing mesenchymal markers like N-cadherin, Snail and Slug (Fig. 3F, G). These results show that JWH-015 attenuates the mesenchymal phenotype of CALU-1 cells.
JWH-015 inhibits M2 macrophage induced EMT in A549 cells
Emerging therapies focus on the tumor microenvironment (TME) as a vital component of cancer progression and metastasis. TME is composed of different kinds of cell types which penetrate into the tumor site and influence changes in ECM by secreting or recruiting tumorigenic factors [8,33]. Macrophages, which reside in most tissues play important role in tumor-stromal interactions. TAMs are a unique population in TME that foster tumorigenesis and metastasis by secreting factors that increase invasion, alter ECM composition, cause immune suppression, imbalance homeostasis, etc. [11,34].
Macrophage activation can be classified into two types- M1 (classical) and M2 (alternative). LPS, IFN-γ trigger macrophages into M1 state, whereas, IL-4, IL-10 trigger macrophages into M2 state. M1 macrophages defend the host against infections and act as tumor suppressors. M2 macrophages suppress M1 mediated functions and promote cancer progression and angiogenesis [10]. Also, macrophage derived growth factors and cytokines alter the composition of tumor population, exhibiting a strong paracrine loop. Reports suggest that macrophage activation and secretion of factors into the tumor site enhances migration, invasiveness, promoting EMT of cancer cells [11-12].
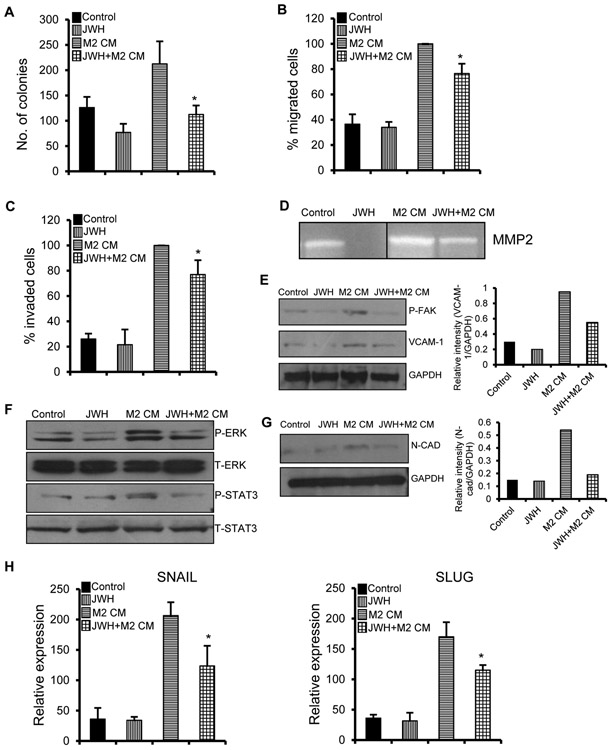
To investigate the effect of macrophage activation and secretion of factors that affect the tumor progression, we performed indirect co-culture assays with human monocyte cell line THP1 and lung adenocarcinoma cell line A549 [33]. We stimulated THP1 cells with PMA together with IL-4 for 24h before indirectly co-culturing with A549 cells. IL-4 induced THP1 cells exibit M2 property (tumor inducing macrophages) which was verified by higher expression of M2 marker Arginase-I (Fig. S2). Co-culture of conditioned media (CM) of M2 macrophages with A549 cells enhanced proliferative (Fig. 4A) and migratory (Fig. 4B, 4C) abilities of A549 cells which were inhibited by treatment of A549 with JWH-015. Also, M2 macrophages increased the invasiveness of A549 which was shown by higher levels of invasive markers like MMP2, pFAK and VCAM-1. These markers were significantly decreased when A549 cells were pre-treated with JWH-015 before co-culture with M2 macrophages (Fig. 4D, 4E).
Figure 4. JWH-015 inhibits M2 macrophage induced EMT in A549 cells.
(A) 1000 individual A549 cells were subjected to colony formation assay by treating with control or JWH-015 (5μM) in the presence of M2 CM for six days. Colonies were stained and counted. A549 cells were treated with control or JWH-015 (5μM) for 48h and subjected to M2-polarized TAM CM-induced migration (B) and invasion (C). Number of cells migrated or invaded were stained and counted. (D) A549 cells were treated with control or JWH-015 (5μM) for 24h in the presence of M2 CM, then conditioned media was replaced by fresh media for another 48h and the supernatants were concentrated and run on zymogram gels. A549 cells were pre-treated with control or JWH-015 (5μM) for 24h, stimulated with M2 CM for 48h and subjected to Immunoblot to determine expression of P-FAK and VCAM-1 (left panel) with quantification (right panel) (E), EGFR signaling pathway like P-STAT3, P-ERK (F) and EMT markers (left panel) with quantification (right panel) (G) and also subjected to Real time PCR (H). GAPDH is loading control. JWH-015 (JWH), M2-polarized TAM CM (M2 CM). P<0.05 (*) as calculated by Student’s t test. Data represent mean ± SD for each experiment repeated three times.
We hypothesize that macrophages secrete factors that activate the EGFR pathway, which promote tumorigenesis of cancer cells by stimulating EMT process. To test our idea, we performed co-culture experiments of M2 macrophages with A549 cells and checked for EGFR targets. We observed that M2 macrophages activated pSTAT3, pERK in A549 cells which were inhibited by treatment of A549 with JWH-015 (Fig. 4F). These data show that macrophages secrete ligands/factors that stimulate EGFR signaling in lung cancer cells that is markedly inhibited by JWH-015 treatment.
To further validate our data, we checked for EMT markers in the M2-macrophage-A549 co-culture experiment. M2 macrophages promoted EMT in A549 cells which was attenuated by JWH-015. This was proved by downregulation of mesenchymal markers like Snail, Slug and N-cadherin after JWH-015 treatment (Fig. 4G, H). Hence, M2 macrophages activated EGFR pathway, thus inducing EMT in A549 cells which was inhibited by JWH-015.
JWH-015 inhibits NSCLC tumor growth in vivo in a subcutaneous mouse model
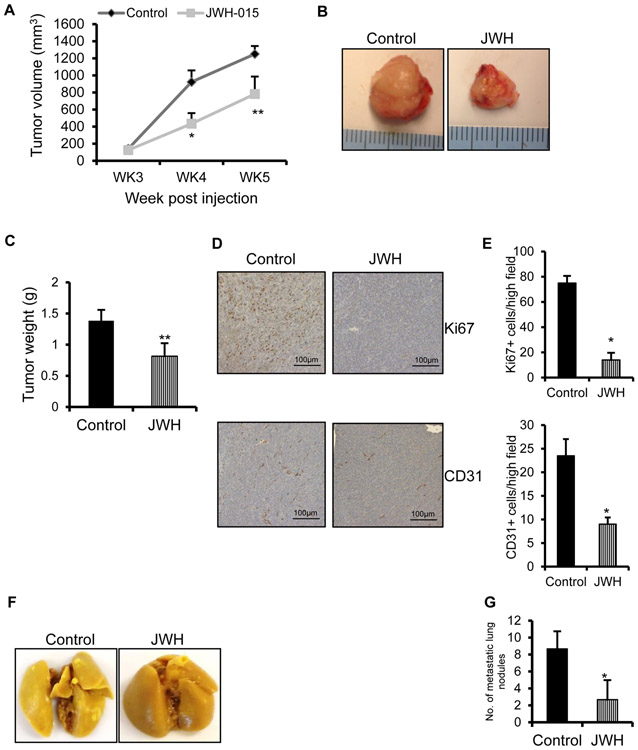
To evaluate the tumor suppressive effects of JWH-015, we used ED1 cells, syngenic with FVB mice. To confirm whether JWH-015 exerts tumor suppressive effects in this cell line, we performed EGF induced proliferation (Fig. S3), which was significantly inhibited by JWH-015. Then, we injected ED1 cells into the right flank of 7 week old male syngenic FVB mice to induce tumor formation. When the tumors reached palpable size, we treated the tumors with either ethanol control or JWH-015 (7.5 mg/kg) (n=5), thrice a week for 3 weeks. Tumor volume was monitored and measured every week. Cannabinoid administration blocked the subcutaneous growth of tumor cells very significantly, as verified by tumor volume (Fig. 5A) and weight (Fig. 5B, C). To correlate the reduction in tumor growth with important cellular parameters, we performed IHC staining of tumors for Ki67 (proliferation marker) and CD31 (vascularization marker) to dissect the effect of JWH-015 on proliferation and angiogenesis. JWH-015 treated tumors expressed lower Ki67 and CD31 levels compared to control (Fig. 5D, E).
Figure 5. JWH-015 inhibits NSCLC growth in in vivo mouse models.
ED1 cells were subcutaneously injected in FVB mice and palpable tumors were treated with ethanol control or JWH-015 (7.5mg/kg) (n-5) every third day for 3 weeks. Tumor volume (A) was calculated using the formula= length x (width)2/2. (B) Representative tumors and (C) tumor weight measured from various experimental groups. (D) Representative photomicrographs of Ki67 and CD31 staining of tumors extracted from various experimental groups. (E) Number of Ki67 and CD31 positive cells was counted in four different fields using bright field microscopy in each experimental group and the average was calculated. ED1 cells were injected by tail vein in FVB mice and after a week, mice were treated with control or JWH-015 (7.5mg/kg) (n-5) for 3 weeks. After treatment, their lungs were isolated (F) and metastatic lesions were counted (G). P<0.05 (*) and P<0.01 (**) as calculated by Student’s t test. Data represent mean ± SD for each experiment repeated three times.
JWH-015 exerts tumor inhibitory effects in in vivo tumorigenicity assay
Since we did not observe any surface lung lesions in the subcutaneous mouse model system, we extended our findings to in vivo tumorigenicity assay in FVB mice. Mouse ED1 lung cancer cells were injected intravenously into immunocompetent FVB mice. After a week of injection, mice were treated with ethanol control or JWH-015 (7.5 mg/kg) (n=5), intraperitoneally for 3 weeks and surface lung metastases were identified (Fig. 5F) and the metastatic lesions were counted. We observed a drastic reduction in lung colonization in mice treated with JWH-015 with respect to control (Fig. 5G). This proves that JWH-015 possesses significant tumor inhibitory effects.
JWH-015 decreases macrophage recruitment to tumor site and inhibits EMT of tumor cells by downregulation of EGFR signaling
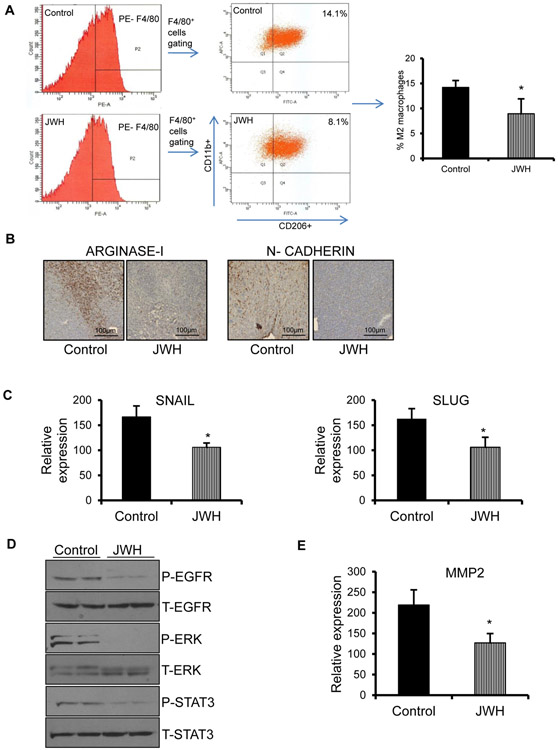
To further confirm our in vitro findings, we performed flow analysis of digested tumor cells harvested from the subcutaneous ED1 tumors. We observed that there was significant reduction in CD11b/F4/80/CD206 M2 macrophage population in JWH-015 treated tumors compared to control (Fig. 6A). There was no significant difference in other immune populations like CD3, CD4, CD8 T cells and CD11b/Gr1 (myeloid derived suppressor cells) (not shown). This was further confirmed by IHC, where there was reduced expression of Arginase-I (M2 macrophage marker) in tumors treated with JWH-015 compared to control (Fig. 6B). To investigate whether reduced macrophage recruitment inhibits EMT of tumor cells, we checked for EMT markers and found that there was downregulation of mesenchymal markers in JWH-015 treated tumors with respect to control (Fig. 6B, C).
Figure 6. JWH-015 decreases macrophage recruitment to tumor site and inhibits EMT of tumor cells by downregulation of EGFR signaling.
(A) Xenograft tumors (n-5) were subjected to flow analysis. F4/80 cells isolated were gated to check for CD11b+CD206+ cells to determine the population of M2 macrophages. (B) Representative photomicrographs of Arginase-1 and N-cadherin staining of tumors extracted from experimental groups. (C) Xenograft tumors (n-5) were subjected to Real Time PCR to determine expression of EMT markers. (D) Xenograft tumors (n-5) isolated were subjected to Western blot to determine expression of EGFR signaling targets. GAPDH is loading control. (E) Xenograft tumors (n-5) were subjected to Real Time PCR to determine expression of MMP2. P<0.05 (*) as calculated by Student’s t test. Data represent mean ± SD for each experiment repeated three times.
To prove that JWH-015 inhibits macrophage induced EMT by downregulation of EGFR pathway, we checked for EGFR targets in tumor lysates and observed marked reduction of pEGFR, pSTAT3 and pERK in tumors treated with JWH-015 with respect to control (Fig. 6D). Also, the JWH-015 treated tumors expressed lesser MMP2 levels compared to control (Fig. 6E).
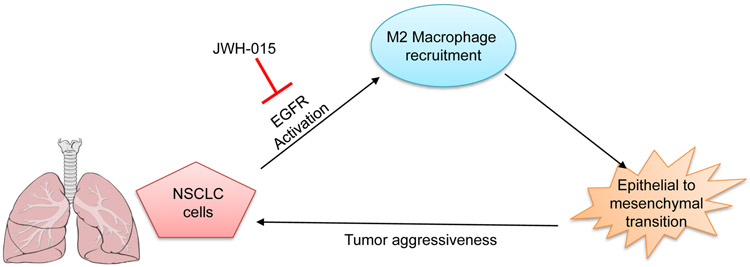
These results show that macrophages are recruited to the tumor site, which enhance EMT process by activation of EGFR pathway. This process is inhibited by treatment of tumor cells with JWH-015 leading to downregulation of EGFR signaling, thereby, reduced macrophage recruitment and attenuation of EMT (Fig.7).
Figure 7. Graphical representation.
JWH-015 inhibits EMT by inhibiting recruitment of macrophages through downregulation of EGFR pathway in NSCLC.
DISCUSSION
Cannabinoids were originally derived from the marijuana plant Cannabis sativa. Currently, there are more than 60 compounds isolated from this plant, apart from the synthetic and endogenous cannabinoids [35-36]. Initially, they were used in patients for palliative care against emesis, pain, etc. Recently, they have been identified and studied for their potent anti-cancer properties. Evidence from the last decade proves that these cannabinoids inhibit tumorigenesis in various cancer types like breast, lung, brain, colon, prostate, etc. These compounds affect various pathways like cell cycle, inflammation, angiogenesis, proliferation, migration, invasion, metastasis, etc. Cannabinoids modulate this complex array of signaling pathways mainly through cannabinoid receptors- CB1 and CB2 [37-38].
JWH-015 is a synthetic compound that is selective for CB2 receptor. Reports suggest that JWH-015 inhibits growth of hepatocellular carcinoma (HCC) via activation of autophagy and apoptosis by AMPK activation and TRB3 regulation [39]. Also, PPARγ plays a major role in JWH-015 induced autophagy in HCC [40]. In prostate cancer cells, JWH-015 exerted anti-proliferative effects by promoting ceramide synthesis induced cell death by regulation of JNK and AKT signaling molecules [16]. JWH-015 exerts anti-tumorigenic effects in other cancer tissue types like breast [15,41], lung [17] and lymphoblastic leukemia [42]. Although the anti-tumorigenic effects of JWH-015 have been established in various cancer types, the mechanism by which JWH-015 exerts these effects is not well known, especially in lung cancer. Our data reveals a novel mechanism by which JWH-015 exibits its tumor suppressive properties in NSCLC.
In our present investigation, we identified that JWH-015 inhibits EGF induced EMT in vitro in an epithelial cell line A549 and a mesenchymal cell line CALU1. EGFR has contributed to lung cancer growth by involving in vital cellular responses like proliferation, migration, invasion, metastasis, etc. [3] Also, EMT, which is activated by receptor tyrosine signaling, oncogenes, etc. contributes to lung cancer development [5,7,43]. Our results confirm these data that EGF induces EMT and JWH-015 significantly inhibits this process. In epithelial cell line A549, EGF induced EMT promoted mesenchymal character that is reversed by JWH-015. This was further strengthened in mesenchymal cell line- CALU1, where JWH-015 inhibits mesenchymal markers and upregulates epithelial markers. Also, JWH-015 inhibits phosphorylation of EGFR and ERK, a downstream target of EGFR pathway [44], thus proving that blockade of EMT is through downregulation of EGFR pathway. Finally, the aggressiveness of EGF induced effects like migration and invasion were reduced by JWH-015 by decreasing the expression of invasive markers like MMP2, VCAM-1 and FAK. This is of great importance considering the fact that EGF induced EMT has been shown in various cancer types and expression of EMT markers like E-cadherin and vimentin may be used as predictive biomarkers in analyzing the efficacy of EGFR inhibitors in NSCLC [6].
Our results indicate that JWH-015 inhibits TAM induced EMT in A549 cells in an indirect co-culture model. The tumor microenvironment is composed of various cellular and non-cellular elements [9,45]. Among these multiple and unique cell types, tumor associated macrophages (TAMs) are crucial components because of their diverse interactions with the cancer cells. TAMs are M2 macrophages, which are tumor promoting macrophages [10-12]. We prove that TAMs secrete EGF like ligands/factors which activate the EGFR pathway in cancer cells, thus promoting EMT. This macrophage induced EMT is significantly inhibited by treating A549 cancer cells with JWH-015, which downregulates the EGFR pathway. This data correlated with our previous result that the effects of EGF induced EMT is reversed by JWH-015. Thus, blockade of tumor progression and malignancy is dependent on the interplay between cancer cells and host cells of the tumor microenvironment.
Mouse models are very important to validate the in vitro results and also to study the efficacy of the anti-tumorigenic compound in vivo. Also, the regulation of the tumor microenvironment is well understood in vivo. We studied the effect of JWH-015 in subcutaneous xenografts of mouse ED1 cells in immunocompetent FVB mice. JWH-015 significantly reduced tumor growth which was confirmed by decrease in proliferation marker- Ki67 and angiogenic marker- CD31. CD11b/ F4/80/ CD206 M2 macrophages which are recruited to the tumor site is effectively blocked by JWH-015. Finally, EMT markers like N-cadherin, Snail and Slug were attenuated in JWH-015 treated tumors. This marked reduction of EMT is due to downregulation of EGFR and its targets like ERK and STAT3 by JWH-015. In a metastatic tail vein model, treatment of mice with JWH-015 reduced the number of metastatic lesions present in the lung. These experiments correlate our in vitro data that JWH-015 modulates the inflammatory microenvironment, thus inhibiting EGF mediated EMT in lung cancer cells.
Overall, the malignant properties of cancer cells can only be well understood if we study the crosstalk between cancer cells and the tumor microenvironment. Thus, a novel approach is required where the anti-tumorigenic compound has to target the tumor cells as well as affect its tumor microenvironment. In our study, JWH-015 was an effective anti-tumorigenic and anti-metastatic cannabinoid agonist. Also, it targeted the inflammatory microenvironment by attenuating the recruitment of tumor associated macrophages, thus inhibiting EMT in cancer cells by downregulation of EGFR pathway. This explores the possibility of crosstalk between CB2 and EGFR receptors and JWH-015 as a novel therapeutic target which is imperative considering the resistance of NSCLC to various chemotherapeutic drugs and its poor prognosis.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Kristin Kovach, Department of Pathology, The Ohio State University, Columbus, Ohio for the immunohistochemical slide analysis of tumor samples.
Funding support: This work was supported by grants from NIH (CA163010 and CA153490) and American Lung Association Discovery Award to RKG and Pelotonia Fellowship to NAW.
Abbreviations:
- NSCLC
Non-Small Cell Lung Cancer
- EGFR
Epidermal Growth Factor Receptor
- MMP
Matrix Metalloproteinase
- ECM
Extra Cellular Matrix
- EMT
Epithelial to Mesenchymal Transition
- TAM
Tumor Associated Macrophage
- TME
Tumor Micro Environment
- STAT3
Signal Transducer and Activator 3
- ERK
Extracellular Regulated Kinase
- FAK
Focal Adhesion Kinase
- VCAM1
Vascular Cell Adhesion Molecule 1
Footnotes
Conflicts of interest: The authors disclose no competing interests.
REFERENCES
- 1.Dearing KR, Sangal A, Weiss GJ. Maintaining clarity: Review of maintenance therapy in non-small cell lung cancer. World J Clin Oncol 2014;5(2):103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper WA, Lam DC, O’Toole SA, Minna JD. Molecular biology of lung cancer. J Thorac Dis 2013;5 Suppl 5:S479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seshacharyulu P, Ponnusamy MP, Haridas D, Jain M, Ganti AK, Batra SK. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin Ther Targets 2012;16(1):15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitsudomi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci 2007;98(12):1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sato M, Shames DS, Hasegawa Y. Emerging evidence of epithelial-to-mesenchymal transition in lung carcinogenesis. Respirology 2012;17(7):1048–1059. [DOI] [PubMed] [Google Scholar]
- 6.Barr S, Thomson S, Buck E et al. Bypassing cellular EGF receptor dependence through epithelial-to-mesenchymal-like transitions. Clin Exp Metastasis 2008;25(6):685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev 2013;27(20):2192–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang H, Declerck YA. Targeting the tumor microenvironment: from understanding pathways to effective clinical trials. Cancer Res 2013;73(16):4965–4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sounni NE, Noel A. Targeting the tumor microenvironment for cancer therapy. Clin Chem 2013;59(1):85–93. [DOI] [PubMed] [Google Scholar]
- 10.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol 2012;33(3):119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu CY, Xu JY, Shi XY et al. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Invest 2013;93(7):844–854. [DOI] [PubMed] [Google Scholar]
- 12.Lin CY, Lin CJ, Chen KH, Wu JC, Huang SH, Wang SM. Macrophage activation increases the invasive properties of hepatoma cells by destabilization of the adherens junction. FEBS Lett 2006;580(13):3042–3050. [DOI] [PubMed] [Google Scholar]
- 13.Vlaicu P, Mertins P, Mayr T et al. Monocytes/macrophages support mammary tumor invasivity by co-secreting lineage-specific EGFR ligands and a STAT3 activator. BMC Cancer 2013;13:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander A, Smith PF, Rosengren RJ. Cannabinoids in the treatment of cancer. Cancer Lett 2009;285(1):6–12. [DOI] [PubMed] [Google Scholar]
- 15.Nasser MW, Qamri Z, Deol YS et al. Crosstalk between chemokine receptor CXCR4 and cannabinoid receptor CB2 in modulating breast cancer growth and invasion. PLoS One 2011;6(9):e23901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olea-Herrero N, Vara D, Malagarie-Cazenave S, Diaz-Laviada I. Inhibition of human tumour prostate PC-3 cell growth by cannabinoids R(+)-Methanandamide and JWH-015: involvement of CB2. Br J Cancer 2009;101(6):940–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Preet A, Qamri Z, Nasser MW et al. Cannabinoid receptors, CB1 and CB2, as novel targets for inhibition of non-small cell lung cancer growth and metastasis. Cancer Prev Res (Phila) 2011;4(1):65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elbaz M, Nasser MW, Ravi J et al. Modulation of the tumor microenvironment and inhibition of EGF/EGFR pathway; novel anti-tumor mechanisms of Cannabidiol in breast cancer. Molecular Oncology 2015; 9(4):906–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nasser MW, Qamri Z, Deol YS et al. S100A7 enhances mammary tumorigenesis through upregulation of inflammatory pathways. Cancer Res 2012;72(3):604–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qamri Z, Preet A, Nasser MW et al. Synthetic cannabinoid receptor agonists inhibit tumor growth and metastasis of breast cancer. Mol Cancer Ther 2009;8(11):3117–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deol YS, Nasser MW, Yu L, Zou X, Ganju RK. Tumor-suppressive effects of psoriasin (S100A7) are mediated through the beta-catenin/T cell factor 4 protein pathway in estrogen receptor-positive breast cancer cells. J Biol Chem 2011;286(52):44845–44854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sneh A, Deol YS, Ganju A et al. Differential role of psoriasin (S100A7) in estrogen receptor alpha positive and negative breast cancer cells occur through actin remodeling. Breast Cancer Res Treat 2013;138(3):727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravi J, Sneh A, Shilo K, Nasser MW, Ganju RK. FAAH inhibition enhances anandamide mediated anti-tumorigenic effects in non-small cell lung cancer by downregulating the EGF/EGFR pathway. Oncotarget 2014;5(9):2475–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wani N, Nasser MW, Ahirwar DK et al. C-X-C motif chemokine 12/C-X-C chemokine receptor type 7 signaling regulates breast cancer growth and metastasis by modulating the tumor microenvironment. Breast Cancer Res 2014;16(3):R54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol 2005;17(5):559–564. [DOI] [PubMed] [Google Scholar]
- 26.Perlikos F, Harrington KJ, Syrigos KN. Key molecular mechanisms in lung cancer invasion and metastasis: a comprehensive review. Crit Rev Oncol Hematol 2013;87(1):1–11. [DOI] [PubMed] [Google Scholar]
- 27.Zhao J, Guan JL. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev 2009;28(1–2):35–49. [DOI] [PubMed] [Google Scholar]
- 28.Ding YB, Chen GY, Xia JG, Zang XW, Yang HY, Yang L. Association of VCAM-1 overexpression with oncogenesis, tumor angiogenesis and metastasis of gastric carcinoma. World J Gastroenterol 2003;9(7):1409–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shintani Y, Maeda M, Chaika N, Johnson KR, Wheelock MJ. Collagen I promotes epithelial-to-mesenchymal transition in lung cancer cells via transforming growth factor-beta signaling. Am J Respir Cell Mol Biol 2008;38(1):95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garofalo M, Romano G, Di Leva G et al. EGFR and MET receptor tyrosine kinase-altered microRNA expression induces tumorigenesis and gefitinib resistance in lung cancers. Nat Med 2012;18(1):74–82. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Shou Y, Hirano T, Gong Y et al. Influence of angiogenetic factors and matrix metalloproteinases upon tumour progression in non-small-cell lung cancer. Br J Cancer 2001;85(11):1706–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol 2011;6:49–69. [DOI] [PubMed] [Google Scholar]
- 33.Dehai C, Bo P, Qiang T et al. Enhanced invasion of lung adenocarcinoma cells after co-culture with THP-1-derived macrophages via the induction of EMT by IL-6. Immunol Lett 2014;160(1):1–10. [DOI] [PubMed] [Google Scholar]
- 34.Goncalves BF, Campos SG, Costa CF, Scarano WR, Goes RM, Taboga SR. Key participants of the tumor microenvironment of the prostate: An approach of the structural dynamic of cellular elements and extracellular matrix components during epithelial-stromal transition. Acta Histochem 2014. [DOI] [PubMed] [Google Scholar]
- 35.Cridge BJ, Rosengren RJ. Critical appraisal of the potential use of cannabinoids in cancer management. Cancer Manag Res 2013;5:301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freimuth N, Ramer R, Hinz B. Antitumorigenic effects of cannabinoids beyond apoptosis. J Pharmacol Exp Ther 2010;332(2):336–344. [DOI] [PubMed] [Google Scholar]
- 37.Chakravarti B, Ravi J, Ganju RK. Cannabinoids as therapeutic agents in cancer: current status and future implications. Oncotarget 2014;5(15):5852–5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarfaraz S, Adhami VM, Syed DN, Afaq F, Mukhtar H. Cannabinoids for cancer treatment: progress and promise. Cancer Res 2008;68(2):339–342. [DOI] [PubMed] [Google Scholar]
- 39.Vara D, Salazar M, Olea-Herrero N, Guzman M, Velasco G, Diaz-Laviada I. Anti-tumoral action of cannabinoids on hepatocellular carcinoma: role of AMPK-dependent activation of autophagy. Cell Death Differ 2011;18(7):1099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vara D, Morell C, Rodriguez-Henche N, Diaz-Laviada I. Involvement of PPARgamma in the antitumoral action of cannabinoids on hepatocellular carcinoma. Cell Death Dis 2013;4:e618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emery SM, Alotaibi MR, Tao Q, Selley DE, Lichtman AH, Gewirtz DA. Combined antiproliferative effects of the aminoalkylindole WIN55,212–2 and radiation in breast cancer cells. J Pharmacol Exp Ther 2014;348(2):293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKallip RJ, Lombard C, Fisher M et al. Targeting CB2 cannabinoid receptors as a novel therapy to treat malignant lymphoblastic disease. Blood 2002;100(2):627–634. [DOI] [PubMed] [Google Scholar]
- 43.Shih JY, Yang PC. The EMT regulator slug and lung carcinogenesis. Carcinogenesis 2011;32(9):1299–1304. [DOI] [PubMed] [Google Scholar]
- 44.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007;26(22):3291–3310. [DOI] [PubMed] [Google Scholar]
- 45.Goubran HA, Kotb RR, Stakiw J, Emara ME, Burnouf T. Regulation of tumor growth and metastasis: the role of tumor microenvironment. Cancer Growth Metastasis 2014;7:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.