Objectives:
In a diverse, multicenter population, to confirm or refute the conclusions that pupillary light reflex changes are associated with increased intracranial pressure.
Design:
Replication study.
Patients:
Within the Establishing Normative Data for Pupillometer Assessments in Neuroscience Intensive Care registry there were 273 patients (16,221 pupillary observations) that included both intracranial pressure and pupillometry values.
Measurements and Main Results:
To evaluate findings by the previous author, we explored for differences among measures of the pulmonary light reflex obtained from automated pupillometry with ICP values dichotomized as < 15 mm Hg (normal) versus ≥ 15 mm Hg (elevated). Analysis of t-test indicates statistically significant differences for all right and left mean pupilometer values, except right latency (p = 0.3000) and repeated measure mixed model (p = 0.0001). In the setting of increased intracranial pressure, mean pupilometer values were lower for both left and right eyes comparing to normal intracranial pressure, except right neurologic pupil index (3.98, 3.92;p = 0.0300) and left latency (0.27, 0.25; p < 0.0001).
Conclusions:
Our findings confirm and extend those of McNett et al Worsening measures of the pupillary light reflex using automated pupillometry are associated with elevated intracranial pressure.
Keywords: brain injuries, critical care, intracranial pressure, neurology, optic nerve, pupil
Examination of the pupillary light reflex (PLR) is essential to a comprehensive neurologic assessment. The traditional pupillary assessment performed using a penlight is being gradually replaced with automated pupilometer assessments (1–3). Increased use of this technology has led to more research. A recent study by McNett et al (4) explored associations between measures of PLR and intracranial pressure (ICP). These results suggest that elevated ICP may be manifest in the PLR as decreased neurologic pupil index (NPi) and constriction velocity (CV), without significant change in pupil size. Having developed a registry (5) of automated pupillometer data that includes over 3,000 subjects, this study aimed to replicate the study by McNett et al (4) with a larger sample set that includes subject data from three different institutions.
BACKGROUND
Examination of the functional status of the optic nerve (cranial nerve [CN]-II) and oculomotor nerve (CN-III) can be performed through the PLR assessment. Impaired PLR is related to conditions that may cause increased risk of central brain herniation or horizontal shift of the intracranial tissues (6). Assessment of the PLR in patients with neurologic conditions has been performed using a penlight (1–3). With this method, specific terminology is employed to describe the PLR and that includes brisk, sluggish, or nonreactive (7). However, these terms are subjective as they lack the standard clinical definition and this leads to poor validity and reliability of the neurologic assessment (8–11). In addition, visual assessment of the practitioners can be affected by ambient light conditions, which impact the inter-examiner agreement. This is related to several factors like examiner’s visual acuity, flashlight stimulus, distance from patient’s eyes, and the lighting conditions of the examination room (12).
Automated pupillometry is a noninvasive tool to monitor the neurologic condition of ICU patients. This method provides reliable and objective measures of pupil constriction and dilation velocities, pupil size, and latency (13, 14). Such measures can be obtained using the NeurOptics NPi-200 (Irvine, CA), a pupilometer implemented to objectively assess the PLR (14, 15). A proprietary algorithm is used to compute an index called “NPi” (16). The NPi values range from 0 to 5, where NPi value equal to or above 3 is considered normal (17). An abnormal NPi can be associated with direct damage to the CN-III or indirect damage of the brain (18–20).
Increased ICP has been associated to several neurologic conditions like herniation with increased risk of irreversible brain damage and eventually death (21–23). Therefore, depending on the reason of increased pressure, treatment should be initiated to lower ICP at pressures above 15–20 mm Hg (22). Furthermore, increased ICP is associated with abnormal pupilometer readings for patients with various neurologic or neurosurgical conditions (24–26). For patients with limited or no serial neurologic examination to follow, ICP monitoring is essential in order to direct therapy for some brain-injured patients (27). According to the American Brain Trauma Foundation, it is suggested to monitor ICP in cases of traumatic brain injury (TBI) where Glasgow Coma Scale (GCS) score less than 9 and abnormal CT scan exist (28).
Several studies found a correlation between pupilometer readings and ICP (1, 4, 17, 29). In 2003, Taylor et al (26) first explored the relationship of PLR and ICP in a cohort of 26 patients with TBI; concluding that mass effect with ICP values greater than 20 mm Hg was associated with slower CV. More recently, Chen et al (17) found that patients with abnormal NPi values (< 3.0) had higher peak ICP readings than those with normal NPi values. McNett et al (4) examined the association between ICP and pupillometry which indicated that increased ICP values result in decreased pupilometer values for NPi and CV, but not for pupil size (4). The study by McNett et al (4) was a single-center study with a total of 76 participants and more than 2,100 ICP and serial pupilometer readings. The work by McNett et al (4) is the most recent to examine serial NPi and ICP readings, and additional research with a larger cohort is warranted. This is a modified replication study of that from McNett et al (4), which investigated if increased ICP affects pupilometer readings on a larger multicenter sample set. The hypothesis of this analysis mirrors the findings of McNett et al (4), such that, it is expected that there will be an inverse relationship between ICP values and NPi, but that pupil size will not be impacted by ICP values.
METHODS
The analysis for this study was carried out using data pooled from the Establishing Normative Data for Pupillometer Assessments in Neuroscience Intensive Care (END-PANIC, NCT02804438) Registry. Partial funding for this registry is provided by NeurOptics, the company that produces the pupillometer used in the study. The registry includes three hospital locations in Texas, Ohio, and California. The design of END-PANIC was described previously (5). Succinctly, END-PANIC is a multicenter prospective registry of pupillometer readings that collects data from patients who are admitted to the neurocritical care unit because of particular neurologic conditions like intracerebral hemorrhage, spinal injury, acute ischemic stroke (AIS), increased ICP, subarachnoid hemorrhage, TBI, and other neurologic conditions.
The data collection centers use pupillometry and ICP management as part of standard of care. All ICP values were obtained from external ventricular drain (EVD) catheters. Hospital practice is to observe the ICP for 5 minutes with the EVD clamped prior to documenting a value. Pupillometry readings can be collected as much as once an hour to every 4 hours, which mirrors the standard of care for the neurologic examination. The highest and average ICP value is recorded daily in the registry data.
This study was carried out following the Institutional Review Board approval. The END-PANIC is ongoing registry that currently has data on over 3,000 subjects. However, only subjects with registered ICP values were included in the study (n = 273). From these, there were 16,221 observations (mean daily values) included in the analysis. Demographic data were collected, which included age, race, sex, and primary diagnosis. Daily prospective data were collected from the patients which included hospital and ICU length of stay (LOS) and GCS.
The study analysis was performed using SAS v9.4 (SAS Institute, Cary, NC) and followed the study analysis by McNett et al (4) for replication purposes. For descriptive statistics, frequencies and percentages for categorical variables (primary diagnosis, race, sex, and ICP) and mean, median, and sd were used for continuous variables. Primary diagnosis was categorized into hemorrhagic stroke, brain tumor, TBI, AIS, spinal injury, and other. Race was categorized as Caucasian, African American, and other. As in the study by McNett et al (4), ICP was dichotomized as normal or elevated; normal included ICP less than 15 mm Hg and elevated was defined as ICP greater than or equal to 15 mm Hg. Brain injury was categorized as severe (GCS, 3–8), moderate (GCS, 9–12), or mild (GCS, 13–15) (30, 31). Pupillometry readings (NPi, CV, dilation velocity [DV], pupil size, and latency) were obtained for both right and left eyes. Examination of differences among several variables based on ICP values (< 15 mm Hg vs ≥ 15 mm Hg) was done by running several student t tests. Additional analyses were run using mixed model to correct for the repeated nature of the data.
RESULTS
Descriptive data were obtained from 273 subjects with 16,221 paired ICP and pupillometer observations. The mean age was 53 years (sd = 16.76). Of those subjects, 142 (52.01%) were female, 209 (76.56%) were Caucasian, 29 (10.62%) African American, and 24 (8.79%) other; 11 (4.03%) subjects did not report race. Primary diagnosis included hemorrhagic stroke (n = 167; 61.17%), brain tumor (n = 35; 12.82%), TBI (n = 14; 5.13%), AIS (n = 7; 2.56%), spinal injury (n = 3; 1.1%), and other (n = 41; 15.02%), and 6 did not report primary diagnosis.
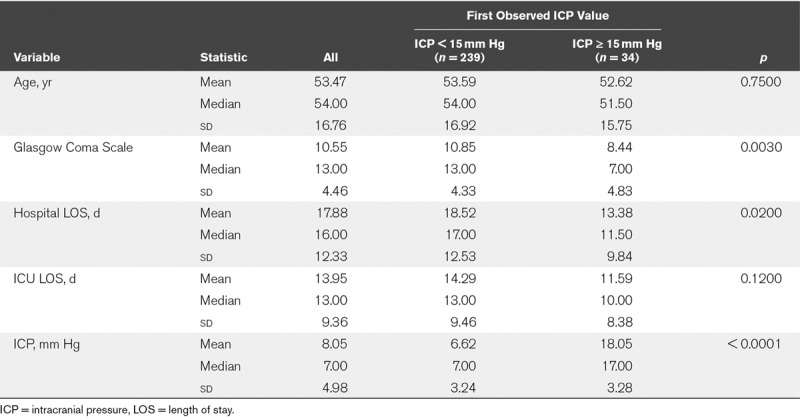
Data were first examined using only one observation per subject (Table 1), and only the first paired observation of pupillometer and ICP data was used. Subjects with ICP less than 15 mm Hg (n = 239) versus those with ICP greater than or equal to 15 mm Hg (n = 34) were similar in age (mean age, 53.59 vs 52.62 yr; p = 0.7500), ICU LOS (mean = 14.29 vs 11.59 d; p = 0.1200), but had significantly lower hospital LOS (mean = 18.52 vs 13.38 d; p = 0.0200), and lower mean GCS (mean = 10.85 vs 8.44; p = 0.0030). The overall mean GCS for both group is 10.55 (sd = 4.66) with a median of 13.00. The difference between mean and the median with most of the GCS scores within 5 sds is an indication that the patients are likely severe or not severe. That is, there are fewer patients in the moderate group. A further subgroup analysis reveals that about 50% of the patients have less severe brain injury, about 14% have mild brain injury and about 36% have severe brain injury. Furthermore, about 30% of the patients with less severe brain injury had a GCS score of 15.
TABLE 1.
Descriptive Statistics for First Observation of Paired Intracranial Pressure and Pupillometer Data
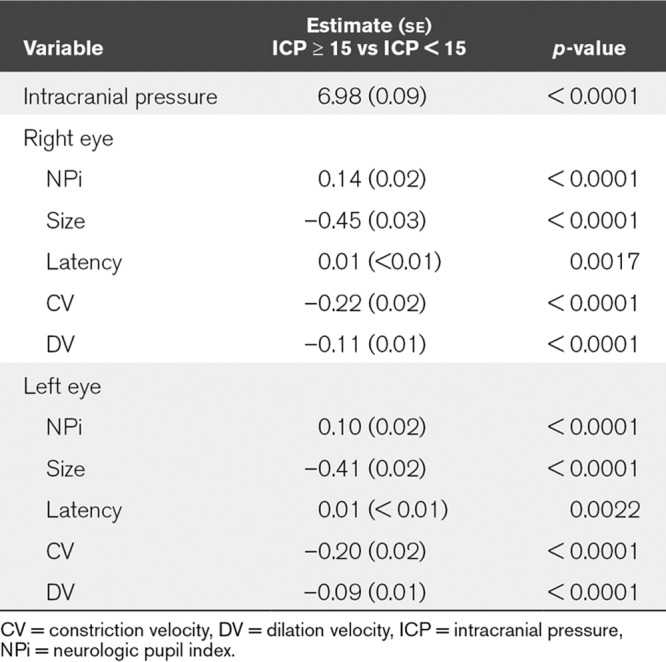
Data were next explored including all paired observations of ICP and pupillometry readings. Student t tests were used to compare PLR values when the ICP was documented as less than 15 versus documented as greater than or equal to 15 mm Hg. As shown in Table 2, in the right eye, an ICP less than 15 mm Hg was associated with statistically significantly lower NPi (p = 0.03), faster CV (p < 0.0001), faster pupil dilation, measured as DV (p < 0.0001), and larger pupil size (p < 0.0001). Similarly, in the left eye, ICP less than 15 mm Hg was associated with statistically significantly higher NPi (p < 0.0001), faster CV (p < 0.0001), faster DV (p < 0.0001), and larger pupil size (p < 0.0001). There was no statistically significant difference in right eye latency (0.30), but a statistically significant difference in left eye latency (p < 0.0001). Furthermore, results from the mixed model showed significant difference between the thresholded IPC based on all parameters considered (Table 3).
TABLE 2.
Pupillometry in Relation to Intracranial Pressure Values
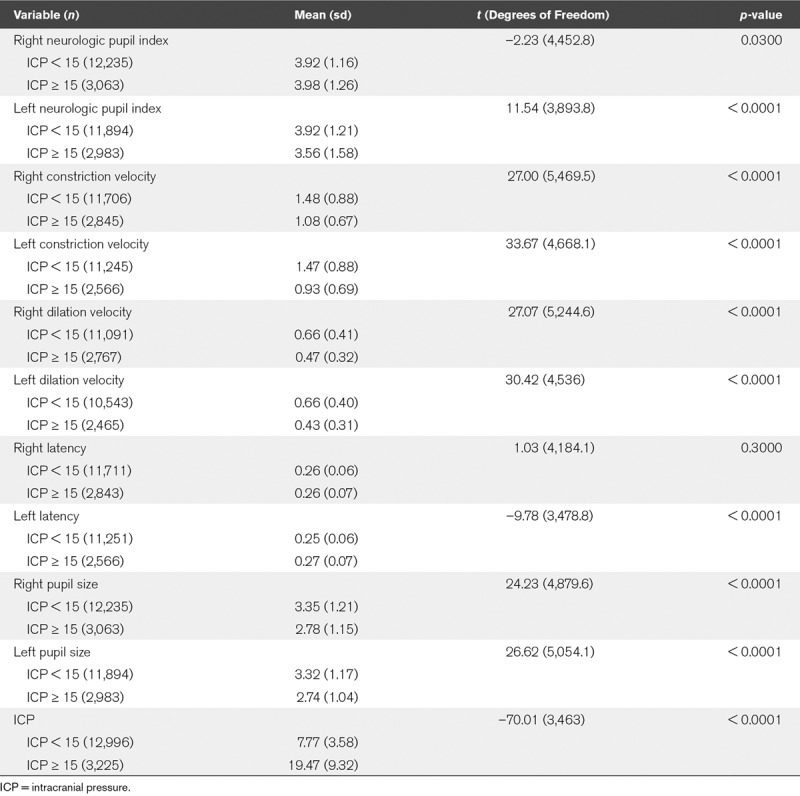
TABLE 3.
Mixed Model Comparing Intracranial Pressure Values for Neurologic Pupil Index, Size, Latency, Constriction Velocity, and Dilation Velocity
DISCUSSION
The results from our analysis confirm and extend the findings published by McNett et al (4). The main finding in our study was that pupillometer values are different in the setting of normal and increased ICP. Such findings are useful in pupillary examinations because it will help practitioners to have a better understanding of the examination trends at the ICU. Patients with increased ICP tend to have lower values for pupillometer readings. Slower right and left CV were associated with increased ICP (p < 0.0001). This is similar to the results reported by McNett et al (4), but only for left CV, as there was no significant difference in right CV (p = 0.1200). However, we also reported changes in DV with increased ICP and found that elevated ICP leads to slower DV for right and left eyes as well (p < 0.0001).
In our study, there was a statistically significant difference both in right and left mean NPi among normal and increased ICP groups. With increased ICP, we had a drop in left NPi values. However, a little higher right mean NPi was associated with increased ICP (increased ICP = 3.98; normal ICP = 3.92; p = 0.0300). This was different from the study by McNett et al (4), as they reported no difference in right mean NPi (increased ICP = 4.2; normal ICP = 4.2; p = 0.4100) although their time series analysis indicated variations within the first 72 hours in paired pupillometer readings for NPi and CV, but not for pupil size. However, our data set included observations that were obtained every 4 hours without a timeline for data collection. Furthermore, we obtained information on latency. Increased ICP was associated with increased latency for left eyes (p < 0.0001). This is consistent with the study of Soeken et al (32) as they reported an increase in latency magnitude with intracranial hypertension group. However, that was not the case in our study for right latency as the difference was not statistically significant among normal and increased ICP groups (p = 0.30).
Our findings were consistent with a recent study done by Freeman et al (29), which examined the association between NPi and ICP (29). The researchers analyzed a total of 951 ICP readings with 67% of patients had ICP of 20 cm H2O or higher throughout the monitoring process. With increased ICP, patients had a drop in the NPi, CV, DV, and pupil size comparing the other group (normal ICP [<15 mm Hg]; p < 0.001). However, the study showed mild to moderate correlations between pupillary readings and ICP before ICP spikes, that some patients have a positive association, whereas others had negative relationship or no association between NPi and ICP. Patients with ICP monitoring may require sedation to help reduce secondary brain injury (33). Although the effect of various sedatives on NPi is not well documented, Shirozu et al (34) found that NPi changes are not associated with fentanyl, nor the combination of propofol and remifentanil.
LIMITATION
A key limitation in comparing results from the END-PANIC registry to those of McNett et al (4) is the temporal relationship between ICP and pupillometry measures. Whereas McNett et al (4) prospectively collected hourly pupillometry values and hourly ICP data, our data are pragmatic and reflect a clinical practice in which ICP values were sampled hourly and NPi values were sampled once every 4 hours (on average). In this sample, all ICP values were obtained from EVD and this may reduce the generalizability of the results (35). Neither the McNett et al (4), nor our analysis examined the PLR finding in relation to the lesion location (e.g., global, infratentorial, ipsilateral), nor is the size/volume of the lesion accounted for. These data were not available in our sample but could provide insight in future prospective studies. Another limitation is the relatively small proportion of subjects with lower GCS values. It is unknown if the same or similar results would be noted in a large cohort of subjects with severe acquired brain injury.
CONCLUSIONS
These findings contribute to the increasing body of literature supporting automated evaluation of the PLR is associated with changes in intracranial dynamics in general and increased ICP in specific. Automated pupillometer is a reliable and objective method used to examine the pupillary changes associated with patients based on their ICP values. Patients with higher ICP demonstrated pupillometer values consistent with lower PLR, such as lower NPi, slower CV, and slower DV. Assessment of pupillometer data is noninvasive method that provides perceptions into the ICP trends to monitor patients with neurologic conditions. The adoption of automated pupillometry must be based on the benefits of objective PLR assessment against equipment costs. Given these findings, it seems reasonable to include measures of PLR when testing for increased ICP.
Footnotes
This work was performed at the University of Texas Southwestern Medical Center, Dallas, TX.
Mr. Al-Obaidi contributed in conceptualization, formal analysis, data curation, and writing original draft. Dr. Atem contributed in conceptualization, formal analysis, data curation, supervision, methodology, writing review, and editing. Dr. Stutzman contributed in resources, writing original draft, and project administration. Dr. Olson contributed in investigation, conceptualization, writing original draft, supervision, and funding acquisition.
Drs. Atem, Stutzman, and Olson received salary support from NeurOptics for the research study. Dr. Al-Obaidi has disclosed that he does not have any potential conflicts of interest.
Supported, in part, by grants from NeurOptics.
References
- 1.McNett M, Moran C, Janki C, et al. Correlations between hourly pupillometer readings and intracranial pressure values. J Neurosci Nurs 201749229–234 [DOI] [PubMed] [Google Scholar]
- 2.Couret D, Boumaza D, Grisotto C, et al. Reliability of standard pupillometry practice in neurocritical care: An observational, double-blinded study. Crit Care. 2016;20:99. doi: 10.1186/s13054-016-1239-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larson MD, Behrends M. Portable infrared pupillometry: A review. Anesth Analg 20151201242–1253 [DOI] [PubMed] [Google Scholar]
- 4.McNett M, Moran C, Grimm D, et al. Pupillometry trends in the setting of increased intracranial pressure. J Neurosci Nurs 201850357–361 [DOI] [PubMed] [Google Scholar]
- 5.Olson DM, Stutzman SE, Atem F, et al. Establishing normative data for pupillometer assessment in neuroscience intensive care: The “END-PANIC” registry. J Neurosci Nurs 201749251–254 [DOI] [PubMed] [Google Scholar]
- 6.Olson DM, Fishel M. The use of automated pupillometry in critical care. Crit Care Nurs Clin North Am 201628101–107 [DOI] [PubMed] [Google Scholar]
- 7.Bader MK, Littlejohns LR. AANN Core Curriculum for Neuroscience Nursing. Philadelphia, Saunders; 2004. [Google Scholar]
- 8.Clark A, Clarke TN, Gregson B, et al. Variability in pupil size estimation. Emerg Med J 200623440–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerr RG, Bacon AM, Baker LL, et al. Underestimation of pupil size by critical care and neurosurgical nurses. Am J Crit Care 201625213–219 [DOI] [PubMed] [Google Scholar]
- 10.Meeker M, Du R, Bacchetti P, et al. Pupil examination: Validity and clinical utility of an automated pupillometer. J Neurosci Nurs 20053734–40 [PubMed] [Google Scholar]
- 11.Olson DM, Stutzman S, Saju C, et al. Interrater reliability of pupillary assessments. Neurocrit Care 201624251–257 [DOI] [PubMed] [Google Scholar]
- 12.Worthley LI. The pupillary light reflex in the critically ill patient. Crit Care Resusc 200027–8 [PubMed] [Google Scholar]
- 13.Chen JW, Vakil-Gilani K, Williamson KL, et al. Infrared pupillometry, the neurological pupil index and unilateral pupillary dilation after traumatic brain injury: Implications for treatment paradigms. Springerplus. 2014;3:548. doi: 10.1186/2193-1801-3-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salah-Mabed I, Saad A, Gatinel D. Assessing repeatability of pupillometric measurements in the eyes of refractive surgery candidates using infrared pupillometer. J Refract Surg 201733552–557 [DOI] [PubMed] [Google Scholar]
- 15.Anderson M, Elmer J, Shutter L, et al. Integrating quantitative pupillometry into regular care in a neurotrauma intensive care unit. J Neurosci Nurs 20185030–36 [DOI] [PubMed] [Google Scholar]
- 16.Zhao W, Stutzman S, Olson D, et al. Inter-device reliability of the NPi-100 pupillometer. J Clin Neurosci 20163379–82 [DOI] [PubMed] [Google Scholar]
- 17.Chen JW, Gombart ZJ, Rogers S, et al. Pupillary reactivity as an early indicator of increased intracranial pressure: The introduction of the neurological pupil index. Surg Neurol Int. 2011;2:82. doi: 10.4103/2152-7806.82248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papangelou A, Zink EK, Chang WW, et al. Automated pupillometry and detection of clinical transtentorial brain herniation: A case series. Mil Med 2018183e113–e121 [DOI] [PubMed] [Google Scholar]
- 19.Truong JQ, Ciuffreda KJ. Objective pupillary correlates of photosensitivity in the normal and mild traumatic brain injury populations. Mil Med 20161811382–1390 [DOI] [PubMed] [Google Scholar]
- 20.Zafar SF, Suarez JI. Automated pupillometer for monitoring the critically ill patient: A critical appraisal. J Crit Care 201429599–603 [DOI] [PubMed] [Google Scholar]
- 21.Gjerris F, Brennum J. Paulson OB, Gjerris F, Sørensen PS. The cerebrospinal fluid, intracranial pressure and herniation of the brain. In: Clinical Neurology and Neurosurgery 2004Copenhagen: FADL Publishing; 179–196 [Google Scholar]
- 22.Smith M. Monitoring intracranial pressure in traumatic brain injury. Anesth Analg 2008106240–248 [DOI] [PubMed] [Google Scholar]
- 23.Treggiari MM, Schutz N, Yanez ND, et al. Role of intracranial pressure values and patterns in predicting outcome in traumatic brain injury: A systematic review. Neurocrit Care 20076104–112 [DOI] [PubMed] [Google Scholar]
- 24.Marshall LF, Barba D, Toole BM, et al. The oval pupil: clinical significance and relationship to intracranial hypertension. J Neurosurg 198358566–568 [DOI] [PubMed] [Google Scholar]
- 25.Chamoun RB, Robertson CS, Gopinath SP. Outcome in patients with blunt head trauma and a Glasgow Coma Scale score of 3 at presentation. J Neurosurg 2009111683–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor WR, Chen JW, Meltzer H, et al. Quantitative pupillometry, a new technology: normative data and preliminary observations in patients with acute head injury. Technical note. J Neurosurg 200398205–213 [DOI] [PubMed] [Google Scholar]
- 27.Saul TG, Ducker TB. Effect of intracranial pressure monitoring and aggressive treatment on mortality in severe head injury. J Neurosurg 198256498–503 [DOI] [PubMed] [Google Scholar]
- 28.Bratton SL, Chestnut RM, Ghajar J, et al. ; Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNS Guidelines for the management of severe traumatic brain injury. VIII. Intracranial pressure thresholds. J Neurotrauma 200724Suppl 1S55–S58 [DOI] [PubMed] [Google Scholar]
- 29.Freeman A, Stockwell J, McCracken C. 804: Assessment of automated pupillometry as a predictor of increased intracranial pressure. Crit Care Med. 2019;47:381. [Google Scholar]
- 30.Odgaard L, Aadal L, Eskildsen M, et al. Nursing sensitive outcomes after severe traumatic brain injury: A nationwide study. J Neurosci Nurs 201850149–154 [DOI] [PubMed] [Google Scholar]
- 31.Hawryluk GW, Manley GT. Classification of traumatic brain injury: Past, present, and future. Handb Clin Neurol 201512715–21 [DOI] [PubMed] [Google Scholar]
- 32.Soeken TA, Alonso A, Grant A, et al. Quantitative pupillometry for detection of intracranial pressure changes during head-down tilt. Aerosp Med Hum Perform 201889717–723 [DOI] [PubMed] [Google Scholar]
- 33.Ortega-Pérez S, Amaya-Rey MC. Secondary brain injury: A concept analysis. J Neurosci Nurs 201850220–224 [DOI] [PubMed] [Google Scholar]
- 34.Shirozu K, Setoguchi H, Tokuda K, et al. The effects of anesthetic agents on pupillary function during general anesthesia using the automated infrared quantitative pupillometer. J Clin Monit Comput 201731291–296 [DOI] [PubMed] [Google Scholar]
- 35.Olson DM, Ortega Peréz S, Ramsay J, et al. Differentiate the source and site of intracranial pressure measurements using more precise nomenclature. Neurocrit Care 201930239–243 [DOI] [PubMed] [Google Scholar]


