Supplemental Digital Content is available in the text.
Keywords: decannulation, extracorporeal membrane oxygenation, ProGlide, vascular complications
Objectives:
Improvements in cannula removal techniques, and in particular a standardized decannulation technique with a suitable closure device, are needed to further improve patients’ outcomes after percutaneous cannulation. The decannulation techniques described so far are neither sufficiently standardized nor proven enough to be used in the large group of venoarterial extracorporeal membrane oxygenation patients. To meet this challenge, we have established a highly standardized and safe decannulation technique based on the Perclose ProGlide closure system (Abbott Vascular, Lake Bluff, IL).
Design:
Establishment of a highly standardized and safe decannulation technique based on the Perclose ProGlide closure system, which is described in detail with comprehensive instructions for the executive clinician and first application in the context of a pilot study.
Measurements and Main Results:
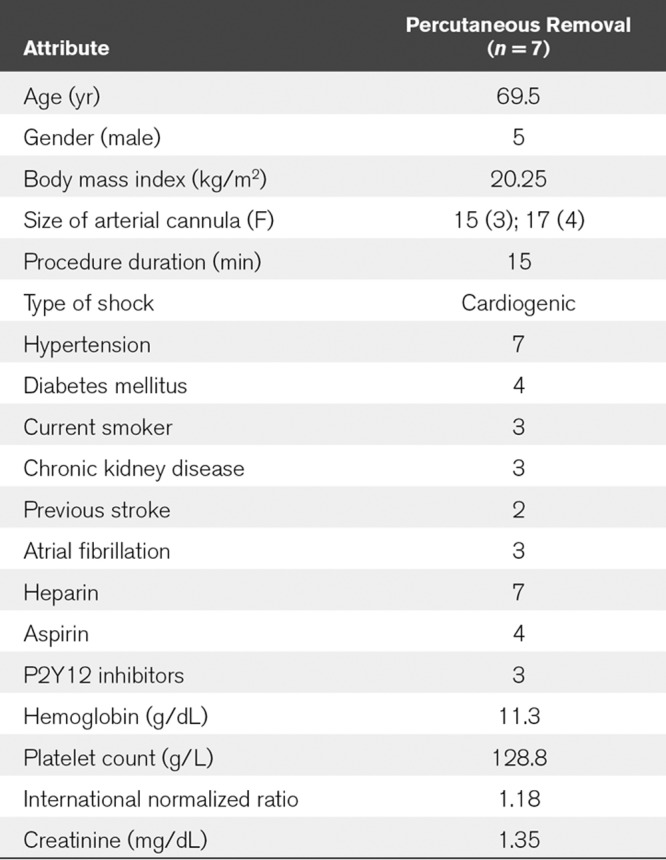
So far our technique has already been used successfully in seven patients since January 2019 as a standard procedure on our ICU with only one minor complication occurred after the first procedure, that is, a small pseudoaneurysm likely originating from antegrade perfusion puncture site which was sealed by thrombin injection.
Conclusions:
Our crossed ProGlide technique using a hemostasis valve Y connector ensuring no blood loss seems to be a very promising decannulation technique.
We read with great interest the article by Danial et al (1), in whose retrospective study the authors compared complication rates and overall survival in a large series of patients who received surgical or percutaneous peripheral venoarterial extracorporeal membrane oxygenation (VA-ECMO). In conclusion, the authors emphasize on the one hand that percutaneous cannulation for peripheral VA-ECMO is associated with fewer local infections, similar rates of ischemia and improved 30-day survival compared with the surgical approach. On the other hand, they point out the higher rate of vascular complications following decannulation, mainly in the percutaneous group (9.4% vs 1.5%; p < 0.001). Danial et al (1) therefore claim that improvements in cannula removal techniques, and in particular a standardized decannulation technique with a suitable closure device, are needed to further improve patients’ outcomes after percutaneous cannulation. The decannulation techniques described so far are neither sufficiently standardized nor proven enough to be used in the large group of VA-ECMO patients (2, 3). For standard multiple device deployment using Perclose ProGlide closure system (Abbott Vascular, Lake Bluff, IL), a sheath for standard guidewire insertion is usually required. The fact that prevents routine usage of this system for VA-ECMO decannulation is a missing standardized procedure to quickly and safely insert a guidewire into the VA-ECMO arterial cannula without significant blood loss.
To meet this challenge, we have established a highly standardized and safe decannulation technique using a hemostasis valve Y connector (Merit Angioplasty Pack; Merit Medical, South Jordan, UT) as modified sheath for quick and safe guidewire insertion in combination with the Perclose ProGlide closure system, which will be detailed below (Supplemental Video 1, Supplemental Digital Content 1, http://links.lww.com/CCX/A58): After a sterile preparation of the cannulation area (Fig. 1A) the arterial and venous cannula must first be clamped shortly behind the selectively hardened proximal venous and arterial cannula body by tubing clamp forceps (e.g., Braun [Melsungen, Germany], 20 cm) (Fig. 1B) before both cannula are cut with a suitable scissor (Fig. 1C). The venous cannula is flushed with a syringe filled with sterile saline solution and clamped again. Subsequently, a hemostasis valve Y connector (Merit Angioplasty Pack) has to be inserted into the proximal arterial cannula end followed by removal of the clamp (Fig. 1, D and E) and flushed with blood. A standard 220 cm 0.035-inch guidewire (AngioKard, Friedeburg, Germany) can then be safely inserted through the hemostasis valve to ascending aorta. With a secure wire position, the arterial cannula can be withdrawn and removed under manual compression of the insertion site by an assistant.
Now the first ProGlide device has to be inserted at about 10 to 11 o´clock and released according to the standard procedure described in instruction for use (IFU, see multiple device deployment at https://vascular.abbott.com/perclose-proglide-intl.html) (Fig. 1F). Next, the guidewire must be reinserted into the side hole of the first ProGlide device to place a second ProGlide device at about 1 to 2 o´clock (Fig. 1G, see IFU). By releasing the second device, two crossed sutures are formed which, according to our previous experience, lead to a secure closure of the vessel. Finally, the knots must be tightened using a knot pusher according to IFU (Fig. 1H) and the guidewire removed after ensuring adequate hemostasis. To prepare for removal of the venous cannula, a Z-suture can be inserted and then the venous cannula withdrawn while firmly tying the sterile swab (Fig. 1I). After final removal of the antegrade perfusion manual compression should then be continued for at least 5 minutes and puncture site covered by pressure bandage for 12 hours according to local standard. A final Doppler ultrasound control be performed to exclude false aneurysm or arteriovenous fistula after release of pressure bandage. So far our technique has already been used successfully in seven patients since January 2019 as a standard procedure on our ICU with only one minor complication occurred after the first procedure, that is, a small pseudoaneurysm likely originating from antegrade perfusion puncture site which was sealed by thrombin injection (Table 1). More data are needed, for example, regarding the rate of ischemic, hemorrhagic, or infectious complications, to further prove our technique. However, our crossed ProGlide technique using a hemostasis valve Y connector ensuring no blood loss seems to be a very promising decannulation technique.
Figure 1.
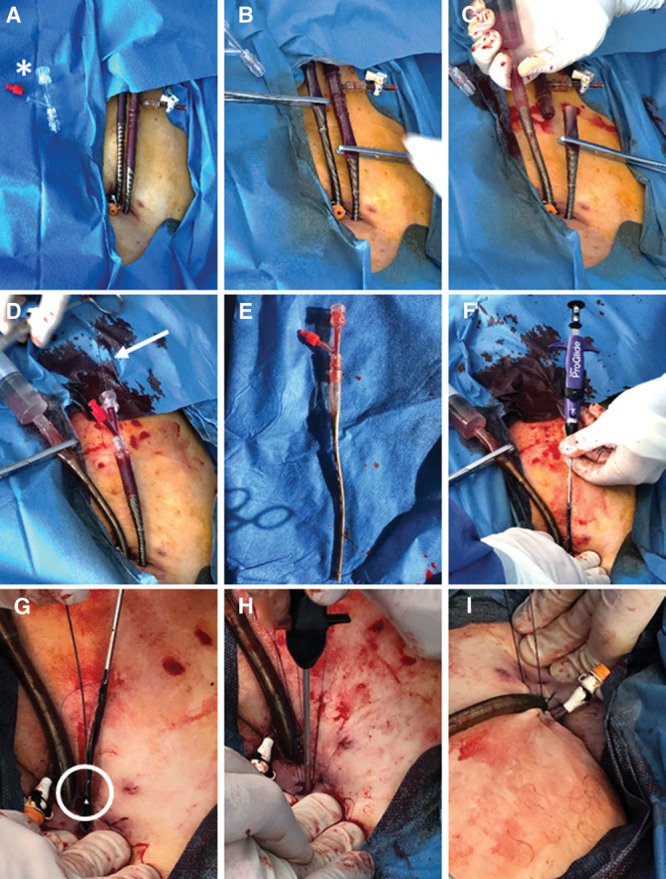
Sterile preparation of the cannulation area with asterisk indicating hemostasis valve Y connector (A). Clamping of arterial and venous cannula shortly behind the selectively hardened proximal venous and arterial cannula body (B) and subsequent cutting by scissors (C). Insertion of hemostasis valve Y connector (Merit Angioplasty Pack, South Jordan, UT) (D) and wire insertion (arrow) into the proximal cannula (E). Insertion and releasing of the first ProGlide via guidewire (Abbott Vascular, Lake Bluff, IL) (F). Reinsertion of the guidewire into the side hole of the first ProGlide device (circle) to place a second ProGlide device (G). Tightening of knots by knot pusher (H). Preparation for removal of the venous cannula by insertion of a Z-suture (I).
TABLE 1.
Baseline Clinical and Laboratory Characteristics
Supplementary Material
Footnotes
Drs. Lüsebrink and Stremmel authors contributed equally
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Danial P, Hajage D, Nguyen LS, et al. Percutaneous versus surgical femoro-femoral veno-arterial ECMO: A propensity score matched study. Intensive Care Med 2018; 44:2153–2161 [DOI] [PubMed] [Google Scholar]
- 2.Hwang JW, Yang JH, Sung K, et al. Percutaneous removal using perclose proglide closure devices versus surgical removal for weaning after percutaneous cannulation for venoarterial extracorporeal membrane oxygenation. J Vasc Surg 2016; 63:998–1003.e1 [DOI] [PubMed] [Google Scholar]
- 3.Majunke N, Mangner N, Linke A, et al. Comparison of percutaneous closure versus surgical femoral cutdown for decannulation of large-sized arterial and venous access sites in adults after successful weaning of veno-arterial extracorporeal membrane oxygenation. J Invasive Cardiol 2016; 28:415–419 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


