ABSTRACT
Mucosal surfaces protect our bodies from pathogens and external irritants using a system of biological barriers. Overcoming these barriers is a significant drug delivery challenge, particularly for immunotherapies that aim to modulate the local immune response. Reaching local lymphoid tissues and draining lymph nodes (LNs) requires crossing the mucus mesh, mucosal epithelium, and either targeting M cells covering lymphoid tissues or utilizing lymphatic transport that shuttles molecules and particulates from the periphery to the LN. We first highlight the barrier properties of mucus and mucosal epithelium, and the function of the mucosal immune system. We then dive into existing drug delivery technologies that have been engineered to overcome each of these barriers. We particularly focus on novel strategies for targeting lymphoid tissues, which has been shown to enhance immunotherapies and vaccinations, via directly targeting LNs, lymphatic vessels, and M cells that transport samples of mucosal content to the lymphoid tissues.
KEYWORDS: Lymphatic endothelial cells, mucus, epithelium, immunoengineering, lymph node, M cells
Introduction
Mucosal surfaces are the largest organs protecting our internal body surfaces from exposure to the external environment, preventing pathogens and macromolecules from reaching the internal surfaces of the body when the entry is undesired.1–4 The mucosal surfaces are made up of a mucus layer covering the mucosa, or mucosal epithelium, mucosal immune cells and lymphoid organs, and underlying blood and lymphatic vasculature. All of these are tightly regulated and form a formidable barrier against pathogens and particulates.3,5–7 Firstly, mucus has a tight mesh structure, and the charged mucins effectively trap positively charged particulates and pathogens.2 Secondly, the mucosal epithelium tightly regulates the transport of molecules, such as, e.g. digested food products in the GI tract, ensuring no pathogen can cross into the body’s interior. And thirdly, the local immune system, including immune cells located in the mucosal-associated lymphoid tissues and lymph nodes, destroys pathogens upon encounter.1 While the mucosal surfaces provide a desired barrier for pathogens, they also form a barrier to nanoparticle-based drug delivery: any particulate needs to first successfully pass through by mucus and epithelium, to reach its therapeutic target. For immunotherapies and vaccination at mucosal surfaces, getting to the local immune cells and lymph nodes is key to induce the desired immune response.1,8–11 Recognizing this, researchers in the past 10+ years have set out to design carriers to overcome the mucus barrier, pass through the epithelium, and target lymph nodes and lymphoid structures through a variety of mechanisms. This review first summarizes the relevant barriers and targets for immunotherapy and vaccination at the mucosal surfaces, and then delves into existing drug delivery technologies that have been engineered to overcome these barriers.
Overview of mucosal immunity
Mucosal surfaces are constantly exposed to microbes – both commensal and pathogenic and a fine balance has to be struck between protecting the body from the pathogenic microbes to not eliminating the commensal ones at the same time. Generally, when no pathogenic microbes are around, the immune response is downregulated at the mucosal surfaces through a variety of immune mechanisms.12 In this section, we will discuss the role of the physical barriers, the immune cell composition, and their various functions at mucosal surfaces (Figure 1).
Figure 1.
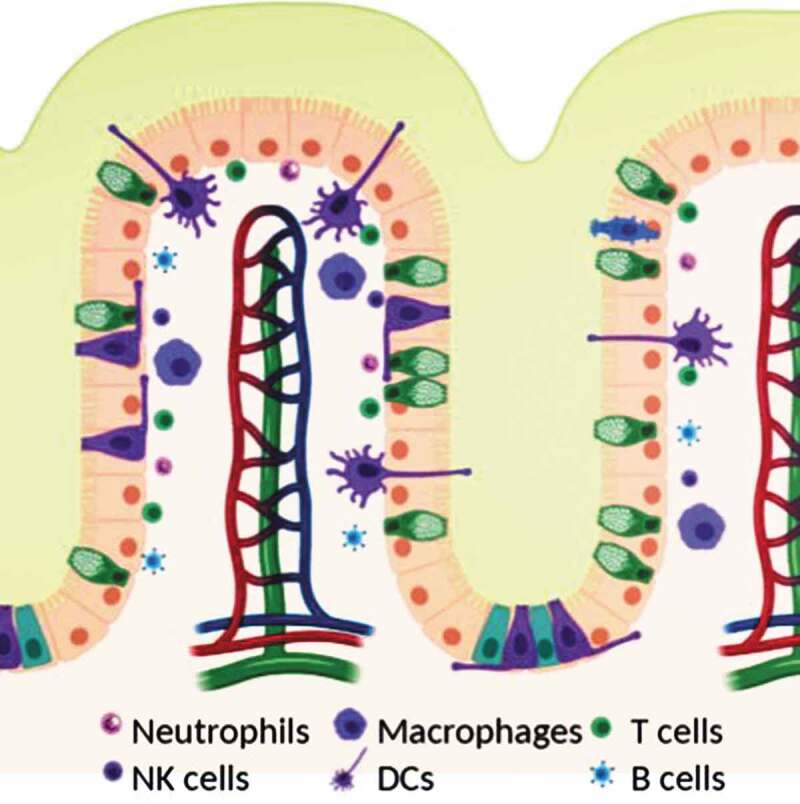
Schematic of mucosal surface including epithelium (orange), blood vessels (red and blue), lymphatic vessels (green), and immune cells (see legend). Created with BioRender.com.
Mucus is our first line of defense at the mucosal surfaces, effectively trapping pathogens and particulates that are rapidly cleared and expelled through the mucus clearance mechanisms that evolved to further prevent pathogens and harmful particulates from reaching the surfaces of our mucosal epithelia. Mucus is a porous hydrogel that takes advantage of size and electrostatic and hydrophobic interactions to trap microbes and particulates, and also some secreted antibodies.5,13,14 Mucus is made up of mucin fibers, peptidoglycans 0.3–2 MDa in size with an overall negative charge.2,5 This effectively traps many pathogens or particulates that have a positive charge. In addition to the glycosylated regions, the mucin peptides also contain hydrophobic regions that bundle mucin fibers into cables, and effectively trap hydrophobic particulates.2,5 Mucin fibers are linked together to form fibers several microns long, leading to a gel-mesh that excludes particulates of larger sizes. The types of components, their ratios, the mucin type, the mucus turnover rate, and the mucus thickness vary at the different mucosal surfaces, and some of the specific composition particularly mucin fibers are summarized in Table 1 and have been discussed in several other review articles.2,5,6,34,49 A second protective property of mucus is its lubrication. Mucus gels are shear-thinning, which occurs when a slippage plane forms between two moving surfaces, such as the epithelium and food bolus moving through the intestine,5 and provides lubrication that serves to prevent any mechanical damage that could be induced, e.g. during digestion, the closing of eyes, or sexual intercourse. Thus, mucus is the first physical barrier at mucosal surfaces, protecting both from mechanical damage, and trapping particulates and pathogens, thus preventing them from reaching and entering the mucosal epithelium.
Table 1.
Mucus composition at different mucosal surfaces.
| Mucosal Surface | Secreted Mucins | Adherent mucins | Other significant components | Reference |
|---|---|---|---|---|
| Respiratory tract | MUC2, MUC5AC, MUC5B, MUC19, MUC7 | MUC1, MUC4, MUC12, MUC15, MUC20 | Microbes, surfactants, phospholipids, immunoglobulins | 15–25 |
| Stomach | MUC5AC, MUC6 | MUC1, MUC4, MUC12, MUC13, MUC15, MUC17 | HCl, phospholipids | 16,20,21,26–28 |
| Small intestine | MUC2, MUC6 | MUC1, MUC3A/B, MUC12, MUC13, MUC15, MUC17 | Bile salts, phospholipids, microbes, immunoglobulins | 21,25,27-33 |
| Large intestine | MUC2 | MUC1, MUC3A/B, MUC4, MUC12, MUC13, MUC15, MUC17, MUC20 | Microbes, phospholipids, immunoglobulins | 16,20,21,25,28–35 |
| Cervicovaginal tract | MUC5AC, MUC5B, MUC6 | MUC1, MUC4, MUC12, MUC16 | Lactobacilli and other microbes, immunoglobulins | 16,17,20,25,27,36–42 |
| Eye | MUC2, MUC5AC, MUC19 | MUC1, MUC4 | Antimicrobial peptides, immunoglobulins | 16,20,25,43–48 |
The mucosal epithelium is the next barrier that needs to be overcome. The mucosal epithelium is a mostly non-keratinized epithelium (except in some areas, such as the oral cavity) that is highly regulated to prevent pathogens from entering the body.3,12 It serves varying functions at the different mucosal surfaces. For instance, in the lungs, the mucosa protects against pathogens in the upper airways, while it allows gas exchange in the lower airways. In the gastrointestinal tract, the mucosa not only protects us from our own microbiome infecting the tissue, but also is responsible for nutrient absorption.4 To perform these functions and regulate the unwanted entry of pathogens or molecules, the epithelium contains numerous tight and cell-cell junctions that form a sort of fence between the material or pathogen attempting to cross to the underlying tissue. Cell-cell and tight junctions serve as the primary barrier for paracellular transport of material or pathogen attempting to cross the mucosal epithelium. Claudins are a key component of epithelial tight junctions and primarily reside at the most apical side of the junction.50 There are over 20 different types of claudins expressed in humans, with many types present on epithelia at mucosal surfaces.51 Claudin-1 can be found in the intestinal epithelium and is widely implicated in strengthening tight-junction barrier properties. In the colon, Claudin-3 serves to limit the transport of solute through this epithelial layer, however, when expressed in the lung alveolar epithelia an opposite effect on the regulation of paracellular transport is shown.52,53 It is naturally unsurprising then that many drug delivery strategies have been designed to interface with these claudin proteins to improve penetration through the epithelial barrier. Two clinically available claudin-disrupting molecules are sodium caprate and mannitol, that when administered enhance paracellular absorption of a drug.54 In addition to being a physical barrier, epithelial cells can sense their microenvironment, e.g. through receptors for pathogen-associated molecular patterns and can secrete ‘danger signals’ to activate an immune cascade such as pro-inflammatory cytokines and also antimicrobial peptides to initially fend off infection. The epithelium thus is the first line of cellular defense against pathogens.
In aid to the epithelial cells come the various immune cells present within the epithelium, also known as intraepithelial lymphocytes (IEL), and the lamina propria (Figure 1). The dominant cell type within the epithelial layer are CD8 + T cells that have an effector or memory phenotype.55,56 In the gut as many as 5–15 lymphocytes can be found for every 100 epithelial cells in this layer. The lamina propria contains a variety of different immune cells including plasma cells, mainly responsible for producing the large quantities of antibodies present in the mucus and throughout the mucosal surfaces, conventional CD4+ and CD8 + T cells, dendritic cells, macrophages, innate lymphoid cells, and mast cells.1,57,58 The dominant cell type here is CD4 + T cells and plasma cells.12 Antibodies produced by plasma cells are secreted into the mucus gel via transcytosis from the basolateral side of the epithelium.58 The predominant type is IgA, which has been shown to interact with the mucus gel.42,59 In fact, antibodies secreted into mucus greatly enhance the ability to trap pathogens and toxins in mucus gel: They diffuse rapidly through the gel, retarded only slightly by transient, low-affinity bonds with the mucus gel.60 However, when they accumulate on the surface of a pathogen they form enough multivalent adhesive interactions with the gel to trap the pathogen, thus serving their purpose of preventing infections.61,62
When foreign bodies are encountered at the mucosal surfaces and they, or their debris make it past the mucus gel, their antigens will be taken up by antigen-presenting cells, including both traditional cells such as dendritic cells and macrophages, as well as non-traditional cells such as M cells on Peyer’s patches in the GI tract. Macrophages at the mucosal surfaces primarily phagocytose and scavenge antigens, and help maintain antigen-specific tolerance locally by the production of tolerogenic factors such as IL-10.1,12,25,58 Unlike in other peripheral tissues, macrophages at mucosal surfaces often do not migrate to the lymph nodes, but instead stay in the tissue to perform their tissue-specific responses.56 These are particularly important for maintaining the ‘down-regulated’ state of the immune response so that there is no response formed to commensal microbes.56 Dendritic cells, in contrast, scavenge antigen and migrate to the local draining lymph nodes for T cell education.63,64 DCs not only phagocytose antigens, but also can acquire antigens from the non-traditional antigen-presenting cells. When an infectious agent is detected, epithelial cells secrete factors that will recruit DCs into the epithelial layer, sample antigens, and proceed to migrate to the LNs.58 In the absence of an infectious agent, the same migrating DCs present antigens and educate T cells to form regulatory T cells that help maintain the tolerogenic environment toward commensal microbes.12,56,58,65 In the lymph nodes, antigen-specific T cells are educated and these begin to expand and circulate back to the mucosal surface. Once pathogens are encountered some of these T cells stay behind as effector/memory lymphocytes.56 In fact, at mucosal surfaces effector and memory lymphocytes as well as plasma B cells are the dominant types found even when no infectious agent is present.55,56 This likely accounts for the ability to mount a quick immune response upon re-encounter of pathogens.
In addition to the classic immune response, mucosal surfaces have secondary lymphoid tissues directly associated with them, usually called mucosal-associated lymphoid tissues, or MALT. These include Peyers patches in the GI tract, bronchus-associated lymphoid tissues in the lungs, tonsils, adenoids, and other gut-associated lymphoid tissues.12,58 These organs serve to rapidly provide an adaptive immune response to pathogens, serving to educate T and B cells into antigen-specific cells that will later on reside in the tissue.66–71 Additionally, the secondary lymphoid tissues are also thought to help maintain the memory T cells that dominate the mucosal lymphocytes. These lymphoid tissues are usually connected to the lymph nodes as well via lymphatic vessels, leading to further distribution of antigens and allowing DC trafficking from the more local lymphoid tissue to the further downstream lymph nodes.63,64 Combined, the local barriers such as mucus, epithelium, and the cellular immune response that is mounted by innate and adaptive cells stemming from the systemic circulation, lymph nodes, and local lymphoid organs, can mount a rapid response to rid the body of the pathogens we are constantly encountering at mucosal surfaces.1,4,57,58 Targeting this response for immunomodulation is of vital interest to prevent and treat a variety of mucosal diseases.
Engineering systems to enhance immunotherapy and vaccination at mucosal surfaces
Therapeutic treatments targeting the immune system are becoming more and more prevalent. They range from classic vaccines and allergen immunotherapy to the cancer immunotherapies that have raised hopes of defeating this devastating disease. New immunotherapy treatments are constantly being developed, and many are applied to treat diseases of mucosal surfaces. These include the above-mentioned allergies and cancer, along with other diseases like inflammatory bowel disease and pulmonary fibrosis. In diseases where the immune response must be controlled and reduced, such as allergies, transplantation, and inflammatory bowel disease, immunosuppressive therapies are generally employed. Immunosuppressive drugs include antibodies that, e.g. block pro-inflammatory cytokines or prevent lymphocyte interaction with antigen-presenting cells, molecules that block cell division of B and T cells (cytostatics), and corticosteroids that prevent transcription of genes of pro-inflammatory cytokines.72 In contrast, pro-inflammatory immunotherapies are used to turn on immune responses that have either been suppressed, like in cancer, or to induce responses to specific antigens such as during vaccination. These immunotherapies include antibodies that activate lymphocytes by, e.g. targeting checkpoint inhibitors that serve to turn off the immune response, pro-inflammatory cytokines that activate immunity, and molecules that activate antigen-presenting cells, e.g. toll-like receptor agonists, and thus cause a down-stream immune cascade.72,73
In addition to these more classic immunotherapies, another application that seeks to take advantage and modulate local immunity at mucosal surfaces are vaccinations. Indeed, effective mucosal vaccination could significantly contribute to global health by protecting against both mucosal infections as well as those entering through the mucosal route but targeting other organs, such as HIV. Mucosal vaccination has several advantages over intramuscular vaccination strategies: it can induce a local immune response through mucosal-associated lymphoid tissues structures; mucosal vaccination at one mucosal surface can induce immune responses at multiple mucosal surfaces; and mucosal vaccination does not require needles and thus both prevents potential spread of blood borne infection by contaminated needles and allows easy dissemination even potentially allowing administration in the comfort of the home.74 Despite these many advantages, not many mucosal vaccines are commercially available. Examples include those using live attenuated virus such as the vaccine for cholera, influenza, polio, rotavirus, and salmonella,10 which are either administered intranasally or orally. For the case of oral administration, one major barrier is the stomach acid that will destroy most infectious and therapeutic agents and thus significantly limits applicability. Additionally, while live attenuated virus appears to be most effective for mucosal vaccines, potential re-activation of the attenuated virus poses a significant problem and could lead to the presentation of the actual disease after vaccination.10 Subunit vaccines, the most common intramuscular vaccines, tend to be less effective because fragmented proteins are less able to withstand stomach acids and digestive enzymes, and do not transport well across epithelium or mucosal-associated lymphoid tissues. Both the potential for ‘digestion’, particularly for oral vaccination, and inability to cross the epithelial barrier are major issues to designing effective mucosal vaccines. These are barriers not only for vaccines but also for traditional immunotherapies. Technologies that have emerged to traverse the sticky mucus mesh and mucosal epithelium, protect vaccination cargo (e.g. via encapsulation in nanoparticles), and more recently to further potentiate vaccination via targeted delivery to lymph nodes or M cells. Figure 2 summarizes some of the known design criteria for vaccine and immunotherapy carriers to cross the mucosal barriers, and also to enter lymphatic vessels, and these technologies are further discussed in the following sections. We further direct the readers to several excellent reviews that include lists of commercially available mucosal-targeting drugs.75–77
Figure 2.
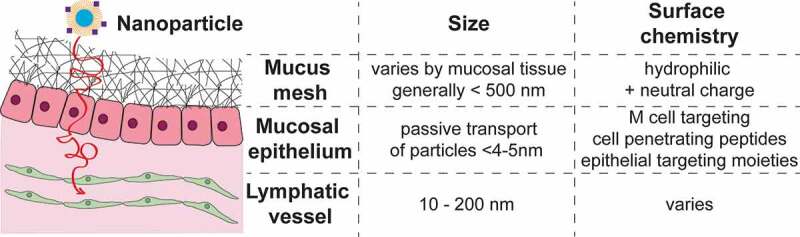
Schematic of mucosal barriers and known design criteria for crossing each barrier.
Traversing the mucus mesh barrier: penetration technologies
The first barrier our technology must overcome is the mucus mesh barrier. The mucus mesh is the first layer of defense at the mucosal surfaces. It is a sticky, mesh-like filter system that effectively traps pollutants, pathogens, and irritants that are subsequently cleared with the mucus. This system also effectively traps nanoparticle systems used to enhance local drug delivery to the mucosal surfaces. Efforts from the last ~10+ years, spear-headed by the Hanes Lab, have led to the development of nanoparticle systems that slip through, rather than adhere to, the mucus mesh. Nanoparticles are able to slip through the mucus mesh by fulfilling two main characteristics: 1) they are smaller than the mucus mesh spacing, and 2) they have an overall hydrophilic and neutrally charged surface.7,78 The size can be controlled by nanoparticle synthesis conditions and a hydrophilic and neutral surface can be achieved by choice of surface coating, e.g. by densely coating nanoparticles with hydrophilic PEG.7,78–85 Nanoparticle systems that do not adhere to the mucus mesh have been shown to improve distribution on mucosal surfaces, including the cervicovaginal tract,78,79,81–83 lungs,84,85 and gastrointestinal (GI) tract,82,83 and have improved drug levels in tissues as well as systemic drug delivery. We refer the reader to more extensive reviews of these systems.86–88
Traversing the mucosal epithelial barrier
The mucosal epithelial layer is a key barrier to the outside environment that putative drug delivery systems must cross. The structure of mucosal epithelial layers in the body vary by location; the GI tract and endocervix contain a simple layer of stratified columnar epithelial cells, the respiratory tract is composed of a pseudostratified columnar layer with the vagina, exocervix, and buccal mucosa comprised of a layer of stratified squamous epithelial cells.2,5 The integrity of these cell layers is maintained through the expression of tight junction proteins including: claudins, E-cadherins, and occludins.4 A large body of work exists on designing delivery systems that attempt to overcome the epithelial barrier for protein delivery,89 and many efforts have focused on two technologies: permeation enhancers, which temporarily affect cell junctions or membranes, and cell-penetrating peptides, which interact with the cell membrane for internalization through various mechanisms. We briefly summarize some of the most promising systems here, including those surface markers that have been targeted for immunomodulation (Table 2), and refer readers to several excellent reviews for more in-depth discussion of permeation enhancers (PE) and cell-penetrating peptides (CPP).89,97–99
Table 2.
Epithelial surface markers targeted for immunomodulation.
| Targeting Agent/Ligand | Target | Application | Reference |
|---|---|---|---|
| P6 Haemophilus influenzae membrane liporprotein |
TLR2 | 1) NF-κB mediated transcription of MUC5AC, 2) Production of β-defensin 2 antimicrobial protein. | 90,91 |
| F-protein from Respiratory Syncytial Virus | TLR4 | Vulnerability to lipopolysacchardide-mediated inflammation | 92 |
| FPS-ZM1 | Inhibitor for receptor for advanced glycan end products (RAGE) | Anti-inflammatory and anti-oxidative stress mediator. | 93 |
| Transferrin | Transferrin receptor | Improves nanoparticle delivery to and across Caco-2 cells | 94 |
| Fc antibody region | Bind to Neonatal Fc Receptor (FcRn) | Mediates transport across epithelial barrier. | 95 |
| alpha-Galactosylceramide | Galactosyl ceramide receptor | Mediates transport across epithelial barrier and modulates immunity | 96 |
Permeation enhancers
PEs promote the delivery of therapeutics across the epithelium by either temporarily modulating tight junction properties or perturbing cell membranes or, in some cases, by both of these methods. PEs have been met with skepticism in terms of their safety, though most have shown little toxicity at concentrations needed to perform their functions in vivo, likely due to the transient nature of their effects.89,100 PEs require proximity of the therapeutic to effectively traffic it across the epithelium, so co-delivery in proximity to the epithelium is key. Paracellular PEs have a variety of mechanisms including targeting tight junction structures such as claudins, E-cadherins, and occludins, while others modulate cytoskeletal reorganization that affects tight junction permeability.89 Transcellular PEs that have been studied are usually varying classes of surfactants that directly disrupt the cellular membrane. Increasing interest lies in administering particulate drug delivery systems orally, though the combinations of particulates with PEs in combination has been limited. The most notable formulation combined insulin-loaded micelles with the surfactant sucrose erurate suspended in soybean oil with sodium cholate and sucrose laurate. The micelles are quite stable in water and effectively reduce blood sugar in rats.101,102 Several other PEs have effectively enhanced the delivery of larger cargo, such as protein-complexes, liposomes, or other nanoparticles including C12E9 and sodium deoxycholate.103–106 Citric acid and other acidifying organic acids have also been shown to improve the delivery of peptides through the GI mucosal epithelium by lowering the optimal pH for proteolysis and while improving solubility.107
Cell-penetrating peptide technologies
CPPs are a class of molecules that improve transcellular or paracellular transport through epithelial cells. Natural and synthetic peptides have been employed for the delivery of genes and protein into and across the epithelial cell barrier.98 CPPs share some unifying characteristics despite having a variety of primary sequences and secondary structures. Generally, CPPs are less than 30 amino acids in length, rich in positively charged amino acid residues, and contain hydrophobic tryptophan residues to help membrane translocation of the CPP. There are several different classes, including protein derived CPPs, such as the HIV transactivator of transcription peptide (TATp), chimeric peptides such as transportan, and synthetic peptides such as octa-arginine.89,100 Most of these operate by initiating endocytosis, direct translocation, tight junction loosening, or formation of channels in the cell membrane. CPP technology has been successfully combined with nanotechnology to enhance systemic absorption of a cargo, often the ‘test cargo’ insulin.89,100 For instance, a cargo of penetrating-modified insulin complexed with a polymer (HMPA) that did not interact with the mucus mesh led to 20-fold higher insulin absorption in vivo, which was followed by a decrease in blood glucose.108 Similarly, a nanoparticle system for insulin delivery that contained a peptide targeting goblet cells (CSKSSDYQC) was shown to co-localize with goblet cells in an in vivo intestinal loop model.109 The hypoglycemic effects were more limited than the penetrating-based system, but still showed a 1.5-fold increase compared to non-targeting nanoparticle controls.109 More recently, systems combining cell and mucus penetration have emerged. For instance, Porsio et al. demonstrated that nanoparticles coated with PEG (to make them mucoinert) and TAT peptide (to enhance cell permeation) enhanced both penetrations through artificial cystic fibrosis mucus, and across lung epithelial cells.110 A system developed by Tan et al. uses PEG-coated 170 nm silica nanoparticles that were loaded with cell-penetrating peptide, penetratin, along with therapeutic peptide.111 These particles not only penetrated mucus, but also showed improved cellular uptake, exocytosis and transcellular permeation across mucosal epithelium compared to particles that were able to either penetrating the mucus barrier or contain CPPs.111
Targeting lymphoid tissue for potentiation of immunomodulatory treatments
Lymphoid tissues, including the lymph nodes (LNs) and mucosal-associated lymphoid tissues have recently become a target for immunotherapeutic treatments and vaccinations, since efforts have demonstrated significant potentiation of treatments when targeting tissues where immune cell education is occurring. In this section, we summarize technologies developed to target both lymph nodes and M cells that coat the mucosal-associated lymphoid tissues for enhanced immunotherapy and vaccination.
Lymph node targeting systems
Usually, LNs are targeted by direct injection into the lymph node or nearby lymphatic vessels. One of the early approaches targeted LNs for allergen immunotherapy, work that has led to a number of clinical trials that demonstrated enhanced efficacy of the treatment while simultaneously requiring much lower dosages, reducing the likelihood of adverse reactions such as anaphylaxis.112,113 Another area that has had considerable developments in lymph node targeting approaches is cancer immunotherapy. Smith et al. demonstrated that intra-lymph node immunization of tumor peptide with adjuvant induces tumor regression and induces antigen-specific T cell response, with ~15% of recirculating CD8 + T cells being tumor antigen-specific.114 Strikingly, 90% of mice immunized with intra-LN vaccines remained protected against tumor induction. Similarly, Liu et all designed cancer vaccines that have albumin ‘hitchhiking’ components, that is adjuvants and peptides conjugated to albumin that natural accumulates in the LN.115 These albumin hitchhiking vaccines were shown to reduce tumor burden in mice and enhance cytolytic activity and cytokines production in antigen-specific CD8 + T cells.
Several studies from the Kündig group have shown that LN targeting injections of CpG enhance its therapeutic potential, yielding a higher CD8 + T cell response to OVA compared to a subcutaneous injection with a 100-fold lower dose, and induced a higher anti-tumor response.116 DNA vaccines also further enhanced the CD8+ cytotoxic T cell antitumor response by 100–1000 fold.117 Furthermore, peptide vaccines injected into LN with MHC class I binding peptides from lymphocytic choriomeningitis virus enhanced the immunogenicity of the peptide by 106 fold compared to subcutaneous or intradermal injection.118 This was evidenced by the significantly stronger CD8 + T cell response with greater cytotoxic activity and IFNy production, as well as long-term protection against viral infections and tumor growth.
Finally, studies in transplantation by Komori et al. demonstrated that the LN can serve as a transplantation site of different tissues and this will reduce the chances of rejection.119 For instance, hepatocytes and thymocytes transplanted into mouse jejunal lymph nodes induced survival in mice with lethal metabolic disease and restored a functional immune system in athymic mice. While these data suggest that tolerance was induced to these cells, there is no mention of how the transplantation into the LN actually altered the immune response.119 Their work suggested that LN transplantation favors vascularization, one of the keys to graft survival, and that transplantation of cell/tissue types into only one lymph node may be sufficient for successful treatment. Taken together, this suggests that targeting lymph nodes directly can significantly enhance the desired immune response, and thus can potentiate immunotherapeutic, vaccination, and even transplantation treatments.
Lymphatic targeting technologies for indirect drug delivery to the lymph nodes
More recently, technologies have emerged that indirectly target lymph nodes by targeting lymphatic transport.120 The rationale behind targeting the lymphatic vessels is due to the transport functions of these vessels. Cells, fluids, protein, and small molecules are transported from peripheral tissues to the draining lymph nodes where adaptive immune responses are formed. Convective fluid flow in the interstitium drives fluid and molecules toward lymphatic vessels.120 This fluid flow becomes increasingly important with increased molecular size, as the opposing movement toward blood capillaries by diffusion decreases for larger molecules.120 At the same time, the extracellular matrix holding together the interstitium needs to be traversed, so particles need to be small enough to get across this extracellular matrix and reach lymphatic vessels and their downstream dLNs.
The size is necessary for particles to enter the lymphatics have been well established, with combined studies suggesting that particles ranging from 10 to 250 nm are ideal for transport into the lymphatics. Work by Reddy et al. demonstrated the optimal nanoparticle size for targeting lymphatics via intradermal injection in mice is between 5 and 50 nm. Intradermally delivered PEG-stabilized poly(propylene sulfide) nanoparticle 20 nm and 45 nm in size efficiently drained to local lymphatics and were recovered in high amounts in the dLNs.121 Manolova et al. showed that larger particles, up to 200 nm, could also freely travel to the dLNs.122 Similarly, Varypataki et al. showed that vaccine response could be enhanced in vivo by using smaller size cationic liposomes (150–200 nm) compared to larger poly-(lactic-co-glycolic-acid) (PLGA) nanoparticle (250–350 nm).123 Interestingly, work by Kobayashi et al. demonstrated that nanoparticle systems smaller than 10 nm in size are inefficiently retained in lymphatic vessels, suggesting that these were too small to take advantage of convective fluid flow in the interstitial space.124
The effects of surface charge on targeting lymphatic transport remains poorly understood. As mentioned, Varypataki et al. showed that cationic liposomes effectively drained into lymphatic vessels.123 Rao et al. demonstrated that hydrophobic, negatively charged PLGA and PLGA-poly(lactic acid)-PEG nanoparticles both accumulated in the dLN after 3h, with the PEGylated nanoparticles having higher accumulation.125 However, this study on PLGA-PEG nanoparticles was far from conclusive. The fairly large injection volume in this work, though, likely led to enhanced convective flow toward lymphatics, and the highly negative ζ-potential of the PEGylated nanoparticles, −30 mV, indicating the particles were not densely PEGylated, may have obscured some of the effects of surface charge and PEGylation on lymphatic transport. Finally, another study by Zeng et al. showed that cationic nanoparticles can also promote LN retention of nanoparticles, though the question remains if this was due to lymphatic transport or enhanced uptake of these nanoparticles by antigen-presenting cells.126 More recently, DeKoker demonstrated that PEGylating 200 nm polymethacrylic acid nanoparticles drastically enhanced nanoparticle accumulation in the lymph nodes after 12 and 48 h, though whether this is by the mechanism of cellular migration or transport via lymphatic vessel remains to be explained.127 Additionally, Mao et al. formulated chylomicron mimicking mesoporous silica nanoparticles containing the antiretroviral drug lopinavir, and demonstrated that these were able to effectively translocate across intestinal epithelium in vitro and in vivo in mice.128 Furthermore, their formulation accumulated in the mesenteric lymph nodes, which could be inhibited using a lymph transport inhibitor, cycloheximide, which interferes with the chylomicron formation pathways, suggesting that chylomicron imitation could indeed be used to target the local gut draining lymph nodes.128 Interestingly, a recent publication from Triacca et al. reveals transcytosis to be a key mode of transport into lymphatic vessels. Inhibiting clathrin and caveolin-mediated uptake was demonstrated to prevent the transport of albumin across lymphatic endothelial cells to a similar extent as tightening of cell-cell junctions using adrenomedullin.129 To date, little is known about the mechanisms of nanoparticle transport across lymphatic vessels, and many more thorough studies are needed to better understand requirements, other than size, for effectively targeting nanoparticles to lymphatic vessels.
M cell-targeted drug delivery
Mucosal associated lymphoid tissues (MALT) are considered an alternate target to lymph nodes at the mucosal surfaces, as these perform similar functions in activating and educating local adaptive immunity. In particular, targeting M cells that cover the GALT, BALT, and NALT have been explored. M cells phagocytose antigens from the apical side of the epithelium and transfer them to the basal side, where the underlying antigen presenting cells take them up for lymphocyte education and activation.12 This specific function makes them a particularly appealing target for immune modulatory treatments. Approaches usually target molecules specifically expressed on M cell surface. Perhaps the most commonly used example is targeting α-L-fucose expressed on M cells specifically using a variety of lectins including Ulex europaeus agglutinin 1 and Aleuria aurantia. Lectins are carbohydrate-binding proteins that specifically recognize certain sugar molecules and have a variety of functions including cell adhesion and receptors for recognition of sugars in the systemic circulation, e.g. by liver cells.8,9,130 Lectins have been used for nanoparticle-based delivery including biodegradable systems, such as PLGA and liposomes, as well as latex particles. These systems induced robust immune responses that included the production of IgA and type 1 cytokines such as IFNy to the antigen ovalbumin.131–135 Despite these promising studies, clinical translation proves difficult due to the immunogenicity inherently associated with these lectins.8 This brought about the development of other molecules to target M cells including RGD peptides that adhere to the integrins expressed by M cells, and antibodies and/or their fragments that have been selected to target M cells specifically. Targeting mechanisms have been exploited for a variety of applications including vaccination against viral or bacterial targets (Table 3), and cancer, targeting responses against biological toxins like botulinum toxin, and induction of antigen-specific antibody responses to induce robust immunity against foreign molecules such as ovalbumin.131–135 Table 3 summarizes targeting molecules and their application.
Table 3.
Summary of materials used to target M cells, their target on the M cell surface, and applications.
| Targeting agent | Type of targeting | Application | Reference |
|---|---|---|---|
| BSK02TM Aleuria aurantia lectin |
Ligand with high affinity to α-L-fucose on M cell surface | Vaccination against ovarian cancer, vaccination reduced/retarded tumor volume and enhanced CD8+ lymphocyte response | 136 |
| M cell homing peptide (CKSTHPLSC) | High M cell affinity | Identified via phage display using in vitro M cells; displayed enhance in vivo transcytosis of cargo, vaccination against pathogenic intestinal spirochete Brachyspira hyodysenteriae | 137,138 |
| Ulex europaeus agglutinin 1 (UEA-1) | Ligand with high affinity to α-L-fucose on M cell surface | Immunization against GP5 antigen; nasal vaccination against Staph aureus protected against acute systemic infection without antibodies | 139,140 |
| OmpH β1α1 domain of Yersinia enterocolitica | Binds to a complement C5a receptor on the M cell surface | Vaccination against Infectious bursal disease virus (IBDV) using viral capsid protein; enhanced delivery of model antigen staph aureas nuclease using lactobacilli lactis expressing omph, | 68,141 |
| Secretory IgA (SIgA) | Binds to M cells (specificity not indicated) | Immunization using HIV p24gag protein induced humoral and cellular response against p24 (both cellular and antibody response) | 142,143 |
| RGD or GRGDS | Targets beta1 integrins on the apical side of M cells | Robust IgG response to OVA after oral delivery; Delivery of PR8 antigen induced antibody production; IgG and cellular response to OVA | 131-133 |
| Monoclonal antibodies NKM 16-2-4 | antibody with high affinity to α-1,2-fucose on M cell surface | Oral delivery of tetanus toxin, botulinum toxoid and cholera toxin induce high levels of IgG and IgA responses and protected against botulinu toxin challenge | 134 |
| Antigen binding fragment from mouse antibody against glycoprotein 2 (GP2) | GP2, transcytotic antigen receptor specifically expressed on M cells | Immunization against OVA and Salmonella enterica lysate induced enhanced protection against bacteria | 135,144 |
| Full transmembrane mRANKL | Induces expression of RANK in M cells, then can target with mRANKL | Intraperitoneal sensitization then targeting induced oral immunization induced strong protective IgA and IgG | 145 |
Conclusion
In summary, the mucosal surfaces form a formidable barrier to pathogens that also need to be overcome for mucosal drug delivery for immunomodulation. A variety of technologies have been developed to cross the mucus mesh and mucosal epithelium for drug delivery, and some have targeted mucosal immunity by, e.g., binding to M cells and targeting lymph nodes via lymphatic vessels. Many of these are quite promising and have been translated into clinical trials or products (Table), but often we are still faced with sub-optimal treatments or vaccines for many mucosal diseases such as cholera, allergies, and inflammatory bowel disease. We expect many more years of research and development of novel technologies are required until we have exhaustively targeted mucosal immunity for local treatment and prevention of diseases.
References
- 1.Brandtzaeg P. Mucosal immunity: induction, dissemination, and effector functions. Scand J Immunol. 2009;70(6):1–17. PubMed PMID: 19906191. doi: 10.1111/j.1365-3083.2009.02319.x. [DOI] [PubMed] [Google Scholar]
- 2.Cone RA. Mucus. Mucosal immunology. 3rded: Academic Press/Elsevier; 2005. 49–72 PubMed PMID: ISI:000311099400008. doi: 10.1016/B978-012491543-5/50008-5. [DOI] [Google Scholar]
- 3.France MM, Turner JR. The mucosal barrier at a glance. J Cell Sci. 2017; 130(2):307–314. Epub 2017/01/06. PubMed PMID: 28062847; PMCID: PMC5278669. doi: 10.1242/jcs.193482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol PubMed PMID: 19855405. 2009;9(11):799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 5.Cone RA. Barrier properties of mucus. Adv Drug Deliv Rev. 2009; 61(2):75–85. Epub 2009/ 01/13. PubMed PMID: 19135107. doi: 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2012;64(6):557–570. Epub 2011/ 12/24 PubMed PMID: 22212900; PMCID: PMC3322271. doi: 10.1016/j.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61(2):158–171. Epub 2008/ 12/13. doi: 10.1016/j.addr.2008.11.002. PubMed PMID: 19133304; PMCID: PMC2667119. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Islam MA, Firdous J, Badruddoza AZM, Reesor E, Azad M, Hasan A, Lim M, Cao W, Guillemette S, Cho CS. M cell targeting engineered biomaterials for effective vaccination. Biomaterials. 2019;192:75–94. Epub 2018/11/12. doi: 10.1016/j.biomaterials.2018.10.041. PubMed PMID: 30439573. [DOI] [PubMed] [Google Scholar]
- 9.Lavelle EC, O’Hagan DT. Delivery systems and adjuvants for oral vaccines. Expert Opin Drug Deliv. 2006;3(6):747–762. PubMed PMID: 17076597 doi: 10.1517/17425247.3.6.747. [DOI] [PubMed] [Google Scholar]
- 10.Miquel-Clopés A, Bentley EG, Stewart JP, Carding SR. Mucosal vaccines and technology. Clin Exp Immunol. 2019;196(2):205–214. Epub 2019/04/08 PubMed PMID: 30963541; PMCID: PMC6468177. doi: 10.1111/cei.13285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srivastava A, Gowda DV, Madhunapantula SV, Shinde CG, Iyer M. Mucosal vaccines: a paradigm shift in the development of mucosal adjuvants and delivery vehicles. APMIS. 2015;123(4):275–288. Epub 2015/ 01/29. doi: 10.1111/apm.12351. PubMed PMID: 25630573. doi:. [DOI] [PubMed] [Google Scholar]
- 12.Murphy K, Travers P, Walport M, Janeway C. Janeway’s immunobiology. 8th ed. New York, NY: Garland Science; 2012. p. xix, 868. [Google Scholar]
- 13.Olmsted SS, Padgett JL, Yudin AI, Whaley KJ, Moench TR, Cone RA. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys J. 2001;81(4):1930–1937. PubMed PMID: 11566767; PMCID: 1301668 doi: 10.1016/S0006-3495(01)75844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeitlin L, Cone RA, Whaley KJ. Using monoclonal antibodies to prevent mucosal transmission of epidemic infectious diseases. Emerg Infect Dis. 1999;5(1):54–64. PubMed PMID: 10081672; PMCID: 2627706 doi: 10.3201/eid0501.990107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dohrman A, Tsuda T, Escudier E, Cardone M, Jany B, Gum J, Kim Y, Basbaum C. Distribution of lysozyme and mucin (MUC2 and MUC3) mRNA in human bronchus. Exp Lung Res. 1994;20(4):367–380. PubMed PMID: 7988497 doi: 10.3109/01902149409064393. [DOI] [PubMed] [Google Scholar]
- 16.Porchet N, Pigny P, Buisine MP, Debailleul V, Degand P, Laine A, Aubert JP. Human mucin genes: genomic organization and expression of MUC4, MUC5AC and MUC5B. Biochem Soc Trans. 1995;23(4):800–805. PubMed PMID: 8654841 doi: 10.1042/bst0230800. [DOI] [PubMed] [Google Scholar]
- 17.Escande F, Porchet N, Aubert JP, Buisine MP. The mouse Muc5b mucin gene: cDNA and genomic structures, chromosomal localization and expression. Biochem J. 2002;363(Pt 3):589–598. PubMed PMID: 11964160; PMCID: PMC1222512 doi: 10.1042/0264-6021:3630589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies JR, Herrmann A, Russell W, Svitacheva N, Wickström C, Carlstedt I. Respiratory tract mucins: structure and expression patterns. Novartis Found Symp. 2002;248:76–88. discussion −93, 277-82. PubMed PMID: 12568489. [PubMed] [Google Scholar]
- 19.Bobek LA, Tsai H, Biesbrock AR, Levine MJ. Molecular cloning, sequence, and specificity of expression of the gene encoding the low molecular weight human salivary mucin (MUC7). J Biol Chem. 1993;268(27):20563–20569. PubMed PMID: 7690757. [PubMed] [Google Scholar]
- 20.Moniaux N, Escande F, Batra SK, Porchet N, Laine A, Aubert JP. Alternative splicing generates a family of putative secreted and membrane-associated MUC4 mucins. Eur J Biochem. 2000;267(14):4536–4544. PubMed PMID: 10880978 doi: 10.1046/j.1432-1327.2000.01504.x. [DOI] [PubMed] [Google Scholar]
- 21.Pallesen LT, Berglund L, Rasmussen LK, Petersen TE, Rasmussen JT. Isolation and characterization of MUC15, a novel cell membrane-associated mucin. Eur J Biochem. 2002;269(11):2755–2763. PubMed PMID: 12047385 doi: 10.1046/j.1432-1033.2002.02949.x. [DOI] [PubMed] [Google Scholar]
- 22.Widdicombe JG. Rôle of lipids in airway function. Eur J Respir Dis Suppl. 1987;153:197–204. PubMed PMID: 3322862. [PubMed] [Google Scholar]
- 23.Creeth JM. Constituents of mucus and their separation. Br Med Bull. 1978;34(1):17–24. PubMed PMID: 342044 doi: 10.1093/oxfordjournals.bmb.a071454. [DOI] [PubMed] [Google Scholar]
- 24.O’Dwyer DN, Dickson RP, Moore BB. The lung microbiome, immunity, and the pathogenesis of chronic lung disease. J Immunol. 2016;196(12):4839–4847. PubMed PMID: 27260767; PMCID: PMC4894335 doi: 10.4049/jimmunol.1600279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mak TW, Saunders ME. Primer to the immune response. Academic Press/Elsevier; 2008. 436. [Google Scholar]
- 26.Toribara NW, Roberton AM, Ho SB, Kuo WL, Gum E, Hicks JW, Gum JR, Byrd JC, Siddiki B, Kim YS. Human gastric mucin. Identification of a unique species by expression cloning. J Biol Chem. 1993;268(8):5879–5885. PubMed PMID: 7680650. [PubMed] [Google Scholar]
- 27.Bartman AE, Buisine MP, Aubert JP, Niehans GA, Toribara NW, Kim YS, Kelly EJ, Crabtree JE, Ho SB. The MUC6 secretory mucin gene is expressed in a wide variety of epithelial tissues. J Pathol. 1998;186(4):398–405. PubMed PMID: 10209489 doi:. [DOI] [PubMed] [Google Scholar]
- 28.Gum JR, Crawley SC, Hicks JW, Szymkowski DE, Kim YS. MUC17, a novel membrane-tethered mucin. Biochem Biophys Res Commun. 2002;291(3):466–475. PubMed PMID: 11855812 doi: 10.1006/bbrc.2002.6475. [DOI] [PubMed] [Google Scholar]
- 29.Gum JR, Byrd JC, Hicks JW, Toribara NW, Lamport DT, Kim YS. Molecular cloning of human intestinal mucin cDNAs. Sequence analysis and evidence for genetic polymorphism. J Biol Chem. 1989;264(11):6480–6487. PubMed PMID: 2703501. [PubMed] [Google Scholar]
- 30.Ogata S, Uehara H, Chen A, Itzkowitz SH. Mucin gene expression in colonic tissues and cell lines. Cancer Res. 1992;52(21):5971–5978. PubMed PMID: 1394223. [PubMed] [Google Scholar]
- 31.Pratt WS, Crawley S, Hicks J, Ho J, Nash M, Kim YS, Gum JR, Swallow DM. Multiple transcripts of MUC3: evidence for two genes, MUC3A and MUC3B. Biochem Biophys Res Commun. 2000;275(3):916–923. PubMed PMID: 10973822 doi: 10.1006/bbrc.2000.3406. [DOI] [PubMed] [Google Scholar]
- 32.Barko PC, McMichael MA, Swanson KS, Williams DA. The gastrointestinal microbiome: a review. J Vet Intern Med. 2018;32(1):9–25. Epub 2017/ 11/24 PubMed PMID: 29171095; PMCID: PMC5787212. doi: 10.1111/jvim.14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brandtzaeg P, Bjerke K, Kvale D, Rognum TO, Scott H, Sollid LM, Valnes K. Production and secretion of immunoglobulins in the gastrointestinal tract. Ann Allergy. 1987;59(5 Pt 2):21–39. PubMed PMID: 3318585. [PubMed] [Google Scholar]
- 34.Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin MA. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008;1(3):183–197. Epub 2008/03/05. doi: 10.1038/mi.2008.5. PubMed PMID: 19079178. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogier EW, Frantz AL, Bruno ME, Kaetzel CS. Secretory IgA is concentrated in the outer layer of colonic mucus along with gut bacteria. Pathogens. 2014;3(2):390–403. Epub 2014/04/29. doi: 10.3390/pathogens3020390. PubMed PMID: 25437806; PMCID: PMC4243452. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gipson IK, Ho SB, Spurr-Michaud SJ, Tisdale AS, Zhan Q, Torlakovic E, Pudney J, Anderson DJ, Toribara NW, Hill JA. Mucin genes expressed by human female reproductive tract epithelia. Biol Reprod. 1997;56(4):999–1011. PubMed PMID: 9096884 doi: 10.1095/biolreprod56.4.999. [DOI] [PubMed] [Google Scholar]
- 37.Gipson IK, Moccia R, Spurr-Michaud S, Argüeso P, Gargiulo AR, Hill JA, Offner GD, Keutmann HT. The Amount of MUC5B mucin in cervical mucus peaks at midcycle. J Clin Endocrinol Metab. 2001;86(2):594–600. PubMed PMID: 11158014 doi: 10.1210/jcem.86.2.7174. [DOI] [PubMed] [Google Scholar]
- 38.Gipson IK, Spurr-Michaud S, Moccia R, Zhan Q, Toribara N, Ho SB, Gargiulo AR, Hill JA. MUC4 and MUC5B transcripts are the prevalent mucin messenger ribonucleic acids of the human endocervix. Biol Reprod. 1999;60(1):58–64. PubMed PMID: 9858486 doi: 10.1095/biolreprod60.1.58. [DOI] [PubMed] [Google Scholar]
- 39.Andersch-Björkman Y, Thomsson KA, Holmén Larsson JM, Ekerhovd E, Hansson GC. Large scale identification of proteins, mucins, and their O-glycosylation in the endocervical mucus during the menstrual cycle. Mol Cell Proteomics. 2007;6(4):708–716. Epub 2007/01/12. doi: 10.1074/mcp.M600439-MCP200. PubMed PMID: 17220477. doi:. [DOI] [PubMed] [Google Scholar]
- 40.Adnane M, Meade KG, O’Farrelly C. Cervico-vaginal mucus (CVM) - an accessible source of immunologically informative biomolecules. Vet Res Commun. 2018;42(4):255–263. Epub 2018/ 08/16. doi: 10.1007/s11259-018-9734-0. PubMed PMID: 30117040; PMCID: PMC6244541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hein M, Petersen AC, Helmig RB, Uldbjerg N, Reinholdt J. Immunoglobulin levels and phagocytes in the cervical mucus plug at term of pregnancy. Acta Obstet Gynecol Scand. 2005;84(8):734–742. PubMed PMID: 16026397 doi: 10.1111/j.0001-6349.2005.00525.x. [DOI] [PubMed] [Google Scholar]
- 42.Wang YY, Schroeder HA, Nunn KL, Woods K, Anderson DJ, Lai SK, Cone RA. Diffusion of immunoglobulin G in shed vaginal epithelial cells and in cell-free regions of human cervicovaginal mucus. PLoS One. 2016;11(6):e0158338. Epub 2016/06/30. doi: 10.1371/journal.pone.0158338. PubMed PMID: 27362256; PMCID: PMC4928780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berry M, Ellingham RB, Corfield AP. Human preocular mucins reflect changes in surface physiology. Br J Ophthalmol. 2004;88(3):377–383. PubMed PMID: 14977773; PMCID: PMC1772032 doi: 10.1136/bjo.2003.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inatomi T, Spurr-Michaud S, Tisdale AS, Zhan Q, Feldman ST, Gipson IK. Expression of secretory mucin genes by human conjunctival epithelia. Invest Ophthalmol Vis Sci. 1996;37(8):1684–1692. PubMed PMID: 8675412. [PubMed] [Google Scholar]
- 45.Spurr-Michaud S, Argüeso P, Gipson I. Assay of mucins in human tear fluid. Exp Eye Res. 2007;84(5):939–950. Epub 2007/02/07. doi: 10.1016/j.exer.2007.01.018. PubMed PMID: 17399701; PMCID: PMC1950265. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu DF, Chen Y, Han JM, Zhang H, Chen XP, Zou WJ, Liang LY, Xu CC, Liu ZG. MUC19 expression in human ocular surface and lacrimal gland and its alteration in Sjögren syndrome patients. Exp Eye Res. 2008;86(2):403–411. Epub 2007/ 11/28. doi: 10.1016/j.exer.2007.11.013. PubMed PMID: 18184611. doi:. [DOI] [PubMed] [Google Scholar]
- 47.Gipson IK. Distribution of mucins at the ocular surface. Exp Eye Res. 2004;78(3):379–388. PubMed PMID: 15106916 doi: 10.1016/s0014-4835(03)00204-5. [DOI] [PubMed] [Google Scholar]
- 48.Hori Y, Spurr-Michaud S, Russo CL, Argüeso P, Gipson IK. Differential regulation of membrane-associated mucins in the human ocular surface epithelium. Invest Ophthalmol Vis Sci. 2004;45(1):114–122. PubMed PMID: 14691162 doi: 10.1167/iovs.03-0903. [DOI] [PubMed] [Google Scholar]
- 49.Bansil R, Turner BS. The biology of mucus: composition, synthesis and organization. Adv Drug Deliv Rev. 2018;124:3–15. Epub 2017/ 09/29. doi: 10.1016/j.addr.2017.09.023. PubMed PMID: 28970050. doi:. [DOI] [PubMed] [Google Scholar]
- 50.Kim DY, Furuta GT, Nguyen N, Inage E, Masterson JC. Epithelial claudin proteins and their role in gastrointestinal diseases. J Pediatr Gastroenterol Nutr. 2019;68(5):611–614. PubMed PMID: 30724794; PMCID: PMC6483856 doi: 10.1097/MPG.0000000000002301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1(2):a002584. PubMed PMID: 20066090; PMCID: PMC2742087 doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia-Hernandez V, Quiros M, Nusrat A. Intestinal epithelial claudins: expression and regulation in homeostasis and inflammation. Ann N Y Acad Sci. 2017;1397(1):66–79. Epub 2017/ 05/10. doi: 10.1111/nyas.13360. PubMed PMID: 28493289; PMCID: PMC5545801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitchell LA, Overgaard CE, Ward C, Margulies SS, Koval M. Differential effects of claudin-3 and claudin-4 on alveolar epithelial barrier function. Am J Physiol Lung Cell Mol Physiol. 2011;301(1):L40–9. Epub 2011/04/22. doi: 10.1152/ajplung.00299.2010. PubMed PMID: 21515662; PMCID: PMC3129905. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hashimoto Y, Tachibana K, Krug SM, Kunisawa J, Fromm M, Kondoh M. Potential for tight junction protein-directed drug development using claudin binders and angubindin-1. Int J Mol Sci. 2019;20(16). Epub 2019/08/17. doi: 10.3390/ijms20164016. PubMed PMID: 31426497; PMCID: PMC6719960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Konjar Š, Ferreira C, Blankenhaus B, Veldhoen M. Intestinal Barrier Interactions with Specialized CD8 T Cells. Front Immunol. 2017;8:1281. Epub 2017/10/11. doi: 10.3389/fimmu.2017.01281. PubMed PMID: 29075263; PMCID: PMC5641586. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takamura S. Niches for the long-term maintenance of tissue-resident memory T cells. Front Immunol. 2018;9:1214. Epub 2018/05/31. doi: 10.3389/fimmu.2018.01214. PubMed PMID: 29904388; PMCID: PMC5990602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McDermott AJ, Huffnagle GB. The microbiome and regulation of mucosal immunity. Immunology. 2014;142(1):24–31. PubMed PMID: 24329495; PMCID: PMC3992044 doi: 10.1111/imm.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McGhee JR, Fujihashi K. Inside the mucosal immune system. PLoS Biol. 2012;10(9):e1001397. Epub 2012/09/25. doi: 10.1371/journal.pbio.1001397. PubMed PMID: 23049482; PMCID: PMC3457930. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fahrbach KM, Malykhina O, Stieh DJ, Hope TJ. Differential binding of IgG and IgA to mucus of the female reproductive tract. PLoS One. 2013;8(10):e76176. Epub 2013/10/02. doi: 10.1371/journal.pone.0076176. PubMed PMID: 24098437; PMCID: PMC3788792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saltzman WM, Radomsky ML, Whaley KJ, Cone RA. Antibody diffusion in human cervical mucus. Biophys J. 1994;66(2 Pt 1):508–515. PubMed PMID: 8161703; PMCID: 1275717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castle PE, Whaley KJ, Hoen TE, Moench TR, Cone RA. Contraceptive effect of sperm-agglutinating monoclonal antibodies in rabbits. Biol Reprod. 1997;56(1):153–159. PubMed PMID: 9002644. [DOI] [PubMed] [Google Scholar]
- 62.Zeitlin L, Cone RA, Moench TR, Whaley KJ. Preventing infectious disease with passive immunization. Microbes Infect. 2000;2(6):701–708. PubMed PMID: 10884621. [DOI] [PubMed] [Google Scholar]
- 63.Platt AM, Randolph GJ. Dendritic cell migration through the lymphatic vasculature to lymph nodes. Adv Immunol. 2013;120:51–68. PubMed PMID: 24070380. doi: 10.1016/B978-0-12-417028-5.00002-8. [DOI] [PubMed] [Google Scholar]
- 64.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5(8):617–628. PubMed PMID: 16056255 doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 65.Steele L, Mayer L, Berin MC. Mucosal immunology of tolerance and allergy in the gastrointestinal tract. Immunol Res. 2012;54(1–3):75–82. PubMed PMID: 22447352; PMCID: PMC3807575 doi: 10.1007/s12026-012-8308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cesta MF. Normal structure, function, and histology of mucosa-associated lymphoid tissue. Toxicol Pathol. 2006;34(5):599–608. PubMed PMID: 17067945 doi: 10.1080/01926230600865531. [DOI] [PubMed] [Google Scholar]
- 67.Baluk P, Adams A, Phillips K, Feng J, Hong YK, Brown MB, McDonald DM. Preferential lymphatic growth in bronchus-associated lymphoid tissue in sustained lung inflammation. Am J Pathol. 2014;184(5):1577–1592. PubMed PMID: 24631179; PMCID: 4005985 doi: 10.1016/j.ajpath.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takahashi K, Yano A, Watanabe S, Langella P, Bermúdez-Humarán LG, Inoue N. M cell-targeting strategy enhances systemic and mucosal immune responses induced by oral administration of nuclease-producing L. lactis. Appl Microbiol Biotechnol. 2018;102(24):10703–10711. Epub 2018/10/11. doi: 10.1007/s00253-018-9427-1. PubMed PMID: 30310964. [DOI] [PubMed] [Google Scholar]
- 69.Randall TD. Bronchus-associated lymphoid tissue (BALT) structure and function. Adv Immunol. 2010;107:187–241. PubMed PMID: 21034975. doi: 10.1016/B978-0-12-381300-8.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Randolph GJ, Bala S, Rahier JF, Johnson MW, Wang PL, Nalbantoglu I, Dubuquoy L, Chau A, Pariente B, Kartheuser A, et al. Lymphoid aggregates remodel lymphatic collecting vessels that serve mesenteric lymph nodes in crohn disease. Am J Pathol. 2016. 12;186:3066–3073. Epub 2016/10/13. doi: 10.1016/j.ajpath.2016.07.026. PubMed PMID: 27746181; PMCID: PMC5225286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Esterházy D, Canesso MCC, Mesin L, Muller PA, de Castro TBR, Lockhart A, ElJalby M, Faria AMC, Mucida D. Compartmentalized gut lymph node drainage dictates adaptive immune responses. Nature. 2019;569(7754):126–130. Epub 2019/ 04/15. doi: 10.1038/s41586-019-1125-3. PubMed PMID: 30988509; PMCID: PMC6587593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nelson RP, Ballow M. 26. Immunomodulation and immunotherapy: drugs, cytokines, cytokine receptors, and antibodies. J Allergy Clin Immunol. 2003;111(2 Suppl):S720–43. PubMed PMID: 12592317 doi: 10.1067/mai.2003.146. [DOI] [PubMed] [Google Scholar]
- 73.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–489. PubMed PMID: 22193102; PMCID: 3967235 doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012. 25;12(8):592–605. Epub 2012/ 07/. doi: 10.1038/nri3251. PubMed PMID: 22828912. doi:. [DOI] [PubMed] [Google Scholar]
- 75.Kammona O, Kiparissides C. Recent advances in nanocarrier-based mucosal delivery of biomolecules. J Controlled Release. 2012;161(3):781–794. PubMed PMID: WOS:000308077000009 doi: 10.1016/j.jconrel.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 76.Kammona O, Bourganis V, Karamanidou T, Kiparissides C. Recent developments in nanocarrier-aided mucosal vaccination. Nanomedicine. 2017;12(9):1057–1074. PubMed PMID: WOS:000401014000009 doi: 10.2217/nnm-2017-0015. [DOI] [PubMed] [Google Scholar]
- 77.Kim S-H, Jang Y-S. The development of mucosal vaccines for both mucosal and systemic immune induction and the roles played by adjuvants. Clin Exp Vaccine Res. 2017;6(1):15. doi: 10.7774/cevr.2017.6.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lai SK, O’Hanlon DE, Harrold S, Man ST, Wang YY, Cone R, Hanes J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc Natl Acad Sci U S A. 2007;104(5):1482–1487. Epub 2007/01/23. doi: 10.1073/pnas.0608611104. PubMed PMID: 17244708; PMCID: PMC1785284. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ensign LM, Tang BC, Wang YY, Tse TA, Hoen T, Cone R, Hanes J. Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus. Sci Transl Med. 2012;4(138):138ra79. PubMed PMID: 22700955; PMCID: PMC3817739 doi: 10.1126/scitranslmed.3003453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ensign LM, Henning A, Schneider CS, Maisel K, Wang YY, Porosoff MD, Cone R, Hanes J. Ex vivo characterization of particle transport in mucus secretions coating freshly excised mucosal tissues. Mol Pharm. 2013;10(6):2176–2182. Epub 2013/05/23. doi: 10.1021/mp400087y. PubMed PMID: 23617606; PMCID: PMC3711090. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lai SK, Wang YY, Hida K, Cone R, Hanes J. Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proc Natl Acad Sci U S A. 2010;107(2):598–603. Epub 2009/12/16. doi: 10.1073/pnas.0911748107. PubMed PMID: 20018745; PMCID: PMC2818964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maisel K, Ensign L, Reddy M, Cone R, Hanes J. Effect of surface chemistry on nanoparticle interaction with gastrointestinal mucus and distribution in the gastrointestinal tract following oral and rectal administration in the mouse. J Control Release. 2015;197:48–57. Epub 2014/ 11/04. doi: 10.1016/j.jconrel.2014.10.026. PubMed PMID: 25449804; PMCID: PMC4272879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maisel K, Reddy M, Xu Q, Chattopadhyay S, Cone R, Ensign LM, Hanes J. Nanoparticles coated with high molecular weight PEG penetrate mucus and provide uniform vaginal and colorectal distribution in vivo. Nanomedicine (Lond). 2016;11(11):1337–1343. Epub 2016/ 05/12. doi: 10.2217/nnm-2016-0047. PubMed PMID: 27171816; PMCID: PMC4897967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schneider CS, Xu Q, Boylan NJ, Chisholm J, Tang BC, Schuster BS, Henning A, Ensign LM, Lee E, Adstamongkonkul P, et al. Nanoparticles that do not adhere to mucus provide uniform and long-lasting drug delivery to airways following inhalation. Sci Adv. 2017;3(4):e1601556. Epub 2017/04/05. doi: 10.1126/sciadv.1601556. PubMed PMID: 28435870; PMCID: PMC5381952. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu Q, Ensign LM, Boylan NJ, Schön A, Gong X, Yang JC, Lamb NW, Cai S, Yu T, Freire E, et al. Impact of Surface Polyethylene Glycol (PEG) density on biodegradable nanoparticle transport in mucus ex vivo and distribution in vivo. ACS Nano. 2015;9(9):9217–9227. Epub 2015/08/31. doi: 10.1021/acsnano.5b03876. PubMed PMID: 26301576; PMCID: PMC4890729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99(Pt A):28–51. Epub 2015/ 10/09. doi: 10.1016/j.addr.2015.09.012. PubMed PMID: 26456916; PMCID: PMC4798869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khutoryanskiy VV. Beyond PEGylation: alternative surface-modification of nanoparticles with mucus-inert biomaterials. Adv Drug Deliv Rev. 2018;124:140–149. Epub 2017/ 07/20. doi: 10.1016/j.addr.2017.07.015. PubMed PMID: 28736302. [DOI] [PubMed] [Google Scholar]
- 88.Huckaby JT, Lai SK. PEGylation for enhancing nanoparticle diffusion in mucus. Adv Drug Deliv Rev. 2018;124:125–139. Epub 2017/ 09/04. doi: 10.1016/j.addr.2017.08.010. PubMed PMID: 28882703. [DOI] [PubMed] [Google Scholar]
- 89.Maher S, Mrsny RJ, Brayden DJ. Intestinal permeation enhancers for oral peptide delivery. Adv Drug Deliv Rev. 2016;106(Pt B):277–319. Epub 2016/06/16. doi: 10.1016/j.addr.2016.06.005. PubMed PMID: 27320643. [DOI] [PubMed] [Google Scholar]
- 90.Chen R, Lim JH, Jono H, Gu -X-X, Kim YS, Basbaum CB, Murphy TF, Li J-D. Nontypeable Haemophilus influenzae lipoprotein P6 induces MUC5AC mucin transcription via TLR2–TAK1-dependent p38 MAPK-AP1 and IKKβ-IκBα-NF-κB signaling pathways. Biochem Biophys Res Commun. 2004;324(3):1087–1094. doi: 10.1016/j.bbrc.2004.09.157. [DOI] [PubMed] [Google Scholar]
- 91.Hertz CJ, Wu Q, Porter EM, Zhang YJ, K-H W, Godowski PJ, Ganz T, Randell SH, Modlin RL. Activation of toll-like receptor 2 on human tracheobronchial epithelial cells induces the antimicrobial peptide human β Defensin-2. J Immunol. 2003;171(12):6820–6826. doi: 10.4049/jimmunol.171.12.6820. [DOI] [PubMed] [Google Scholar]
- 92.Monick MM, Yarovinsky TO, Powers LS, Butler NS, Carter AB, Gudmundsson G, Hunninghake GW. Respiratory syncytial virus up-regulates TLR4 and sensitizes airway epithelial cells to endotoxin. J Biol Chem. 2003;278(52):53035–53044. doi: 10.1074/jbc.m308093200. [DOI] [PubMed] [Google Scholar]
- 93.Lee H, Lee J, Hong S-H, Rahman I, Yang S-R. Inhibition of RAGE attenuates cigarette smoke-induced lung epithelial cell damage via RAGE-mediated Nrf2/DAMP signaling. Front Pharmacol. 2018:9. doi: 10.3389/fphar.2018.00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yong JM, Mantaj J, Cheng Y, Vllasaliu D. Delivery of nanoparticles across the intestinal epithelium via the transferrin transport pathway. Pharmaceutics. 2019;11(7):298. doi: 10.3390/pharmaceutics11070298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sockolosky JT, Szoka FC. The neonatal Fc receptor, FcRn, as a target for drug delivery and therapy. Adv Drug Deliv Rev. 2015;91:109–124. doi: 10.1016/j.addr.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hong S, Wilson MT, Serizawa I, Wu L, Singh N, Naidenko OV, Miura T, Haba T, Scherer DC, Wei J, et al. The natural killer T-cell ligand α-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat Med. 2001;7(9):1052–1056. doi: 10.1038/nm0901-1052. [DOI] [PubMed] [Google Scholar]
- 97.Maher S, Brayden DJ, Casettari L, Illum L. Application of permeation enhancers in oral delivery of macromolecules: an update. Pharmaceutics. 2019;11(1). Epub 2019/ 01/19. doi: 10.3390/pharmaceutics11010041. PubMed PMID: 30669434; PMCID: PMC6359609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boisguérin P, Deshayes S, Gait MJ, O’Donovan L, Godfrey C, Betts CA, Wood MJ, Lebleu B. Delivery of therapeutic oligonucleotides with cell penetrating peptides. Adv Drug Deliv Rev. 2015;87:52–67. Epub 2015/03/04. doi: 10.1016/j.addr.2015.02.008. PubMed PMID: 25747758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Komin A, Russell LM, Hristova KA, Searson PC. Peptide-based strategies for enhanced cell uptake, transcellular transport, and circulation: mechanisms and challenges. Adv Drug Deliv Rev. 2017;110-111:52–64. Epub 2016/ 06/13. doi: 10.1016/j.addr.2016.06.002. PubMed PMID: 27313077. doi:. [DOI] [PubMed] [Google Scholar]
- 100.Malhaire H, Gimel JC, Roger E, Benoît JP, Lagarce F. How to design the surface of peptide-loaded nanoparticles for efficient oral bioavailability? Adv Drug Deliv Rev. 2016;106(Pt B):320–336. Epub 2016/04/04. doi: 10.1016/j.addr.2016.03.011. PubMed PMID: 27058155. doi:. [DOI] [PubMed] [Google Scholar]
- 101.Toorisaka E, Ono H, Arimori K, Kamiya N, Goto M. Hypoglycemic effect of surfactant-coated insulin solubilized in a novel solid-in-oil-in-water (S/O/W) emulsion. Int J Pharm. 2003. PubMed PMID: 12550804;252(1–2):271–274. doi: 10.1016/S0378-5173(02)00674-9. [DOI] [PubMed] [Google Scholar]
- 102.Toorisaka E, Hashida M, Kamiya N, Ono H, Kokazu Y, Goto M. An enteric-coated dry emulsion formulation for oral insulin delivery. J Control Release. 2005;107(1):91–96. PubMed PMID: 16039746 doi: 10.1016/j.jconrel.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 103.Sha X, Yan G, Wu Y, Li J, Fang X. Effect of self-microemulsifying drug delivery systems containing Labrasol on tight junctions in Caco-2 cells. Eur J Pharm Sci. 2005;24(5):477–486. PubMed PMID: 15784337 doi: 10.1016/j.ejps.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 104.Venkatesan N, Yoshimitsu J, Ito Y, Shibata N, Takada K. Liquid filled nanoparticles as a drug delivery tool for protein therapeutics. Biomaterials. 2005;26(34):7154–7163. PubMed PMID: 15967493 doi: 10.1016/j.biomaterials.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 105.Merisko-Liversidge E, McGurk SL, Liversidge GG. Insulin nanoparticles: a novel formulation approach for poorly water soluble Zn-insulin. Pharm Res. 2004. PubMed PMID: 15497677;21(9):1545–1553. doi: 10.1023/B:PHAM.0000041446.14569.e2. [DOI] [PubMed] [Google Scholar]
- 106.Cadete A, Figueiredo L, Lopes R, Calado CC, Almeida AJ, Gonçalves LM. Development and characterization of a new plasmid delivery system based on chitosan-sodium deoxycholate nanoparticles. Eur J Pharm Sci. 2012;45(4):451–458. Epub 2011/ 10/01. doi: 10.1016/j.ejps.2011.09.018. PubMed PMID: 21986445. doi:. [DOI] [PubMed] [Google Scholar]
- 107.Welling SH, Hubálek F, Jacobsen J, Brayden DJ, Rahbek UL, Buckley ST. The role of citric acid in oral peptide and protein formulations: relationship between calcium chelation and proteolysis inhibition. Eur J Pharm Biopharm. 2014;86(3):544–551. Epub 2013/ 12/31. doi: 10.1016/j.ejpb.2013.12.017. PubMed PMID: 24384069. [DOI] [PubMed] [Google Scholar]
- 108.Zhu X, Shan W, Zhang P, Jin Y, Guan S, Fan T, Yang Y, Zhou Z, Huang Y. Penetratin derivative-based nanocomplexes for enhanced intestinal insulin delivery. Mol Pharm. 2014;11(1):317–328. Epub 2013/ 11/25. doi: 10.1021/mp400493b. PubMed PMID: 24255985. [DOI] [PubMed] [Google Scholar]
- 109.Jin Y, Song Y, Zhu X, Zhou D, Chen C, Zhang Z, Huang Y. Goblet cell-targeting nanoparticles for oral insulin delivery and the influence of mucus on insulin transport. Biomaterials. 2012;33(5):1573–1582. Epub 2011/ 11/16. doi: 10.1016/j.biomaterials.2011.10.075. PubMed PMID: 22093292. doi:. [DOI] [PubMed] [Google Scholar]
- 110.Porsio B, Craparo EF, Mauro N, Giammona G, Cavallaro G. Mucus and cell-penetrating nanoparticles embedded in nano-into-micro formulations for pulmonary delivery of ivacaftor in patients with cystic fibrosis. ACS Appl Mater Interfaces. 2018. 10(1):165–181. Epub 2017/ 12/26. doi: 10.1021/acsami.7b14992. PubMed PMID: 29235345. [DOI] [PubMed] [Google Scholar]
- 111.Tan X, Zhang Y, Wang Q, Ren T, Gou J, Guo W, Yin T, He H, Zhang X, Tang X. Cell-penetrating peptide together with PEG-modified mesostructured silica nanoparticles promotes mucous permeation and oral delivery of therapeutic proteins and peptides. Biomater Sci. 2019;7(7):2934–2950. PubMed PMID: 31094367 doi: 10.1039/c9bm00274j. [DOI] [PubMed] [Google Scholar]
- 112.Martínez-Gómez JM, Johansen P, Erdmann I, Senti G, Crameri R, Kündig TM. Intralymphatic injections as a new administration route for allergen-specific immunotherapy. Int Arch Allergy Immunol. 2009;150(1):59–65. Epub 2009/04/02. doi: 10.1159/000210381. PubMed PMID: 19339803. [DOI] [PubMed] [Google Scholar]
- 113.Senti G, Prinz Vavricka BM, Erdmann I, Diaz MI, Markus R, McCormack SJ, Simard JJ, Wüthrich B, Crameri R, Graf N, et al. Intralymphatic allergen administration renders specific immunotherapy faster and safer: a randomized controlled trial. Proc Natl Acad Sci U S A. 2008;105(46):17908–17912. Epub 2008/ 11/10. doi: 10.1073/pnas.0803725105. PubMed PMID: 19001265; PMCID: PMC2582048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Smith KA, Meisenburg BL, Tam VL, Pagarigan RR, Wong R, Joea DK, Lantzy L, Carrillo MA, Gross TM, Malyankar UM, et al. Lymph node-targeted immunotherapy mediates potent immunity resulting in regression of isolated or metastatic human papillomavirus-transformed tumors. Clin Cancer Res. 2009;15(19):6167–6176. Epub 2009/09/29. doi: 10.1158/1078-0432.CCR-09-0645. PubMed PMID: 19789304; PMCID: PMC2756704. doi: 10.1158/1078-0432.CCR-09-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, Irvine DJ. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507(7493):519–522. Epub 2014/ 02/16. doi: 10.1038/nature12978. PubMed PMID: 24531764; PMCID: PMC4069155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.von Beust BR, Johansen P, Smith KA, Bot A, Storni T, Kündig TM. Improving the therapeutic index of CpG oligodeoxynucleotides by intralymphatic administration. Eur J Immunol. 2005;35(6):1869–1876. PubMed PMID: 15909311 doi: 10.1002/eji.200526124. [DOI] [PubMed] [Google Scholar]
- 117.Maloy KJ, Erdmann I, Basch V, Sierro S, Kramps TA, Zinkernagel RM, Oehen S, Kündig TM. Intralymphatic immunization enhances DNA vaccination. Proc Natl Acad Sci U S A. 2001;98(6):3299–3303. Epub 2001/02/27. doi: 10.1073/pnas.051630798. PubMed PMID: 11248073; PMCID: PMC30648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Johansen P, Häffner AC, Koch F, Zepter K, Erdmann I, Maloy K, Simard JJ, Storni T, Senti G, Bot A, et al. Direct intralymphatic injection of peptide vaccines enhances immunogenicity. Eur J Immunol. 2005;35(2):568–574. PubMed PMID: 15682446 doi: 10.1002/eji.200425599. [DOI] [PubMed] [Google Scholar]
- 119.Komori J, Boone L, DeWard A, Hoppo T, Lagasse E. The mouse lymph node as an ectopic transplantation site for multiple tissues. Nat Biotechnol. 2012;30(10):976–983. Epub 2012/09/23. doi: 10.1038/nbt.2379. PubMed PMID: 23000933; PMCID: PMC3469750. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maisel K, Sasso MS, Potin L, Swartz MA. Exploiting lymphatic vessels for immunomodulation: rationale, opportunities, and challenges. Adv Drug Deliv Rev. 2017;114:43–59. PubMed PMID: 28694027; PMCID: 6026542. doi: 10.1016/j.addr.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J Control Release. 2006;112(1):26–34. PubMed PMID: 16529839 doi: 10.1016/j.jconrel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 122.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38(5):1404–1413. PubMed PMID: 18389478 doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 123.Varypataki EM, Silva AL, Barnier-Quer C, Collin N, Ossendorp F, Jiskoot W. Synthetic long peptide-based vaccine formulations for induction of cell mediated immunity: A comparative study of cationic liposomes and PLGA nanoparticles. J Control Release. 2016;226:98–106. Epub 2016/ 02/11. doi: 10.1016/j.jconrel.2016.02.018. PubMed PMID: 26876760. doi:. [DOI] [PubMed] [Google Scholar]
- 124.Kobayashi H, Kawamoto S, Bernardo M, Brechbiel MW, Knopp MV, Choyke PL. Delivery of gadolinium-labeled nanoparticles to the sentinel lymph node: comparison of the sentinel node visualization and estimations of intra-nodal gadolinium concentration by the magnetic resonance imaging. J Control Release. 2006;111(3):343–351. Epub 2006/ 02/21. doi: 10.1016/j.jconrel.2005.12.019. PubMed PMID: 16490277. [DOI] [PubMed] [Google Scholar]
- 125.Rao DA, Forrest ML, Alani AW, Kwon GS, Robinson JR. Biodegradable PLGA based nanoparticles for sustained regional lymphatic drug delivery. J Pharm Sci. 2010;99(4):2018–2031. PubMed PMID: 19902520; PMCID: PMC5178132 doi: 10.1002/jps.21970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zeng Q, Jiang H, Wang T, Zhang Z, Gong T, Sun X. Cationic micelle delivery of Trp2 peptide for efficient lymphatic draining and enhanced cytotoxic T-lymphocyte responses. J Control Release. 2015;200:1–12. Epub 2014/ 12/23. doi: 10.1016/j.jconrel.2014.12.024. PubMed PMID: 25540903. [DOI] [PubMed] [Google Scholar]
- 127.De Koker S, Cui J, Vanparijs N, Albertazzi L, Grooten J, Caruso F, De Geest BG. Engineering Polymer hydrogel nanoparticles for lymph node-targeted delivery. Angew Chem Int Ed Engl. 2016;55(4):1334–1339. Epub 2015/ 12/15. doi: 10.1002/anie.201508626. PubMed PMID: 26666207. [DOI] [PubMed] [Google Scholar]
- 128.Mao Y, Feng S, Li S, Zhao Q, Di D, Liu Y, Wang S. Chylomicron-pretended nano-bio self-assembling vehicle to promote lymphatic transport and GALTs target of oral drugs. Biomaterials. 2019;188:173–186. Epub 2018/ 10/18. doi: 10.1016/j.biomaterials.2018.10.012. PubMed PMID: 30359884. [DOI] [PubMed] [Google Scholar]
- 129.Triacca V, Guc E, Kilarski WW, Pisano M, Swartz MA. Transcellular pathways in lymphatic endothelial cells regulate changes in solute transport by fluid stress. Circ Res. 2017;120(9):1440–1452. PubMed PMID: 28130294 doi: 10.1161/CIRCRESAHA.116.309828. [DOI] [PubMed] [Google Scholar]
- 130.Jackson RJ, Fujihashi K, Xu-Amano J, Kiyono H, Elson CO, McGhee JR. Optimizing oral vaccines: induction of systemic and mucosal B-cell and antibody responses to tetanus toxoid by use of cholera toxin as an adjuvant. Infect Immun. 1993;61(10):4272–4279. PubMed PMID: 8406816; PMCID: PMC281154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Garinot M, Fiévez V, Pourcelle V, Stoffelbach F, Des Rieux A, Plapied L, Theate I, Freichels H, Jérôme C, Marchand-Brynaert J, et al. PEGylated PLGA-based nanoparticles targeting M cells for oral vaccination. J Control Release. 2007;120(3):195–204. Epub 2007/05/22. doi: 10.1016/j.jconrel.2007.04.021. PubMed PMID: 17586081. [DOI] [PubMed] [Google Scholar]
- 132.Lee DY, Nurunnabi M, Kang SH, Nafiujjaman M, Huh KM, Lee YK, Kim YC. Oral gavage delivery of PR8 Antigen with β-glucan-conjugated GRGDS carrier to enhance M-cell targeting ability and induce immunity. Biomacromolecules. 2017;18(4):1172–1179. Epub 2017/03/21. doi: 10.1021/acs.biomac.6b01855. PubMed PMID: 28278374. [DOI] [PubMed] [Google Scholar]
- 133.Fievez V, Plapied L, Des Rieux A, Pourcelle V, Freichels H, Wascotte V, Vanderhaeghen ML, Jerôme C, Vanderplasschen A, Marchand-Brynaert J, et al. Targeting nanoparticles to M cells with non-peptidic ligands for oral vaccination. Eur J Pharm Biopharm. 2009;73(1):16–24. Epub 2009/05/04. doi: 10.1016/j.ejpb.2009.04.009. PubMed PMID: 19409989. [DOI] [PubMed] [Google Scholar]
- 134.Nochi T, Yuki Y, Matsumura A, Mejima M, Terahara K, Kim DY, Fukuyama S, Iwatsuki-Horimoto K, Kawaoka Y, Kohda T, et al. A novel M cell-specific carbohydrate-targeted mucosal vaccine effectively induces antigen-specific immune responses. J Exp Med. 2007;204(12):2789–2796. Epub 2007/11/05. doi: 10.1084/jem.20070607. PubMed PMID: 17984304; PMCID: PMC2118513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shima H, Watanabe T, Fukuda S, Fukuoka S, Ohara O, Ohno H. A novel mucosal vaccine targeting Peyer’s patch M cells induces protective antigen-specific IgA responses. Int Immunol. 2014;26(11):619–625. Epub 2014/06/07. doi: 10.1093/intimm/dxu061. PubMed PMID: 24908678. [DOI] [PubMed] [Google Scholar]
- 136.Mattila JP, Mirandola L, Chiriva-Internati M. Development of a M cell-targeted microparticulate platform, BSK02™, for oral immunization against the ovarian cancer antigen, sperm protein 17. J Biomed Mater Res B Appl Biomater. 2019;107(1):29–36. Epub 2018/ 03/04. doi: 10.1002/jbm.b.34092. PubMed PMID: 29504239. [DOI] [PubMed] [Google Scholar]
- 137.Yoo MK, Kang SK, Choi JH, Park IK, Na HS, Lee HC, Kim EB, Lee NK, Nah JW, Choi YJ, et al. Targeted delivery of chitosan nanoparticles to Peyer’s patch using M cell-homing peptide selected by phage display technique. Biomaterials. 2010;31(30):7738–7747. Epub 2010/07/24. doi: 10.1016/j.biomaterials.2010.06.059. PubMed PMID: 20656343. doi:. [DOI] [PubMed] [Google Scholar]
- 138.Singh B, Maharjan S, Jiang T, Kang SK, Choi YJ, Cho CS. Combinatorial approach of antigen delivery using M cell-homing peptide and mucoadhesive vehicle to enhance the efficacy of oral vaccine. Mol Pharm. 2015;12(11):3816–3828. Epub 2015/10/02. doi: 10.1021/acs.molpharmaceut.5b00265. PubMed PMID: 26394158. [DOI] [PubMed] [Google Scholar]
- 139.Du L, Yu Z, Pang F, Xu X, Mao A, Yuan W, He K, Li B. Targeted Delivery of GP5 Antigen of PRRSV to M cells enhances the antigen-specific systemic and mucosal immune responses. Front Cell Infect Microbiol. 2018;8:7. Epub 2018/01/25. doi: 10.3389/fcimb.2018.00007. PubMed PMID: 29423381; PMCID: PMC5788884. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Misstear K, McNeela EA, Murphy AG, Geoghegan JA, O’Keeffe KM, Fox J, Chan K, Heuking S, Collin N, Foster TJ, et al. Targeted nasal vaccination provides antibody-independent protection against Staphylococcus aureus. J Infect Dis. 2014;209(9):1479–1484. Epub 2013/ 11/22. doi: 10.1093/infdis/jit636. PubMed PMID: 24273045; PMCID: PMC4813749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu L, Zhang W, Song Y, Wang W, Zhang Y, Wang T, Li K, Pan Q, Qi X, Gao Y, et al. Recombinant Lactococcus lactis co-expressing OmpH of an M cell-targeting ligand and IBDV-VP2 protein provide immunological protection in chickens. Vaccine. 2018;36(5):729–735. Epub 2017/ 12/27. doi: 10.1016/j.vaccine.2017.12.027. PubMed PMID: 29289381. [DOI] [PubMed] [Google Scholar]
- 142.Rochereau N, Pavot V, Verrier B, Ensinas A, Genin C, Corthésy B, Paul S. Secretory IgA as a vaccine carrier for delivery of HIV antigen to M cells. Eur J Immunol. 2015;45(3):773–779. Epub 2015/01/14. doi: 10.1002/eji.201444816. PubMed PMID: 25412898. [DOI] [PubMed] [Google Scholar]
- 143.Rochereau N, Pavot V, Verrier B, Jospin F, Ensinas A, Genin C, Corthésy B, Paul S. Delivery of antigen to nasal-associated lymphoid tissue microfold cells through secretory IgA targeting local dendritic cells confers protective immunity. J Allergy Clin Immunol. 2016;137(1):214–22.e2. Epub 2015/ 09/26. doi: 10.1016/j.jaci.2015.07.042. PubMed PMID: 26414879. [DOI] [PubMed] [Google Scholar]
- 144.Hase K, Kawano K, Nochi T, Pontes GS, Fukuda S, Ebisawa M, Kadokura K, Tobe T, Fujimura Y, Kawano S, et al. Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature. 2009;462(7270):226–230. PubMed PMID: 19907495 doi: 10.1038/nature08529. [DOI] [PubMed] [Google Scholar]
- 145.Maharjan S, Singh B, Jiang T, Yoon SY, Li HS, Kim G, Gu MJ, Kim SJ, Park OJ, Han SH, et al. Systemic administration of RANKL overcomes the bottleneck of oral vaccine delivery through microfold cells in ileum. Biomaterials. 2016;84:286–300. Epub 2016/01/23 PubMed PMID: 26851393. doi: 10.1016/j.biomaterials.2016.01.043. [DOI] [PubMed] [Google Scholar]


