Abstract
Background
A cholesterol‐lowering diet and several other dietary interventions have been suggested as a management approach either independently or as an adjuvant to drug therapy in children and adults with familial hypercholesterolaemia (FH). However, a consensus has yet to be reached on the most appropriate dietary treatment. Plant sterols are commonly used in FH although patients may know them by other names like phytosterols or stanols.
Objectives
To examine whether a cholesterol‐lowering diet is more effective in reducing ischaemic heart disease and lowering cholesterol than no dietary intervention in children and adults with familial hypercholesterolaemia. Further, to compare the efficacy of supplementing a cholesterol‐lowering diet with either omega‐3 fatty acids, soya proteins, plant sterols or plant stanols.
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Inborn Errors of Metabolism Trials Register, which is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated with each new issue of The Cochrane Library), quarterly searches of MEDLINE and the prospective handsearching of one journal ‐ Journal of Inherited Metabolic Disease. Most recent search of the Group's Inborn Errors of Metabolism Trials Register: 22 August 2013. We also searched PubMed to 05 February 2012.
Selection criteria
Randomised controlled trials, both published and unpublished, where a cholesterol‐lowering diet in children and adults with familial hypercholesterolaemia has been compared to other forms of dietary treatment or to no dietary intervention were included.
Data collection and analysis
Two authors independently assessed the trial eligibility and risk of bias and one extracted the data, with independent verification of data extraction by a colleague.
Main results
In the 2014 update of the review, 15 trials have been included, with a total of 453 participants across seven comparison groups. The included trials had either a low or unclear risk of bias for most of the parameters used for risk assessment. Only short‐term outcomes could be assessed due to the short duration of follow up in the included trials. None of the primary outcomes, (incidence of ischaemic heart disease, number of deaths and age at death) were evaluated in any of the included trials. No significant differences were noted for the majority of secondary outcomes for any of the planned comparisons. However, a significant difference was found for the following comparisons and outcomes: for the comparison between plant sterols and cholesterol‐lowering diet (in favour of plant sterols), total cholesterol levels, mean difference 0.30 mmol/l (95% confidence interval 0.12 to 0.48); decreased serum LDL cholesterol, mean difference ‐0.60 mmol/l (95% CI ‐0.89 to ‐0.31). Fasting serum HDL cholesterol levels were elevated, mean difference ‐0.04 mmol/l (95% CI ‐0.11 to 0.03) and serum triglyceride concentration was reduced, mean difference ‐0.03 mmol/l (95% CI ‐0.15 to ‐0.09), although these changes were not statistically significant. Similarly, guar gum when given as an add on therapy to bezafibrate reduced total cholesterol and LDL levels as compared to bezafibrate alone.
Authors' conclusions
No conclusions can be made about the effectiveness of a cholesterol‐lowering diet, or any of the other dietary interventions suggested for familial hypercholesterolaemia, for the primary outcomes: evidence and incidence of ischaemic heart disease, number of deaths and age at death,due to the lack of data on these. Large, parallel, randomised controlled trials are needed to investigate the effectiveness of a cholesterol‐lowering diet and the addition of omega‐3 fatty acids, plant sterols or stanols, soya protein, dietary fibers to a cholesterol‐lowering diet.
Plain language summary
Dietary modifications for managing familial hypercholesterolaemia
Familial hypercholesterolaemia is an inherited disorder characterised by a raised blood cholesterol, and premature ischaemic heart disease. Changing diet is an important management option to reduce low‐density lipoprotein cholesterol (the bad cholesterol) levels. Recently, certain lipid‐lowering drugs have shown to be safe and effective for the treatment of children with familial hypercholesterolaemia. However, dietary management remains important either on its own or combined with drug therapy. Several strategies are used to modify diet. This review aimed to compare cholesterol‐lowering dietary interventions either in combination with each other or alone. These interventions included adding omega‐3 fatty acids or plant sterols or plant stanols or soya proteins to diet. Fifteen trials were included in this updated review. The included trials had either a low or unclear risk of bias for most of the domains used for risk assessment. All the trials were short term and the majority were cross‐over in design. For most of the comparisons there was no significant difference in the various intervention strategies when compared to cholesterol‐lowering diet. However, for total cholesterol levels, serum low density lipoprotein (LDL) concentrations, a significant benefit was obtained with plant sterols. However, before drawing any conclusions, methodological problems with pooling results from cross‐over trials should be considered. There is a need for long‐term trials with parallel group design to assess the potential benefits and harms of a cholesterol‐lowering diet.
Background
Description of the condition
Familial hypercholesterolaemias are a group of genetic disorders causing severe elevations of blood cholesterol levels. Total cholesterol concentrations in heterozygous familial hypercholesterolemia (FH) patients are in the range of 9 to 14.2 mmol/l (350 to 550 mg/dL) and in homozygotes range from 16.8 to 25.9 mmol/l (650 to 1000 mg/dL) (Goldberg 2011; SBRG 1991). This disorder is one of the most common congenital metabolic disorders; the prevalence of heterozygous FH is approximately 1 in 300 to 500 with much higher incidence in certain populations, such as the Afrikaners, Christian Lebanese, Finns, and French‐Canadians (Marais 2004). The characteristic features of FH include elevated levels of low density lipoprotein cholesterol (LDL‐C) and total cholesterol (TC) in the circulation, deposits of cholesterol in peripheral tissues, presence of tendon xanthomas and accelerated atherosclerosis, leading to premature cardiovascular events (Goldstein 1995). Primary mutations causing FH are either due to defects in the low density lipoprotein‐receptor gene (LDLR), apolipoprotein B‐100 gene (APOB), or proprotein convertase subtilisin/kexin type 9 gene (PCSK9), singly or in combination (Rader 2003). The most prevalent of these genetic defects are defects in the LDLR gene with approximately 1600 known (till date) mutations in the LDLR gene causing almost 85% to 90% cases of FH. Defects in the APOB gene account for 5% to 10% of FH in northern European population (less in other populations). The PCSK9 gene defects account for about 5% of cases of FH (Hopkins 2011).The most severe form is related to total lack of receptors (receptor‐negative mutations), while ‘receptor‐defective’ mutations that comprise most of the mutations are usually accompanied by lesser symptoms (Austin 2004).
It is recommended that in children under 16 years of age, diagnosis of FH is based on a total cholesterol level of above 6.7 mmol/l (260 mg/dl) and a LDL cholesterol of above 4.0 mmol/l (155 mg/dl) on two measurements taken one month apart (Wray 1996). In the 1994 revision of the Simon Broome Register Group definition, cases are categorised as 'definite' or 'possible' (Marks 2003). According to the revision, 'DNA based evidence of an LDL‐receptor mutation or familial defective apoB‐100' was added as a sufficient criteria for 'definite' familial hypercholesterolaemia diagnosis. The aim of treatment in children and adults is the reduction of blood LDL cholesterol concentrations in order to reduce the risk of ischaemic heart disease.
Management of FH aims at lowering LDL by ≥ 50% or to < 3.36 mmol/l (130mg/dL). Statins are the most preferred pharmacological agents recommended for the treatment of FH along with diet and physical activity management in all age groups (Avis 2007; Shafiq 2007). Four statins (lovastatin, simvastatin, pravastatin and atorvastatin) have also been approved by U.S. Food and Drug Administration (US FDA) for use in children with familial hypercholesterolaemia. Children who do not achieve the LDL cholesterol goal after prescribed initial statin dosing need higher dose of statin or addition of another lipid lowering agent. Ezetimibe, a cholesterol absorption inhibitor, is recommended as a monotherapy or in combination with statins in children and adolescents (Yeste 2009). Bile acid sequestrants cholestyramine and cholestipol are not recommended for use in pediatric age group due to severe gastrointestinal side effects and poor palatability. Colesvelam, another bile acid sequastrant, can be used in boys aged 7 to 10 years and in postmenarchal girls as monotherapy or as adjuvant to statins. Niacin and fibrates are not recommended in the pediatric age group due to their adverse effects (O'Connor 1990; Tonstad 1997a).
Description of the intervention
A cholesterol‐lowering diet based on the following principles is recommended in the United Kingdom, the USA and elsewhere for the dietary treatment of FH (Maclean 1994; Goldstein 1995; AHA Statement 2007):
reduction in the intake of saturated fatty acids (fatty acids are components of fats);
reduction in dietary cholesterol intake;
reduction in total fat intake;
manipulation of carbohydrate intake to replace the energy deficit of the low fat diet;
increasing the intake of certain dietary components, e.g. garlic, onions, soy protein, plant sterols and stanols and omega‐3 (ω‐3) fatty acids, barley, psyllium, oat bran and rice bran.
How the intervention might work
The currently prescribed diet is sometimes considered to be monotonous and can lead to problems with compliance. The reduction in fat intake, if taken to the extreme, could potentially lead to a deficiency of essential fatty acids and fat soluble vitamins and a reduction in the overall energy content of the diet which has implications for satiety and growth in children who have relatively high energy requirements. An increase in the carbohydrate content of the diet may lead to raised blood levels of another type of fat, called triglyceride, which is also a risk factor for ischaemic heart disease. In addition, the aim of the diet is to decrease the total cholesterol concentration in the blood. However this form of dietary treatment may result in not only a reduction of LDL cholesterol but also high density lipoprotein (HDL) levels (Howell 1997). HDL is thought be involved in the removal of cholesterol from the blood and therefore any intervention which lowers the levels of HDL in the blood could have a detrimental effect. In view of this, a number of other dietary therapies have been considered for the treatment of FH, including:
manipulation of different types of fatty acids whilst maintaining normal total fat intake;
increasing dietary intake of soluble fibre;
increasing the dietary intake of antioxidants;
increasing the intake of certain dietary components, e.g. garlic, onions, soy protein, plant sterols and stanols and omega‐3 (ω‐3) fatty acids (AHA Statement 2007).
Several scientific and authoritative bodies recommend the daily consumption of 2 g plant stanols or plant sterols for improving blood lipid levels (US FDA 2010). Phytosterols, found in fat‐soluble fractions of plants, chemically resemble cholesterol and inhibit cholesterol absorption in the small intestine. They reduce plasma total and LDL‐C levels (Nigon 2001).
Oral omega‐3 ethyl esters improve the lipid profile principally by reducing TG levels. However, changes in TC and HDL‐C were generally not found to be clinically significant, with a small net increase in LDL‐C associated with a shift toward less atherogenic LDL subfractions (Levantesi 2010)
β‐glucan contained in soy protein has been shown to slow gastric emptying, digestion, and absorption (Schneeman 1998). This causes increased excretion of bile acids and neutral sterols, increasing catabolism of cholesterol, and reduced absorption of cholesterol and fat (Marlett 1997).
Various soluble fibers reduce total and LDL Cholesterol as has been previously shown (Brown 1999). However, this effect was only modest. The lipid lowering effects of soluble dietary fibers acts through its ability to increase intraluminal viscosity thereby affecting the entero‐hepatic recirculation ob bile acids and lipid metabolism.
Why it is important to do this review
The majority of these interventions do not appear to have been adequately assessed and consensus has yet to be reached on the most appropriate dietary treatment for FH. The aim of this review was to assess the effectiveness of the currently recommended cholesterol lowering diet compared to no dietary treatment or to other forms of dietary intervention.
Objectives
The aims of this review were to examine in children and adults with familial hypercholesterolaemia.
Whether manipulating the fat, protein or carbohydrate content of the diet influences serum lipid levels and the risk of ischaemic heart disease;
What effect does adding ω‐3 fatty acids (or their ethyl esters) to the background diet have on serum lipid levels and the risk of ischaemic heart disease?
What effect does adding plant sterols or stanols (both usually given in the form of esters) to the background diet have on serum lipid levels and the risk of ischaemic heart disease? Is there any dose response effect?
Does adding soy protein to the background diet influence serum lipid levels and the incidence of ischaemic heart disease?
Does adding dietary fibers such as barley, oat bran, rice bran , flax seeds or psyllium influence serum lipid levels and the incidence of ischemic heart disease?
Does using any of the above dietary strategies in addition to lipid lowering drugs have any added benefit?
Post hoc change:these comparisons in the current update have been changed from the previous review, in lieu with the growing knowledge about the effects of dietary supplements in altering blood lipid levels.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), both published and unpublished. Trials where quasi‐randomisation methods such as alternation were included if there was sufficient evidence that the treatment and comparison groups were comparable in terms of clinical and nutritional status.
Types of participants
Children and adults with familial hypercholesterolaemia. Trials which included people with familial hypercholesterolaemia alongside those with non‐familial hypercholesterolaemia were only included if the group of familial individuals was well defined and the results for this group were available.
Types of interventions
Cholesterol‐lowering diet or any other dietary intervention intended to lower serum total and LDL cholesterol, for a period of at least six months. When dietary treatment had been used as a control in a trial of cholesterol‐lowering drugs, these trials were excluded. However, trials were included in the review when the only difference between the control and treatment groups was the diet, for example, if a drug treatment alone was compared to the same drug treatment in combination with dietary treatment. Trials where one form of modified dietary intake was compared to another form of dietary intake were included if the comparison was done in a head‐to‐head comparison.
Types of outcome measures
Primary outcomes
Evidence and incidence of ischaemic heart disease
Number of deaths
Age at death
Secondary outcomes
Fasting serum total cholesterol concentration
Fasting serum LDL cholesterol
Fasting serum HDL cholesterol
Fasting serum triglyceride concentration
Fasting apolipoprotein A‐1concentration,
Fasting apolipoprotein B‐100 concentration
Quality of life
Compliance
Morbidity
Weight, height and other measures of nutritional status
Micronutrient intake
Search methods for identification of studies
Electronic searches
Relevant trials were identified by searching the Inborn Errors of Metabolism Trials Register using the term: diet*.
The Inborn Errors of Metabolism Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of The Cochrane Library), quarterly searches of MEDLINE and the prospective handsearching of one journal ‐ Journal of Inherited Metabolic Disease. Unpublished work is identified by searching through the abstract books of the Society for the Study of Inborn Errors of Metabolism conference and the SHS Inborn Error Review Series. For full details of all searching activities for the register, please see the relevant section of the Cystic Fibrosis and Genetic Disorders Group Module.
Date of the most recent search of the Cochrane Cystic Fibrosis and Genetic Disorders Group's Inborn Errors of Metabolism Register: 22 August 2013.
An additional search of of the Cochrane Central Register of Controlled Trials (CENTRAL) was undertaken (05 March 2012) (Appendix 1).
Searching other resources
Clinicaltrials.gov (www.clinicaltrials.gov) was also searched. Five trials were identified, none of them was completed and the recruitment status was unknown .
Additional trials were identified from handsearching the Journal of Inherited Metabolic Disease (from inception (1978) to 5 March 2012).
Additional trials were identified from the reference lists of identified trials. Unpublished work was identified through the searching of the abstract books of the major conference on inborn errors of metabolism and metabolic disease.
Data collection and analysis
Selection of studies
At initial review stage and for each update, two authors independently selected the trials to be included in the review.
Data extraction and management
Two review authors (AR and AM) independently extracted data using a pre‐designed data extraction form that contained publication details, patient population, randomisation, allocation concealment, details of blinding measures, description of interventions and results (Zavala 2006). They resolved any differences in the extracted data by consulting the other review authors (NS, SM and MS). The data entered into Review Manager software (RevMan 5.1) for was rechecked for accuracy (RevMan 2011).
Due to the diverse range of dietary interventions suggested for FH, the authors divided the trials into the following comparisons ‐ cholesterol lowering diet with no intervention; ω‐3 fatty acids added to cholesterol lowering diet with cholesterol lowering diet alone; plant sterols added to cholesterol lowering diet with cholesterol lowering diet alone; plant stanols added to cholesterol lowering diet with cholesterol lowering diet alone; soya protein added to cholesterol lowering diet with cholesterol lowering diet alone; barley added to cholesterol lowering diet with cholesterol lowering diet alone; and dietary modification with lipid lowering drugs; and lipid lowering drugs alone.
The authors planned to group outcome data into those measured at up to one, three, six and twelve months and annually thereafter. However, as was the case, if outcome data were recorded at other time periods (e.g. one‐ to two‐month data) then the authors planned to consider examining these as well. Between a fortnight and one month is generally the time when the treatment effects of dietary intervention on lipids become visible. In order to see how the effects are maintained, analyses at longer periods are desirable. For the primary outcomes, analysing the results of longer follow‐up is necessary. For outcomes relating to weight, it may not be apparent in the trials whether an increase or decrease in weight would be desirable. For example, in a group of overweight adults with FH, a reduction in body weight may be desirable. However, for adults of normal body weight, such a reduction may not be desirable. Therefore, unless a judgement can be made on whether it is desirable for weight to increase, decrease or remain static during a trial, the authors planned to discuss data on body weight and related outcomes in the results section of the review, but not include these in the meta‐analysis.
Assessment of risk of bias in included studies
The authors independently assessed the following domains as either low, unclear or high risk of bias:
sequence generation;
allocation concealment;
blinding (of participants, personnel and outcome assessors);
incomplete outcome data addressed;
free of selective outcome reporting;
free of other bias.
Overall, trials were considered at high‐risk of bias if we could only assess the majority of domains as having a high or unclear risk. The authors resolved any differences by consultation.
Measures of treatment effect
For future updates of the review, if we have data for the two primary outcomes of incidence of ischaemic heart disease and death, the number of events and the total number randomised in each group will be taken to calculate the odds ratio and 95% confidence intervals (CIs).
We analysed continuous outcomes using the mean difference (MD) and associated 95% CIs. For future updates, we will calculate the standardised mean difference (SMD) if different scales of measurement have been used. When only the standard error (SE) was provided, we converted this to the SD by multiplying the SE by the square root of the number of participants.
Unit of analysis issues
Where we obtained data from cross‐over trials, we would have undertaken a paired analysis if possible, to allow a within‐individual comparison of the treatment intervention. This is the preferred method of analysis of data from cross‐over trials (Elbourne 2002). As these data were not available from any of the trials included in the review, we used data from the first arm of two of the trials in the analysis (Laurin 1991; Wolfe 1992). For the remaining trials, data presented in the original papers were combined from both treatments and control arms of the trials, thereby ignoring the cross‐over design. We included such data in the meta‐analysis, which may be considered to be justified but unsatisfactory. This should be taken into account when considering the results of this review. We attempted to contact the authors but no response was obtained.
Dealing with missing data
In order to allow an intention‐to‐treat analysis, the authors would have sought data on the number of participants with each outcome event, by allocated treatment group, irrespective of compliance and whether or not the participant was later thought to be ineligible or otherwise excluded from treatment or follow up.
Data for the Guardamagna trial have not been included in the present analysis since the authors have not reported the results of subgroup of patients with familial hypercholesterolaemia (Guardamagna 2011a). The authors were requested to supply these data through electronic communication. At the time of writing this review, these data have not been received.
Assessment of heterogeneity
We tested for heterogeneity between trial results using a standard chi‐square test, P < 0.1 was considered statistically significant.
We also used the I2 statistic as a measure of heterogeneity (Higgins 2003). This describes the percentage of the variability in effect estimates that is due to heterogeneity rather than chance. We used the following ranges and descriptions:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
The authors planned to assess publication bias with the means of a funnel plot. The primary outcome measure was to be the main outcome for generation of the funnel plot. In the absence of an adequate number of trials reporting the primary outcome, any secondary outcome for which three or more trials were available, would have been used for funnel plot construction.
The authors intended to assess outcome reporting bias ideally by comparing the original trial protocols with the final published papers. If the protocols were not available the authors planned to compare the outcomes that were described as being measured in the 'Methods' section of the final papers with the 'Results' section to identify any outcomes that were not reported. In addition, the authors clinical knowledge would help them identify any outcomes they would normally expect to be measured, but which were not reported.
Data synthesis
We performed a fixed‐effect meta‐analysis where we observed no statistically significant heterogeneity. Otherwise, we used a random‐effects model.
Subgroup analysis and investigation of heterogeneity
Where heterogeneity did exist between the trials (P < 0.1 (chi‐square test) or substantial to considerable heterogeneity as defined by values of I2 above, was used as evidence of statistical heterogeneity), we investigated this and performed a random‐effects meta‐analysis.
We planned to undertake a subgroup analysis on those trials carried out in children.
Sensitivity analysis
We planned to perform a sensitivity analysis based on the risk of bias of the trials, including and excluding quasi‐randomised trials; however, we identified insufficient trials to allow such analysis.
Results
Description of studies
Results of the search
In a previous version of this review 377 references were identified from the electronic and manual search strategies. In this updated version of the review, we detected 397 references. We identified and retrieved 17 new and potentially relevant trials, of which 13 were excluded as they did not meet our inclusion criteria (seeCharacteristics of excluded studies), therefore, there are now a total of 375 excluded studies. For this update, four new trials were included (Guardamagna 2011a; Ketomäki 2004a; Nigon 2001; Wirth 1982), bringing the total number of included trials to 15.
A further two studies are listed as 'Studies awaiting classification' (Fuentes 2008; Stein 2007). We identified one ongoing study (Párraga ongoing).
Included studies
In total, 15 trials met the criteria for inclusion in the review with a total of 453 participants (Amundsen 2002; Balestrieri 1996; Chisholm 1992; Engler 2004; Guardamagna 2011a; Gylling 1995; Ketomäki 2003; Ketomäki 2004a; Ketomäki 2005; Laurin 1991; Neil 2001; Nigon 2001; O'Neill 2004; Wolfe 1992;Wirth 1982). None of the included trials reported data on the primary outcomes of this review. Long‐term data were not available for any of the outcomes. After consultation with experts in the field of FH, it was decided to include short‐term trials as information on change in serum lipid levels; nutritional status and nutritional intake from such trials could be considered useful.
We report on ten different interventions separately.
01 Cholesterol‐lowering diet compared with no dietary intervention or nutritional advice
We included two trials in this intervention (Chisholm 1992; Guardamagna 2011a). The first of which was conducted in adults with FH (Chisholm 1992). The trial was a short‐term randomised controlled cross‐over trial in 19 patients with three eight‐week treatment periods in each arm (high‐fat diet followed by low‐fat diet followed by high‐fat diet compared to low‐fat diet followed by high‐fat diet followed by low‐fat diet). All participants continued with lipid‐lowering medication (simvastatin) throughout the trial. In the second trial 40 children were randomised to receive either cholesterol lowering diet comprising of yeast rice extract, monacolins, policosanaols, folic acid coenzyme Q10, astaxanthin or placebo (Guardamagna 2011a). Patients either had familial hypercholesterolemia (n = 24 ) or combined familial hyperlipidaemia (n = 16). No results from this trial could be included in the review as no subgroup analysis for patients with FH was undertaken separately.
02 Supplementation with omega‐3 fatty acids given with a cholesterol‐lowering diet compared to a cholesterol‐lowering diet alone
Two trials assessed the effect of adding ω‐3 fatty acids to a cholesterol‐lowering diet (Balestrieri 1996; Engler 2004). The Balestrieri trial assessed the impact of increasing the intake of fish oils in adults (n = 16) with FH (Balestrieri 1996). The Engler trial investigated the effect of docosahexaenoic acid (DHA) supplementation to a cholesterol‐lowering diet in children with FH (n = 20) (Engler 2004). Both trials were of cross‐over design, of short duration and provided data which we were able to include in a meta‐analysis.
03 Cholesterol‐lowering diet compared with dietary interventions to increase intake of plant stanols
We included two cross‐over trials, one of which reported on children with FH (Gylling 1995) and one on adults (Ketomäki 2004a). The Gylling trial (n = 14), which was a short‐term trial, evaluated the effect of sitostanol (3 g/day) ester dissolved in rapeseed oil‐rich margarine for six weeks in a double‐blind cross‐over design (Gylling 1995). The Ketomaki trial (n = 5) studied the effect of both plant stanol and sterol ester spreads on triglyceride‐rich lipoprotien (TRL) removal in statin‐treated patients with FH using intravenous intralipid‐squalene fat tolerance test (Ketomäki 2004a).
04 Cholesterol‐lowering diet compared with dietary interventions to increase intake of plant sterols
For this comparison, five trials have been included (Amundsen 2002; Guardamagna 2011a; Ketomäki 2004a; Neil 2001; Nigon 2001). Three of the trials were on adults (Ketomäki 2004a; Neil 2001; Nigon 2001) and two on children (Amundsen 2002; Guardamagna 2011a). All five trials were cross‐over in design. The Neil trial presented data on the trials FH subgroup (n = 30) (type IIa patients were also included (n = 26)) as a parallel group (Neil 2001). All five trials were short‐term with each arm of the trial lasting between one and two months (with variable washout periods).
The Amundsen (n = 41), Neil and Nigon (n = 53) trials compared a plant sterol‐enriched fat spread with a control fat spread not enriched with plant sterols (Amundsen 2002; Neil 2001; Nigon 2001). The Ketomaki trial (n = 5) compared low fat plant sterol ester spread or low fat plant stanol ester spread over and above ongoing drug therapy (Ketomäki 2004a). The Guardamagna trial (n = 24) compared a dietary supplement containing 200 mg red yeast rice extract, corresponding to 3 mg of monacolins, and 10 mg policosanols once‐daily versus placebo (Guardamagna 2011a).
05 Soy protein as a form of dietary intervention compared to another form of dietary intervention or drug or no treatment
No trials were identified.
06 Dietary fibers as a form of dietary intervention compared to another form of dietary intervention or drug or no treatment
The dietary fibers which were considered were barley, guar gum, psyllium, oat bran, flax seed and rice bran. Only one trial (n = 12) was found which satisfied inclusion criteria (Wirth 1982). In this trial guar gum was administered with bezafibrate and this was compared with bezafibrate given alone. The trial included adult patients with familial hypercholesterolemia.
07 Dietary modification with lipid‐lowering drugs
No trials were identified.
08 Lipid‐lowering drugs alone
No trials were identified.
09 Cholesterol‐lowering diet compared to a high‐protein diet
We were able to include two cross‐over trials for this intervention (Laurin 1991; Wolfe 1992). One trial assessed the effect of soy products in children (n = 10) with FH (Laurin 1991), while the other assessed the effect of a high‐protein diet in adults with FH (n = 5) (Wolfe 1992). Both were short‐term trials with each arm of the trial lasting between one and three months.
10 Comparing one form of dietary intervention with another, where cholesterol‐lowering diet is not the control group
Three short‐term trials of adults with FH were included in this intervention group (Ketomäki 2003; Ketomäki 2005; O'Neill 2004). In the 2005 Ketomaki trial, adults with FH (n = 18) receiving hypolipidaemic drugs were randomised to receive either plant sterols or stanols. The trial was of cross‐over design and did not allow the comparison of the addition of plant sterols or stanols supplementation to drug treatment (Ketomäki 2005). In another trial by Ketomaki, 16 out of 23 children had FH (Ketomäki 2003). The data from these children were not reported separately and the authors were contacted for this information. This trial compared plant sterol and sterol ester spreads added to low‐fat diet given to all the participants. The third trial was of parallel design with three separate treatment groups: plant sterols versus low‐dose plant stanols versus high‐dose plant stanols (O'Neill 2004). This trial had 69 FH participants who were included alongside unaffected individuals (O'Neill 2004). The authors did provide the data for the 69 individuals with FH. However, these data were not in the format which could be used for analysis; percentage change in the lipid levels were given instead of actual values.
Excluded studies
There were over 300 studies excluded for the following reasons: not being an RCT; not being trials of dietary interventions; not including participants with FH; and including familial participants alongside non‐familial participants but not as a well‐defined subgroup.
Studies awaiting classification
In addition two studies were classified as studies awaiting classification for reasons that include non‐availability of required data or inadequacy of dietary intervention (Fuentes 2008; Stein 2007).
Risk of bias in included studies
Please refer to the additional figure for a summary of the risk of bias (Figure 1).
1.
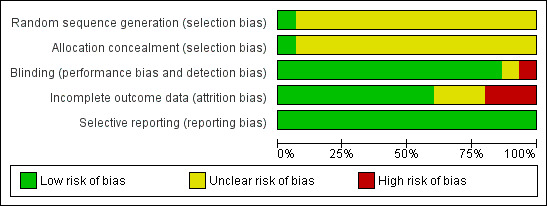
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Generation of the randomisation sequence was adequate in one (low risk of bias), where authors have stated that computer‐generated random numbers were used to assign the participants to either test or the control group with equal probability (Neil 2001) and unclear in the remaining 13 trials.
Concealment of allocation was adequate in one trial (low risk of bias) where the trial reports have described the methods adopted for assuring allocation concealment (Neil 2001). Tamper‐proof block randomisation was used and clinic and lab staff remained unaware of the assigned treatment throughout the trial. However, concealment of allocation was unclear for the remainder of the trials (unclear risk of bias) (Amundsen 2002; Balestrieri 1996; Chisholm 1992; Engler 2004; Guardamagna 2011a; Gylling 1995; Ketomäki 2003; Ketomäki 2004a; Ketomäki 2005; Laurin 1991; O'Neill 2004; Wolfe 1992; Wirth 1982).
Blinding
Twelve trials were reported as being double‐blinded (Amundsen 2002; Balestrieri 1996; Engler 2004; Guardamagna 2011a; Gylling 1995; Ketomäki 2003; Ketomäki 2004a; Ketomäki 2005; Laurin 1991; Neil 2001; Nigon 2001; O'Neill 2004). Information regarding blinding was not given in the other trials (Chisholm 1992; Laurin 1991; Wirth 1982). Assessment bias could therefore not be ruled out for these trials.
Incomplete outcome data
It was unclear if an intention‐to‐treat analysis was carried out in one of the trials, giving an unclear risk of bias (Chisholm 1992). In eight trials intention‐to‐treat analysis was considered adequate giving a low risk of bias (Engler 2004; Gylling 1995; Ketomäki 2003; Ketomäki 2005; Neil 2001; Wolfe 1992; Ketomäki 2004a; Nigon 2001); and in three trials participants were withdrawn from the trials and not included in the final analysis, therefore intention‐to‐treat analysis was not applied (Amundsen 2002; Balestrieri 1996; Laurin 1991). Two participants withdrew from one trial due to medical reasons (one suffered a heart attack and one required vascular surgery) (Balestrieri 1996). Two participants were withdrawn from another trial for lack of compliance and elevated serum lipid levels (Laurin 1991). Two trials undertook a per protocol analysis (Guardamagna 2011a; O'Neill 2004). In the former, six of them discontinued the trial before visit 1: two had difficulties in drinking the yogurt; two had poor adherence to the diet program; one was unable to attend visits and only one reported recurrent abdominal discomfort. No sample attrition occurred in the Wirth trial (Wirth 1982).
Selective reporting
Selective reporting was not noted in any of the include trials (Amundsen 2002; Balestrieri 1996; Chisholm 1992; Engler 2004; Guardamagna 2011a; Gylling 1995; Ketomäki 2003; Ketomäki 2004a; Ketomäki 2005; Laurin 1991; Neil 2001; Nigon 2001; O'Neill 2004; Wirth 1982; Wolfe 1992).
Effects of interventions
All the trials from which data were extracted and included in the meta‐analysis employed a cross‐over design except one which was a parallel trial (O'Neill 2004). Only seven of the trials presented data in such a way that the preferred method of analysis could be conducted (Engler 2004; Ketomäki 2004a; Laurin 1991; Neil 2001; Nigon 2001; O'Neill 2004; Wolfe 1992). However, such data were not provided for all of the outcomes assessed in these trials. Re‐analysis of the data will be undertaken at a future date if we are successful in contacting the authors for individual patient data.
Only those comparison groups for which there are eligible trials are listed below.
Only short‐term outcomes are included in this review due to the length of the trials identified from the searches. For primary outcomes, incidence of ischaemic or atheromatous disease and deaths were not reported on in any of the included trials. For secondary outcomes, quality of life, compliance with treatment and morbidity were not assessed in any of the included trials. Therefore we have not listed these outcomes below.
Comparison 01: Cholesterol‐lowering diet compared with no dietary intervention or nutritional advice
Two trials were identified (Chisholm 1992; Guardamagna 2011a). In the first trial which included 19 participants (Chisholm 1992). No significant differences were found between the two interventions for any of the outcomes assessed in which data could be entered into the meta‐analysis. None of the results from the second trial could be included in the meta‐analysis since no subgroup analysis for patients with familial hypercholesterolemia was undertaken separately from those individuals with combined familial hyperlipidaemia (Guardamagna 2011a).
Primary outcomes
The primary outcomes of ischaemic heart disease, number of deaths and or age at death were not reported in the included trial (Chisholm 1992).
Secondary outcomes
1. Serum total cholesterol concentration (fasting)
There was no significant difference between treatments for this outcome, MD ‐0.40 (95% CI ‐0.95 to 0.15) mmol/l (Analysis 1.1) (Chisholm 1992).
1.1. Analysis.

Comparison 1 Cholesterol‐lowering diet compared to no dietary intervention or nutritional advice, Outcome 1 Fasting serum total cholesterol concentration (mmol/l).
2. Serum LDL cholesterol (fasting)
There was no significant difference between treatments for his outcome, MD ‐0.27 (95% CI ‐0.79 to 0.25) mmol/l (Analysis 1.2) (Chisholm 1992).
1.2. Analysis.
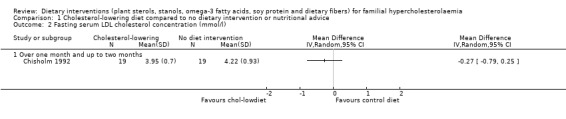
Comparison 1 Cholesterol‐lowering diet compared to no dietary intervention or nutritional advice, Outcome 2 Fasting serum LDL cholesterol concentration (mmol/l).
3. Serum HDL cholesterol (fasting)
There was no significant difference between treatments for his outcome, MD ‐0.11 (95% CI ‐0.34 to 0.12)mmol/l (Analysis 1.3) (Chisholm 1992).
1.3. Analysis.
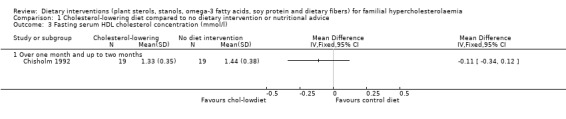
Comparison 1 Cholesterol‐lowering diet compared to no dietary intervention or nutritional advice, Outcome 3 Fasting serum HDL cholesterol concentration (mmol/l).
4. Serum triglyceride concentration (fasting)
There was no significant difference between treatments for his outcome, MD 0.06 (95% CI ‐0.43 to 0.55) mmol/l (Analysis 1.4) (Chisholm 1992).
1.4. Analysis.
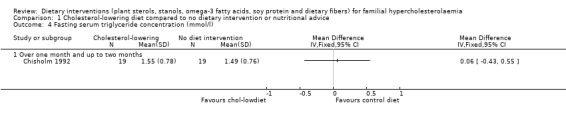
Comparison 1 Cholesterol‐lowering diet compared to no dietary intervention or nutritional advice, Outcome 4 Fasting serum triglyceride concentration (mmol/l).
5. Apolipoprotein A1concentration, the protein component of HDL cholesterol (fasting)
There was a significant difference in favouring the cholesterol‐lowering diet, MD ‐0.06 (95% CI ‐0.12 to ‐0.01) g/L (Analysis 1.5) (Chisholm 1992).
1.5. Analysis.
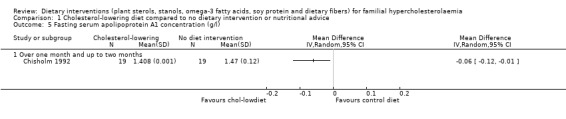
Comparison 1 Cholesterol‐lowering diet compared to no dietary intervention or nutritional advice, Outcome 5 Fasting serum apolipoprotein A1 concentration (g/l).
6. Apolipoprotein B‐100 concentration, the protein component of LDL cholesterol (fasting)
There was no significant difference in favouring the cholesterol‐lowering diet, MD ‐0.01 (95% CI ‐0.05 to 0.03) g/L (Analysis 1.6) (Chisholm 1992).
1.6. Analysis.
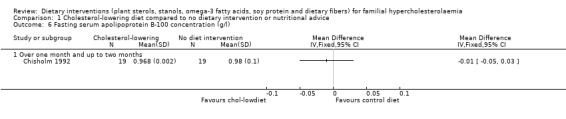
Comparison 1 Cholesterol‐lowering diet compared to no dietary intervention or nutritional advice, Outcome 6 Fasting serum apolipoprotein B‐100 concentration (g/l).
7. Quality of life
This outcome was not reported in the included trial (Chisholm 1992).
8. Compliance
This outcome was not reported in the included trial (Chisholm 1992).
9. Morbidity
This outcome was not reported in the included trial (Chisholm 1992).
10. Weight, height and other measures of nutritional status
Insufficient data (weight, height, body mass index (BMI)) were provided to allow inclusion of these outcomes in the meta‐analysis (Chisholm 1992). However, weight and BMI appeared to remain static during the two arms of the trial (mean BMI was 29.2 on the cholesterol‐lowering diet and 29.3 on a free diet, although no SDs were provided).
11. Micronutrient intake
Not assessed in the included trial (Chisholm 1992).
COMPARISON 02: Supplementation with ω‐3 fatty acids given with cholesterol‐lowering diet compared to cholesterol‐lowering diet alone
Two trials including 34 participants were identified (Balestrieri 1996; Engler 2004). No significant differences were found between the two interventions for any of the outcomes assessed.
Primary outcomes
The primary outcomes of ischaemic heart disease, number of death and or age at death were not reported in either of the trials (Balestrieri 1996; Engler 2004).The follow‐up period was too short to have any effect on primary outcomes (four weeks in the Baestrieri trial and six weeks in the Engler trial).
Secondary outcomes
1. Serum total cholesterol concentration (fasting)
Both trials reported on this outcome. There was no significant difference between treatment groups, MD ‐0.06 (95% CI ‐ 0.80 to 0.69) mmol/l (Analysis 2.1) (Balestrieri 1996; Engler 2004).
2.1. Analysis.
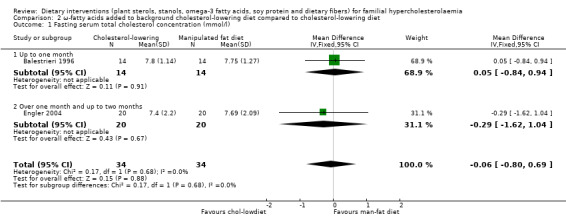
Comparison 2 ω‐fatty acids added to background cholesterol‐lowering diet compared to cholesterol‐lowering diet, Outcome 1 Fasting serum total cholesterol concentration (mmol/l).
2. Serum LDL cholesterol (fasting)
Both trials reported on this outcome. There was no significant difference between treatment groups, MD ‐0.12 (95% CI ‐0.93 to 0.69) mmol/l (Analysis 2.2) (Balestrieri 1996; Engler 2004).
2.2. Analysis.
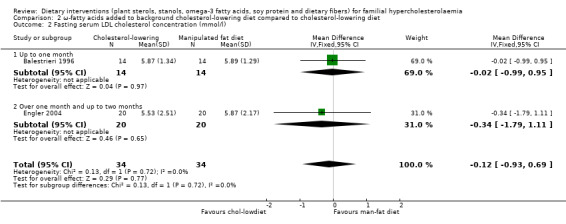
Comparison 2 ω‐fatty acids added to background cholesterol‐lowering diet compared to cholesterol‐lowering diet, Outcome 2 Fasting serum LDL cholesterol concentration (mmol/l).
3. Serum HDL cholesterol (fasting)
Both trials reported on this outcome. There was no significant difference between treatment groups, MD 0.02 (95% CI ‐0.10 to 0.13) mmol/l (Analysis 2.3) (Balestrieri 1996; Engler 2004).
2.3. Analysis.
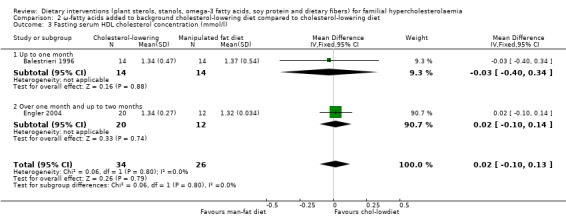
Comparison 2 ω‐fatty acids added to background cholesterol‐lowering diet compared to cholesterol‐lowering diet, Outcome 3 Fasting serum HDL cholesterol concentration (mmol/l).
4. Serum triglyceride concentration (fasting)
Both trials reported on this outcome. There was no significant difference between treatment groups, MD 0.18 (95% CI ‐0.07 to 0.43) mmol/l (Analysis 2.4) (Balestrieri 1996; Engler 2004).
2.4. Analysis.
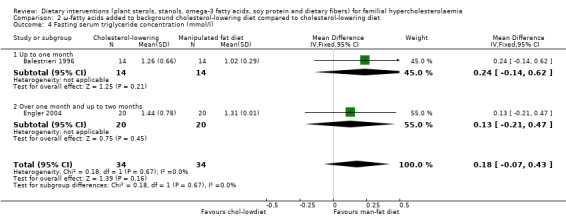
Comparison 2 ω‐fatty acids added to background cholesterol‐lowering diet compared to cholesterol‐lowering diet, Outcome 4 Fasting serum triglyceride concentration (mmol/l).
5. Apolipoprotein A1concentration, the protein component of HDL cholesterol (fasting)
This outcome was assessed by one trial (Balestrieri 1996). There was no significant difference between treatment groups, MD ‐0.02 (95% CI ‐0.35 to 0.31) g/L (Analysis 2.5).
2.5. Analysis.
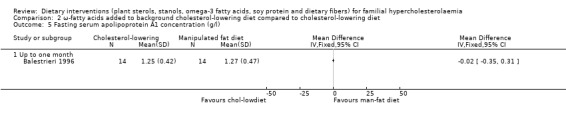
Comparison 2 ω‐fatty acids added to background cholesterol‐lowering diet compared to cholesterol‐lowering diet, Outcome 5 Fasting serum apolipoprotein A1 concentration (g/l).
6. Apolipoprotein B‐100 concentration, the protein component of LDL cholesterol (fasting)
This outcome was assessed by one trial (Balestrieri 1996). There was no significant difference between treatment groups, MD 0.01 (95% CI ‐21.99 to 22.01 g/L (Analysis 2.6).
2.6. Analysis.
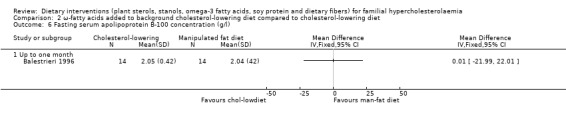
Comparison 2 ω‐fatty acids added to background cholesterol‐lowering diet compared to cholesterol‐lowering diet, Outcome 6 Fasting serum apolipoprotein B‐100 concentration (g/l).
7. Quality of life
Neither trial reported on this outcome (Balestrieri 1996; Engler 2004).
8. Compliance
Neither trial reported on this outcome (Balestrieri 1996; Engler 2004).
9. Morbidity
Neither trial reported on this outcome (Balestrieri 1996; Engler 2004).
10. Weight, height and other measures of nutritional status
Neither trial reported on this outcome (Balestrieri 1996; Engler 2004).
11. Micronutrient intake
Neither trial reported on this outcome (Balestrieri 1996; Engler 2004).
COMPARISON 03: Cholesterol‐lowering diet compared with dietary interventions to increase intake of plant stanols
Two trials were included, one on children (N = 14) (Gylling 1995) and one on adults (N = 5) (Ketomäki 2004a).
Primary outcomes
The authors did not evaluate any of the primary outcomes of ischaemic heart disease and number of deaths and or age at death in either of these trials (Gylling 1995; Ketomäki 2004a).
Secondary outcomes
1. Serum total cholesterol concentration (fasting)
There was a significant difference in cholesterol level between the stanol treatment and cholesterol‐lowering diet group favouring stanol treatment, MD 0.51 (95% CI 0.07 to 0.96) mmol/l after pooling the two trials (Analysis 3.1) (Gylling 1995; Ketomäki 2004a).
3.1. Analysis.
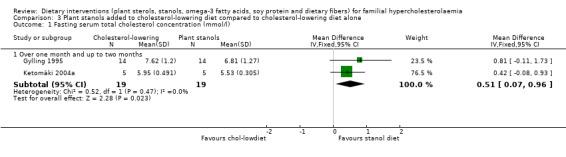
Comparison 3 Plant stanols added to cholesterol‐lowering diet compared to cholesterol‐lowering diet alone, Outcome 1 Fasting serum total cholesterol concentration (mmol/l).
2. Serum LDL cholesterol (fasting)
There was a significant difference between stanol treatment and the cholesterol‐lowering diet group favouring stanol treatment, MD 0.71 (95% CI 0.43 to 0.99) mmol/l after pooling (Analysis 3.2) (Gylling 1995; Ketomäki 2004a).
3.2. Analysis.
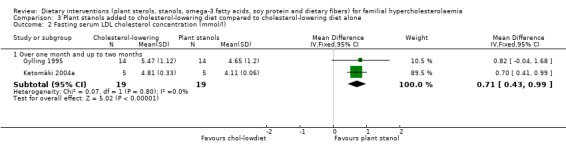
Comparison 3 Plant stanols added to cholesterol‐lowering diet compared to cholesterol‐lowering diet alone, Outcome 2 Fasting serum LDL cholesterol concentration (mmol/l).
3. Serum HDL cholesterol (fasting)
The difference between treatment groups after pooling did not quite reach statistical significance, MD ‐0.08 (95% CI ‐0.15 to ‐0.00) mmol/l (Analysis 3.3) (Gylling 1995; Ketomäki 2004a).
3.3. Analysis.
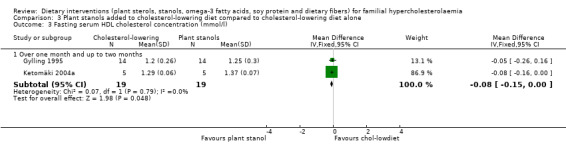
Comparison 3 Plant stanols added to cholesterol‐lowering diet compared to cholesterol‐lowering diet alone, Outcome 3 Fasting serum HDL cholesterol concentration (mmol/l).
4. Serum triglyceride concentration (fasting)
There was no significant difference between treatment groups after pooling, SMD 0.12 (95% CI ‐0.52 to 0.76) mmol/l (Analysis 3.4) (Gylling 1995; Ketomäki 2004a).
3.4. Analysis.
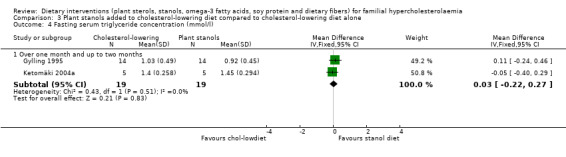
Comparison 3 Plant stanols added to cholesterol‐lowering diet compared to cholesterol‐lowering diet alone, Outcome 4 Fasting serum triglyceride concentration (mmol/l).
5. Apolipoprotein A1concentration, the protein component of HDL cholesterol (fasting)
This was not assessed in the included trials (Gylling 1995; Ketomäki 2004a).
6. Apolipoprotein B‐100 concentration, the protein component of LDL cholesterol (fasting)
This was not assessed in the included trials (Gylling 1995; Ketomäki 2004a).
7. Quality of life
Neither trial reported on this outcome (Gylling 1995; Ketomäki 2004a).
8. Compliance
Neither trial reported on this outcome (Gylling 1995; Ketomäki 2004a).
9. Morbidity
Neither trial reported on this outcome (Gylling 1995; Ketomäki 2004a).
10. Weight, height and other measures of nutritional status
Neither trial reported on this outcome (Gylling 1995; Ketomäki 2004a).
11. Micronutrient intake
Neither trial reported on this outcome (Gylling 1995; Ketomäki 2004a).
COMPARISON 04: Cholesterol‐lowering diet compared with dietary interventions to increase intake of plant sterols
Data from four trials with 129 participants were included in this comparison (Amundsen 2002; Ketomäki 2004a; Neil 2001; Nigon 2001). A fifth trial was also eligible, but the data from the trial done by Guardamagna have not been included in the present analysis since the authors have not reported the results of subgroup of patients with familial hypercholesterolaemia (Guardamagna 2011a). The authors were requested to supply these data through electronic communication. At the time of writing this review, these data have not been received. However, in their manuscript do mention that the dietary supplementation did favourably alter the lipid profile in patients with familial hypercholesterolaemia similar to that seen in the combined analysis of all the patients.
Primary outcomes
The authors did not evaluate any of the primary outcomes of ischaemic heart disease and number of deaths and or age at death in any of the trials.
Secondary outcomes
1.Serum total cholesterol concentration (fasting)
All the four trials reported on this outcome. There was a significant difference between sterol treated participants as compared to cholesterol‐lowering diet alone favour of sterol, MD 0.30 (95% CI 0.12 to 0.48) mmol/l (Analysis 4.1) (Amundsen 2002; Ketomäki 2004a; Neil 2001; Nigon 2001).
4.1. Analysis.
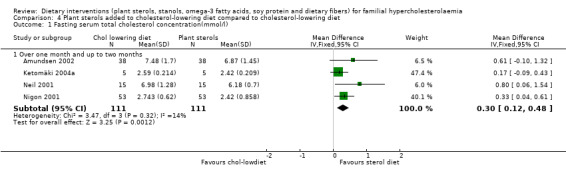
Comparison 4 Plant sterols added to cholesterol‐lowering diet compared to cholesterol‐lowering diet, Outcome 1 Fasting serum total cholesterol concentration(mmol/l).
2. Serum LDL cholesterol (fasting)
All the four trials reported on this outcome. The LDL was significantly lower with the sterol treatment, MD ‐0.60 (95% CI ‐0.89 to ‐0.31) mmol/l (Analysis 4.2) (Amundsen 2002; Ketomäki 2004a; Neil 2001; Nigon 2001).
4.2. Analysis.
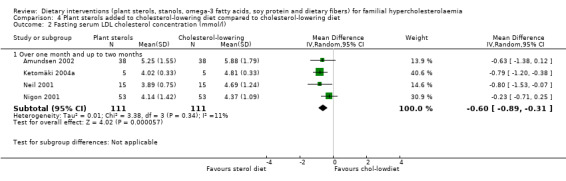
Comparison 4 Plant sterols added to cholesterol‐lowering diet compared to cholesterol‐lowering diet, Outcome 2 Fasting serum LDL cholesterol concentration (mmol/l).
3. Serum HDL cholesterol (fasting)
All the four trials reported on this outcome. The HDL levels were not significantly different between plant sterol treated and cholesterol‐lowering diet alone, MD ‐0.04 (95% CI ‐0.11 to 0.03) mmol/l (Analysis 4.3) (Amundsen 2002; Ketomäki 2004a; Neil 2001; Nigon 2001).
4.3. Analysis.
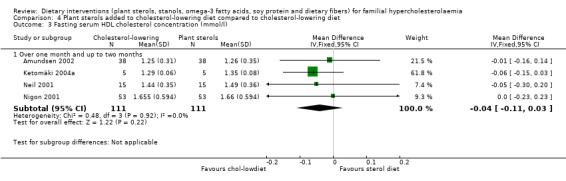
Comparison 4 Plant sterols added to cholesterol‐lowering diet compared to cholesterol‐lowering diet, Outcome 3 Fasting serum HDL cholesterol concentration (mmol/l).
4. Serum triglyceride concentration (fasting)
All the four trials reported on this outcome. The TG levels were not significantly lower with sterol treatment, MD ‐0.03 (95% CI ‐0.15 to 0.09) mmol/l, although there was considerable heterogeneity (Analysis 4.4) (Amundsen 2002; Ketomäki 2004a; Neil 2001; Nigon 2001).
4.4. Analysis.
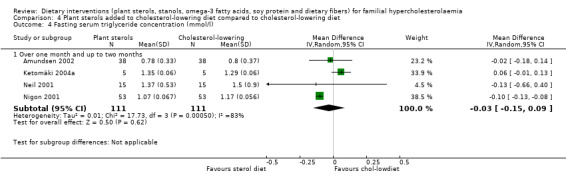
Comparison 4 Plant sterols added to cholesterol‐lowering diet compared to cholesterol‐lowering diet, Outcome 4 Fasting serum triglyceride concentration (mmol/l).
5. Apolipoprotein A1concentration, the protein component of HDL cholesterol (fasting)
This outcome was assessed by two trials (Amundsen 2002; Nigon 2001). There was no significant difference between treatment groups, MD 0.03 (95% CI ‐0.08 to 0.14) g/L (Analysis 4.5) (Amundsen 2002; Nigon 2001).
4.5. Analysis.
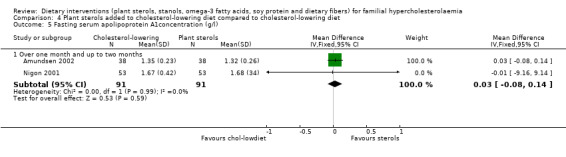
Comparison 4 Plant sterols added to cholesterol‐lowering diet compared to cholesterol‐lowering diet, Outcome 5 Fasting serum apolipoprotein A1concentration (g/l).
6. Apolipoprotein B‐100 concentration, the protein component of LDL cholesterol (fasting)
This outcome was assessed by two trials (Amundsen 2002, Nigon 2001). There was no significant difference between treatment groups, MD 0.02 (95% CI ‐0.09 to 0.13) g/L (Analysis 4.6).
4.6. Analysis.
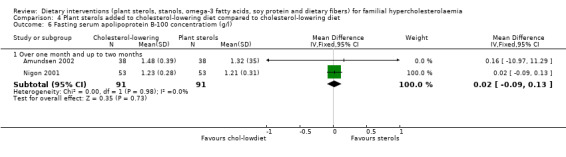
Comparison 4 Plant sterols added to cholesterol‐lowering diet compared to cholesterol‐lowering diet, Outcome 6 Fasting serum apolipoprotein B‐100 concentratiom (g/l).
7. Quality of life
None of the trials reported on this outcome (Amundsen 2002; Ketomäki 2004a; Neil 2001; Nigon 2001).
8. Compliance
None of the trials reported on this outcome (Amundsen 2002; Ketomäki 2004a; Neil 2001; Nigon 2001).
9. Morbidity
None of the trials reported on this outcome (Amundsen 2002; Ketomäki 2004a; Neil 2001; Nigon 2001).
10. Weight, height and other measures of nutritional status
None of the trials reported on this outcome (Amundsen 2002; Ketomäki 2004a; Neil 2001; Nigon 2001).
11. Micronutrient intake
None of the trials reported on this outcome (Amundsen 2002; Ketomäki 2004a; Neil 2001; Nigon 2001).
COMPARISON 06: Dietary fibers as a form of dietary intervention compared to another form of dietary intervention or drug or no treatment
One trial in adults was included (N = 12) (Wirth 1982).
Primary outcomes
The authors did not evaluate any of the primary outcomes of ischaemic heart disease and number of deaths and or age at death in any of the trials (Wirth 1982).
Secondary outcomes
1.Serum total cholesterol concentration (fasting)
There was no significant difference between guar gum and bezafibrate treated group as compared to group of patients treated with bezafibrate alone, MD ‐0.57 (95% CI ‐2.08 to 0.94) mmol (Analysis 6.1) (Wirth 1982).
6.1. Analysis.
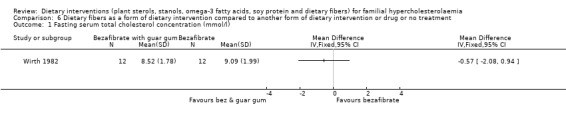
Comparison 6 Dietary fibers as a form of dietary intervention compared to another form of dietary intervention or drug or no treatment, Outcome 1 Fasting serum total cholesterol concentration (mmol/l).
2. Serum LDL cholesterol (fasting)
The LDL was significantly lower when guar gum was combined with bezafibrate compared to bezafibrate alone, MD ‐1.83 (95% CI ‐3.32 to ‐0.34) mmol (Analysis 6.2) (Wirth 1982).
6.2. Analysis.
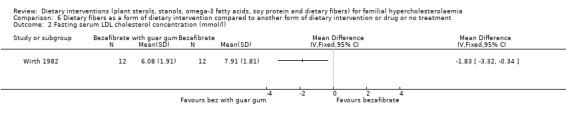
Comparison 6 Dietary fibers as a form of dietary intervention compared to another form of dietary intervention or drug or no treatment, Outcome 2 Fasting serum LDL cholesterol concentration (mmol/l).
3. Serum HDL cholesterol (fasting)
There was no change in the level of HDL in the group receiving guar gum in addition to bezafibrate, MD ‐0.18 (95% CI ‐0.46 to 0.10) mmol (Analysis 6.3) (Wirth 1982).
6.3. Analysis.
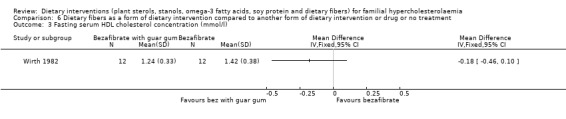
Comparison 6 Dietary fibers as a form of dietary intervention compared to another form of dietary intervention or drug or no treatment, Outcome 3 Fasting serum HDL cholesterol concentration (mmol/l).
4. Serum triglyceride concentration (fasting)
There was no significant change in this outcome with the use of guar gum, MD ‐0.41 (95% CI ‐0.12 to 0.94) mmol (Analysis 6.4) (Wirth 1982).
6.4. Analysis.
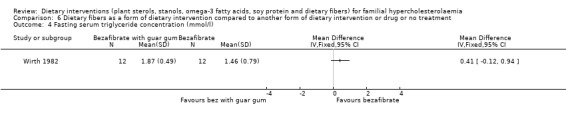
Comparison 6 Dietary fibers as a form of dietary intervention compared to another form of dietary intervention or drug or no treatment, Outcome 4 Fasting serum triglyceride concentration (mmol/l).
5. Apolipoprotein A1concentration, the protein component of HDL cholesterol (fasting)
No significant change was noted for this outcome, MD ‐0.04 (95% CI ‐6.75 to 6.83) gm/L (Analysis 6.5) (Wirth 1982).
6.5. Analysis.
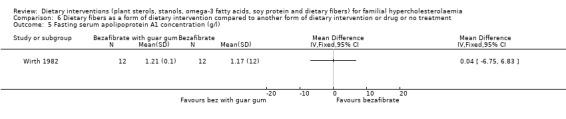
Comparison 6 Dietary fibers as a form of dietary intervention compared to another form of dietary intervention or drug or no treatment, Outcome 5 Fasting serum apolipoprotein A1 concentration (g/l).
6. Apolipoprotein B‐100 concentration, the protein component of LDL cholesterol (fasting)
There was a significant change noted for this outcome in favour of the use of guar gum with bezafibrate, MD ‐0.50 (95% CI ‐0.65 to ‐0.35) gm/L (Analysis 6.6) (Wirth 1982).
6.6. Analysis.
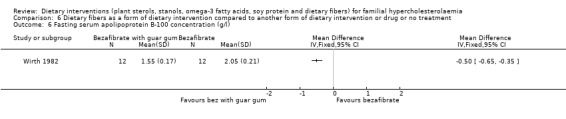
Comparison 6 Dietary fibers as a form of dietary intervention compared to another form of dietary intervention or drug or no treatment, Outcome 6 Fasting serum apolipoprotein B‐100 concentration (g/l).
7. Quality of life
This outcome was not reported in the included trial (Wirth 1982).
8. Compliance
The trial reported that none of the patients were excluded for inadequate compliance (Wirth 1982).
9. Morbidity
This was not reported in the included trial (Wirth 1982).
10. Weight, height and other measures of nutritional status
Only weight was reported in the trial. There was no significant difference in the weights in the two groups at the end of the trial period, MD ‐0.40 (95% CI ‐5.09 to 5.89) gm/L (Analysis 6.7) (Wirth 1982).
6.7. Analysis.
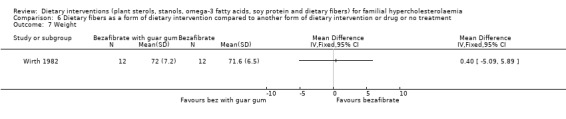
Comparison 6 Dietary fibers as a form of dietary intervention compared to another form of dietary intervention or drug or no treatment, Outcome 7 Weight.
11. Micronutrient intake
This was not evaluated in the included trial (Wirth 1982).
COMPARISON 09: Cholesterol‐lowering diet compared to a high‐protein diet
Two trials were included in this comparison (Laurin 1991; Wolfe 1992). One of a cholesterol‐lowering diet compared with a high‐protein diet (five participants) (Wolfe 1992) and one of a cholesterol‐lowering diet compared with a soy‐protein diet (10 participants) (Laurin 1991). No significant differences were found between the two interventions for any of the outcomes assessed.
Primary outcomes
The authors did not evaluate any of the primary outcomes of ischaemic heart disease and number of deaths and or age at death (Laurin 1991; Wolfe 1992).
Secondary outcomes
1. Serum total cholesterol concentration (fasting and non‐fasting)
Both trials reported on this outcome. There was no significant difference between treatment groups, MD 0.08 (95% CI ‐ 0.65 to 0.81) mmol/l (Analysis 5.1) (Laurin 1991; Wolfe 1992).
5.1. Analysis.
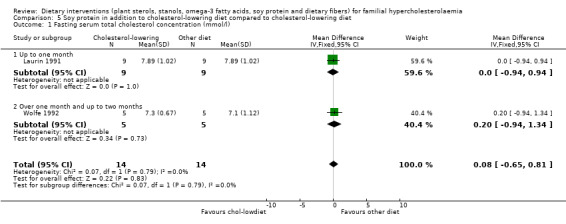
Comparison 5 Soy protein in addition to cholesterol‐lowering diet compared to cholesterol‐lowering diet, Outcome 1 Fasting serum total cholesterol concentration (mmol/l).
2. Serum LDL cholesterol (fasting and non‐fasting)
Both trials reported on this outcome. There was no significant difference between treatment groups, MD 0.12 (95% CI ‐0.46 to 0.69) mmol/l (Analysis 5.2) (Laurin 1991; Wolfe 1992).
5.2. Analysis.
Comparison 5 Soy protein in addition to cholesterol‐lowering diet compared to cholesterol‐lowering diet, Outcome 2 Fasting serum LDL cholesterol concentration (mmol/l).
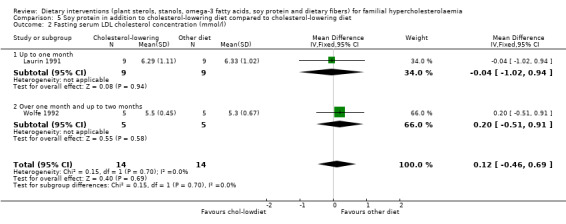
3. Serum HDL cholesterol (fasting and non‐fasting)
Both trials reported on this outcome. There was no significant difference between treatment groups, MD ‐0.07 (95% CI ‐0.23 to 0.08) mmol/l (Analysis 5.3) (Laurin 1991; Wolfe 1992).
5.3. Analysis.
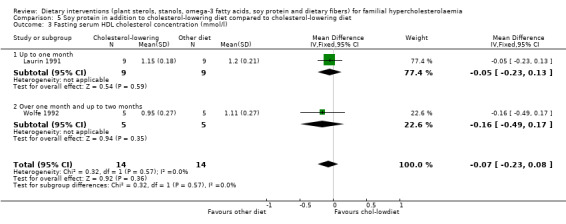
Comparison 5 Soy protein in addition to cholesterol‐lowering diet compared to cholesterol‐lowering diet, Outcome 3 Fasting serum HDL cholesterol concentration (mmol/l).
4. Serum triglyceride concentration (fasting and non‐fasting)
Both trials reported on this outcome. There was no significant difference between treatment groups, MD 0.25 (95% CI ‐0.01 to 0.50) mmol/l (Analysis 5.4) (Laurin 1991; Wolfe 1992).
5.4. Analysis.
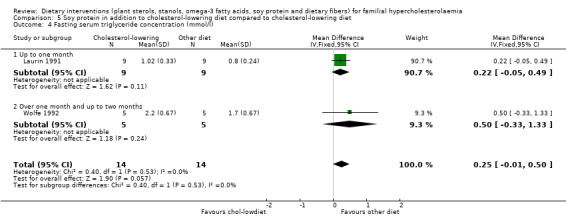
Comparison 5 Soy protein in addition to cholesterol‐lowering diet compared to cholesterol‐lowering diet, Outcome 4 Fasting serum triglyceride concentration (mmol/l).
5. Apolipoprotein A1concentration, the protein component of HDL cholesterol (fasting and non‐fasting)
This outcome was assessed by one trial (Laurin 1991). There was no significant difference between treatment groups, MD 0.04 (95% CI ‐5.84 to 5.92) g/L (Analysis 5.5) (Laurin 1991; Wolfe 1992).
5.5. Analysis.
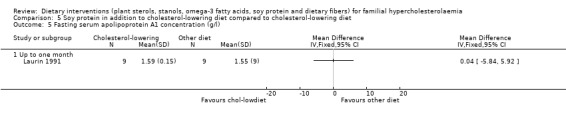
Comparison 5 Soy protein in addition to cholesterol‐lowering diet compared to cholesterol‐lowering diet, Outcome 5 Fasting serum apolipoprotein A1 concentration (g/l).
6. Apolipoprotein B‐100 concentration, the protein component of LDL cholesterol (fasting and non‐fasting)
This outcome was assessed by one trial (Laurin 1991). There was no significant difference between treatment groups, MD 0.00 (95% CI ‐3.92 to 3.92) mg/dL (Analysis 5.6).
5.6. Analysis.
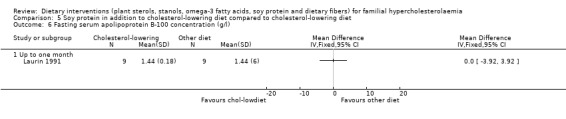
Comparison 5 Soy protein in addition to cholesterol‐lowering diet compared to cholesterol‐lowering diet, Outcome 6 Fasting serum apolipoprotein B‐100 concentration (g/l).
7. Quality of life
This outcome was not assessed in either trial (Laurin 1991; Wolfe 1992).
8. Compliance
This outcome was not assessed in either trial (Laurin 1991; Wolfe 1992).
9. Morbidity
This outcome was not assessed in either trial (Laurin 1991; Wolfe 1992).
10. Weight, height and other measures of nutritional status
These outcomes were assessed in one trial (Laurin 1991). There was no significant difference between treatment groups for any of these outcomes: weight, MD 0.00 (95% CI ‐7.58 to 7.58) kg (Analysis 5.7); height, MD 0.00 (95% CI ‐7.63 to 7.63) cm (Analysis 5.8); and BMI, MD ‐0.09 (95% CI ‐2.77 to 2.95) (Analysis 5.9). No pooling of data were possible as the results are from one trial only.
5.7. Analysis.
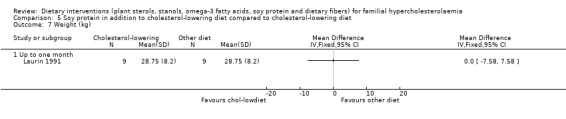
Comparison 5 Soy protein in addition to cholesterol‐lowering diet compared to cholesterol‐lowering diet, Outcome 7 Weight (kg).
5.8. Analysis.
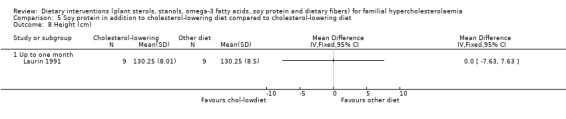
Comparison 5 Soy protein in addition to cholesterol‐lowering diet compared to cholesterol‐lowering diet, Outcome 8 Height (cm).
5.9. Analysis.
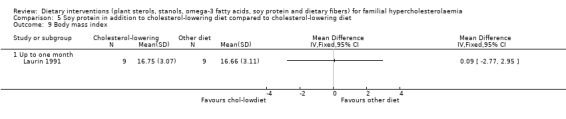
Comparison 5 Soy protein in addition to cholesterol‐lowering diet compared to cholesterol‐lowering diet, Outcome 9 Body mass index.
11. Micronutrient intake
This outcome was not assessed in either trial (Laurin 1991; Wolfe 1992).
COMPARISON 10: One form of dietary intervention compared to another form of dietary intervention
Three short‐term trials of adults with FH were included in this intervention group (Ketomäki 2003; Ketomäki 2005; O'Neill 2004). Two trials included participants with and without FH, but data are not presented separately in either trial for the subset of participants with FH (Ketomäki 2003; O'Neill 2004).
Primary outcomes
The authors did not evaluate any of the primary outcomes of ischaemic heart disease and number of deaths and or age at death (Ketomäki 2003; Ketomäki 2005; O'Neill 2004).
Secondary outcomes
1. Serum total cholesterol concentration (fasting and non‐fasting)
The results of 134 participants in the O'Neill trial demonstrated a significant reduction in serum total cholesterol (TC) from baseline at the end of two months in both the high‐dose (2.6 g) stanol group (HSTA) and the low‐dose (1.6 g) stanol group (LSTA) (O'Neill 2004). In the HSTA group, serum total cholesterol levels decreased from mean (SD) 6.1 (0.20) mmol/l at baseline to 5.3 (0.15) mmol/l (P < 0.001). This was also the case in the LSTA group after two months; serum total cholesterol levels showed a significant reduction from mean (SD) 5.8 (0.19) mmol/l at baseline to 5.5 (18.00) mmol/l (P < 0.001). In the sterol (STE) group (1.6 g), cholesterol levels were significantly reduced at one month from mean (SD) 5.8 (0.17) mmol (baseline) to 5.4 (0.15) mmol/l (P < 0.001) at one month. A subgroup analysis of the 69 FH participants was not presented.
In the second trial, the authors noted a significant reduction in total cholesterol levels following a five‐week intervention period by both stanol and sterol esters (percentage change from baseline (mean (SE of the mean (SEM)) ‐ 9 (3) and ‐6 (2), respectively) (Ketomäki 2003). The data for 16 participants with FH was not presented separately.
In the third trial, in the plant stanol group, the serum total cholesterol values reduced from mean (SD) 6.30 (0.24) at baseline to 5.65 (0.22) mmol/l while in STE group, TC reduced to 5.7 (0.21) mmol/l following two consecutive four‐week intervention periods (Ketomäki 2005). This reduction was significant as compared to baseline values. In both the groups the participants were on statins.
2. Serum LDL cholesterol (fasting and non‐fasting)
O' Neill observed a significant reduction from baseline in LDL cholesterol in all the three groups: HSTA mean (SD) 3.77 (0.18) to 3.30 (0.14) mmol/l (P < 0.001); LSTA mean (SD) 3.83 (0.16) to 3.54 (0.14) (P = 0.03); and STE mean (SD) 3.81 (0.15) to 3.63 (0.15) (P = 0.003) (O'Neill 2004). The data for 69 FH participants were not available.
In the earlier Ketomaki trial, a significant percentage reduction from baseline in LDL cholesterol was noted in both stanol and sterol groups, mean (SEM) ‐12% (3%) and ‐9% (3%) respectively (Ketomäki 2003). The data were expressed only as percentage reduction. Additionally, data for 16 FH participants were not given separately.
In the later Ketomaki trial, when given in addition to statins, a significant reduction from baseline in LDL cholesterol levels was noted in both the stanol group (mean (SD) 4.50 (0.21) to 3.81 (0.18))mmol/l and the sterol group (mean (SD) 4.50 (0.21) to 3.86 (0.19) mmol/l (Ketomäki 2005).
3. Serum HDL cholesterol (fasting and non‐fasting)
While O’Neill reported no statistically significant changes in HDL cholesterol levels in the LSTA and STE groups, a significant reduction in HDL cholesterol levels was noted after two months in the HSTA group (O'Neill 2004). Again, the data for 69 FH patients were not presented separately.
In the earlier Ketomaki trial, no significant difference in HDL cholesterol level was noted in any of the groups (Ketomäki 2003). In the later Ketomaki trial, when given over and above statins, sterols caused a significant increase in HDL cholesterol; from mean (SD) 1.26 (0.05) mmol/l at baseline to 1.37 (0.04) mmol/l (Ketomäki 2005).
4. Serum triglyceride concentration (fasting and non‐fasting)
O'Neill demonstrated significant decrease in triglyceride levels at two months in the HSTA group (‐15.0%) and no changes in the LSTA and the STE groups (O'Neill 2004).
No significant difference in triglyceride (TG) levels was observed in either the stanol or the sterol groups in the second trial (Ketomäki 2003). In the third trial, the authors concluded significant decrease in serum TG only in the sterol group from mean (SD) 1.19 (0.10) at baseline to 1.05 (0.09) following two consecutive four‐week intervention periods (Ketomäki 2005).
5. Apolipoprotein A1concentration, the protein component of HDL cholesterol (fasting and non‐fasting)
None of the three trials evaluated apolipoprotein A1 concentration (Ketomäki 2003; Ketomäki 2005; O'Neill 2004).
6. Apolipoprotein B‐100 concentration, the protein component of LDL cholesterol (fasting and non‐fasting)
O' Neill reported a significant reduction in Apo B‐100 levels in the LSTA, HSTA and STE groups at two months; ‐6.6%, ‐8.5% and ‐5.9% respectively) (O'Neill 2004). These data were not separately presented for FH participants. The other two trials did not report this outcome (Ketomäki 2003; Ketomäki 2005).
7. Quality of life
This outcome was not assessed in any of the trials (Ketomäki 2003; Ketomäki 2005; O'Neill 2004).
8. Compliance
This outcome was not assessed in any of the trials (Ketomäki 2003; Ketomäki 2005; O'Neill 2004).
9. Morbidity
This outcome was not assessed in any of the trials (Ketomäki 2003; Ketomäki 2005; O'Neill 2004).
10. Weight, height and other measures of nutritional status
No significant reduction from baseline was noted for weight in any of the three trials (Ketomäki 2003; Ketomäki 2005; O'Neill 2004).
11. Micronutrient intake
This outcome was not evaluated in any of the trials (Ketomäki 2003; Ketomäki 2005; O'Neill 2004).
Discussion
In the present update of this review, 14 trials have been included with a total of 441 participants across seven comparison groups. The sample size of included trials is a concern as inadequately‐powered trials seldom lead to meaningful conclusions. Further, the majority of trials included in this review were cross‐over in design. One trial which used a parallel group design included FH participants only as a subgroup (O'Neill 2004). For another trial, the authors provided the data of FH subgroup as a parallel group, although the trial had a cross‐over design (Neil 2001). This does make it important that caution be exercised in interpreting the pooled results.
Only one trial was identified which assessed the effect of a cholesterol‐lowering diet compared to no dietary intervention (Chisholm 1992). This is disappointing as a cholesterol‐lowering diet is the recommended dietary treatment for FH. Although no significant differences have been found between the comparisons assessed, this does not mean that the diets are not effective, rather that the data available are insufficient to reach any conclusions on the efficacy of the different dietary treatments. There is a wide range of dietary treatment options that have been suggested for FH; however, for each of these options there appear to have been very few or no trials carried out.
The use of plant sterols and stanols has received renewed attention in recent years and seven trials assessing this intervention were included in the analysis (Amundsen 2002; Ketomäki 2003; Ketomäki 2004a; Ketomäki 2005; Neil 2001; Nigon 2001; O'Neill 2004). The last one of these compared sterol and stanol substitution on the background of lipid lowering drugs, mainly statins. Both the interventions significantly improved the lipid profile. However, the trial design did not allow analysis of the effect of adding plant sterols or stanols to lipid lowering drugs when compared to lipid lowering drugs given alone. There was no effect in other outcomes like apolipoproteins A and B100.
Fish oils containing omega‐3 fatty acids have also been evaluated as an option for dietary intervention (Balestrieri 1996; Engler 2004). However, much needs to be clarified on this issue as regards to the optimal dose and the ratio of docosahexaenoic acid (DHA) and eicosopentanoic acid (EPA) which should be used. More trials are needed to investigate this dietary intervention strategy.
All the trials identified were short term and did not assess long‐term outcomes. It is disappointing that no long‐term trials were identified as long‐term outcomes may be more relevant to people with familial hypercholesterolaemia and their care givers. None of the primary outcomes could be evaluated for the same reason.
Again, there is the problem of combining the results of familial hypercholesterolaemia with hypercholesterolaemia. A priori subgroup analysis was not planned by any of the authors for the trials in which this problem was noted. Two of the authors provided the data for FH participants and these trials have thus been included in the present update (Neil 2001; O'Neill 2004). For one of the trials, however, the data could not be incorporated for meta‐analysis as the authors did not provide the data in the required format for evaluation by RevMan (O'Neill 2004). Hopefully, in a future version of this review these data may also be included.
Further, in view of evolving approaches in the management of familial hypercholesterolaemia, we had planned to undertake additional analysis. One of the aims of the review was to assess the usefulness of cholesterol‐lowering diet over and above the drug therapy (statins, bile acid sequestrants, fenofibrate and anion exchange resins). Trials designed to evaluate various dietary modifications over and above lipid‐lowering drugs are needed.
Trials in which head‐to‐head comparisons of various dietary intervention strategies are carried out were remarkably absent with a few exceptions. In one trial, the effect of low‐dose and high‐dose stanol was compared to sterol esters in a head‐to‐head manner (O'Neill 2004). The authors noted that, despite a trend, the improvement in serum lipid profile with a high‐dose stanol ester was no better than that of low‐dose stanol esters. In the other trial, sterol esters were compared with stanol esters (O'Neill 2004). The authors analysed the change from baseline in the levels of total cholesterol, LDL, HDL and triglycerides. However, a comparison between sterol and stanol ester groups was not made.
The new comparisons included in this update were soluble fibers such as barley, oat bran, rice bran, guar gum, psyllium or flax seeds and soy protein as form of dietary intervention compared to another form of dietary intervention or drug or no dietary intervention. Only one trial satisfying inclusion criteria was identified. The intervention used was guar gum with bezafibrate. It was in 12 adult familial hypercholesterolemic patients. Though statistically significant reduction in total and LDL cholesterol levels were noted, the utility of this finding in the current era of statins is suspect. A meta‐analysis (Brown 1999) has shown beneficial effect of soluble dietary fibers in hypercholesterolemia and thus there is a case for studying dietary fibers in an adequately powered trial.
People with FH may be more susceptible to potentially detrimental psychological and nutritional consequences of their dietary treatment. For this reason, it is disappointing to note that very few of the trials included in this review assessed measures of nutritional status and none assessed quality of life or nutritional intake.
Publication bias cannot be ruled out. A funnel plot could not be constructed as the data required were not available in sufficient quantities. We hope that with new possibilities opening up for publishing negative trials, more trials will come to the fore and possibly be included in the review.
Authors' conclusions
Implications for practice.
No conclusions can be made about the short‐ or long‐term effectiveness of the cholesterol‐lowering diet.
Considering the fact that the pooled trials did not conform completely to the requirements for pooling, careful attention should be paid when implementing the conclusions for practice. Sterol treatment did significantly lower total cholesterol; however, more evidence is required in the form of large, good quality controlled trials before sterols are recommended for people with FH.
Implications for research.
A large, parallel, randomised controlled trial is needed to investigate the effectiveness of the cholesterol‐lowering diet and no dietary intervention. Due to the relatively small numbers of people with this condition, it is recommended that a multi‐centre approach is adopted and that participants should not be recruited to small scale trials which may preclude them from being involved in larger trials. It is also important that future trials consider the long‐term outcomes in addition to short‐term ones. Since drug therapy , particularly statins, is the standard of care in FH, dietary intervention would need to be studied as adjuvant. In addition, as the effect of statins on hard outcomes would be large, the effect of dietary interventions on these outcomes would be very difficult to study and effect on surrogate outcomes (LDL) would be important. To assist in ensuring the appropriateness of future trials, it would be useful to involve people with FH and their carers in the design of the trial.
What's new
| Date | Event | Description |
|---|---|---|
| 2 May 2014 | New citation required but conclusions have not changed | Four new trials have been included in the update (Guardamagna 2011a; Ketomäki 2004a; Nigon 2001; Wirth 1982). Additional interventions (e.g. dietary fibers) have been added separately. No major changes have been made to the conclusions of the review. |
| 2 May 2014 | New search has been performed | A search of the Group's Inborn Errors of Metabolism Trials Regitser and PubMed identified four eligible trials for inclusion (Guardamagna 2011a; Ketomäki 2004a; Nigon 2001; Wirth 1982) which showed that the addition of plant sterols to the diet significantly reduced the total cholesterol, serum LDL and serum total triglycerides for patients with FH. The title has been changed from: Dietary treatment for familial hypercholesterolaemia. |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 2, 2001
| Date | Event | Description |
|---|---|---|
| 20 September 2010 | New search has been performed | Contact details updated. Three trials have been added to the previous update ( Ketomäki 2004a , Guardamagna 2011a and Nigon 2001). The scientific statement from the American Heart Association (AHA) for the treatment of high‐risklipid abnormalities in childr en and adolescents, which advocated the use of dietary treatment as adjuvant to pharmacolog ical treatment, was published in 2007. No revision of this statement has been published subsequently. F ollowing our updated review (2010), we are updating this review with additional information. |
| 19 October 2009 | New search has been performed | Four additional trials have been included in the current update (Engler 2004; Ketomäki 2003; Ketomäki 2005; O'Neill 2004); and three trials are listed as 'Awaiting classification' (Fuentes 2008; Retterstol 2009; Stein 2007). |
| 19 October 2009 | New citation required but conclusions have not changed | The team of review authors has changed. Vanessa Poustie and Patricia Rutherford are no longer active authors on this review. New comparisons between groups have been added. A previous comparison of cholesterol lowering diet and all other dietary interventions has been removed in the current update. Instead a new outcome for the evaluation of the effect of adding dietary intervention to drug therapy has been added in the present review. |
| 31 October 2008 | Amended | Converted to new review format. |
| 23 September 2008 | New citation required and conclusions have changed | Substantive amendment |
Notes
The review was first published in Issue 2, 2001 by Vanessa Poustie and Patricia Rutherford. The review team changed to the current team from Issue 1, 2010.
Appendices
Appendix 1. Search strategy for CENTRAL, The Cochrane Library
#1 MeSH descriptor Dietary fiber explode all trees
#2 diet*
#3 MeSH descriptor Plant sterols explode all trees
#4 plant next sterol
#5 MeSH descriptor Plant stanol ester explode all trees
#6 stanol*
#7 MeSH descriptor Soy protein explode all trees
#8 Barley
#9 Guar gum
#10 Rice bran
# 11oat bran
# 12 rice bran
#13 flax seeds
#14 psyllium
#15 MeSH descriptor Omega 3 fatty acids explode all trees
#16 (#1 OR #2 OR #3 OR #4 OR #5 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15)
#17 MeSH descriptor familial hypercholesterolemia explode all trees
#18 MeSH descriptor familial hyperlipoproteinemia explode all trees
#19 (#17 OR #18)
#20 (#16 AND #19)
Data and analyses
Comparison 1. Cholesterol‐lowering diet compared to no dietary intervention or nutritional advice.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fasting serum total cholesterol concentration (mmol/l) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Over one month and up to two months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Fasting serum LDL cholesterol concentration (mmol/l) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Over one month and up to two months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Fasting serum HDL cholesterol concentration (mmol/l) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Over one month and up to two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Fasting serum triglyceride concentration (mmol/l) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Over one month and up to two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Fasting serum apolipoprotein A1 concentration (g/l) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Over one month and up to two months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Fasting serum apolipoprotein B‐100 concentration (g/l) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Over one month and up to two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. ω‐fatty acids added to background cholesterol‐lowering diet compared to cholesterol‐lowering diet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fasting serum total cholesterol concentration (mmol/l) | 2 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.80, 0.69] |
| 1.1 Up to one month | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.84, 0.94] |
| 1.2 Over one month and up to two months | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐1.62, 1.04] |
| 2 Fasting serum LDL cholesterol concentration (mmol/l) | 2 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.93, 0.69] |
| 2.1 Up to one month | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.99, 0.95] |
| 2.2 Over one month and up to two months | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐1.79, 1.11] |
| 3 Fasting serum HDL cholesterol concentration (mmol/l) | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.10, 0.13] |
| 3.1 Up to one month | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.40, 0.34] |
| 3.2 Over one month and up to two months | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.10, 0.14] |
| 4 Fasting serum triglyceride concentration (mmol/l) | 2 | 68 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.07, 0.43] |
| 4.1 Up to one month | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.24 [‐0.14, 0.62] |
| 4.2 Over one month and up to two months | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.21, 0.47] |
| 5 Fasting serum apolipoprotein A1 concentration (g/l) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Fasting serum apolipoprotein B‐100 concentration (g/l) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Plant stanols added to cholesterol‐lowering diet compared to cholesterol‐lowering diet alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fasting serum total cholesterol concentration (mmol/l) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Over one month and up to two months | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.51 [0.07, 0.96] |
| 2 Fasting serum LDL cholesterol concentration (mmol/l) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Over one month and up to two months | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.71 [0.43, 0.99] |
| 3 Fasting serum HDL cholesterol concentration (mmol/l) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Over one month and up to two months | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.15, ‐0.00] |
| 4 Fasting serum triglyceride concentration (mmol/l) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Over one month and up to two months | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.22, 0.27] |
Comparison 4. Plant sterols added to cholesterol‐lowering diet compared to cholesterol‐lowering diet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fasting serum total cholesterol concentration(mmol/l) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Over one month and up to two months | 4 | 222 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [0.12, 0.48] |
| 2 Fasting serum LDL cholesterol concentration (mmol/l) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Over one month and up to two months | 4 | 222 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.89, ‐0.31] |
| 3 Fasting serum HDL cholesterol concentration (mmol/l) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Over one month and up to two months | 4 | 222 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.11, 0.03] |
| 4 Fasting serum triglyceride concentration (mmol/l) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Over one month and up to two months | 4 | 222 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.15, 0.09] |
| 5 Fasting serum apolipoprotein A1concentration (g/l) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Over one month and up to two months | 2 | 182 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.08, 0.14] |
| 6 Fasting serum apolipoprotein B‐100 concentratiom (g/l) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Over one month and up to two months | 2 | 182 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.09, 0.13] |
Comparison 5. Soy protein in addition to cholesterol‐lowering diet compared to cholesterol‐lowering diet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fasting serum total cholesterol concentration (mmol/l) | 2 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.65, 0.81] |
| 1.1 Up to one month | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.94, 0.94] |
| 1.2 Over one month and up to two months | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.94, 1.34] |
| 2 Fasting serum LDL cholesterol concentration (mmol/l) | 2 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.46, 0.69] |
| 2.1 Up to one month | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐1.02, 0.94] |
| 2.2 Over one month and up to two months | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.51, 0.91] |
| 3 Fasting serum HDL cholesterol concentration (mmol/l) | 2 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.23, 0.08] |
| 3.1 Up to one month | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.23, 0.13] |
| 3.2 Over one month and up to two months | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.49, 0.17] |
| 4 Fasting serum triglyceride concentration (mmol/l) | 2 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.01, 0.50] |
| 4.1 Up to one month | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.05, 0.49] |
| 4.2 Over one month and up to two months | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐0.33, 1.33] |
| 5 Fasting serum apolipoprotein A1 concentration (g/l) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Fasting serum apolipoprotein B‐100 concentration (g/l) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Weight (kg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Height (cm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Body mass index | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Dietary fibers as a form of dietary intervention compared to another form of dietary intervention or drug or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fasting serum total cholesterol concentration (mmol/l) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Fasting serum LDL cholesterol concentration (mmol/l) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Fasting serum HDL cholesterol concentration (mmol/l) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Fasting serum triglyceride concentration (mmol/l) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Fasting serum apolipoprotein A1 concentration (g/l) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Fasting serum apolipoprotein B‐100 concentration (g/l) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Weight | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amundsen 2002.
| Methods | Randomised controlled cross‐over trial. | |
| Participants | 41 children with confirmed or suspected FH (mean age 10.5 years +/‐ 1.7 years). Three dropped out (2 because of family problems and one because she found the amount of spread was too large | |
| Interventions | Plant sterol‐enriched fat spread (8 weeks) versus a control spread not enriched with plant sterols (8 weeks). 4‐week washout period. | |
| Outcomes | Total, LDL, HDL cholesterol, triacylglycerol, apolipoprotein A1 and B, carotenoids, retinol and alpha‐tocopherol concentrations, and albumin and other blood biochemistry. | |
| Notes | 3 children were withdrawn from the trial and not followed up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but method not described. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double‐blind, but not stated who was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 participants were withdrawn from the trials and not included in the final analysis. 2 girls dropped out during the trial because of family problems not related to the project, and a third because she found that the amount of spread was too large. |
| Selective reporting (reporting bias) | Low risk | All the outcomes stated in methods were reported. |
Balestrieri 1996.
| Methods | Randomised controlled cross‐over trial. | |
| Participants | 16 participants (7 females, 9 males) with heterozygous FH (mean age 45.2 +/‐ 15.0 years). | |
| Interventions | Lipid lowering diet & 6 g fish oil ethylester/day for 4 weeks versus lipid lowering diet & 6 g olive oil/day for 4 weeks. 4 week washout period. All patients maintained on lipid lowering medication (simvastatin, dose range 10 ‐ 40 mg) throughout both arms of the trial. | |
| Outcomes | Total cholesterol, HDL, LDL, triglycerides, apolipoprotein A1 &B and Lp(a) concentrations. | |
| Notes | NB. short‐term outcomes only will be assessed. 2 patients were withdrawn from the trial and not included in the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but method not described. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double‐blind, but not stated who was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2 participants withdrew from one trial due to medical reasons (1 suffered a heart attack and one required vascular surgery) and not included in the final analysis. |
| Selective reporting (reporting bias) | Low risk | All the outcomes stated in methods were reported |
Chisholm 1992.
| Methods | Randomised controlled cross‐over trial. | |
| Participants | 19 participants (11 females, 8 males) with FH, mean age 51 +/‐ 10yrs. | |
| Interventions | 3 x 8 weeks periods (high fat, low fat, high fat verses low fat, high fat, low fat). Maintained on lipid lowering medication (simvastatin) throughout all arms of trial. Low fat diet = 27% energy from total fat, 8% energy from saturated fat, high fat diet = 38% energy from total fat, 14% energy from saturated fat. | |
| Outcomes | Total & HDL cholesterol, triglycerides and apolipoprotein A1 & B. | |
| Notes | NB. short‐term outcomes only will be assessed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but method not described. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if an intention‐to‐treat analysis was carried out |
| Selective reporting (reporting bias) | Low risk | Selective reporting of outcomes was not found |
Engler 2004.
| Methods | Randomised controlled cross‐over trial. | |
| Participants | 20 participants (9 ‐ 19 years) with FH or familial combined hyperlipidemia. | |
| Interventions | DHA (1.2 gm/day) was combined with NCEP‐II diet (6 weeks) followed by placebo after a washout period of 2 weeks. | |
| Outcomes | Total, LDL, VLDL, HDL and triglyceride levels were measured. | |
| Notes | Short‐term outcomes only will be assessed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but method not described. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double‐blind, but not stated who was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis was considered adequate. No dropouts. |
| Selective reporting (reporting bias) | Low risk | All the outcomes stated in methods were reported |
Guardamagna 2011a.
| Methods | Double‐blind, randomised, placebo‐controlled, cross‐over trial. | |
| Participants | Children with familial hypercholesterolemia (n = 24 ) and combined familial hyperlipidemia (n = 16). | |
| Interventions | Dietary supplement containing 200 mg red yeast rice extract, corresponding to 3 mg of monacolins, and 10 mg policosanols once‐daily (for 8 weeks) versus placebo (for 8 weeks). 4‐week washout period. | |
| Outcomes | Total cholesterol, LDL‐C, apolipoprotein B, HDL‐C. | |
| Notes | No subgroup analysis presented by type of hypercholesterolemia. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Arbitrary allocation was done but randomisation procedure unclear. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double blind. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear. |
| Selective reporting (reporting bias) | Low risk | All the outcomes stated in methods were reported |
Gylling 1995.
| Methods | Randomised controlled cross‐over trial. | |
| Participants | 14 children (7 females, 7 males) with heterozygous FH, mean age 9.1 +/‐ 1.1 years. | |
| Interventions | Rapeseed oil margarine with or without sitostanol ester for 6 weeks in addition to low fat, low cholesterol diet. | |
| Outcomes | Total, VLDL, HDL & LDL cholesterol, phospholipids & triglycerides & apolipoprotein E. | |
| Notes | NB. Short‐term outcomes only will be assessed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but method not described. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double‐blind, but not stated who was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis was considered adequate. |
| Selective reporting (reporting bias) | Low risk | All the outcomes stated in methods were reported |
Ketomäki 2003.
| Methods | Randomised, controlled, cross‐over trial. | |
| Participants | Pediatric patients with hypercholesterolaemia with a subgroup of 16 patients who had FH. | |
| Interventions | Low fat diet with plant stanol spread compared with low fat diet with sterol ester spread for 5 weeks; separated by a 5‐week washout period. | |
| Outcomes | Total, VLDL, HDL & LDL cholesterol, triglycerides, Apo‐B and non‐cholesterol sterols. | |
| Notes | NB: Only short term outcomes were assessed, data for the 17 patients with FH not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but method not described. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double‐blind, but not stated who was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis was considered adequate. No dropouts. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was noted |
Ketomäki 2004a.
| Methods | Randomised, cross‐over trial. | |
| Participants | 5 adult participants with FH. | |
| Interventions | Sterol and stanol for 4 weeks for each treatment period. No washout period described. | |
| Outcomes | Total, HDL & LDL cholesterol, triglycerides. | |
| Notes | Only short‐term outcomes were assessed in small number of participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as a randomised trial. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Included all participants. |
| Selective reporting (reporting bias) | Low risk | All the outcomes stated in methods were reported |
Ketomäki 2005.
| Methods | Randomised, controlled, cross‐over trial. | |
| Participants | 18 adults (6 males, 12 females) with FH on cholesterol‐lowering drug therapy. Mean (SEM) age of 48 (2) years). | |
| Interventions | Low fat plant sterol ester spread or low fat plant stanol ester spread over over and above ongoing drug therapy. | |
| Outcomes | Total, VLDL, HDL & LDL cholesterol, triglycerides, Apo‐B and non‐cholesterol sterols. | |
| Notes | NB: Only short term outcomes were assessed; No treatment period involved a phase of lipid‐lowering drug given alone. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but method not described. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double‐blind, but not stated who was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis was considered adequate. |
| Selective reporting (reporting bias) | Low risk | All the outcomes stated in methods were reported |
Laurin 1991.
| Methods | Randomised controlled cross‐over trial. | |
| Participants | 10 children (4 females, 6 males) with heterozygous FH, aged 6 ‐ 12 years (mean (SD) 8 (1) year). | |
| Interventions | Cows milk diet + regular diet (4 weeks) followed by soy‐beverage diet + regular diet (4 weeks) or vice versa. 4 week washout period on regular diet between treatment arms. | |
| Outcomes | Total, HDL, VLDL, LDL cholesterol, triglycerides, phospholipids & apolipoprotein A‐1 & B. | |
| Notes | NB. 2 children were excluded from the final analysis. Short term outcomes only will be assessed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but method not described. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double‐blind, but not stated who was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2 participants were withdrawn from the trial for lack of compliance and elevated serum lipid levels and not included in the final analysis. |
| Selective reporting (reporting bias) | Low risk | All the outcomes stated in methods were reported |
Neil 2001.
| Methods | Randomised controlled cross‐over trial. | |
| Participants | 30 adults with heterozygous FH and 32 with type IIa primary hypercholesterolaemia (26 females), (mean age 51.6 years). | |
| Interventions | A plant sterol‐enriched fat spread (8 weeks) versus a control fat spread not enriched with plant sterols (8 weeks). No washout period. | |
| Outcomes | Serum total, HDL, LDL cholesterol, apolipoprotein A‐1 and B, liver function tests and plant sterol and cholesterol precursor sterol levels. | |
| Notes | Due to evidence of significant carry‐over effect , analysis was restricted to first treatment period only. The authors provided data for only 15 patients with FH. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers were used to assign the participants to either test or the control group with equal probability. |
| Allocation concealment (selection bias) | Low risk | Tamper proof block randomisation was used and clinic and lab staff remained unaware of the assigned treatment throughout the trial. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double‐blind, but not stated who was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis was considered adequate. No dropouts. |
| Selective reporting (reporting bias) | Low risk | All the outcomes stated in methods were reported |
Nigon 2001.
| Methods | Randomised, double‐blind,placebo‐controlled two‐period cross‐over trial with 2 treatments and 3 periods. | |
| Participants | 53 primary hypercholesterolaemia participants (22 men and 31 women). | |
| Interventions | Spread enriched with plant sterols was compared to non‐enriched control spread. 1.6 g/day of plant sterols . The plant sterol content consisted of sitosterol esters (50%), campesterol esters (25%), stigmasterol esters (20%) and 10% of other esters. Treatment periods: 2 months each, with a washout period of 2 months between them. | |
| Outcomes | Total and LDL‐C concentrations. | |
| Notes | Fibrates were used additionally in 19 participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated randomised, method not described. |
| Allocation concealment (selection bias) | Unclear risk | A physician allocated each person to one of the treatments for each period such that the design was balanced, in the following order: non‐enriched control spread was followed by phytosterol enriched spread or the reverse order. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient did not consume the full amount of intervention and another stopped it for 10 days. Both were included in the analysis. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was observed. |
O'Neill 2004.
| Methods | Radnomised double blind, parallel trial. | |
| Participants | 145 participants recruited, 11 excluded, 134 completed trial. 69 participants with FH (57% female, mean age 53 years), 65 unaffected (69% female, mean age 46 years). | |
| Interventions | 3 treatment groups: STE: 1.6g + 1 placebo cereal bar daily; LSTA: 1.6g + 1 placebo cereal bar daily; HSTA: 1.6 g+ 1 g stanol ester daily. Each treatment lasted 2 months following a 1 month run‐in on placebo margarine and followed by a 1‐month washout on placebo margarine. | |
| Outcomes | Changes in serum lipids, total and LDL cholesterol, serum apoB and LDL cholesterol: apo B ratios, changes in non‐cholesterol sterols (lathosterol, sitosterol, campesterol), changes in 7 alpha OH‐cholestenone. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomised" but method not described. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double‐blind, but not stated who was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were included in the analysis. |
| Selective reporting (reporting bias) | Low risk | Reporting bias was not present. |
Wirth 1982.
| Methods | Randomized, cross‐over trial. | |
| Participants | 12 adult patients with familial hypercholesterolemia, 11 out of 12 had an isolated hypercholesterolemia (type IIa). | |
| Interventions | Bezafibrate at a dose of 200 mg t.i.d was prescribed for 2 months during the initial period. First group was given 5.2 g guar t.i.d in a granulate form in addition to the 200 mg bezafibrate t.i.d during the second 2‐month period. They were then subsequently treated in the next period of 2 month with bezafibrate alone. In the other group the sequence was reversed. | |
| Outcomes | Serum lipids and lipoprotein levels. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details not given. |
| Allocation concealment (selection bias) | Unclear risk | Details not given. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Details not given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned. |
| Selective reporting (reporting bias) | Low risk | All the outcomes stated in methods were reported. |
Wolfe 1992.
| Methods | Randomised controlled cross‐over trial. | |
| Participants | 5 patients with FH (3 females, 2 males) mean (SD) age 48 (6) years. | |
| Interventions | 4 ‐ 5 weeks of high‐protein, low‐fat diet versus 4 ‐ 5 weeks of low‐protein, low‐fat diet. | |
| Outcomes | Fasting blood lipoprotein levels (LDL, HDL, VLDL, triglycerides). | |
| Notes | NB. Short term outcomes only will be assessed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but method not described. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double‐blind, but not stated who was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis was considered adequate.There were no dropouts. |
| Selective reporting (reporting bias) | Low risk | All the outcomes stated in methods were reported. |
FH: familial hypercholesterolaemia HDL: high density lipoprotein HSTA: high‐dose stanol group LDL: low density lipoprotein LSTA: low‐dose stanol group SEM: standard error of the mean STE: plant sterol ester VLDL: very low density lipoprotein
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbey 1990 | Not FH |
| Abbey 1993a | Not FH |
| Abbey 1993b | Not FH |
| Abrahamson 1974 | Not FH, not an RCT |
| Ahmed 1984 | Not FH, not an RCT |
| Ahrens 1954 | Not FH, not an RCT |
| Ahrens 1957 | Not an RCT |
| Ahrens 1959 | Not an RCT |
| Am Acad Paed 1972 | Not an RCT |
| Amundsen 2004 | Non‐randomized |
| Anderson 1957 | Not FH, not an RCT |
| Anderson 1976 | Not FH |
| Anderson 1980 | Not FH, not an RCT |
| Anderson 1984a | Not defined FH |
| Anderson 1984b | Not defined FH |
| Asherio 1995 | Not FH, not an RCT |
| Atkinson 1987 | Not FH, not an RCT |
| Baggio 1988 | Not an RCT |
| Bartram 1992 | Not an RCT |
| Becker 1983 | Not FH, not an RCT |
| Becker 1993 | Not an RCT |
| Beil 1991 | Not FH |
| Beitz 1981 | Not FH, not an RCT |
| Berg 1991 | Not an RCT |
| Berge 1959 | Not an RCT |
| Berry 1991 | Not FH |
| Best 1954 | Not defined FH |
| Best 1955 | Not defined FH |
| Best 1956 | Not an RCT |
| Beveridge 1955 | Not FH, not an RCT |
| Beveridge 1957 | Not FH, not an RCT |
| Beveridge 1959 | Not FH, not an RCT |
| Beynen 1985 | Not FH, not an RCT |
| Bierenbaum 1963 | Not FH |
| Bierenbaum 1970 | Not defined FH |
| Blair 2000 | No patients with familial hypercholesterolemia |
| Blankenhorn 1990 | Not diet vs diet |
| Blaton 1984 | Not an RCT |
| Boberg 1986 | Not FH |
| Bonanome 1988 | Not defined FH |
| Bonanome 1992 | Not FH |
| Bowry 1993 | Not an RCT |
| Boyd 1990 | Not FH |
| Braden 1990 | Animal study |
| Brensike 1982 | Not defined FH |
| Brensike 1984 | Not defined FH |
| Brinton 1990 | Not in patients with FH |
| Briones 1984 | Drug study |
| Brongeest 1979a | Not FH |
| Brongeest 1979b | Not FH |
| Brongeest 1979c | Not FH, not an RCT |
| Bronte‐Stewart 1956 | Not FH, not an RCT |
| Brown 1991 | Not FH, not an RCT |
| Brox 1981 | Not FH |
| Brox 1983 | Not randomized |
| Bruckner 1987 | Not defined FH |
| Brude 1997 | Not defined FH |
| Brussaard 1980 | Not FH, not an RCT |
| Burr 1989 | Not FH |
| Canetti 1995 | Not FH, not diet |
| Carlson 1971 | Not RCT |
| Carmen‐Ramon 1998 | Not RCT |
| Carranza 1997 | Not defined FH |
| Carroll 1975 | Not RCT |
| Carroll 1978 | Animal studies |
| Carroll 1982 | Review article |
| Carroll 1991 | Not RCT |
| Chait 1974 | Not FH |
| Chang 1990 | Not FH, not RCT |
| Chen 1979 | Animal studies |
| Chenoweth 1981 | Not FH, not RCT |
| Childs 1981 | Not an RCT |
| Clevidence 1992 | Not FH, not RCT |
| Clifton 1992 | Not defined FH |
| Cobb 1991 | Not defined FH |
| Cole 1992 | Not an RCT |
| Colquhoun 1992 | Not FH, not RCT |
| Connor 1961a | Not FH, not RCT |
| Connor 1961b | Not FH, not RCT |
| Connor 1964 | Not FH, not RCT |
| Connor 1982 | Not FH, not RCT |
| Corder 1989 | Drug study |
| Cortese 1983 | Not defined FH |
| Crouse 1979 | Not FH, not RCT |
| Da Col 1984 | Drug study |
| Dattilo 1992 | Not an RCT |
| Davidson 1991 | Not FH |
| Davis 1985 | Drug study |
| de Groot 1963 | Animal study |
| De Jong 2008 | No F H pts |
| Demke 1988 | Not defined FH |
| Demke 1991 | Not defined FH |
| Denke 1995 | Not defined FH |
| Descovich 1980 | Not an RCT |
| Detre 1985 | Not defined FH |
| Dieber 1991 | Not FH, not RCT |
| Dreon 1990 | Not FH, not RCT |
| Dreon 1994 | Not FH |
| Dreon 1997 | Not RCT |
| Durrington 1977 | Not FH, not RCT |
| Dyerberg 1978 | Not RCT |
| East 1988 | Drug study |
| Edington 1989 | Not RCT |
| Ehnholm 1982 | Not RCT |
| Ehnholm 1984 | Not in patients with FH |
| Elkeles 1988 | Not RCT |
| Erickson 1964 | Not FH, not RCT |
| Eritsland 1995 | Not FH |
| Ernst 1980 | Not RCT |
| Failor 1986 | Not FH |
| Falko 1980 | Not FH, not RCT |
| Fallat 1979 | Not RCT |
| Farquhar 1956 | Not RCT |
| Farquhar 1958 | Not defined FH |
| Fehily 1983 | Not FH |
| Fernandes 1977 | Not RCT, drug study |
| Fernandes 1981 | Not RCT |
| Ferro‐Luzzi 1984 | Not FH, not RCT |
| Fisher 1983 | Not FH, not RCT |
| Flaim 1981 | Not FH |
| Flynn 1981 | Not FH, not RCT |
| Follick 1984 | Not FH, not RCT |
| Forsythe 1986 | Review article, not RCT |
| Frank 1978 | Not RCT |
| Frankel 1994 | Not FH |
| Frantz 1975 | Not FH |
| Frantz 1989 | Not FH |
| Friday 1991 | Not cholesterol‐lowering diet |
| Fumagalli 1978 | Animal study |
| Fumagalli 1982 | Not RCT, not defined FH |
| Gaddi 1987 | Not RCT |
| Galvan 1996 | Drug study |
| Gardner 1995 | Not RCT |
| Gardner 2005 | Not in patients with FH |
| Glueck 1972 | Not RCT |
| Glueck 1977 | Not RCT |
| Glueck 1978 | Not RCT |
| Glueck 1979 | Not RCT |
| Glueck 1983 | Not RCT |
| Glueck 1991 | Not RCT |
| Goldberg 1982 | Not defined FH |
| Goodnight 1981 | Not FH |
| Gordon 1977 | Not FH, not RCT |
| Gordon 1982 | Non‐interventional study |
| Grande 1970 | Not FH |
| Grande 1972 | Not FH |
| Gries 1990 | Not FH, not RCT |
| Groot 1980 | Drug study |
| Grundy 1970 | Not RCT |
| Grundy 1986 | Not defined FH |
| Grundy 1990 | Not RCT |
| Guardamagna 2011b | Natural HMGCoA inhibitor used |
| Gustafsson 1982 | Not RCT |
| Gustafsson 1983 | Not defined FH |
| Gustafsson 1985 | Not defined FH |
| Gustafsson 1992 | Not defined FH |
| Gustafsson 1994 | Not defined FH |
| Gylling 1988 | Not RCT |
| Gylling 2010 | Not in patients with FH |
| Gylling 2011 | Not in patients with FH |
| Hansen 1989 | Study in healthy volunteers |
| Harris 1983a | Not FH |
| Harris 1988 | Not FH |
| Harris 1989 | Not RCT |
| Hashim 1960 | Not RCT |
| Hay 1982 | Not RCT |
| Hegsted 1965 | Not FH, not RCT |
| Hegsted 1986 | Not RCT |
| Hegsted 1993 | Not RCT |
| Heinemann 1986 | Not RCT |
| Helms 1977 | Not RCT |
| Hennekens 1996 | Not FH |
| Herold 1986 | Not RCT |
| Hodges 1967 | Not FH, not RCT |
| Hoeg 1984 | Drug study |
| Holme 1990 | Not RCT |
| Holmes 1980 | Not in patients with FH. |
| Hooper 1980 | Not FH, not RCT |
| Hopkins 1992 | Not RCT |
| Huff 1984 | Not defined FH |
| Hunninghake 1993 | Not defined FH |
| Iacono 1991 | Not FH |
| Illingworth 1991 | Not defined FH |
| Jackson 1984 | Not FH, not RCT |
| Jakubowski 1978 | Not FH, not RCT |
| Jenkins 1975a | Not FH, not RCT |
| Jenkins 1975b | Not FH, not RCT |
| Jenkins 1980 | Not RCT |
| Jialal 1992 | Not FH |
| Jialal 1993 | Not FH |
| Joyner 1955 | Not defined FH |
| Judd 1988 | Participants did not have familial hypercholesterolemia |
| Kane 1981 | Drug study |
| Kane 1990 | Drug study |
| Kestin 1989 | Not FH |
| Ketomäki 2004b | Not RCT |
| Keys 1957 | Not RCT |
| Keys 1965a | Not RCT |
| Keys 1965b | Not RCT |
| Keys 1984 | Not RCT |
| Khan 1981 | Not FH |
| Kingsbury 1961 | Not FH, not RCT |
| Kinsell 1952 | Not RCT |
| Kirby 1981 | Not defined FH |
| Kok 1987 | Not RCT |
| Kris‐Etherton 1993 | Not FH |
| Kromhout 1985 | Not RCT |
| Kudchodkar 1976 | Not RCT |
| Kuo 1979 | Drug study |
| Kuo 1981 | Not RCT |
| Kuusi 1985 | Not FH |
| Laine 1982 | Not FH, not RCT |
| Lambert 1996 | Drug study |
| Leaf 1988 | Not RCT |
| Leelarthaepin 1974 | Not RCT |
| Lees 1977 | Not RCT |
| Leibman 1983 | Not FH, not RCT |
| LeLorier 1977 | Drug study |
| Lewis 1981 | Not FH |
| Lichtenstein 1994 | Not RCT |
| Lifshitz 1989 | Not RCT |
| Lindgard 1984 | Not defined FH |
| Linnebur 2007 | No FH pts |
| Lithell 1984 | Not defined FH |
| Lorenz 1983 | Not FH, not RCT |
| Lovati 1987 | Not defined FH |
| LRCP 1984a | Drug study |
| LRCP 1984b | Drug study |
| Macdonald 1967 | Not FH, not RCT |
| Mackness 1993 | Not RCT Not FH |
| Malmros 1957 | Not RCT |
| Mannarinno 2009 | No F H pts |
| Maranhao 1983 | Not RCT |
| Marshall 1986 | Not FH |
| Mata 1992 | Not FH, not RCT |
| Mathur 1968 | Not FH, not RCT |
| Mattson 1972 | Not FH |
| Mattson 1975 | Not FH, not RCT |
| Mattson 1977 | Animal study |
| Mattson 1982 | Not RCT |
| Mattson 1985 | Not defined FH |
| McCombs 1994 | Not RCT |
| McGill 1979 | Not RCT |
| Mellies 1983 | Not defined FH |
| Mensink 1992 | Not RCT |
| Miettinen 1972 | Not FH, not RCT |
| Miettinen 1977 | Not RCT |
| Miettinen 1989a | Not FH, not RCT |
| Miettinen 1989b | Not FH, not RCT |
| Miettinen 1992a | Study not relevant |
| Miettinen 1992b | Not FH |
| Miettinen 1994 | Not defined FH |
| Miller 1988 | Not FH |
| Mokino 1990 | Not RCT |
| Morita 1983 | Animal study |
| MRC 1965 | Not FH |
| MRC 1968 | Not FH |
| Nagakawa 1983 | Not FH, not RCT |
| Napoli 1998 | Not defined FH |
| NDHSRG 1968 | Not FH |
| Neil 1995 | Not FH |
| Nenseter 1992 | Not FH |
| Nessim 1983 | Not defined FH, drug study |
| Nestel 1973 | Not RCT |
| Nestel 1976 | Not FH, not RCT |
| Nestel 1984 | Not FH, not RCT |
| Nestel 1986 | Not FH, not RCT |
| Nestel 1992a | Not defined FH |
| Nestel 1992b | Not FH |
| Nilson 1991 | Not FH |
| Nydahl 1994 | Not defined FH |
| Nyyssonen 1994 | Not FH |
| Olszewski 1993 | Not defined FH |
| Omenn 1996 | Not FH |
| Ordovas 1995 | Not RCT |
| Parks 1990 | Animal study |
| Parthasarathy 1990 | Animal study |
| Peto 1985 | Not RCT |
| Phillipson 1985 | Not defined FH |
| Pirich 1999 | Not defined FH |
| Princen 1995 | Not RCT, not FH |
| Quintao 1971 | Not RCT |
| Radack 1989 | Not defined FH |
| Radack 1990 | Not defined FH |
| Rapola 1996 | Not defined FH |
| Reaven 1991 | Not FH |
| Reaven 1993a | Not FH |
| Reaven 1993b | Not defined FH |
| Reaven 1993c | Not FH, not RCT |
| Reaven 1994 | Not FH |
| Reiser 1985 | Not FH |
| Retzloff 1991 | Not defined FH |
| Riccardi 1987 | Review article |
| Rivellese 1994 | Not defined FH |
| Roberts 1994 | Not RCT |
| Rona 1985 | Not FH, not RCT |
| Rose 1976 | Not RCT |
| Rosenthal 1985 | Not RCT |
| Sacks 1986 | Not FH |
| Sanders 1981 | Not FH |
| Sanders 1983 | Not FH |
| Sanders 1984 | Not in patients with FH |
| Saynor 1982a | Not RCT |
| Saynor 1984a | Not RCT |
| Saynor 1984b | Not FH |
| Schaefer 1981 | Not RCT |
| Schectman 1989 | Not FH, not RCT |
| Schlierf 1982 | Drug study |
| Schonfeld 1982 | Not FH, not RCT |
| Schwandt 1982 | Not FH, not RCT |
| Segall 1970 | Not RCT |
| Seppanen‐Laakso 1992 | Not FH |
| Seppanen‐Laakso 1993 | Not FH, not RCT |
| Shepherd 1978 | Not FH |
| Shepherd 1980 | Not FH, not RCT |
| Shorey 1981 | Not FH, not RCT |
| Siess 1980 | Not FH, not RCT |
| Simons 1985a | Not defined FH |
| Simons 1985b | Not defined FH |
| Singer 1983 | Not FH, not RCT |
| Singer 1984 | Not defined FH |
| Singer 1986 | Not defined FH |
| Sirtori 1977 | Not FH, not RCT |
| Sirtori 1979 | Not defined FH |
| Sirtori 1981 | Not RCT |
| Sirtori 1986 | Not defined FH |
| Sirtori 1992 | Not FH |
| Sperling 1987 | Not FH, not RCT |
| Stein 1975 | Not FH, not RCT |
| Stein 1982 | Not RCT |
| Stephens 1996 | Not defined FH |
| Subbaiah 1989 | Not RCT |
| Sucic 1998 | Not defined FH |
| Szczeklik 1985 | Not defined FH |
| Thorngren 1981 | Not FH, not RCT |
| Thorngren 1984 | Not FH, not RCT |
| Thorngren 1986 | Not FH, not RCT |
| Tonstad 1997b | Not RCT |
| Ullmann 1990 | Drug study |
| Uusitupa 1991 | Comparison not per protocol |
| Valsta 1992 | Not FH, not RCT |
| Van Gent 1979 | Not FH, not RCT |
| Van Horn 1986 | Not FH |
| Vanhanen 1991 | Not RCT |
| Vanhanen 1993 | Not defined FH |
| Vanhanen 1994 | Not defined FH |
| Varady 2007 | No FH pts |
| Vega 1982 | Not RCT |
| Verrillo 1985 | Not defined FH |
| Vessby 1980a | Not RCT |
| Vessby 1980b | Not RCT |
| Vessby 1982 | Not RCT |
| Von Schacky 1985 | Not FH, not RCT |
| Von Schacky 1987 | Not RCT |
| Vuorio 2000 | Non‐randomized |
| Wardlaw 1990 | Not FH |
| Weisweiler 1983 | Not FH, not RCT |
| Weisweiler 1985 | Not FH, not RCT |
| Widhalm 1978 | Not defined FH |
| Widhalm 1993 | Not defined FH |
| Williams 1986 | Not FH, not RCT |
| Wilson 1971 | Not RCT |
| Wilt 1989 | Not defined FH |
| Witters 1976 | Not RCT |
| Wolf 1983 | Not RCT |
| Wolfe 1991 | Not FH |
| Worne 1959 | Not defined FH |
| Yacowitz 1965 | Not FH, not RCT |
| Zhao 1993 | Not defined FH, drug study |
| Zimmerman 1986 | Not FH, not RCT |
| Zino 1987 | Not FH |
| Zock 1992 | Not FH |
| Zucker 1983 | Not defined FH, drug study |
| Zucker 1988 | Not defined FH |
RCT=Randomised controlled trial FH=Familial hypercholesterolaemia
Characteristics of studies awaiting assessment [ordered by study ID]
Fuentes 2008.
| Methods | Randomised and cross‐over dietary intervention trial. |
| Participants | 38 participants with FH were recruited, but only 30 were subjected to 4 low‐fat dietary intervention periods. |
| Interventions | 4 low‐fat dietary intervention periods, each of 4 weeks. Each intervention had a different content of cholesterol (< 150 or 300 mg/day) and sitosterol (< 1 or 2 g/day). |
| Outcomes | Plasma sitosterol/cholesterol ratio, basal sitosterol concentrations, change in LDL‐C. |
| Notes | Duration of intervention 4 weeks. |
Stein 2007.
| Methods | Open‐label, non‐comparative, multicenter study. |
| Participants | 1380 individuals with severe hypercholesterolaemia, including heterozygous familial hypercholesterolaemia. Individuals were 18 years old or over with fasting LDL‐C greater than or equal to 190 and less than or equal to 260 mg/dl and triglycerides under 400 mg/dl. |
| Interventions | Rosuvastatin 40 mg for 48 weeks. An optional additional 48‐week treatment period followed. |
| Outcomes | The initial period had 2 primary end points: percentage of patients achieving National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III LDL cholesterol goals at 12 weeks, and long‐term safety, assessed during 48 weeks by incidence and severity of adverse events and abnormal laboratory values. Safety was the primary end point in the extension period. |
| Notes | 6‐week dietary lead‐in. |
FH: familial hypercholesterolaemia LDL‐C: low density lipoprotein cholesterol
Characteristics of ongoing studies [ordered by study ID]
Párraga ongoing.
| Trial name or title | Párraga 2011. |
| Methods | Randomised, double‐blind, placebo controlled trial. |
| Participants | Adults with "limit" or "defined" hypercholesterolaemia and who have LDL cholesterol levels of 130 mg/dl or over. |
| Interventions | Yoghurt containing 2 g of plant sterol ester per container versus yoghurt without sterol for 24 months. |
| Outcomes | Change in lipid profile at 1, 3, 6, 12, 18 and 24 months. |
| Starting date | |
| Contact information | iparraga@sescam.jccm.es |
| Notes |
Differences between protocol and review
The comparisons listed in Objectives in the current update have been changed from the previous review, in line with the growing knowledge about the effects of dietary supplements in altering blood lipid levels.
May 2014: the title has been changed from 'Dietary treatment for familial hypercholesterolaemia'.
Contributions of authors
From 1999 to October 2008
The review authors were Vanessa Poustie and Patricia Rutherford. Each of these review authors participated in the writing of the text, the selection of eligible studies and the assessment of methodological quality. Vanessa Poustie undertook the searching for additional studies and extracted the data.
From October 2008:
Designing the review: Dr Nusrat Shafiq, Dr Meenu Singh Dr S. Malhotra Data collection for the review: Dr Nusrat Shafiq Designing search strategies: Dr Nusrat Shafiq, Natalie Yates (Trials Search Co‐ordinator, Cochrane Cystic Fibrosis & Genetic Disorders Group) Undertaking searches: Natalie Yates Screening search results:Dr Nusrat Shafiq, Dr Pratibha Khosla, Dr Sharonjeet Kaur Organising retrieval of papers: Natalie Yates Screening retrieved papers against inclusion criteria:Dr Pratibha Khosla, Dr Nusrat Shafiq Appraising quality of papers: Dr Pratibha Khosla, Dr Sharonjeet Kaur Writing to authors of papers for additional information: Dr Nusrat Shafiq,Dr Meenu Singh Obtaining and screening data on unpublished studies: Natalie Yates Data management for the review: Dr Nusrat Shafiq, Dr Samir Malhotra, Dr Pratibha Khosla Entering data into RevMan:Dr Nusrat Shafiq Analysis of data: Dr Nusrat Shafiq, Dr Samir Malhotra Interpretation of data:Dr Nusrat Shafiq, Dr Samir Malhotra, Dr Meenu Singh Providing a methodological perspective:Dr Nusrat Shafiq, Dr Samir Malhotra Providing a clinical perspective: Dr Nusrat Shafiq, Dr Meenu Singh, Dr Samir Malhotra, Providing a policy perspective: Dr Samir Malhotra, Dr Meenu Singh Writing the review: Dr Nusrat Shafiq, Dr Samir Malhotra Providing general advice on the review: Dr Meenu Singh, Dr Samir Malhotra
From March 2012:
Designing the review: Dr Nusrat Shafiq, Dr Samir Malhotra Data collection for the review: Dr. Anita Malhotra, Anjuman Arora and Dr Nusrat Shafiq Designing search strategies: Dr Nusrat Shafiq, Natalie Yates and Ms Neelima Chaddha (Trials Search Co‐ordinator, Cochrane Cystic Fibrosis & Genetic Disorders Group) Undertaking searches: Natalie Yates and Ms Neelima Chaddha Screening search results:Dr Nusrat Shafiq and Dr Anita Malhotra Organising retrieval of papers: Natalie Yates and Ms. Neelima Chaddha Screening retrieved papers against inclusion criteria: Dr Nusrat Shafiq and Dr. Anita Malhotra Appraising quality of papers: Dr Nusrat Shafiq, Dr Anita Malhotra, Dr Samir Malhotra, Anjuman Arora, Rajendra Kumar Writing to authors of papers for additional information: Dr Nusrat Shafiq, Dr Meenu Singh Obtaining and screening data on unpublished studies: Natalie Yates Data management for the review: Dr Nusrat Shafiq Entering data into RevMan:Dr Anita Malhotra Analysis of data: Dr Nusrat Shafiq and Dr, Anita Malhotra Interpretation of data:Dr Nusrat Shafiq, Dr Samir Malhotra Providing a methodological perspective:Dr Nusrat Shafiq Providing a clinical perspective: Dr Meenu Singh and Dr Samir Malhotra Providing a policy perspective: Dr Nusrat Shafiq, Dr Samir Malhotra Writing the review: Dr Anita Malhotra, Anjuman Arora Providing general advice on the review: Dr Nusrat Shafiq, Dr Meenu Singh and Dr. Samir Malhotra
Declarations of interest
None declared.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Amundsen 2002 {published data only}
- Amundsen AL, Ose L, Nenseter MS, Ntanios FY. Plant sterol ester‐enriched spread lowers plasma total and LDL cholesterol in children with familial hypercholesterolemia. American Journal of Clinical Nutrition 2002;76(2):338‐44. [DOI] [PubMed] [Google Scholar]
Balestrieri 1996 {published data only}
- Balestrieri GP, Maffi V, Sleiman I, Spandrio S, Stefano O, Salvi A, et al. Fish oil supplementation in patients with heterozygous familial hypercholesterolemia. Recenti Progressi in Medicina 1996;87(3):102‐5. [PubMed] [Google Scholar]
Chisholm 1992 {published data only}
- Chisholm A, Mann J, Sutherland W, Williams S, Ball M. Dietary management of patients with familial hypercholesterolaemia treated with Simvastatin. Quarterly Journal of Medicine 1992;85(2‐3):825‐31. [PubMed] [Google Scholar]
- Chisholm A, Sutherland W, Ball M. The effects of dietary fat content on plasma noncholesterol sterol concentrations in patients with familial hypercholesterolaemia treated with simvastatin. Metabolism 1994;43(3):310‐4. [DOI] [PubMed] [Google Scholar]
Engler 2004 {published data only}
- Engler MM, Engler MG, Malloy M, Chiu E, Besio D, Paul S, et al. Docosahexaenoic acid restores endothelial function in children with hyperlipidemia: results from the EARLY Study. International Journal of Clinical Pharmacology and Therapeutics 2004;42(12):672‐9. [DOI] [PubMed] [Google Scholar]
Guardamagna 2011a {published data only}
- Guardamagna O, Abello F, Baracco V, Stasiowska B, Martino F. The treatment of hypercholesterolemic children: efficacy and safety of a combination of red yeast rice extract and policosanols. Nutrition, Metabolism and Cardiovascular Diseases 2011;21(6):424‐9. [DOI] [PubMed] [Google Scholar]
Gylling 1995 {published data only}
- Gylling H, Siimes MA, Miettinen TA. Sitostanol ester margarine in dietary treatment of children with familial hypercholesterolemia. Journal of Lipid Research 1995;36:1807‐12. [PubMed] [Google Scholar]
Ketomäki 2003 {published data only}
- Ketomaki AM, Gyllling H, Antikaimen M, Simes MA, Miettinen TA. Red cell and plasma plant sterols are related during consumption of plant sterols are related during consumption of plant stanol ester spreads in children with hypercholesterolemia. Journal of Pediatrics 2003;142(5):524‐31. [DOI] [PubMed] [Google Scholar]
Ketomäki 2004a {published data only}
- Ketomäki A, Gylling H, Miettinen TA. Removal of intravenous Intralipid in patients with familial hypercholesterolemia during inhibition of cholesterol absorption and synthesis. Clinica Chimica Acta 2004;344(1‐2):83‐93. [DOI] [PubMed] [Google Scholar]
Ketomäki 2005 {published data only}
- Ketomäki A, Gylling H, Miettinen TA. Non‐cholesterol sterols in serum, lipoproteins and red cells in statin‐treated familial hypercholesterolemia subjects off and on plant stanol and sterol ester spreads. Clinica Chimica Acta 2005;353(1‐2):75‐86. [DOI] [PubMed] [Google Scholar]
Laurin 1991 {published data only}
- Jacques H, Laurin D, Moorjani S, Steinke M, Gagne C, Brun D, et al. Influence of diets containing cow's milk or soy protein beverage on plasma lipids in children with familial hypercholesterolaemia. Journal of American College of Nutrition 1992;11 Suppl:69‐73. [DOI] [PubMed] [Google Scholar]
- Laurin D, Jacques H, Moorjani S, Steinke F, Gagne C, Brun D, et al. Effects of a soy‐protein beverage on plasma lipoproteins in children with familial hypercholesterolemia. American Journal of Clinical Nutrition 1991;54:98‐103. [DOI] [PubMed] [Google Scholar]
Neil 2001 {unpublished data only}
- Neil HA, Meijer GW, Roe LS. Randomised controlled trial of use by hypercholesterolaemic patients of a vegetable oil sterol‐enriched fat spread. Atherosclerosis 2001;156(2):329‐37. [DOI] [PubMed] [Google Scholar]
Nigon 2001 {published data only}
- Nigon F, Serfaty‐Lacrosnière C, Beucler I, Chauvois D, Neveu C, Giral P, et al. Plant sterol‐enriched margarine lowers plasma LDL in hyperlipidemic subjects with low cholesterol intake: effect of fibrate treatment. Clinical Chemistry and Laboratory Medicine 2001;39(7):634‐40. [DOI] [PubMed] [Google Scholar]
O'Neill 2004 {published and unpublished data}
- O'Neill FH, Brynes A, Mandeno R, Rendell N, Taylor G, Seed M, et al. Comparison of the effects of dietary plant sterol and stanol esters on lipid metabolism. Nutrition, Metabolism, and Cardiovascular Diseases 2004;14(3):133‐42. [DOI] [PubMed] [Google Scholar]
Wirth 1982 {published data only}
- Wirth A, Middelhoff G, Braeuning Ch, Schlierf G. Treatment of familial hyperholesterolemia with combination of bezafibrate and guar. Atherosclerosis 1982;45:291‐7. [DOI] [PubMed] [Google Scholar]
Wolfe 1992 {published data only}
- Wolfe BM. Potential role of raising dietary protein intake for reducing risk of atherosclerosis. Canadian Journal of Cardiology 1995;11 Suppl G:127G‐31G. [PubMed] [Google Scholar]
- Wolfe BM, Giovannetti PM. High protein diet complements resin therapy of familial hypercholesterolemia. Clinical and Investigative Medicine 1992;15(4):349‐59. [PubMed] [Google Scholar]
References to studies excluded from this review
Abbey 1990 {published data only}
- Abbey M, Clifton P, Kestin M, Belling B, Nestel P. Effect of fish oil on lipoproteins, lecithin: cholesterol acyltransferase and lipid transfer protein activity in humans. Arteriosclerosis 1990;10(1):85‐94. [DOI] [PubMed] [Google Scholar]
Abbey 1993a {published data only}
- Abbey M, Belling B, Noakes M, Hirata F, Nestel P. Oxidation of low‐density lipoproteins: intraindividual variability and the effect of dietary linoleate supplementation. American Journal of Clinical Nutrition 1993;57(3):391‐8. [DOI] [PubMed] [Google Scholar]
Abbey 1993b {published data only}
- Abbey M, Nestel P, Baghurst PA. Antioxidant vitamins and low‐density lipoprotein oxidation. American Journal of Clinical Nutrition 1993;58(4):525‐32. [DOI] [PubMed] [Google Scholar]
Abrahamson 1974 {published data only}
- Abrahamson H, Gustafson A, Ohlson R. Polyunsaturated fatty acids in hyperlipoproteinemia. Nutrition Metabolism 1974;17(6):337‐9. [DOI] [PubMed] [Google Scholar]
Ahmed 1984 {published data only}
- Ahmed AA, Holub BJ. Alteration and recovery of bleeding times, platelet aggregation and fatty acid composition of individual phospholipids in platelets of human subjects receiving a supplement of cod liver oil. Lipids 1984;19(8):617‐24. [DOI] [PubMed] [Google Scholar]
Ahrens 1954 {published data only}
Ahrens 1957 {published data only}
- Ahrens EH, Insull W, Blomstrand R, Hirsch J, Tsaltas TT, Peterson ML. The influence of dietary fats on serum lipid levels in man. Lancet 1957;86:943‐53. [DOI] [PubMed] [Google Scholar]
Ahrens 1959 {published data only}
- Ahrens EH, Insull W, Hirsch J, Stoffel W, Peterson ML, Farquhur JW, et al. The effect on human serum lipids of a dietary fat, highly unsaturated, but poor in essential fatty acids. Lancet 1959;i:115. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Am Acad Paed 1972 {published data only}
- American Academy of Pediatrics Committee on Nutrition. Childhood diet and coronary heart disease. Pediatrics 1972;49(2):305‐7. [PubMed] [Google Scholar]
Amundsen 2004 {published data only}
- Amundsen AL, Ntanios F, Put N, Ose L. Long‐term compliance and changes in plasma lipids, plant sterols and carotenoids in children and parents with FH consuming plant sterol ester‐enriched spread. European Journal of Clinical Nutrition 2004;58(12):1612‐20. [DOI] [PubMed] [Google Scholar]
Anderson 1957 {published data only}
- Anderson JT, Keys A, Grande F. The effects of different food fats on serum cholesterol concentrations in man. Journal of Nutrition 1957;62(3):421. [DOI] [PubMed] [Google Scholar]
Anderson 1976 {published data only}
- Anderson JT, Grande F, Keys A. Independence of the effects of cholesterol and degree of saturation of the fat diet on serum cholesterol in man. American Journal of Clinical Nutrition 1976;29(11):1784‐9. [DOI] [PubMed] [Google Scholar]
Anderson 1980 {published data only}
- Anderson JT, Chen WL, Sieling B. Hypolipidemic effects of high‐carbohydrate, high‐fibre diets. Metabolism 1980;29:551‐8. [DOI] [PubMed] [Google Scholar]
Anderson 1984a {published data only}
- Anderson JW, Story L, Sieling B, Chen W‐JL, Petro MS, Story J. Hypercholesterolemic effects of oat‐bran or bean intake for hypercholesterolemic men. American Journal of Clinical Nutrition 1984;40:1146‐55. [DOI] [PubMed] [Google Scholar]
Anderson 1984b {published data only}
- Anderson JW, Story L, Sieling B, Chen WL. Hypocholesterolemic effects of high fibre diets rich in water soluble plant fibres. Journal of the Canadian Dietetic Association 1984;45:140‐9. [Google Scholar]
Asherio 1995 {published data only}
- Asherio A, Rimm EB, Stampfer MJ, Giovannucci EL, Willet WC. Dietary intake of marine n‐3 fatty acids and the risk of coronary disease among men. New England Journal of Medicine 1995;332:977‐82. [DOI] [PubMed] [Google Scholar]
Atkinson 1987 {published data only}
- Atkinson PM, Wheeler MC, Mendelsohn D, Pienaar N, Chetty N. Effects of a four week fresh water fish (trout) diet on platelet aggregation, platelet fatty acids, serum lipids and coagulation factors. American Journal of Hematology 1987;24:143‐9. [DOI] [PubMed] [Google Scholar]
Baggio 1988 {published data only}
- Baggio G, Pagnan A, Muraca M, Martini S, Opportuno A, Bonanome A, et al. Olive oil enriched diet: effect on serum lipoprotein and biliary cholesterol saturation. American Journal of Clinical Nutrition 1988;47:960‐4. [DOI] [PubMed] [Google Scholar]
Bartram 1992 {published data only}
- Bartram P, Geriarch S, Scheppach W, Keller F, Kasper H. Effect of single oat bran cereal breakfast on serum cholesterol, lipoproteins and apolipoproteins in patients with hyperlipoproteiemia type IIa. JPEN J Parenter Enteral Nutr 1992;16:533‐7. [DOI] [PubMed] [Google Scholar]
Becker 1983 {published data only}
- Becker N, Illingworth DR, Alaupovic P, Connor WE, Sundberg EE. Effects of saturated, monounsaturated and n‐6 polyunsaturated fatty acids on plasma lipids, lipoproteins and apolipoproteins in humans. American Journal of Clinical Nutrition 1983;37:355‐60. [DOI] [PubMed] [Google Scholar]
Becker 1993 {published data only}
- Becker M, Staab D, Bergmann K. Treatment of severe familial hypercholesterolemia in childhood with sitosterol and sitostanol. Journal of Pediatrics 1993;122:292‐6. [DOI] [PubMed] [Google Scholar]
Beil 1991 {published data only}
- Beil FU, Terres W, Orgass M, Heimer G. Dietary fish oils lower lipoprotein(a) in primary hypertriglyceridemia. Atherosclerosis 1991;90:95‐7. [DOI] [PubMed] [Google Scholar]
Beitz 1981 {published data only}
- Beitz J, Mest HJ, Forster W. Influence of linseed oil diet on the pattern of serum phospholipids in man. Acta Biologica Medica Germanica 1981;40:31‐5. [PubMed] [Google Scholar]
Berg 1991 {published data only}
- Berg Schmidt E, Klausen IC, Kristensen SD, Lervang HH, Faergeman O, Dyerberg J. The effect of n‐3 polyunsaturated fatty acids on Lp(a). Clinica Chimica Acta 1991;198:271‐6. [DOI] [PubMed] [Google Scholar]
Berge 1959 {published data only}
- Berge GK, Achor RWP, Barker NW, Power MH. Comparison of the treatment of hypercholesterolemia with nicotinic acid, sitosterol and safflower oil. American Heart Journal 1959;58:849. [DOI] [PubMed] [Google Scholar]
Berry 1991 {published data only}
- Berry EM, Eisenberg S, Haratz D, Friedlander Y, Norman Y, Kaufmann NA, et al. Effects of diets rich in monounsaturated fatty acids on plasma lipoproteins ‐ The Jerusalem Nutritional Study: High MUFAs vs high PUFAs. American Journal of Clinical Nutrition 1991;53(4):899‐907. [DOI] [PubMed] [Google Scholar]
Best 1954 {published data only}
- Best MM, Duncan CH, Vanloon EJ, Wathen JD. Lowering of serum cholesterol by the administration of a plant sterol. Circulation 1954;10:201. [DOI] [PubMed] [Google Scholar]
Best 1955 {published data only}
- Best MM, Duncan CH, Loon EJ, Wathen JD. The effects of sitosterol on serum lipids. American Journal of Medicine 1995;19:61. [DOI] [PubMed] [Google Scholar]
Best 1956 {published data only}
- Best MM, Duncan CH. Modification of abnormal serum lipid patterns in atherosclerosis by administration of sitosterol. Annals of Internal Medicine 1956;45:614. [DOI] [PubMed] [Google Scholar]
Beveridge 1955 {published data only}
- Beveridge JMR, Connell WF, Mayer GA, Firstbrook JB, DeWolfe MS. The effects of certain vegetable and animal fats on the plasma lipids of humans. Journal of Nutrition 1955;56:311‐9. [DOI] [PubMed] [Google Scholar]
Beveridge 1957 {published data only}
- Beveridge JMR, Connell WF, Mayer GA. Plasma cholesterol depressant factor in corn oil. Federation Proceedings 1957;16:11. [Google Scholar]
Beveridge 1959 {published data only}
- Beveridge JMR, Connell WF, Haust HL, Mayer GA. Dietary cholesterol and plasma cholesterol levels in man. Canadian Journal of Biochemistry and Physiology 1959;37:575‐82. [PubMed] [Google Scholar]
Beynen 1985 {published data only}
- Beynen AC, Katan MB. Reproducibility of the variations between humans in the response of serum cholesterol to cessation of egg consumption. Atherosclerosis 1985;57:19‐31. [DOI] [PubMed] [Google Scholar]
Bierenbaum 1963 {published data only}
- Bierenbaum ML, Green DP, German JB, et al. The effects of two low fat dietary patterns on the blood cholesterol level of young male coronary patients. Journal of Chronic Diseases 1963;16:1073. [DOI] [PubMed] [Google Scholar]
Bierenbaum 1970 {published data only}
- Bierenbaum ML, Fleischman AI, Green DP, Raichelson RI, Hayton T, Watson PB, et al. The 5‐years experience of a modified fat diet on younger men with coronary heart disease. Circulation 1970;42:943‐52. [DOI] [PubMed] [Google Scholar]
Blair 2000 {published data only}
- Blair SN, Capuzzi DM, Gottlieb SO, Nguyen T, Morgan JM, Cater NB. Incremental reduction of serum total cholesterol and low‐density lipoprotein cholesterol with the addition of plant stanol ester‐containing spread to statin therapy. American Journal of Cardiology 2000;86(1):46‐52. [DOI] [PubMed] [Google Scholar]
Blankenhorn 1990 {published data only}
- Blankenhorn DH, Johnson RL, Mack WJ, Zein HA, Vailas LI. The influence of diet on the appearance of new lesions in human coronary arteries. Journal of the American Medical Association 1990;263:1646‐52. [PubMed] [Google Scholar]
Blaton 1984 {published data only}
- Blaton V, Buyzere M, Declercq B, et al. Effect on polyunsaturated isocaloric fat diets on plasma lipids, apolipoproteins and fatty acids. Atherosclerosis 1984;53:9‐20. [DOI] [PubMed] [Google Scholar]
Boberg 1986 {published data only}
- Boberg M, Vessby B, Selinus I. Effects of dietary supplementation with n‐6 and n‐3 long‐chain polyunsaturated fatty acids on serum lipoproteins and platelet function in hypertriglyceridaemic patients. Acta Medica Scandinavia 1986;220:153‐60. [DOI] [PubMed] [Google Scholar]
Bonanome 1988 {published data only}
- Bonanome A, Grundy SM. Effects of dietary stearic acid on plasma cholesterol and lipoprotein levels. New England Journal of Medicine 1988;318:1244‐8. [DOI] [PubMed] [Google Scholar]
Bonanome 1992 {published data only}
- Bonanome A, Pagnan A, Biffanti S, Opportuno A, Sorgato F, Dorella M, et al. Effect of dietary monounsaturated and polyunsaturated fatty acids on the susceptibility of plasma low density lipoproteins to oxidative modification. Arteriosclerosis Thrombosis 1992;12:529‐33. [DOI] [PubMed] [Google Scholar]
Bowry 1993 {published data only}
- Bowry VW, Stocker R. Tocopherol‐mediated peroxidation: the effect of vitamin E on the radical‐initiated oxidation of human low‐density lipoprotein. Journal of the American Chemical Society 1993;115:6029‐44. [Google Scholar]
Boyd 1990 {published data only}
- Boyd NF, Cousins M, Beaton M, Kriukov, Lockwood G, Tritchler D. Quantitative changes in dietary fat intake and serum cholesterol on women: results of a randomised controlled trial. American Journal of Clinical Nutrition 1990;52:470‐6. [DOI] [PubMed] [Google Scholar]
Braden 1990 {published data only}
- Braden GA, Knapp HR, Fitzgerald DJ, Fitzgerald GA. Dietary fish oil accelerates the response to coronary thrombolysis with tissue‐type plasminogen activator. Circulation 1990;82:178‐87. [DOI] [PubMed] [Google Scholar]
Brensike 1982 {published data only}
- Brensike JF. National heart, lung and blood institute type II coronary intervention study. Controlled Clinical Trials 1982;3:91‐111. [DOI] [PubMed] [Google Scholar]
Brensike 1984 {published data only}
- Brensike JF, Levy RI, Kelsey SF, Passamani ER, Richardson JM, Loh IK, et al. Effects of therapy with cholestyramine on progression of coronary arteriosclerosis: results of the NHLBI Type II Coronary Intervention Study. Circulation 1984;69:313‐24. [DOI] [PubMed] [Google Scholar]
Brinton 1990 {published data only}
- Brinton EA, Eisenberg S, Breslow JL. A low fat diet decreases high density lipoprotein (HDL) cholesterol levels by decreasing HDL apolipoprotein transport rates. Journal of Clinical Investigation 1990;85:144‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Briones 1984 {published data only}
- Briones ER, Steiger D, Palumbo PJ, Kottke BA. Primary hypercholesterolaemia: effect of treatment on serum lipids, lipoprotein fractions, cholesterol absorption, sterol balance, and platelet aggregation. Mayo Clinic Proceedings 1984;59:251‐7. [DOI] [PubMed] [Google Scholar]
Brongeest 1979a {published data only}
- Brongeest‐Schoute HO, Hautvast JGAJ, Hermus RJJ. Dependence of the effects of dietary cholesterol and experimental conditions on serum lipids in man.I. Effects of dietary cholesterol in a linoleic‐acid rich diet. American Journal of Clinical Nutrition 1979;32:2183‐7. [DOI] [PubMed] [Google Scholar]
Brongeest 1979b {published data only}
- Brongeest‐Schoute HO, Brongeest‐Schoute DC, Hermus RJ, Dallinga‐Thie GM, Hautvast JG. Dependence of the effects of dietary cholesterol and experimental conditions on serum lipids in man.II. Effects of dietary cholesterol in a linoleic acid‐poor diet. American Journal of Clinical Nutrition 1979;32:2188‐92. [DOI] [PubMed] [Google Scholar]
Brongeest 1979c {published data only}
- Bronsgeest‐Schoute DC, Hermus RJ, Dallinga‐Thie GM, et al. Dependence of the effects of dietary cholesterol and experimental conditions on serum lipids in man.III. The effect on serum cholesterol of removal of eggs from the diet of free‐living, habitually egg‐eating people. American Journal of Clinical Nutrition 1979;32(11):2193‐7. [DOI] [PubMed] [Google Scholar]
Bronte‐Stewart 1956 {published data only}
- Bronte‐Stewart B, Antonis A Eales L, Brock JF. Effects of feeding different fats on serum cholesterol levels. Lancet 1956;1:521‐7. [DOI] [PubMed] [Google Scholar]
Brown 1991 {published data only}
- Brown SA, Morrisett J, Patsch JR, Reeves R, Gotto AM, Patsch W. Influence of short term dietary cholesterol and fat on human plasma Lp[a] and LDL levels. Journal of Lipid Research 1991;32:1281‐9. [PubMed] [Google Scholar]
Brox 1981 {published data only}
- Brox JH, Killie JE, Gunnes S, Nordoy A. The effect of cod liver oil and corn oil on platelets and vessel wall in man. Thrombosis and Haemostasis 1981;46:604. [PubMed] [Google Scholar]
Brox 1983 {published data only}
- Brox JH, Killie JE, Osterud B, Holme S, Nordoy A. Effects of cod liver oil on platelets and coagulation in familial hypercholesterolemia (type A). Acta Medica Scandinavica 1983;213:137. [DOI] [PubMed] [Google Scholar]
Bruckner 1987 {published data only}
- Bruckner G, Webb P, Greenwell L, Chow C, Richardson D. Fish oil increases peripheral capillary blood cell velocity in humans. Atherosclerosis 1987;66:237‐45. [DOI] [PubMed] [Google Scholar]
Brude 1997 {published data only}
- Brude IR, Drevon CA, Hjermann I, Seljeflot I, Lund‐Katz S, Saarem K, et al. Peroxidation of LDL from combined‐hyperlipidemic male smokers supplied with omega‐3 fatty acids and antioxidants. Arteriosclerosis, Thrombosis, and Vascualr Biology 1997;17:2576‐88. [DOI] [PubMed] [Google Scholar]
Brussaard 1980 {published data only}
- Brussaard JH, Dallinga‐Thie G, Grott PH, Katan MB. Effects of amount and type of dietary fat on serum lipids, lipoproteins and apolipoproteins in man: a controlled 8‐week trial. Atherosclerosis 1980;36:515‐27. [DOI] [PubMed] [Google Scholar]
Burr 1989 {published data only}
- Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, et al. Effects of changes in fat, fish and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet 1989;2:757‐61. [DOI] [PubMed] [Google Scholar]
Canetti 1995 {published data only}
- Canetti M, Moreira M, Mas R, Illnait J, Fernandez L, Fernandez J. A two year study on the efficacy and tolerability of policosanol in patients with type II hyperlipoproteinaemia. International Journal of Clinical Pharmacology Research 1995;15:159‐65. [PubMed] [Google Scholar]
Carlson 1971 {published data only}
- Carlson LA, Olsson AG, Oro L, Rossner S. Effects of oral calcium upon serum cholesterol and triglycerides in patients with hyperlipidemia. Atherosclerosis 1971;14:391‐400. [DOI] [PubMed] [Google Scholar]
Carmen‐Ramon 1998 {published data only}
- Carmen‐Ramon R, Ascaso JF, Real JT, Ordovas JM, Carmen R. Genetic variation at the ApoA‐IV gene locus and response to diet in familial hypercholesterolemia. Arteriosclerosis, Thrombosis, and Vascualr Biology 1998;18:1266‐74. [DOI] [PubMed] [Google Scholar]
Carranza 1997 {published data only}
- Carranza‐Madrigal J, Herrera‐Abarca JE, Alvizouri‐Munza M, Alvarado‐Jimenez MR, Chavez‐Carbajal F. Effects of a vegetarian diet vs a vegetarian diet enriched with avocado in hypercholesterolemic patients. Archives of Medical Research 1997;28(4):537‐41. [PubMed] [Google Scholar]
Carroll 1975 {published data only}
- Carroll KK, Hamilton RMG. Effects of dietary protein and carbohydrate on plasma cholesterol levels in relation to atherosclerosis. Journal of Food Science 1975;40:18. [Google Scholar]
Carroll 1978 {published data only}
- Carroll KK. The role of dietary protein in hypercholesterolemia and atherosclerosis. Lipids 1978;13:360. [DOI] [PubMed] [Google Scholar]
Carroll 1982 {published data only}
- Carroll KK. Hypercholesterolemia and atherosclerosis: effects of dietary protein. Federation Proceedings 1982;41:2792‐6. [PubMed] [Google Scholar]
Carroll 1991 {published data only}
- Carroll KK. Review of clinical studies on cholesterol‐lowering response to soy protein. Journal of the American Dietetic Association 1991;91:820‐7. [PubMed] [Google Scholar]
Chait 1974 {published data only}
- Chait A, Onitiri A, Nicoll A, Rabaya E, Davies J, Lewis B. Reduction of serum triglyceride levels by polyunsaturated fat. Studies on the mode of action and on very low density lipoprotein composition. Atherosclerosis 1974;20:347‐64. [DOI] [PubMed] [Google Scholar]
Chang 1990 {published data only}
- Chang NW, Huang PC. Effects of dietary monounsaturated fatty acids on plasma lipids in humans. Journal of Lipid Research 1990;31:2141‐7. [PubMed] [Google Scholar]
Chen 1979 {published data only}
- Chen WL, Anderson JT. Effects of plant fibre in decreasing plasma total cholesterol and increasing high density lipoprotein cholesterol. Proceedings of the Society for Experimental Biology and Medicine 1979;162:310‐3. [DOI] [PubMed] [Google Scholar]
Chenoweth 1981 {published data only}
- Chenoweth W, Ullmann M, Simpson R, Leveille G. Influence of dietary cholesterol and fat on serum lipids in man. Journal of Nutrition 1981;111:2069‐80. [DOI] [PubMed] [Google Scholar]
Childs 1981 {published data only}
- Childs MT, Bowlin JA, Ogilvie JT, Hazzard WR, Albers JJ. The contrasting effects of a dietary soya lecithin product and corn oil on lipoprotein lipids in normolipidemic and familial hypercholesterolemic subjects. Atherosclerosis 1981;38:217‐28. [DOI] [PubMed] [Google Scholar]
Clevidence 1992 {published data only}
- Clevidence BA, Judd JT, Schatzkin A, et al. Plasma lipid and lipoprotein concentrations of men consuming a low‐fat, high fibre diet. American Journal of Clinical Nutrition 1992;55:689‐94. [DOI] [PubMed] [Google Scholar]
Clifton 1992 {published data only}
- Clifton PM, Wight MB, Nestel PJ. Is fat restriction needed with HMGCoA reductase inhibitor treatment?. Atherosclerosis 1992;93:59‐70. [DOI] [PubMed] [Google Scholar]
Cobb 1991 {published data only}
- Cobb M, Teitelbaum H, Breslow J. Lovestatin efficiency in reducing low density lipoprotein cholesterol levels on high vs low fat diets. Journal of the American Medical Association 1991;265:997‐1001. [PubMed] [Google Scholar]
Cole 1992 {published data only}
- Cole TG, Bowen PE, Schmeisser D, Prewitt TE, Aye P, Langenberg P, et al. Differential reduction of plasma cholesterol by the American Heart Association Phase 3 Diet in moderately hypercholesterolemic, premenopausal women with different body mass indexes. American Journal of Clinical Nutrition 1992;55(22):385‐94. [DOI] [PubMed] [Google Scholar]
Colquhoun 1992 {published data only}
- Colquhoun DM, Moores D, Somerset SM, Humphries JA. Comparison of the effects on lipoproteins and apolipoproteins of a diet high in monounsaturated fatty acids enriched with avocado, and a high carbohydrate diet. American Journal of Clinical Nutrition 1992;56:671‐7. [DOI] [PubMed] [Google Scholar]
Connor 1961a {published data only}
- Connor WE, Hodges RE, Blieler RE. Effect of dietary cholesterol upon serum lipids in man. Journal of Laboratory and Clinical Medicine 1961;57:331‐42. [PubMed] [Google Scholar]
Connor 1961b {published data only}
- Connor WE, Hodges RE, Blieler RE. The serum lipids in men receiving high cholesterol and cholesterol‐free diets. Journal of Clinical Investigation 1961;40:894‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Connor 1964 {published data only}
- Connor WE, Stone DB, Hodges RE. The interrelated effects of dietary cholesterol and fat upon human serum lipid levels. Journal of Clinical Investigation 1964;43:1691‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Connor 1982 {published data only}
- Connor WE, Connor SL. The dietary treatment of hyperlipidemia. Rationale, technique and efficacy. Medical Clinics of North America 1982;66:485‐518. [DOI] [PubMed] [Google Scholar]
Corder 1989 {published data only}
- Corder CN, Kloer HU, Price MD. CI‐924 effects on plasma lipids in patients with type II and type IV hyperlipoproteinaemia. European Journal of Clinical Pharmacology 1989;37:477‐81. [DOI] [PubMed] [Google Scholar]
Cortese 1983 {published data only}
- Cortese C, Levy Y, Janus ED, Turner PR, Rao SN, Miller NE, et al. Modes of action of lipid‐lowering diets in man: studies of apolipoprotein B kinetics in relation to fat consumption and dietary fatty acid composition. European Journal of Clinical Investigation 1983;13:79‐85. [DOI] [PubMed] [Google Scholar]
Crouse 1979 {published data only}
- Crouse JR, Grundy SM. Effects of sucrose polyester on cholesterol metabolism in man. Metabolism 1979;28:994‐1000. [DOI] [PubMed] [Google Scholar]
Da Col 1984 {published data only}
- Col PG, Cattin L, Fonda M, Mameli MG, Feruglio FS. Pantethine in the treatment of hypercholesterolemia: A randomised double blind trial versus tiadenol. Current Therapeutic Research, Clinical and Experimental 1984;36:314‐22. [Google Scholar]
Dattilo 1992 {published data only}
- Dattilo AM, Kris‐Etherton PM. effects of weight reduction on blood lipids and lipoproteins: a meta‐analysis. American Journal of Clinical Nutrition 1992;56:320‐8. [DOI] [PubMed] [Google Scholar]
Davidson 1991 {published data only}
- Davidson MH, Burns JH, Papasani VS, Conn ME, Dremman KB. Marine oil capsule therapy for the treatment of hyperlipidemia. Archives of Internal Medicine 1991;151:1732‐40. [PubMed] [Google Scholar]
Davis 1985 {published data only}
- Davis TA, Anderson EC, Ginsburg AV, Goldberg AP. Weight loss improves lipoprotein lipid profiles in patients with hypercholesterolemia. Journal of Laboratory and Clinical Medicine 1985;106:447‐54. [PubMed] [Google Scholar]
de Groot 1963 {published data only}
- Groot AP, Luyken R, Pikaar NA. Cholesterol lowering effect of rolled oats. Lancet 1963;2:303‐4. [DOI] [PubMed] [Google Scholar]
De Jong 2008 {published data only}
- Jong A, Plat J, Lütjohann D, Mensink RP. Effects of long‐term plant sterol or stanol ester consumption on lipid and lipoprotein metabolism in subjects on statin treatment. British Journal of Nutrition 2008;100(5):937‐41. [DOI] [PubMed] [Google Scholar]
Demke 1988 {published data only}
- Demke DM, Peters GR, Linet OI, Metzler CM, Klott KA. Effects of a fish oil concentrate in patients with hypercholesterolemia. Atherosclerosis 1988;70:73‐80. [DOI] [PubMed] [Google Scholar]
Demke 1991 {published data only}
- Demke DM, Grundy SM. Lauric acid is hypercholesterolemic in man [abstract]. Circulation 1991;84 (Suppl II):218. [Google Scholar]
Denke 1995 {published data only}
- Denke M. Lack of efficacy of low‐dose sitostanol therapy as an adjunct to a cholesterol‐lowering diet in men with moderate hypercholesterolemia. American Journal of Clinical Nutrition 1995;61:392‐6. [DOI] [PubMed] [Google Scholar]
Descovich 1980 {published data only}
- Descovich GC, Gaddi A, Mannino G. Multicentre study of soybean‐protein diet for outpatient hypercholesterolemic patients. Lancet 1980;2:709‐12. [DOI] [PubMed] [Google Scholar]
Detre 1985 {published data only}
- Detre KM, Levy RI, Kelsey SF, Epstein SE, Brensike JF, Passamani ER, et al. Secondary prevention and lipid lowering: results and implications. American Heart Journal 1985;110:1123‐7. [DOI] [PubMed] [Google Scholar]
Dieber 1991 {published data only}
- Dieber‐Rotheneder M, Puhl H, Waeg G, Striegl G, Esterbaur H. Effect of oral supplementation with D‐alpha‐tocopherol on the vitamin e content of human low density lipoproteins and resistance to oxidation. Journal of Lipid Research 1991;32:1325‐32. [PubMed] [Google Scholar]
Dreon 1990 {published data only}
- Dreon DM, Vranizan KM, Krauss RM, Austin MA, Wood PD. The effects of polyunsaturated versus monounsaturated fat on plasma lipoproteins. Journal of American Medical Association 1990;263:2462‐6. [PubMed] [Google Scholar]
Dreon 1994 {published data only}
- Dreon DM, Fernstrom HA, Miller B, Krauss RM. Low density subclass patterns and lipoprotein response to a reduced fat diet in man. The FASEB journal: official publication of the Fedeation of American Societies for Experimental Bioogy 1994;8:121‐6. [PubMed] [Google Scholar]
Dreon 1997 {published data only}
- Dreon DM, Krauss RM. Diet‐gene interactions in human lipoprotein metabolism. Journal of the American College of Nutrition 1997;4:313‐24. [DOI] [PubMed] [Google Scholar]
Durrington 1977 {published data only}
- Durrington PN, Bolton CH, Hartog M, Angelinetta R, Emmett P, Furniss S. The effect of a low‐cholesterol, high‐polyunsaturate diet on serum lipid levels, apolipoprotein B levels and triglyceride fatty acid composition. Atherosclerosis 1977;27:465‐75. [DOI] [PubMed] [Google Scholar]
Dyerberg 1978 {published data only}
- Dyerberg J, Bang HO, Stoffersen E. Eicosapentanoic acid and prevention of thrombosis and atherosclerosis. Lancet 1978;2:117‐9. [DOI] [PubMed] [Google Scholar]
East 1988 {published data only}
- East C, Bilheimer DW, Grundy SM. Combination drug therapy for familial combined hyperlipidaemia. Annals of Internal Medicine 1988;109:25‐32. [DOI] [PubMed] [Google Scholar]
Edington 1989 {published data only}
- Edington JD, Geekie M, Carter R, Benfield L, Ball M, Mann J. Serum lipid response to dietary cholesterol in subjects fed a low‐fat, high fibre diet. American Journal of Clinical Nutrition 1989;50:58‐62. [DOI] [PubMed] [Google Scholar]
Ehnholm 1982 {published data only}
- Ehnholm C, Huttunen JK, Pietinen P, et al. Effect of diet on serum lipoproteins in a population with a high risk of coronary heart disease. New England Journal of Medicine 1982;307:850‐5. [DOI] [PubMed] [Google Scholar]
Ehnholm 1984 {published data only}
- Ehnholm C, Huttien JK, Peitinen P, et al. Effect of a diet low in saturated fatty acids on plasma lipids, lipoproteins and HDL subfractions. Arteriosclerosis 1989;4:265‐9. [DOI] [PubMed] [Google Scholar]
Elkeles 1988 {published data only}
- Elkeles RS, Chakrabarti R, Vickers MV, Stirling Y, Meade TW. Effect of treatment of hyperlipidaemia on haemostatic variables. British Medical Journal 1980;281:973‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Erickson 1964 {published data only}
- Erickson BA, Coots RH, Mattson FH, Kligman AM. The effect of partial hydrogenation of dietary fats, of the ratio of polyunsaturated to saturated fatty acids, and of dietary cholesterol upon plasma lipids in man. Journal of Clinical Investigation 1964;43:2017‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eritsland 1995 {published data only}
- Eritsland J, Arnesen H, Seljeflot I, Hostmark AT. Long‐term metabolic effects of n‐3 polyunsaturated fatty acids in patients with coronary artery disease. American Journal of Clinical Nutrition 1995;61:831‐6. [DOI] [PubMed] [Google Scholar]
Ernst 1980 {published data only}
- Ernst N, Fisher M, Bowen P, Schaefer EJ, Levy RI. Changes in plasma lipids and lipoproteins after a fat modified diet. Lancet 1980;2:111‐3. [DOI] [PubMed] [Google Scholar]
Failor 1986 {published data only}
- Failor RA, Childs MT, Bierman EL. The effect of Omega‐3 and Omega‐6 fatty acid enriched diets upon plasma lipoproteins in subjects with familial combined hyperlipoproteinemia. Clinical Research 1986;34:103. [Google Scholar]
Falko 1980 {published data only}
- Falko JM, Schofeld G, Witztum JL, Kolar JB, Weidman SW, Steelman R. Effects of diet on apoprotein E levels and on the apoprotein E subspecies in human plasma lipoproteins. Journal of Clinical Endocrinology and Metabolism 1980;50:521‐8. [DOI] [PubMed] [Google Scholar]
Fallat 1979 {published data only}
- Fallat RW, Glueck CJ, Lutmer FH. Short term study of sucrose polyester, a non‐absorbable fat‐like material as a dietary agent for lowering plasma cholesterol. American Journal of Clinical Nutrition 1979;29:1204‐15. [DOI] [PubMed] [Google Scholar]
Farquhar 1956 {published data only}
- Farquhar JW, Smith RE, Dempsey M. The effect of beta‐sitosterol on the serum lipids of young men with arteriosclerotic heart disease. Circulation 1956;14:77. [DOI] [PubMed] [Google Scholar]
Farquhar 1958 {published data only}
- Farquhar JW, Sokolow M. Response of serum lipids and lipoproteins of man to beta‐sitosterol and safflower oil. Circulation 1958;17:890. [DOI] [PubMed] [Google Scholar]
Fehily 1983 {published data only}
- Fehily AM, Burr ML, Phillips MC, Deadman NM. The effect of fatty fish on plasma lipid and lipoprotein concentrations. American Journal of Clinical Nutrition 1983;38:349‐51. [DOI] [PubMed] [Google Scholar]
Fernandes 1977 {published data only}
- Fernandes J, Dijkhuis‐Stoffelsma R, Grose WFA. The effect of cholestyramine on serum lipids and platelet aggregation of hypercholesterolemic children (Type IIA) while on high linoleic acid diet. Acta Paediatric Scandinavia 1977;66:621‐4. [DOI] [PubMed] [Google Scholar]
Fernandes 1981 {published data only}
- Fernandes J, Dijkhuis‐Stoffelsma R, Groot PHE, Grose WFA, Ambaptsheer JJ. The effect of a virtually cholesterol‐free, high linoleic acid vegetarian diet on serum lipoproteins of children with hypercholesterolemia (type IIa). Acta Paediatrica Scandinavica 1981;70:677‐82. [DOI] [PubMed] [Google Scholar]
Ferro‐Luzzi 1984 {published data only}
- Ferro‐Luzzi A, Strazzullo P, Scaccini PC, Siani A, Sette S, Mariani MA, et al. Changing the Mediterranean diet: effects on blood lipids. American Journal of Clinical Nutrition 1984;40(5):1027‐37. [DOI] [PubMed] [Google Scholar]
Fisher 1983 {published data only}
- Fisher EA, Blum CB, Zanni VI, Breslow JL. Independent effects of dietary saturated fat and cholesterol on plasma lipids, lipoproteins and apolipoprotein E. Journal of Lipid Research 1983;24:1039‐48. [PubMed] [Google Scholar]
Flaim 1981 {published data only}
- Flaim E, Ferrei LF, Thye FW, Hill JE, Ritchey SJ. Plasma lipid and lipoprotein cholesterol concentrations in adult males consuming normal and high cholesterol diets under controlled conditions. American Journal of Clinical Nutrition 1981;34:1103‐8. [DOI] [PubMed] [Google Scholar]
Flynn 1981 {published data only}
- Flynn MA, Heine B, Nolph GB, Naumann HD, Parisi E, Ball D, et al. Serum lipids in humans fed diets containing beef or fish and poultry. American Journal of Clinical Nutrition 1981;34(12):2734‐41. [DOI] [PubMed] [Google Scholar]
Follick 1984 {published data only}
- Follick MO, Abrams DB, Smith TW, Henderson LO, Herbert PN. Contrasting short and long term effects of weight loss on lipoprotein levels. Archives of Internal Medicine 1984;144:1571‐4. [PubMed] [Google Scholar]
Forsythe 1986 {published data only}
- Forsythe WA, Green MS, Anderson JJB. Dietary protein effects on cholesterol and lipoprotein concentrations: A review. Journal of the American College of Nutrition 1986;5:533‐49. [DOI] [PubMed] [Google Scholar]
Frank 1978 {published data only}
- Frank GC, Berenson GS, Webber LS. Dietary studies and the relationship of diet to cardiovascular disease risk factor variables in 10yr old children (The Bogalusa Heart Study). American Journal of Clinical Nutrition 1978;31:328‐40. [DOI] [PubMed] [Google Scholar]
Frankel 1994 {published data only}
- Frankel EN, Parks EJ, Xu R, Schneeman BO, Davis PA, German JB. Effect of n‐3 fatty acid rich fish oil supplementation on the oxidation of low density lipoproteins. Lipids 1994;29:233‐6. [DOI] [PubMed] [Google Scholar]
Frantz 1975 {published data only}
- Frantz ID, Dawson EA, Kuba K, Brewer ER, Gatewood LC, Bartch GE. The Minnesota Coronary Survey: Effect of diet on cardiovascular events and deaths [abstract]. Circulation 1975;51‐52:ii‐4‐. [Google Scholar]
Frantz 1989 {published data only}
- Frantz ID Jr, Dawson EA, Ashman PL, Gatewood LC, Bartsch GE, Kuba K, et al. Test of effect of lipid lowering by diet on cardiovascular risk. The Minnesota Coronary Survey. Arteriosclerosis 1989;9(1):129‐35. [DOI] [PubMed] [Google Scholar]
Friday 1991 {published data only}
- Friday KE, Failor RA, Childs MT, Bierman EL. Effects of n‐3 and n‐6 fatty acid‐enriched diets on plasma lipoproteins and apoliproteins in heterozygous familial hypercholesterolaemia. Arteriosclerosis & Thrombosis 1991;11:47‐54. [DOI] [PubMed] [Google Scholar]
Fumagalli 1978 {published data only}
- Fumagalli R, Paoletti R, Howard AN. Hypocholesterolemic effect of soya. Life Sciences 1978;22:947. [DOI] [PubMed] [Google Scholar]
Fumagalli 1982 {published data only}
- Fumagalli R, Soleri L, Farina R, Musanti R, Mantero O, Noseda G, et al. Fecal cholesterol excretion studies in type II hypercholesterolemic patients treated with the soybean protein diet. Atherosclerosis 1982;43(2‐3):341‐53. [DOI] [PubMed] [Google Scholar]
Gaddi 1987 {published data only}
- Gaddi A, Descovich GC, Noseda G, Fragiacomo C, Nicolini A, Montanari G, et al. Hypercholesterolemia treated by soybean protein diet. Archives of Disease in Childhood 1987;62:274‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Galvan 1996 {published data only}
- Galvan AQ, Natali A, Baldi S, Frascerra S, Sampietro T, Galetta F, et al. Effect of a reduced fat diet with or without pravastatin on glucose tolerance and insulin sensitivity in patients with primary hypercholesterolemia. Journal of Cardiovascular Pharmacology 1996;28(4):595‐602. [DOI] [PubMed] [Google Scholar]
Gardner 1995 {published data only}
- Gardner CD, Kraemar HC. Monounsaturated versus polyunsaturated dietary fat and serum lipids. A meta‐analysis. Arteriosclerosis, Thrombosis, and Vascular Biology 1995;15:1917‐27. [DOI] [PubMed] [Google Scholar]
Gardner 2005 {published data only}
- Gardner CD, Coulston A, Chatterjee L, Rigby A, Spiller G, Farquhar JW. The effect of a plant‐based diet on plasma lipids in hypercholesterolemic adults. Annals of Internal Medicine 2005;142(9):725‐33. [DOI] [PubMed] [Google Scholar]
Glueck 1972 {published data only}
- Glueck CJ, Fallat R, Tsang R. Pediatric familial type II hyperlipoproteinemia: therapy with diet and cholestyramine resin. Pediatrics 1972;52:669‐79. [PubMed] [Google Scholar]
Glueck 1977 {published data only}
- Glueck CJ, Reginald C, Tsang R, Ronald W, Fallat R, Mellies MJ. Diet in children heterozygous for familial hypercholesterolemia. American Journal in Diseases in Children 1977;131:162‐6. [DOI] [PubMed] [Google Scholar]
Glueck 1978 {published data only}
- Glueck CJ, McGill HC Jr, Shank RE, Lauer RN. Value and safety of diet modification to control hyperlipidemia in childhood and adolescence. A statement for physicians. Circulation 1978;58:381A‐5A. [PubMed] [Google Scholar]
Glueck 1979 {published data only}
- Glueck CJ, Mattson FH, Jandacek RJ. The lowering of plasma cholesterol by sucrose polyester in subjects consuming diets with 800, 300 or less than 50mg of cholesterol per day. American Journal of Clinical Nutrition 1979;32:1636‐44. [DOI] [PubMed] [Google Scholar]
Glueck 1983 {published data only}
- Glueck CJ, Jandacek RJ, Hogg E, Allen C, Baehler L, Tewksbury MB. Sucrose polyester: substitution for dietary fats in hypocaloric diets in the treatment of familial hypercholesterolemia. American Journal of Clinical Nutrition 1983;37:347‐54. [DOI] [PubMed] [Google Scholar]
Glueck 1991 {published data only}
- Glueck CJ, Speirs J, Tracy T, Streicher P, Illig E, Vandegrift J. Relationships of serum plant sterols (phytosterols) and cholesterol in 595 hypercholesterolemic subjects, and familial aggregation of phytosterols, cholesterol and premature coronary heart disease in hypercholesterolemic probands and their first degree relatives. Metabolism 1991;40:842‐8. [DOI] [PubMed] [Google Scholar]
Goldberg 1982 {published data only}
- Goldberg AP, Lim A, Kolar JB, Grundhauser JJ, Steinke FH, Schonfeld G. Soybean protein independently lowers plasma cholesterol levels in primary hypercholesterolemia. Atherosclerosis 1982;43:355‐68. [DOI] [PubMed] [Google Scholar]
Goodnight 1981 {published data only}
- Goodnight SH Jr, Harris WS, Connor WE. The effects of dietary omega‐3 fatty acids on platelet composition and function in man: a prospective, controlled study. Blood 1981;58:880‐5. [PubMed] [Google Scholar]
Gordon 1977 {published data only}
- Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. American Journal of Medicine 1977;62:707‐14. [DOI] [PubMed] [Google Scholar]
Gordon 1982 {published data only}
- Gordon DJ, Salz KM, Roggenkamp KJ, Franklin FA. Dietary determinants of plasma cholesterol change in the recruitment phase of the Lipid Research Clinics Coronary Primary Prevention Trial. Arteriosclerosis 1982;2:537‐48. [DOI] [PubMed] [Google Scholar]
Grande 1970 {published data only}
- Grande F, Anderson JT, Keys A. Comparison of effects of palmitic and stearic acids in the diet on serum cholesterol in man. American Journal of Clinical Nutrition 1970;23:1184‐93. [DOI] [PubMed] [Google Scholar]
Grande 1972 {published data only}
- Grande F, Anderson JT, Keys A. Diets of different fatty acid composition producing identical serum cholesterol levels in man. American Journal of Clinical Nutrition 1972;25:53‐60. [DOI] [PubMed] [Google Scholar]
Gries 1990 {published data only}
- Gries A, Malle E, Wurm H, Kostner GM. Influence of dietary fish oils on Lp(a) levels. Thrombosis Research 1990;58:667‐8. [DOI] [PubMed] [Google Scholar]
Groot 1980 {published data only}
- Groot PH, Grose WFA, Dijkhuis‐Stoffelsma R, Fernandes J, Ambagtsheer JJ. The effect of oral calcium carbonate administration on serum lipoproteins of children with familial hypercholesterolaemia (type II‐A). European Journal of Pediatrics 1980;135:81‐4. [DOI] [PubMed] [Google Scholar]
Grundy 1970 {published data only}
- Grundy SM, Ahrens EH. The effects of unsaturated dietary fats on absorption, excretion, synthesis and distribution of cholesterol in man. Journal of Clinical Investigation 1970;49:1135‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grundy 1986 {published data only}
- Grundy SM. Comparison of monounsaturated fatty acids and carbohydrates for lowering plasma cholesterol. New England Journal of Medicine 1986;314:745‐8. [DOI] [PubMed] [Google Scholar]
Grundy 1990 {published data only}
- Grundy SM, Denke MA. Dietary influences on serum lipids and lipoproteins. Journal of Lipid Research 1990;31:1149‐72. [PubMed] [Google Scholar]
Guardamagna 2011b {published data only}
- Guardamagna O, Abello F, Baracco V, Federici G, Bertucci P, Mozzi A, et al. Primary hyperlipidemias in children: effect of plant sterol supplementation on plasma lipids and markers of cholesterol synthesis and absorption. Acta Diabetologica 2011;48(2):127‐33. [DOI] [PubMed] [Google Scholar]
Gustafsson 1982 {published data only}
- Gustafsson IB, Vessby B, Boberg J, Karlstrom B, Lithell H. Effects of lipid lowering diets on patients with hyperlipoproteinemia. Journal of the American Dietetic Association 1982;80:426‐32. [PubMed] [Google Scholar]
Gustafsson 1983 {published data only}
- Gustafsson I, Boberg J, Karlstrom B, Lithell H, Vessby B. Similar serum lipoprotein reductions by lipid‐lowering diets with different polyunsaturated: saturated fat values. British Journal of Nutrition 1983;50:531‐7. [DOI] [PubMed] [Google Scholar]
Gustafsson 1985 {published data only}
- Gustafsson I, Vessby B, Karlstrom B, Boberg J, Boberg M, Lithell H. Effects on the serum lipoprotein concentrations by lipid‐lowering diets with different fatty acid compositions. Journal of the American College of Nutrition 1985;4:241‐8. [DOI] [PubMed] [Google Scholar]
Gustafsson 1992 {published data only}
- Gustafsson IB, Vessby B, Nydahl M. Effects of lipid lowering diets enriched with monounsaturated and polyunsaturated fatty acids on serum lipoprotein composition in patients with hypolipoproteinemia. Atherosclerosis 1992;96:109‐18. [DOI] [PubMed] [Google Scholar]
Gustafsson 1994 {published data only}
- Gustafsson I, Vessby B, Ohrvall M, Nydahl M. A diet rich in monounsaturated rapeseed oil reduces the lipoprotein cholesterol concentration and increases the relative content of n‐3 fatty acids in serum in hyperlipidemic subjects. American Journal of Clinical Nutrition 1994;59:667‐74. [DOI] [PubMed] [Google Scholar]
Gylling 1988 {published data only}
- Gylling H, Miettinen TA. Serum non‐cholesterol sterols related to cholesterol metabolism in familial hypercholesterolemia. Clinica Chimica Acta; International Journal of Clinical Chemistry 1988;178:41‐50. [DOI] [PubMed] [Google Scholar]
Gylling 2010 {published data only}
- Gylling H, Hallikainen M, Nissinen MJ, Simonen P, Miettinen TA. Very high plant stanol intake and serum plant stanols and non‐cholesterol sterols. European Journal of Nutrition 2010;49(2):111‐7. [DOI] [PubMed] [Google Scholar]
Gylling 2011 {published data only}
- Gylling H, Hallikainen M, Simonen P, Miettinen HE, Nissinen MJ, Miettinen TA. Serum and lipoprotein sitostanol and non‐cholesterol sterols after an acute dose of plant stanol ester on its long‐term consumption. European Journal of Nutrition 2011;51(5):615‐22. [DOI] [PubMed] [Google Scholar]
Hansen 1989 {published data only}
- Hansen JB, Olsen JO, Wilsgard L, Osterud P. Effects of dietary supplementation with cod liver oil on monocyte thromboplastin synthesis, coagulation and fibrinolysis. Journal of Internal Medicine Suppl 1989;731:133‐9. [DOI] [PubMed] [Google Scholar]
Harris 1983a {published data only}
- Harris WS, Connor WE, McMurry MP. The comparative reductions of the plasma lipids and lipoproteins by dietary polyunsaturated fats: salmon oil versus vegetable oil. Metabolism 1983;32:179‐84. [DOI] [PubMed] [Google Scholar]
Harris 1988 {published data only}
- Harris WS, Dujovne CA, Zucker M, Johnson B. Effects of a low saturated fat, low cholesterol fish oil supplement in hypertriglyceridemic patients. Annals of Internal Medicine 1988;109:465‐70. [DOI] [PubMed] [Google Scholar]
Harris 1989 {published data only}
- Harris WS. Fish oils and plasma lipid and lipoprotein metabolism in humans: a critical review. Journal of Lipid Research 1989;30:785‐807. [PubMed] [Google Scholar]
Hashim 1960 {published data only}
- Hashim SA, Arteaga A, Itallie TB. Effect of a saturated medium‐chain triglyceride on serum lipids in man. Lancet 1930;1:1105‐8. [DOI] [PubMed] [Google Scholar]
Hay 1982 {published data only}
- Hay CRM, Durber AP, Saynor R. Effect of fish oil on platelet kinetics in patients with ischaemic heart disease. Lancet 1982;1:1269‐72. [DOI] [PubMed] [Google Scholar]
Hegsted 1965 {published data only}
- Hegsted DM, McGandy RB, Myers ML, Stare FJ. Quantitative effects of dietary fats on serum cholesterol in man. American Journal of Clinical Nutrition 1965;17:281‐90. [DOI] [PubMed] [Google Scholar]
Hegsted 1986 {published data only}
- Hegsted DM. Serum cholesterol response to dietary cholesterol: a re‐evaluation. American Journal of Clinical Nutrition 1986;44:299‐305. [DOI] [PubMed] [Google Scholar]
Hegsted 1993 {published data only}
- Hegsted DM, Ausman LM, Johnson JA, Dallal GE. Dietary fat and serum lipids: an evaluation of the experimental data. American Journal of Clinical Nutrition 1993;57(6):875‐83. [DOI] [PubMed] [Google Scholar]
Heinemann 1986 {published data only}
- Heinemann T, Leiss O, Bergmann K. Effect of low‐dose sitostanol on serum cholesterol in patients with hypercholesterolemia. Atherosclerosis 1986;61:219‐23. [DOI] [PubMed] [Google Scholar]
Helms 1977 {published data only}
- Helms P, Gatti E, Sirtori CR. Soybean protein diet and plasma cholesterol. Lancet 1977;1:805‐6. [DOI] [PubMed] [Google Scholar]
Hennekens 1996 {published data only}
- Hennekens CH, Buring JE, Manson JE, Stampfer MJ, Rosner B, Cook NR, et al. Lack of effect of long term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. New England Journal of Medicine 1996;334:1145‐9. [DOI] [PubMed] [Google Scholar]
Herold 1986 {published data only}
- Herold PM, Kinsella JE. Fish oil consumption and decreased risk of cardiovascular disease: a comparison of findings from animal and human feeding trials. American Journal of Clinical Nutrition 1986;43:566‐98. [DOI] [PubMed] [Google Scholar]
Hodges 1967 {published data only}
- Hodges RE, Krehl WA, Stone DB, Lopez A. Dietary carbohydrates and low cholesterol diets ‐ Effects on serum lipids in man. American Journal of Clinical Nutrition 1967;20:198. [DOI] [PubMed] [Google Scholar]
Hoeg 1984 {published data only}
- Hoeg JM, Maher MB, Bou E, Zech LA, Bailey KR, Gregg RE, et al. Normalization of plasma lipoprotein concentrations in patients with type II hyperlipoproteinemia by combined use of neomycin and niacin. Circulation 1984;70(6):1004‐11. [DOI] [PubMed] [Google Scholar]
Holme 1990 {published data only}
- Holme I. An analysis of randomised trials evaluating the effect of cholesterol reduction on total mortality and coronary heart disease incidence. Circulation 1990;82:1916‐24. [DOI] [PubMed] [Google Scholar]
Holmes 1980 {published data only}
- Holmes WL, Rubel GB, Hood SS. Comparison of the effect of dietary meat versus dietary soybean protein on plasma lipids of hyperlipidemic individuals. Atherosclerosis 1980;36:379‐387. [Google Scholar]
Hooper 1980 {published data only}
- Hooper PL, Visconti L, Garry PJ, Johnson GE. Zinc lowers high density lipoprotein cholesterol levels. Journal of the American Medical Association 1980;244:1960‐1. [PubMed] [Google Scholar]
Hopkins 1992 {published data only}
- Hopkins PN. Effects of dietary cholesterol on serum cholesterol: a meta‐analysis and review. American Journal of Clinical Nutrition 1992;55:1060‐70. [DOI] [PubMed] [Google Scholar]
Huff 1984 {published data only}
- Huff MW, Giovannetti PM, Wolfe BM. Turn‐over of very low‐density lipoprotein‐apoprotein B is increased by substitution of soybean protein for meat and dairy protein in the diets of hypercholesterolemic men. American Journal of Clinical Nutrition 1984;39:888‐97. [DOI] [PubMed] [Google Scholar]
Hunninghake 1993 {published data only}
- Hunninghake DB, Stein EA, Dujovne CA, Harris WS, Feldman EB, Miller VT, et al. The efficacy of intensive dietary therapy alone or combined with lovastatin in outpatients with hypercholesterolemia. New England Journal of Medicine 1993;328(17):1213‐9. [DOI] [PubMed] [Google Scholar]
Iacono 1991 {published data only}
- Iacono JM, Dougherty RM. Lack of effect of linoleic acid on the high‐density‐lipoprotein‐cholesterol fraction of plasma lipoproteins. American Journal of Clinical Nutrition 1991;53:660‐4. [DOI] [PubMed] [Google Scholar]
Illingworth 1991 {published data only}
- Illingworth DR, Hatcher LF, Newcomb KC, Connor WE. Response to dietary cholesterol on a monounsaturated enriched low‐fat diet [abstract]. Arteriosclerosis Thrombosis 1991;11:1602(a). [Google Scholar]
Jackson 1984 {published data only}
- Jackson RL, Kashyap ML, Barnhart RL, Allen C, Hogg E, Glueck CJ. Influence of polyunsaturated and saturated fats on plasma lipids and lipoproteins in man. American Journal of Clinical Nutrition 1984;39:589‐97. [DOI] [PubMed] [Google Scholar]
Jakubowski 1978 {published data only}
- Jakubowski JA, Ardlie NG. Modification of human platelet function by a diet enriched in saturated or polyunsaturated fat. Atherosclerosis 1978;31:335‐44. [DOI] [PubMed] [Google Scholar]
Jenkins 1975a {published data only}
- Jenkins DJA, Leeds AR, Newton C, Cummings JH. Effect of pectin, guar gum and wheat fibre on serum cholesterol. Lancet 1975;1:1116. [DOI] [PubMed] [Google Scholar]
Jenkins 1975b {published data only}
- Jenkins DJA, Hill MS, Cummings JH. Effect of wheat fiber on blood lipids, fecal steroid excretion and serum iron. American Journal of Clinical Nutrition 1975;28:1408. [DOI] [PubMed] [Google Scholar]
Jenkins 1980 {published data only}
- Jenkins DJA, Reynold D, Slavin B, Leeds AR, Jenkins AL, Jepson EM. Treatment of hypercholesterolemia with guar crispbread. American Journal of Clinical Nutrition 1980;33:575. [DOI] [PubMed] [Google Scholar]
Jialal 1992 {published data only}
- Jialal I, Grundy SM. Effect of dietary supplementation with alpha‐tocopherol on the oxidative modification of low‐density lipoprotein. Journal of Lipid Research 1992;33:899‐906. [PubMed] [Google Scholar]
Jialal 1993 {published data only}
- Jialal I, Grundy SM. Effect of combined supplementation with alpha‐tocopherol, ascorbate and beta‐carotene on low‐density lipoprotein oxidation. Circulation 1993;88:2780‐6. [DOI] [PubMed] [Google Scholar]
Joyner 1955 {published data only}
- Joyner CP, Kuo PT. The effect of sitosterol administration upon the serum cholesterol level and lipoprotein pattern. American Journal of Medical Science 1955;230:636. [DOI] [PubMed] [Google Scholar]
Judd 1988 {published data only}
- Judd JT, Oh SY, Hennig B, Dupont J, Marshall MW. Effects of low fat diets differing in degree of fat unsaturation on plasma lipids, lipoproteins and apolipoproteins in adult men. Journal of the American College of Nutrition 1988;7:223‐34. [DOI] [PubMed] [Google Scholar]
Kane 1981 {published data only}
- Kane JP, Malloy MJ, Tun P, Phillips NR, Freedman DD, Williams ML, et al. Normalization of low‐density‐lipoprotein levels in heterozygous familial hypercholesterolemia with a combined drug regimen. New England Journal of Medicine 1981;304(5):251‐8. [DOI] [PubMed] [Google Scholar]
Kane 1990 {published data only}
- Kane JP, Malloy MJ, Ports TA, Phillips NR, Diehl JC, Havel RJ. Regression of coronary atherosclerosis during treatment of familial hypercholesterolemia with combined drug regimes. Journal of the American Medical Association 1990;264:3007‐12. [PubMed] [Google Scholar]
Kestin 1989 {published data only}
- Kestin M, Rouse IL, Correll RA, Nestel PJ. Cardiovascular disease risk factors in free‐living men: comparison of two prudent diets, one based on lactoovovegetarianism and the other allowing lean meat. American Journal of Clinical Nutrition 1989;50:280‐7. [DOI] [PubMed] [Google Scholar]
Ketomäki 2004b {published data only}
- Ketomaki A, Gylling H, Miettinen TA. Effects of plant stanol and sterol esters on serum phytosterols in a family with familial hypercholesterolemia including a homozygous subject. Journal of Laboratory and Clinical Clin Med 2004;143(4):255‐62. [DOI] [PubMed] [Google Scholar]
Keys 1957 {published data only}
- Keys A, Anderson JT, Grande F. Essential fatty acids, degree of unsaturation and effect of corn (maize) oil on the serum cholesterol level in man. Lancet 1957;1:66. [DOI] [PubMed] [Google Scholar]
Keys 1965a {published data only}
- Keys A, Anderson JT, Grande F. Serum cholesterol response to changes in the diet IV. Particular saturated fatty acids in the diet. Metabolism 1965;14:776‐87. [DOI] [PubMed] [Google Scholar]
Keys 1965b {published data only}
- Keys A, Anderson JT, Grande E. Serum cholesterol response to changes in the diet. I. Iodine value of dietary fat vs 2S‐P. Metabolism 1965;14:747‐58. [DOI] [PubMed] [Google Scholar]
Keys 1984 {published data only}
- Keys A. Serum cholesterol response to dietary cholesterol. American Journal of Clinical Nutrition 1984;40:351‐9. [DOI] [PubMed] [Google Scholar]
Khan 1981 {published data only}
- Khan AR, Khan GY, Mitchel A, Quadeer MA. Effect of guar gum on blood lipids. American Journal of Clinical Nutrition 1981;34:2446. [DOI] [PubMed] [Google Scholar]
Kingsbury 1961 {published data only}
- Kingsbury KJ, Morgan DM, Aylott C, Emmerson R. Effects of ethyl arachidonate, cod liver oil and corn oil on the plasma cholesterol level. Lancet 1961;1:739‐41. [DOI] [PubMed] [Google Scholar]
Kinsell 1952 {published data only}
- Kinsell LW, Partridge J, Boling L, Margen S, Michaels G. Dietary modification of serum cholesterol and phospholipid levels. Journal of Clinical Endocrinology and Metabolism 1952;12:909‐13. [DOI] [PubMed] [Google Scholar]
Kirby 1981 {published data only}
- Kirby RW, Anderson JW, Sieling B, Rees ED, Chen WJ, Miller RE, et al. Oat‐bran selectively lowers serum low‐density lipoprotein cholesterol concentrations of hypercholesterolemic men. American Journal of Clinical Nutrition 1981;34(5):824‐9. [DOI] [PubMed] [Google Scholar]
Kok 1987 {published data only}
- Kok FJ, Bruijn AM, Vermeeren R, Hofman A, Laar A, Bruin M, et al. Serum selenium, vitamin antioxidants, and cardiovascular mortality: a 9‐year follow‐up study in the Netherlands. American Journal of Clinical Nutrition 1987;45(2):462‐8. [DOI] [PubMed] [Google Scholar]
Kris‐Etherton 1993 {published data only}
- Kris‐Etherton PM, Derr J, Mitchell DC, Mustad VA, Russell ME, McDonnell ET, et al. The role of fatty acid saturation on plasma lipids, lipoproteins and apolipoproteins. I. Effects of whole food diets high in cocoa butter, olive oil, soybean oil, dairy butter and milk chocolate on the plasma lipids of young men. Metabolism 1993;42(1):121‐9. [DOI] [PubMed] [Google Scholar]
Kromhout 1985 {published data only}
- Kromhout D, Bosschieter EB, Coulnader CL. The inverse relation between fish consumption and 20‐year mortality from coronary heart disease. New England Journal of Medicine 1985;312(19):1205‐9. [DOI] [PubMed] [Google Scholar]
Kudchodkar 1976 {published data only}
- Kudchodkar BJ, Horlick L, Sodhi HS. Effect of plant sterols on cholesterol metabolism in man. Atherosclerosis 1976;23:239‐48. [Google Scholar]
Kuo 1979 {published data only}
- Kuo PT, Hayase K, Kostis JB, Moreyra AE. Use of combined diet and colestipol in long‐term (7‐7.5 years) treatment of patients with type II hyperlipoproteinemia. Circulation 1979;59:199‐211. [DOI] [PubMed] [Google Scholar]
Kuo 1981 {published data only}
- Kuo PT, Kostis JB, Morayra AE, Hayes JA. Familial type II hyperlipoproteinemia with coronary heart disease. Effect of diet‐cholesterol‐nicotinic acid treatment. Chest 1981;79:286‐91. [DOI] [PubMed] [Google Scholar]
Kuusi 1985 {published data only}
- Kuusi T, Ehnholm C, Huttunen JK, Kostiainen E, Pietinen P, Leino U, et al. Concentration and composition of serum lipoproteins during a low fat diet at two levels of polyunsaturated fat. Journal of Lipid Research 1985;26(3):360‐7. [PubMed] [Google Scholar]
Laine 1982 {published data only}
- Laine DC, Snodgrass CM, Dawson EA, Ener MA, Kuba K, Frantz ID. Lightly hydrogenated soy oil versus other vegetable oils as a lipid lowering dietary constituent. American Journal of Clinical Nutrition 1982;35:683‐90. [DOI] [PubMed] [Google Scholar]
Lambert 1996 {published data only}
- Lambert M, Lupien PJ, Gagne C, Levy E, Blaichman S, Langlois S, et al. Treatment of familial hypercholesterolemia in children and adolescents: effect of lovastatin. Canadaian Lovastatin in Children Study Group. Pediatrics 1996;97:619‐28. [PubMed] [Google Scholar]
Leaf 1988 {published data only}
- Leaf A, Weber PC. Cardiovascular effects of n‐3 fatty acids. New England Journal of Medicine 1988;318:549‐57. [DOI] [PubMed] [Google Scholar]
Leelarthaepin 1974 {published data only}
- Leelarthaepin B, Palmer AJ, Woodhill JM, Blacket RB. Obesity, diet and type II hyperlipidemia. Lancet 1974;2:1217‐21. [DOI] [PubMed] [Google Scholar]
Lees 1977 {published data only}
- Lees AM, Mok HYI, McCluskey MA, Grundy SM. Plant sterols as cholesterol lowering agents: clinical trials in patients with hypercholesterolemia and studies of sterol balance. Atherosclerosis 1977;28:325‐38. [DOI] [PubMed] [Google Scholar]
Leibman 1983 {published data only}
- Leibman M, Smith MC, Iverson J, Thye FW, Hinkle DE, Herbert WG, et al. Effects of coarse wheat bran fiber and exercise on plasma lipids and lipoproteins in moderately overweight men. American Journal of Clinical Nutrition 1983;37:71. [DOI] [PubMed] [Google Scholar]
LeLorier 1977 {published data only}
- LeLorier J, DuBreuil‐Quidoz S, Lussier‐Cacan S, Huang YS, Davignon J. Additive effects on plasma cholesterol concentrations in patients with familial type II hyperlipoproteinemia. Archives of Internal Medicine 1977;137:1429‐34. [PubMed] [Google Scholar]
Lewis 1981 {published data only}
- Lewis B, Hammett F, Katan M, Merkx I, Miller NE, Kay RM, et al. Towards an improved lipid lowering diet: additive effects of changes in nutrient intake. Lancet 1981;ii:1310. [DOI] [PubMed] [Google Scholar]
Lichtenstein 1994 {published data only}
- Lichtenstein AH, Ausman LM, Carrasco W, Jenner JL, Ordovas JM, Schaefer EJ. Short term consumption of a reduced fat diet beneficially affects plasma lipid concentrations only when accompanied by weight loss. Arteriosclerosis Thrombosis 1994;14:1751‐60. [DOI] [PubMed] [Google Scholar]
Lifshitz 1989 {published data only}
- Lifshitz F, Moses N. Growth failure: a complication of dietary treatment of hypercholesterolemia. American Journal of Diseases of Children 1989;143:537‐42. [PubMed] [Google Scholar]
Lindgard 1984 {published data only}
- Lindgard F, Larsson L. Effects of a concentrated bran fibre preparation on HDL‐cholesterol in hypercholesterolaemic men. Human Nutrition Clinical Nutrition 1984;38C:39. [PubMed] [Google Scholar]
Linnebur 2007 {published data only}
- Linnebur SA, Capell WH, Saseen JJ, Wolfe P, Eckel RH. Plant sterols added to combination statin and colesevelam hydrochloride therapy failed to lower low‐density lipoprotein cholesterol concentrations. Journal of Clinical Lipidology 2007;1(6):626‐33. [DOI] [PubMed] [Google Scholar]
Lithell 1984 {published data only}
- Lithell H, Selinus I, Vessby B. Lack of effect of a purified bran preparation in men with low HDL cholesterol. Human Nutrition Clinical Nutrition 1984;38:309‐13. [PubMed] [Google Scholar]
Lorenz 1983 {published data only}
- Lorenz R, Spengler U, Fischer S, Drum J, Weber PC. Platelet function, thromboxane formation and blood pressure control during supplementation of the western diet with cod liver oil. Circulation 1983;67:504‐11. [DOI] [PubMed] [Google Scholar]
Lovati 1987 {published data only}
- Lovati MR, Manzoni C, Canavesi A, Sirtori M, Vaccarino V, Marchi M, et al. Soybean protein diet increases low density lipoprotein receptor activity in mononuclear cells from hypercholesterolemic patients. Journal of Clinical Investigation 1987;80(5):1498‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
LRCP 1984a {published data only}
- Lipid Research Clinics Program. The Lipid Research Clinics Coronary Primary Intervention Trials results II. The relationship in incidence of coronary heart disease to cholesterol lowering. Journal of the American Medical Association 1984;251:351‐74. [Google Scholar]
LRCP 1984b {published data only}
- Lipid Research Clinics Program. The Lipid Research Clinics Coronary Prevention Trial results I. Reduction in incidence of coronary heart disease. Journal of the American Medical Association 1984;251:351. [DOI] [PubMed] [Google Scholar]
Macdonald 1967 {published data only}
- Macdonald I. Interrelationships between the influence of dietary carbohydrates and fats on fasting serum lipids. American Journal of Clinical Nutrition 1967;20:345‐52. [DOI] [PubMed] [Google Scholar]
Mackness 1993 {published data only}
- Mackness MI, Abbott C, Arrol S, Durrington PN. The role of high density lipoprotein and lipid soluble antioxidant vitamins in inhibiting low‐density lipoprotein oxidation. Biochemical Journal 1993;294:829‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malmros 1957 {published data only}
- Malmros H, Wigand G. The effect on serum cholesterol of diets containing different fats. Lancet 1957;2:1‐8. [DOI] [PubMed] [Google Scholar]
Mannarinno 2009 {published data only}
- Mannarino E, Pirro M, Cortese C, Lupattelli G, Siepi D, Mezzetti A, et al. Effects of a phytosterol‐enriched dairy product on lipids, sterols and 8‐isoprostane in hypercholesterolemic patients: a multicenter Italian study. Nutrition, Metabolism & Cardiovascular Diseases 2009;19(2):84‐90. [DOI] [PubMed] [Google Scholar]
Maranhao 1983 {published data only}
- Maranhao RC, Quintao E. Long term steroid metabolism balance studies in subjects on cholesterol‐free and cholesterol‐rich diets: comparison between normal and hypercholesterolemic individuals. Journal of Lipid Research 1983;24:167‐73. [PubMed] [Google Scholar]
Marshall 1986 {published data only}
- Marshall MW, Judd JT, Matusik EJ, Church J, Canary JJ. Effects of low fat diets varying in P/S ratio on nutrient intakes, fecal excretion, blood chemistry profiles, and fatty acids of adult men. Journal of the American College of Nutrition 1986;5:263‐79. [DOI] [PubMed] [Google Scholar]
Mata 1992 {published data only}
- Mata P, Garrido JA, Ordovas JM, Blazquez E, Alvarez‐Sala LA, Rubio MJ, et al. Effect of dietary monounsaturated fatty acids on plasma lipoproteins and apolipoproteins in women. American Journal of Clinical Nutrition 1992;56(1):77‐83. [DOI] [PubMed] [Google Scholar]
Mathur 1968 {published data only}
- Mathur KS, Khan MA, Sharma RD. Hypocholesterolemic effect of Bengal Gram: A long term study in man. British Medical Journal 1968;1:30‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mattson 1972 {published data only}
- Mattson FH, Erickson BA, Kligman AM. Effect of dietary cholesterol on serum cholesterol in man. American Journal of Clinical Nutrition 1972;25:589‐94. [DOI] [PubMed] [Google Scholar]
Mattson 1975 {published data only}
- Mattson FH, Hollenback EJ, Kligman AM. Effect of hydrogenated fat on the plasma cholesterol and triglyceride levels of man. American Journal of Clinical Nutrition 1975;28:726‐31. [DOI] [PubMed] [Google Scholar]
Mattson 1977 {published data only}
- Mattson FH, Volpenhein RA, Erickson BA. Effect of plant sterol esters on the absorption of dietary cholesterol. Journal of Nutrition 1977;107:1139‐46. [DOI] [PubMed] [Google Scholar]
Mattson 1982 {published data only}
- Mattson FH, Grundy SM, Crouse JR. Optomizing the effect of plant sterols on cholesterol absorption in man. American Journal of Clinical Nutrition 1982;35:697‐700. [DOI] [PubMed] [Google Scholar]
Mattson 1985 {published data only}
- Mattson FH, Grundy SM. Comparison of effects of dietary saturated, monounsaturated and polyunsaturated fatty acids on plasma lipids and lipoproteins in man. Journal of Lipid Research 1985; Vol. 26:194‐202. [PubMed]
McCombs 1994 {published data only}
- McCombs RJ, Marcadis DE, Ellis J, Weinberg RB. Attenuated hypercholesterolemic response to a high cholesterol diet in subjects heterozygous for the apolipoprotein A‐IV‐2 allele. New England Journal of Medicine 1994;331:706‐10. [DOI] [PubMed] [Google Scholar]
McGill 1979 {published data only}
- McGill HC. The relationship of dietary cholesterol to serum cholesterol concentration and to atherosclerosis in man. American Journal of Clinical Nutrition 1979;32:2664‐702. [DOI] [PubMed] [Google Scholar]
Mellies 1983 {published data only}
- Mellies JM, Jandacek RJ, Taulbee JD, Tewksbury MB, Lamkin G, Baehler L, et al. A double‐blind, placebo‐controlled study of sucrose polyester in hypercholesterolemic outpatients. American Journal of Clinical Nutrition 1983;37:339‐46. [DOI] [PubMed] [Google Scholar]
Mensink 1992 {published data only}
Miettinen 1972 {published data only}
- Miettinen M, Turpeinen O, Karvonen MJ, Elosno R, Paavilainen E. Effect of cholesterol‐lowering diet on mortality from coronary heart disease and other causes. A twelve‐year clinical trial in men and women. Lancet 1972;2:835. [DOI] [PubMed] [Google Scholar]
Miettinen 1977 {published data only}
Miettinen 1989a {published data only}
- Miettinen TA, Tilvis RS, Kesaniemi YA. Serum cholesterol and plant sterol levels in relation to cholesterol metabolism in middle aged men. Metabolism 1989;38:136‐40. [DOI] [PubMed] [Google Scholar]
Miettinen 1989b {published data only}
- Miettinen TA. Serum plant sterols and their relation to cholesterol absorption. American Journal of Clinical Nutrition 1989;49:629‐35. [DOI] [PubMed] [Google Scholar]
Miettinen 1992a {published data only}
- Miettinen TA, Gylling H, Vanhanen HT, Ollus A. Cnolesterol absorption, elimination and synthesis related to LDL kinetics during varying fat intake in men with differentapoprotein E phenotypes. Arteriosclerosis Thrombosis 1992;12:1044‐52. [DOI] [PubMed] [Google Scholar]
Miettinen 1992b {published data only}
- Miettinen TA, Gylling H, Vanhanen HT, Ollus A. Cholesterol absorption, elimination and synthesis related to LDL kinetics during varying fat intake in men with different apoprotein E phenotypes. Arteriosclerosis Thrombosis 1992;12(9):1044‐52. [DOI] [PubMed] [Google Scholar]
Miettinen 1994 {published data only}
- Miettinen TA, Vanhanen HT. Dietary sitostanol related to absorption, synthesis and serum level of cholesterol in different apolipoprotein E phenotypes. Atherosclerosis 1994;105:217‐26. [DOI] [PubMed] [Google Scholar]
Miller 1988 {published data only}
- Miller JP, Heath ID, Choraria SK, Shepherd NW, Cajendragadkar RV, Harcus AW, et al. Triglyceride lowering effect of MaxEPA fish lipid concentrate: a multicentre placebo controlled double blind study. Clinica Chimica Acta 1988;178(3):251‐60. [DOI] [PubMed] [Google Scholar]
Mokino 1990 {published data only}
- Mokino H, Yamada N, Sugimoto T, et al. Cholesterol free diet with a high ratio of polyunsaturated to saturated fatty acids in heterozygous familial hypercholesterolemia: significant lowering effect on plasma cholesterol. Hormone and Metabolic Research 1990;22:246‐51. [DOI] [PubMed] [Google Scholar]
Morita 1983 {published data only}
- Morita I, Saito Y, Chang WC, Murota S. Effects of purified eicosapentaenoic acid or arachidonic acid metabolism in cultured murine aortic smooth muscle cells, vessel walls and platelets. Lipids 1983;18(1):1842‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MRC 1965 {published data only}
- Research Committee to the MRC. Low fat diet in myocardial infarction: A controlled trial. Lancet 1965;2:501‐4. [PubMed] [Google Scholar]
MRC 1968 {published data only}
- Research Committee to the MRC. Controlled trial of soybean oil in myocardial infarction. Lancet 1968;2:693‐700. [PubMed] [Google Scholar]
Nagakawa 1983 {published data only}
- Nagakawa Y, Orimo H, Harasawa M, Morita I, Yashiri K, Murota S. Effect of eicosapentaenoic acid on the platelet aggregation and composition of fatty acid in man. Atherosclerosis 1983;47:71‐5. [DOI] [PubMed] [Google Scholar]
Napoli 1998 {published data only}
- Napoli C, Leccese M, Palumbo G, Nigris F, Chiariello P, Zuliani P, et al. Effects of vitamin E and HMG‐CoA reductase inhibition on cholesteryl ester transfer protein and lecithin‐cholesterol acyltransferase in hypercholesterolemia. Coronary Artery Disease 1998;9:257‐64. [DOI] [PubMed] [Google Scholar]
NDHSRG 1968 {published data only}
- National Diet‐Heart Study Research Group. The National Diet‐Heart Study Final Report. Circulation 1968;1 Suppl 1:1‐428. [PubMed] [Google Scholar]
Neil 1995 {published data only}
- Neil HA, Roe L, Godlee RL, Moore JW, Clark GM, Brown J. Randomised trial of lipid lowering dietary advice in general practice: the effects on serum lipids, lipoproteins and antioxidants. British Medical Journal 1995;310:569‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nenseter 1992 {published data only}
- Nenseter MS, Rustan AC, Lund‐Katz S, Maelandsmo G, Phillips MC, Drevon CA. Effect of dietary supplementation with n‐3 polyunsaturated fatty acids on physical properties and metabolism of low density lipoprotein in humans. Arteriosclerosis Thrombosis 1992;12:369‐79. [DOI] [PubMed] [Google Scholar]
Nessim 1983 {published data only}
- Nessim SA, Chin HP, Alaupovic P, Blankenhorn DH. Combined therapy of niacin, colestipol and fat controlled diet in men with coronary bypass. Effect on blood lipids and apolipoproteins. Arteriosclerosis 1983;3:568‐73. [DOI] [PubMed] [Google Scholar]
Nestel 1973 {published data only}
- Nestel P, Havenstein N, Whyte HM, Scott TJ, Cook LJ. Lowering of plasma cholesterol and enhanced sterol excretion with the consumption of polyunsaturated ruminant fats. New England Journal of Medicine 1973;288:379‐82. [DOI] [PubMed] [Google Scholar]
Nestel 1976 {published data only}
- Nestel P, Poyser A. Changes in cholesterol synthesis and excretion when cholesterol intake is increased. Metabolism 1976;25:1591‐9. [DOI] [PubMed] [Google Scholar]
Nestel 1984 {published data only}
- Nestel PJ, Connor WE, Reardon MF, Connor S, Wong S, Boston R. Suppression by diets rich in fish oil of very low density lipoprotein production in man. Journal of Clinical Investigation 1984;74:82‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nestel 1986 {published data only}
- Nestel P. Fish oil attenuates the cholesterol rise in lipoprotein cholesterol. American Journal of Clinical Nutrition 1986;43:752‐7. [DOI] [PubMed] [Google Scholar]
Nestel 1992a {published data only}
- Nestel P, Noakes M, Belling B. Plasma lipoprotein lipid and Lp[a] changes with substitution of elaidic acid for oleic acid in the diet. Journal of Lipid Research 1992;33:1029‐36. [PubMed] [Google Scholar]
Nestel 1992b {published data only}
- Nestel PJ, Noakes M, Belling GB, MacArthur R, Clifton PM, Abbey M. Plasma cholesterol‐lowering potential of edible‐oil blends suitable for commercial use. American Journal of Clinical Nutrition 1992;55:46‐50. [DOI] [PubMed] [Google Scholar]
Nilson 1991 {published data only}
- Nilson DW, Dalaker K, Nordoy A, Osterud B, Ingedretsen OC, Lyngmo V, et al. Influence of a concentrated ethylester compound of n‐3 fatty acids on lipids, platelets and coagulation in patients undergoing coronary bypass surgery. Thrombosis and Haemostasis 1991;66:195‐201. [PubMed] [Google Scholar]
Nydahl 1994 {published data only}
- Nydahl M, Gustafsson IB, Vessby B. Lipid‐lowering diets enriched with monounsaturated and polyunsaturated fatty acids but low in saturated fatty acids have similar effects on serum lipid concentrations in hyperlipidemic patients. American Journal of Clinical Nutrition 1994;59:115‐22. [DOI] [PubMed] [Google Scholar]
Nyyssonen 1994 {published data only}
- Nyyssonen K, Porkkala E, Salonen R, Korpela H, Salonen JT. Increase in oxidation resistance of atherogenic serum lipoproteins following antioxidant supplementation: a randomised double‐blind placebo controlled clinical trial. European Journal of Clinical Nutrition 1994;48:633‐42. [PubMed] [Google Scholar]
Olszewski 1993 {published data only}
- Olszewski AJ, McCully KS. Fish oil decreases serum homocysteine in hyperlipemic men. Coronary Artery Disease 1993;4:53‐60. [DOI] [PubMed] [Google Scholar]
Omenn 1996 {published data only}
- Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, et al. Effects of a combination of beta‐carotene and vitamin A on lung cancer and cardiovascular disease. New England Journal of Medicine 1996;334:1150‐5. [DOI] [PubMed] [Google Scholar]
Ordovas 1995 {published data only}
- Ordovas JM, Lopez‐Miranda J, Mata P, Perez‐Jimenez F, Lichtenstein AH, Schaefer EJ. Gene‐diet interaction in determining plasma lipid response to dietary intervention. Atherosclerosis 1995;118 (Suppl):S11‐27. [PubMed] [Google Scholar]
Parks 1990 {published data only}
- Parks JS, Rudel LL. Effect of fish oil on atherosclerosis and lipoprotein metabolism. Atherosclerosis 1990;84:83‐4. [DOI] [PubMed] [Google Scholar]
Parthasarathy 1990 {published data only}
- Parthasarathy S, Khoo JC, Miller E, Barnett J, Witztum JL, Steinberg D. Low density lipoprotein rich in oleic acid is protected against oxidative modification: implications for dietary prevention of atherosclerosis. Proceedings of the National Academy of Sciences 1990;87:3894‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peto 1985 {published data only}
- Peto R, Yusuf S, Collins R. Cholesterol lowering trial results in their epidemiological context [abstract]. Circulation 1985;72 (Suppl III):III‐451. [Google Scholar]
Phillipson 1985 {published data only}
- Phillipson BE, Rothrock DW, Connor WE, Harris WS, Illingworth DR. Reduction of plasma lipids, lipoproteins and apoproteins by dietary fish oils in patients with hypertriglyceridemia. New England Journal of Medicine 1985;312:1210‐6. [DOI] [PubMed] [Google Scholar]
Pirich 1999 {published data only}
- Pirich C, Caszo A, Granegger S, Sinzinger H. Effects of fish oil supplementation on platelet survival and ex vivo platelet function in hypercholesterolemic patients. Thrombosis Research 1999;96:219‐27. [DOI] [PubMed] [Google Scholar]
Princen 1995 {published data only}
- Princen HM, Duyvenvoorde W, Buytenhek R, Laarse A, Popple G, Gevers Leuven JA, et al. Supplementation with low doses of vitamin E protects LDL from lipid peroxidation in men and women. Arteriosclerosis, Thrombosis, and Vascular Biology 1995;15:325‐33. [DOI] [PubMed] [Google Scholar]
Quintao 1971 {published data only}
- Quintao E, Grundy SM, Ahrens EH. Effects of dietary cholesterol on the regulation of total body cholesterol in man. Journal of Lipid Research 1971;12:233‐47. [PubMed] [Google Scholar]
Radack 1989 {published data only}
- Radack K, Deck C, Huster D. Dietary supplementation with low‐dose fish oils lowers fibrinogen levels: a randomized, double‐blind controlled study. Annals of Internal Medicine 1989;111:757‐8. [DOI] [PubMed] [Google Scholar]
Radack 1990 {published data only}
- Radack K, Deck C, Huster D. The comparative effects of n‐3 and n‐6 polyunsaturated fatty acids on plasma fibrinogen levels: a controlled clinical trial in hypertriglyceridemic subjects. Journal of the American College of Nutrition 1990;9:352‐7. [DOI] [PubMed] [Google Scholar]
Rapola 1996 {published data only}
- Rapola JM, Virtamo J, Haukka JK, Heinonen OP, Albanes D, Taylor PR, et al. Effect of vitamin E and beta‐carotene on the incidence of angina pectoris: a randomised, double blind controlled trial. Journal of the American Medical Association 1996;275:693‐8. [DOI] [PubMed] [Google Scholar]
Reaven 1991 {published data only}
- Reaven PD, Parthasarathy S, Grasse BJ, Miller E, Almazan F, Mattson FH, et al. Feasibility of using an oleate‐rich diet to reduce the susceptibility of low‐density lipoprotein to oxidative modification in humans. American Journal of Clinical Nutrition 1991;54:701‐6. [DOI] [PubMed] [Google Scholar]
Reaven 1993a {published data only}
- Reaven PD, Witztum JL. Comparison of supplementation of RRR‐alpha‐tocopherol and racemic alpha‐tocopherol in humans: effects on lipid levels and lipoprotein susceptibility to oxidation. Arteriosclerosis Thrombosis 1993;13:601‐8. [DOI] [PubMed] [Google Scholar]
Reaven 1993b {published data only}
- Reaven PD, Parthasarathy S, Grasse BJ, Miller E, Steinberg D, Witztum JL. Effects of oleate‐rich and linoleate‐rich diets on the susceptibility of low‐density lipoprotein to oxidative modification in mildly hypercholesterolaemic subjects. Journal of Clinical Investigation 1993;91:668‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reaven 1993c {published data only}
- Reaven PD, Khouw A, Beltz WF, Parthasarathy S, Witztum JL. Effect of dietary antioxidant combinations in humans: protection of LDL by vitamin E but not by beta‐carotene. Arteriosclerosis Thrombosis 1993;13:590‐600. [DOI] [PubMed] [Google Scholar]
Reaven 1994 {published data only}
- Reaven PD, Grasse BJ, Tribble DL. Effects of linoleate‐enriched and oleate‐enriched diets in combination with alpha‐tocopherol on the susceptibility of LDL and LDL subfractions to oxidative modification in humans. Arteriosclerosis Thrombosis 1994;14:557‐66. [DOI] [PubMed] [Google Scholar]
Reiser 1985 {published data only}
- Reiser R, Probstfield JL, Silvers A, Scott LW, Shorney ML, Wood RD, et al. Plasma lipid and lipoprotein response of humans to beef fat, coconut oil and safflower oil. American Journal of Clinical Nutrition 1985;42:190‐7. [DOI] [PubMed] [Google Scholar]
Retzloff 1991 {published data only}
- Retzloff BM, Dowdy AA, Walden CE, McCann BS, Gey G, Cooper M, et al. Changes in vitamin and mineral intakes and serum concentrations among free living men on cholesterol lowering diets. American Journal of Clinical Nutrition 1991;53(4):890‐8. [DOI] [PubMed] [Google Scholar]
Riccardi 1987 {published data only}
- Riccardi G, Rivellese AA, Mancini M. The use of diet to lower plasma cholesterol levels. European Heart Journal 1987;8 Suppl:79‐85. [DOI] [PubMed] [Google Scholar]
Rivellese 1994 {published data only}
- Rivellese AA, Auletta P, Marotta G, Saldalamacchia G, Giacco A, Mastrilli V, et al. Long term metabolic effects of two dietary methods of treating hyperlipidaemia. British Medical Journal 1994;308:227‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 1994 {published data only}
- Roberts WC. The ineffectiveness of a commonly recommended lipid lowering diet in significantly lowering the serum total and low‐density lipoprotein cholesterol levels. American Journal of Cardiology 1994;73:623‐4. [DOI] [PubMed] [Google Scholar]
Rona 1985 {published data only}
Rose 1976 {published data only}
- Rose V, Allen DM, Pearse RG, Chapell J. Primary hyperlipoproteinemia in childhood and adolescence: identification and treatment of persons at risk for premature atherosclerosis. Canadian Medical Association Journal 1976;115:753‐7. [PMC free article] [PubMed] [Google Scholar]
Rosenthal 1985 {published data only}
- Rosenthal MB, Barnard RJ, Rose DP, Inkeles S, Hall J, Pritikin N. Effects of a high complex carbohydrate, low fat, low cholesterol diet on levels of serum lipids and estradiol. American Journal of Medicine 1985;78:23‐7. [DOI] [PubMed] [Google Scholar]
Sacks 1986 {published data only}
- Sacks FM, Handysides GH, Marais GE, Rosner B, Kass EH. Effects of a low fat diet on plasma lipoprotein levels. Archives of Internal Medicine 1986;146:1573‐7. [PubMed] [Google Scholar]
Sanders 1981 {published data only}
- Sanders TA, Younger KM. The effect of dietary supplements of w‐3 polyunsaturated fatty acids on the fatty acid composition of platelets and plasma choline phosphoglycerides. British Journal of Nutrition 1981;45:613‐6. [DOI] [PubMed] [Google Scholar]
Sanders 1983 {published data only}
- Sanders TA, Hochland MC. A comparison of the influence on plasma lipids and platelet function of supplements of w‐3 and w‐6 polyunsaturated fatty acids. British Journal of Nutrition 1983;50:521‐9. [DOI] [PubMed] [Google Scholar]
Sanders 1984 {published data only}
- Sanders TA, Mistry M. Controlled trials of fish oils supplements on plasma lipid concentrations. British Journal of Clinical Practice Supplement 1984;31:78‐84. [PubMed] [Google Scholar]
Saynor 1982a {published data only}
- Saynor R, Verel D. Eicosapentaenoic acid, bleeding time and serum lipids. Lancet 1982;2:272. [DOI] [PubMed] [Google Scholar]
Saynor 1984a {published data only}
- Saynor R, Verel D, Gillott T. The long‐term effect of dietary supplementation with fish lipid concentrate on serum lipids, bleeding time, platelets and angina. Atherosclerosis 1984;50:3‐10. [DOI] [PubMed] [Google Scholar]
Saynor 1984b {published data only}
- Saynor R. Effects of w‐3 fatty acids on serum lipids. Lancet 1984;2:696‐7. [DOI] [PubMed] [Google Scholar]
Schaefer 1981 {published data only}
- Schaefer EJ, Levy RI, Ernst ND, Sant FD, Brewer HB Jr. The effects of low cholesterol, high polyunsaturated fat and low fat diets on plasma lipids and lipoprotein cholesterol levels in normal and hypercholesterolemic subjects. American Journal of Clinical Nutrition 1981;34(9):1758‐63. [DOI] [PubMed] [Google Scholar]
Schectman 1989 {published data only}
- Schectman G, Kaul S, Cherayil GD, Lee M, Kissebah A. Can the hypotriglyceridemia effect of fish oil concentrate be sustained. Annals of Internal Medicine 1989;110:346‐52. [DOI] [PubMed] [Google Scholar]
Schlierf 1982 {published data only}
- Schlierf G, Mrozik K, Heuck CC, Middlehoff G, Oster P, Riesen W, et al. "Low dose" colestipol in children, adolescents and young adults with familial hypercholesterolaemia. Atherosclerosis 1982;41(1):133‐8. [DOI] [PubMed] [Google Scholar]
Schonfeld 1982 {published data only}
- Schonfeld G, Patsch W, Rudel LL, Nelson C, Epstein M, Olson RE. Effects of dietary cholesterol and fatty acids on plasma lipoproteins. Journal of Clinical Investigation 1982;69(5):1072‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwandt 1982 {published data only}
- Schwandt P, Janetschek P, Weisweiler P. High density lipoproteins unaffected by dietary fat modification. Atherosclerosis 1982;44:9‐17. [DOI] [PubMed] [Google Scholar]
Segall 1970 {published data only}
- Segall MM, Fosbrooke AS, Lloyd JK, Wolff OH. Treatment of familial hypercholesterolaemia in children. Lancet 1970;1:641‐4. [DOI] [PubMed] [Google Scholar]
Seppanen‐Laakso 1992 {published data only}
- Seppanen‐Laakso T, Vanhanen HT, Laakso I, Kohtamaki H, Viikari J. Replacement of butter on bread by rapeseed oil and rapeseed oil containing margarine: effects on plasma fatty acid composition and serum cholesterol. British Journal of Nutrition 1992;68:677‐92. [DOI] [PubMed] [Google Scholar]
Seppanen‐Laakso 1993 {published data only}
- Seppanen‐Laakso T, Vanhanen HT, Laakso I, Kohtamaki H, Viikari J. Replacement of margarine on bread by rapeseed and olive oil effects on plasma fatty acid composition and serum cholesterol. Annals of Nutrition and Metabolism 1993;37:161‐74. [DOI] [PubMed] [Google Scholar]
Shepherd 1978 {published data only}
- Shepherd J, Packard CJ, Patsch JR, Gotto AM Jr, Taunton OD. Effects of dietary polyunsaturated and saturated fat on the properties of high density lipoprotein and the metabolism of apolipoprotein A‐1. Journal of Clinical Investigation 1978;61:1582‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shepherd 1980 {published data only}
- Shepherd J, Packard CJ, Grundy SM, Yeshurun D, Gotto AM Jr, Taunton OD. Effects of saturated and polyunsaturated fat diets on the chemical composition and metabolism of low density lipoproteins in man. Journal of Lipid Research 1980;21:91‐9. [PubMed] [Google Scholar]
Shorey 1981 {published data only}
Siess 1980 {published data only}
- Siess W, Roth P, Scherer B, Kurzmann I, Bohlig B, Weber PC. Platelet‐membrane fatty acids, platelet aggregation, and thromboxane formation during a mackerel diet. Lancet 1980;1:441‐4. [DOI] [PubMed] [Google Scholar]
Simons 1985a {published data only}
- Simons LA, Hickie JB, Balasubramaniam S. On the effects of dietary n‐3 fatty acids (MaxEPA) on plasma lipids and lipoproteins in patients with hyperlipidaemia. Atherosclerosis 1985;54:75‐88. [DOI] [PubMed] [Google Scholar]
Simons 1985b {published data only}
- Simons LA, Hickie JB. On the effects of dietary n‐3 fatty acids (Maxepa) on plasma lipids and lipoproteins in patients with hyperlipidaemia. Atherosclerosis 1985;54:75‐88. [DOI] [PubMed] [Google Scholar]
Singer 1983 {published data only}
- Singer P, Jaeger W, Wirth M, Voight S, Naumann, Zimontkowski S, et al. Lipid and blood pressure‐lowering effect of mackerel diet in man. Atherosclerosis 1983;49(1):99‐108. [DOI] [PubMed] [Google Scholar]
Singer 1984 {published data only}
- Singer P, Voigt S, Wirth M, et al. Effect of eicosapentaenoic acid‐rich diet on risk factors of atherosclerosis in healthy subject and patients with essential hypertension and hyperlipoproteinemia [Zur Beeinflussung von Risikofaktoren der Arteriosklerose durch eicosapentaensourereiche Diet bei klinisch Gesunden sowie Patienten mit essentieller Hypertonie und Hyperlipoproteinomie]. Das Deutsche Gesundheitswesen 1984;39:219‐23. [Google Scholar]
Singer 1986 {published data only}
- Singer P, Wirth M, Godlicke W, Jaeger W, Voight S. Slow desaturation and elongation of linoleic and alpha‐linolenic acids as a rationale of eicosapentaenoic acid‐rich diet to lower blood pressure and serum lipids in normal, hypertensive and hyperlipemic subjects. Leukotrienes & Medicine 1986;24:173‐93. [DOI] [PubMed] [Google Scholar]
Sirtori 1977 {published data only}
- Sirtori CR, Agradi E, Conti F, Mantero O, Gatti E. Soybean protein diet in the treatment of type II hypercholesterolaemia. Lancet 1977;1:275‐7. [DOI] [PubMed] [Google Scholar]
Sirtori 1979 {published data only}
- Sirtori CR, Gatti E, Mantero O, Conti F, Agradi E, Tremoli E, et al. Clinical experience with the soybean protein diet in the treatment of hypercholesterolemia. American Journal of Clinical Nutrition 1979;32(8):1645‐58. [DOI] [PubMed] [Google Scholar]
Sirtori 1981 {published data only}
- Sirtori CR, Descovich GC, Noseda G. The soybean protein diet does not lower plasma cholesterol?. Atherosclerosis 1981;38:423. [DOI] [PubMed] [Google Scholar]
Sirtori 1986 {published data only}
- Sirtori CR, Tremoli E, Gatti E, Montanari G, Sirtori M, Colli S, et al. Controlled evaluation of fat intake in the Mediterranean diet: comparative activities of olive oil and corn oil on plasma lipids and platelets in high‐risk patients. American Journal of Clinical Nutrition 1986; Vol. 44, issue 5:635‐42. [DOI] [PubMed]
Sirtori 1992 {published data only}
- Sirtori CR, Gatti E, Tremoli E, Gatti C, Gianfranceschi G, Franceschini G, et al. Olive oil, corn oil and n‐3 fatty acids differently affect lipids, lipoproteins, platelets and superoxide formation in type II hypercholesterolemia. American Journal of Clinical Nutrition 1992;56(1):113‐22. [DOI] [PubMed] [Google Scholar]
Sperling 1987 {published data only}
- Sperling RI, Robin JL, Kylander KA, Lee TH, Lewis RA, Austen KF. The effects of n‐3 polyunsaturated fatty acids on the generation of platelet‐activating factor‐acether by human monocytes. Journal of Immunology 1987;139(12):4186‐91. [PubMed] [Google Scholar]
Stein 1975 {published data only}
- Stein EA, Mendelsohn D, Fleming M, Barnard CD, Carter KJ, du Toit PS, et al. Lowering of plasma cholesterol levels in free‐living adolescent males: use of natural and synthetic polyunsaturated foods to provide balanced fat diets. American Journal of Clinical Nutrition 1975;28(11):1204‐16. [DOI] [PubMed] [Google Scholar]
Stein 1982 {published data only}
- Stein EA, Shapero J, McNerney C, Glueck CJ, Tracy T, Gartside P. Changes in plasma lipid and lipoprotein fractions after alteration in dietary cholesterol, polyunsaturated, saturated and total fat in free‐living normal and hypercholesterolemic children. American Journal of Clinical Nutrition 1982;35:1375‐90. [DOI] [PubMed] [Google Scholar]
Stephens 1996 {published data only}
- Stephens NG, Parsons A, Schofield PM, Kelly F, Cheeseman K, Mitchinson MJ. Randomised controlled trial of vitamin E in patients with coronary heart disease: Cambridge Heart Antioxidant Study (CHAOS). Lancet 1996;347(9004):781‐6. [DOI] [PubMed] [Google Scholar]
Subbaiah 1989 {published data only}
- Subbaiah PV, Davidson MH, Ritter MC, Buchanan W, Bagdade JD. Effects of dietary supplementation with marine lipid concentrate on the plasma lipoprotein composition of hypercholesterolemic patients. Atherosclerosis 1989;79:157‐66. [DOI] [PubMed] [Google Scholar]
Sucic 1998 {published data only}
- Sucic M, Katica D, Kovacevic V. Effect of dietary fish supplementation on lipiprotein levels in patients with hyperlipoproteinemia. Collegium Antropologicum 1998;22(1):77‐83. [PubMed] [Google Scholar]
Szczeklik 1985 {published data only}
- Szczeklik A, Gryglewski RJ, Domagala B, Dworski R, Basista M. Dietary supplementation with vitamin E in hyperlipoproteinaemias: effects on plasma lipid peroxides, antioxidant activity, prostacyclin generation and platelet aggregability. Thrombosis and Haemostasis 1985;54:425‐30. [PubMed] [Google Scholar]
Thorngren 1981 {published data only}
- Thorngren M, Gustafson A. Effects of 11 week increase in dietary eicosapentaenoic acid on bleeding time, lipids and platelet aggregation. Lancet 1981;2:1190‐3. [DOI] [PubMed] [Google Scholar]
Thorngren 1984 {published data only}
- Thorngren M, Shafi M, Born GVR. Delay in primary haemostasis produced by a fish diet without change in local thromboxane A2. British Journal of Haematology 1984;58:567‐78. [DOI] [PubMed] [Google Scholar]
Thorngren 1986 {published data only}
- Thorngren M, Nilsson E, Gustafson A. Plasma lipoproteins and fatty acid composition during moderate EPA diet. Acta Medica Scandinavica 1986;219:23‐8. [DOI] [PubMed] [Google Scholar]
Tonstad 1997b {published data only}
- Tonstad S, Aksnes L. Fat soluble vitamin levels in familial hypercholesterolemia. Journal of Pediatrics 1997;130:274‐80. [DOI] [PubMed] [Google Scholar]
Ullmann 1990 {published data only}
- Ullmann D, Connor WE, Illingworth DR, Hagemenas FC, Pappu A. Additive effects of Lovastatin and fish oil in familial hypercholesterolaemia. Arteriosclerosis 1990;10(5):846a. [Google Scholar]
Uusitupa 1991 {published data only}
- Uusitupa M, Ebeling T, Happonen P, Vartilainen E, Turtola H, Parviainen M, Pyorala K. Combination therapy with lovastatin and guar gum versus lovastatin and cholestyramine in treatment of hypercholesterolemia. J Cardiovasc Pharmacol 1991;18:496‐503. [DOI] [PubMed] [Google Scholar]
Valsta 1992 {published data only}
- Valsta L, Jauhiainen M, Aro A, Katan MB, Mutanen M. Effects of a monounsaturated rapeseed oil and a polyunsaturated sunflower oil diet on lipoprotein levels in humans. Arteriosclerosis Thrombosis 1992;12:50‐7. [DOI] [PubMed] [Google Scholar]
Van Gent 1979 {published data only}
- Gent CM, Luten JB, Brongeest‐Schoute HO, Ruiter A. Effect on serum lipid levels of w‐3 fatty acids, of ingesting fish oil concentrate. Lancet 1979;2:1249‐50. [DOI] [PubMed] [Google Scholar]
Vanhanen 1991 {published data only}
- Vanhanen HT, Miettinen TA. Effects of sitostanol ester, dissolved in dietary oil, on serum cholesterol, plant sterols and cholesterol precursors. Circulation 1991;84 (Suppl II):601. [Google Scholar]
Vanhanen 1993 {published data only}
- Vanhanen HT, Blomqvist S, Ehnholm C, Hyvonen M, Jauhiainen M, Torstila I, et al. Serum cholesterol, cholesterol precursors, and plant sterols in hypercholesterolemic subjects with different apoE phenotypes during dietary sitostanol ester treatment. Journal of Lipid Research 1993;34:1535‐44. [PubMed] [Google Scholar]
Vanhanen 1994 {published data only}
- Vanhanen HT, Kajander J, Lehtovirta H, Miettinen TA. Serum levels, absorption efficiency, faecal elimination and synthesis of cholesterol during increasing doses of dietary sitostanol esters in hypercholesterolaemic subjects. Clinical Science 1994;87:61‐7. [DOI] [PubMed] [Google Scholar]
Van Horn 1986 {published data only}
- Horn LV, Liu K, Parker D, Emdiy L, Liao YL, Pan WH, et al. Serum lipid response to oat product intake with a fat modified diet. Journal of the American Dietetic Association 1986;86(6):759‐64. [PubMed] [Google Scholar]
Varady 2007 {published data only}
- Varady KA, Houweling AH, Jones PJ. Effect of plant sterols and exercise training on cholesterol absorption and synthesis in previously sedentary hypercholesterolemic subjects. Translational Research 2007;149(1):22‐30. [DOI] [PubMed] [Google Scholar]
Vega 1982 {published data only}
- Vega GL, Groszek E, Wolfe R, Grundy SM. Influence of polyunsaturated fats on plasma lipoprotein composition and apolipoprotein. Journal of Lipid Research 1982; Vol. 23:811‐22. [PubMed]
Verrillo 1985 {published data only}
Vessby 1980a {published data only}
- Vessby B, Gustafsson IB, Boberg J, Karlstrom B, Lithell H, Werner I. Substituting polyunsaturated fat for saturated fat as a single change in a Swedish diet: effects on serum lipoprotein metabolism and glucose tolerance in patients with hyperlipoproteinemia. European Journal of Clinical Investigation 1980;10:193‐202. [DOI] [PubMed] [Google Scholar]
Vessby 1980b {published data only}
- Vessby B, Boberg J, Gustafsson IB, Karlstrom B, Lithell H, Ostlund‐Lindqvist AM. Reduction of high density lipoprotein cholesterol and apolipoprotein A‐1 concentrations by a lipid‐lowering diet. Atherosclerosis 1980;35:21‐7. [DOI] [PubMed] [Google Scholar]
Vessby 1982 {published data only}
- Vessby B, Karlstrom B, Lithell H, Gustafsson IB, Werner I. The effects on lipid and carbohydrate metabolism of replacing some animal protein by soy‐protein in a lipid‐lowering diet for hypercholesterolaemic patients. Human Nutrition Applied Nutrition 1982;36A:179‐89. [PubMed] [Google Scholar]
Von Schacky 1985 {published data only}
- Schacky C, Fischer S, Weber PC. Long‐term effects of dietary marine omega‐3 fatty acids upon plasma and cellular lipids, platelet function and eicosanoid formation in humans. Journal of Clinical Investigation 1985;76:1626‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Von Schacky 1987 {published data only}
- Schacky C. Prophylaxis of atherosclerosis with marine omega‐3 fatty acids. Annals of Internal Medicine 1987;107:890‐9. [DOI] [PubMed] [Google Scholar]
Vuorio 2000 {published data only}
- Vuorio AF, Gylling H, Turtola H, Kontula K, Ketonen P, Miettinen TA. Stanol ester margarine alone and with simvastatin lowers serum cholesterol in families with hypercholesterolemia caused by the FH‐Karelia mutation. Arteriosclerosis, Thrombosis and Vascular Biology 2000;20(2):500‐6. [DOI] [PubMed] [Google Scholar]
Wardlaw 1990 {published data only}
- Wardlaw GM, Snook JT. Effects of diets high in butter, corn oil or high‐oleic acid sunflower oil on serum lipids and apolipoproteins in men. American Journal of Clinical Nutrition 1990;51:815‐21. [DOI] [PubMed] [Google Scholar]
Weisweiler 1983 {published data only}
- Weisweiler P, Drosner M, Janetschek P, Schwandt P. Changes in very low and low density lipoproteins with dietary fat modification. Atherosclerosis 1983;49:325‐32. [DOI] [PubMed] [Google Scholar]
Weisweiler 1985 {published data only}
- Weisweiler P, Janetschek P, Schwandt P. Influence of polyunsaturated fats and fat restriction on serum lipoproteins in humans. Metabolism 1985;34:83‐7. [DOI] [PubMed] [Google Scholar]
Widhalm 1978 {published data only}
- Widhalm K, Maxa E, Zyman H. Effect of diet and exercise upon the cholesterol and triglyceride content of plasma lipoproteins in overweight children. European Journal of Pediatrics 1978;127:121‐6. [DOI] [PubMed] [Google Scholar]
Widhalm 1993 {published data only}
- Widhalm K, Brazada G. Effect of soy protein diet versus standard low fat, low cholesterol diet on lipid and lipoprotein levels in children with familial or polygenic hypercholesterolaemia. Journal of Pediatrics 1993;123:30‐4. [DOI] [PubMed] [Google Scholar]
Williams 1986 {published data only}
- Williams PT, Krauss RM, Kindel‐Joyce S, Dreon DM, Vranizan KM, Wood PD. Relationship of dietary fat, protein, cholesterol and fiber intake to atherogenic lipoproteins in men. American Journal of Clinical Nutrition 1986;44:788‐97. [DOI] [PubMed] [Google Scholar]
Wilson 1971 {published data only}
- Wilson WS, Hulley SB, Burrows M, Nichaman MZ. Serial lipid and lipoprotein responses to the American Heart Association fat controlled diet. American Journal of Medicine 1971;51:491‐503. [DOI] [PubMed] [Google Scholar]
Wilt 1989 {published data only}
- Wilt TJ, Lofgren RP, Nichol KS, Schorer AE, Crespin L, Downes D, et al. Fish oil supplementation does not lower plasma cholesterol in men with hypercholesterolemia. Annals of Internal Medicine 1989;111:900‐5. [DOI] [PubMed] [Google Scholar]
Witters 1976 {published data only}
- Witters LA, Herbert PN, Shulman RS, Krauss RM, Levy RI. Theraputic failure in familial Type II hyperlipoproteinemia. Metabolism 1976;25:1017‐26. [DOI] [PubMed] [Google Scholar]
Wolf 1983 {published data only}
- Wolf RN, Grundy SM. Influence of exchanging carbohydrate for saturated fatty acids on plasma lipids and lipoproteins in men. Journal of Nutrition 1983;113:1521‐8. [DOI] [PubMed] [Google Scholar]
Wolfe 1991 {published data only}
- Wolfe BM, Giovannetti PM. Short‐term effects of substituting protein for carbohydrate in the diets of moderately hypercholesterolemic human subjects. Metabolism 1991;40:338‐43. [DOI] [PubMed] [Google Scholar]
Worne 1959 {published data only}
- Worne HE, Smith LW. Effects of certain pure long chain polyunsaturated fatty acid esters on blood lipids in man. American Journal of Medical Science 1959;237:710‐21. [DOI] [PubMed] [Google Scholar]
Yacowitz 1965 {published data only}
- Yacowitz H, Fleischman AI, Bierenbaum ML. Effects of oral calcium upon serum lipids in man. British Medical Journal 1965;1:1352‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 1993 {published data only}
Zimmerman 1986 {published data only}
- Zimmerman J, Eisenberg S, Kaufmann NA, Fainaru M, OSchry Y, Friedlander Y, et al. Effect of moderate isocaloric modification of dietary carbohydrate on high density lipoprotein composition and apolipoprotein A‐1 turnover in humans. Israel Journal of Medical Sciences 1986;22(2):95‐104. [PubMed] [Google Scholar]
Zino 1987 {published data only}
- Zino S, Skeaff M, Williams S, Mann J. Randomised controlled trial of effect of fruit and vegetable consumption on plasma concentrations of lipids and antioxidants. British Medical Journal 1997;314:1787‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zock 1992 {published data only}
- Zock PL, Katan MB. Hydrogenation alternatives: effects of trans fatty acids and stearic acid versus linoleic acid on serum lipids and lipoproteins in humans. Journal of Lipid Research 1992;33:399‐410. [PubMed] [Google Scholar]
Zucker 1983 {published data only}
- Zucker ML, Woodroof J, DeCoursey S, Jackson B, Dujovne CA. The effect of variable fat diets and cholesterol‐lowering drugs on Antithrombin III levels in hyperlipoproteinemic and normal subjects. Thrombosis Research 1983;30:661‐9. [DOI] [PubMed] [Google Scholar]
Zucker 1988 {published data only}
- Zucker ML, Bilyeu DS, Helmkamp GM, Harris WS, Dujovne CA. Effects of dietary fish oil on platelet function and plasma lipids in hyperlipoproteinemic and normal subjects. Atherosclerosis 1988;73:13‐22. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Fuentes 2008 {published data only}
- Fuentes F, Lopez‐Miranda J, Garcia A, Perez‐Martinez P, Moreno J, Cofan M, et al. Basal plasma concentrations of plant sterols can predict LDL‐C response to sitosterol in patients with familial hypercholesterolemia. European Journal of Clinical Nutrition 2008;62(4):495‐501. [DOI] [PubMed] [Google Scholar]
Stein 2007 {published data only}
- Stein EA, Amerena J, Ballantyne CM, Brice E, Farnier M, Guthrie RM, et al. Long‐term efficacy and safety of rosuvastatin 40 mg in patients with severe hypercholesterolemia. American Journal of Cardiology 2007;100(9):1387‐96. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Párraga ongoing {published data only}
- Párraga I, López‐Torres J, Andrés F, Navarro B, Campo JM, García‐Reyes M, et al. Effect of plant sterols on the lipid profile of patients with hypercholesterolaemia. Randomised, experimental study. www.biomedcentral.com/1472‐6882/11/73 (BMC Complementary and Alternative Medicine) (accessed 01 March 2012). [DOI] [PMC free article] [PubMed]
Additional references
AHA Statement 2007
- McCrindle BW, Urbina EM, Dennison BA, Jacobson MC, Steinberger J, Rocchin AP, et al. AHA Scientific Statement. Drug therapy of high risk lipid abnormalities in children and adolescents. A scientific statement from the American Heart Association Atherosclerosis, hypertension and obesity in Youth Committee, Council of Cardiovascular disease in the Young with the Council on Cardiovascular Nursing. Circulation 2007;115(14):1948‐67. [DOI] [PubMed] [Google Scholar]
Austin 2004
- Austin MA, Hutter CM, Zimmern RL, Humphries SE. Familial hypercholesterolemia and coronary heart disease: a HuGE association review. American Journal of Epidemiology 2004;160(5):421‐9. [DOI] [PubMed] [Google Scholar]
Avis 2007
- Avis HJ, Vissers MN, Stein EA, Wijburg FA, Frip MD, Kastelein JJ, et al. A systematic review and meta‐analysis of statin therapy in children with familial hypercholesterolemia. Arteriosclerosis, Thrombosis, and Vascular Biology 2007;27(8):1803‐10. [DOI] [PubMed] [Google Scholar]
Brown 1999
- Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol‐lowering effects of dietary fiber: a meta‐analysis. American Journal of Clinical Nutrition 1999;69(1):30‐42. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Goldberg 2011
- Goldberg AC, Hopkins PN, Toth PP, Ballantyne CM, Rader DJ, Robinson JG, et al. Familial Hypercholesterolemia: Screening, diagnosis and management of pediatric and adult patients. Journal of Clinical Lipidology 2011;5(3 suppl):51‐8. [Goldberg 2011] [DOI] [PubMed] [Google Scholar]
Goldstein 1995
- Goldstein JL, Hobbs HH, Brown MS. Familial hypercholesterolemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D editor(s). The metabolic and molecular bases of inherited disease. 7th Edition. Vol. 2, New York: McGraw‐Hill, 1995:1981‐2030. [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hopkins 2011
- Hopkins PN, Toth PP, Ballantyne CM, Rader DJ. Familial Hypercholesterolemias:prevalence, genetics, diagnosis and screening recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. Journal of Clinical Lipidology 2011;5(3 suppl):S9–S17. [Hopkins 2011] [DOI] [PubMed] [Google Scholar]
Howell 1997
- Howell WH, McNamara DJ, Tosca MA, Smith BT, Gaines JA. Plasma lipid and lipoprotein response to dietary fat and cholesterol: a meta‐analysis. American Journal of Clinical Nutrition 1997;65:747‐64. [DOI] [PubMed] [Google Scholar]
Levantesi 2010
- Levantesi G, Silletta MG, Marchioli R. Uses and benefits of omega‐3 ethyl esters in patients with cardiovascular disease. Journal of Multidisciplinary Healthcare 2010;3:79–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maclean 1994
- Maclean M. Lipid disorders. In: Shaw V, Lawson M editor(s). Clinical Paediatric Dietetics. Oxford: Blackwell Science, 1994:239‐45. [Google Scholar]
Marais 2004
- Marais AD. Familial hypercholesterolaemia. Clinical Biochemist Reviews 2004;25(1):49‐68. [Marais 2004] [PMC free article] [PubMed] [Google Scholar]
Marks 2003
- Marks D, Thorogood M, Neil AW, Humphries SE. A review on the diagnosis, natural history, and treatment of familial hypercholesterolaemia. Atherosclerosis 2003;168(1):1‐14. [DOI] [PubMed] [Google Scholar]
Marlett 1997
- Marlett JA. Sites and mechanism for the hypocholesterolemic actions of soluble dietary fiber sources. Advances in Experimental Medicine and Biology 1997;427:109‐21. [DOI] [PubMed] [Google Scholar]
O'Connor 1990
- O'Connor P, Feely J, Shepherd J. Lipid lowering drugs. BMJ 1990;300(6725):667‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rader 2003
- Rader DJ, Cohen J, Hobbs HH. Monogenic hypercholesterolemia: new insights in pathogenesis and treatment. Journal of Clinical Investigation 2003;111(12):1795‐1803. [Rader 2003] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
SBRG 1991
- Scientific Steering Committee on behalf of the Simon Broome Register Group. Risk of fatal coronary heart disease in familial hypercholesterolaemia. BMJ 1991;303(6807):893‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schneeman 1998
- Schneeman BO. Dietary fiber and gastrointestinal function. Nutrition Research 1998;18:625‐32. [DOI] [PubMed] [Google Scholar]
Shafiq 2007
- Shafiq N, Bhasin B, Pattanaik S, Pandhi P, Venkateshan SP, Singh M, et al. A meta‐analysis to evaluate the efficacy of statins in children with familial hypercholesterolemia. International Journal of Clinical Pharmacology and Therapeutics 2007;45(10):548‐55. [DOI] [PubMed] [Google Scholar]
Tonstad 1997a
- Tonstad S. A rational approach to treating hypercholesterolaemia in children. Weighing the risks and benefits. Drug safety 1997;16(5):330‐41. [DOI] [PubMed] [Google Scholar]
US FDA 2010
- U.S. Food, Drug Administration. Food Labeling; Health Claim; Phytosterols and Risk of Coronary Heart Disease. http://www.gpo.gov/fdsys/pkg/FR‐2010‐12‐08/pdf/2010‐30386.pdf (accessed 01 March 2012).
Wray 1996
- Wray R, Neil H, Rees J. Screening for hyperlipidaemia in childhood. Recommendations of the British Hyperlipidaemia Association. Journal of Royal College of Physicians London 1996;30(2):115‐8. [PMC free article] [PubMed] [Google Scholar]
Yeste 2009
- Yeste D, Chacon P, Clemente M, Albisu MA, Gussinye M, Carracosa A. Ezetimibe as monotherapy in the treatment of hypercholesterolemia in children and adolescents. Journal of Pediatric Endocrinology and Metabolism 2009;22(6):487‐92. [Yeste 2009] [DOI] [PubMed] [Google Scholar]
Zavala 2006
- Zavala D, Martí‐Carvajal A, Peña‐Martí G, Comunián G. Data extraction sheet to help manage the characteristics of included studies in Cochrane reviews. Valencia: Universidad de Carabobo, 2006.. www.cochrane.fcs.uc.edu.ve/hrs (accessed 01 March 2012).
References to other published versions of this review
Shafiq 2010
- Shafiq N, Singh M, Kaur S, Khosla P, Malhotra S. Dietary treatment for familial hypercholesterolaemia. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD001918.pub2] [DOI] [PubMed] [Google Scholar]


