Supplemental Digital Content is available in the text.
Keywords: aneurysmal subarachnoid hemorrhage, cerebral vasospasm, CT-angiography, delayed cerebral ischemia, transcranial Doppler
Objectives:
Cerebral vasospasm in the first 2 weeks after aneurysmal subarachnoid hemorrhage is recognized as a major predictor of delayed cerebral ischemia. The routine screening for cerebral vasospasm with either transcranial Doppler or CT angiography has been advocated, although its diagnostic value has not yet been determined. Our study investigated the diagnostic accuracy of detecting vasospasm by transcranial Doppler and CT angiography for the prediction of delayed cerebral ischemia and functional outcome. Additionally, agreement between transcranial Doppler and CT angiography was determined.
Design:
Prospective diagnostic accuracy study.
Settings:
Neurocritical care unit and neurosurgical ward at a tertiary academic medical center.
Patients:
Between 2013 and 2016, 59 consenting patients were included.
Intervention:
Patients undergo both transcranial Doppler and CT angiography for detection of cerebral vasospasm on days 5 and 10 after aneurysmal subarachnoid hemorrhage. Delayed cerebral ischemia was defined as secondary neurologic deterioration, not explained otherwise. Unfavorable outcome was defined modified Rankin Scale > 2 at 6 months.
Measurements and Main Results:
On transcranial Doppler, cerebral vasospasm was observed in 26 patients (45%). On CT angiography, vasospasm was observed in 54 patients (95%). The agreement between transcranial Doppler and CT angiography was 0.47. Delayed cerebral ischemia occurred in 16 patients (27%); unfavorable outcome in 12 patients (20%). Transcranial Doppler predicted delayed cerebral ischemia with a sensitivity of 0.44 (day 5) and 0.50 (day 10), with a specificity of 0.67 (day 5) and 0.57 (day 10). CT angiography predicted delayed cerebral ischemia with a sensitivity of 0.81 (day 5 and 10) and with a specificity of 0.070 (day 5) and 0.00 (day 10). The highest accuracy for predicting unfavorable outcome was on day 5 (0.61 for transcranial Doppler vs 0.27 for CT angiography).
Conclusion:
The diagnostic accuracy of both CT angiography and transcranial Doppler for detection of cerebral vasospasm as well as prediction of delayed cerebral ischemia and functional outcome is limited. The agreement between CT angiography and transcranial Doppler is low.
INTRODUCTION
Delayed cerebral ischemia (DCI) after aneurysmal subarachnoid hemorrhage (aSAH) is a dreaded secondary complication. DCI is commonly defined as any neurologic deterioration (new focal neurologic deficits or decline in Glasgow Coma Scale) or ischemic lesions on follow-up imaging (CT or MRI) that may not be explained otherwise (1–4). Prevention of DCI is challenging because its pathophysiology is unclear. A prominent role of cerebral vasospasm (CVS) in DCI is generally accepted in the literature, although CVS is not proven to be a causative factor. On conventional angiography, CVS occurs in about 70% of aSAH patients; 30% of all patients develop clinical symptoms (5, 6). It is hypothesized that CVS develops from blood degradation products and may thereby lead to ischemic strokes due to insufficient cerebral blood flow (2, 7, 8). Therefore, it is common practice in many neurovascular centers to use TCD or CTA for CVS detection in an early phase to apply strategies to prevent DCI (e.g., induced hypertension and hypervolemia). Not all patients (i.e., comatose patients) can be monitored on clinical signs. Moreover, waiting for clinical signs of DCI might be late to perform an intervention to prevent DCI. Nevertheless, evidence for these strategies is lacking (3, 9, 10). As a result, DCI causes about 15% morbidity and 2.5% additional mortality (4, 10).
The optimal screening modality for detecting symptomatic CVS is a matter of debate. The gold standard is digital subtraction angiography (DSA), but that is an invasive procedure that carries a complication risk up to 2.6% (11, 12). Transcranial Doppler (TCD) and/or CT-angiography (CTA) are often used as noninvasive screening tools. CTA has a good agreement with DSA (10, 13), but there are limited data about the agreement between CTA and TCD in the detection of CVS. Hence, a direct comparison of CTA versus TCD was deemed useful. The current study aimed to compare the diagnostic accuracy of TCD and CTA for the prediction of DCI and functional outcome. The diagnostic agreement between CTA versus TCD is also compared.
MATERIALS AND METHODS
Patients
This study was designed as a prospective diagnostic accuracy study. All aSAH patients admitted to the neurovascular unit of the University Medical Center Groningen (UMCG), The Netherlands, between August 2013 and March 2016 were eligible for enrollment. Inclusion criteria were: 1) patients with a proven aSAH within 4 days after onset; 2) age 18 years or older; 3) written informed consent from the patient or a legally authorized representative. The following patients were excluded: 1) moribund patients; 2) contraindications for iodine contrast agents; 3) unsuitable temporal bone-window for TCD. The study was registered in the Dutch trial register (NTR4157) and was approved by local research ethical board.
Baseline characteristics were collected including, age, gender, clinical status on admission according to the World Federation of Neurological Surgeons (WFNS) scale (14), Fisher CT rating scale (15), type of treatment, and aneurysm location and repair modality (clipping or coiling). Patients were treated according to the national aSAH management guidelines, comparable to the standard American Heart Association (AHA) protocol (9). The protocol included oral nimodipine, fluid management to prevent hypovolemia, and frequent evaluation of neurologic condition. In case of neurologic deterioration, common causes such as hydrocephalus, re-bleeding, seizures, and metabolic disturbances were excluded. Pressure augmentation was induced in the ICU if DCI was considered the cause of deterioration blood.
Imaging
Following standard protocol, all patients were screened for CVS with TCD three times a week. TCD was performed by an experienced neurophysiology technician. The following vessels were examined by TCD: extracranial internal carotid artery (ICA); proximal and distal middle cerebral artery (MCA); anterior cerebral artery (ACA); basilar artery (BA); and posterior cerebral artery (PCA).
CVS in the MCA was defined as Lindegaard ratio > 3 and mean flow velocity (MFV) > 120 cm/sec. Lindegaard ratio was calculated as MCA-MFV divided by the distal ipsilateral extracranial ICA-MFV. Severe CVS in the MCA was defined as a MFV > 200 and LR > 6. CVS of the PCA and BA was defined as MFV > 85. A MFV > 80 with a Sloan ratio > 4.0 was considered CVS for the ACA. Sloan ratio is calculated as ACA-MFV divided by ipsilateral ICA-MFV (16, 17). Any CVS on TCD in the PCA, BA, and ACA was regarded as severe because TCD does not differentiate on severity.
Following study protocol, additional CTA was performed on days 5 and 10 (± 1 d) post aSAH and TCD and CTA were performed on the same day. CTA was only used for research purposes unless there was clinical reason. The scan protocol for CTA consisted of a combination of non-contrast CT followed by CTA with iodine contrast agent (Iomeron). CTA contained 0.60–0.75 mm slices and maximum intensity projection (MIPS) reconstructions in coronal, sagittal, and axial planes. Two neuroradiologists (O.S.E.,A.M.) were blinded for TCD and clinical information and independently evaluated the CTA. The following 17 arterial segments were assessed for presence of vasospasm on CTA: ICA; first and second segment of MCA (M1, M2); first and second segment ACA (A1, A2); vertebral artery (VA); BA; and first and second segment of PCA (P1, P2).
CVS on CTA was assessed in accordance with that presented by Dankbaar et al (18). A visual comparison of the admission CTA and the follow-up CTA on days 5 and 10 was made (assuming that CVS was absent at admission) (18). On CTA, CVS was defined as luminal reduction relative to the proximal and distal segment, assessed by eyeballing, in comparison to the contralateral vessels. As such, atherosclerotic narrowing and hypoplasia were excluded as CVS. If technical causes such as coil or clip artifacts made evaluation of vessels impossible, that was noted as not assessable.
CVS was categorized as: 1) none; 2) mild (<50% decrease in luminal diameter); 3) severe (>50% decrease in luminal diameter); 4) not assessable; or 5) hypoplasia. Consensus was achieved for discordant results. The most severely affected vessel on TCD and CTA was used for analysis. MRI, including MR angiography, was performed at 6 months after aSAH as part of the standard follow-up protocol, in conjunction with a clinical evaluation of functional outcome, as assessed with the modified Rankin Scale (mRS).
Outcome Measures
Diagnostic agreement between TCD and CTA was assessed. A comparison of angiographic vasospasm examined with TCD and CTA were made on the following main outcomes: 1) the occurrence of DCI; and 2) functional outcome assessed by the modified Rankin Scale (mRS) after 6 months.
DCI was defined as neurologic deterioration (new focal neurologic deficits and/or decline in Glasgow Coma Scale) and/or ischemic lesions on follow-up imaging (CT or MRI), not explained otherwise. Unfavorable clinical outcome was defined as mRS > 2, which reflects dependency in activities of daily care.
Statistical Analysis
Contingency tables were used to assess agreement and diagnostic accuracy. The term agreement was used for comparison between CTA and TCD, which means the fraction correct in a contingency table (e.g., [A+D]/total group). The term diagnostic accuracy was used for the prediction of DCI and poor clinical outcome for both screening methods. That is also the fraction correct (e.g., [A+D]/total group) in the contingency tables, but then with CTA against DCI or functional outcome and TCD against DCI or functional outcome. Agreement between CVS on CTA and TCD was expressed as the maximum fraction correct on day 5 and 10. Predictive value of CTA and TCD for DCI and functional outcome at 6 months was reported by the sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) for each test. Univariate group comparisons between DCI and non-DCI for nominal and ordinal variables were performed with chi-square or Fisher’s exact test where appropriate. Mann-Whitney U tests were performed for continuous variables. Additional sensitivity analyses for TCD and CTA were performed with severe vasospasm instead of any vasospasm.
RESULTS
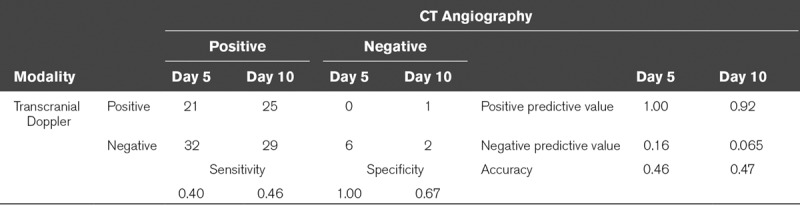
The baseline characteristics of the 59 included patients are presented in Table 1. All patients underwent CTA on day 5 and CTA on day 10 was performed in 57 patients; one patient died and another patient withdrew consent before the second CTA. TCD revealed CVS in 21 patients (35%) at day 5 and in 26 patients (45%) at day 10. CTA showed vasospasm in 53 patients (90%) at day 5 and in 54 patients (95%) at day 10. Table 2 shows that the agreement between TCD and CTA was comparably low at day 5 (0.46) and day 10 (0.47).
TABLE 1.
Baseline Characteristics

TABLE 2.
Contingency Table for Transcranial Doppler Vasospasm Versus CT Angiography Vasospasm at Day 5 and 10
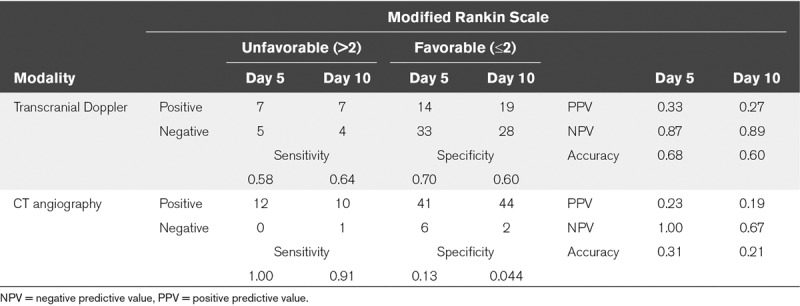
Sixteen patients (27%) had DCI and 12 patients (20%) had a poor functional outcome. The diagnostic accuracy of both CTA and TCD for prediction of DCI and unfavorable outcome was low. Accuracy of CTA was lower than that of TCD. The sensitivity of CTA was higher than TCD, but its specificity was much lower for CTA (Tables 3–4; and Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/CCX/A0). Additional analyses were performed on severe CVS and yielded nonclinical significant results regarding sensitivity, specificity, PPV, and NPV (Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/CCX/A0).
TABLE 3.
Contingency Table for Transcranial Doppler, CT Angiography Vasospasm, and Presence/Absence of Delayed Cerebral Ischemia at Day 5 and 10
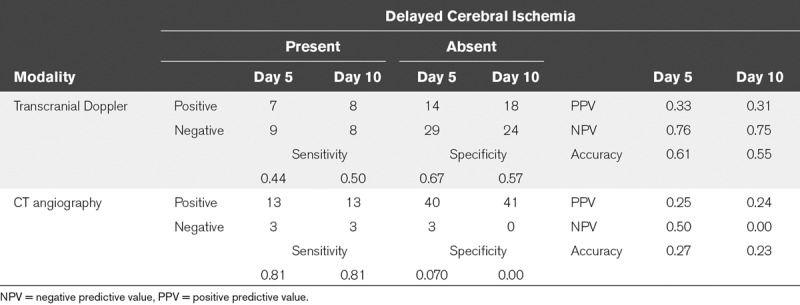
TABLE 4.
Contingency Table for Transcranial Doppler and CT Angiography Vasospasm at Day 5 and 10, and Functional Outcome After Six Months
DISCUSSION AND CONCLUSIONS
In this study, nearly all aSAH patients showed CVS on CTA in one or more vessels. TCD showed CVS less often than CTA and diagnostic agreement between both modalities was low. Although CTA had a high sensitivity for prediction of DCI, its specificity was extremely low compared with TCD. Other studies found a much higher agreement (0.85) between TCD and CTA, but this study only took severe CVS into account (19). The rate of CVS on CTA in our study is higher than other studies, in which a rate between 50–65% is reported (20, 21). However, the rate is similar to the findings of a study that included any degree of CVS on CTA and DSA (22). A plausible reason for the high prevalence of CVS is that 17 arterial segments were rated and moderate vasospasm was included. The incidence of CVS on TCD was 64%, which is in accordance with previous reports, although we found a slightly lower sensitivity and specificity of TCD predicting DCI (23, 24).
In contrast to other studies, we also assessed the predictive value of TCD and CTA for unfavorable outcome. In our study, 27% of patients developed DCI, in agreement with other studies that report 21–33% of the patients developing symptomatic CVS or DCI (4, 19, 25, 26). Twenty percent of patients had an unfavorable outcome, which is also in agreement with other studies (27). Different cutoff scores for favorable versus unfavorable outcome have been used in previous studies. In this study unfavorable clinical outcome was defined as mRS > 2 defining functional dependence for activities of daily living. This cutoff is a clinically relevant endpoint and appears to be a more powerful dichotomization then at higher cutoff scores (28).
Limitations
There are several limitations in our study that are important to point out. First, aSAH patients with WFNS grades 3–5 were underrepresented and this could point toward a selection bias. Good-grade patients (WFNS grade 1–3) could also imply a lower chance of developing DCI, and a better functional outcome after six months (as was seen in other studies) (25, 29). First, our study had relative high Fisher grades. About 90% of patients had a Fisher 3 or 4, which is associated with a higher chance for developing DCI (25, 29). Second, in our study any degree of CVS seen on TCD or CTA was considered, instead of only severe vasospasm. Nevertheless, sensitivity analyses with only severe vasospasm did not ultimately signify the final result. Third, we used the usual cutoff values of TCD for absolute MFV and LR. There are studies with good results with using relative changes in MFV. Controversy exists about the contribution of these methods in making better predictions (30–32). It might be useful to take these relative changes in MFV into account in future studies. Fourth, there could be an inter-rater difference in the assessment of DCI. Fifth, the number of patients might be low but it is not expected that larger numbers make these screening methods more predictive because the CTA demonstrated vasospasm in nearly all patients (95%) with regard to the predictive value for DCI and unfavorable outcome. Sixth, we cannot rule out that any kind of intervention that was initiated based on TCD results could have influenced the outcome in some patients, still no predictive value on DCI was observed. Moreover, there is still no evidence-based treatment to effectively prevent DCI.
Alternative Techniques
Several other studies used CT-perfusion for prediction of CVS resulting in DCI. CT perfusion was found to have a sensitivity of 0.84 and a specificity of 0.79, obviously better than CTA and TCD (33). The advantage of CT perfusion is that it provides information on both the cerebral micro- and macrovascular circulation; combination with CTA is desirable to be optimal informed about macrovascular vasospasm (34). There is currently a lack of knowledge about how microvascular and macrovascular dysfunction after SAH result in ischemia. It is plausible that at a point macrovascular spasm has an effect on the microvascular circulation. It is important to not only predict DCI but also to find screening methods to select the right patients at the right moment to prevent irreversible cerebral ischemia. It might be that, at the time that perfusion CT detects low perfusion, intervention to prevent ischemia is too late. Near-infrared spectroscopy (NIRS) is a promising, relatively new technique that brings more continuous bedside noninvasive monitoring of aSAH patients within reach. NIRS measures cerebral blood oxygenation changes in a quantitative manner, thereby providing indirect information about the cerebral vasculature. Studies using NIRS in aSAH patients are limited, but a good agreement with CTA is reported (35). The potential added value of NIRS is however only confirmed in small studies (36).
The guideline from the American Heart Association/American Stroke Association for the management of aSAH does not state which technique should be used for the detection of CVS, in the absence of trials that compare the impact of screening methods on patient outcome. The use of TCD is considered reasonable for monitoring the development of CVS. The role of CTA as a detection method for CVS is not determined in that guideline (9). An important requirement for good screening methods is the detection of CVS at an early stage that later becomes clinically manifest (3, 19). Our study does not support a prominent role of screening with TCD or CTA. Detection of CVS that does not become clinically manifest likely leads to overtreatment and prolonged hospital stay. Considering the low accuracy of our study, it would not be rational to initiate therapy based on CVS detected by TCD or CTA alone.
In conclusion, CVS is frequently present on CTA and TCD, lacking accurate prediction of DCI or unfavorable outcome after 6 months. Although there may be a role for TCD or CTA in confirmatory testing in cases of suspected CVS, the role for screening of CVS by either of both imaging techniques is of limited value.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Dr. Bokkers receives financial support from the Dutch Heart Foundation (grant number 2013T047). The study was registered in the Dutch trial register (NTR4157) and was approved by local research ethical board.
Dr. van der Harst’s institution received funding from the Efficacy Fund of the University Medical Center Groningen. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Vergouwen MD. Participants in the International Multi-Disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage: Vasospasm versus delayed cerebral ischemia as an outcome event in clinical trials and observational studies. Neurocrit Care. 2011;15:308–311 [DOI] [PubMed] [Google Scholar]
- 2.Al-Tamimi YZ, Orsi NM, Quinn AC, et al. A review of delayed ischemic neurologic deficit following aneurysmal subarachnoid hemorrhage: Historical overview, current treatment, and pathophysiology. World Neurosurg. 2010;73:654–667 [DOI] [PubMed] [Google Scholar]
- 3.Kistka H, Dewan MC, Mocco J. Evidence-based cerebral vasospasm surveillance. Neurol Res Int. 2013;2013:256713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frontera JA, Fernandez A, Schmidt JM, et al. Defining vasospasm after subarachnoid hemorrhage: What is the most clinically relevant definition? Stroke. 2009;40:1963–1968 [DOI] [PubMed] [Google Scholar]
- 5.Washington CW, Zipfel GJ. Participants in the International Multi-disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage: Detection and monitoring of vasospasm and delayed cerebral ischemia: A review and assessment of the literature. Neurocrit Care. 2011;15:312–317 [DOI] [PubMed] [Google Scholar]
- 6.Schmidt JM, Wartenberg KE, Fernandez A, et al. Frequency and clinical impact of asymptomatic cerebral infarction due to vasospasm after subarachnoid hemorrhage. J Neurosurg. 2008;109:1052–1059 [DOI] [PubMed] [Google Scholar]
- 7.van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369:306–318 [DOI] [PubMed] [Google Scholar]
- 8.Suhardja A. Mechanisms of disease: Roles of nitric oxide and endothelin-1 in delayed cerebral vasospasm produced by aneurysmal subarachnoid hemorrhage. Nat Clin Pract Cardiovasc Med. 2004;1:110–116 quiz 2 p following 116 [DOI] [PubMed] [Google Scholar]
- 9.Connolly ES, Jr, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2012;43:1711–1737 [DOI] [PubMed] [Google Scholar]
- 10.Ferguson SD, Rosen DS, Bardo D, et al. Arterial diameters on catheter and computed tomographic angiography. World Neurosurg. 2010;73:165–73 discussion e25 [DOI] [PubMed] [Google Scholar]
- 11.Kaufmann TJ, Huston J, 3rd, Mandrekar JN, et al. Complications of diagnostic cerebral angiography: Evaluation of 19,826 consecutive patients. Radiology. 2007;243:812–819 [DOI] [PubMed] [Google Scholar]
- 12.Willinsky RA, Taylor SM, TerBrugge K, et al. Neurologic complications of cerebral angiography: Prospective analysis of 2,899 procedures and review of the literature. Radiology. 2003;227:522–528 [DOI] [PubMed] [Google Scholar]
- 13.Anderson GB, Ashforth R, Steinke DE, et al. CT angiography for the detection of cerebral vasospasm in patients with acute subarachnoid hemorrhage. AJNR Am J Neuroradiol. 2000;21:1011–1015 [PMC free article] [PubMed] [Google Scholar]
- 14.Teasdale GM, Drake CG, Hunt W, et al. A universal subarachnoid hemorrhage scale: Report of a committee of the world federation of neurosurgical societies. J Neurol Neurosurg Psychiatry. 1988;51:1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1–9 [DOI] [PubMed] [Google Scholar]
- 16.Kirsch JD, Mathur M, Johnson MH, et al. Advances in transcranial doppler US: Imaging ahead. Radiographics. 2013;33:E1–E14 [DOI] [PubMed] [Google Scholar]
- 17.Naqvi J, Yap KH, Ahmad G, et al. Transcranial doppler ultrasound: A review of the physical principles and major applications in critical care. Int J Vasc Med. 2013;2013:629378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dankbaar JW, de Rooij NK, Rijsdijk M, et al. Diagnostic threshold values of cerebral perfusion measured with computed tomography for delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Stroke. 2010;41:1927–1932 [DOI] [PubMed] [Google Scholar]
- 19.Ionita CC, Graffagnino C, Alexander MJ, et al. The value of CT angiography and transcranial doppler sonography in triaging suspected cerebral vasospasm in SAH prior to endovascular therapy. Neurocrit Care. 2008;9:8–12 [DOI] [PubMed] [Google Scholar]
- 20.Dankbaar JW, Rijsdijk M, van der Schaaf IC, et al. Relationship between vasospasm, cerebral perfusion, and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Neuroradiology. 2009;51:813–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aralasmak A, Akyuz M, Ozkaynak C, et al. CT angiography and perfusion imaging in patients with subarachnoid hemorrhage: Correlation of vasospasm to perfusion abnormality. Neuroradiology. 2009;51:85–93 [DOI] [PubMed] [Google Scholar]
- 22.Binaghi S, Colleoni ML, Maeder P, et al. CT angiography and perfusion CT in cerebral vasospasm after subarachnoid hemorrhage. AJNR Am J Neuroradiol. 2007;28:750–758 [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar G, Shahripour RB, Harrigan MR. Vasospasm on transcranial doppler is predictive of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. Neurosurg. 2016;124:1257–1264 [DOI] [PubMed] [Google Scholar]
- 24.Mastantuono JM, Combescure C, Elia N, et al. Transcranial doppler in the diagnosis of cerebral vasospasm: An updated meta-analysis. Crit Care Med. 2018;46:1665–1672 [DOI] [PubMed] [Google Scholar]
- 25.Frontera JA, Claassen J, Schmidt JM, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: The modified fisher scale. Neurosurgery. 2006;59:21–27; discussion 21 [DOI] [PubMed] [Google Scholar]
- 26.Charpentier C, Audibert G, Guillemin F, et al. Multivariate analysis of predictors of cerebral vasospasm occurrence after aneurysmal subarachnoid hemorrhage. Stroke. 1999;30:1402–1408 [DOI] [PubMed] [Google Scholar]
- 27.Hop JW, Rinkel GJ, Algra A, et al. Case-fatality rates and functional outcome after subarachnoid hemorrhage: A systematic review. Stroke. 1997;28:660–664 [DOI] [PubMed] [Google Scholar]
- 28.Banks JL, Marotta CA. Outcomes validity and reliability of the modified rankin scale: Implications for stroke clinical trials: A literature review and synthesis. Stroke. 2007;38:1091–1096 [DOI] [PubMed] [Google Scholar]
- 29.de Oliveira Manoel AL, Jaja BN, Germans MR, et al. The VASOGRADE: A simple grading scale for prediction of delayed cerebral ischemia after subarachnoid hemorrhage. Stroke. 2015;46:1826–1831 [DOI] [PubMed] [Google Scholar]
- 30.Wardlaw JM, Offin R, Teasdale GM, et al. Is routine transcranial doppler ultrasound monitoring useful in the management of subarachnoid hemorrhage? J Neurosurg. 1998;88:272–276 [DOI] [PubMed] [Google Scholar]
- 31.Naval NS, Thomas CE, Urrutia VC. Relative changes in flow velocities in vasospasm after subarachnoid hemorrhage: A transcranial doppler study. Neurocrit Care. 2005;2:133–140 [DOI] [PubMed] [Google Scholar]
- 32.Malhotra K, Conners JJ, Lee VH, et al. Relative changes in transcranial doppler velocities are inferior to absolute thresholds in prediction of symptomatic vasospasm after subarachnoid hemorrhage. Stroke Cerebrovasc Dis. 2014;23:31–36 [DOI] [PubMed] [Google Scholar]
- 33.Dankbaar JW, de Rooij NK, Velthuis BK, et al. Diagnosing delayed cerebral ischemia with different CT modalities in patients with subarachnoid hemorrhage with clinical deterioration. Stroke. 2009;40:3493–3498 [DOI] [PubMed] [Google Scholar]
- 34.Greenberg ED, Gobin YP, Riina H, et al. Role of CT perfusion imaging in the diagnosis and treatment of vasospasm. Imaging Med. 2011;3:287–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yokose N, Sakatani K, Murata Y, et al. Bedside monitoring of cerebral blood oxygenation and hemodynamics after aneurysmal subarachnoid hemorrhage by quantitative time-resolved near-infrared spectroscopy. World Neurosurg. 2010;73:508–513 [DOI] [PubMed] [Google Scholar]
- 36.Maslehaty H, Krause-Titz U, Petridis AK, et al. Continuous measurement of cerebral oxygenation with near-infrared spectroscopy after spontaneous subarachnoid hemorrhage. ISRN Neurol. 2012;2012:907187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


