ABSTRACT
Tendons connect muscles to bones to transfer the forces necessary for movement. Cell-cell junction proteins, cadherins and connexins, may play a role in tendon development and injury. In this review, we begin by highlighting current understanding of how cell-cell junctions may regulate embryonic tendon development and differentiation. We then examine cell-cell junctions in postnatal tendon, before summarizing the role of cadherins and connexins in adult tendons. More information exists regarding the role of cell-cell junctions in the formation and homeostasis of other musculoskeletal tissues, namely cartilage and bone. Therefore, to inform future tendon studies, we include a brief survey of cadherins and connexins in chondrogenesis and osteogenesis, and summarize how cell-cell junctions are involved in some musculoskeletal tissue pathologies. An enhanced understanding of how cell-cell junctions participate in tendon development, maintenance, and disease will benefit future regenerative strategies.
KEYWORDS: Tendon, cadherin, connexin, development, tissue engineering
Introduction
Tendons are musculoskeletal tissues that transfer mechanical forces from muscles to bones and are vital for skeletal movement. A major clinical challenge is the limited healing ability of tendon. If ruptured, tendon healing is characterized by the formation of scar tissue1,2 and inferior mechanical properties, compared to uninjured tissues.3 Even with surgical repair, re-rupture rates range from 3.6% to 94% depending on the tendon, size of the tear, age, and other factors.4–6 In addition, tendon injury rates are climbing, with a 10-fold increase in the incidence of Achilles tendon ruptures from 1979 to 2011.7 The limitations of existing treatment options emphasize the need for tissue engineering and regenerative strategies to improve tendon healing and repair.
Regenerative tissue engineering approaches are challenged by a limited understanding of how tendon cells respond to mechanical and biochemical signals to enable initial tenogenic differentiation during embryonic development, and the formation and maintenance of tendon’s highly organized extracellular matrix (ECM).8 The ECM in tendon is composed primarily of collagen type (Col) I that accounts for nearly 65–80% of the dry mass.9 The collagen plays a critical role in transmitting tensile forces and is hierarchically arranged, with collagen fibrils (nm scale) bundled into collagen fibers and fascicles (mm scale).9 In the adult tendon, tendon cells reside within the fascicles and interfascicular membranes, and these resident cells are thought to play a role in maintaining the collagen matrix10 and the tendon length by cell-mediated contraction.11 Though mature tendon is typically considered to have a relatively sparse distribution of cells, tendon cells are known to possess direct cell-to-cell connections.12
Developing embryonic and postnatal tendons are characterized by a dense cell network13,14 with adjacent cells in direct contact.15 These direct cell-to-cell adhesions in tendon cells occur through cadherins and gap junctions. For tendon, cadherins and gap junctions may be particularly relevant, as they are known mechanotransducers,16 and mechanical stimuli impact many aspects of tendon formation, injury, and homeostasis.17–28 This review examines the cadherins and gap junctions identified in developing and mature tendon, and discusses what role these cell-cell junction proteins might be playing in the formation, maintenance, and mechanoregulation of tendon. Like tendon, cartilage and bone are musculoskeletal tissues of mesenchymal lineage, but the cell-cell junctions in these tissues have been more extensively studied. Therefore, to inform future tendon studies, we also include a brief review of cadherins and gap junctions in chondrogenesis and osteogenesis, and in some musculoskeletal tissue pathologies. Understanding the specific role of cell-cell junctions in tendon cells and their impacts on cellular responses may ultimately advance strategies to prevent tendon injury and improve regenerative medicine and tissue engineering.
Cell-cell junctions in developing tendon
Embryonic tendon arises from a condensation of mesenchymal cells that are tightly packed and are in direct cell-to-cell contact.15 These embryonic tendon cells are distinguishable from other musculoskeletal cells of mesenchymal lineage (e.g., cartilage and bone cells) by their expression of scleraxis,29 mohawk,30 and tenomodulin.31 The transcription factor scleraxis is a regulator and early marker of tenogenesis,29,32–34 as is the transcription factor mohawk.30 Scleraxis regulates tenomodulin, a late stage tendon marker.31,35 Tendon cells constitute a significant portion of the embryonic tendon structure.36 The amount of DNA (as a measure of cell density) present in the calcaneus tendons of embryonic chickens from Hamburger-Hamilton stages (HH) 28 to 43 (e.g., embryonic day (E) 5.5 to 18) accounted for between 3% and 9% of the tendon dry mass, whereas hydroxyproline (a measure of collagen) accounted for less than 5% of the dry mass at HH43.36
The organization of embryonic tendon cells appears unique, with highly ordered cells tightly packed and aligned to the long-axis of the tendon, as observed in E13 chick metatarsal tendon, and E15.5 mouse tail tendon.37 A different study found that cells in chick calcaneal tendon from HH34 to HH37 possessed a highly aligned and well-organized actin cytoskeleton network, with actin filaments that appeared continuous between cells (Figure 1a).13 When the actin cytoskeleton of the embryonic tendon cells was disrupted with blebbistatin, a small molecule inhibitor of non-muscle myosin contraction, the elastic modulus of the tendon decreased significantly, suggesting that the cells contribute to the embryonic tendon mechanical properties.13 Overall, embryonic tendon is highly cellular, with a well-organized and apparently interconnected network of cells. While inherent differences between avian and mammalian tendon development may exist, embryonic tendons across species as diverse as chick, mouse, and horse appear to share the characteristics of high cellularity, alignment, and direct cell-cell contact.37,38 Given this high cellularity and cell alignment, cell-cell junction proteins are likely candidates as regulators of embryonic tendon cell organization. Therefore, we discuss the cell-cell junction proteins (mainly cadherins and connexins) that have been identified in developing tendons and explore their potential roles in tendon development.
Figure 1.
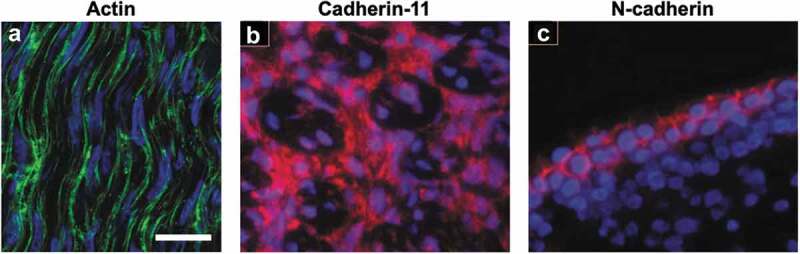
Embryonic tendons possess an organized actin cytoskeleton network, as well as cadherin-11 and N-cadherin cell-cell junctions. (a) The high cell density and an organized actin cytoskeleton network are visible in the midsubstance of E11 chick calcaneal tendons. Actin filaments (green) in embryonic tendon appear to form a continuous network between adjacent cells. (b) Cadherin-11 (red) and (c) N-cadherin (red) are present in E13 chick metatarsal tendons, but N-cadherin is localized to the exterior of the midsubstance of the tendon, while cadherin-11 appears in the interior of the midsubstance. Cell nuclei are labeled with blue. Figure 1A used with publisher’s permission from Schiele et al. 2015.13 Scale bar = 10 μm. Figure 1B and 1C adapted with publisher’s permission from Richardson et al. 2007.37
Cadherins in embryonic tendon
To identify possible cell-cell junction proteins, a gene microarray analysis of E13 chick metatarsal tendon was conducted.37 Gene expression for cadherin-11, N-cadherin, R-cadherin, and connexin-32 and −43 was identified, but expression levels were highest for cadherin-11.37 The presence of cadherin-11 in embryonic tendon was also found using RT-PCR and immunofluorescence staining. Specifically, cadherin-11 was present in the fascicles and in the interfascicular space (Figure 1b), as well as in the cell-cell contacts made by the embryonic tendon cells that had been isolated and cultured in vitro.37 N-cadherin was also found by immunofluorescence but appeared to be produced mainly by tendon cells on the tendon surface and not in the mid-substance of the tendon (Figure 1c).37 When cadherin-11 expression was knocked down using siRNA, the embryonic tendon cells appeared to have disrupted cell-cell contacts and collagen fibril organization.37 This study suggests that cadherin-11 may regulate tendon cell condensation and may even play a role in alignment of collagen fibrils. Cadherin-11 may also contribute to limb patterning, since cadherin-11 expression was shown by in situ hybridization to be restricted to the distal, but not proximal, regions of developing mouse limbs at E9.5 and E13.5.39 This same study demonstrated that mouse embryonic fibroblasts transfected with cadherin-11 cDNA adhered to other cadherin-11-transfected cells, but did not co-aggregate with cells transfected to express N-, E-, P-, or R-cadherin.39 The proximal or distal restriction of cadherin expression and the timing of expression of multiple cadherins relative to cell condensation may ensure correct tissue patterning during development.
N-cadherin is a regulator of cell adhesion and connective tissue morphogenesis that has also been explored in patterning of the musculoskeletal tissues in the limbs. N-cadherin-null mice do not survive in utero unless rescued with transgenic expression of a cardiac cadherin.40 While non-rescued N-cadherin-null mice survive to form forelimb buds at E9.5, they are not viable by E11-E12 due to cardiac malformations, and further limb development cannot be assessed.40 To address this limitation, a follow-up study cultured forelimbs from rescued E10.5 N-cadherin-null mice ex vivo for 7 days (d), and found that the limbs developed and did not differ significantly from wild-type forelimbs in overall morphology, size, and cellular condensation of chondrogenic precursors.41 Although N-cadherin expression was absent in the mutant limbs, expression of cadherin-11 was not affected, indicating that cadherin-11 and other cadherins may drive limb development in the absence of N-cadherin.41
The cardiac, neural, and connective tissue malformations in N-cadherin-null mice are likely due to the role of N-cadherin in cell adhesion. Cell adhesion is necessary for patterning in early development and is controlled upstream of the cadherins by T-box transcription factors.42 In mouse E16.5 forelimbs with deletion of the T-box transcription factor (Tbx)5, and E15.5 hindlimbs with deletion of Tbx4, muscle patterning was disrupted, and ectopic splitting of muscles of the zeugopod, the region of the developing limb encompassing the forearm but excluding the digits, was observed.42 In the forearms of E15.5 Scleraxis-Green Fluorescent Protein (Scx-GFP)-expressing mice, Tbx5 deletion led to changes in tendon morphology. Specifically, there were fewer tendon fibers present, fibers were thinner than normal, and some fibers had fused with each other.42 Despite the changes observed in the tendons, the muscles still made myotendinous attachments, and tendons developed entheses (tendon-to-bone attachments) on the forming skeleton, indicating that crosstalk between the developing muscles, bones, and tendons was still intact.42 The same study also found that N-cadherin expression was significantly lower in Tbx5 null mice,42 as was expression of β-catenin, a protein that couples with cadherins to facilitate cytoplasmic anchoring to the actin cytoskeleton and participates in both cell adhesion and signaling via the wingless/integrated (Wnt)/β-catenin pathway.43 Although N-cadherin and β-catenin expression was reduced, expression of cadherin-11 and Tcf4, a downstream Wnt target, were unaffected, suggesting that Tbx5 deletion specifically affects N-cadherin and β-catenin, but does not globally disrupt cadherins or Wnt signaling.42 These findings suggest that N-cadherin and regulation by Tbx5 are necessary for early embryonic tendon development and patterning, but more research is needed to understand how N-cadherin is participating in early tendon formation. In a different study, differentiation of dermal fibroblasts toward a myofibroblast phenotype was characterized by a transition from N-cadherin to cadherin-11 expression.44 This process may occur when stronger bonds are needed between cells, as cadherin-11 bonds were found to have twice the strength as N-cadherin bonds.45 Therefore, it is possible that tenogenically differentiating embryonic tendon cells express specific cadherins that have different bond strengths during specific developmental stages, though this will need further study. Taken together, both N-cadherin and cadherin-11 are found in embryonic tendons and appear to be involved in cell condensation and early tissue formation and patterning. A deeper understanding of how these cadherins contribute to tenogenic differentiation and ultimately functional tendon formation will be immensely valuable.
Other cadherins may also be regulating tendon development. The protocadherin Fat-1 is expressed in tissues of mesenchymal origin during early embryonic development.44 Fat-1 controls cell proliferation during early musculoskeletal tissue development and cell condensation,46 and has been shown to regulate both transforming growth factor beta (TGFβ)47 and Wnt/β-catenin signaling.48 Genetic ablation and in situ hybridization in E12.5 mice showed Fat-1 is required in mesenchyme-derived connective tissue formation.46 Conditional Fat-1 knockouts displayed abnormal morphology of the cutaneous maximus muscle and innervating motor neurons.46 Muscle formation is needed for subsequent tendon development,49 but Fat-1 expression persisted in Pax3 cre/cre knockout mice, which lack skeletal muscle cells, suggesting that Fat-1 expression may be driven by mesenchymal or connective tissue cells, rather than muscle cells.46 Effects of Fat-1 disruption were further shown to vary by cell and tissue type: extensive muscle-shape patterning defects resulting from Fat-1 ablation were seen in mouse mesenchymal cells, but not motor neuron cells.46 In situ hybridization showed overlapping Fat-1 and scleraxis expression, and Fat-1 was required for the developing connective tissue precursors to interact with the cutaneous maximus muscle progenitors that were concurrently forming.46 As tendon progenitors arise from embryonic mesenchyme,50 correct patterning of the mesenchyme and the contribution of Fat-1 to this patterning may be important for tendon development. Furthermore, the reciprocal signals exchanged between different progenitor populations (muscle, tendon, cartilage, bone) during limb patterning are essential for the correct development of the limb and associated connective tissues. Several distinct subtypes of connective tissue are derived from progenitors influenced by Fat-1 expression, including tendons, making Fat-1 a useful target for further research in tendon development.
Fat-1 expression may also contribute to proximodistal orientation during limb patterning. In E9.5-E13.5 mouse limb buds, proximal restriction of Fat-1, coupled with distal expression of cadherin-11, may ensure correct patterning by regionally inhibiting Wnt signaling.39 In HH16-HH24 chick forelimb buds, whole mount in situ hybridization showed that Fat-1 expression was restricted to tendon primordia of proximal regions, but expression extended to the proximal and intermediate regions of the forelimb at HH27, and was detectable in mesenchymal cells between the developing digits at HH28.51 In contrast, hindlimbs had extended stretches of expression into the interdigit mesenchyme at HH27. Fat-1 was expressed diffusely in regions of developing tendon cells, but only proximally in developing chondrocytes, suggesting that expression of Fat-1 may be grouping specific cells together to form and maintain digit patterning and distinct musculoskeletal connective tissues.51
A few potential regulators of cadherins during differentiation have emerged. Decorin, a proteoglycan involved in cell signaling,52 collagen fibrillogenesis,53 and aging of tendon,54 may be important for the cell aggregation phase of musculoskeletal tissue development, before various cell precursors have differentiated into tendon, muscle, cartilage, or bone. Micromass cultures of limb mesodermal cell precursors from HH25 chicks showed significant upregulation of Sox9 and cadherin-11, but no changes in scleraxis expression when cells were treated with human decorin for 1 d, and gene expression was analyzed at 2 d.55 Cadherin-4, −7, and −13, and N-cadherin, were unaffected. Interestingly, there was no upregulation of Sox9, cadherin-11, and scleraxis when decorin was added after 4 d of culture and gene expression analyzed at 5 d.55 While Sox9 is considered a chondrogenic marker, it promotes the common cell aggregation step necessary for the cartilage/tendon/ligament specification that takes place in early embryonic development.56 In this case, the upregulation of Sox9 may be due to decorin influencing the cell aggregation and condensation step that precedes differentiation.55 The same study showed via in situ hybridization of whole limb mounts from HH20-31 chicks that the expression patterns for decorin, cadherin-11, and TGFβ2 overlapped at HH30 in the joint and tendon blastemas of the embryonic digits.55 Addition of TGFβ2 to HH22 limb bud explants upregulated decorin but increased decorin expression was lost when TGFβ2 signaling was blocked using the Smad2 inhibitor SB431542.55 Finally, in decorin-silenced mesenchymal cells, expression of Sox9 was significantly decreased, and expression of scleraxis was significantly increased, compared to control cells with functional decorin, though cadherin-11 expression was not examined.55 Collectively, these results highlight overlapping expression of cadherin-11, TGFβ2, and decorin during differentiation of musculoskeletal tissues. The increase in decorin expression seen with the addition of TGFβ2 suggests that decorin and cadherin-11 are downstream targets of TGFβ2 signaling. Lack of decorin may lead to tendon pathologies via cadherin reduction. Decreased decorin expression has been proposed as a cause of abnormal collagen formation and tendon hypoplasia in mohawk-null mice.30 The observed disruptions of the collagen matrix and gross morphology may be due to disruption of cadherin-11-mediated cell aggregation driven by the decorin deficiency. More research is needed to understand how decorin responds to TGFβ2 signaling and mediates expression of cadherin-11 and, potentially, other cadherins in developing tendons.
The cytoplasmic anchor of the cadherins, β-catenin, may also be involved in tendon development. β-catenin facilitates the formation of cell-cell junctions by modulating actin cytoskeleton-cadherin coupling through association with α-catenin and vinculin.57,58 β-catenin was found to be necessary for this process, as the α-catenin/vinculin complex is only capable of binding the actin cytoskeleton directly, and thus requires β-catenin to form linkages with cellular cadherins and the actin cytoskeleton.57 β-catenin regulates cell growth through the Wnt pathway, and is constitutively expressed in the cytosol, where it is regulated via Axin, casein kinase (Ck)1, and glycogen synthase kinase (GSK)3β.59,60 In E13 chick metatarsal tendon, relatively high levels of β-catenin gene expression were identified by microarray analysis, along with cadherin-11.37 β-catenin upregulation was observed in tendon cells adjacent to a 14-gauge needle puncture injury in the Achilles tendon of 6-week old rats.61 Interestingly, tendon cells had decreased gene expression for scleraxis, mohawk, and tenomodulin when Wnt/β-catenin signaling was chemically activated during in vitro cell culture.61 The same study found that activation of Wnt/β-catenin signaling decreased cellular levels of Smad2 and 3, and that Wnt/β-catenin activation suppressed tenogenic genes.61 However, in a different study, equine bone marrow-derived mesenchymal stem cells (MSCs) encapsulated in collagen gels in vitro upregulated tenomodulin and decorin gene expression with β-catenin activation.62 Taken together, β-catenin interacts with cadherins and regulates cell behavior, and maybe both pro- and anti-tenogenic, depending on specific culture conditions or cell phenotypes. Future studies are needed to investigate how β-catenin may be regulating cadherins in tendon and how β-catenin impacts tenogenic differentiation and tendon formation.
Connexins in embryonic tendon
In addition to cadherins, gap junction proteins (connexins) are expressed in embryonic tendon.37 The involvement of connexin-43 and another gap junction protein, connexin-32, in the differentiation of multiple stem cell lineages, and particularly in bone development and homeostasis, has been highlighted in recent reviews.63,64 Connexin-32 and −43 have been found in murine,12 rat,65 avian,37,66 equine,38 and ovine tendons.67 However, there is limited information on the role of connexins in tendon development. While both connexin-32 and −43 are expressed in tendon, their localization is different. In adult rat digital flexor tendons, connexin-43 has been found in the tips of cell processes and between cell bodies, while connexin-32 appears to be confined between the bodies of adjacent cells.12 Though the functional significance of this pattern is not fully understood, it is possible that connexin-32 and connexin-43 enable a differential response to mechanical loading.66 As embryonic tendon responds to mechanical stimuli,26 gap junction-mediated mechanotransduction may be involved, but the effects of blocking this communication during embryonic development have not been explored.
To further elucidate the role of gap junctions in developing tendons, connexin-32 and −43 expression during development has been examined by several studies. Using immunofluorescence staining, one study identified connexin-32 and −43 in the superficial digital flexor tendon (SDFT) and common digital extensor tendon (CDET) of fetal and mature horses.38 Expression of connexin-32 and −43 peaked in developing tendons, and decreased in the first 6 months of life. Fetal tendons had significantly larger areas of labeled connexin-32 and −43, compared to tendons from young (1–6 months), young adult (2–7 y), and old (18–33 y) horses (Figure 2). There were no differences in expression of either connexin between the SDFT and CDET at any age.38 While both connexins were present throughout development, their patterns of expression and localization differed between ages, with more widely distributed expression and overall larger expression areas observed in fetal tendons compared to all older stages.38
Figure 2.
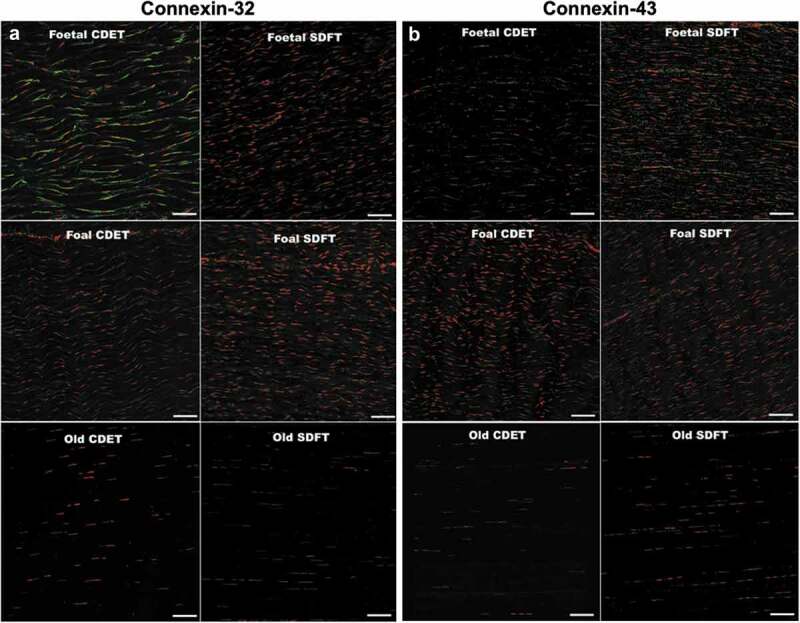
Longitudinal cryosections from equine CDET and SDFT tendons labeled for connexin-32 and connexin-43. (a) Connexin-32 (green) labeled in tendons from fetal, foal, and mature horses. (b) Connexin-43 (green) labeled in tendons from fetal, foal, and mature horses. Cell nuclei are labeled with red. Scale bar = 80 μm. Figure used with publisher’s permission from Stanley et al. 2007.38.
Another study examined the expression of connexin-32 and −43 during ovine tendon development. Ovine calcaneal tendons at two different fetal stages (mid-gestation and late gestation, when the tendons were 14 cm and 40 cm long, respectively) had a more developed endotenon, as well as differences in cell shape.67 Cells from mid-fetal tendons had a rounded morphology, which became fusiform and regionally aligned in late-gestation cells. Adult cells were elongated and displayed a parallel arrangement along the longitudinal axis of the tendon.67 Only cells from mid-fetal stage tendons expressed regenerative growth factors and pluripotent stem cell markers.67 Cellularity was higher in mid-fetal tendons compared to both late-fetal and adult tendons, with increased cell proliferation compared to the two later stages. Connexin-32 and −43 were produced at similar levels during both fetal stages but decreased significantly in adult tendons. Gene expression revealed significant decreases in Col I, Col III, scleraxis, tenomodulin, thrombospondin 4, osteocalcin, and TGFβ1 between the mid-fetal tendon cells and both the late fetal and adult tendon cells.67 Taken together, these results highlight a progressive and rapid decrease of tendon marker gene expression throughout development, and show that this decrease begins in utero in an ovine model. The decreased production of connexins is noteworthy, since gap junction communication may be needed during development to coordinate the response to mechanical load and other stimuli driving tendon formation. The decrease in connexins with age may reflect a reduced ability to regenerate adult tendon, as embryonic tendon is known to heal scarlessly.1 Embryonic tendon expresses both connexin-32 and −43, and this expression rapidly decreases following birth. However, it is unknown if changes in connexin expression are consistent across species or tendons (e.g., calcaneus vs digital flexor). It is also unknown how these gap junctions may be regulating tenogenic differentiation.
Connexin-43 expression was found to overlap with growth/differentiation factor (GDF)-5 expression during embryonic mouse limb development.68 GDF-5 and connexin-43 expression were localized to the condensing digit and long bones of the hindlimb at E12.5, the perichondral regions of the forelimb at E13.5, and to the elbow and hip joint surrounding the femoral head from E12.5 to E14.5.68 Colocalized expression of GDF-5 and connexin-43 was also detected in tendons of the hip at E14.5, and around the tendons of each toe at E15.5, indicating that GDF-5 and connexin-43 are both present in embryonic tendon.68 Together these results highlight that connexin-32 and −43 gap junction proteins are present in embryonic tendon. However, the specific contributions of connexins to embryonic tendon development remain unknown, and future studies correlating connexin expression with functional cellular communication and tenogenesis are needed.
Cell-cell junctions in postnatal tendon
In the developing mouse, early postnatal tendon appears to resemble embryonic tendon structure, with high cell density and low collagen content.14 For example, the Achilles tendon of a mouse at postnatal day (P)4 contains less than 3% collagen and by P28 is still only 36% collagen.69 In an ovine model, tendon cells accounted for 33.4% of the digital flexor tendon volume after 1 week of development, whereas at 40 weeks tendon cells accounted for less than 6% of the volume.70 This high cell density in postnatal tendon suggests that the tendon cells have the potential to maintain direct cell-to-cell connections. Postnatally, mechanical forces significantly increase from the development of weight-bearing locomotor behavior,71 as do tendon mechanical properties and tenogenesis. Gene expression for scleraxis and tenomodulin was found to peak at P7 in the Achilles tendons of mice, when compared to P1 or later ages.72 However, relationships between postnatal tendon formation and cell-cell junction proteins have not been extensively investigated. One study conducted a proteomic analysis of P1 mouse tendon cells from the flexor digitorum longus to determine the proteins in the pericellular regions. Potential cell-cell junction proteins were identified and include Fat-4, protocadherin-15, cadherin-13, and catenin alpha-1, a protein involved in linking cadherins to the actin cytoskeleton.73 Additional studies are needed to understand how these proteins change throughout development and contribute to postnatal tendon formation.
Though few studies have examined cell-cell junctions in the early postnatal stages, connexins have been shown to decrease postnatally in horses. As described above, connexin-32 and −43 were significantly reduced from the fetal to the postnatal (1 to 6 months) stage in horse SDFT and CDET tendons, though these gap junctions were still present at all ages.38 However, the reduction in connexin-32 and −43 from the fetal to the postnatal (e.g., foal) stage is noteworthy, since tendon development continues after birth and is exposed to increasing mechanical stimuli from the onset of weight-bearing locomotion.
Taken together, tendon cells express gap junction proteins and a range of cadherins during embryonic and postnatal development (Table 1), but more work is needed to identify their specific contributions to tendon formation. Further studies are also needed to identify novel cell-cell junction proteins that may be participating in tendon development, and to elucidate their tenogenic influence. Looking to other developing musculoskeletal tissues may provide potential proteins to explore. However, tendons have so far been found to lack pannexins, the large transmembrane channels that link the cytosol and extracellular matrix, even though they are present in bone and cartilage cells.64 Based on the overlapping expression of cell-cell junction proteins and various growth factors,55,68 and the apparent redundancy of signaling pathways previously thought to be necessary for limb development,40 other proteins may be participating in tendon development, and characterizing them will provide new targets for treating tendon injury and disease.
Table 1.
Cell-cell junction proteins in developing tendon.
| Cell-Cell Junction Type | Possible Functions In Developing Tendon | References | |
|---|---|---|---|
| Cadherins | N-cadherin | Cellular condensation, cell adhesion, limb patterning | Hasson 201042 Luo 200541 Luo 200140 Richardson 200737 |
| R-cadherin | Unknown role in embryonic tendon, but expression identified in E13 chick metatarsal tendon | Kimura 199539 Richardson 200737 |
|
| Cadherin-4 | Unknown role in embryonic tendon, but expression identified in mesodermal cell precursors in chick at HH25 | Lorda-Diez 201455 | |
| Cadherin-7 | Unknown role in embryonic tendon, but required for cell condensation and migration in chick limb mesenchymal cells | Kim 200974 | |
| Cadherin-11 | Cellular condensation, distinction of cartilage and tendon precursors, limb patterning, collagen fibril alignment | Kimura 199539 Richardson 200737 |
|
| Cadherin-13 | Unknown role, but identified in tendon cells at P1 | Smith 201273 | |
| Protocadherin-15 | Unknown role, but identified in tendon cells at P1 | Smith 201273 | |
| Fat-1 | Cell proliferation, patterning of muscle and tendon precursors, overlapping expression domain with scleraxis, limb patterning. | Helmbacher 201846 Smith 200751 |
|
| Fat-4 | Unknown role, but identified in tendon cells at P1 | Smith 201273 | |
| Connexins | Connexin-32 | Mechanotransduction, stimulation of collagen production | McNeilly 199612 Russo 201567 Stanley 200738 Wagget 200666 |
| Connexin-43 | Mechanotransduction, inhibition of collagen production, separation of cartilage and tendon precursors, cell condensation, tissue patterning | Coleman 2003a68 Coleman 2003b75 McNeilly 199612 Russo 201567 Stanley 200738 Wagget 200666 |
|
| Tight Junctions | ZO-1 and Claudin-1 | Epithelium surrounding tendons – containment of tendon cells within tendon and prevention of tendinopathic adhesions | Taylor 201176 |
Cell-cell junctions in adult tendons
While not as abundant as embryonic tendon cells, adult tendon cells are found within the tendon fascicles and interfascicular membranes. Adult tendon cells respond to mechanical and biochemical stimuli, and may play a role in tendon homeostasis.10,11 Various signaling mechanisms beyond cell-cell junctions may be involved, and these have been summarized in a recent book chapter.77 Here, we examine the current understanding of cell-cell junction proteins in adult tendon (Table 2), with an emphasis on the response to mechanical load.
Table 2.
Cell-cell junction proteins in adult tendon.
| Cell-Cell Junction Type | Possible Functions In Adult Tendon | References | |
|---|---|---|---|
| Cadherins | N-cadherin | Response to mechanical load and growth factors, coupling with α-catenin | Barry 201478 Desai 201379 Keller 201180 Ralphs 200281 |
| Cadherin-11 | Unknown role in adult tendon, but associated with strong cell adhesions in myofibroblasts | Pittet 200845 | |
| Connexins | Connexin-26 | Mechanotransduction; other functions unknown | Maeda 201282 |
| Connexin-32 | Mechanotransduction, stimulation of collagen production; other functions unknown | Maeda 201282 Ralphs 199865 Wagget 200666 |
|
| Connexin-43 | Intercellular communication, movement of small molecules between cells in response to strain, coupling with actin cytoskeleton to facilitate mechanotransduction, tissue maintenance, suppression of collagen production, downregulation of IL-1β during inflammatory response | Banes 199983 Maeda 201282 Maeda 201584 Maeda 2017a85 Maeda 2017b86 Ralphs 199865 Wagget 200666 Wall 200787 |
|
| Tight Junctions | ZO-1 and Claudin-1 | Membrane surrounding tendon; adhesions form following disruption of these junctions after tendon injury | Taylor 201176 |
Cadherins in adult tendon
Only a few studies have investigated the presence of cadherins in adult tendon cells. Immunofluorescence staining showed that digital flexor tendons from adult chickens possess N-cadherin in vivo.81 N-cadherin appeared to mirror the distribution of actin filaments and vinculin, which form parallel rows along the tendon long axis. In the same study, isolated adult tendon cells grown in culture produced N-cadherin, as observed by immunofluorescence and western blotting. When cells were cyclically strained (75 millistrain, 1 Hz, 8 h/d for 4 d), N-cadherin protein levels increased significantly compared to static controls,81 suggesting that mechanical stimulation may impact the cell-cell adhesions in tendon cells. However, how cadherins may regulate or impact the tendon cell response to loading is unknown.
N-cadherin in adult tendon cells responds to growth factor treatment. Tendon cells isolated from the Achilles tendons of young adult (10-week-old) rats and treated with 1, 10, and 1000 ng/mL GDF-5 for 4 d had significant decreases in N-cadherin gene expression at all GDF-5 concentrations, compared to untreated controls.80 However, when treated with 100 ng/mL of GDF-5 over 12 d, N-cadherin gene expression was increased at 3, 6, and 9 d compared to untreated controls, though at 12 d gene expression levels were similar to controls.80 GDF-5 is known to impact tendon formation88 and healing,89 but N-cadherin has been shown to decrease during tenogenic differentiation of mouse MSCs.90 These variations suggest that N-cadherin expression may depend on the timing and intensity of GDF-5 signaling, but more studies are needed to assess how N-cadherin responds to growth factors in adult tendon cells.
Connexins in adult tendon
Connexins have been identified in adult tendon cells from a range of species.12,38,65,66 Although connexin levels decrease with increasing age,38,67 their presence in adult tendons suggests they are facilitating communication between cells, and may be involved in maintaining the tissue. Using fluorescence recovery after photobleaching (FRAP) to quantitatively evaluate the diffusion-dependent redistribution of a gap junction-permeable fluorescent dye, one study showed that adult tendon cells from human hamstrings formed functional gap junctions with connexin-43 in both 2 and 3 dimensional (D) in vitro culture, and rapidly redistributed calcein acetoxymethylester (AM), a non-fluorescent dye that is converted to green-fluorescent and membrane-impermeable calcein by intracellular esterases.91 HeLa cells, which do not communicate via gap junctions, did not redistribute calcein AM. Dye redistribution was impaired in tendon cells when gap junctions were chemically inhibited using 18 β-glycyrrhetinic acid and carbenoxolone, indicating that intercellular communication was disrupted.91 Additionally, isolated tendon cells with fewer cell-to-cell contacts recovered more slowly from the photobleaching and could not redistribute the calcein, compared to cells that formed many cell-to-cell contacts, suggesting that adult tendon cells depended on their gap junction-linked network to rapidly move small molecules.91
Gap junction communication may also modulate inflammatory and catabolic responses in adult tendon, including the response to overuse and exercise. Adult rabbit Achilles tendon cells subjected to 30 min of heat (37°C, 41°C or 43°C, to simulate the heating of tendon that can occur following intense exercise) had decreased cell viability and increased expression of the proinflammatory markers matrix metalloproteinase (MMP)-1, interleukin (IL)-1β and IL-6, and decreased expression of Col I, 24 h after heat stimulation.85 Connexin-43 expression was not affected by heat treatment, but inhibiting gap junctions using 18 β-glycyrrhetinic acid resulted in further increases in expression of MMP-1, IL-1β, and IL-6, compared to cells subjected to heat but with functional gap junctions.85 Interestingly, when connexin-43 was overexpressed, expression of Col I and IL-1β was significantly lower, and MMP-1 and IL-6 expression trended lower in the heated tendon cells, compared to cells heated without overexpression of connexin-43.85 Taken together, these findings show that connexins may influence inflammatory markers in tendon cells following intense exercise that results in heat.
In addition to heat, gap junctions are sensitive to mechanical signals, and respond to mechanical loading. In one study, gap junctions were quantified in the tail tendon fascicles of adult rats using FRAP and calcein AM.82 Fascicles were statically loaded to 1 N and FRAP was carried out after 10 min or 1 h of loading to assess junction permeability between groups.82 A mathematical compartment model was also generated to calculate a permeability constant for intercellular communication. Loading for 10 min did not change the permeability, while loading for 1 h significantly reduced intercellular permeability, compared to unloaded controls.82 Strong gene expression of connexin-26 and connexin-43 was observed following 1 h of loading, while connexin-32 was undetectable. Connexin-43 gene expression increased significantly after 24 h of loading, while no differences were seen in connexin-26 levels.82 Protein production of connexin-26 and connexin-43 was observed following 1 h of loading, though production of connexin-43 was decreased, and the localization of connexin-43 changed from the contact points of neighboring cells in the same row to a more discrete, punctate pattern.82 In contrast, neither the protein levels nor the localization of connexin-26 were affected by the loading. Connexin-32 was not found in loaded or unloaded fascicles.82 The decrease in connexin-43 protein production is interesting given the increase in connexin-43 gene expression, and suggests that tendon cells attempt to remodel their gap junctions in response to load. However, actual production of gap junctions may be compromised with loading, and hence overall permeability is suppressed, further inhibiting intercellular communication. Overall, functional gap-junction intercellular communication may influence tendon cell behavior by propagating mechanical signals throughout cell networks to stimulate a coordinated response to loading.
In addition to the duration of loading, gap junctions may also be sensitive to the magnitude of mechanical strain. Adult tendon cells from rabbit Achilles tendons showed that intermediate tensile strain (4%) for 1 h enhanced intercellular communication, illustrated through increased transport of fluorescent tracer molecules between cells.84 High strain (8%) significantly decreased intercellular gap junction communication, while neither 4 or 8% strain affected intracellular communication, which was mediated by diffusion instead of by gap junctions.84 Connexin-43 was localized to the cytoplasm and cell-cell boundaries in the unstrained cells, and was also detected near the nucleus in the 4% strain condition, while overall connexin-43 production was reduced at 8% strain.84 Gap junction communication appears to be enhanced by moderate strain, but inhibited by high strain, possibly reflecting the physiologically relevant range of strain magnitudes.92 Higher strain magnitudes may mechanically disrupt the cell-cell junctions and lead to the increased inflammation that follows gap junction inhibition.85 Strain magnitudes were further assessed in a follow-up study. Cells from adult rabbit Achilles tendons were left unstrained, or subjected to 4% or 8% strain for 24 h, and an intercellular diffusion coefficient was used to quantify gap junction communication.86 The intercellular diffusion coefficient was significantly increased following application of 4% strain for 1 h, compared to 0% strain controls, and its level was maintained for 6 h, but returned to pre-strain levels at 24 h.86 This was accompanied by a transient increase in connexin-43 gene expression and localization at the cell membrane and within the cell bodies at 1 h and 6 h following 4% strain.86 In contrast, application of 8% strain reduced the intercellular diffusion coefficient to the levels of the unstrained control group or below at 6 h, and inhibited connexin-43 expression, but did not change connexin-43 localization.86 Moderate mechanical loading may be therapeutically useful for preventing or repairing tendon injuries,10,93,94 while the effects of high strains on gap junctions may explain the presentation of certain tendon pathologies. Taken together, the results of these studies suggest that disruption of cell-to-cell communication by tensile loading may account for some of the damage tendons can sustain from mechanical loading. Gap junctions are part of a complex cellular communication mechanism in tendon cells that responds to load and other stimuli, such as heat, by altering permeability of the cells and enhancing or inhibiting production of connexins, and possibly modulating the message the cells receive.
In addition to mechanotransduction, gap junctions in adult tendon cells may be actively involved in the mechanoregulation of collagen production. Blocking gap junctions with octanol in adult chicken flexor digitorium profundus tendons ex vivo inhibited the increase in DNA and collagen synthesis that was observed following 3 d of cyclic loading to 0.65% strain, and was reversible when the octanol was removed.83 Gap junctions may be initiating the cellular communication that coordinates collagen production in response to mechanical loading. The unequal distribution of connexins within tendons may reflect the different magnitudes and modes of mechanical loading associated with specific regions. In the rat, the distal Achilles tendon midsubstance, sesamoid fibrocartilage, and enthesial fibrocartilage regions were shown to have different expression of connexin-32 and −43.65 Immunofluorescence revealed prominent connexin-32 and −43 junctions in the tendon midsubstance, with both connexins localized between cells and rows, and connexin-43 predominantly occurring between lateral cell processes.65 Connexin-32 formed plaques between cells with many small foci, while connexin-43 displayed a more punctate labeling pattern. Within the enthesis, both connexin-32 and −43 were significantly reduced. Connexin-43 was not detected in the enthesis, but connexin-32 was detected in the cells that stopped forming rows, and adopted a rounded morphology associated with mineralized fibrocartilage.65 Finally, connexin-32 and −43 labeling were weak in the sesamoid fibrocartilage, with connexin-32 labeling localized mainly to the points of contact between cells, and with no visible connexin-43 labeling.65 In addition to variations in connexin expression, cells in the different regions displayed distinct morphologies, with elongated, narrow cells observed in the midsubstance, and more rounded cells with fewer opportunities for cell-cell contact seen in the enthesis and sesamoid areas.65 Cell shape affects the contacts cells can make with one another, and may enhance or suppress expression of certain gap junction proteins. It is worth noting that some bone cells in the calcified enthesis fibrocartilage expressed connexin-43,65 demonstrating the wide-ranging distribution of connexin-43 throughout multiple musculoskeletal tissues. The variations in expression patterns of connexins between bone, tendon, and fibrocartilage may also help guide the differentiation process to ensure correct formation of separate tissues.
Gap junctions may also couple with the actin cytoskeleton and together play a role in mechanoregulation of tendon cells. Connexin-43 was found to colocalize with actin filaments in both avian and human tendon cells. While only ~4% of avian tendon cells had colocalization of actin and connexin-43 in an unloaded control group, cells subjected to 5% cyclic strain had significantly increased colocalization of actin and connexin-43.87 The same study also investigated COS-7 cells (CV-1 in Origin with SV40 genes, derived from the kidney of the African green monkey). Colocalization of actin and connexin-43 was significantly increased following cyclic strain in the tendon cells but was not observed in the COS-7 cells, suggesting that COS-7 cells are not as mechanosensitive or that connexin-43 is playing a different role.87 Additionally, inhibiting non-muscle myosin II activity to block actin contractility, and hence mechanotransduction, greatly reduced the detectable connexin-43 on actin filaments, suggesting that connexin-43 is not produced when the cell cannot contract. The colocalization of connexin-43 and actin filaments with mechanical loading, and subsequent loss of this colocalization with inhibited actin-myosin contractility indicates that gap junction production in tendon cells is mechanically regulated and partially dependent on the actin cytoskeleton.
Localization of connexin-32 and connexin-43 has been shown to vary within tendon cells as they reside in the tendon tissue: tendon cells are linked by both connexins longitudinally (e.g., within rows), but only by connexin-43 laterally (e.g., between rows).12,65,66 3D reconstructions of sections from adult rat deep digital flexor tendons stained for connexin-32 and −43 showed that, in addition to the processes between cells, connexin-43 was prominent in the periphery of the tendon, between the epitenon and the outermost layer of tendon.12 Connexin-32 was also prominent in the periphery, but with a more diffuse distribution than connexin-43, and with many foci between cells that appeared as plaques.12 This reconstruction identified the precise locations of connexin-32 and −43 in tendon, and showed that the 3D structure of tendon cells is complex, with collagen bundles distributed throughout the tendon, and different localization patterns of connexin-32 and −43. The differences in positioning between these two connexins may be attributed to their different mechanoresponsive functions in Col I secretion, a hypothesis investigated by selectively blocking specific connexins in avian tendon cells. Tendon cells from chicken deep digital flexor tendons strained to 75 millistrain for 8 h/d for 3 d increased Col I secretion by 23%, compared to unstrained controls.66 When connexin-32 and −43 were both inhibited via the biomimetic peptide gap27, Col I production was reduced compared to untreated cultures, in both strained and unstrained cells.66 When connexin-43 was selectively inhibited via antisense oligonucleotides, Col I secretion was further increased with loading, while antisense inhibition of connexin-32 resulted in reduced Col I secretion.66 These results suggest that mechanical signals are integrated by tendon cells to produce a coordinated response to load and that connexin-32 may be stimulatory and connexin-43 may be inhibitory.66 The differential actions of connexin-32 and −43, and possibly other gap junctions, could contribute to the overall cell response to mechanical loading in tendon. Excess collagen production is responsible for scarring, making targeted inhibition of stimulatory gap junction proteins a potential clinical treatment to prevent fibrosis following tendon injury. The anabolic and catabolic cellular processes that maintain the adult tendon ECM are likely mediated in part by gap junction communication, but a deeper understanding of the relationships between mechanical signals and cell responses is needed.
Cell-cell junctions in tendon injury and disease
Adult tendons heal poorly, and the roles of gap junctions in injury and healing have been explored. Gap junction involvement in tendinopathies may be driven in part by inflammatory cytokines. The inflammatory cytokine IL-1β is produced following tendon injury95,96 and directly impacts tendon cells.97,98 To evaluate this, cells from human flexor digitorum profundus tendons were cultured in 3D and subjected to 3.5% cyclic strain at 1 Hz for 1 h/d for up to 5 d with or without IL-1β treatment. All strained cells underwent increased apoptosis, and had upregulated gene expression of Col I, fibronectin, biglycan, TGFβ1, cyclooxygenase-2, MMP-27, and a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS5), genes which are upregulated in human tendinopathies.99 IL-1β decreased expression of all target genes to the levels of unstrained controls, with the exception of cyclooxygenase-2 and ADAMTS5, which were both increased by addition of IL-1β alone, though strain with IL-1β did not further increase their expression.99 Cells seeded more densely made more connections and survived cyclic straining, while cells at a lower seeding density did not survive. Enhanced cell survival was lost when gap junctions were inhibited, indicating that cell survival was mediated by gap junctions.99 Indeed, IL-1β treatment upregulated connexin-43 expression in a time- and dose-dependent manner, as early as 8 h after its addition to cell cultures. IL-1β also altered cell morphology, with treated cells forming multiple long, axon-like processes and connecting to one another.99 Although high levels of IL-1β likely contribute to tendon pathology via upregulation of inflammatory proteins such as cyclooxygenase-2 and ADAMTS5, IL-1β may also be part of the adaptive response to loading. In particular, the ability for IL-1β to upregulate connexin-43, shown in other studies to attenuate cell death caused by strain,85,86 may be useful for preventing or treating strain-induced tendon pathologies. However, more work is needed to determine how the progression of tendinopathies may differ between in vivo and in vitro conditions, and how connexin-43 may mediate strain-induced injury.
Fluoroquinolone antibiotics, such as ciprofloxacin, are known to cause tendinopathies and ruptures in some patients.100 These tenotoxic side effects of ciprofloxacin may be due to altered expression of cadherins and connexins by the tendon cells. To assess this, adult human tendon cells from the tendons of the rectus femoris, gracilis, and semitendinosus muscles were cultured for 2 d and treated with 10, 20 or 50 µg/mL ciprofloxacin. Control cells were cultured without ciprofloxacin. Ciprofloxacin administration significantly decreased expression of N-cadherin at all concentrations, and connexin-43 expression trended downwards.101 Col I, Col III, and MMP-2 expression were unaffected by ciprofloxacin, but MMP-1 expression increased, and tissue inhibitor of MMP (TIMP)-1 decreased.101 Expression of long lysyl hydroxylase 2 (LH2b), an enzyme involved in collagen crosslinking, trended lower, though the decrease was not statistically significant. No changes were observed in the actin, vimentin, and microtubule network between control and ciprofloxacin-treated cells.101 The reduction in LH2b, coupled with the decrease in N-cadherin and downward trend in connexin-43, suggests that ciprofloxacin may inhibit collagen crosslinking in some patients and impact the cell-cell contact in tendon cells, possibly leading to tendon ruptures. However, the mechanisms through which N-cadherin and connexin-43 in adult tendon cells influence tissue maintenance in normal and tendinopathic tendons need further study.
Ectopic ossification is associated with Achilles tendon injuries and repairs.102 The exact causes are unknown, but cytokines such as bone morphogenetic protein (BMP)2 and 4, and TGFβ2 may be driving an endothelial-to-mesenchymal transition of vascular endothelial cells, which subsequently undergo osteogenic differentiation, resulting in mineralization within the tendons.103 Smad proteins are the main signal transducers of the TGFβ pathway, and Smad2 and 3 are necessary for TGFβ2-induced tenogenesis during embryonic limb development.104–106 Conversely, Smad7 has been shown to inhibit Smad2 signaling, and Smad7 overexpression blocks myofibroblast activation and transformation,107 inhibiting endothelial-to-mesenchymal transition. To assess the potential of Smad7 as a preventative for heterotropic ossification following tendon repair, adult rat Achilles tendons were fully transected and injected with a Smad7 overexpressing lentivirus. Histological and gene expression analysis showed that, compared to the control groups, injured tendons transduced with the Smad7 lentivirus increased production of VE-cadherin and the endothelial marker cluster-of-differentiation 31 (CD31), and decreased production of N-cadherin and vimentin, especially at 6- and 10-weeks post-surgery.103 Histological and X-ray analysis at 10-weeks post-surgery revealed that rats treated with the Smad7 lentivirus did not show signs of heterotropic ossification, while control rats showed ossifying regions of their Achilles tendons in both X-rays and histological sections.103 The decrease in N-cadherin in the Smad7 treated rats, coupled with the robust N-cadherin production seen in injured tendons, suggests that N-cadherin is involved in the endothelial-to-mesenchymal transition that may be underlying heterotropic ossification of injured tendons. As tenogenic differentiation of stem cells is accompanied by decreases in N-cadherin production,90 Smad7 overexpression may be creating a more tenogenic environment to promote healing in injured tendons. Cadherins have remained underexplored as targets for regenerative therapies for tendon injuries. The extensive involvement of cadherins in tissue development, patterning, and injury response makes them worthy of future investigations.
Tight junctions in tendon
Tendons must be able to slide over other tissues during movement to carry out their function, and both aging and injury have been shown to result in fibrous adhesions.108 The surface of E14 chick metatarsal and 4-week-old mouse flexor tendons was recently found to be coated in a Col IV/laminin basement membrane and contained a keratinized epithelium that was located on the outermost aspect of the tendon.76 Immunofluorescence imaging showed that the basement membrane surrounding the tendon was composed of ZO-1 and claudin-1 tight junctions (Figure 3), as well as the epithelial markers keratin 1 and 10.76 The epithelial cells of the basement membrane had a flattened morphology and interdigitated cell processes.76 This epithelial layer was proposed to facilitate tendon sliding, as well as prevent tendon cell migration away from the tissue. Cutting 4-week-old mouse flexor tendons and incubating them ex vivo for 5 d showed cells migrating from the cut ends, but not from the tendon midsubstance surrounded by the intact epithelial layer.76 When this epithelial layer was digested by trypsin prior to 5 d of culture, cells migrated away from all the trypsin damaged surfaces, indicating that they were no longer contained by the tight junctions around the tendon.76 Adhesions that form following injury may therefore originate from damage to this basement membrane and epithelium, a hypothesis tested in a Col IV mutant mouse line, which is not able to form intact basement membranes due to its mutated collagen.76 Histological examination of the deep flexor tendons in the hind limbs of mutant mice showed evidence of adhesions between the tendon and the tendon synovium, and these adhesions were not present in wild-type mice.76 Finally, when the flexor tendons of 4-week-old mice were severed and allowed to heal for 21 d, adhesions were seen between the tendon and tendon synovium and skin, though the tendon epithelium flanking the injury was intact.76 Overall, these observations suggest that the epithelial layer surrounding tendon is crucial to preventing adhesions, and repairing damage to the epithelial layer to prevent adhesions may help mitigate the effects of injury.
Figure 3.
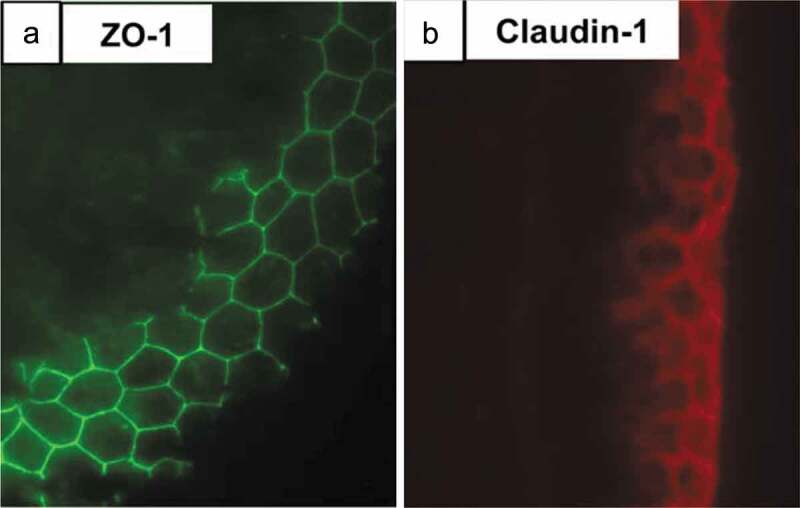
ZO-1 and claudin-1 are seen in a transverse section of E14 chick metatarsal tendon. Immunofluorescence staining showed the presence of an epithelium containing (a) ZO-1 (green) and (b) claudin−1 (red) tight junctions on the surface of the tendon. This epithelium may prevent tendon cell migration and tendinopathic adhesion formation. Figure used with publisher’s permission from Taylor et al. 2011.76.
Cell-cell junctions as regulators of musculoskeletal tissue formation
Regulation of cell-cell junctions with tenogenesis
Differentiation of stem cells toward the tendon lineage (tenogenesis) may be regulated by mechanical,26 biochemical,109 and combinatorial factors.110 Differentiation has been characterized by increases in expression of tendon markers such as scleraxis and tenomodulin. However, few studies have investigated how the profile of cell-cell junctions may be influenced by tenogenic differentiation. The growth factor TGFβ2 has been identified in embryonic tendon development as an important tenogenic factor34,111 and has been explored in tenogenesis of stem cells.110,112,113 In vitro, N-cadherin and cadherin-11 protein production were significantly decreased in C3H10T1/2 mouse MSCs treated with TGFβ2 for 3, 7, 14, and 21 d, while scleraxis and tenomodulin production were significantly increased after 14 and 21 d.90 Cell morphology also changed with TGFβ2 treatment. As early as 3 d and throughout 21 d of TGFβ2 treatment, MSCs appeared fibroblastic, with an elongated actin cytoskeleton and a high degree of local actin alignment, compared to untreated controls.90 The decrease of N-cadherin to almost undetectable levels, as well as the sustained decrease of cadherin-11, and simultaneous increase in tenogenic markers scleraxis and tenomodulin, suggests that suppression of these cadherins may occur during tenogenic induction. In the same study, connexin-43 increased significantly following 3 d of TGFβ2 treatment but did not differ significantly from untreated controls at later timepoints.90 Additional studies are needed to understand if N-cadherin, cadherin-11, and connexin-43 are regulators of the tenogenic stem cell response to TGFβ2. To better understand how cadherins and connexins may regulate tendon development and tenogenesis, we look to other musculoskeletal tissues of mesenchymal lineage. For example, chondrogenic differentiation is associated with changes in N-cadherin levels,114,115 and cadherin-11 plays a role in osteogenesis.116 Therefore, we briefly survey the cadherins and connexins involved in chondrogenesis and osteogenesis.
Cadherin regulation of chondrogenesis and osteogenesis
In MSCs, N-cadherin and cadherin-11 are regulators of differentiation toward cartilage and bone.117 The timing of N-cadherin expression appears especially critical for chondrogenesis. N-cadherin was initially shown to have a specific spatiotemporal expression pattern during limb bud chondrogenesis in chick.115 Shell-less embryos injected during the cell condensation stage at HH22-24 with N-cadherin blocking antibodies failed to undergo chondrogenesis, and had other gross developmental and pattern deformities, likely due to widespread disruption of cell adhesion.115 The same study also treated in vitro micromass cultures of chick limb mesenchymal cells with N-cadherin blocking antibodies. As in the in vivo experiments, cells treated with N-cadherin blocking antibodies did not undergo aggregation and condensation, and chondrogenesis was inhibited.115 As N-cadherin expression is highest during the cellular condensation phase, blocking N-cadherin during cellular condensation is especially detrimental to chondrogenesis.
The in vivo increase in N-cadherin during cellular condensation and chondrogenesis may be driven by BMP2, a growth factor and member of the TGFβ signaling family.118 BMP2-induced chondrogenesis of mouse MSCs resulted in a significant upregulation of N-cadherin after 24 h and 5 d in culture.118 Blocking N-cadherin interactions using a N-cadherin mimicking peptide inhibited chondrogenesis, and overall loss of chondrogenesis was greater with higher concentrations of the peptide.118 Finally, mouse MSCs transfected to overexpress N-cadherin augmented the effect of BMP2 when N-cadherin expression was doubled, but BMP2-induced chondrogenesis was inhibited when N-cadherin expression was quadrupled.118 Cells transfected with a mutant N-cadherin failed to undergo BMP2-induced chondrogenesis. Collectively, these results suggest that the timing of N-cadherin expression is critical to chondrogenesis, and while high levels are needed initially, N-cadherin levels must decrease in order for chondrogenesis to proceed. The initial increase and subsequent reduction of N-cadherin was further investigated using micromass cultures of embryonic chick mesenchymal cells. Cells from HH23-24 were transfected with mutant N-cadherin or wild-type N-cadherin, and in all transfected cells a transient increase in N-cadherin expression was observed for the first 2 d of culture, followed by a decrease at 3 d,114 mirroring the expression pattern documented in mouse MSCs chondrogenesis.118 Overexpression of N-cadherin resulted in enhanced cell condensation and chondrogenesis after 2 and 3 d of culture, while micromass cultures transfected with mutant N-cadherin showed suppressed cell condensation and cartilaginous matrix generation, with reduced Col II and proteoglycan production.114
Potential intracellular regulators of N-cadherin, such as Rho guanosine triphosphate (RhoGTP)-ases, specifically the kinase Rac1, have also been explored in chondrogenesis.119 Inhibiting Rac1 in micromass cultures of E11.5 mouse mesenchymal limb bud cells led to reduced cell numbers, size, and organization of cellular condensations, and decreased expression of N-cadherin. Overexpression of Rac1 in cultured chondrogenic ATDC5 cells resulted in increased expression of the cartilage markers Sox9, 5, and 6, collagen II, and aggrecan.119 In micromass cultures, genetic ablation of Rac1 led to reduced expression of chondrogenic markers, including Sox9,119 and Rac1 overexpression resulted in increased N-cadherin expression.119 Based on these findings, it is possible that Rac1 affects the N-cadherin expression needed for cellular condensation during chondrogenesis.
TGFβ signaling may also modulate chondrogenesis via N-cadherin. TGFβ1 initiated and maintained chondrogenesis of human mesenchymal progenitor cells in addition to a corresponding initial upregulation and subsequent downregulation of N-cadherin.120 Wnt7, a target of β-catenin, had an expression pattern that mirrored N-cadherin, with a significant increase after 1 d of TGFβ1 treatment, and a return to control levels at 3 d.120 This suggests Wnt7 may regulate N-cadherin during TGFβ1-mediated chondrogenesis. Alongside TGFβ1, TGFβ3 is also a chondrogenic growth factor.121 TGFβ3-induced chondrogenesis in human MSCs was accompanied by increases in N-cadherin.122 Chondrogenic differentiation was further enhanced when cells were prevented from spreading and flattening (flattened cells differentiated into smooth muscle), suggesting cell-shape may be mediated by cadherin cell-cell adhesions, and may affect subsequent differentiation.122 Finally, cadherin-7 has been shown to be necessary for cartilage condensation in HH22-23 chick limb mesenchymal cells.74 Knockdown of cadherin-7 resulted in impaired cell migration and failed precursor condensation, while overexpression of β-catenin also resulted in inhibition of cadherin-7 and suppression of cell migration.74 Thus, in addition to N-cadherin, cadherin-7 may control cellular condensation during chondrogenesis.
N-cadherin and cadherin-11 are also involved in osteogenic differentiation and modulation of bone growth. At 6 months of age, N-cadherin± and cadherin-11−/− double-knockout mice had severe phenotypic deficiencies, including smaller body mass and reduced trabecular bone mass, bone strength, and bone formation rate, and smaller diaphyses, compared to either single-knockout or wild-type mice.123 Knocking out both N-cadherin and cadherin-11 resulted in decreased β-catenin in both the nucleus and the cytoplasm and cell membrane, leading to an overall downregulation of β-catenin signaling when cell adhesion was disrupted.123 N-cadherin promoted osteogenic induction of human MSCs that were cultured in hydrogels functionalized with N-cadherin mimetic peptides,124 though a different study found that N-cadherin mimetic peptides enhanced chondrogenesis of human MSCs,125 highlighting N-cadherin’s multifaceted roles during differentiation and development of bone and cartilage. Mature osteoblasts also express N-cadherin,126 but prolonged N-cadherin overexpression limited osteogenic differentiation of mouse MSCs by downregulating β-catenin and extracellular signal-regulated kinase (ERK)1/2 signaling.127 Additionally, ectopic bone formation in nude mice was prevented by N-cadherin overexpression,127 suggesting that N-cadherin levels are tightly regulated during mesenchymal cell condensation to ensure correct tissue differentiation, and offering a potential explanation for the drop in N-cadherin levels seen following cell condensation.115,118 Cadherin-11 also appears to be osteogenic during development, with reduced osteoblast differentiation and bone density observed in cadherin-11 knockout mice.116 However, just as N-cadherin is needed for cell condensation during chondrogenesis, cadherin-11 may also be needed for cell condensation during tendon development.37,128
In addition to their chondrogenic and osteogenic roles, N-cadherin and cadherin-11 may be involved in the organization and maintenance of the synovial lining, the thin membrane between the joint cavity and the fibrous joint capsule that facilitates low-friction movement at the joint, and becomes inflamed during osteoarthritis.129 Fibroblast-like synoviocytes from cadherin-11-null and wild-type mice were cultured and stained to determine the presence of cadherins. Cell-cell contacts in the wild-type synoviocytes contained cadherin-11, while cadherin-11-null cells still made cell-cell contacts that contained β-catenin.130 Wild-type cells expressed both N-cadherin and cadherin-11, while cadherin-11-null cells expressed only N-cadherin and the mutated form of cadherin-11, though interestingly, cadherin-11-null cells expressed higher levels of N-cadherin than wild-type cells.130 Furthermore, immunoprecipitation using β-catenin, which binds the cytoplasmic tail of cadherins, indicated that the two cadherins are not part of the same molecular complex in synoviocytes.130 As more information emerges about the role of cadherins in the synovium, novel cadherin contributions to tendon formation may become evident.
Connexin regulation of chondrogenesis and osteogenesis
In addition to cadherins, connexins are involved in the early differentiation and patterning of other musculoskeletal tissues. Connexin-43 has been shown to be necessary for chondrogenesis and osteogenesis, and its participation in both of these processes has been summarized in a recent review.131 While less is known about the role of connexins compared to cadherins in cartilage differentiation, adult articular chondrocytes express both connexin-43 and −45 in vitro.132 In one study, connexin-43 was shown to be involved in GDF-5-induced chondrogenesis.75 Chick limb MSCs expressing mutated GDF-5 and treated with human GDF-5 had increased Col II expression in 1 d cultures and increased Alcian blue staining (indicative of glycosaminoglycan production and chondrogenic differentiation) in 3 d cultures compared to wild-type control cells, but the enhanced chondrogenesis was lost when gap junction communication was chemically blocked by oleamide.75 Overexpressed GDF-5 functioned independently of N-cadherin, as cells with mutated N-cadherin still underwent GDF-5-induced chondrogenesis, while connexin-43 protein levels remained constant between cells expressing mutated GDF-5 and controls.75 As GDF-5 and connexin-43 are also both present in embryonic tendon,68 it is possible that tendon and cartilage share similar developmental mechanisms. The potential involvement of connexin-43 in chondrogenesis also comes from a study that blocked gap junction communication in bone marrow-derived mesenchymal stromal cells and human chondrocytes with 18-α glycyrrhetinic acid, and observed a decrease in Col II and extracellular adenosine triphosphate (ATP).133 When cells were cultured farther away from each other in an engineered tissue scaffold, differentiation was not impacted, but blocking connexins again led to decreases in chondrogenic markers, suggesting functional gap junctions rather than general cell-cell contacts were needed for chondrogenesis.133
During osteogenesis, the expression of connexin-43 has been shown to parallel osteoblastic differentiation, and inhibiting connexin-43 reduces osteoblast differentiation in human fetal osteoblastic cells.134 In human osteoblasts, connexin-43 may regulate alkaline phosphatase activity, as well as osteocalcin and osteopontin expression, all of which contribute to osteoblastic differentiation.134 In contrast, 2-month-old mice with a conditional knockout of connexin-43 had increased periosteal bone formation in vivo, and increased periosteal and endocortical bone mass in response to axial compressive load at lower strains, indicating an overall increased sensitivity to mechanical load compared to their wild-type littermates.135 These contradictory results are likely due to the differential effects of inhibiting connexin-43 at various developmental stages and in specific tissues rather than globally. Complete loss of connexin-43 earlier in development leads to disruption of osteoblastic differentiation, while targeted disruptions at later stages, such as those achieved in osteoblasts on the endocortical surface, osteocytes, and periosteal cells by a conditional knockout,135 do not cause severe phenotypic deficiencies. While the causal mechanism of connexin-43-mediated disruption of osteogenesis remains unknown, the c-terminal domain of connexin-43 was recently found to bind several signaling proteins, including β-catenin.136 Deletion of the c-terminal domain of connexin-43 in a mouse model resulted in disruption of osteoblast proliferation, differentiation, and collagen processing and organization,136 suggesting connexin-43-mediated intracellular signaling may be needed for bone formation.
Collectively, there is a need to better understand how chondrogenesis and osteogenesis are mediated by cadherins and gap junctions. Not only will a more thorough understanding of these differentiation processes point to potential tenogenic mechanisms, but aberrant differentiation of tendon cells into bone or cartilage is frequently observed in human tendinopathies.102 Ectopic ossification or chondrogenesis within tendons implies the processes are related, and communication deficiencies or incorrect cell signaling may induce aberrant differentiation and lead to disease.
Cell-cell junctions in musculoskeletal system pathologies
Cadherins in musculoskeletal and skin diseases
Cadherin-11 has been shown to participate in the inflammatory mechanisms of rheumatoid arthritis in the synovium.137 Although cadherin-11 is required for synovial lining formation,129 it also contributed to inflammation and mediated cartilage degradation, possibly via recruitment of inflammatory molecules to the synovium.137 Another study found that IL-17, an inflammatory cytokine involved in rheumatoid arthritis, increased cadherin-11 expression in the knee joints of mice with induced arthritis.138 The same study found increased cadherin-11 expression in the synovium of mice with arthritis, as well as in mice with deficient IL-1 receptors, and human patients with rheumatoid arthritis. Cultured fibroblast-like synoviocytes also had significantly increased cadherin-11 when treated with IL-17, but increases were blocked in cells treated with nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) inhibitors to suppress the inflammatory response.138 Blocking cadherin-11 during rheumatoid arthritis may attenuate some of the symptoms, but future studies must determine other side effects of disrupting cadherin-mediated cell adhesion in the synovium. Nevertheless, cadherins may be promising targets for preventing or reversing inflammation within joints and are worth exploring in tendon and other musculoskeletal tissues.
Cadherin-11 is also implicated in pathologies of the mouth and skin. Human periodontal ligament cells express cadherin-11, but expression was suppressed by mechanical stress, as cultured cells weighted under glass coverslips from 0 to 2 g/cm2 for up to 24 h showed increased cadherin-11 suppression with higher stress and loading time.139 Col I and β-catenin expression were also suppressed by loading, and reducing cadherin-11 expression with β-catenin knockdown led to rounding of periodontal ligament cells and an overall change in collagen matrix deposition.139 Cadherin-11 was also found on the surface of fibroblasts and macrophages isolated from the skin of systemic sclerosis patients.140,141 Blood samples from patients with widespread skin symptoms of systemic sclerosis showed significantly increased cadherin-11 expression, compared to samples from patients with limited systemic sclerosis or other connective tissue diseases.140 Furthermore, logistic regression analysis showed a significant correlation of increased cadherin-11 expression with extensive skin symptoms of systemic sclerosis.140 Together, these findings illustrate that cadherin-11 may play a role in some musculoskeletal pathologies.
Connexins in musculoskeletal and skin diseases
Connexins are involved in many human pathologies, and the mechanisms by which connexins contribute to various disease states have been summarized in a few recent reviews,131,142 though it was noted that fundamental knowledge of connexin signaling in cartilage, tendon, and muscle is lacking.131 However, connexin-43 has emerged as a potential mediator of synovium pathologies. Elevated connexin-43 expression was seen in the synovial lining of patients with osteoarthritis. Connexin-43 expression was 50% higher and MMP-1 production was increased with osteoarthritis, possibly in response to elevated IL-1β.143 IL-1β was also detected in diseased synovia of human osteoarthritis patients,143 and IL-1β was shown to significantly increase connexin-43 production and localization to the cell membrane in adult rabbit synovial fibroblasts.144 In a different study, human chondrocytes from the articular cartilage of the femoral head and knee of patients with osteoarthritis also had elevated expression of connexin-43 and −45, and increased gap junction plaques.132 Together, there is evidence of connexin-43 involvement in inflammation and osteoarthritis in synovial lining and cartilage. However, future studies are needed to identify the contributions of connexin-43 and other connexins, such as connexin-32, to tendon and other musculoskeletal pathologies.
Conclusions and future directions
Tendon cells in immature and mature tendons possess an array of cell-cell junction proteins, including cadherins and connexins. It is likely that additional cell-cell junction proteins are present and have yet to be explored. Improving our understanding of how the currently identified cell-cell junctions (e.g., cadherin-11, N-cadherin, connexin-32 and −43) and potential downstream signaling pathways (e.g., β-catenin) (Figure 4) regulate tenogenesis may result in novel strategies to direct engineered tendon formation and differentiation. A potential approach is designing engineered tissue scaffolds that are functionalized with peptides that mimic specific cell-cell junctions, similar to what has been demonstrated with N-cadherin mimicking peptides for chondrogenesis125 and osteogenesis.124 Tendon tissue engineering strategies that exploit the natural ability of the cells to form tissues through cadherin and connexin mediated self-assembly, such as scaffold-free techniques that found cadherin-11 in the early stages of fiber formation,128 could also be guided by a more complete understanding of the cell-cell adhesions found in developing tendon. A different approach may be to use novel biomaterials to control spatiotemporal siRNA application to stem cells to regulate cell-cell junction levels and ultimately tendon formation.145 Future engineered tissue constructs with the appropriate types and ratios of cadherins and connexins may also acquire self-organizational capacity as the cell–cell interactions mimic in vivo development. Constructs that can mimic the developmental processes more closely can offer new insights into cell-cell junction-associated signaling pathways that remain underexplored in tendon development (e.g., Wnt/β-catenin). Furthermore, β-catenin may be particularly relevant in tenogenesis as it is needed for recruitment of vinculin and actin to mechanically stressed cadherin complexes,78 and β- and γ-catenin are critical to the formation of adherens junctions in Drosophila, but are unexplored in the developing tendons of mammals.79
Figure 4.
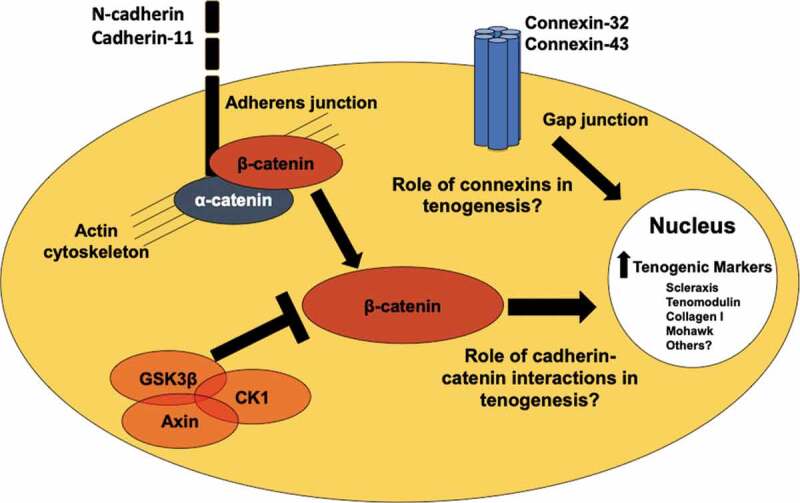
Potential cell-cell junction and cell signaling regulators of tenogenesis. N-cadherin and cadherin-11 are found in embryonic and adult tendon cells,37,81 and are regulated in tenogenically differentiating stem cells.90 Cadherin-β-catenin coupling and β-catenin signaling may regulate tendon and stem cell behavior.61,62 Connexin-32 and connexin-43 are present in embryonic, postnatal, and adult tendon cells.38,66 Future studies are needed to understand what the roles these cell-cell junction proteins and β-catenin are playing in tenogenesis.
Other regulators of cell-cell junctions in tendon may also emerge. The tumor suppressor protein p53, which is mutated in several cancers that also involve cadherin mutations,146 was found elevated in partially torn human supraspinatus tendons, compared to intact reference subscapularis tendons.147 Injured tendons displayed increased apoptosis and proliferation compared to intact controls,147 and it is possible that p53 affects cadherins or other cell-cell junctions in tendons following injury, though its potential involvement has yet to be explored. In addition to p53, NF-κB was found to be elevated in tendinopathy and tendon injury, and presents a possible target for reducing inflammation in tendinopathies.148,149 This upregulation of NF-κB during tendinopathy may potentially affect cell-cell junction proteins, though any relationship between NF-κB and cell-cell junctions in tendon has yet to be investigated. Overall, a comprehensive understanding of potential regulators of cell-cell junction proteins in tendon will provide new targets for clinical treatment of tendinopathies.
Our current understanding of the roles of cell-cell junctions in bone and cartilage highlights the diverse contributions of cadherins and connexins to the development of musculoskeletal tissues. In comparison, the regulation of tendon formation by cell-cell junctions is poorly understood. Given that bone and cartilage form concurrently with tendon during limb development, and their development is spatiotemporally regulated by cell-cell junctions, it is possible cell-cell junctions are also critical regulators of tenogenic differentiation. Ultimately, improved understanding of the roles of cell-cell junctions in tendon differentiation, development, and maintenance has implications for advancing tissue engineering approaches and regenerative tendon healing. Additionally, cell-cell junctions are involved in pathologies of the musculoskeletal system and deserve further investigation in tendon.
Funding Statement
This work was supported by the INBRE Program, NIH Grant No. [P20 GM103408] (National Institute of General Medical Sciences), the John F. Keegan Fellowship (to SKT), and the Beckman Scholars Award from the Arnold and Mabel Beckman Foundation (to JBM).
Acknowledgments
The authors would like to thank Nicholas Pancheri for reading the manuscript.
Disclosure of Interest
The authors report no conflict of interest.
References
- 1.Galatz LM, Gerstenfeld L, Heber-Katz E, Rodeo SA.. Tendon regeneration and scar formation: the concept of scarless healing. J. Orthop. Res. 2015;33(6):1–26. doi: 10.1002/jor.22853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang G, Rothrauff BB, Tuan RS. Tendon and ligament regeneration and repair: clinical relevance and developmental paradigm. Birth Defects Res. C Embryo Today. 2013;99(3):203–222. doi: 10.1002/bdrc.v99.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin TW, Cardenas L, Soslowsky LJ. Biomechanics of tendon injury and repair. J. Biomech. 2004;37(6):865–877. doi: 10.1016/j.jbiomech.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Wilkins R, Bisson LJ. Operative versus nonoperative management of acute Achilles tendon ruptures: a quantitative systematic review of randomized controlled trials. Am. J. Sports Med. 2012;40(9):2154–2160. doi: 10.1177/0363546512453293. [DOI] [PubMed] [Google Scholar]
- 5.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J. Bone Joint Surg. Am. 2004;86-A(2):219–224. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Heuberer PR, Smolen D, Pauzenberger L, Plachel F, Salem S, Laky B, Kriegleder B, Anderl W. Longitudinal long-term magnetic resonance imaging and clinical follow-up after single-row arthroscopic rotator cuff repair: clinical superiority of structural tendon integrity. Am. J. Sports Med. 2017;45(6):1283–1288. doi: 10.1177/0363546517689873. [DOI] [PubMed] [Google Scholar]
- 7.Lantto I, Heikkinen J, Flinkkila T, Ohtonen P, Leppilahti J. Epidemiology of Achilles tendon ruptures: increasing incidence over a 33-year period. Scand. J. Med. Sci. Sports. 2015;25(1):e133–8. doi: 10.1111/sms.2015.25.issue-1. [DOI] [PubMed] [Google Scholar]
- 8.Subramanian A, Schilling TF. Tendon development and musculoskeletal assembly: emerging roles for the extracellular matrix. Development. 2015;142:4191–4204. doi: 10.1242/dev.114777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kastelic J, Galeski A, Baer E. The multicomposite structure of tendon. Connect. Tissue. Res. 1978;6(1):11–23. doi: 10.3109/03008207809152283. [DOI] [PubMed] [Google Scholar]
- 10.Arnoczky SP, Lavagnino M, Egerbacher M. The mechanobiological aetiopathogenesis of tendinopathy: is it the over-stimulation or the under-stimulation of tendon cells? Int. J. Exp. Pathol. 2007;88(4):217–226. doi: 10.1111/j.1365-2613.2007.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavagnino M, Bedi A, Walsh CP, Enselman ERS, Sheibani-Rad S, Arnoczky SP. Tendon contraction after cyclic elongation is an age-dependent phenomenon in vitro and in vivo comparisons. Am. J. Sport Med. 2014;42(6):1471–1477. doi: 10.1177/0363546514526691. [DOI] [PubMed] [Google Scholar]
- 12.McNeilly CM, Banes AJ, Benjamin M, Ralphs JR. Tendon cells in vivo form a three dimensional network of cell processes linked by gap junctions. J. Anat. 1996;189:593–600. [PMC free article] [PubMed] [Google Scholar]
- 13.Schiele NR, von Flotow F, Tochka ZL, Hockaday LA, Marturano JE, Thibodeau JJ, Kuo CK. Actin cytoskeleton contributes to the elastic modulus of embryonic tendon during early development. J. Orthop. Res. 2015;33(6):874–881. doi: 10.1002/jor.22880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grinstein M, Dingwall HL, O’Connor LD, Zou K, Capellini TD, Galloway JL. A distinct transition from cell growth to physiological homeostasis in the tendon. eLife. 2019;8:e48689. doi: 10.7554/eLife.48689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaplin DM, Greenlee TK Jr.. The development of human digital tendons. J. Anat. 1975;120:253–274. [PMC free article] [PubMed] [Google Scholar]
- 16.Leckband DE, de Rooij J. Cadherin adhesion and mechanotransduction. Annu. Rev. Cell Dev. Biol. 2014;30:291–315. doi: 10.1146/annurev-cellbio-100913-013212. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz AG, Long F, Thomopoulos S. Enthesis fibrocartilage cells originate from a population of hedgehog-responsive cells modulated by the loading environment. Development. 2015;142(1):196–206. doi: 10.1242/dev.112714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott A, Danielson P, Abraham T, Fong G, Sampaio AV, Underhill TM. Mechanical force modulates scleraxis expression in bioartificial tendons. J. Musculoskelet Neuronal Interact. 2011;11:124–132. [PubMed] [Google Scholar]
- 19.Chokalingam K, Juncosa-Melvin N, Hunter SA, Gooch C, Frede C, Florert J, Bradica G, Wenstrup R, Butler DL. Tensile stimulation of murine stem cell-collagen sponge constructs increases collagen type I gene expression and linear stiffness. Tissue Eng. Part A. 2009;15(9):2561–2570. doi: 10.1089/ten.tea.2008.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juncosa-Melvin N, Matlin KS, Holdcraft RW, Nirmalanandhan VS, Butler DL. Mechanical stimulation increases collagen type I and collagen type III gene expression of stem cell-collagen sponge constructs for patellar tendon repair. Tissue Eng. 2007;13(6):1219–1226. doi: 10.1089/ten.2006.0339. [DOI] [PubMed] [Google Scholar]
- 21.Kalson NS, Holmes DF, Herchenhan A, Lu Y, Starborg T, Kadler KE. Slow stretching that mimics embryonic growth rate stimulates structural and mechanical development of tendon-like tissue in vitro. Dev. Dyn. 2011;240(11):2520–2528. doi: 10.1002/dvdy.22760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuo CK, Tuan RS. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng. Part A. 2008;14(10):1615–1627. doi: 10.1089/ten.tea.2006.0415. [DOI] [PubMed] [Google Scholar]
- 23.Mubyana K, Corr DT. Cyclic uniaxial tensile strain enhances the mechanical properties of engineered Scaffold-Free Tendon Fibers. Tissue Eng. Part A. 2018;24:1807–1817. [DOI] [PubMed] [Google Scholar]
- 24.Qin T-W, Sun Y-L, Thoreson AR, Steinmann SP, Amadio PC, An K-N, Zhao C. Effect of mechanical stimulation on bone marrow stromal celleseeded tendon slice constructs: A potential engineered tendon patch for rotator cuff repair. Biomaterials. 2015;51:43–50. doi: 10.1016/j.biomaterials.2015.01.070. [DOI] [PubMed] [Google Scholar]
- 25.Nirmalanandhan VS, Dressler MR, Shearn JT, Juncosa-Melvin N, Rao M, Gooch C, Bradica G, Butler DL. Mechanical stimulation of tissue engineered tendon constructs: effect of scaffold materials. J. Biomech. Eng. 2007;129(6):919–923. doi: 10.1115/1.2800828. [DOI] [PubMed] [Google Scholar]
- 26.Schiele NR, Marturano JE, Kuo CK. Mechanical factors in embryonic tendon development: potential cues for stem cell tenogenesis. Curr. Opin. Biotechnol. 2013;24(5):834–840. doi: 10.1016/j.copbio.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shearn JT, Juncosa-Melvin N, Boivin GP, Galloway MT, Goodwin W, Gooch C, Dunn MG, Butler DL. Mechanical stimulation of tendon tissue engineered constructs: effects on construct stiffness, repair biomechanics, and their correlation. J. Biomech. Eng-T Asme. 2007;129(6):848–854. doi: 10.1115/1.2800769. [DOI] [PubMed] [Google Scholar]
- 28.Subramony SD, Dargis BR, Castillo M, Azeloglu EU, Tracey MS, Su A, Lu HH. The guidance of stem cell differentiation by substrate alignment and mechanical stimulation. Biomaterials. 2013;34(8):1942–1953. doi: 10.1016/j.biomaterials.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. [DOI] [PubMed] [Google Scholar]
- 30.Ito Y, Toriuchi N, Yoshitaka T, Ueno-Kudoh H, Sato T, Yokoyama S, Nishida K, Akimoto T, Takahashi M, Miyaki S, et al. The mohawk homeobox gene is a critical regulator of tendon differentiation. Proc. Natl. Acad. Sci. U.S.A. 2010;107(23):10538–10542. doi: 10.1073/pnas.1000525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Docheva D, Hunziker EB, Fassler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol. Cell Biol. 2005;25(2):699–705. doi: 10.1128/MCB.25.2.699-705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113(2):235–248. doi: 10.1016/S0092-8674(03)00268-X. [DOI] [PubMed] [Google Scholar]
- 33.Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007;134(14):2697–2708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- 34.Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dunker N, Schweitzer R. Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development. 2009;136(8):1351–1361. doi: 10.1242/dev.027342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shukunami C, Takimoto A, Oro M, Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev. Biol. 2006;298(1):234–247. doi: 10.1016/j.ydbio.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 36.Marturano JE, Arena JD, Schiller ZA, Georgakoudi I, Kuo CK. Characterization of mechanical and biochemical properties of developing embryonic tendon. Proc. Natl. Acad. Sci. U.S.A. 2013;110(16):6370–6375. doi: 10.1073/pnas.1300135110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richardson SH, Starborg T, Lu Y, Humphries SM, Meadows RS, Kadler KE. Tendon development requires regulation of cell condensation and cell shape via cadherin-11-mediated cell-cell junctions. Mol. Cell Biol. 2007;27(17):6218–6228. doi: 10.1128/MCB.00261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanley RL, Fleck RA, Becker DL, Goodship AE, Ralphs JR, Patterson-Kane JC. Gap junction protein expression and cellularity: comparison of immature and adult equine digital tendons. J. Anat. 2007;211:325–334. doi: 10.1111/joa.2007.211.issue-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kimura Y, Matsunami H, Inoue T, Shimamura K, Uchida N, Ueno T, Miyazaki T, Takeichi M. Cadherin-11 expressed in association with mesenchymal morphogenesis in the head, somite, and limb bud of early mouse embryos. Dev. Biol. 1995;169(1):347–358. doi: 10.1006/dbio.1995.1149. [DOI] [PubMed] [Google Scholar]
- 40.Luo Y, Ferreira-Cornwell M, Baldwin H, Kostetskii I, Lenox J, Lieberman M, Radice G. Rescuing the N-cadherin knockout by cardiac-specific expression of N- or E-cadherin. Development. 2001;128:459–469. [DOI] [PubMed] [Google Scholar]
- 41.Luo Y, Kostetskii I, Radice GL. N-cadherin is not essential for limb mesenchymal chondrogenesis. Dev Dyn. 2005;232(2):336–344. doi: 10.1002/(ISSN)1097-0177. [DOI] [PubMed] [Google Scholar]
- 42.Hasson P, DeLaurier A, Bennett M, Grigorieva E, Naiche LA, Papaioannou VE, Mohun TJ, Logan MP. Tbx4 and tbx5 acting in connective tissue are required for limb muscle and tendon patterning. Dev. Cell. 2010;18(1):148–156. doi: 10.1016/j.devcel.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brembeck FH, Rosario M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr. Opin. Genet. Dev. 2006;16(1):51–59. doi: 10.1016/j.gde.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 44.Hinz B, Pittet P, Smith-Clerc J, Chaponnier C, Meister JJ. Myofibroblast development is characterized by specific cell-cell adherens junctions. Mol. Biol. Cell. 2004;15(9):4310–4320. doi: 10.1091/mbc.e04-05-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pittet P, Lee KM, Kulik AJ, Meister JJ, Hinz B. Fibrogenic fibroblasts increase intercellular adhesion strength by reinforcing individual OB-cadherin bonds. J. Cell Sci. 2008;121(6):877–886. doi: 10.1242/jcs.024877. [DOI] [PubMed] [Google Scholar]
- 46.Helmbacher F. Tissue-specific activities of the Fat1 cadherin cooperate to control neuromuscular morphogenesis. PLoS Biol. 2018;16(5):e2004734. doi: 10.1371/journal.pbio.2004734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmed A, de Bock C, Lincz L, Pundavela J, Zouikr I, Sontag E, Hondermarck H, Thorne R. FAT1 cadherin acts upstream of hippo signalling through TAZ to regulate neuronal differentiation. Cell Mol. Life Sci. 2015;72:4653–4669. doi: 10.1007/s00018-015-1955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morris LG, Kaufman AM, Gong Y, Ramaswami D, Walsh LA, Turcan S, Eng S, Kannan K, Zou Y, Peng L, et al. Recurrent somatic mutation of FAT1 in multiple human cancers leads to aberrant Wnt activation. Nat. Genet. 2013;45(3):253–261. doi: 10.1038/ng.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang AH, Riordan TJ, Pryce BA, Weibel JL, Watson SS, Long F, Lefebvre V, Harfe BD, Stadler HS, Akiyama H, et al. Musculoskeletal integration at the wrist underlies the modular development of limb tendons. Development. 2015;142(14):2431–2441. doi: 10.1242/dev.122374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev. Dyn. 2005;233(3):706–720. doi: 10.1002/(ISSN)1097-0177. [DOI] [PubMed] [Google Scholar]
- 51.Smith TG, Van Hateren N, Tickle C, Wilson SA. The expression of Fat-1 cadherin during chick limb development. Int. J. Dev. Biol. 2007;51(2):173–176. doi: 10.1387/ijdb.062221tg. [DOI] [PubMed] [Google Scholar]
- 52.Seidler DG, Dreier R. Decorin and its galactosaminoglycan chain: extracellular regulator of cellular function? IUBMB Life. 2008;60(11):729–733. doi: 10.1002/iub.v60:11. [DOI] [PubMed] [Google Scholar]
- 53.Ruhland C, Schonherr E, Robenek H, Hansen U, Iozzo RV, Bruckner P, Seidler DG. The glycosaminoglycan chain of decorin plays an important role in collagen fibril formation at the early stages of fibrillogenesis. Febs J. 2007;274(16):4246–4255. doi: 10.1111/j.1742-4658.2007.05951.x. [DOI] [PubMed] [Google Scholar]
- 54.Dunkman AA, Buckley MR, Mienaltowski MJ, Adams SM, Thomas SJ, Kumar A, Beason DP, Iozzo RV, Birk DE, Soslowsky LJ. The injury response of aged tendons in the absence of biglycan and decorin. Matrix Biol. 2014;35:232–238. doi: 10.1016/j.matbio.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lorda-Diez CI, Garcia-Porrero JA, Hurle JM, Montero JA. Decorin gene expression in the differentiation of the skeletal connective tissues of the developing limb. Gene. Expr. Patterns. 2014;15:52–60. doi: 10.1016/j.gep.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 56.Akiyama H, Chaboissier M, Martin JF, Schedl A, De Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Gene Dev. 2002;16:2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123(5):889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gottardi CJ, Gumbiner BM. Distinct molecular forms of β-catenin are targeted to adhesive or transcriptional complexes. J. Cell Biol. 2004;167(2):339–349. doi: 10.1083/jcb.200402153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valenta T, Hausmann G, Basler K. The many faces and functions of β-catenin. Embo J. 2012;31(12):2714–2736. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verheyen EM, Gottardi CJ. Regulation of Wnt/β-catenin signaling by protein kinases. Dev. Dyn. 2010;239(1):34–44. doi: 10.1002/dvdy.22019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kishimoto Y, Ohkawara B, Sakai T, Ito M, Masuda A, Ishiguro N, Shukunami C, Docheva D, Ohno K. Wnt/β-catenin signaling suppresses expressions of Scx, Mkx, and Tnmd in tendon derived cells. PLoS One. 2017;12(7):e0182051. doi: 10.1371/journal.pone.0182051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miyabara S, Yuda Y, Kasashima Y, Kuwano A, Arai K. Regulation of tenomodulin expression Via Wnt/beta-catenin signaling in equine bone marrow-derived mesenchymal stem cells. J. Equine Sci. 2014;25(1):7–13. doi: 10.1294/jes.25.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Genet N, Bhatt N, Bourdieu A, Hirschi KK. Multifaceted roles of connexin 43 in stem cell niches. Curr. Stem Cell Rep. 2018;4(1):1–12. doi: 10.1007/s40778-018-0110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Plotkin LI, Stains JP. Connexins and pannexins in the skeleton: gap junctions, hemichannels and more. Cell. Mol. Life Sci. 2015;72(15):2853–2867. doi: 10.1007/s00018-015-1963-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ralphs JR, Benjamin M, Waggett AD, Russell DC, Messner K, Gao J. Regional differences in cell shape and gap junction expression in rat Achilles tendon: relation to fibrocartilage differentiation. J. Anat. 1998;193:215–222. doi: 10.1046/j.1469-7580.1998.19320215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Waggett AD, Benjamin M, Ralphs JR. Connexin 32 and 43 gap junctions differentially modulate tenocyte response to cyclic mechanical load. Eur. J. Cell Biol. 2006;85(11):1145–1154. doi: 10.1016/j.ejcb.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 67.Russo V, Mauro A, Martelli A, Di Giacinto O, Di Marcantonio L, Nardinocchi D, Berardinelli P, Barboni B. Cellular and molecular maturation in fetal and adult ovine calcaneal tendons. J. Anat. 2015;226:126–142. doi: 10.1111/joa.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coleman CM, Loredo GA, Lo CW, Tuan RS. Correlation of GDF5 and connexin 43 mRNA expression during embryonic development. Anatomical Record Part B. 2003;275A:1117–1121. doi: 10.1002/(ISSN)1097-0185. [DOI] [PubMed] [Google Scholar]
- 69.Ansorge HL, Adams S, Birk DE, Soslowsky LJ. Mechanical, compositional, and structural properties of the post-natal mouse Achilles tendon. Ann. Biomed. Eng. 2011;39(7):1904–1913. doi: 10.1007/s10439-011-0299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meller R, Schiborra F, Brandes G, Knobloch K, Tschernig T, Hankemeier S, Haasper C, Schmiedl A, Jagodzinski M, Krettek C, et al. Postnatal maturation of tendon, cruciate ligament, meniscus and articular cartilage: a histological study in sheep. Ann. Anat. 2009;191(6):575–585. doi: 10.1016/j.aanat.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 71.Theodossiou SK, Bozeman AL, Burgett N, Brumley MR, Swann HE, Raveling AR, Becker JJ, Schiele NR. Onset of neonatal locomotor behavior and the mechanical development of Achilles and tail tendons. J. Biomech. 2019;96:109354. doi: 10.1016/j.jbiomech.2019.109354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yin Z, Hu JJ, Yang L, Zheng ZF, An CR, Wu BB, Zhang C, Shen WL, Liu HH, Chen JL, et al. Single-cell analysis reveals a nestin+ tendon stem/progenitor cell population with strong tenogenic potentiality. Sci. Adv. 2016;2(11):e1600874. doi: 10.1126/sciadv.1600874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith SM, Thomas CE, Birk DE. Pericellular proteins of the developing mouse tendon: a proteomic analysis. Connect. Tissue. Res. 2012;53(1):2–13. doi: 10.3109/03008207.2011.602766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim D, Kang SS, Jin EJ. Alterations in the temporal expression and function of cadherin-7 inhibit cell migration and condensation during chondrogenesis of chick limb mesenchymal cells in vitro. J. Cell Physiol. 2009;221(1):161–170. doi: 10.1002/jcp.21840. [DOI] [PubMed] [Google Scholar]
- 75.Coleman CM, Tuan RS. Functional role of growth/differentiation factor 5 in chondrogenesis of limb mesenchymal cells. Mech. Dev. 2003;120:823–836. doi: 10.1016/S0925-4773(03)00067-4. [DOI] [PubMed] [Google Scholar]
- 76.Taylor SH, Al-Youha S, Van Agtmael T, Lu Y, Wong J, McGrouther DA, Kadler KE. Tendon is covered by a basement membrane epithelium that is required for cell retention and the prevention of adhesion formation. PLoS One. 2011;6(1):e16337. doi: 10.1371/journal.pone.0016337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wall ME, Dyment NA, Bodle J, Volmer J, Loboa E, Cederlund A, Fox AM, Banes AJ. Cell signaling in tenocytes: response to load and ligands in health and disease. In: Ackerman PW, Hart DA, editors. Metabolic influences on risk for tendon disorders. Switzerland: Springer International Publishing; 2016. p. 79–95. [DOI] [PubMed] [Google Scholar]
- 78.Barry AK, Tabdili H, Muhamed I, Wu J, Shashikanth N, Gomez GA, Yap AS, Gottardi CJ, de Rooij J, Wang N, et al. alpha-catenin cytomechanics–role in cadherin-dependent adhesion and mechanotransduction. J. Cell Sci. 2014;127(Pt 8):1779–1791. doi: 10.1242/jcs.139014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Desai R, Sarpal R, Ishiyama N, Pellikka M, Ikura M, Tepass U. Monomeric alpha-catenin links cadherin to the actin cytoskeleton. Nat. Cell Biol. 2013;15(3):261–273. doi: 10.1038/ncb2685. [DOI] [PubMed] [Google Scholar]
- 80.Keller TC, Hogan MV, Kesturu G, James R, Balian G, Chhabra AB. Growth/differentiation factor-5 modulates the synthesis and expression of extracellular matrix and cell-adhesion-related molecules of rat Achilles tendon fibroblasts. Connect. Tissue. Res. 2011;52(4):353–364. doi: 10.3109/03008207.2010.534208. [DOI] [PubMed] [Google Scholar]
- 81.Ralphs JR, Waggett AD, Benjamin M. Actin stress fibres and cell-cell adhesion molecules in tendons: organisation in vivo and response to mechanical loading of tendon cells in vitro. Matrix Biol. 2002;21(1):67–74. doi: 10.1016/S0945-053X(01)00179-2. [DOI] [PubMed] [Google Scholar]
- 82.Maeda E, Ye SJ, Wang W, Bader DL, Knight MM, Lee DA. Gap junction permeability between tenocytes within tendon fascicles is suppressed by tensile loading. Biomech. Model Mechan. 2012;11(3–4):439–447. doi: 10.1007/s10237-011-0323-1. [DOI] [PubMed] [Google Scholar]
- 83.Banes AJ, Weinhold P, Yang X, Tsuzaki M, Bynum D, Bottlang M, Brown T. Gap junctions regulate responses of tendon cells ex vivo to mechanical loading. Clin. Orthop. Relat. R. 1999;367:S356–S370. doi: 10.1097/00003086-199910001-00034. [DOI] [PubMed] [Google Scholar]
- 84.Maeda E, Ohashi T. Mechano-regulation of gap junction communications between tendon cells is dependent on the magnitude of tensile strain. Biochem. Bioph. Res. Co. 2015;465(2):281–286. doi: 10.1016/j.bbrc.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 85.Maeda E, Kimura S, Yamada M, Tashiro M, Ohashi T. Enhanced gap junction intercellular communication inhibits catabolic and pro-inflammatory responses in tenocytes against heat stress. J. Cell Commun. Signal. 2017;11(4):369–380. doi: 10.1007/s12079-017-0397-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maeda E, Pian H, Ohashi T. Temporal regulation of gap junctional communication between tenocytes subjected to static tensile strain with physiological and non-physiological amplitudes. Biochem. Biophys. Res. Commun. 2017;482(4):1170–1175. doi: 10.1016/j.bbrc.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 87.Wall ME, Otey C, Qi J, Banes AJ. Connexin 43 is localized with actin in tenocytes. Cell Motil. Cytoskel. 2007;64(2):121–130. doi: 10.1002/(ISSN)1097-0169. [DOI] [PubMed] [Google Scholar]
- 88.Mikic B, Schalet BJ, Clark RT, Gaschen V, Hunziker EB. GDF-5 deficiency in mice alters the ultrastructure, mechanical properties and composition of the Achilles tendon. J. Orthop. Res. 2001;19(3):365–371. doi: 10.1016/S0736-0266(00)90018-4. [DOI] [PubMed] [Google Scholar]
- 89.Chhabra A, Tsou D, Clark RT, Gaschen V, Hunziker EB, Mikic B. GDF-5 deficiency in mice delays Achilles tendon healing. J. Orthop. Res. 2003;21(5):826–835. doi: 10.1016/S0736-0266(03)00049-4. [DOI] [PubMed] [Google Scholar]
- 90.Theodossiou SK, Tokle J, Schiele NR. TGFbeta2-induced tenogenesis impacts cadherin and connexin cell-cell junction proteins in mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2019;508(3):889–893. doi: 10.1016/j.bbrc.2018.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kuzma-Kuzniarska M, Yapp C, Pearson-Jones TW, Jones AK, Hulley PA. Functional assessment of gap junctions in monolayer and three-dimensional cultures of human tendon cells using fluorescence recovery after photobleaching. J. Biomed. Opt. 2014;19(1). doi: 10.1117/1.JBO.19.1.015001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Screen HRC, Bader DL, Lee DA, Shelton JC. Local strain measurement within tendon. Strain. 2004;40:157–163. doi: 10.1111/str.2004.40.issue-4. [DOI] [Google Scholar]
- 93.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. Biomech. Eng. 2003;125:106–113. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- 94.Arnoczky SP, Tian T, Lavagnino M, Gardner K. Ex vivo static tensile loading inhibits MMP-1 expression in rat tail tendon cells through a cytoskeletally based mechanotransduction mechanism. J. Orthop. Res. 2004;22(2):328–333. doi: 10.1016/S0736-0266(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 95.Manning CN, Havlioglu N, Knutsen E, Sakiyama-Elbert SE, Silva MJ, Thomopoulos S, Gelberman RH. The early inflammatory response after flexor tendon healing: a gene expression and histological analysis. J. Orthop. Res. 2014;32(5):645–652. doi: 10.1002/jor.v32.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang G, Im HJ, Wang JH. Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene. 2005;363:166–172. doi: 10.1016/j.gene.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qi J, Fox AM, Alexopoulos LG, Chi L, Bynum D, Guilak F, Banes AJ. IL-1beta decreases the elastic modulus of human tenocytes. J. Appl. Physiol. 2006;101(1):189–195. doi: 10.1152/japplphysiol.01128.2005. [DOI] [PubMed] [Google Scholar]
- 98.Tsuzaki M, Guyton G, Garrett W, Archambault JM, Herzog W, Almekinders L, Bynum D, Yang X, Banes AJ. IL-1 beta induces COX2, MMP-1, −3 and −13, ADAMTS-4, IL-1 beta and IL-6 in human tendon cells. J. Orthop. Res. 2003;21(2):256–264. doi: 10.1016/S0736-0266(02)00141-9. [DOI] [PubMed] [Google Scholar]
- 99.Qi J, Chi L, Bynum D, Banes AJ. Gap junctions in IL-1β-mediated cell survival response to strain. J. Appl. Physiol. 2011;110(5):1425–1431. doi: 10.1152/japplphysiol.00477.2010. [DOI] [PubMed] [Google Scholar]
- 100.Khaliq Y, Zhanel GG. Fluoroquinolone-associated tendinopathy: a critical review of the literature. Clin. Infect. Dis. 2003;36:1404–1410. doi: 10.1086/375078. [DOI] [PubMed] [Google Scholar]
- 101.Menon A, Pettinari L, Martinelli C, Colombo G, Portinaro N, Dalle-Donne I, d’Agostino MC, Gagliano N. New insights in extracellular matrix remodeling and collagen turnover related pathways in cultured human tenocytes after ciprofloxacin administration. Muscles Ligaments Tendons J. 2013;3:122–131. [PMC free article] [PubMed] [Google Scholar]
- 102.O’Brien EJO, Frank CB, Shrive NG, Hallgrimsson B, Hart DA. Heterotropic mineralization (ossification or calcification) in tendinopathy or following surgical tendon trauma. Int. J. Exp. Pathol. 2012;93(5):319–331. doi: 10.1111/j.1365-2613.2012.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang C, Zhang Y, Zhong B, Luo CF. SMAD7 prevents heterotopic ossification in a rat Achilles tendon injury model via regulation of endothelial-mesenchymal transition. Febs J. 2016;283(7):1275–1285. doi: 10.1111/febs.13667. [DOI] [PubMed] [Google Scholar]
- 104.Havis E, Bonnin MA, de Lima JE, Charvet B, Milet C, Duprez D. TGFβ and FGF promote tendon progenitor fate and act downstream of muscle contraction to regulate tendon differentiation during chick limb development. Development. 2016;143:3839–3851. doi: 10.1242/dev.136242. [DOI] [PubMed] [Google Scholar]
- 105.Havis E, Bonnin MA, Olivera-Martinez I, Nazaret N, Ruggiu M, Weibel J, Durand C, Guerquin MJ, Bonod-Bidaud C, Ruggiero F, et al. Transcriptomic analysis of mouse limb tendon cells during development. Development. 2014;141(19):3683–3696. doi: 10.1242/dev.108654. [DOI] [PubMed] [Google Scholar]
- 106.Lorda-Diez CI, Montero JA, Diaz-Mendoza MJ, Garcia-Porrero JA, Hurle J. M.. βig-h3 potentiates the profibrogenic effect of tgfβ signaling on connective tissue progenitor cells through the negative regulation of master chondrogenic genes. Tissue Eng. Part A. 2013;19(3 and 4):448–457. doi: 10.1089/ten.tea.2012.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sobral LM, Montan PF, Zecchin KG, Martelli-Junior H, Vargas PA, Graner E, Coletta RD. Smad7 blocks transforming growth factor‐β1–induced gingival fibroblast–myofibroblast transition via inhibitory regulation of smad2 and connective tissue growth factor. J. Periodontol. 2011;82(4):642–651. doi: 10.1902/jop.2010.100510. [DOI] [PubMed] [Google Scholar]
- 108.Khanna A, Friel M, Gougoulias N, Longo UG, Maffulli N. Prevention of adhesions in surgery of the flexor tendons of the hand: what is the evidence? Br. Med. Bull. 2009;90:85–109. doi: 10.1093/bmb/ldp013. [DOI] [PubMed] [Google Scholar]
- 109.Goncalves AI, Rodrigues MT, Lee SJ, Atala A, Yoo JJ, Reis RL, Gomes ME. Understanding the role of growth factors in modulating stem cell tenogenesis. PLoS One. 2013;8(12):e83734. doi: 10.1371/journal.pone.0083734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brown JP, Galassi TV, Stoppato M, Schiele NR, Kuo CK. Comparative analysis of mesenchymal stem cell and embryonic tendon progenitor cell response to embryonic tendon biochemical and mechanical factors. Stem Cell Res. Ther. 2015;6(1):89. doi: 10.1186/s13287-015-0043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kuo CK, Petersen BC, Tuan RS. Spatiotemporal protein distribution of TGF-betas, their receptors, and extracellular matrix molecules during embryonic tendon development. Dev. Dyn. 2008;237(5):1477–1489. doi: 10.1002/dvdy.21547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brown JP, Finley VG, Kuo CK. Embryonic mechanical and soluble cues regulate tendon progenitor cell gene expression as a function of developmental stage and anatomical origin. J. Biomech. 2014;47(1):214–222. doi: 10.1016/j.jbiomech.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chien C, Pryce B, Tufa SF, Keene DR, Huang AH. Optimizing a 3D model system for molecular manipulation of tenogenesis. Connect. Tissue. Res. 2018;59(4):295–308. doi: 10.1080/03008207.2017.1383403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Delise AM, Tuan RS. Analysis of N-cadherin function in limb mesenchymal chondrogenesis in vitro. Dev. Dyn. 2002;225(2):195–204. doi: 10.1002/(ISSN)1097-0177. [DOI] [PubMed] [Google Scholar]
- 115.Oberlender SA, Tuan RS. Expression and functional involvement of N-cadherin in embryonic limb chondrogenesis. Development. 1994;120:177–187. [DOI] [PubMed] [Google Scholar]
- 116.Kawaguchi J, Azuma Y, Hoshi K, Kii I, Takeshita S, Ohta T, Ozawa H, Takeichi M, Chisaka O, Kudo A. Targeted disruption of cadherin-11 leads to a reduction in bone density in calvaria and long bone metaphyses. J. Bone Miner. Res. 2001;16(7):1265–1271. doi: 10.1359/jbmr.2001.16.7.1265. [DOI] [PubMed] [Google Scholar]
- 117.Alimperti S, Andreadis ST. CDH2 and CDH11 act as regulators of stem cell fate decisions. Stem Cell Res. 2015;14(3):270–282. doi: 10.1016/j.scr.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Haas AR, Tuan RS. Chondrogenic differentiation of murine C3H10T1/2 multipotential mesenchymal cells: II. Stimulation by bone morphogenetic protein-2 requires modulation of N-cadherin expression and function. Differentiation. 1999;64(2):77–89. doi: 10.1046/j.1432-0436.1999.6420077.x. [DOI] [PubMed] [Google Scholar]
- 119.Woods A, Wang G, Dupuis H, Shao Z, Beier F. Rac1 signaling stimulates N-cadherin expression, mesenchymal condensation, and chondrogenesis. J. Biol. Chem. 2007;282(32):23500–23508. doi: 10.1074/jbc.M700680200. [DOI] [PubMed] [Google Scholar]
- 120.Tuli R, Tuli S, Nandi S, Huang X, Manner PA, Hozack WJ, Danielson KG, Hall DJ, Tuan RS. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J. Biol. Chem. 2003;278(42):41227–41236. doi: 10.1074/jbc.M305312200. [DOI] [PubMed] [Google Scholar]
- 121.Bian L, Zhai DY, Tous E, Rai R, Mauck RL, Burdick JA. Enhanced MSC chondrogenesis following delivery of TGF-beta3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials. 2011;32(27):6425–6434. doi: 10.1016/j.biomaterials.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gao L, McBeath R, Chen CS. Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N-cadherin. Stem Cells. 2010;28(3):564–572. doi: 10.1002/stem.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Di Benedetto A, Watkins M, Grimston S, Salazar V, Donsante C, Mbalaviele G, Radice GL, Civitelli R. N-cadherin and cadherin 11 modulate postnatal bone growth and osteoblast differentiation by distinct mechanisms. J. Cell Sci. 2010;123(Pt 15):2640–2648. doi: 10.1242/jcs.067777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhu M, Lin S, Sun Y, Feng Q, Li G, Bian L. Hydrogels functionalized with N-cadherin mimetic peptide enhance osteogenesis of hMSCs by emulating the osteogenic niche. Biomaterials. 2016;77:44–52. doi: 10.1016/j.biomaterials.2015.10.072. [DOI] [PubMed] [Google Scholar]
- 125.Bian L, Guvendiren M, Mauck RL, Burdick JA. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc. Natl. Acad. Sci. U.S.A. 2013;110(25):10117–10122. doi: 10.1073/pnas.1214100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cheng SL, Lecanda F, Davidson MK, Warlow PM, Zhang SF, Zhang L, Suzuki S, St John T, Civitelli R. Human osteoblasts express a repertoire of cadherins, which are critical for BMP-2-induced osteogenic differentiation. J. Bone Miner. Res. 1998;13(4):633–644. doi: 10.1359/jbmr.1998.13.4.633. [DOI] [PubMed] [Google Scholar]
- 127.Xu L, Meng F, Ni M, Lee Y, Li G. N-cadherin regulates osteogenesis and migration of bone marrow-derived mesenchymal stem cells. Mol. Biol. Rep. 2013;40(3):2533–2539. doi: 10.1007/s11033-012-2334-0. [DOI] [PubMed] [Google Scholar]
- 128.Schiele NR, Koppes RA, Chrisey DB, Corr DT. Engineering cellular fibers for musculoskeletal soft tissues using directed self-assembly. Tissue Eng. Part A. 2013;19(9–10):1223–1232. doi: 10.1089/ten.tea.2012.0321. [DOI] [PubMed] [Google Scholar]
- 129.Kiener HP, Brenner MB. Building the synovium: cadherin 11 mediates fibroblaset-like synoviocyte cell-cell adhesion. Arthritis Res. Ther. 2005;7:49–54. doi: 10.1186/ar1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Agarwal SK, Lee DM, Kiener HP, Brenner MB. Coexpression of two mesenchymal cadherins, cadherin 11 and N-cadherin, on murine fibroblast-like synoviocytes. Arthritis Rheum-Us. 2008;58(4):1044–1054. doi: 10.1002/art.23369. [DOI] [PubMed] [Google Scholar]
- 131.Donahue HJ, Qu RW, Genetos DC. Joint diseases: from connexins to gap junctions. Nat. Rev. 2018;14:42–51. [DOI] [PubMed] [Google Scholar]
- 132.Mayan MD, Carpintero-Fernandez P, Gago-Fuentes R, Martinez-de-Ilarduya O, Wang H, Valiunas V, Brink P, Blanco FJ. Human articular chondrocytes express multiple gap junction proteins. Am. J. Pathol. 2013;182(4):1337–1346. doi: 10.1016/j.ajpath.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schrobback K, Klein TJ, Woodfield TB. The importance of connexin hemichannels during chondroprogenitor cell differentiation in hydrogel versus microtissue culture models. Tissue Eng. Part A. 2015;21(11–12):1785–1794. doi: 10.1089/ten.tea.2014.0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li Z, Zhou Z, Saunders MM, Donahue HJ. Modulation of connexin 43 alters expression of osteoblastic differentiation markers., American journal of physiology. Cell Physiology. 2006;290(4):1248–1255. doi: 10.1152/ajpcell.00428.2005. [DOI] [PubMed] [Google Scholar]
- 135.Grimston SK, Watkins MP, Brodt MD, Silva MJ, Civitelli R. Enhanced periosteal and endocortical responses to axial tibial compression loading in conditional connexin 43 deficient mice. PLoS One. 2012;7(9):e44222. doi: 10.1371/journal.pone.0044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moorer MC, Hebert C, Tomlinson RE, Iyer SR, Chason M, Stains JP. Defective signaling, osteoblastogenesis and bone remodeling in a mouse model of connexin 43 C-terminal truncation. J. Cell Sci. 2017;130(3):531–540. doi: 10.1242/jcs.197285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chang SK, Gu ZZ, Brenner MB. Fibroblast-like synoviocytes in inflammatory arthritis pathology: the emerging role of cadherin-11. Immunol. Rev. 2010;233:256–266. doi: 10.1111/imr.2009.233.issue-1. [DOI] [PubMed] [Google Scholar]
- 138.Park YE, Woo YJ, Park SH, Moon YM, Oh HJ, Kim JI, Jin HS, Baek SH, Kim GT, Lee JH, et al. IL-17 increases cadherin-11 expression in a model of autoimmune experimental arthritis and in rheumatoid arthritis. Immunol. Lett. 2011;140(1–2):97–103. doi: 10.1016/j.imlet.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 139.Feng L, Zhang Y, Kou X, Yang R, Liu D, Wang X, Song Y, Cao H, He D, Gan Y, et al. Cadherin-11 modulates cell morphology and collagen synthesis in periodontal ligament cells under mechanical stress. Angle Orthod. 2017;87(2):193–199. doi: 10.2319/020716-107.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Christopoulos PF, Bournia VK, Panopoulos S, Vaiopoulos A, Koutsilieris M, Sfikakis PP. Increased messenger RNA levels of the mesenchymal cadherin-11 in the peripheral blood of systemic sclerosis patients correlate with diffuse skin involvement. Clin. Exp. Rheumatol. 2015;33:S36–S39. [PubMed] [Google Scholar]
- 141.Wu MH, Pedroza M, Lafyatis R, George AT, Mayes MD, Assassi S, Tan FK, Brenner MB, Agarwal SK. Identification of cadherin 11 as a mediator of dermal fibrosis and possible role in systemic sclerosis. Arthritis Rheum. 2014;66(4):1010–1021. doi: 10.1002/art.38275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Srinivas M, Verselis VK, White TW. Human diseases associated with connexin mutations. Biochim. Biophys. Acta. 2018;1860(1):192–201. doi: 10.1016/j.bbamem.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Marino AA, Waddell DD, Kolomytkin OV, Meek WD, Wolf R, Sadasivan KK, Albright JA. Increased intercellular communication through gap junctions may contribute to progression of osteoarthritis. Clin. Orthop. Relat. Res. 2004;422:224–232. doi: 10.1097/01.blo.0000129346.29945.3b. [DOI] [PubMed] [Google Scholar]
- 144.Niger C, Howell FD, Stains JP. Interleukin-1β increases gap junctional communication among synovial fibroblasts via the extracellular signal regulated kinase pathway. Biol. Cell. 2009;102(1):37–49. doi: 10.1042/BC20090056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang Y, Zhang S, Benoit DSW. Degradable poly(ethylene glycol) (PEG)-based hydrogels for spatiotemporal control of siRNA/nanoparticle delivery. J. Control Release. 2018;287:58–66. doi: 10.1016/j.jconrel.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yu Y, Elble RC. Homeostatic signaling by cell–cell junctions and its dysregulation during cancer progression. J. Clin. Med. 2016;5(26):5020026. doi: 10.3390/jcm5020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lundgreen K, Lian O, Scott A, Engerbretsen L. Increased levels of apoptosis and p53 in partial-thickness supraspinatus tendon tears. Knee Surg. Sports Traumatol. Arthrosc. 2013;21:1636–1641. doi: 10.1007/s00167-012-2226-9. [DOI] [PubMed] [Google Scholar]
- 148.Abraham AC, Shah SA, Golman M, Song L, Li X, Kurtaliaj I, Akbar M, Millar NL, Abu-Amer Y, Galatz LM, et al. Targeting the NF-kB signaling pathway in chronic tendon disease. Sci. Transl. Med. 2019;11:eav4319. doi: 10.1126/scitranslmed.aav4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Best KT, Lee FK, Knapp E, Awad HA, Loiselle AE. Deletion of NFKB1 enhances canonical NF-κB signaling and increases macrophage and myofibroblast content during tendon healing. Sci. Rep. 2019;9:10926. doi: 10.1038/s41598-019-47461-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


