Supplemental Digital Content is available in the text.
Keywords: acute lung injury, alveolar macrophages, bronchoalveolar lavage, N-acetyl cysteine, pulmonary function test, vaping
Abstract
Objectives:
Vaping-associated lung injury has rapidly become a nationwide epidemic and a threat to public health. In this case series, we describe unique clinical features of severe vaping-associated lung injury, defined as respiratory failure due to vaping that requires mechanical ventilation.
Data Sources:
Clinical observation of four patients.
Study Selection:
Case series.
Data Extraction:
Data and images were extract from medical records after approval was obtained from the institutional review board.
Data Synthesis:
Four patients were admitted to the ICU with severe manifestation of vaping-associated lung injury. Although every case required mechanical ventilatory support (venovenous extracorporeal membrane oxygenation in one patient), all patients survived and were discharged without supplemental oxygen. Systemic corticosteroids were administered in three patients and N-acetyl cysteine in one. A postdischarge pulmonary function test in one patient was normal except for mildly decreased diffusing capacity.
Conclusions:
Based on our experience, prognosis of severe vaping-associated lung injury appears favorable with aggressive supportive care, although there is evidence from existing literature that mortality rate might rise with increasing disease severity. Underlying mechanism of lung injury might be similar between vaping-associated lung injury and amiodarone pneumonitis. Foamy or lipid-laden macrophages, seen in both conditions, might be a marker of cytotoxicity from substances contained in e-cigarettes, such as vitamin E acetate. Systemic corticosteroids, and possibly N-acetyl cysteine, could be considered as therapeutic adjuncts in vaping-associated lung injury. Serial pulmonary function tests should be obtained in these patients to monitor for potential long-term complications. The primary limitations of this case series are its small sample and lack of longitudinal follow-up data.
Initially a regional outbreak that began in summer of 2019 from Wisconsin and Illinois (1), vaping-associated lung injury (VALI) has rapidly evolved into a national epidemic (2). Although numerous pathophysiologic mechanisms have been discussed in recent literature (3–9), the underlying disease process has not been defined yet. Recent discovery of vitamin E acetate in the bronchoalveolar lavage (BAL) samples from 29 patients shed some light on a possible causative agent, although so far this is merely an associative finding (2). The significance of foamy and lipid-laden macrophages in BAL fluid remains unclear (1, 3, 5, 7–10). Although one case series proposed exogenous lipoid pneumonia as a mechanism (5), this notion was challenged in other reports (3, 8). No explanation has been proposed in regards to heterogeneity of disease severity either, which has varied from mild respiratory symptoms managed as outpatient to severe respiratory failure requiring mechanical ventilation and in some cases, venovenous extracorporeal membrane oxygenation (ECMO) (1, 3, 5–9).
This study examines four cases of severe VALI, defined as respiratory failure due to vaping that requires mechanical ventilation, at an acute quaternary care academic medical center in Los Angeles during August and September of 2019. We believe this case series will make valuable contributions to the existing body of knowledge by discussing the following: 1) the results of first-ever postdischarge pulmonary function test (PFT), 2) the role of early systemic corticosteroids, 3) mortality outcome data for mechanically ventilated patients, 4) the role of N-acetyl cysteine (NAC) as a therapeutic modality, and 5) pathogenesis of VALI with analogies drawn from amiodarone pneumonitis.
CASE SERIES
Our patients’ age ranged from 18 to 29 years with a 1:1 male-to-female ratio. All patients endorsed respiratory and constitutional symptoms at presentation. Pleuritic chest pain, gastrointestinal symptoms, myalgia, congestion, and anorexia were also present in some. All four patients had a recent history of vaping prior to symptom onset. Three patients endorsed extensive use of both nicotine- and marijuana-based flavored e-cigarettes for 1–2 years. Two of these three patients also intermittently engaged in practice known as dabbing, a form of vaping that involves inhalation of heated, vaporized tetrahydrocannabinol extract admixed with liquid butane (11). Two patients reported obtaining their vaping devices off the street. Detailed vaping history was unavailable for one patient due to incomplete records. Medical history was notable for history of asthma in one patient and a recent pulmonary contusion in another. One patient was a construction worker with possible inhalational exposure(s) but without any symptoms or limitations at baseline. Time from symptom onset to presentation ranged from 2 to 5 days.
Tachycardia, tachypnea, hypoxemia, and abnormal pulmonary examination were present in all patients. Fever was noted in three patients initially and eventually developed in everyone.
All patients required intubation between 22 and 62 hours after presentation and remained on mechanical ventilation for 4 to 18 days. Due to refractory hypoxemia, paralytic agents were administered in three patients. Out of these three patients, one patient underwent prone positioning and another required 14 days of venovenous ECMO.
Chest imaging exhibited bilateral pulmonary infiltrates in every case (Fig. 1). Everyone had peripheral leukocytosis with neutrophil predominance but no eosinophilia. Testing for HIV was negative in all cases. Results of bronchoscopy, which was performed in all patients, did not reveal a clear infectious or a malignant etiology. BAL respiratory viral polymerase chain reaction panel was negative in all except for rhino-enterovirus in one patient, which the clinical care team did not believe was the primary etiology of lung injury. BAL cell count showed neutrophil predominance in all but one patient who had macrophage predominance. No eosinophilia was noted. Cytopathology demonstrated lipid-laden macrophages in two cases in which oil red O stain was performed. The remaining two cases showed vacuolated (“foamy”) macrophages. (Fig. 2)
Figure 1.
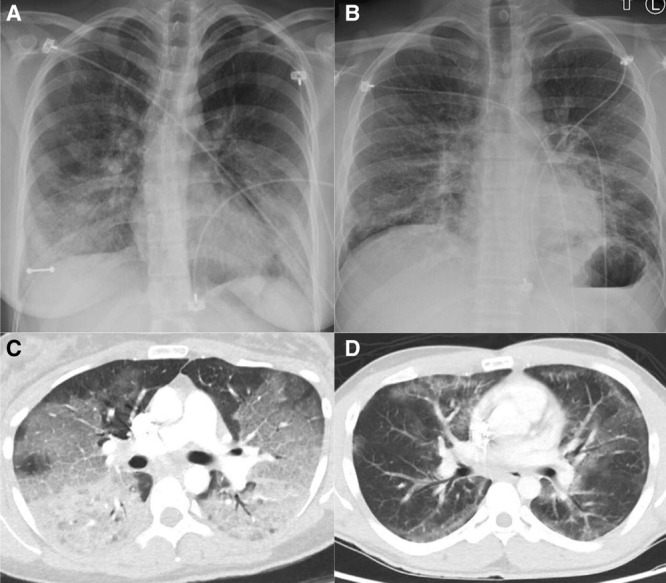
Chest radiograph and CT angiogram obtained from two patients with severe vaping-associated lung injury. Initial anterior-posterior chest radiograph (A) from patient A demonstrates basilar predominant interstitial and superimposed airspace opacities. An axial CT angiogram image (C) obtained from patient A 19 hr later shows diffuse ground glass and interstitial opacities. Initial posterior-anterior chest radiograph (B) from patient B shows bilateral mid- and lower-lung zone predominant infiltrates. An axial CT angiogram image (D) obtained from patient B 5 hr later demonstrates patchy ground glass and reticular opacities.
Figure 2.
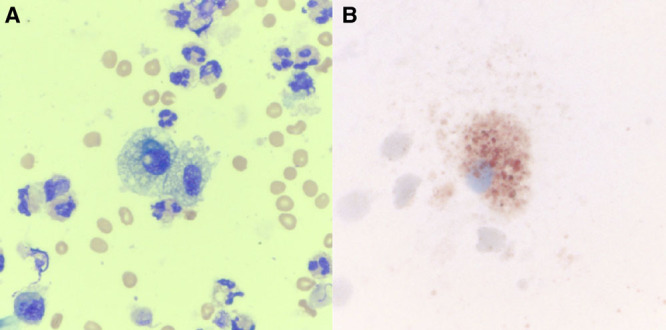
Cytopathology of bronchoalveolar lavage samples from two patients with severe vaping-associated lung injury in our case series. A, Wright’s stain demonstrates vacuolated alveolar macrophages admixed with multiple adjacent neutrophils from patient A (×40). B, A prominent lipid-laden macrophage is highlighted with oil red O stain (×60) from patient C.
Every patient was treated with empiric broad-spectrum antibiotics. Three patients received systemic corticosteroids. One of these three patients was treated with inhaled NAC for mucolytic indication. All patients were successfully liberated from mechanical ventilation and discharged without supplemental oxygen. A 1-week postdischarge chest radiograph in one patient demonstrated complete resolution of bilateral infiltrates. At 2 weeks postdischarge, the same patient endorsed mild hoarseness of voice but otherwise had complete resolution of symptoms, with PFT revealing mildly reduced diffusing capacity but no obstructive or restrictive defect.
Detailed information on patient characteristics, laboratory data, and clinical course can be found in Supplemental Table 1 (Supplemental Digital Content 1, http://links.lww.com/CCX/A131).
DISCUSSION
Our case series offers an excellent opportunity to delve into features of severe VALI. Remarkably, everyone in our case series was discharged without supplemental oxygen despite the severity of their hypoxemia. Notwithstanding excellent short-term clinical outcomes in all of our cases, abnormal diffusing capacity in the postdischarge PFT of one of our patients suggests that physiologic abnormalities might persist despite symptomatic and radiologic recovery. To date, no other follow-up PFTs have been reported in these patients.
Although aggressive supportive measures should be used at all stages of VALI, early employment of anti-inflammatory therapy such as corticosteroids might help prevent progression into fulminant respiratory failure, despite only anecdotal evidence supporting its use (1, 3, 5, 7–9). The only patient in our cohort who did not receive systemic corticosteroids underwent venovenous ECMO. In recently published case series, mortality rate for mechanically ventilated and ECMO subgroups were 11.5% (3/26, two unknown outcomes) and 50% (2/4), respectively (1, 3, 5, 7, 9). We excluded one case series (8) from this analysis due to incomplete data on mechanical ventilation. Overall mortality rate nationwide so far is 1.9% (39/2,051) (2). Although direct outcome comparison between our cases, other case series, and nationwide data are challenging due to numerous reasons (our small sample size, possible selection bias in case series, incomplete national data on mechanical ventilation, etc.), it seems that once fulminant respiratory failure develops, mortality rate increases.
Another potential treatment that could be considered in VALI is NAC, as was used in one of our patients albeit for its mucolytic property. One in vitro study found that reactive oxygen species production was markedly increased in alveolar macrophages exposed to e-cigarette vapors (12). When macrophages were treated with NAC, cytotoxic and pro-apoptotic effects of e-cigarette vapors on macrophages returned to baseline and phagocytic activity was restored (12).
The role of macrophages in pathogenesis of VALI has been one of the centerpieces of discussion since early on in the epidemic, specifically in regards to the source and significance of foamy and lipid-laden macrophages seen on lung biopsy and BAL samples, including ours (1, 3, 5, 7–10). Exogenous lipoid pneumonia was proposed early in the epidemic as the pathogenic mechanism in a case series from North Carolina (5). However, recently published lung biopsy results did not show any histopathologic findings consistent with exogenous lipoid pneumonia, but instead demonstrated patterns of nonspecific acute lung injury (3, 8). Furthermore, 29 BAL samples tested by the Centers for Disease Control and Prevention did not identify lipid-based substances such as plant oils or petroleum distillates (2).
In amiodarone pneumonitis, another disease in which foamy and lipid-laden macrophages can be found, lipid accumulation within alveolar macrophages is thought to represent drug-induced cytotoxicity (13). The suggestion of a similar mechanism of lung injury between amiodarone pneumonitis and VALI was made by one group of authors (3). In amiodarone pneumonitis, foamy and lipid-laden macrophages were found both in BAL samples of those with amiodarone-induced pneumonitis and in those with exposure to amiodarone but without pulmonary disease (13). The presence of foamy and lipid-laden macrophages in amiodarone pneumonitis might reflect drug-induced cytotoxicity that is insufficient to cause lung injury until a certain inflammatory threshold is crossed (perhaps in the setting of other exacerbating factors such as high Fio2 and ensuing toxic oxygen free radicals) (14), as might also be the pathogenic mechanism in VALI. If there is a possibility of a similar mechanism of lung injury between VALI and amiodarone pneumonitis, high Fio2 should perhaps be used with caution in VALI. The presence of foamy or lipid-laden macrophages in BAL samples of vapers without lung injury could be helpful in discerning their true role in pathogenesis of VALI.
Vitamin E acetate recently emerged as a possible culprit (2), although more research is needed to elucidate pathogenic relationships linking vitamin E acetate, formation of foamy or lipid-laden macrophages, and mechanism(s) of lung injury in VALI. It is also possible that vitamin E acetate is not the only causative agent or sufficient to cause VALI by itself. There is evidence that various substances contained in e-cigarette vapor and marijuana smoke can modify intra-pulmonary environment by altering gene expression, protein synthesis, mitochondrial function, and ciliary motility in bronchial epithelium as well as by changing the permeability of alveolar epithelium (15–18). E-cigarette vapors can also exert direct cytotoxic effect on alveolar macrophages with a subsequent increase in apoptosis, necrosis, reactive oxygen species production, inflammatory cytokines release, and inhibition of phagocytosis (12). These modifications in cellular functions might make the lungs more susceptible to acute injury. By the time vitamin E acetate came under the spotlight as a possible causative agent in November of 2019 (2), our BAL samples, which were collected in August and September of 2019, were no longer available for vitamin E acetate testing.
The primary limitations of this case series are small sample size and lack of longitudinal follow-up data. More cases combined with serial symptom assessment, imaging, and PFTs would let us truly gauge the short- and long-term outcomes of this disease. Long-term complications such as obliterative bronchiolitis or giant-cell interstitial pneumonia are a concern in VALI and therefore (3, 6), patients should be longitudinally followed with serial PFTs and imaging.
In summary, we present four patients with severe VALI, all of whom survived with aggressive respiratory support, corticosteroids, and possibly NAC. Foamy and lipid-laden macrophages, perhaps a marker of cytotoxic injury from vaping, do not seem to be a manifestation of exogenous lipoid pneumonia as proposed earlier in the epidemic. Postdischarge PFT in one patient showed mildly reduced diffusing capacity but was otherwise normal.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Drs. Chen and Parimon are researchers funded by the National Institutes of Health. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Institutional Review Board Approval: Cedars-Sinai institutional review board gave approval for this research. Approval letter and study protocol were submitted as supplemental material.
Drs. Choe, Chen, Falk, Ng, Parimon, and Ghandehari contributed to initial conception of the work. Dr. Choe drafted the article with Drs. Parimon and Ghandehari. Dr. Nguyen provided critical input for cytopathology. All authors took part in data collection, analysis, article revision and made unique intellectual contributions. All authors take full responsibility for the content of the article, including integrity and accuracy of the data and its analysis. All authors gave final approval for publication of the article.
REFERENCES
- 1.Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin - preliminary report. N Engl J Med. 2019 Sep 6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention: Outbreak of Lung Injury Associated With E-Cigarette Use, or Vaping. 2019.. Available at: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html. Accessed November 10, 2019
- 3.Butt YM, Smith ML, Tazelaar HD, et al. Pathology of vaping-associated lung injury. N Engl J Med. 2019; 381:1780–1781 [DOI] [PubMed] [Google Scholar]
- 4.Christiani DC. Vaping-induced lung injury. N Engl J Med. 2019 Sep 6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Davidson K, Brancato A, Heetderks P, et al. Outbreak of electronic-cigarette-associated acute lipoid pneumonia - North Carolina, July-August 2019 MMWR Morb Mortal Wkly Rep. 2019; 68:784–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henry TS, Kanne JP, Kligerman SJ. Imaging of vaping-associated lung disease. N Engl J Med. 2019; 381:1486–1487 [DOI] [PubMed] [Google Scholar]
- 7.Maddock SD, Cirulis MM, Callahan SJ, et al. Pulmonary lipid-laden macrophages and vaping. N Engl J Med. 2019; 381:1488–1489 [DOI] [PubMed] [Google Scholar]
- 8.Mukhopadhyay S, Mehrad M, Dammert P, et al. Lung biopsy findings in severe pulmonary illness associated with e-cigarette use (vaping) Am J Clin Pathol. 2020; 153:30–39 [DOI] [PubMed] [Google Scholar]
- 9.Triantafyllou GA, Tiberio PJ, Zou RH, et al. Vaping-associated acute lung injury: A case series. Am J Respir Crit Care Med. 2019; 200:1430–1431 [DOI] [PubMed] [Google Scholar]
- 10.Schier JG, Meiman JG, Layden J, et al. ; CDC 2019 Lung Injury Response Group. Severe pulmonary disease associated with electronic-cigarette-product use - interim guidance. MMWR Morb Mortal Wkly Rep. 2019; 68:787–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson RP, Zechar K. Lung injury from inhaling butane hash oil mimics pneumonia. Respir Med Case Rep. 2019; 26:171–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott A, Lugg ST, Aldridge K, et al. Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax. 2018; 73:1161–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Israël-Biet D, Venet A, Caubarrère I, et al. Bronchoalveolar lavage in amiodarone pneumonitis. Cellular abnormalities and their relevance to pathogenesis. Chest. 1987; 91:214–221 [DOI] [PubMed] [Google Scholar]
- 14.Hughes M, Binning A. Intravenous amiodarone in intensive care. Time for a reappraisal? Intensive Care Med. 2000; 26:1730–1739 [DOI] [PubMed] [Google Scholar]
- 15.Clapp PW, Lavrich KS, van Heusden CA, et al. Cinnamaldehyde in flavored e-cigarette liquids temporarily suppresses bronchial epithelial cell ciliary motility by dysregulation of mitochondrial function. Am J Physiol Lung Cell Mol Physiol. 2019; 316:L470–L486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh A, Coakley RC, Mascenik T, et al. Chronic E-cigarette exposure alters the human bronchial epithelial proteome. Am J Respir Crit Care Med. 2018; 198:67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gil E, Chen B, Kleerup E, et al. Acute and chronic effects of marijuana smoking on pulmonary alveolar permeability. Life Sci. 1995; 56:2193–2199 [DOI] [PubMed] [Google Scholar]
- 18.Park HR, O’Sullivan M, Vallarino J, et al. Transcriptomic response of primary human airway epithelial cells to flavoring chemicals in electronic cigarettes. Sci Rep. 2019; 9:1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


