Abstract
Objectives:
To describe a pediatric case of cytokine release syndrome secondary to chimeric antigen receptor-modified T cells associated with acute respiratory distress syndrome.
Design:
Case report.
Setting:
PICU.
Patients:
A 14-year-old boy with refractory B cell precursor acute lymphoblastic leukemia given chimeric antigen receptor cells developed severe cytokine release syndrome 7 days after the drug product infusion with progressive respiratory failure. He was admitted to PICU with a clinical picture of acute respiratory distress syndrome, requiring mechanical ventilation, and secondary hemophagocytic lymphohistiocytosis.
Interventions:
Hemoadsorption with cartridge column (Cytosorb) in combination with continuous renal replacement therapy was associated to the anti-cytokine therapy (tocilizumab, a monoclonal antibody targeting interleukin-6 receptor).
Measurements and Main Results:
Decrease of the inflammatory biomarkers (ferritin, interleukin-6, interleukin-10) in the first 96 hours associated with a progressive improvement of acute respiratory distress syndrome (Pao2/Fio2 ratio) 7 day after the start of the multimodal treatment.
Conclusions:
This case suggests that hemoadsorption with cartridge column in combination with continuous renal replacement therapy and tocilizumab is safe and potentially effective in pediatric patients with severe cytokine release syndrome.
Keywords: acute respiratory distress syndrome, blood purification, chimeric antigen receptor-modified T cells, cytokine release syndrome, cytokines, Cytosorb
Chimeric antigen receptor-modified T cells (CAR-Ts) are a promising approach for the treatment of chemotherapy-refractory B-cells malignancies (1). This therapy can induce a generalized hyperinflammatory syndrome, secondary to CAR-Ts expansion, known as cytokine release syndrome (CRS) (1). CRS severity spans on different grades and may become life threatening or even fatal for vasodilatory shock with capillary leak, hypoxia, and multiple organ dysfunction (2). The recently proposed standard treatment of CRS is tocilizumab, an antibody targeting the interleukin (IL)–6 receptor, which was shown to be effective in most patients, with a clinical response within hours, although refractory cases have been observed (2). Corticosteroids and other immunosuppressive drugs are less frequently used because of concerns related to their detrimental effect on CAR-Ts function (3, 4).
CASE REPORT
We report the case of a 14-year-old boy with B cell precursor acute lymphoblastic leukemia refractory to chemotherapy and immunotherapy (inotuzumab and blinatumomab). He was enrolled into an academic trial on the use of CD19-directed CAR-Ts. Seven days after CAR-Ts infusion, he developed severe CRS characterized by progressive respiratory failure; after 3 days from CRS onset, he was admitted to the PICU because of acute respiratory distress syndrome (ARDS) (Pao2/Fio2 ratio, 45 mm Hg; oxygen index, 5.9) with need of invasive mechanical ventilation. No microbiological pathogen was identified from material of respiratory tract and blood cultures; (C-reactive protein, 17 mg/dL procalcitonin, 4.16 ng/mL). Hemodynamic clinical picture was characterized by moderate low resistance (systemic vascular resistance index, 1,500) with a vasopressor support of noradrenaline 0.06 μg/kg/min and a preserved myocardial function (ejection fraction, 50%). ARDS was managed with a protective ventilation strategy, calculation of the best positive end-expiratory pressure, prone positioning, and low-dose methylprednisolone. Considering the diagnosis of grade 4 CRS associated with secondary hemophagocytic lymphohistiocytosis (s-HLH) (ferritin, 146.095 ng/mL), a blood purification strategy with a cartridge column (Cytosorb) in combination with continuous renal replacement therapy was initiated despite the fact that the child conserved a urinary output greater than 2 mL/kg/hr. The therapeutic schema of blood purification with Cytosorb in correlation with the ferritin and cytokines time course is shown in Figure 1. Five cartridge columns were used the first two were changed every 12 hours and then every 24 hours. Tocilizumab was administered 6 hours before the admission in PICU and at the 3rd and 4th days after the start of the hemoadsorption treatment (Fig. 1).
Figure 1.
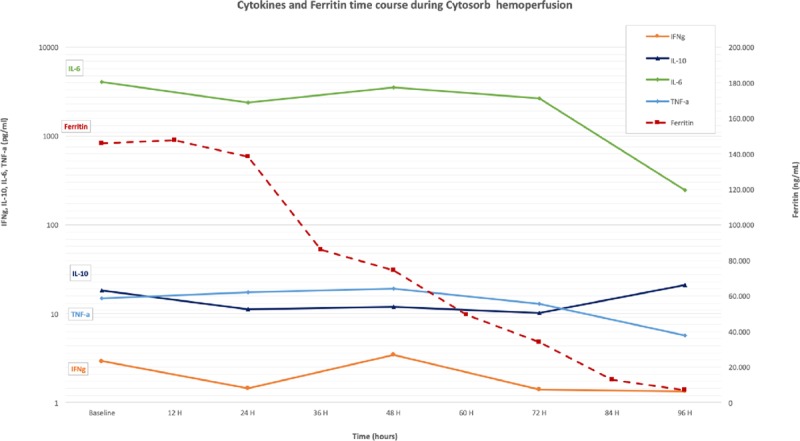
Cytokines and ferritin time course during Cytosorb hemoperfusion. Red arrows indicate the cartridge column change during the extracorporeal blood purification therapy. IFNγ = interferon gamma, IL = interleukin, TNF-α = tumor necrosis factor-α.
During this multimodal approach, we assisted in the first 96 hours to a dramatic reduction of ferritin levels to 6,934 ng/mL. The cytokines dosage has showed the same trend with a significant reduction of IL-6 (time 0 = T0 4048,082 pg/mL; 96 hr = 96 H 247,682 pg/mL), interferon gamma (T0 2,919 pg/mL; 96 H 1,347 pg/mL), and tumor necrosis factor-alpha (T0 14,827 pg/mL; 96 H 5,74 pg/mL). The reduction inflammatory biomarkers was paralleled by a gradual and progressive increase of the Pao2/Fio2 ratio and an improvement of the chest radiographs (Fig. 2) reversing the exudative phase of ARDS. On day 12 from the admission in PICU, he was weaned from invasive mechanical ventilation and on day 19, he was discharged from PICU.
Figure 2.
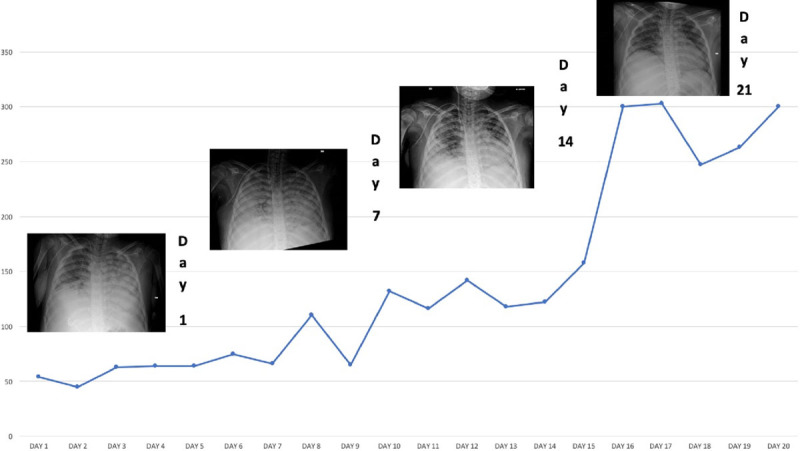
Pao2/Fio2 ratio and improvement of the chest radiographs from the PICU admission (day 1) to the PICU discharge (day 19). The hemoperfusion with Cytosorb has been performed from day 2 to day 5.
DISCUSSION
CRS is a severe complication of CAR-Ts therapy characterized by uncontrolled systemic inflammation. This new emerging syndrome could be a challenging clinical scenario in intensive care and clinicians should be aware of the physiopathology of this complication in order to plan the best intensive therapeutic approach (5).
To the best of our knowledge, this is the first case of application in a clinical setting of a hemoadsorption with Cytosorb to manage severe CRS secondary to CAR-Ts infusion. Cytosorb cartridge columns have a large surface area that adsorbs middle molecular weight molecules, such as cytokines and other inflammatory mediators (cutoff < 75 kilodalton [kDa]) (6). The blood purification strategy in this setting has important therapeutic implications: first, it does not interfere with the first-line pharmacologic therapy based on tocilizumab administration (molecular weight > 100 kDa); second, Cytosorb could represent an interesting therapeutic resource in grade 3/4 CRS in order to dampen the hyperinflammatory reaction without affecting CAR-Ts efficacy (3, 4).
In our case, the relation between the hemoadsorption treatment and inflammatory biomarkers changes supports the efficacy of this blood purification strategy to manage the systemic hyperinflammatory syndrome induced by CRS. Remarkably, our patient developed also s-HLH with high levels of ferritin and the blood purification has probably played a role in preventing the need of high doses of steroids or other immunosuppressive agents as suggested from some authors in case of refractory s-HLH associated with CRS (7, 8).
Insufficient evidences exist about the efficacy of hemoadsorption in ARDS (9): although a casual relationship cannot be proven, we presume that controlling the excessive inflammatory reaction with Cytosorb helped reverse the clinical picture of ARDS in CRS, which recognizes a pure inflammatory pathophysiology (4).
CAR-Ts infusion and other immunotherapeutic approach have opened important therapeutic perspectives for a broad range of cancers. The growing use of these agents increases the occurrence rate of CRS, which can represent one of the future challenge in intensive care. A key point in the management of CRS is to minimize CRS-related toxicity while maintaining the efficacy of CAR-Ts. The combination of hemoadsorption with Cytosorb and tocilizumab might have a synergistic effect in this setting: this association has not yet been explored in the treatment of CRS. Further studies are needed to assess the safety and efficacy of the approach; furthermore, they will help identifying the optimal timing of initiation of such an approach in children suffering from severe CRS.
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018; 378:439–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frey N, Porter D. Cytokine release syndrome with chimeric antigen receptor T cell therapy. Biol Blood Marrow Transplant. 2019; 25:e123–e127 [DOI] [PubMed] [Google Scholar]
- 3.Frey N. Cytokine release syndrome: Who is at risk and how to treat. Best Pract Res Clin Haematol. 2017; 30:336–340 [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald JC, Weiss SL, Maude SL, et al. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit Care Med. 2017; 45:e124–e131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gödel P, Shimabukuro-Vornhagen A, von Bergwelt-Baildon M. Understanding cytokine release syndrome. Intensive Care Med. 2018; 44:371–373 [DOI] [PubMed] [Google Scholar]
- 6.Poli EC, Rimmelé T, Schneider AG. Hemoadsorption with Cytosorb®. Intensive Care Med. 2019; 45:236–239 [DOI] [PubMed] [Google Scholar]
- 7.Neelapu S, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy-assessment and management of toxicities. Nat Rev Clin Oncol. 2018; 15:47–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teachey DT, Bishop M, Maloney D, et al. Toxicity management after chimeric antigen receptor T cell therapy: One size not fit “ALL.” Nat Rev Clin Oncol. 2018; 15:218. [DOI] [PubMed] [Google Scholar]
- 9.Putzu A, Fang MX, Boscolo Berto M, et al. Blood purification with continuous veno-venous hemofiltration in patients with sepsis or ARDS: A systematic review and meta-analysis. Minerva Anestesiol. 2017; 83:867–877 [DOI] [PubMed] [Google Scholar]


