Objectives:
The key to further improving outcomes in sepsis lies in understanding and abrogating the dysfunctional immune response that leads to organ failure. Activation of gasdermin-D, a pore-forming protein within the inflammasome cascade, has recently been recognized as the critical step in pyroptosis and organ dysfunction. In this study, we sought to investigate the presence of gasdermin-D in critically ill subjects.
Design, Setting, and Patients:
Prospective pilot study comparing microparticulate active gasdermin-D levels in critically ill patients admitted to the medical ICU at The Ohio State University Medical Center to healthy donors and clinical outcomes.
Interventions:
None.
Measurements and Main Results:
Plasma was collected from subjects upon consent and microparticles were isolated by ultracentrifugation. Proteins of interest were identified by immunoblot analysis of microparticle lysates. Quantification was accomplished by densitometry using ImageJ software (National Institutes of Health, Bethesda, MD). Investigators were then unblinded and compared microparticulate active gasdermin-D levels to physician adjudicated clinical diagnoses and outcomes. No appreciable levels of active gasdermin-D were observed in microparticles from healthy volunteers and nonseptic critically ill patients. However, elevated levels of gasdermin-D were noted in microparticles from the septic cohort of critically ill patients. Furthermore, a significant positive correlation by linear regression was noted when microparticulate active gasdermin-D levels were compared with microparticulate levels of CD63, an exosomal marker, CD14, a monocyte marker, and CD69, a marker of monocyte activation (R2 = 0.37, p = 0.0011, R2 = 0.85, p < 0.0001, and R2 = 0.43, p = 0.0003, respectively).
Conclusions:
This is the first study to demonstrate circulating active gasdermin-D in septic patients in the intensive care setting. Our findings also suggest that active gasdermin-D in septic patients is encapsulated in exosomes derived from activated monocytes. Further characterization in the clinical setting is warranted.
Keywords: caspase-1, gasdermin-D, microparticle, monocyte, sepsis
The sepsis syndrome has been recognized since the time of Hippocrates yet it remains a significant healthcare challenge and burden. Sepsis afflicts up to 1.7 million people annually and one out of every three patients who die during their hospitalizations in the United States are septic. In 2009, the aggregate hospital expenditure cost for sepsis was 15.4 billion dollars (1). A dysregulated immune response to infection leading to organ dysfunction has been key to sepsis outcomes (2). Although there have been many advances in antimicrobial therapy and hemodynamic resuscitation, the holy grail of sepsis management lies in understanding and abrogating the process that results in the initial maladaptive immune response. Targeting the immune system with anti-inflammatory caspase inhibitors has been shown to provide a survival benefit in animal models. However, immune based pharmacologic therapies for sepsis have remained elusive due to the complexity of the human innate immune response and the need to identify specific molecular targets that are strongly linked to causality and clinical outcomes (3).
Early on in the septic process, host detection of pathogen leads to assembly and activation of the inflammasome which regulates caspase-1 activation and cytokine release (4). Work by our group and others has shown that microparticulate caspase-1 regulates pyroptosis and that microparticle encapsulated active gasdermin-D (GSDM-D) is the critical step in caspase-1 mediated cell injury (5, 6). Per Ding et al (7) the N-terminus of p30 kD GSDM-D is directly responsible for programmed cell death through membrane disruption. GSDM-D knockout mice and cell lines have also been shown to resist pyroptosis when exposed to a variety of inflammasome activators (8, 9). Furthermore, chemical inhibitors of GSDM-D including necrosulfonamide, have recently been discovered and have been shown to abrogate pyroptosis in sepsis models (10).
Based on these observations, we set out to expand on our limited prior attempt at investigating the presence of GSDM-D in a clinical model of critically ill patients (6). We hypothesized that higher levels of circulating microparticulate active GSDM-D would be associated with worse clinical outcomes. In this prospective pilot study, we collected blood samples from patients as they were admitted to the medical ICU (MICU) and measured circulating microparticulate active GSDM-D levels. GSDM-D levels were then correlated to clinical diagnoses, markers of severity of illness, and clinical outcomes.
METHODS
After institution review board approval, 40 critically ill patients with new ventilator or pressor requirements were consecutively enrolled early into their admission to the MICU at The Ohio State University Wexner Medical Center. Exclusion criteria included inability to provide consent, pregnancy, incarceration, active hematologic malignancy, or status post stem cell transplantation. A separate group of 13 healthy volunteers were enrolled as a control. Ten milliliters of blood were collected within 24 hours of admission to the MICU and obtaining consent. Investigators were blinded in regards to clinical data. Microparticles were isolated by differential ultracentrifugation of plasma using previously published protocols (5). Peripheral blood mononuclear cells were isolated via Corning lymphocyte separation medium followed by purification with anti_CD14-coated magnetic microbeads loaded onto a MACS column following manufacturer's recommendations (Miltenyi Biotec, Bergisch Gladbach, Germany). Monocyte, lymphocytes, and microparticle fractions were lysed and normalized for protein. Active GSDM-D was identified by immunoblot analysis using a specific GSDM-D antibody from Abnova (Taipei, Taiwan). Whole blood from healthy donors was also separately subjected to lipopolysaccharide (LPS) (1 μg/mL) for 24 hours and monocyte and lymphocyte fractions were analyzed for GSDM-D activation to help distinguish the cellular origin of active GSDM-D. Quantification was completed using densitometry with ImageJ software. Microparticles were further probed for CD63 (exosome marker), CD14 (monocyte maker), and CD69 (marker of monocyte activation). Active GSDM-D levels were correlated with clinical diagnoses and markers of severity illness including Sequential Organ Failure Assessment (SOFA) scores, lactic acid concentrations, and mortality. Inter-protein associations were made using linear regression with JMP statistical software (SAS Institute, Cary, NC). p values of less than 0.05 were considered significant.
RESULTS
Forty MICU patients were enrolled resulting in a yield of 36 samples with sufficient material for analysis. After adjudication by two blinded physicians using Sepsis-3 criteria (3), 26 septic patients, and 10 critically ill nonseptic patients were identified. Of the septic patients, 18 were in septic shock (69%). Infection was mostly attributable to pulmonary (73%) or urinary tract (23%) sources. Five patients were bacteremic (19%). The most commonly encountered infectious organisms were Staphylococcal species (34%) followed by Escherichia coli (19%). There was no statistically significant difference between groups in terms of age, gender, SOFA scores, lactic acid, and procalcitonin concentrations or survival. Acute respiratory distress syndrome (ARDS) was only present in septic patients (Table 1).
TABLE 1.
General Cohort Demographics, Diagnoses, and Markers of Severity of Illness
Active p30 kD GSDM-D was detected in circulating plasma microparticles from the critically ill patients with considerable heterogeneity among patients. However, elevated active GSDM-D levels were only detected in septic patients. Healthy patients and critically ill nonseptic patients did not have appreciable levels of microparticulate active GSDM-D (Fig. 1, A and B). Microparticulate active GSDM-D was found to have a significant positive correlation with CD63 (R2 = 0.37; p = 0.0011), CD14 (R2 = 0.85; p < 0.0001), and CD69 (R2 = 0.43; p = 0.0003) (Fig. 1C–E). There was no significant correlation between the levels of microparticulate active GSDM-D and SOFA scores, lactic acid or procalcitonin levels, source of infection, infectious organism, or survival.
Figure 1.
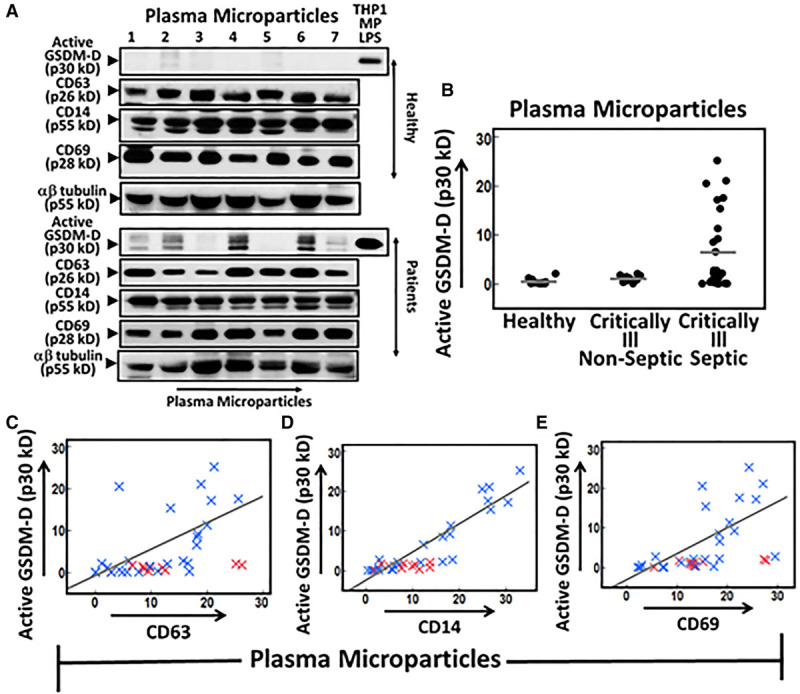
Plasma exosomal active gasdermin-D (GSDM-D) is elevated in septic patients. Ten milliliters of blood were collected within 24 hr of admission to the medical ICU and obtaining consent. Microparticles (MPs) were isolated from the plasma of critically ill patients and healthy donors using sequential ultracentrifugation. MPs were treated with inhibitors of protein degradation and then lysed. Proteins of interest were identified using immunoblot analysis. Loading was controlled for total protein. Quantification was performed by densitometry using ImageJ software. A, Representative MP blots from the plasma of healthy and critically ill subjects. Lipopolysaccharide (LPS) stimulated monocytic cell line THP-1 MP lysates were used as a positive control to assist with p30 KD GSDM-D band identification (10 ug of LPS THP-1 MP was used in the top and 15 ug in the bottom). No detectable levels of active p30 kD GSDM-D were observed in the MPs of healthy volunteers. Critically ill patient samples did demonstrate heterogeneity in p30 kD GSDM-D band signal. B, MP active GSDM-D densitometric values versus clinical diagnosis. Elevated active GSDM-D levels were only evident in the septic cohort. C–E, MP active GSDM-D densitometric values versus densitometric values of CD63 (exosomal marker), CD14 (monocyte marker), and CD69 (marker of monocyte activation) levels in critically ill patients (R2 = 0.37, p = 0.0011, R2 = 0.85, p < 0.0001, and R2 = 0.43, p = 0.0003, respectively). Blue “x” denotes a septic patient, whereas a red “x” denotes a nonseptic patient. Nonseptic patient values were excluded from linear regression analysis using JMP statistical software and the line of best fit. p values of less than 0.05 were considered significant.
Separate analysis of whole blood from healthy donors revealed inactive GSDM-D in both monocyte and lymphocyte fractions, but active GSDM-D was only detected in the monocyte fraction after stimulation with LPS (Fig. 2A). Preliminary analysis of monocyte and lymphocytes from our patient cohort also detected active GSDM-D only in monocyte fractions (Fig. 2B). In contrast to microparticle active GSDM-D, active GSDM-D levels from the monocyte lysates appeared to be lower in the septic state with the exception of a single outlier (Fig. 2C), supporting our observation of increased release of active GSDM-D in circulating CD14 positive microparticles from septic patients (Fig. 1B). There was a significant positive correlation between monocyte active GSDM-D and monocyte CD14 (R2 = 0.83; p < 0.0001), CD69 (R2 = 0.52; p = 0.0005), and CD63 (R2 = 0.41; p = 0.0034) (Fig. 2, D and E).
Figure 2.
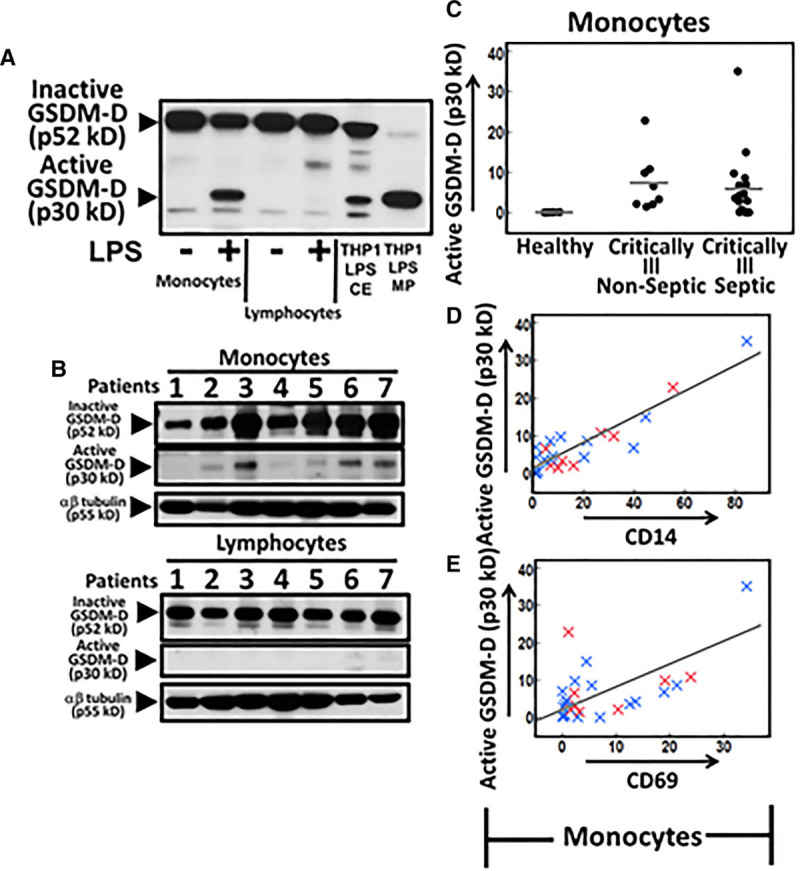
Active gasdermin-D (GSDM-D) levels in cells from patient cohort. A, Monocyte and lymphocyte cell extract (CE) from whole blood stimulated with lipopolysaccharide (LPS) (1 μg/mL) for 24 hr or not were analyzed for presence of active GSDM-D. LPS stimulated THP1 CE and microparticles (MPs) were used as marker for inactive (p52kD) and active GSDM-D (p30kD). B, GSDM-D levels in monocyte and lymphocyte cell lysates from patients. C, Monocyte active GSDM-D densitometric values versus clinical diagnosis. D and E, Monocyte active GSDM-D densitometric values versus densitometric values of CD14 and CD69 levels in patient cohort (R2 = 0.83; p < 0.0001 and R2 = 0.52; p = 0.0005, respectively). Blue “x” denotes a septic patient, whereas a red “x” denotes a nonseptic patient. p values of less than 0.05 were considered significant.
DISCUSSION
This is the first study to demonstrate that the active form of GSDM-D is found exclusively in the circulation of septic critically ill patients raising its potential as an agent of dysregulated immunity in systemic infection. This is a significant expansion over limited work in our own laboratory that had detected circulating active GSDM-D in association with active caspase 1 while investigating caspase 1 mediated pyroptosis in seven patients with ARDS (6). The significant positive correlation between active GSDM-D and CD63 (exosome marker), CD14 (monocyte marker), and CD69 (marker of activated monocytes) lead us to conclude that the circulating active GSDM-D in septic patients is encapsulated in exosomes that originate from activated monocytes.
There are several limitations of this study. Although the mechanistic role of GSDM-D in pyroptosis and cell injury has been well described in vitro, the identification of circulating active GSDM-D in septic patients is mostly correlative. Second, we did not assess the kinetics of expression of GSDM-D throughout the evolution of the septic syndrome. Current ongoing work in the laboratory is focused on longitudinal analysis of GSDM-D levels in our critically ill patient population. Although GSDM-D containing exosomes were associated with CD14 positivity indicating a monocytic origin, we cannot rule out a contribution from other cell types to circulating microparticulate GSDM-D at this time. Initial analysis of monocytes and lymphocyte fractions detected active GSDM-D only in activated monocytes (monocyte p30 GSDM-D vs CD69; R2 = 0.33; p = 0.0018). Finally, our pilot analysis was not sufficiently powered to assess the association of GSDM-D with important clinical outcomes including length of stay, organ dysfunction, and mortality. Future direction includes the characterization of the kinetics of GSDM-D throughout the evolution of the septic syndrome and analyzing how that correlates with clinical outcomes.
Footnotes
Dr. Sarkar was involved in conception of hypothesis, design and acquisition of data, analysis and interpretation, and drafting article for intellectual content. Drs. Homsy and Exline were involved in design and acquisition of patient samples, analysis and interpretation, and drafting article for intellectual content. Drs. Wewers and Mallampalli were involved in analysis and interpretation and drafting of article. Drs. Das and Consiglio involved in design and acquisition of data. Drs. McAtee and Zachman involved in acquisition of patient samples and clinical data. Dr. Nagaraja involved in statistical analysis.
Supported, in part, by grants from National Institutes of Health (NIH) (RO1HL24325 to Dr. Sarkar and RO1GM108928 to Drs. Wewers and Sarkar). The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Elixhauser A, Friedman B, Stranges E. Septicemia in U.S. Hospitals, 2009: Statistical Brief #122. Rockville, MD: Agency for Healthcare Research and Quality; 2006. [PubMed] [Google Scholar]
- 2.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016315801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Tinsley KW, Swanson PE, et al. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci U S A 19999614541–14546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi J, Gao W, Shao F. Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci 201742245–254 [DOI] [PubMed] [Google Scholar]
- 5.Mitra S, Wewers MD, Sarkar A. Mononuclear phagocyte-derived microparticulate caspase-1 induces pulmonary vascular endothelial cell injury. PLoS One. 2015;10:e0145607. doi: 10.1371/journal.pone.0145607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitra S, Exline M, Habyarimana F, et al. Microparticulate caspase 1 regulates gasdermin D and pulmonary vascular endothelial cell injury. Am J Respir Cell Mol Biol 20185956–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding J, Wang K, Liu W, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 2016535111–116 [DOI] [PubMed] [Google Scholar]
- 8.Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015526660–665 [DOI] [PubMed] [Google Scholar]
- 9.Kayagaki N, Stowe IB, Lee BL, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015526666–671 [DOI] [PubMed] [Google Scholar]
- 10.Rathkey JK, Zhao J, Liu Z, et al. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol. 2018;3:eaat2738. doi: 10.1126/sciimmunol.aat2738. [DOI] [PMC free article] [PubMed] [Google Scholar]


