Supplemental Digital Content is available in the text.
Keywords: acute kidney injury, kidney injury molecule-1, neutrophil gelatinase–associated lipocalin, sepsis, subtypes
Objectives:
To identify mechanisms associated with sepsis-acute kidney injury based on the expression levels of renal injury biomarkers, neutrophil gelatinase–associated lipocalin, and kidney injury molecule-1 in renal biopsies which may allow the identification of sepsis-acute kidney injury patient subtypes.
Design:
Prospective, clinical laboratory study using “warm” human postmortem sepsis-acute kidney injury kidney biopsies.
Setting:
Research laboratory at university teaching hospital.
Subjects:
Adult patients who died of sepsis in the ICU and control patients undergoing tumor nephrectomy.
Measurements and Main Results:
Reverse transcription quantitative polymerase chain reaction and immunohistochemical staining were used to quantify messenger RNA and protein expression levels of neutrophil gelatinase–associated lipocalin and kidney injury molecule-1 in the kidney of sepsis-acute kidney injury patients and control subjects. Morphometric analysis was used to quantify renal and glomerular neutrophil gelatinase–associated lipocalin and kidney injury molecule-1 protein levels. Neutrophil gelatinase–associated lipocalin and kidney injury molecule-1 messenger RNA and protein levels were increased in kidneys of sepsis-acute kidney injury patients compared with control kidney tissue. Neutrophil gelatinase–associated lipocalin was localized in the distal tubules, collecting ducts, the adventitia of the renal arterioles, and in the glomerular tufts of renal biopsies from sepsis-acute kidney injury patients. In contrast, kidney injury molecule-1 was localized at the brush border of the proximal tubules. There was no correlation between neutrophil gelatinase–associated lipocalin and kidney injury molecule-1 levels. Furthermore, renal neutrophil gelatinase–associated lipocalin and kidney injury molecule-1 levels were not associated with the extent of renal injury, the severity of critical illness, or serum creatinine levels at either ICU admission or day of expiration. By laser microdissecting glomeruli, followed by reverse transcription quantitative polymerase chain reaction, we identified heterogenous glomerular neutrophil gelatinase–associated lipocalin production in the kidney of sepsis-acute kidney injury patients.
Conclusion:
We found differences in the expression of neutrophil gelatinase–associated lipocalin and kidney injury molecule-1 in patients with the same syndrome “sepsis-acute kidney injury” meaning there is no single pathway leading to sepsis-acute kidney injury. This underscores the beliefs that there are many/different pathophysiological pathways that can cause sepsis-acute kidney injury. Hence, patients with criteria that meet the definitions of both acute kidney injury and sepsis can be divided into subtypes based on pathophysiological features.
Acute kidney injury (AKI) in critically ill patients is a common heterogeneous syndrome, which, due to the lack of pharmacological therapies, is associated with poor short and long-term outcome (1, 2). Because we do not yet fully understand the pathophysiological mechanisms associated with AKI, we still rely on a definition to make a clinical diagnosis. The use of (Risk, Injury, Failure, Loss of kidney function, End-stage renal disease [RIFLE], Acute Kidney Injury Network, or Kidney Disease Improving Global Outcomes [KDIGO]) definitions alone is inadequate (3) because they do not address underlying, potentially heterogenous pathophysiological mechanisms, which, once better understood, may allow individualized therapy. Furthermore, these definitions are based on serum creatinine levels, which increase only 24–48 hours after declining renal function (4). Sepsis-associated AKI (sepsis-AKI) is even more enigmatic because it is clinically diagnosed based on a set of symptoms (5). Sepsis-AKI can be caused by different pathogens in patients of different ages and with diverse comorbidities and, therefore, can assume different pathophysiologies, severities of illness, response to treatment, and outcome. Recently, two AKI subphenotypes based on plasma biomarkers and clinical variables were found to respond differently to vasopressin therapy (6). These subphenotypes are strikingly similar to the acute respiratory distress syndrome (ARDS) subphenotypes also recently identified (7–9). Because plasma biomarkers are an accumulation of proteins from different cells and organs, the identified AKI and ARDS phenotypes may more accurately represent subphenotypes of critical illness with multiple organ failure, rather than AKI or ARDS, specifically. If we are ever to find a therapy that will benefit AKI patients by reversing and/or limiting organ injury, it must be directed toward the pathophysiology of renal injury because this offers the best chance of success.
The quest for AKI biomarkers has revealed a plethora of molecules increased in the plasma and/or urine of patients (10), yet none are currently used in clinical practice. neutrophil gelatinase–associated lipocalin (NGAL) and kidney injury molecule (KIM)-1 are promising markers of both sepsis severity and renal injury (11), and in response to an acute insult are involved in renal protection and recovery (12). Plasma NGAL and KIM-1 increase a few hours after the initial insult and can persist for days to weeks (13). However, NGAL and KIM-1 are not increased to a similar extent in all AKI patients (13). Similarly, unique renal transcriptional patterns were found in mice with a similar AKI stage but due to differing etiologies (14). Experimental animal models have shown that renal NGAL is increased after both lipopolysaccharide (LPS) and ischemia-reperfusion injury (IRI), whereas KIM-1 was increased as a result of IRI only (15). Furthermore, NGAL and KIM-1 staining in renal allograft biopsies showed a different distribution and localization (16), with similar findings observed in children with prerenal and intrinsic AKI (17). Thus, the production of NGAL and KIM-1 appears to be etiology dependent.
We hypothesized that heterogenous renal responses to sepsis would result in variable expression of injury-related biomarkers which may be associated with specific pathophysiological mechanisms. The aim of this study was, therefore, to identify possible mechanisms associated with sepsis-AKI based on the expression levels of the renal injury biomarkers, NGAL, and KIM-1, which may allow identification of sepsis-AKI patient subtypes.
MATERIALS AND METHODS
Patients
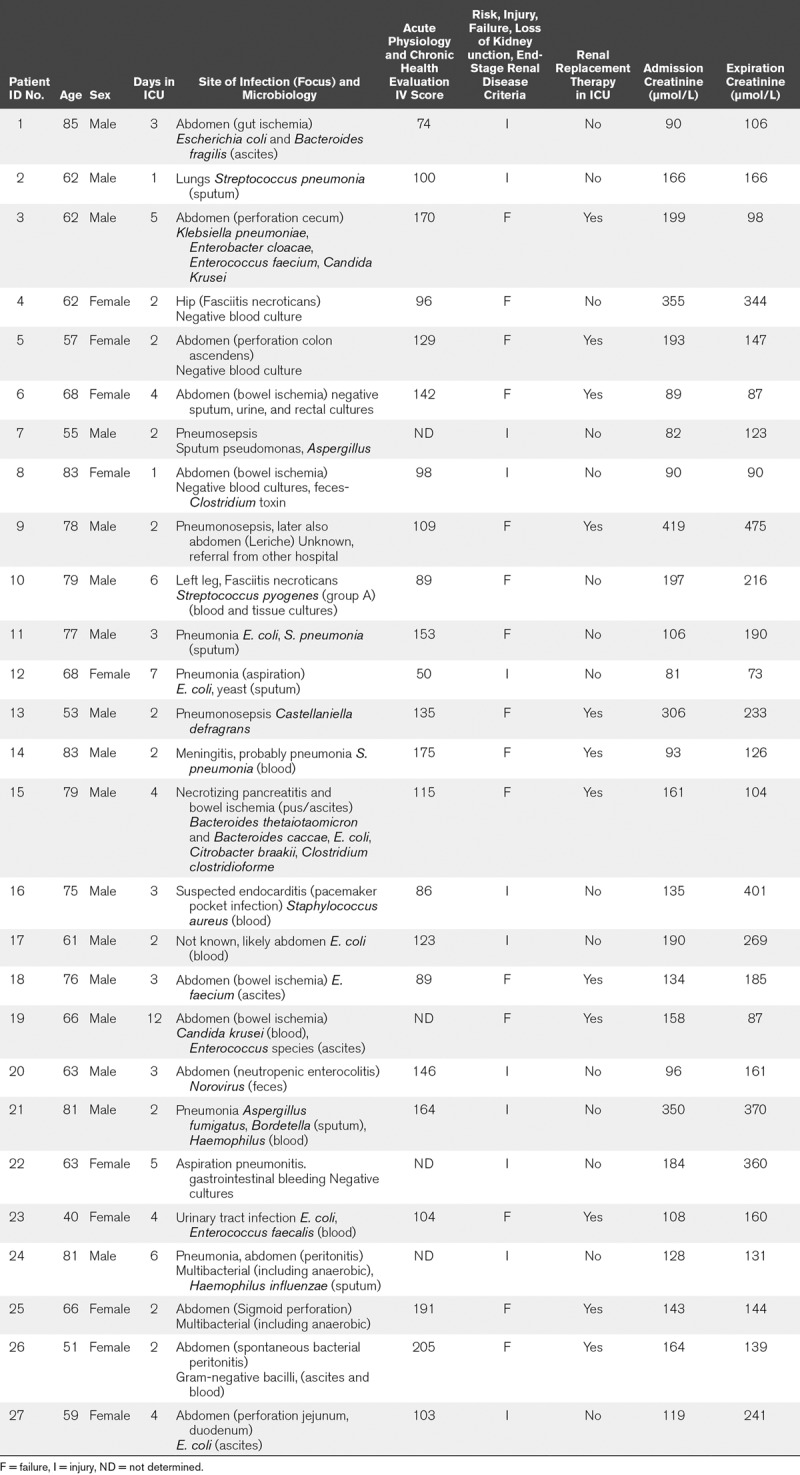
Postmortem kidney biopsies were collected from patients with sepsis and AKI, as described elsewhere (18). Patients 18 years old and older who died of sepsis in the ICU were included in the study. Patients with pre-existing chronic kidney disease, active autoimmune disorders with renal involvement, and treatment with immune-suppressive medication were excluded from this study. All patients were classified as having septic shock according to the International Sepsis Definitions (19). In addition, AKI in all patients was classified according to the RIFLE criteria, using serum creatinine and urine output (20). The severity of critical illness was defined upon admission to the ICU using the Acute Physiology and Chronic Health Evaluation (APACHE) IV and the Simplified Acute Physiology Score (SAPS) II scoring system (21, 22). In patients undergoing complete nephrectomy as a result of kidney cancer, a healthy part of tissue was isolated from the kidney cortex as far away as possible from the tumor. A dedicated renal pathologist at our hospital assessed these biopsies and considered them to be normal. Thus, we forthwith refer to these biopsies as healthy controls. Patients with previous renal function loss were excluded. All biopsies were taken within an average of 33 minutes after death at the bedside in our ICU, or in the operating theater, immediately after removal of the kidney in nephrectomy patients, and are therefore considered “warm.” These measures were taken to avoid necrosis. Immediate postmortem biopsies are performed by definition in deceased patients. Therefore, legal regulations for studies in alive patients do not apply. We considered our immediate postmortem biopsies as a limited autopsy. Full autopsy was also offered to the relatives of the patients. The limited autopsy was performed under the responsibility of the clinicians and pathologist with the purpose to explore the cause of renal failure. Permission and written informed consent for this limited autopsy were asked for and obtained in the final family meeting before or just after death. The limited autopsy procedure was explained in detail, and we mentioned that we would try to make the cause of death clearer and that we furthermore had a research purpose. An autopsy report of the routine histological findings was added to the patient chart and was discussed during a meeting with the family 6 weeks after discharge (death). Control biopsies were obtained from patients who underwent total nephrectomy as a result of kidney cancer. All renal cancer patients gave preoperative consent. The Medical Ethics Review Committee (METC) of the University Medical Center Groningen reviewed and waived this study (METc 2011/372). Patient characteristics and clinical and laboratory details can be found in Tables 1 and 2.
TABLE 1.
Control Patients: Clinical, Laboratory, and Renal Function Details
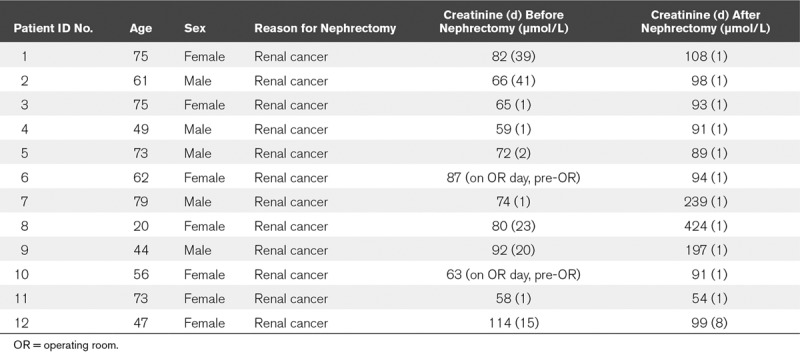
TABLE 2.
Sepsis-Associated Acute Kidney Injury Patients: Clinical, Laboratory, and Renal Function Details
Gene Expression Analysis by Reverse Transcription Quantitative Polymerase Chain Reaction
RNA was isolated from 20 × 5 µm kidney cryosections using the RNeasy Mini Plus Kit (Qiagen, Leusden, The Netherlands) according to the manufacturer’s instructions. RNA integrity was analyzed, complementary DNA synthesized, and quantitative reverse transcription polymerase chain reaction performed as described (Supplemental Digital Content 1, http://links.lww.com/CCX/A103).
Immunohistochemistry and Morphometric Analysis
Immunohistochemical staining of NGAL, KIM-1, and Neutrophil Elastase on formalin-fixed paraffin-embedded human kidney tissue was performed as described (Supplemental Digital Content 1, http://links.lww.com/CCX/A103). To quantify NGAL and KIM-1 immunostaining, the sections were first scanned using a Nanozoomer HT (Hamamatsu Photonics, Hamamatsu, Japan). Morphometric analysis was performed using the Aperio Imagescope positive pixel analysis v9.1 algorithm (Aperio Technologies, Vista, CA) as described previously (23). Neutrophil infiltration was quantified by counting the number of neutrophils (neutrophil Elastase positive) present in all glomeruli of the kidney sections.
Laser Microdissection
Cryosections (9 µm) were mounted on PolyEthylene Naphthalate–membrane slides (Carl Zeiss B.V., Breda, The Netherlands), fixed, stained with Mayer hematoxylin, washed with diethylpyrocarbonate-treated water, and air-dried. Glomeruli were laser microdissected using the LMD6500 system (Leica Microsystems, Wetzlar, Germany) using LMD6500 software v7.0 (Leica Microsystems). Glomeruli (80–100) with a total area of 2 × 106 µm2 were dissected and collected in a 0.5-mL adhesive cap (Carl Zeiss B.V.) and stored at –80°C until gene expression analysis by reverse transcription quantitative polymerase chain reaction (RT-qPCR).
Statistical Analysis
All statistical analyses were performed using GraphPad Prism Software v8 (San Diego, CA). Data are presented as mean ± sd. Statistical analysis was performed using a two-tailed unpaired Student t test, assuming unequal variances to compare two replicate means. Correlations between selected groups were assessed by Pearson tests. Differences were considered significant when p values were less than 0.05.
RESULTS
Renal NGAL and KIM-1 Levels Are Increased in Critically Ill Patients With Sepsis-AKI
Early detection of AKI and subtyping of AKI patients based on early pathophysiological parameters is critical to enable fast individualized therapeutic options for patients. We found significantly higher renal NGAL and KIM-1 messenger RNA levels in sepsis-AKI biopsies when compared with control subjects (Fig. 1A). However, NGAL and KIM-1 messenger RNA levels did not correlate with each other (Fig. 1B).
Figure 1.
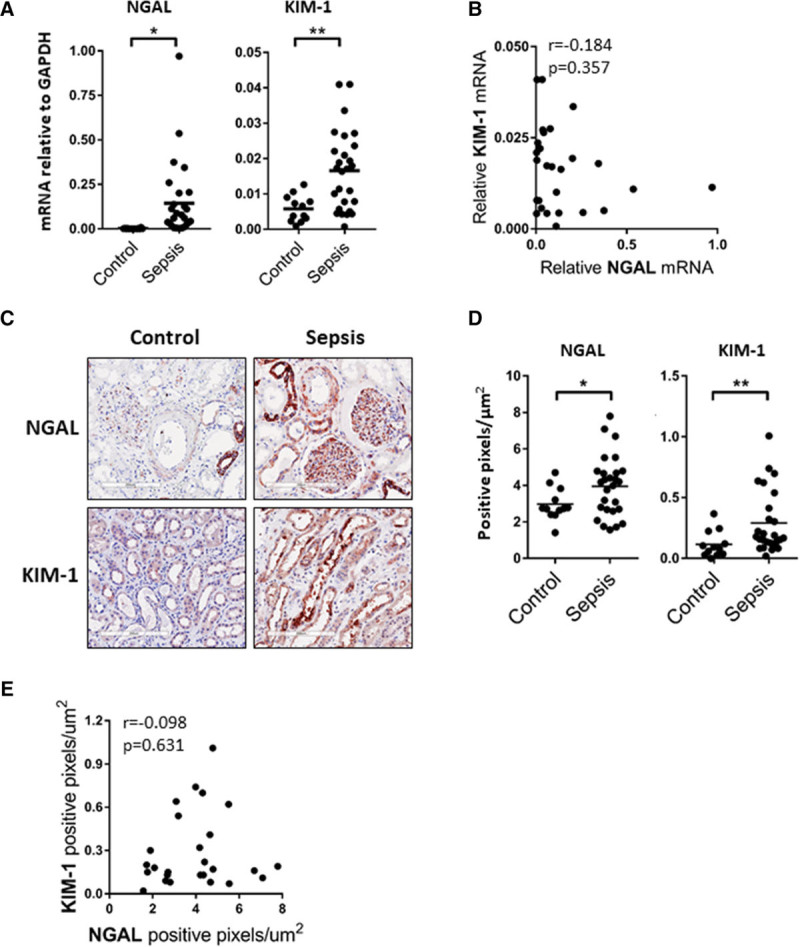
Renal neutrophil gelatinase–associated lipocalin (NGAL) and[citation f1] kidney injury molecule (KIM)-1 levels are increased in critically ill patients with sepsis-associated acute kidney injury (Sepsis-AKI). A, Postmortem kidney biopsies were collected from patients with sepsis-AKI (n = 27). Kidney tissue was also obtained from control subjects (n = 12). NGAL and KIM-1 messenger RNA (mRNA) expression was determined by reverse transcription quantitative polymerase chain reaction using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a housekeeping gene. Each dot represents an individual subject, *p < 0.05, **p < 0.005. B, NGAL mRNA levels do not correlate with KIM-1 mRNA levels as determined by Spearman correlation testing, r = –0.0184, p = 0.357.C, Representative immunohistochemical staining of NGAL (red) and KIM-1 (red) in a postmortem kidney biopsy from a sepsis-AKI patient compared with control renal tissue, original magnification ×400. D, Morphometric quantification of NGAL and KIM-1 staining in kidney biopsies from control (n = 12) and sepsis-AKI(n = 27) subjects. Graphs represent the total number of positive pixels per μm2. Each dot represents an individual subject, *p < 0.05, **p < 0.005. E, Renal NGAL protein levels do not correlate with KIM-1 protein levels as determined by Spearman correlation testing, r = –0.098, p = 0.631.
We proceeded by investigating the localization of NGAL and KIM-1 staining in sepsis-AKI and control biopsies. NGAL staining was present in the distal tubules, collecting ducts, adventitia of the renal arterioles, and, surprisingly, in the glomerular tuft of renal biopsies from Sepsis-AKI patients. In control biopsies, NGAL was absent in the glomeruli and present in the distal tubules and collecting ducts, but to a lesser extent than in Sepsis-AKI biopsies (Fig. 1C; and Supplemental Fig. 1, Supplemental Digital Content 1, http://links.lww.com/CCX/A103). NGAL was virtually absent in proximal tubules in Sepsis-AKI and control biopsies (Fig. 1C; and Supplemental Fig. 1, Supplemental Digital Content 1, http://links.lww.com/CCX/A103). KIM-1 staining was primarily localized at the brush border of the proximal tubular epithelium (Fig. 1C; and Supplemental Fig, 1, Supplemental Digital Content 1, http://links.lww.com/CCX/A103). Morphometric analysis of the kidney biopsy sections found a clear increase in renal NGAL and KIM-1 protein levels in sepsis-AKI biopsies when compared with control (Fig. 1D). Thus, both increased NGAL and KIM-1 messenger RNA and protein levels were found in sepsis-AKI compared with control biopsies, yet both injury markers were distributed differently within the kidney. No correlation was found between NGAL and KIM-1 messenger RNA or protein levels (Fig. 1, B and E) suggesting a heterogeneous and independent up-regulation of both markers in patients with sepsis.
Renal NGAL and KIM-1 Protein Levels Do Not Correlate With the Severity of Critical Illness or the Extent of Renal Injury
Using the APACHE IV or the SAPS II scoring system, we found that NGAL and KIM-1 levels did not correlate with the severity of critical illness (Fig. 2A and data not shown). Additionally, the NGAL and KIM-1 protein levels were not dependent on the degree of renal injury, as characterized in the sepsis-AKI patients using the RIFLE criteria (Fig. 2B). They were also not related to serum creatinine levels at ICU admission (Fig. 2C) or on the day of expiration (Fig. 2D). The origin and type of infection also had no influence on the extent of renal NGAL or KIM-1 protein levels (Supplemental Fig. 2, Supplemental Digital Content 1, http://links.lww.com/CCX/A103). Taken together, these findings indicate that renal NGAL and KIM-1 expression levels are increased in sepsis-AKI patients but are not associated with the severity of critical illness, AKI severity, or the type of infection.
Figure 2.
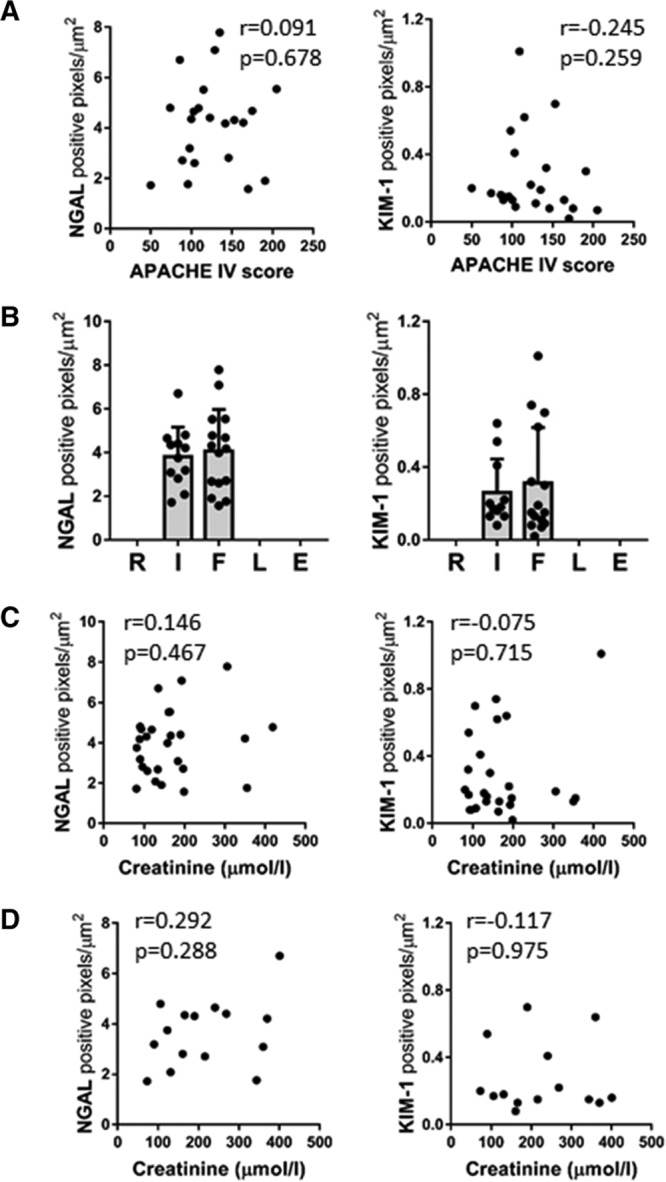
Renal neutrophil gelatinase–associated lipocalin (NGAL) and kidney injury molecule (KIM)-1 protein levels do not correlate with the severity of critical illness or the extent of renal injury. A, Renal NGAL and KIM-1 protein levels do not correlate with the severity of critical illness (Acute Physiology and Chronic Health Evaluation [APACHE IV] score) as determined by Spearman correlation analysis, r = 0.091, p = 0.678 (NGAL),r = –0.245, p = 0.259 (KIM-1). B, Renal NGAL and KIM-1 protein levels, represented by the total number of positive pixels per μm2 from sepsis-AKI patients (n = 27), were also categorized by the extent of renal injury, Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease (RIFLE) criteria. C, Serum creatinine levels from sepsis-AKI patients at ICU admission do not correlate with the amount of renal NGAL and KIM-1 protein levels in biopsies from the same patients as determined by Spearman correlation analysis, r = 0.146, p = 0.467 (NGAL), r = –0.075, p = 0.715 (KIM-1). D, Serum creatinine levels from sepsis-AKI patients that did not receive renal replacement therapy, determined on the day of expiration, did not correlate with renal NGAL and KIM-1 protein levels as determined by Spearman correlation testing, r = 0.292, p = 0.288 (NGAL), r = –0.117, p = 0.975 (KIM-1).
Glomerular NGAL Protein Levels Identify Glomerular Heterogeneity in Sepsis-AKI Patients
We found NGAL staining in the glomeruli of sepsis-AKI patients (Fig. 3A). This was an unexpected finding, which, to our knowledge, has not been described before. We morphometrically determined the amount of NGAL staining in the glomerular tuft for all glomeruli in all biopsies. The size of the glomerular tufts in the control and sepsis-AKI biopsies did not significantly differ (Fig. 3B). Glomerular NGAL staining intensity was significantly higher in sepsis-AKI patients than in control subjects (Fig. 3C). Minimal glomerular NGAL staining intensity was found in control subjects (Fig. 3D). In contrast, glomerular NGAL staining intensity in kidneys of sepsis-AKI patients was highly variable (Fig. 3D). Some sepsis-AKI biopsies presented similar glomerular NGAL levels as control subjects (n = 9), whereas very high glomerular NGAL levels were found in 11 sepsis-AKI patients. Intermediate levels of glomerular NGAL were found in seven sepsis-AKI patients (Fig. 3D). Glomerular NGAL appeared to be associated with sepsis in particular because staining of renal biopsies from patients with acute and chronic organ rejection, in which inflammation plays an important role, was devoid of NGAL positive glomeruli (data not shown).
Figure 3.
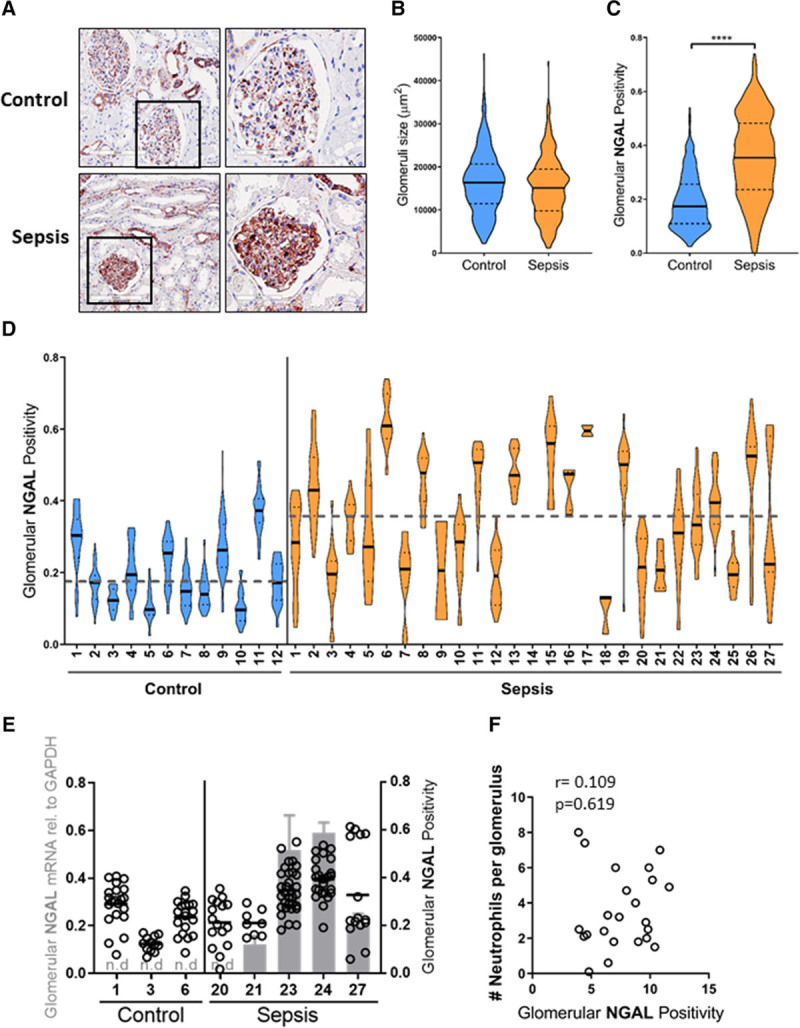
Glomerular neutrophil gelatinase–associated lipocalin (NGAL) protein levels identifies glomerular heterogeneity in sepsis-acute kidney injury (AKI) patients. A, Representative immunohistochemical staining of NGAL (red) in a postmortem kidney biopsy from a sepsis-AKI patient compared with control renal tissue, original magnification ×400. B, Violin plot of glomerulus size of all individual glomeruli per biopsy in control (n = 469 from 12 control biopsies) and sepsis-AKI patients (n = 406 from 27 sepsis-AKI biopsies). The solid lines indicate the median size of all glomeruli in control and sepsis-AKI subjects, respectively. C, Violin plot of glomerular NGAL positivity in all glomeruli tufts from all control (n = 12) and sepsis-AKI patients (n = 27). The solid lines indicate the median glomerular positivity in control and sepsis-AKI subjects, respectively. D, Glomerular NGAL positivity was determined in all glomeruli tufts within a renal biopsy from control subjects (n = 12) and sepsis-AKI (n = 27). Each dot represents the NGAL positivity within a single glomerulus within a single renal biopsy. Solid lines indicate the median glomerular positivity in that particular biopsy. The dashed lines indicate the mean size of all glomeruli in control and sepsis-AKI subjects, respectively. E, Glomerular NGAL messenger RNA levels were determined within the whole biopsy by reverse transcription quantitative polymerase chain reaction in control (n = 3) and sepsis-AKI patients (n = 5) using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a housekeeping gene (gray bars, left axis). Dots indicate the individual glomerular NGAL positivity from the same control and sepsis-AKI patient (right axis). F, Glomerular NGAL protein levels determined in biopsies from sepsis-AKI patients do not correlate with the average number of neutrophils infiltrating glomeruli in the same sepsis-AKI patients. r = 0.109, p = 0.619.
To elucidate whether NGAL is trapped in glomeruli, for example, due to impaired filtration, or, alternatively, is produced by endogenous cells, we laser microdissected glomeruli and subjected them to RT-qPCR. NGAL messenger RNA levels were not detectable in glomeruli of control subjects but could be detected in four of the five sepsis-AKI patients (Fig. 3E). Furthermore, high NGAL messenger RNA levels paralleled high glomerular NGAL protein staining (Fig. 3E). Intriguingly, NGAL protein levels did not correlate with the number of neutrophils present in the glomeruli of sepsis-AKI patients (Fig. 3F), which suggests that glomerular NGAL expression is not derived from neutrophils present in the glomeruli. Taken together, our data indicate that NGAL protein is produced in the glomeruli of sepsis-AKI patients to varying degrees within one kidney and is not associated with the number of infiltrating neutrophils.
DISCUSSION
In critically ill patients, sepsis-AKI is associated with high morbidity and mortality (1). Many patients leaving the ICU are dialysis dependent or at risk of developing or accelerating progression of chronic kidney disease (24). In sepsis, many processes in multiple cell types act in unison to cause kidney injury. Among these processes are acute inflammation resulting from bacteria or cellular products due to end-organ damage, microvascular dysfunction, and ischemic injury due to low blood pressure, blood shunting, and intravascular coagulation (25). Paradoxically, therapies such as antibiotics and high chloride resuscitation fluid might also be nephrotoxic (26). However, not all of these detrimental processes will occur in all sepsis patients and to the same extent. Furthermore, not all of these injurious cellular processes will induce the same renal response because damage can be inflicted at the glomerular, microvascular, and/or tubular level (25). For example, renal tubules are known to respond differently to ischemia than to toxin or LPS exposure, all of which are prevalent in sepsis (15).
We investigated whether NGAL and KIM-1 could differentiate AKI patient phenotypes. NGAL is a N-glycosylated protein with partly elucidated functions (27). It has iron-chelating capacity and might play a role in iron-mediated bacteriostasis (28), and in endogenous metabolic processes mediating cell growth (29), apoptosis (30), metabolism, and rescue from ischemia (31). There is a low-grade continuous NGAL production in the liver (32), yet most organs can produce NGAL in response to toxic, inflammatory, or infectious cellular injury. Serum NGAL is significantly increased in patients with sepsis-AKI when compared with patients with nonseptic AKI (33). We found increased NGAL expression in the kidney from sepsis-AKI patients compared with control subjects. This expression was localized in the distal tubules and collecting ducts, corroborating previous findings (34). Surprisingly, we also found that glomeruli in kidneys from sepsis-AKI patients produced NGAL. However, not all glomeruli expressed NGAL to the same extent, even when they were adjacent. This observation implies that even within a short distance range, glomerular heterogeneity in human kidneys exists. It is well known that adjacent glomeruli vary in structure and possibly also in function. Previous studies have shown that some glomeruli appear normal, whereas others are sclerotic, despite a clinical picture of increased proteinuria and diminished glomerular filtration rate (35). Our data indicate that NGAL staining in the glomerulus was not a result of impaired glomerular filtration but a consequence of cells localized within the glomerulus producing NGAL. Neutrophils are known to produce and secrete NGAL; however, our previous study found only a few neutrophils in the glomerulus (18). Furthermore, we found no correlation between the amount of glomerular NGAL and the number of neutrophils localized in the glomerulus. Hence, glomerulus-specific cells, endothelial cells, podocytes, or mesangial cells may produce NGAL under sepsis conditions. LPS was previously found to induce NGAL expression in podocytes in culture and in glomeruli in vivo (36). Likewise, NGAL expression was previously found in macrophages, smooth muscle cells, and endothelial cells in human carotid atherosclerotic arteries (37). Hence, glomerular endothelial cells and podocytes may be able to produce NGAL under sepsis conditions.
KIM-1 is a transmembrane glycoprotein that is undetectable in normal healthy kidneys. However, the expression of KIM-1 is specifically induced in the kidney after ischemic or toxic injury and can be detected in the plasma and urine, highlighting the specificity of KIM-1 for kidney injury (38, 39). KIM-1 is thought to be important for the removal of dead cells and the regeneration of tubular epithelial cells after injury (13). We found increased and considerable diverse expression of both KIM-1 messenger RNA and protein in sepsis-AKI patients. KIM-1 was found primarily localized on the apical surface of a limited number of proximal tubular epithelial cells as well as in the cytoplasm in flat and stretched tubular epithelial cells within dilated tubules, corroborating previous findings (40).
Both renal injury markers were increased in the kidney of patients with sepsis, but a correlation between NGAL and KIM-1 messenger RNA or protein expression was absent. The trigger inducing up-regulation of these biomarkers is known to be different (15), and the lack of correlation suggests that in different patients with the same consensus diagnosis, the balance between both response mechanisms is different which may be related to the kinetics of biomarker production. Hence, the pathophysiological mechanisms of kidney injury likely differ between sepsis-AKI patients.
A strength of our study is the use of kidney biopsies from sepsis-AKI patients, which has allowed us to make associations among clinical data, pathology, and molecular changes albeit in a small cohort of patients. However, several limitations need to be considered. We analyzed a subpopulation of sepsis-AKI patients, namely nonsurvivors, which probably represent the most severe critically ill patients. Furthermore, the onset and duration of sepsis varied per patient, as did the length of ICU stay. Hence, the kinetics of renal biomarker production in the kidney is unknown. Ideally, we would have correlated plasma and urine levels of NGAL and KIM-1 with the data presented here because this would have provided an insight into the relationship between the amounts of NGAL and KIM-1 found in the serum and/or urine and kidney pathophysiology. Unfortunately, we currently do not have plasma or urine samples from this sepsis cohort, yet we plan to expand this cohort and will include blood and urine samples from the same patients.
CONCLUSIONS
We have shown that there is a difference in the expression of renal NGAL and KIM in patients with the same syndrome “sepsis-AKI.” The fact that the expression differs means that there is no single pathway leading to sepsis-AKI. This underscores the beliefs that there are many/different pathophysiological pathways that can cause sepsis-AKI. Hence, patients with criteria that meet the definitions of both AKI and sepsis can be divided into subtypes based on pathophysiological features.
Supplementary Material
Footnotes
Ms. Jou-Valencia and Dr. Koeze share equal first authorship.
Supplemental digital content is available for this article. Direct URL citations appear in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Supported, in part, by grants from Department of Critical Care Research foundation, University Medical Center Groningen, Groningen, The Netherlands.
The authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Poston JT, Koyner JL. Sepsis associated acute kidney injury. BMJ. 2019;364:k4891. doi: 10.1136/bmj.k4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kellum JA, Lameire N; KDIGO AKI Guideline Work Group Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (part 1). Crit Care 201317204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koeze J, Keus F, Dieperink W, et al. Incidence, timing and outcome of AKI in critically ill patients varies with the definition used and the addition of urine output criteria. BMC Nephrol. 2017;18:70. doi: 10.1186/s12882-017-0487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol 200920672–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016315801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatraju PK, Zelnick LR, Herting J, et al. Identification of acute kidney injury subphenotypes with differing molecular signatures and responses to vasopressin therapy. Am J Respir Crit Care Med 2019199863–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Famous KR, Delucchi K, Ware LB, et al. ; ARDS Network Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med 2017195331–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delucchi K, Famous KR, Ware LB, et al. ; ARDS Network Stability of ARDS subphenotypes over time in two randomised controlled trials. Thorax 201873439–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bos LD, Schouten LR, van Vught LA, et al. ; MARS consortium Identification and validation of distinct biological phenotypes in patients with acute respiratory distress syndrome by cluster analysis. Thorax 201772876–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang K, Xie S, Xiao K, et al. Biomarkers of sepsis-induced acute kidney injury. Biomed Res Int. 2018;2018:6937947. doi: 10.1155/2018/6937947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lentini P, de Cal M, Clementi A, et al. Sepsis and AKI in ICU patients: The role of plasma biomarkers. Crit Care Res Pract. 2012;2012:856401. doi: 10.1155/2012/856401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haase-Fielitz A, Haase M, Devarajan P. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: A critical evaluation of current status. Ann Clin Biochem 201451335–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alge JL, Arthur JM. Biomarkers of AKI: A review of mechanistic relevance and potential therapeutic implications. Clin J Am Soc Nephrol 201510147–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu K, Rosenstiel P, Paragas N, et al. Unique transcriptional programs identify subtypes of AKI. J Am Soc Nephrol 2017281729–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mar D, Gharib SA, Zager RA, et al. Heterogeneity of epigenetic changes at ischemia/reperfusion- and endotoxin-induced acute kidney injury genes. Kidney Int 201588734–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bansal N, Carpenter MA, Weiner DE, et al. Urine injury biomarkers and risk of adverse outcomes in recipients of prevalent kidney transplants: The folic acid for vascular outcome reduction in transplantation trial. J Am Soc Nephrol 2016272109–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westhoff JH, Fichtner A, Waldherr S, et al. Urinary biomarkers for the differentiation of prerenal and intrinsic pediatric acute kidney injury. Pediatr Nephrol 2016312353–2363 [DOI] [PubMed] [Google Scholar]
- 18.Aslan A, van den Heuvel MC, Stegeman CA, et al. Kidney histopathology in lethal human sepsis. Crit Care. 2018;22:359. doi: 10.1186/s13054-018-2287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med 2003311250–1256 [DOI] [PubMed] [Google Scholar]
- 20.Bellomo R, Ronco C, Kellum JA, et al. Acute dialysis quality initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The second international consensus conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care 20048R204–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knaus WA, Zimmerman JE, Wagner DP, et al. APACHE-acute physiology and chronic health evaluation: A physiologically based classification system. Crit Care Med 19819591–597 [DOI] [PubMed] [Google Scholar]
- 22.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American Multicenter Study. Jama 19932702957–2963 [DOI] [PubMed] [Google Scholar]
- 23.Jou-Valencia D, Molema G, Popa E, et al. Renal Klotho is reduced in septic patients and pretreatment with recombinant klotho attenuates organ injury in lipopolysaccharide-challenged mice. Crit Care Med 201846e1196–e1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neyra JA, Mescia F, Li X, et al. Impact of acute kidney injury and CKD on adverse outcomes in critically ill septic patients. Kidney Int Rep 201831344–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zarbock A, Gomez H, Kellum JA. Sepsis-induced acute kidney injury revisited: Pathophysiology, prevention and future therapies. Curr Opin Crit Care 201420588–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yunos NM, Bellomo R, Hegarty C, et al. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. Jama 20123081566–1572 [DOI] [PubMed] [Google Scholar]
- 27.Chakraborty S, Kaur S, Guha S, et al. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta 20121826129–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flo TH, Smith KD, Sato S, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 2004432917–921 [DOI] [PubMed] [Google Scholar]
- 29.Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 20053651231–1238 [DOI] [PubMed] [Google Scholar]
- 30.Mishra J, Mori K, Ma Q, et al. Neutrophil gelatinase-associated lipocalin: A novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol 200424307–315 [DOI] [PubMed] [Google Scholar]
- 31.Bao GH, Barasch J, Xu J, et al. Purification and structural characterization of “simple catechol”, the NGAL-siderocalin siderophore in human urine. RSC Adv 2015528527–28535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charlton JR, Portilla D, Okusa MD. A basic science view of acute kidney injury biomarkers. Nephrol Dial Transplant 2014291301–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bagshaw SM, Bennett M, Haase M, et al. Plasma and urine neutrophil gelatinase-associated lipocalin in septic versus non-septic acute kidney injury in critical illness. Intensive Care Med 201036452–461 [DOI] [PubMed] [Google Scholar]
- 34.Mori K, Lee HT, Rapoport D, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest 2005115610–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma K, Paša-Tolić L. Toward individual glomerular phenotyping: Advent of precision medicine in kidney biopsies. Kidney Int 2018931265–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SJ, Borsting E, Declèves AE, et al. Podocytes express IL-6 and lipocalin 2/neutrophil gelatinase-associated lipocalin in lipopolysaccharide-induced acute glomerular injury. Nephron Exp Nephrol 2012121e86–e96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eilenberg W, Stojkovic S, Piechota-Polanczyk A, et al. Neutrophil gelatinase-associated lipocalin (NGAL) is associated with symptomatic carotid atherosclerosis and drives pro-inflammatory state in vitro. Eur J Vasc Endovasc Surg 201651623–631 [DOI] [PubMed] [Google Scholar]
- 38.Urbschat A, Gauer S, Paulus P, et al. Serum and urinary NGAL but not KIM-1 raises in human postrenal AKI. Eur J Clin Invest 201444652–659 [DOI] [PubMed] [Google Scholar]
- 39.Vaidya VS, Ozer JS, Dieterle F, et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol 201028478–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takasu O, Gaut JP, Watanabe E, et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med 2013187509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


