Supplemental Digital Content is available in the text.
Keywords: electrical impedance tomography, mechanical ventilation, pediatric acute respiratory distress syndrome, personalized ventilation
Abstract
Objectives:
To provide proof-of-concept for a protocol applying a strategy of personalized mechanical ventilation in children with acute respiratory distress syndrome. Positive end-expiratory pressure and inspiratory pressure settings were optimized using real-time electrical impedance tomography aiming to maximize lung recruitment while minimizing lung overdistension.
Design:
Prospective interventional trial.
Setting:
Two PICUs.
Patients:
Eight children with early acute respiratory distress syndrome (< 72 hr).
Interventions:
On 3 consecutive days, electrical impedance tomography-guided positive end-expiratory pressure titration was performed by using regional compliance analysis. The Acute Respiratory Distress Network high/low positive end-expiratory pressure tables were used as patient’s safety guardrails. Driving pressure was maintained constant. Algorithm includes the following: 1) recruitment of atelectasis: increasing positive end-expiratory pressure in steps of 4 mbar; 2) reduction of overdistension: decreasing positive end-expiratory pressure in steps of 2 mbar until electrical impedance tomography shows collapse; and 3) maintaining current positive end-expiratory pressure and check regional compliance every hour. In case of derecruitment start at step 1.
Measurements and Main Results:
Lung areas classified by electrical impedance tomography as collapsed or overdistended were changed on average by –9.1% (95% CI, –13.7 to –4.4; p < 0.001) during titration. Collapse was changed by –9.9% (95% CI, –15.3 to –4.5; p < 0.001), while overdistension did not increase significantly (0.8%; 95% CI, –2.9 to 4.5; p = 0.650). A mean increase of the positive end-expiratory pressure level (1.4 mbar; 95% CI, 0.6–2.2; p = 0.008) occurred after titration. Global respiratory system compliance and gas exchange improved (global respiratory system compliance: 1.3 mL/mbar, 95% CI [–0.3 to 3.0], p = 0.026; Pao2: 17.6 mm Hg, 95% CI [7.8–27.5], p = 0.0039; and Pao2/Fio2 ratio: 55.2 mm Hg, 95% CI [27.3–83.2], p < 0.001, all values are change in pre vs post).
Conclusions:
Electrical impedance tomography-guided positive end-expiratory pressure titration reduced regional lung collapse without significant increase of overdistension, while improving global compliance and gas exchange in children with acute respiratory distress syndrome.
There is sufficient evidence from randomized controlled trials in adult patients with acute lung injury (ALI) suggesting that delivering small tidal volumes (Vts), adequate levels of positive end-expiratory pressure (PEEP) and a restrictive fluid strategy improve outcome (1–3). However, there is also evidence that there is a dichotomy in outcome in patients with ALI and not all patients respond uniformly (4–8). Concurrent with these data and with the common bedside experience that individual patients may or may not respond to interventions, such as escalation of PEEP or positional changes, there may be a role for a more personalized ventilator strategy. This strategy could account for the unique individual morphology of lung disease, such as the amount of atelectasis and overdistension as a percentage of total lung tissue, the exact location of atelectasis, and whether positional changes or elevation of PEEP produce lung recruitment or overdistension.
Electrical impedance tomography (EIT), a bedside monitor to describe regional lung volume changes, displays a real-time cross-sectional image of the lung. Regional volume changes during EIT have been highly correlated with volume changes detected on CT (9–11). Only few studies have investigated whether this technology has the capability of improving outcome in patients (12) and in animal models (11, 13) with ALI.
The aim of this study was to provide proof-of-concept for a protocol applying a strategy of EIT-guided mechanical ventilation in children with ALI. Although adhering to accepted strategies of lung protection, such as low Vts and permissive hypercarbia, personalized PEEP settings were determined by continuously monitoring the amount of lung overdistension and atelectasis using EIT in real time at the bedside. Individual ventilator settings were chosen with the aim to minimize lung overdistension and maximize lung recruitment. Data from a recent animal study showing a decrease in ventilator-induced lung injury (VILI) during EIT-guided ventilation (11) were directly translated to this study.
MATERIALS AND METHODS
Subjects
The study protocol was approved by the ethics committee of Ludwig-Maximilians-University Munich (institutional review board) and written informed consent was obtained from a parent or guardian prior to enrollment. Intubated and mechanically ventilated patients with a chest circumference greater than 70 cm (smallest available EIT-belt), younger than 18 years old, and meeting the Berlin definition of acute respiratory distress syndrome (ARDS) (14) for less than 72 hours were considered eligible. Patients were either deeply sedated or were receiving neuromuscular blocking agents. They received a chest radiograph in the first 12 hours after enrollment. Exclusion criteria are presented in the supplement (Supplemental Digital Content 1, http://links.lww.com/CCX/A59) (4).
Setting
Two academic PICUs (Children’s Hospital Traunstein and Dr. von Haunersches Kinderspital, both Ludwig-Maximilians-University, Munich, Germany).
Cardiopulmonary Monitoring
Vital signs, transcutaneous oxygen saturations (Spo2), and respiratory variables were monitored continuously and recorded at every PEEP alteration. Respiratory variables were monitored at the airway opening with a mainstream pneumotachometer and capnometer. All arterial blood gas measurements (0.1 mL microsample per measurement) were performed 15 minutes following each PEEP change to allow for the equilibration of physiologic responses and were analyzed in the ICU (Rapidlab; Siemens Healthcare, Erlangen, Germany).
Electrical Impedance Tomography
EIT measurements were performed using the Dräger PulmoVista 500 EIT-device (Dräger Medical GmbH, Lübeck, Germany). The 16-electrode belt was placed between the 4th and 5th intercostal space. Ventilator data were continuously recorded by the EIT-device through a serial interface (Medibus, Dräger Medical, Lübeck, Germany) from the ventilator. EIT data were recorded continuously and recording was interrupted only for data transmission and processing. All EIT data processing was performed at the bedside utilizing the EITeasy software application (Dräger Medical GmbH, Lübeck, Germany). In order to assess the optimal PEEP, the regional compliance per pixel, as described by Costa et al (15), was calculated by the EITeasy software. The display of “WIN/LOSS” summarizes all pixels with compliance win and all pixels with compliance loss, comparing the current PEEP level to a reference level (start of PEEP titration). The display of “OD/CL” refers to the measurement of lung overdistension and collapse in percent of scanned lung, also described by Costa et al (15). Figure 1 shows both options. More details are presented in the supplement (Supplemental Digital Content 1, http://links.lww.com/CCX/A59).
Figure 1.
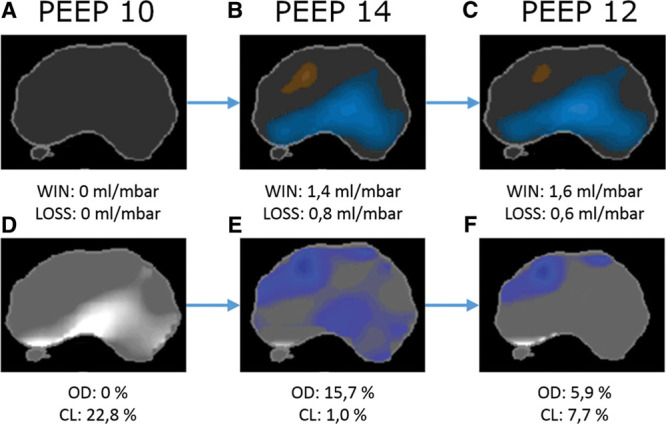
Display options “WIN/LOSS” (all pixels with compliance win and all pixels with compliance loss [A, B, C]) and “OD/CL” (lung overdistension and collapse [D, E, F]) at three different positive end-expiratory pressure (PEEP) levels during PEEP titration. PEEP 12 would be chosen as optimal PEEP level.
Experimental Protocol
Patients were ventilated with the Dräger Evita Infinity V500 or the Dräger Evita XL ventilator (all Dräger Medical, Lübeck, Germany). Baseline ventilation was provided using pressure-controlled mode with a target Vt of 6 mL/kg of ideal body weight. Goal inspiratory pressure (Pinsp) was less than or equal to 30 mbar. If Pinsp increased greater than or equal to 30 mbar, then Vts were reduced to 4 mL/kg. Pinsp greater than 35 mbar were only allowed for a brief period of time (< 15 min). Respiratory rate was set to achieve a target Pao2 above 55 mm Hg and a pH goal of 7.25–7.40. Elevated Paco2 levels were tolerated (permissive hypercapnia). During PEEP changes, the driving pressure (ΔP) was maintained constant. As at the end of PEEP titration, improved global respiratory system compliance often occurred, the respiratory rate and ΔP were often reduced at the very end of the titration protocol to prevent hyperventilation.
Patients received EIT-guided PEEP titration at the following time points; on day 1 (opening and optimizing phase) for 6 consecutive hours and on days 2 and 3 (re-opening and maintenance phase) for 3 hours.
The Acute Respiratory Distress Network (ARDSnet) low-PEEP and high-PEEP tables (16, 17) were used as patients’ safety guardrails. The patients received minimally the lowest PEEP from the low-PEEP table and maximally the highest PEEP from the high-PEEP table. EIT-guidance was used to optimize PEEP by the following strategy:
The patient was started at the PEEP level set by the treating physician.
1) PEEP was increased by steps of 4 mbar every 15 minutes: If there was evidence of lung recruitment, PEEP was again increased by 4 mbar until a stopping criterion was met or the maximal PEEP was reached. If there was no more evidence of lung recruitment, then:
2) PEEP was gradually reduced by steps of 2 mbar: If the patient’s PEEP at start of titration was the maximally allowed PEEP, the titration started at this point.
3) PEEP was maintained at the first evidence of lung collapse during PEEP decrease, and the regional compliance was checked every hour. If collapse increased or outweighed recruitment at any point of the 6 hours measurement, the protocol was restarted at point 1 by again increasing the PEEP by 4 mbar and ended 2 mbar above the level where lung collapse occurred.
Stopping criteria were as follows: reaching minimal/maximal PEEP according to the ARDSnet grid, pH less than 7.0, drop of the mean arterial pressure (MAP) greater than 20%, 50% increase in vasopressor dosing, Spo2 less than 80%, and constant Pinsp greater than 35 mbar while Vt 4 mL/kg.
Statistical Analysis
All three measurement days of each patient were included in the analysis. Response to recruitment was identified by decreased overdistension/collapse. PEEP titration effects on EIT variables (overdistension and collapse), respiratory variables (PEEP, global respiratory system compliance, ΔP), gas exchange (Pao2, Paco2, Pao2/Fio2 ratio, oxygenation index [OI]), and hemodynamic variables (heart rate [HR], blood pressure) were analyzed with a linear mixed-effects model with a random effect. p values of less than 0.05 are considered statistically significant. Statistical analysis was performed with the software IBM SPSS Statistics Version 25 (IBM Corp., Armonk, NY) and R Version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). The analysis was overseen by statisticians of the Institute of Social Pediatrics and Adolescent Medicine, Ludwig Maximilian University Munich. Details of the statistical analysis are described in the supplement (Supplemental Digital Content 1, http://links.lww.com/CCX/A59).
RESULTS
Patient Characteristics
Eight patients met eligibility criteria and were enrolled between January 2015 and May 2018. One patient had no measurement on day 3 due to staff shortage. A total of 23 titrations was performed. Four patients briefly met stopping criteria of the protocol (arterial hypotension) but were able to complete the protocol. One patient with pulmonary hemorrhage met the stopping criterion of high Pinsp at the start of titration. The ventilator variables were considered clinically justified, and the patient was allowed to complete the protocol and was included in the analysis.
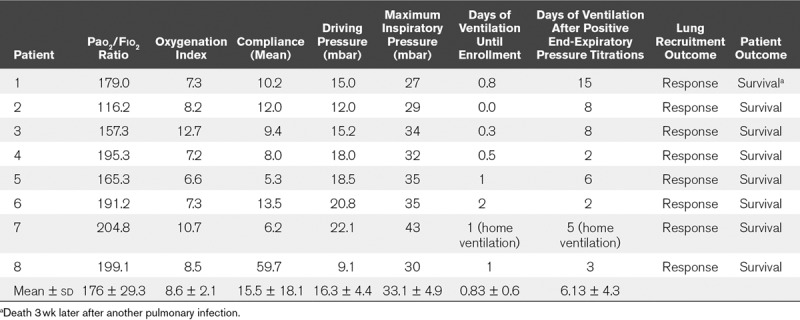
Demographic data for all patients are shown in Table 1. Baseline respiratory variables, response to lung recruitment, and outcome are shown in Table 2.
TABLE 1.
Demographic Data
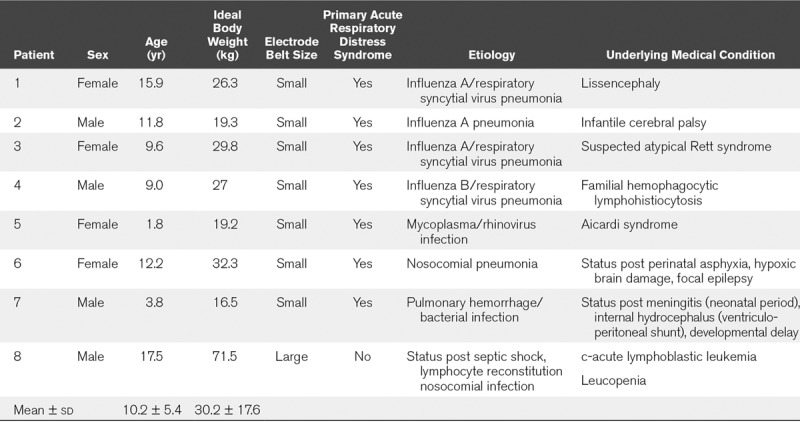
TABLE 2.
Response to Lung Recruitment and Outcome
Response to Recruitment
EIT measured collapse was changed during titration by –9.9% (95% CI, –15.3 to –4.5; p < 0.001) on average, while overdistension was not significantly increased at the end of titration (mean of differences, 0.8%; 95% CI, –2.9 to 4.5; p = 0.650). Overdistension and collapse is a composite variable indicating the percentage of lung which is not optimally ventilated. Overdistension/collapse was decreased by –9.1% (95% CI, –13.7 to –4.4; p < 0.001) through the titration. Figure 2A shows the development of overdistension and collapse during PEEP titration at three time points; at start of titration (T1), maximum PEEP level (T2), and end of titration/optimal PEEP (T3). On average, collapse drops to zero at the highest PEEP level, while overdistension increases to its maximum. At the optimal PEEP level, mean collapse and mean overdistension converge. Figure 2B shows the difference of overdistension/collapse before and after titration for all performed measurements.
Figure 2.
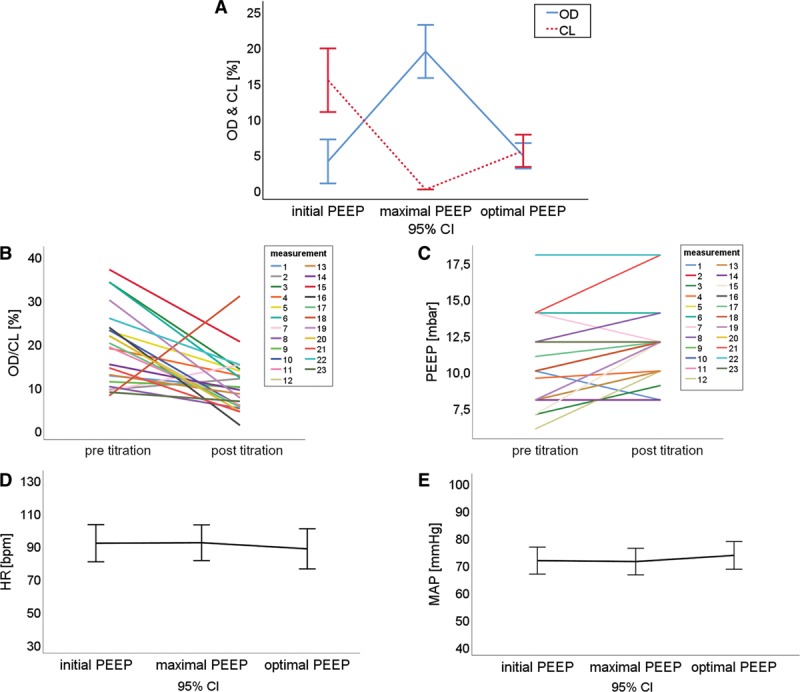
Overdistension and collapse (OD/CL) (B) and positive end-expiratory pressure (PEEP) levels (C) before and after the PEEP titration. OD and CL (A), heart rate (HR) (D) and mean arterial pressure (MAP) (E) at the start, the maximum PEEP level and the end of titration. bpm = beats/min.
Respiratory Variables
EIT-guided PEEP titration on average led to higher PEEP levels (mean of differences, 1.4 mbar; 95% CI, 0.6–2.2; p = 0.008). Figure 2C shows the difference of the PEEP levels before and after the titration for all performed measurements. In three cases, the optimal PEEP identified by EIT was equal to the PEEP level set by the treating physician. In two titrations, the PEEP level was reduced. Global respiratory system compliance was increased after titration (1.3 mL/mbar; 95% CI, –0.3 to 3.0; p = 0.026). To avoid hyperventilation after recruitment and improved lung compliance, ΔP was reduced at the end of titration if possible. The reduction of ΔP was significant (–1.2 mbar; 95% CI, –2.0 to –0.4; p = 0.0088).
Gas Exchange
Pao2 levels were significantly higher after EIT-guided PEEP titration (17.6 mm Hg; 95% CI, 7.8–27.5; p = 0.0039). Pao2/Fio2 ratio increased by 55.2 mm Hg (95% CI, 27.3–83.2; p < 0.001). OI was lower after PEEP titration (–0.8; 95% CI, –1.4 to –0.1) not reaching the level of significance (p = 0.085). Paco2 levels did not differ (1.6 mm Hg; 95% CI, –3.1 to 6.3; p = 0.5045).
Hemodynamics
HR and MAP were compared at the three critical points during a PEEP titration (T1, T2, T3). No significant difference of MAP at the start of titration compared with the maximum PEEP level (p = 0.893) or the start of titration compared with the end of titration (p = 0.461) occurred. No significant difference of HR at the start of titration compared with the maximum PEEP level (p = 0.958) or the start of titration compared with the end of titration (p = 0.555) occurred. Arithmetic mean and 95% CI are shown in Figure 2, D and E.
DISCUSSION
The main findings of this study are as follows: 1) EIT-guided PEEP titration with the provided algorithm was feasible in children with mild-to-moderate ARDS. Lung recruitment during the titration phase was accompanied by only temporary overdistension. At the end of titration, collapse was significantly reduced while overdistension was not significantly increased; 2) EIT-guided titration on average lead to higher PEEP levels than the PEEP levels set by the treating physician; 3) Global respiratory system compliance improved after EIT-guided titration and ΔP could be reduced; and 4) Gas exchange improved during EIT-guided titration, as evidenced by improved Pao2 levels and improved Pao2/Fio2 ratios.
The present algorithm was derived from a previous randomized study in an animal model of ARDS (11), comparing EIT-guided PEEP titration with ARDSnet ventilation. In that study, EIT-guided ventilation produced histopathologic evidence of lung protection, as evidenced by lower amounts of hyaline membranes and airway fibrin. Similar to the present study, EIT-guided ventilation led to higher PEEP levels, improved compliance, and improved oxygenation. Another recent study, however, investigating an EIT-guided optimized PEEP strategy in a porcine model of ARDS, showed no differences in gas exchange or histopathology between the groups (13). In that study, EIT-derived optimal PEEP was determined only once during a fixed incremental-decremental PEEP maneuver and that PEEP was applied throughout the study. In the present human and the previous animal study, real-time EIT-guidance was achieved by repeated analysis of EIT measurements and subsequent PEEP- adjustments. Adjustments were based on the actual assessments of overdistension and collapse during the entire course of the ventilation protocol, which may explain the diverging results. In a study involving adult patients with ARDS, adjusting PEEP based on EIT variables led to increases in lung compliance and improvement of oxygenation, in accordance with our results (18).
In the present study, PEEP titration resulted in lung recruitment as measured by EIT. However, this recruitment method is not a classic recruitment maneuver. An open lung approach (OLA) commonly leads to very high Pinsp and PEEP levels in contrast to our protocol, limiting ΔPs and relying on a stepwise increase in PEEP. A recent multicenter randomized clinical trial reports the risks of an aggressive recruitment strategy. In this trial, OLA improved hypoxemia, but increased the risk of adverse effects, including mortality (19). The results could indicate the need for a personalized ventilation strategy, as one approach may not fit all patients.
The ARDS network PEEP tables are considered to be safe to apply (17), but suggest a wide range of possible options (e.g., Fio2 0.4 leads to PEEP 5–16 cm H2O), without measures to avoid overdistension. Previous studies used EIT to identify the optimal PEEP level by retrospective analysis (20). When EIT was used prospectively, standardized decremental or incremental-decremental PEEP trials were performed (21–25). The approach in this study provides an individualized opportunity to find the “optimal” PEEP level, relying on regional variables of ventilation and specifically including variables of atelectasis and overdistension. Importantly, only CT may provide better characterization of ventilation heterogeneity, but rarely is feasible in the acute setting.
All patients responded to recruitment. However, not all patients showed response to recruitment at all three measurement days (Fig. 2B, measurement 2, 7, 18). One patient with alveolar hemorrhage did not respond to recruitment on day 1, but showed good response the next day. This case illustrates the dynamic nature of ARDS. Identifying patients responding to recruitment strategies, while protecting nonresponders from aggressive recruitment maneuvers, may add lung protection.
Four patients briefly experienced arterial hypotension during the protocol. In two of these cases, brief dose adjustments of vasopressors were necessary. Despite these findings, there was no significant difference in MAP or HR at the three critical points of a titration (T1, T2, T3). Therefore, we consider this approach to be hemodynamically safe to apply.
The “optimal” EIT-derived variable to be used for EIT-guided ventilation is still subject of debate. Ideally, the optimal EIT-variable should indicate a state of optimal recruitment with minimized lung collapse and overdistension, while accurately quantifying overdistended and atelectatic lung areas. A recent study suggests, that using the composite variable of overdistension and collapse may be the best variable, as collapsed lung areas were significantly smaller when overdistension/collapse was used to set the PEEP level, compared with global compliance (21). Simultaneously quantifying the fraction of overdistended and atelectatic lung regions has been previously used in an experimental ARDS model (26) and in patients with ARDS (18, 23, 27). Other studies have used broader measures of regional ventilation, measuring where the majority of ventilation occurs (center of ventilation) (22, 25, 28), or the global inhomogeneity index, indicating the variation of Vt distribution throughout the lung, to define optimal (homogeneous) ventilation (20, 22, 24, 25).
The present study has important limitations. As the study had no control group and no histopathologic analysis was performed, a conclusion on the outcome or the occurrence of VILI is precluded. Improved compliance and gas exchange could also occur during a PEEP trial without EIT-guidance. The number of cases was low, attributed to the fact that true pediatric ARDS is a rare disease, and the group was heterogeneous as the patients had various underlying medical conditions leading to the occurrence of ARDS. There was no confirming method like CT scans used due to the consequences of radiation exposure, but validity of EIT images in comparison to CT images have been shown in various previous studies (9–11). Regional impedance changes were only measured within one cross-sectional plane, covering 10–30% of the entire lung (29), but not along the craniocaudal axis.
CONCLUSIONS
This pilot trial demonstrated proof-of-concept that an EIT-guided PEEP titration protocol is feasible in children with mild-to-moderate ARDS. Significant recruitment could be achieved while lung overdistension was not significantly increased. EIT-guided PEEP titration resulted in improved respiratory system mechanics and gas exchange. This method may help identify the individual level of PEEP that produces both a maximal resolution of atelectasis and minimal lung overdistension.
ACKNOWLEDGMENTS
We acknowledge all the staff members of the PICUs at the Children’s Hospital in Traunstein and Dr. von Haunersches Kinderspital in Munich for their help and support during this study. We thank Eckard Teschner and Dr. Yvo Gärber from Dräger Medical (Lübeck, Germany) for their help regarding the technical support for the electrical impedance tomography (EIT) device and software. We thank Dräger Medical (Lübeck, Germany) for providing the EIT devices and software during this study.
Supplementary Material
Footnotes
Part of the data in this article have been previously published in form of an abstract at the Conference of the German Interdisciplinary Association for Intensive Care and Emergency Medicine (DIVI) in Leipzig, Germany, December 6, 2017, as part of Dr. Rosemeier’s doctoral thesis (http://react-profile.org/ebook/DIVI2017/Abstractbuch/index.html#8–9/z).
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Supported, in part, by grant from the departmental funds. The electrical impedance tomography devices and software were provided by Dräger Medical (Lübeck, Germany) during the time of the study. Dräger had no influence in the data collection, interpretation of the data, or the writing of this article.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Brower RG, Matthay MA, Morris A, et al. ; Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 20003421301–1308 [DOI] [PubMed] [Google Scholar]
- 2.Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: Systematic review and meta-analysis. JAMA 2010303865–873 [DOI] [PubMed] [Google Scholar]
- 3.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 20063542564–2575 [DOI] [PubMed] [Google Scholar]
- 4.Wolf GK, Gómez-Laberge C, Kheir JN, et al. Reversal of dependent lung collapse predicts response to lung recruitment in children with early acute lung injury. Pediatr Crit Care Med 201213509–515 [DOI] [PubMed] [Google Scholar]
- 5.Gattinoni L, Caironi P, Cressoni M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 20063541775–1786 [DOI] [PubMed] [Google Scholar]
- 6.Papazian L, Forel JM, Gacouin A, et al. ; ACURASYS Study Investigators Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 20103631107–1116 [DOI] [PubMed] [Google Scholar]
- 7.Gattinoni L, Tognoni G, Pesenti A, et al. ; Prone-Supine Study Group Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med 2001345568–573 [DOI] [PubMed] [Google Scholar]
- 8.Guérin C, Reignier J, Richard JC, et al. ; PROSEVA Study Group Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 20133682159–2168 [DOI] [PubMed] [Google Scholar]
- 9.Victorino JA, Borges JB, Okamoto VN, et al. Imbalances in regional lung ventilation: A validation study on electrical impedance tomography. Am J Respir Crit Care Med 2004169791–800 [DOI] [PubMed] [Google Scholar]
- 10.Wrigge H, Zinserling J, Muders T, et al. Electrical impedance tomography compared with thoracic computed tomography during a slow inflation maneuver in experimental models of lung injury. Crit Care Med 200836903–909 [DOI] [PubMed] [Google Scholar]
- 11.Wolf GK, Gómez-Laberge C, Rettig JS, et al. Mechanical ventilation guided by electrical impedance tomography in experimental acute lung injury. Crit Care Med 2013411296–1304 [DOI] [PubMed] [Google Scholar]
- 12.Zhao Z, Chang MY, Chang MY, et al. Positive end-expiratory pressure titration with electrical impedance tomography and pressure-volume curve in severe acute respiratory distress syndrome. Ann Intensive Care. 2019;9:7. doi: 10.1186/s13613-019-0484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hochhausen N, Biener I, Rossaint R, et al. Optimizing PEEP by electrical impedance tomography in a porcine animal model of ARDS. Respir Care 201762340–349 [DOI] [PubMed] [Google Scholar]
- 14.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: The Berlin definition. JAMA 20123072526–2533 [DOI] [PubMed] [Google Scholar]
- 15.Costa EL, Borges JB, Melo A, et al. Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. Intensive Care Med 2009351132–1137 [DOI] [PubMed] [Google Scholar]
- 16.National Heart, Lung, and Blood Institute ARDS Clinical Trials Network: Mechanical Ventilation Protocol Summary. 2008. Available at: http://www.ardsnet.org/files/ventilator_protocol_2008-07.pdf. Accessed November 1, 2015.
- 17.Brower RG, Lanken PN, MacIntyre N, et al. ; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004351327–336 [DOI] [PubMed] [Google Scholar]
- 18.Heines SJH, Strauch U, van de Poll MCG, et al. Clinical implementation of electric impedance tomography in the treatment of ARDS: A single centre experience. J Clin Monit Comput 201933291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavalcanti AB, Suzumura ÉA, Laranjeira LN, et al. ; Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: A randomized clinical trial. JAMA 20173181335–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Z, Steinmann D, Frerichs I, et al. PEEP titration guided by ventilation homogeneity: A feasibility study using electrical impedance tomography. Crit Care. 2010;14:R8. doi: 10.1186/cc8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karsten J, Voigt N, Gillmann HJ, et al. Determination of optimal positive end-expiratory pressure based on respiratory compliance and electrical impedance tomography: A pilot clinical comparative trial. Biomed Tech (Berl) 201964135–145 [DOI] [PubMed] [Google Scholar]
- 22.Blankman P, Hasan D, Erik G, et al. Detection of ‘best’ positive end-expiratory pressure derived from electrical impedance tomography parameters during a decremental positive end-expiratory pressure trial. Crit Care. 2014;18:R95. doi: 10.1186/cc13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franchineau G, Bréchot N, Lebreton G, et al. Bedside contribution of electrical impedance tomography to setting positive end-expiratory pressure for extracorporeal membrane oxygenation-treated patients with severe acute respiratory distress syndrome. Am J Respir Crit Care Med 2017196447–457 [DOI] [PubMed] [Google Scholar]
- 24.Long Y, Liu DW, He HW, et al. Positive end-expiratory pressure titration after alveolar recruitment directed by electrical impedance tomography. Chin Med J (Engl) 20151281421–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karsten J, Grusnick C, Paarmann H, et al. Positive end-expiratory pressure titration at bedside using electrical impedance tomography in post-operative cardiac surgery patients. Acta Anaesthesiol Scand 201559723–732 [DOI] [PubMed] [Google Scholar]
- 26.Liu S, Tan L, Möller K, et al. Identification of regional overdistension, recruitment and cyclic alveolar collapse with electrical impedance tomography in an experimental ARDS model. Crit Care. 2016;20:119. doi: 10.1186/s13054-016-1300-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gómez-Laberge C, Arnold JH, Wolf GK. A unified approach for EIT imaging of regional overdistension and atelectasis in acute lung injury. IEEE Trans Med Imaging 201231834–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eichler L, Mueller J, Grensemann J, et al. Lung aeration and ventilation after percutaneous tracheotomy measured by electrical impedance tomography in non-hypoxemic critically ill patients: A prospective observational study. Ann Intensive Care. 2018;8:110. doi: 10.1186/s13613-018-0454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teschner E, Imhoff M, Leonhardt S. Elektrische Impedanztomographie: Von der Idee zur Anwendung des regionalen Beatmungsmonitorings. Second Edition. Lübeck, Germany: Drägerwerk AG & Co. KGaA; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


