Abstract
Objectives:
Restrictive transfusion policies have been adopted in critical care, although these have not included patients receiving extracorporeal membrane oxygenation. We aimed to assess survival outcomes, adverse events related to RBC transfusion, and cost implications following a change from a “liberal” to a “restrictive” RBC transfusion practice in patients receiving extracorporeal membrane oxygenation.
Design:
Retrospective observational study.
Setting:
Single high-volume tertiary critical care department at a university hospital.
Patients:
Patients 16 years old or greater receiving venovenous extracorporeal membrane oxygenation between 2011 and 2017 for more than 24 hours.
Interventions:
None.
Measurements and Main Results:
Clinical diagnoses, complications, outcomes, median hemoglobin, and hematocrit levels were obtained from patients’ electronic records. All laboratory results for hemoglobin and hematocrit were included. RBC transfusions were obtained from prescription charts. We included 402 patients: 99 during a “liberal” transfusion practice (2011–2014)—when the target hemoglobin level was greater than 100 g/L; and 303 treated during a “restrictive” transfusion practice (2014–2017) when the target hemoglobin level was greater than 80 g/L. We found that survival outcomes did not change following the implementation of a “restrictive” transfusion policy. There was also a decrease in the extracorporeal blood flow rates with restrictive transfusion of 0.5 L/min. Nonsurvivors of venovenous extracorporeal membrane oxygenation had higher usage of RBC units following a change in transfusion practice. The restrictive strategy allowed a cost saving of £454 per patient.
Conclusions:
These results suggest that the adoption of a more restrictive approach to RBC transfusion during venovenous extracorporeal membrane oxygenation is more cost-effective and associated with similar survival outcomes, than when compared with a more liberal approach.
Keywords: blood transfusion, extracorporeal membrane oxygenation, hemoglobin
The use of venovenous extracorporeal membrane oxygenation (ECMO) has increased dramatically over the last 10 years. Over 100,000 patients have been entered into the Extracorporeal Life Support Organization (ELSO) registry from 1990 to 2018, specifically there was a surge of adult patients since the influenza pandemic of 2009 (1). Since 2013, more adult patients have been added to the ELSO registry than pediatric and neonatal patients. Venovenous ECMO is considered for patients with potentially reversible severe acute respiratory failure. Specific examples include those patients with adequate gas exchange but who are unable to achieve lung-protective ventilation, patients with hypercarbia (pH < 7.2), severe hypoxia (Pao2:Fio2 < 13.3 kilopascal), or life-threatening asthma. Four variables affect the state of oxygenation and determine the oxygen saturation (Sao2) and mixed venous oxygen saturation during venovenous ECMO support: oxygen consumption (Vo2), cardiac output, hemoglobin concentration, and ECMO blood flow (ECBF) (2).
RBC transfusion is routinely exploited to improve oxygen delivery to tissues. The current guidelines suggest targeting a hemoglobin level greater than 150 g/L (3, 4), although in clinical practice, practitioners may use lower thresholds. This threshold is proposed as an optimal cutoff balancing the risks of transfusion and increased circuit flow rates (3). Patients receiving ECMO require higher usage of RBC transfusions (3). The benefits of transfusion include improved oxygen delivery at lower ECBF (5). The major risks of transfusion include volume overload, transfusion-associated acute lung injury, immunomodulation, human leukocyte antigen (HLA), RBC antigen sensitization, and transfusion-transmitted infection (6). High ECBF can result in excessive negative pressure on the drainage side causing hemolysis and in the extreme, pump-related cavitation. Practically, ECBF is frequently limited by the size and position of the drainage cannula and the patient’s intravascular volume (5).
Several large randomized control trials (TRIC, TRICS-III, and TITRe2) have consistently demonstrated that a “restrictive” RBC transfusion strategy typically maintaining hemoglobin concentration between 70 and 90 g/L is noninferior to a “liberal” transfusion strategy maintaining hemoglobin between 100 and 120 g/L in critically ill adults (7–9). These findings are supported and endorsed by national guidelines (10). However, patients receiving ECMO were not included in these studies. Retrospective studies have suggested that lower hematocrit and hemoglobin concentration may provide acceptable clinical outcomes during ECMO (11, 12). RBC transfusion during ECMO has been associated with an increased mortality and morbidity including thrombosis, acute renal impairment, and sepsis, although the causality of this relationship has not been shown (11, 13, 14).
Our local policy changed from a “liberal” transfusion strategy of hemoglobin concentration greater than 100 g/L in January 2014 to follow a “restrictive” approach to transfusion in our adults receiving venovenous ECMO with a target hemoglobin greater than 80 g/L. The aim of this study was to compare the short-term survival outcomes of adults receiving venovenous ECMO between those having a “restrictive” approach to transfusion to recipients of a “liberal” approach. We anticipated that there would be no significant impact following this change in practice.
MATERIALS AND METHODS
Subjects and Study Design
The study was registered as a clinical audit at Guy’s & St Thomas’ NHS Foundation Trust (Audit Reference Number—6741). The study was reviewed by the local Institutional Review Board and waivered further ethical approval. Data were collected retrospectively from electronic medical records and hospital records. All patients greater than 16 years old receiving venovenous ECMO support for more than 24 hours, between January 1, 2011, and December 31, 2017, were identified from a prospectively kept electronic registry.
Demographics
Subject clinical details were extracted using R Version 3.5.1 (R Foundation for Statistical Computing, Auckland, New Zealand; http://www.r-project.org/) from electronic records on Phillips ICIP Intellispace CCA (Amsterdam, The Netherlands). Clinical details including gender, age at initiation of ECMO, cause of respiratory failure, Acute Physiology and Chronic Health Evaluation (APACHE) II score at initiation, duration of ECMO, and the use of renal replacement therapy were recorded.
ECMO Details
All patients reviewed in this study received venovenous ECMO. The oxygenator circuit used at our center is the Cardiohelp system (Getinge, Gothenburg, Sweden) and BE HLS 7.0 oxygenator (Getinge). Cannulation is routinely performed using bifemoral Biomedicus cannulae (Medtronic, Dublin, Ireland) using a previously described method (15). Anticoagulation at the time of the study was with an IV unfractionated heparin infusion (UFH) with 50 IU/kg as a bolus at the time of cannulation with 2,500 units in the priming fluid for the circuit. A subsequent UFH infusion is monitored by the activated partial thromboplastin time ratio targeting a level of 1.5–2.0—unless clinical or radiological evidence of bleeding. This was also guided by a preceding history bleeding or thrombotic complications and whole-body CT performed at the time of cannulation.
RBC Transfusion Indications and Targets
Patients were transfused due to anemia or due to bleeding. The transfusion targets in the patient with stable parameters recommended in our center reduced from a hemoglobin concentration of 100–120 g/L to 80–90 g/L in January 2014 but for stable patients and particularly in those where the concerns about HLA sensitization are important a transfusion target of 70 g/L or less is used. For clinically unstable patients with difficult oxygenation, higher RBC transfusion triggers were defined by the attending physicians. For patients with active bleeding, a pragmatic target hemoglobin concentration of 80–90 g/L is used in our unit.
Outcome Variables
The primary outcomes were survival to decannulation, survival to discharge from the ICU and survival at 6 months after discharge. Patients with incomplete follow-up at 6 months were not included in the outcome frequencies at that time point. The data was collected prospectively for entry to our local registry and reviewed retrospectively for the purpose of this study. We restricted our analysis to include those patients who survived greater than 24 hours after cannulation and for their first run on ECMO.
RBC transfusion was identified from our electronic health record which records RBC units transfused by pack serial number to prevent double counting of transfusions. All serious adverse events, which occur following transfusion, are recorded under the Trust’s transfusion reaction vigilance system and then reported to the Serious Hazards of Transfusion (SHOT) Organization, the UK’s independent hemovigilance scheme. Databases containing adverse event and SHOT reports were reviewed.
Detection of intracranial hemorrhage (ICH) by cranial CT is routinely performed at the commencement of ECMO at our center and subsequent cranial CT is performed if clinically indicated (16). Bleeding assessment is performed qualitatively during ECMO by the clinical care team and recorded contemporaneously in the electronic medical record. Major hemorrhage for the purpose of this study was defined as the transfusion of four or more units of RBC within a 24-hour period with clinical evidence of bleeding. Large volume RBC transfusion for reasons not related to bleeding were not included in this definition. A cost of £129 (July 2019) per packed RBC unit was used to perform a limited cost analysis comparing the two time periods. Calculation of a mean cost per patient allowed a basic comparison of RBC transfusion costs under “liberal” and more “restrictive” transfusion strategies.
Laboratory Blood Testing Values and Assays
A full blood count (FBC) is routinely performed once daily for patients receiving ECMO and additionally if there are other clinical indications such as bleeding, preoperatively or following circuit change. All FBC results from venous samples were included in this study to give a median hemoglobin concentration and hematocrit during ECMO for further statistical analysis. Results were reviewed for outliers and these were then reviewed manually with clinical records for errors.
FBC testing is performed using DxH 900 Hematology (Beckman Coulter, Brea, CA) to provide hemoglobin concentration and hematocrit results at our center. Laboratory testing is fully CPD accredited and takes part in routine internal and external quality assessment as mandated by the United Kingdom Accreditation Service.
Statistical Considerations
Statistical analysis was performed using R Statistics Version 3.5.1 (R Foundation for Statistical Computing). Descriptive analysis of continuous variables was given as medians and interquartile ranges. Categorical variables were given as numbers of individuals/events and percentages. We compared those patients initiated on ECMO in our institution between January 1, 2011, and December 31, 2013, to those between January 1, 2014, and December 31, 2017.
Null hypothesis significance testing was employed to examine variable distributions between the two time periods. Mann-Whitney U tests were performed to assess continuous variables, and chi-square goodness-of-fit tests were performed to assess categorical variables. A multiple logistic regression was performed to examine variables associated with survival to decannulation from ECMO. The regression model covariates were selected with consideration for plausible and known predictors of survival; thus, gender, age, illness severity, and length of ECMO provision were included with the study-specific variables of median hemoglobin concentration, number of RBC transfusions during ECMO, and the time period of ECMO provision. A significance level of alpha equals to 0.05 was used for all statistical tests.
RESULTS
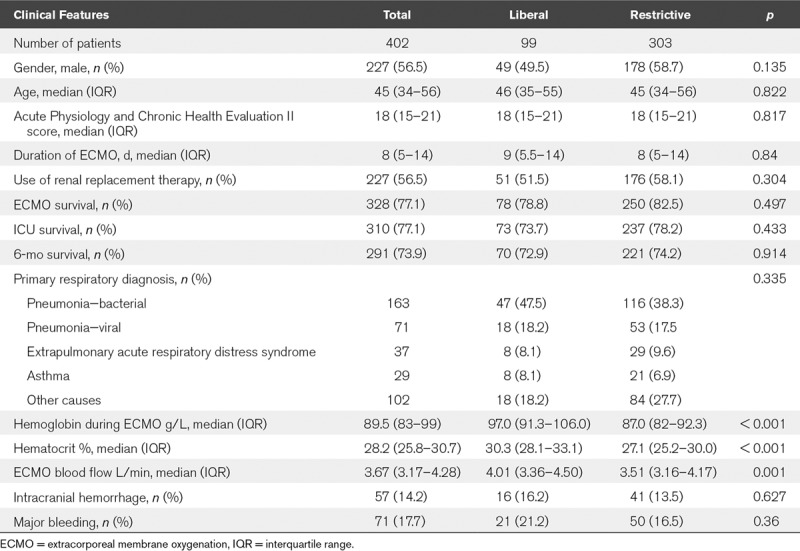
During the study period, 411 patients received venovenous ECMO for severe respiratory failure. Nine patients were excluded from the analysis: six patients survived less than 24 hours and two patients were under 16 years old. One patient was placed on ECMO twice, their second run was not included in the analysis. Ninety-nine patients were treated between 2011 and 2013, 303 patients between 2014 and 2017 (Fig. 1). The characteristics of the groups are shown in Table 1. The characteristics between the groups are similar with no statistically significant differences in age, APACHE score at admission to our unit, use of renal replacement therapy, and survival outcomes. The most common reason for patients to require venovenous ECMO was bacterial pneumonia and thereafter viral pneumonia. The occurrence rate of ICH was 16 patients (16.2%) receiving venovenous ECMO between 2011 and 2013 and 41 (13.5%) between 2014 and 2017. Major hemorrhage occurred in 21 (21.2%) and 50 (16.5%) respectively with no statistically significant difference with both bleeding events in the two time periods.
Figure 1.
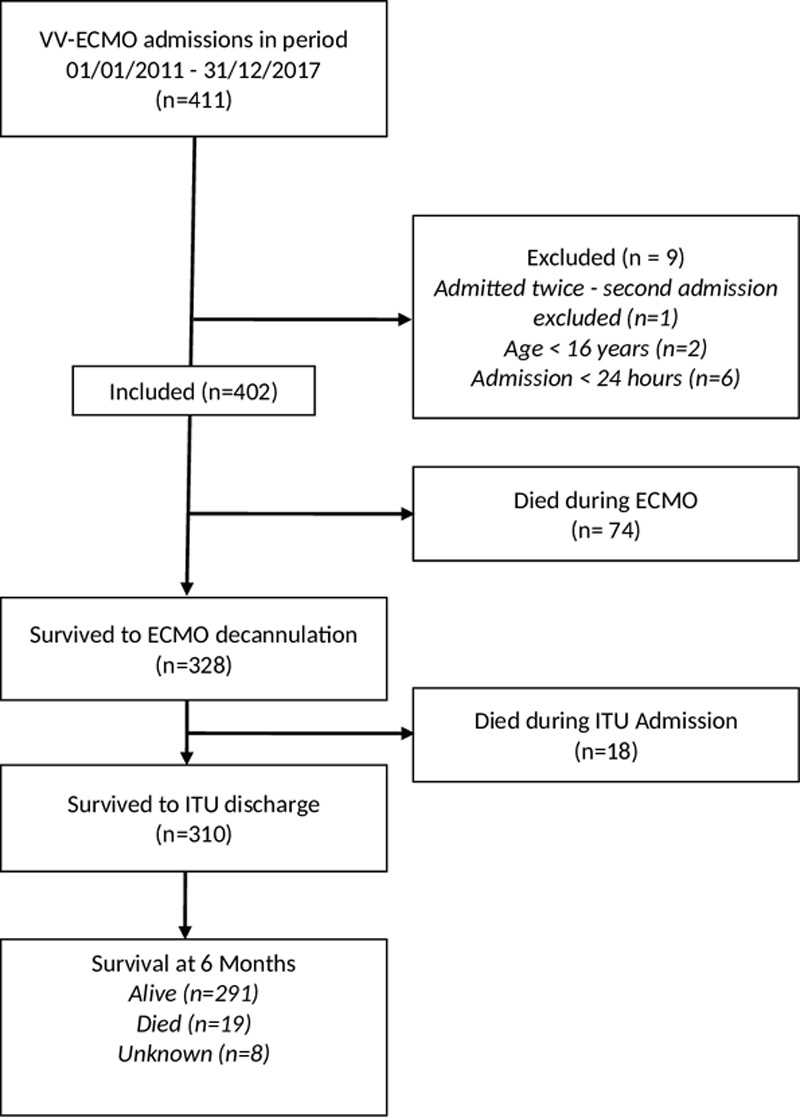
Flow diagram of study inclusion and survival outcomes of patients receiving venovenous extracorporeal membrane oxygenation (VV-ECMO) to decannulation, discharge from the intensive therapy unit (ITU) and at 6 mo.
TABLE 1.
Clinical Features of Patients Receiving Extracorporeal Membrane Oxygenation Comparing Patients Between 2011 and 2013 (“Liberal” RBC Transfusion Approach) to 2014–2017 (“Restrictive” RBC Transfusion Approach)
After the introduction of “restrictive” transfusion practice in 2014, the median hemoglobin concentration decreased from 97 to 87 g/L (p < 0.001) shown in Figure 2. The average number of units transfused to patients when a restrictive approach was used fell from 0.66 RBC units per ECMO day to 0.44 (p < 0.001). Thereafter, we compared the median flow rate on ECMO between the groups as ECBF is a key determinant of systemic oxygenation. The mean ECBF during the first 7 days of those receiving ECMO was higher in the initial group at 4.0 L/min (range, 3.4–4.5 L/min) receiving a “liberal” transfusion approach and lower at 3.5 L/min (3.2–4.2 L/min) (p < 0.05) in the “restrictive” transfusion period (Fig. 2).
Figure 2.
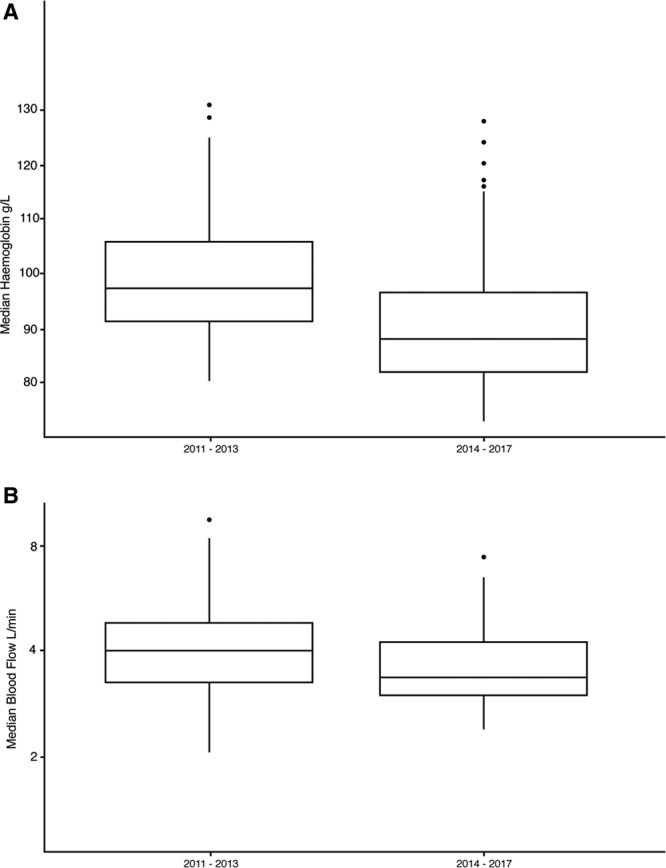
Comparison of hemoglobin and extracorporeal membrane oxygenation (ECMO) blood flow rates between "liberal" and "restrictive" transfusion practices. A, Median hemoglobin concentration; and B, ECMO blood flow (ECBF) during the first 7 d during venovenous ECMO between 2011 and 2013 and 2014–2017 (p ≤ 0.001).
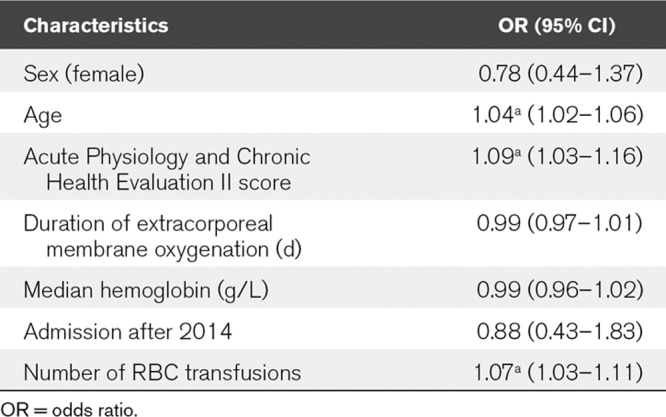
Multiple logistic regression analysis for all patients in the study showed that APACHE II score, age at cannulation, and number of RBC transfusions were factors independently associated to survival to decannulation, whereas the period that they received ECMO was not (Table 2).
TABLE 2.
Multiple Logistic Regression Showing the Odds Ratio and CIs for Variables for Mortality While Receiving Extracorporeal Membrane Oxygenation (ap < 0.01)
Thereafter, we examined the number of RBC units transfused in patients who had survived or died to ECMO decannulation in the two time periods shown in Figure 3. The rates of transfusion among survivors were similar in the two groups. Patients in the later time period with a “restrictive” approach showed that those patients who died received more RBC transfusions compared with those who survived. When reviewing the implications of this change in practice, the approximate cost saving between the liberal and restrictive strategies was £454 less per patient who received the “restrictive” approach.
Figure 3.
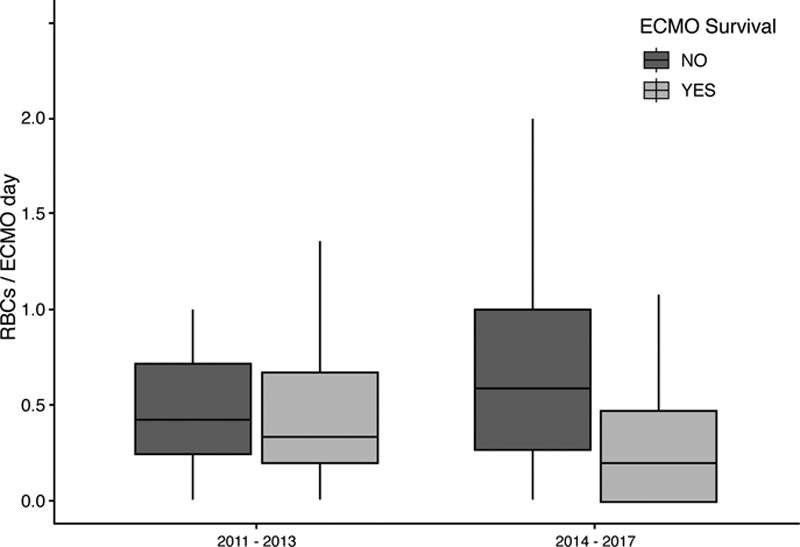
Rate of RBC transfusion and survival to decannulation from extracorporeal membrane oxygenation (ECMO) over the two study time periods (p = 0.38).
Finally, we reviewed transfusion databases to identify adverse incidents related to RBC transfusion over the study period. There were 2,296 RBC units transfused and eight reported episodes of adverse events or reactions related to RBC transfusion giving an incident rate of 0.35% over 7 years. Three were related to storage of blood products, three related to incorrect patient identifying details, one related to delay in blood product provision, and one related to the incompatible transfusion of a clinically significant minor blood group positive to a patient with alloantibodies. Two cases were reported to SHOT (incompatible transfusion with alloantibodies and inappropriate storage for a prolonged period). There were no cases reported of transfusion-related acute lung injury (TRALI), transfusion-associated cardiac overload (TACO), or anaphylaxis in patients receiving ECMO over a 7-year period.
DISCUSSION
To our knowledge, this is the largest single-center retrospective study examining transfusion practice in adults receiving venovenous ECMO for severe respiratory failure. We adopted a restrictive approach largely on the basis of the known risks of RBC transfusions and the increasing number of studies demonstrating transfusion is linked with significantly increased mortality and morbidity in critical illness (7, 9, 17, 18). However, these studies did not include patients using ECMO and “restrictive” transfusion practice is outside of current guidance (4). Our study demonstrated that a “restrictive” approach to transfusion in ECMO is not associated with an inferior survival outcome to decannulation from ECMO, survival to ICU discharge and survival at 6 months. Our mortality rates are comparable to those of the ELSO registry and national and international studies (16, 19, 20).
The average number of units transfused to patients when a restrictive approach was used fell by 0.2 RBC units per ECMO day. This has the potential to generate a significant cost saving and minimize unnecessary blood product use.
Interestingly, despite a lower transfusion threshold, the average ECBF in the first 7 days was 500 mL/min higher in those patients where a “liberal” transfusion practice was used. This finding was unexpected and in direct contradiction to what we had expected when we considered the model suggested by Spinelli and Bartlett (2). We are uncertain of the reason for this observation. However, it is our practice to leave the fractional delivery (Fd) of oxygen at 100% on our ECMO circuits and aim to maintain blood flows between 3 and 4.5 L/min to target an Sao2 of greater than 88%. The Fd of oxygen is weaned when we commence studies to liberate patients from ECMO. We do not routinely allow ECBF below 3 L/min as we believe this may be associated with an increased risk of thrombosis and circuit loss. The discrepancy in our practice and the model described by Spinelli and Bartlett (5) may account for some of the differences observed between expected and observed flow rate.
Our results are consistent with two other retrospective studies of ECMO that have shown noninferiority survival for patients lower hemoglobin concentration and hematocrit targets with a small proportion being maintained with an average hematocrit above the targeted 40% during treatment (11, 12).
There was a higher use of RBC transfusion in nonsurvivors at all survival time points following a change to a restrictive transfusion approach. This is consistent with previous studies (6, 21). Although age and severity of illness at presentation were associated with an increased risk of mortality prior to decannulation, the mean hemoglobin level during ECMO was not. Associated events such as bleeding and increased efforts to improve cardiovascular stability in those not responding to ECMO support may have contributed to a higher use of RBC transfusions in nonsurvivors.
Rates of transfusion-related reactions were low and may suggest under-reporting, which has previously been shown in critical care (22, 23). Adverse transfusion-related reactions such as TACO or TRALI may not be identified during ECMO due to concurrent cardiopulmonary support, sedation, and external temperature regulation.
This is study is a single-center retrospective analysis and is therefore prone to bias and physician selection, which we may not have been able to identify. There is also substantial physiologic rationale to support a higher transfusion threshold in patients with severe respiratory failure, who in the majority have approached or exceeded a critical threshold of oxygen delivery (Do2) (4). Indeed, a hemoglobin concentration measurement does not inform a clinician of a patient’s Do2 and a single hemoglobin concentration measurement has limited utility as a transfusion trigger. It is important to emphasize that we did not include any cases of venoarterial ECMO in this analysis. Another limitation is our inability to differentiate if survival with respect to hemoglobin concentrations were confounded by major bleeding in individual cases, although these rates were not statistically different between the two time periods. Our analysis of the APACHE II score is a potential confounder as they were calculated using data collected from our center once patients have been retrieved from another hospital. Therefore, the majority of patients had already been placed on ECMO before the initial APACHE II score was calculated impacting upon recorded parameters.
CONCLUSIONS
This retrospective study showed that “restrictive” transfusion practice (hemoglobin concentration target 80–90 g/L) had comparable survival rates to a “liberal” transfusion strategy (target 100–120 g/L) with regards to decannulation from ECMO, discharge from ICU and survival at 6 months. Our findings now need to be validated in a prospective controlled trial for there is an urgent need to develop better transfusion practice in patients receiving ECMO.
Footnotes
Dr. Barrett received research and educational support from Mitsubishi-Tanabe Pharmaceuticals, Maquet, Alung Incorporated, Draeger, and Fisher & Paykel. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Extracorporeal Life Support Organization: ECLS Registry Report - International Summary. 2019. Available at: https://www.elso.org/Registry/Statistics/InternationalSummary.aspx. Accessed April 10, 2019
- 2.Spinelli E, Bartlett RH. Anemia and transfusion in critical care: Physiology and management. J Intensive Care Med. 2016; 31:295–306 [DOI] [PubMed] [Google Scholar]
- 3.Bartlett RH, Conrad SA. Brogan TV, Lequier L, Lorusso R. The physiology of extracorporeal life support. Extracorporeal Life Support: The ELSO Red Book. 2017. Fifth Edition Ann Arbor, MI: Extracoporeal Life Support Organisation, 31–47 [Google Scholar]
- 4.ELSO Guidelines for Cardiopulmonary Extracorporeal Life Support: Extracorporeal Life Support Organization (ELSO) Guidelines for Adult Respiratory Failure. ELSO Guidel Adult Respir Fail Guidel. 20171–32. Available at: www.elso.org/Resources/Guidelines.aspx. Accessed April 10, 2019
- 5.Spinelli E, Bartlett RH. Relationship between hemoglobin concentration and extracorporeal blood flow as determinants of oxygen delivery during venovenous extracorporeal membrane oxygenation: A mathematical model. ASAIO J. 2014; 60:688–693 [DOI] [PubMed] [Google Scholar]
- 6.Corwin HL, Napolitano LM. Anemia in the critically ill: Do we need to live with it?* Crit Care Med. 2014; 42:2140–2141 [DOI] [PubMed] [Google Scholar]
- 7.Mazer CD, Whitlock RP, Fergusson DA, et al. TRiCS III - an international multicenter randomized trial of transfusion requirements in cardiac surgery. Circulation. 2017; 136:e449 [Google Scholar]
- 8.Reeves BC, Pike K, Rogers CA, et al. A multicentre randomised controlled trial of transfusion indication threshold reduction on transfusion rates, morbidity and health-care resource use following cardiac surgery (TITRe2) Health Technol Assess. 2016; 20:1–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion requirements in critical care investigators, Canadian critical care trials group. N Engl J Med. 1999; 340:409–417 [DOI] [PubMed] [Google Scholar]
- 10.Retter A, Wyncoll D, Pearse R, et al. ; British Committee for Standards in Haematology. Guidelines on the management of anaemia and red cell transfusion in adult critically ill patients. Br J Haematol. 2013; 160:445–464 [DOI] [PubMed] [Google Scholar]
- 11.Swol J, Marschall C, Strauch JT, et al. Hematocrit and impact of transfusion in patients receiving extracorporeal life support. Perfusion. 2018; 33:546–552 [DOI] [PubMed] [Google Scholar]
- 12.Voelker MT, Busch T, Bercker S, et al. Restrictive transfusion practice during extracorporeal membrane oxygenation therapy for severe acute respiratory distress syndrome. Artif Organs. 2015; 39:374–378 [DOI] [PubMed] [Google Scholar]
- 13.Lo Pinto H, Allyn J, Persichini R, et al. Predictors of red blood cell transfusion and its association with prognosis in patients undergoing extracorporeal membrane oxygenation. Int J Artif Organs. 2018; 41:644–652 [DOI] [PubMed] [Google Scholar]
- 14.Smith A, Hardison D, Bridges B, et al. Red blood cell transfusion volume and mortality among patients receiving extracorporeal membrane oxygenation. Perfusion. 2013; 28:54–60 [DOI] [PubMed] [Google Scholar]
- 15.Burns J, Cooper E, Salt G, et al. Retrospective observational review of percutaneous cannulation for extracorporeal membrane oxygenation. ASAIO J. 2016; 62:325–328 [DOI] [PubMed] [Google Scholar]
- 16.Lockie CJA, Gillon SA, Barrett NA, et al. Severe respiratory failure, extracorporeal membrane oxygenation, and intracranial hemorrhage. Crit Care Med. 2017; 45:1642–1649 [DOI] [PubMed] [Google Scholar]
- 17.Mazer CD, Whitlock RP, Fergusson DA, et al. ; TRICS Investigators and Perioperative Anesthesia Clinical Trials Group. Restrictive or liberal red-cell transfusion for cardiac surgery. N Engl J Med. 2017; 377:2133–2144 [DOI] [PubMed] [Google Scholar]
- 18.Murphy GJ, Reeves BC, Rogers CA, et al. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007; 116:2544–2552 [DOI] [PubMed] [Google Scholar]
- 19.Paden ML, Conrad SA, Rycus PT, et al. ; ELSO Registry. Extracorporeal life support organization registry report 2012 ASAIO J. 2013; 59:202–210 [DOI] [PubMed] [Google Scholar]
- 20.Combes A, Hajage D, Capellier G, et al. ; EOLIA Trial Group, REVA, and ECMONet. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018; 378:1965–1975 [DOI] [PubMed] [Google Scholar]
- 21.Vincent JL, Sakr Y, Sprung CL, et al. ; Sepsis Occurrence in Acutely Ill Patients Investigators. Sepsis in European intensive care units: Results of the SOAP study. Crit Care Med. 2006; 34:344–353 [DOI] [PubMed] [Google Scholar]
- 22.Li G, Rachmale S, Kojicic M, et al. Incidence and transfusion risk factors for transfusion-associated circulatory overload among medical intensive care unit patients. Transfusion. 2011; 51:338–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar R, Gupta M, Gupta V, et al. Acute transfusion reactions (ATRs) in intensive care unit (ICU): A retrospective study. J Clin Diagn Res. 2014; 8:127–129 [DOI] [PMC free article] [PubMed] [Google Scholar]


