Abstract
Background
Spinal cord injury (SCI) is a serious nervous system injury, causing extremely low quality of life and immensurable economic losses. However, there is few therapies that can effectively cure the injury. The goal of the present study was to explore the potential therapeutic effects of dihydrotanshinone I (DI) for SCI and the involving mechanism.
Material/Methods
A SCI rat model was structured to investigate the effects of DI on recovery of SCI. Tarlov’s scale was employed to assess the neuronal function and histopathological examination was carried out by hematoxylin and eosin staining. In addition, tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-1β, inducible nitric oxide synthase (iNOS), total oxidant status (TOS) and total antioxidant status (TAS) levels were detected. Tunel assay and western blot analysis were performed to evaluate cell apoptosis. Furthermore, western blot assay was used to measure the protein expressions.
Results
The results demonstrated that the treatment of DI alleviated the pathological damage induced by SCI and promoted the neuronal functional recovery. DI suppressed TNF-α, IL-1β, IL-6, iNOS, and TOS levels while improved the TAS level. Moreover, increased cell apoptosis in SCI rats was inhibited by administration of DI. Most importantly, DI reserved the soaring of TLR4, MyD88, HMGB1, and NOX4 level after induction of SCI. Thus, the observation revealed that the HMGB1/TLR4/NOX4 pathway may be involved in the protective effects of DI on SCI.
Conclusions
In conclusion, the findings suggest that DI alleviates SCI by restraining secretion of inflammatory factors, and occurrence of oxidative stress and apoptosis in vivo. DI may be developed into an effective alternative therapy for SCI in clinic.
MeSH Keywords: Inflammation, Oxidative Stress, Rats, Spinal Cord Injuries
Background
Spinal cord injury (SCI) is a serious complication of spine injury, putting patients in debilitating pathological conditions physically and psychologically, and may cause huge economic challenges to society [1–3]. SCI leads to physical damages, including infarction of the spinal cord, dyskinesia, and sensory deficits in the area below the injury, muscle wasting, chronic pain, and paralysis [4,5], resulting in acceleration of tissue edema, neural cell apoptosis, and the inflammatory cytokine release [6]. Nowadays, supportive measures are still the primary treatment because of the complex pathophysiology of SCI [7]. Pharmacological therapies, such as high-dose corticosteroids, GM-1 ganglioside, and mecobalamin, have been widely performed while the therapeutic effects for SCI of these treatments are uncertain and unexpected side effects are reported [8]. Therefore, to develop more effective and stable therapies with fewer side effects is urgent need.
Traditional Chinese medicine (TCM) has been proven to play multiple therapeutic roles in various diseases. Salvia miltiorrhiza (Danshen), a well-known TCM, has been used in treatment of cardiovascular diseases, amenorrhea, hemorrhage, and hepatitis for several hundred years [9]. The active components of Salvia miltiorrhiza can be divided into 2 components: hydrophilic and lipophilic fractions [10]. The major active constituents in hydrophilic fractions are salvianolic acid derivatives and diterpenoids, while those in lipophilic fractions are the diversified tanshinones [11]. Dihydrotanshinone I (DI) is one of the tanshinones extracted from Salvia miltiorrhiza and has been widely researched for its extensive biological activities. DI has exhibited anti-inflammatory effects in vitro and in vivo [12], reverses the multidrug resistance [13], promotes vasorelaxation of coronary arteries in rats [14], and even acts as a tumor suppressor by inducing cytotoxicity and inhibiting angiogenesis [15]. However, little study has investigated the ability and the underlying mechanism of DI to protect from spinal cord injury.
In the current study, we established the spinal cord injury rat model and explained the protection of DI against spinal cord injury by inhibition of inflammatory cytokine response, decreases of oxidative stress and cell apoptosis.
Material and Methods
DI preparation
DI powder (HPLC ≥95.0%) was obtained from Sigma–Aldrich Corporation (St. Louis, MO, USA) and purity of the red powder was greater than 95.0% according to the detection of HPLC–UV. It was dissolved with absolute alcohol and stored at −20°C.
Animals
Sprague-Dawley (SD) rats weighing 250–270 g were purchased from Comparative Medicine Centre of Yangzhou University. The rats were raised under a controllable condition of 22±3°C, relative humidity 50±10%, and 12-hour light and 12-hour darkness, supplied with standard laboratory feed. All experiments were approved by the Animal Care and Use Committee of Dajiangdong Hospital.
SCI model and group
The SCI rat model was constructed as previously described [16,17]. Briefly, the SD rats were injected intraperitoneally with 100 mg/kg ketamine and 1 mg/kg chlorpromazine for anesthesia. Then the T8 and T9 vertebral peduncles of the rats were removed by a laminectomy. Following this, all rats were individually housed in a room with the aforementioned conditions. After inducing the SCI model for 24 hours, rats were orally administered DI treatment. The 40 SD rats were divided into 5 groups (n=8). In the sham group, rats were subjected to surgical procedures of laminectomy without the removal of T8 and T9 vertebral peduncles, and given normal saline orally. In the SCI group, rats underwent the laminectomy and were treated with normal saline. In the SCI+DI group, SCI rats were administered orally with 2 mg/kg and 4 mg/kg of DI. All groups were administrated once a day for 28 days and were sacrificed at 24 hours after the last dose.
Histopathological examination
Spinal cord samples from rats of the 5 groups were fixed with 10% neutral formaldehyde buffer overnight, dehydrated in different concentrations of ethanol, embedded in paraffin, and cut into 4 μm sections. The sections were then stained with Hematoxylin and eosin (H and E) staining. The pathological changes of the samples after SCI or treatment of DI were observed by a light microscope
Assessment of neuronal function
Neurological function of SCI rats was evaluated after treatment of DI for 28 day after surgery in accordance to the Tarlov scale: 0 point, no lower extremity movement and could not bear gravity; 1 point, lower extremity motion and could not bear gravity; 2 point, lower extremity motion against gravity; 3 point, lower extremity could support the weight and walk for 1 to 2 steps; 4 point, walk with mild obstacles; 5 point, walk normally.
Measurement of TNF-α, IL-6, and IL-1β levels
To measure effects of DI on pro-inflammatory cytokine in rats with SCI, blood was harvested and curdled. After centrifugation at 1000 g for 20 minutes, the supernatant was collected and the levels of serum TNF-α, IL-6, and IL-1β were examined by enzyme-linked immunosorbent assay (ELISA). All assays were replicated for 3 times and 6 replicates were established in each group.
Western blot assay
Spinal cord tissues from rats were collected and homogenized in a cold RIPA lysis buffer. The concentration of extracted total proteins was measured with a BCA protein assay kit (Thermo, USA). Sample proteins were then loaded onto 12% SDS-PAGE and electrotransferred to nitrocellulose membranes. The membranes was used to blocked with 5% non-fat milk at 37°C for 1 hour, and incubated with primary antibodies (IL-6, 1: 1000, ab6672; IL-1β, 1: 1000, ab2105; TNF-α, 1: 1000, ab6671; iNOS, 1: 1000, ab15323;Bcl-2, 1: 1000, ab32124; Bax, 1: 1000, ab32503; cleaved caspase 3, 1μg/ml, ab2302; TLR4, 1: 500, ab13556; MyD88, 1: 1000, ab133739; HMGB1, 1: 1000, ab79823; and NOX4, 1: 2000, ab133303. All were purchased from Abcam) at 4°C overnight. Following that, the membranes were washed 3 times and incubated with secondary goat anti-rabbit (1: 2000, ab6721) or anti-mouse IgG (1: 1000, ab7068) antibodies for 2 hours at 37°C. The bands were visualized with enhanced chemiluminescent (ECL) detection kit.
Measurement of oxidative stress
The levels of serum total oxidant status (TOS), serum total antioxidant status (TAS) and nitric oxide (NO) were detected by a commercially available kit (Jiancheng Bio., Nanjing, China) following the manufacturer’s protocol, respectively. Each group was replicated for 5 times and all assays were independently repeated 3 times.
Tunel assay
TUNEL assay was carried out to evaluate the cell apoptosis induced by SCI. Spinal cord cells were washed 3 times with DPBS and fixed with 4% paraformaldehyde for 30 minutes without light. The cells were then incubated with 15 μg/mL proteinase K for 15 minutes and 3% H2O2 for 15 minutes at 37°C. After washing several times in PBS, cells were stained with 3,3′-diaminobenzidine (DAB). Positive stained cells were counted with a fluorescence microscopy.
Statistical analysis
All data were analyzed by SPSS 20.0 software and are presented as mean ± standard deviation (SD). The comparison of the 2 groups was conducted by Student’s t-test and the significance of multiple group differences were analyzed using one-way analysis of variance (ANOVA). A P value <0.05 was considered as the significant difference.
Results
DI alleviated pathological changes of spinal cord in rats with SCI
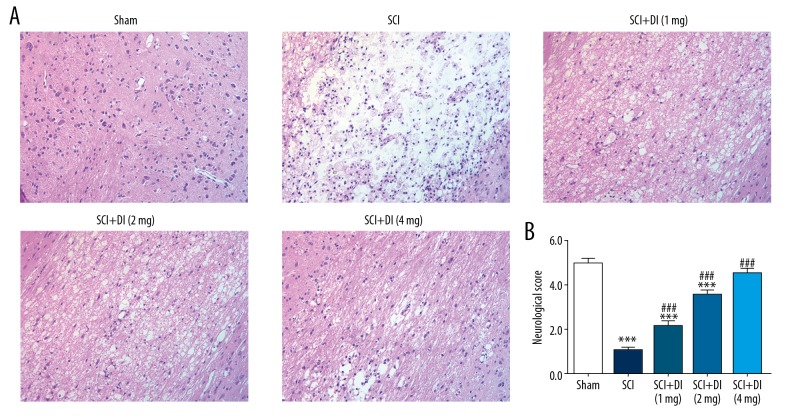
SCI results in multiple alteration of pathology and morphology in spinal cord tissues [18]. To investigate the influence of DI on histopathology of spinal cord segments in SCI rats, SCI rat model was established. As presented in Figure 1A, the SCI rats showed the significant alteration of neuron’s morphology, degeneration of gray matter, infiltration of leukocytes and increased cavitation areas in spinal cord tissue compared with sham-operated rats. However, with administration of different dose of DI, pathological lesions induced by SCI were rescued in a dose-dependent manner. In addition to, the scope of cavitation areas was shrunken as well. These data indicated that DI may reduce alteration of spinal cord morphology in SCI rats.
Figure 1.
DI reduced histopathological changes in spinal cord tissues and recovered motor function after SCI. (A) H and E staining of spinal cord section was performed with or without DI. Original magnification: 200×. (B) Neurological function of the SCI rats in each group was evaluated by Tarlov scores. Data are expressed as mean±SD. *** P<0.001 versus sham group; ### P<0.001 versus SCI group. DI – dihydrotanshinone I; SCI – spinal cord injury; H and E – hematoxylin and eosin; SD – standard deviation.
DI promoted neurological functional recovery
To explore the role of DI in functional recovery after SCI, neurological examination was performed with a Tarlov scale. As shown in Figure 1B, the average neurological functional scale in the SCI group was significantly lower than that in sham group. The score of neurological function was remarkably increased after the treatment of DI compared with the group with induction of SCI. Besides, we found that there was no significant difference between the scores of the DI-treated SCI rats and the sham-operated rats, suggesting that enough dose of DI had obvious effects on accelerating neurological and motor functional recovery in rats subjected to SCI.
DI reduced inflammatory cytokines in serum and spinal cord tissues
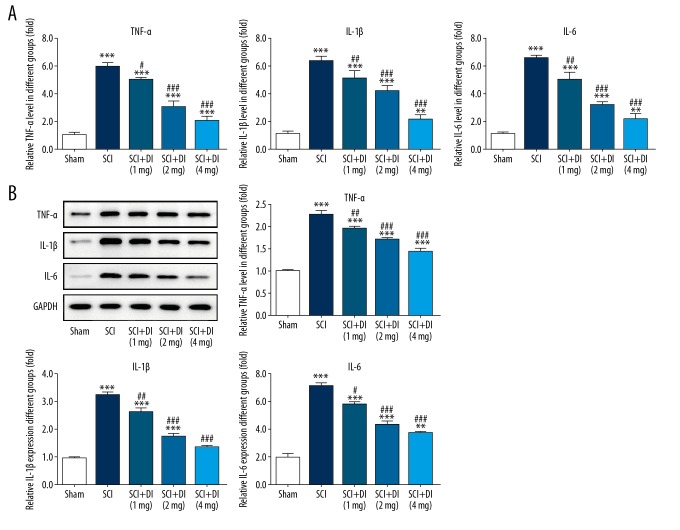
To identify the effects of DI on SCI-induced inflammatory response, inflammatory cytokines in both serum and spinal cord tissues were evaluated. The results from ELISA assay revealed that SCI dramatically increased the secretions of IL-6, IL-1β, and TNF-α in serum in comparison with the sham group. However, diminishing levels of IL-6, IL-1β, and TNF-α were observed in DI-treated SCI group (Figure 2A). In addition, western blot analysis result demonstrated that the production of IL-6, IL-1β, and TNF-α were induced after SCI while DI treatment reversed the increases in spinal cord tissues (Figure 2B). These results indicated the suppressive effects of DI on the generation of pro-inflammatory factors in SCI-induced serum and spinal cord tissues.
Figure 2.
Effects of DI on inflammatory cytokines in rat spinal cord. (A) Levels of IL-6, IL-1β and TNF-α in serum were assessed by ELISA assay. (B) Western blot assay was employed to determine the generation of TNF-α, IL-6, and IL-1β in spinal cord tissues of rats after sham surgery, and SCI and DI treatment. Data are expressed as mean±SD. ** P<0.01, *** P<0.001 versus sham group; # P<0.05, ## P<0.01, ### P<0.001 versus SCI group. DI – dihydrotanshinone I; IL – interleukin; TNF – tumor necrosis factor; ELISA – enzyme-linked immunosorbent assay; SCI – spinal cord injury; SD – standard deviation.
DI suppressed SCI induced oxidative stress and iNOS generation
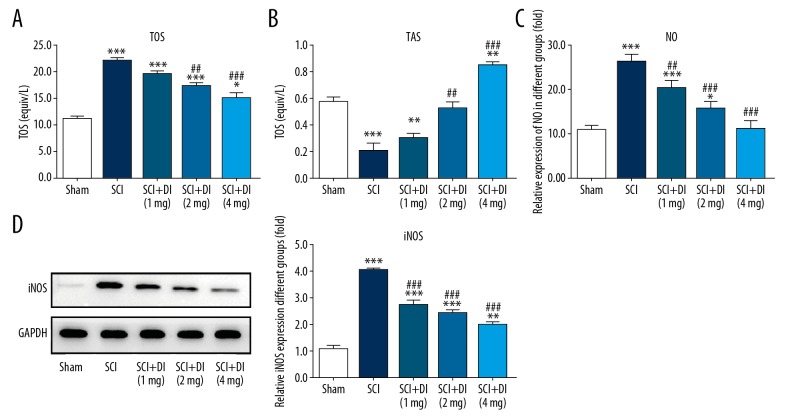
In addition to inflammatory response, serum oxidative stress level after SCI was measured. As shown in Figure 3A and 3B, SCI markedly enhanced TOS while reduced TAS in comparison with the sham group. Meanwhile, DI treatment prevented the aberrant alteration and elevated the capacity of antioxidant after SCI. In addition, NO level was found to be increased in SCI-stimulated rats and the excessive expression of NO was suppressed by treatment of DI (Figure 3C). Western blot assay showed that increased protein level of iNOS after SCI was significantly reduced by DI treatment (Figure 3D). The results suggested that DI exerted strong anti-oxidative activity in SCI rat model.
Figure 3.
Effects of DI on oxidative stress in rat spinal cord. TOS (A), TAS (B), and NO level (C) in serum of SCI-treated rats were detected in the absence or presence of DI. (D) iNOS protein level was assessed by western blot analysis. Data are expressed as mean±SD. * P<0.05, ** P<0.01, *** P<0.001 versus sham group; ## P<0.01, ### P<0.001 versus SCI group. DI – dihydrotanshinone I; TOS – total oxidant status (TOS); TAS – total antioxidant status; iNOS – inducible nitric oxide synthase; SD – standard deviation.
DI exerted anti-apoptosis activity in SCI rats
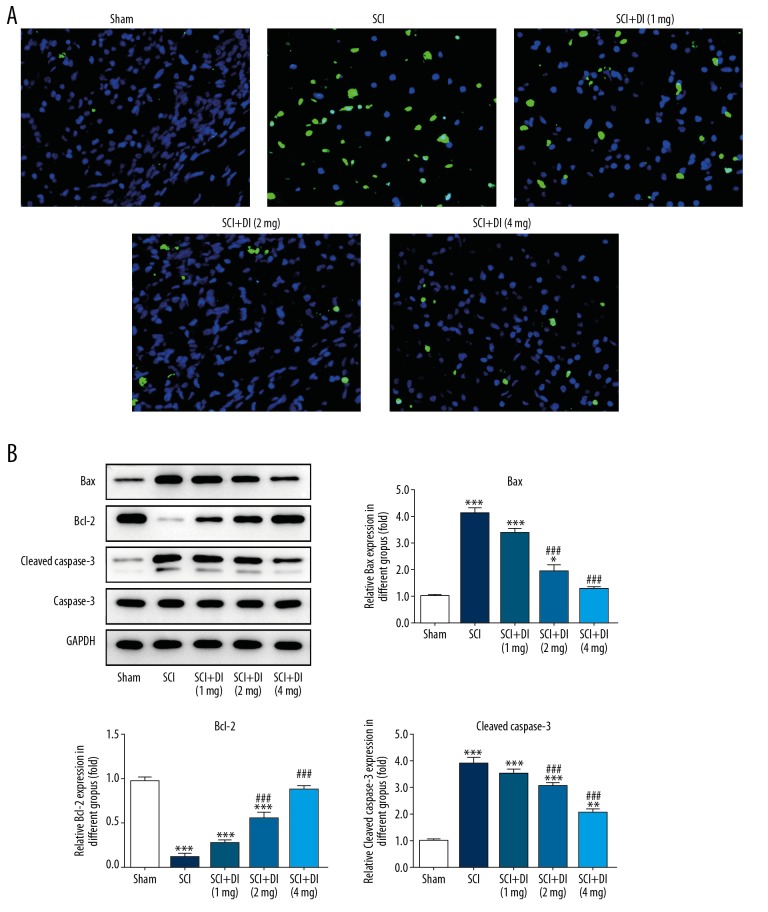
Neural cell apoptosis stimulated by SCI affects the physiological activities of central nervous system (CNS) and represses the recovery of white matter and neurological functions [19]. To investigate the role of DI in SCI-induced cell apoptosis, Tunel assay and western blot analysis were performed. As shown in Figure 4A, SCI induced an obviously increased apoptosis rate whereas DI treatment greatly reduced cell apoptosis compared with the cells treated with SCI. Additionally, western blot results showed that protein levels of Bax and cleaved caspase-3 were significantly increased and Bcl-2 level was decreased in SCI rats. DI treatment reduced the expression levels of Bax and cleaved caspase-3, and enhanced Bcl-2 level after SCI (Figure 4B). The data indicated DI evidently suppressed cell apoptosis after SCI lesions.
Figure 4.
DI suppressed SCI-induced cell apoptosis in rats. (A) Tunel assay was carried out to assess the apoptosis after SCI in the absence or presence of DI. Original magnification: 400×. (B) Western blot assay was implemented to measure the levels of proteins involved in cell apoptosis. Data are expressed as mean±SD. * P<0.05, ** P<0.01, *** P<0.001 versus sham group; ## P<0.01, ### P<0.001 versus. SCI group. DI – dihydrotanshinone I; SCI – spinal cord injury; SD – standard deviation.
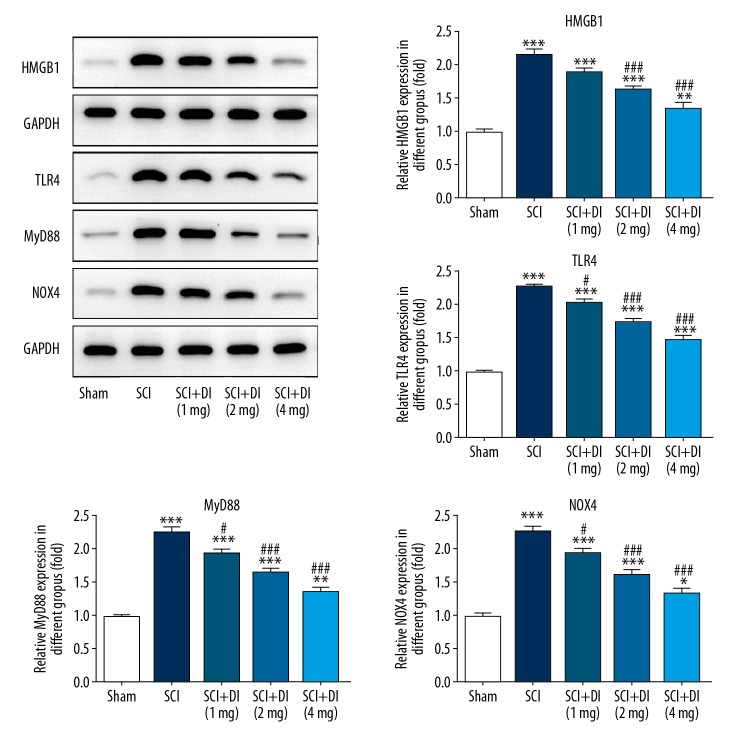
DI inhibited the HMGB1/TLR4/NOX4 signaling pathway
Further, we examined the underlying mechanism by which DI protects rats against SCI. As shown in Figure 5, when compared with the sham group, the contents of HMGB1, TLR4, MyD88, and NOX4 in SCI-treated spinal cord tissues were extremely increased. However, SCI-induced increase levels of 4 proteins were notably inhibited by DI treatment in a concentration-dependent manner, which indicate that DI may exert its protective effects on SCI rats via inactivating the HMGB1/TLR4/NOX4 signaling pathway.
Figure 5.
Effect of DI on HMGB1/TLR4/NOX4 activation in rat spinal cord. Levels of MGB1, TLR4, MyD88 and NOX4 were decreased in SCI rats after administration of DI. Data are expressed as mean±SD. * P<0.05, ** P<0.01, *** P<0.001 versus sham group; # P<0.05, ### P<0.001 versus SCI group. DI – dihydrotanshinone I; SCI – spinal cord injury; SD – standard deviation.
Discussion
In the present study, we observed that DI ameliorated pathological damages by reversing histological changes in spinal cord and recovered neurological and motor functions with an improved Tarlov scale. Moreover, we also revealed that DI alleviated inflammatory response by reduction of TNF-α, IL-6 and IL-1β in serum and spinal cord, inhibited oxidative stress by a decreased TOS and an increased TAS, and suppressed cell apoptosis following the SCI. Furthermore, HMGB1/TLR4/NOX4 signaling pathway was found to be associated with the protective effects of DI on SCI-treated rats.
Dihydrotanshinone I (DI), as a potent active component of Salvia miltiorrhiza (Danshen), have been extensively used for gynecological and cardiovascular diseases [20–22]. It is well established that DI shows strong anti-oxidative and anti-inflammatory properties in many inflammation and other diseases. Yuan et al. found that DI showed anti-inflammatory effects by inhibited the secretions of inflammatory cytokines and activation of NF-κB pathway in vitro and alleviating LPS-induced acute kidney injury in vitro [12]. Wang et al. revealed that DI remarkably suppressed the expression of NF-κB reporter gene and phosphorylation of IκBα and p65 induced by TNF-α, and also repressed the expressions of NF-κB target genes TNF-α, IL-6, and MCP1 [23]. In addition, previous study demonstrated that lipophilic fractions of Salvia miltiorrhiza inhibited the oxidative stress level in diabetic nephropathy by abolishing high glucose-induced reactive oxygen species and enhancing the Nrf2 level [24]. Dai et al. reported that Tanshinone I exerted neuroprotection through ameliorating motor and cognitive functions damaged by hypoxia-ischemia via inhibiting oxidative stress in the neonatal rats [25]. In the current study, our results showed that DI not only blocked the production of IL-6, IL-1β, TNF-α, and iNOS improved anti-oxidative level and suppressed the NO level after SCI, but reduced tissue lesions and rescued neurological function, which is consistent with previous reports. These data indicate the suppressive effects of DI on SCI-stimulated inflammation and oxidative stress in spinal cord tissues.
It is widely accepted that inflammatory reaction under abnormal conditions may cause cell apoptosis [26,27]. Neutrophils release reactive oxygen free radicals, damage the vascular endothelium cells, which accelerate tissue edema, necrosis and apoptosis after SCI [28,29]. Previous study has showed that DI exerted anti-tumor activity in colon cancer by promoting caspase dependent apoptosis and autophagy in vitro, meanwhile increasing the induction of apoptosis and caspase-3 activation in xenograft rats [30]. Cao et al. elucidated that DI increased the activation of caspase-3/9 and the release of cytochrome c induces apoptosis and inhibits proliferation so as to induce apoptosis and inhibit proliferation in glioma cells [31]. However, Wei et al. revealed that DI reduced myocardial apoptosis by inhibiting the expressions of Bax, cleaved caspase-3 and cleaved caspase-9 while promoting the Bcl-2 expression after myocardial ischemia–reperfusion injury (MIRI) [32]. In our study, results from Tunel assay demonstrated that DI evidently diminished SCI-induced apoptosis, with a decrease level of Bax and cleaved caspase-3 and an increased level of Bcl-2. Based on the aforementioned result, we speculate that DI plays different roles in the apoptosis associated with various diseases and the underlying molecular mechanism remains to be further illustrated.
Studies have shown that acute SCI resulted in an increase of HMGB1 level which is related to neuronal apoptosis [33]. For example, glycyrrhizin protected rats against ischemic spinal cord injury by inhibiting the secretions of inflammatory cytokines and HMGB1 [34]. Besides, TLR4 is intimately linked with the autoimmune and non-infectious immune response. Kang et al. reported that the intervention of hyperbaric oxygen alleviated secondary spinal cord injury by reduced the levels of HMGB1, NF-κB and TLR4 in vivo [35]. Another study revealed that Shikonin alleviated tissue injury and recovered motor function induced by spinal cord injury through regulating the inflammation response via the inactivation of HMGB1/TLR4/NF-κB pathway [36]. NOX4 has been also regarded as a strong proinflammatory factor for its role of the downstream of TLR4 under certain circumstances and the interaction with TLR4 in both endogenous and exogenous TLR4 ligand-induced ROS generation [37–39]. In the present study, the expressions of HMGB1/TLR4/NOX4 pathway in SCI rats were detected following SCI in the presence and absence of DI. We found that stimulation of SCI induced the HMGB1/TLR4/NOX4 signaling while DI suppressed the high expressions of this signaling after SCI. These results indicate HMGB1/TLR4/NOX4 pathway is involved in the protection of DI against SCI in vivo.
Conclusions
Our study demonstrated that DI ameliorated spinal cord injury through suppressing the progression of inflammatory response and oxidative stress, as well as apoptosis in rats. The beneficial effects of DI may be associate with blockages of HMGB1/TLR4/NOX4 signaling. Collectively, the data indicate that DI has the potential to serve as a therapeutic candidate for patients with SCI.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Chen D, Pan D, Tang S, et al. Administration of chlorogenic acid alleviates spinal cord injury via TLR4/NFkappaB and p38 signaling pathway anti-inflammatory activity. Mol Med Rep. 2018;17(1):1340–46. doi: 10.3892/mmr.2017.7987. [DOI] [PubMed] [Google Scholar]
- 2.Pei JP, Fan LH, Nan K, et al. HSYA alleviates secondary neuronal death through attenuating oxidative stress, inflammatory response, and neural apoptosis in SD rat spinal cord compression injury. J Neuroinflammation. 2017;14(1):97. doi: 10.1186/s12974-017-0870-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang R, Cai X, Li J, et al. Protective effects of MiR-129-5p on acute spinal cord injury rats. Med Sci Monit. 2019;25:8281–88. doi: 10.12659/MSM.916731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng B, Hao D, Guo H, He B. Melatonin alleviates acute spinal cord injury in rats through promoting on progenitor cells proliferation. Saudi Pharm J. 2017;25(4):570–74. doi: 10.1016/j.jsps.2017.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Jiang SH, Tu WZ, Zou EM, et al. Neuroprotective effects of different modalities of acupuncture on traumatic spinal cord injury in rats. Evid Based Complement Alternat Med. 2014;2014 doi: 10.1155/2014/431580. 431580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kjell J, Olson L. Rat models of spinal cord injury: From pathology to potential therapies. Dis Model Mech. 2016;9(10):1125–37. doi: 10.1242/dmm.025833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Shea TM, Burda JE, Sofroniew MV. Cell biology of spinal cord injury and repair. J Clin Invest. 2017;127(9):3259–70. doi: 10.1172/JCI90608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karsy M, Hawryluk G. Pharmacologic management of acute spinal cord injury. Neurosurg Clin North Am. 2017;28(1):49–62. doi: 10.1016/j.nec.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Wang JW, Wu JY. Tanshinone biosynthesis in Salvia miltiorrhiza and production in plant tissue cultures. Appl Microbiol Biotechnol. 2010;88(2):437–49. doi: 10.1007/s00253-010-2797-7. [DOI] [PubMed] [Google Scholar]
- 10.Adams JD, Wang R, Yang J, Lien EJ. Preclinical and clinical examinations of Salvia miltiorrhiza and its tanshinones in ischemic conditions. Chin Med. 2006;1:3. doi: 10.1186/1749-8546-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng YY, Fong SM, Chang HM. [Protective action of Salvia miltiorrhiza aqueous extract on chemically induced acute myocardial ischemia in rats]. Zhong Xi Yi Jie He Za Zhi. 1990;10(10):609–11. [in Chinese] [PubMed] [Google Scholar]
- 12.Yuan R, Huang L, Du L, et al. Dihydrotanshinone exhibits an anti-inflammatory effect in vitro and in vivo through blocking TLR4 dimerization. Pharmacol Res. 2019;142:102–14. doi: 10.1016/j.phrs.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Hu T, To KK, Wang L, et al. Reversal of P-glycoprotein (P-gp) mediated multidrug resistance in colon cancer cells by cryptotanshinone and dihydrotanshinone of Salvia miltiorrhiza. Phytomedicine. 2014;21(11):1264–72. doi: 10.1016/j.phymed.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Lam FF, Yeung JH, Chan KM, Or PM. Dihydrotanshinone, a lipophilic component of Salvia miltiorrhiza (Danshen), relaxes rat coronary artery by inhibition of calcium channels. J Ethnopharmacol. 2008;119(2):318–21. doi: 10.1016/j.jep.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Bian W, Chen F, Bai L, et al. Dihydrotanshinone I inhibits angiogenesis both in vitro and in vivo. Acta Biochim Biophys Sinica. 2008;40(1):1–6. doi: 10.1111/j.1745-7270.2008.00370.x. [DOI] [PubMed] [Google Scholar]
- 16.Ersahin M, Toklu HZ, Erzik C, et al. Ghrelin alleviates spinal cord injury in rats via its anti-inflammatory effects. Turk Neurosurg. 2011;21(4):599–605. [PubMed] [Google Scholar]
- 17.Yang W, Yang Y, Yang JY, et al. Treatment with bone marrow mesenchymal stem cells combined with plumbagin alleviates spinal cord injury by affecting oxidative stress, inflammation, apoptosis and the activation of the Nrf2 pathway. Int J Mol Med. 2016;37(4):1075–82. doi: 10.3892/ijmm.2016.2498. [DOI] [PubMed] [Google Scholar]
- 18.Didangelos A, Puglia M, Iberl M, et al. High-throughput proteomics reveal alarmins as amplifiers of tissue pathology and inflammation after spinal cord injury. Sci Rep. 2016;6:21607. doi: 10.1038/srep21607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beattie MS, Hermann GE, Rogers RC, Bresnahan JC. Cell death in models of spinal cord injury. Prog Brain Res. 2002;137:37–47. doi: 10.1016/s0079-6123(02)37006-7. [DOI] [PubMed] [Google Scholar]
- 20.Su CY, Ming QL, Rahman K, et al. Salvia miltiorrhiza: Traditional medicinal uses, chemistry, and pharmacology. Chin J Nat Med. 2015;13(3):163–82. doi: 10.1016/S1875-5364(15)30002-9. [DOI] [PubMed] [Google Scholar]
- 21.Li CM, Dong XL, Fan XD, et al. Aqueous extract of Danshen (Salvia miltiorrhiza Bunge) protects ovariectomized rats fed with high-fat diet from endothelial dysfunction. Menopause (New York, NY) 2013;20(1):100–9. doi: 10.1097/gme.0b013e31825b512d. [DOI] [PubMed] [Google Scholar]
- 22.MElm XD, Cao YF, Che YY, et al. Danshen: A phytochemical and pharmacological overview. Chin J Nat Med. 2019;17(1):59–80. doi: 10.1016/S1875-5364(19)30010-X. [DOI] [PubMed] [Google Scholar]
- 23.Wang F, Ma J, Wang KS, et al. Blockade of TNF-alpha-induced NF-kappaB signaling pathway and anti-cancer therapeutic response of dihydrotanshinone I. Int Immunopharmacol. 2015;28(1):764–72. doi: 10.1016/j.intimp.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 24.An L, Zhou M, Marikar F, et al. Salvia miltiorrhiza lipophilic fraction attenuates oxidative stress in diabetic nephropathy through activation of nuclear factor erythroid 2-related factor 2. Am J Chin Med. 2017;45(7):1441–57. doi: 10.1142/S0192415X17500781. [DOI] [PubMed] [Google Scholar]
- 25.Dai C, Liu Y, Dong Z. Tanshinone I alleviate motor and cognitive impairments via suppressing oxidative stress in the neonatal rats after hypoxic-ischemic brain damage. Mol Brain. 2017;10(1):52. doi: 10.1186/s13041-017-0332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolb JP, Oguin TH, 3rd, Oberst A, Martinez J. Programmed cell death and inflammation: Winter is coming. Trends Immunol. 2017;38(10):705–18. doi: 10.1016/j.it.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rock KL, Kono H. The inflammatory response to cell death. Ann Rev Pathol. 2008;3:99–126. doi: 10.1146/annurev.pathmechdis.3.121806.151456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelissen S, Vangansewinkel T, Geurts N, et al. Mast cells protect from post-traumatic spinal cord damage in mice by degrading inflammation-associated cytokines via mouse mast cell protease 4. Neurobiol Dis. 2014;62:260–72. doi: 10.1016/j.nbd.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Genovese T, Esposito E, Mazzon E, et al. Absence of endogenous interleukin-10 enhances secondary inflammatory process after spinal cord compression injury in mice. J Neurochem. 2009;108(6):1360–72. doi: 10.1111/j.1471-4159.2009.05899.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Hu T, Shen J, et al. Dihydrotanshinone I induced apoptosis and autophagy through caspase dependent pathway in colon cancer. Phytomedicine. 2015;22(12):1079–87. doi: 10.1016/j.phymed.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Cao Y, Huang B, Gao C. Salvia miltiorrhiza extract dihydrotanshinone induces apoptosis and inhibits proliferation of glioma cells. Bosn J Basic Med Sci. 2017;17(3):235–40. doi: 10.17305/bjbms.2017.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei Y, Xu M, Ren Y, et al. The cardioprotection of dihydrotanshinone I against myocardial ischemia-reperfusion injury via inhibition of arachidonic acid omega-hydroxylase. Can Jo Physiol Pharmacol. 2016;94(12):1267–75. doi: 10.1139/cjpp-2016-0036. [DOI] [PubMed] [Google Scholar]
- 33.Kawabata H, Setoguchi T, Yone K, et al. High mobility group box 1 is upregulated after spinal cord injury and is associated with neuronal cell apoptosis. Spine. 2010;35(11):1109–15. doi: 10.1097/BRS.0b013e3181bd14b6. [DOI] [PubMed] [Google Scholar]
- 34.Gong G, Yuan LB, Hu L, et al. Glycyrrhizin attenuates rat ischemic spinal cord injury by suppressing inflammatory cytokines and HMGB1. Acta Pharmacol Sin. 2012;33(1):11–18. doi: 10.1038/aps.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang N, Hai Y, Yang J, et al. Hyperbaric oxygen intervention reduces secondary spinal cord injury in rats via regulation of HMGB1/TLR4/NF-kappaB signaling pathway. Int J Clin Exp Pathol. 2015;8(2):1141–53. [PMC free article] [PubMed] [Google Scholar]
- 36.Bi Y, Zhu Y, Zhang M, et al. Effect of Shikonin on spinal cord injury in rats via regulation of HMGB1/TLR4/NF-κB signaling pathway. Cell Physiol Biochem. 2017;43(2):481–91. doi: 10.1159/000480474. [DOI] [PubMed] [Google Scholar]
- 37.Ogier-Denis E, Mkaddem SB, Vandewalle A. NOX enzymes and Toll-like receptor signaling. Semin Immunopathol. 2008;30(3):291–300. doi: 10.1007/s00281-008-0120-9. [DOI] [PubMed] [Google Scholar]
- 38.Park HS, Jung HY, Park EY, et al. Cutting edge: Direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol (Baltimore, MD: 1950) 2004;173(6):3589–93. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 39.Ben Mkaddem S, Pedruzzi E, Werts C, et al. Heat shock protein gp96 and NAD(P)H oxidase 4 play key roles in Toll-like receptor 4-activated apoptosis during renal ischemia/reperfusion injury. Cell Death Differ. 2010;17(9):1474–85. doi: 10.1038/cdd.2010.26. [DOI] [PubMed] [Google Scholar]