Supplemental Digital Content is available in the text.
Keywords: cardiac arrest team, critical care, rapid response system, rapid response team
Objectives:
Despite improvements in the management of in-hospital cardiac arrest over the past decade, in-hospital cardiac arrest continues to be associated with poor prognosis. This has led to the development of rapid response systems, hospital-wide efforts to improve patient outcomes by centering on prompt identification of decompensating patients, expert clinical management, and continuous quality improvement of processes of care. The rapid response system may include cardiac arrest teams, which are centered on identification and treatment of patients with in-hospital cardiac arrest. However, few evidence-based guidelines exist to guide the formation of such teams, and the degree of their variation across the United States has not been well described.
Design:
Descriptive cross-sectional, internet-based survey.
Setting:
Cohort of preidentified clinicians involved in their hospital’s adult rapid response system across the United States.
Subjects:
Clinicians who had been identified by study team members using personal and professional contacts over a 7-month period from June 2018 to December 2018.
Interventions:
An 80-item survey was developed by the investigators. It sought information on the afferent (identification and notification of providers) and efferent (response of providers to patient) limbs of the rapid response system, as well as management of patients post in-hospital cardiac arrest.
Measurements and Main Results:
One-hundred fourteen surveys were distributed. Of these, 109 (96%) were completed. Six were duplicates and were excluded, leaving a total of 103 surveys from 103 hospitals in 30 states. Seventy-six percent of hospitals were academic, 30% were large hospitals (> 750 inpatient beds), and 58% had large ICUs (> 50 ICU beds). We found wide variation in the structure and function in both the afferent and efferent limbs of the rapid response system. The majority of hospitals had a rapid response team and a cardiac arrest team. Most rapid response teams contained a provider, a critical care nurse, and a respiratory therapist. In hospitals with training programs in internal medicine, anesthesia, emergency medicine, or critical care, 45% of rapid response teams and 75% of cardiac arrest teams were led by trainees, with inconsistent attending presence. Targeted temperature management and coronary catheterization were widely used post in-hospital cardiac arrest, but indications varied considerably.
Conclusions:
We have demonstrated substantial variation in the structure and function of rapid response systems as well as in management of patients during and after in-hospital cardiac arrest.
Despite continued improvements in the management of in-hospital cardiac arrest (IHCA) over the past decade, adult IHCA continues to be associated with poor outcomes (1–3). IHCA is frequently preceded by worsening vital signs, mental status changes, and other signs of physiologic deterioration (4–7). The recognition that delayed intervention in this population leads to worse outcomes has led to the development of rapid response systems (RRSs), which are hospital-wide efforts to identify such patients promptly and to intervene in order to prevent further decompensation (7, 8). Each RRS comprises of an afferent limb, responsible for detecting deterioration and triggering a response, an efferent limb who respond to the adverse event, as well as administrative and quality improvement limbs to support and improve the RRS respectively (9, 10).
RRSs have become widely implemented across the United States due to their inclusion in the Institute for Healthcare Improvement’s (IHI) 5 Million Lives Campaign, as well as regulatory guidance from the National Quality Forum (11, 12).
Despite widespread adoption, implementation of RRSs have been inconsistently linked to improved patient outcomes (13–18). This heterogeneity is likely in part explained by variations in the implementation and acceptance of the RRS as well as the skills and ability of the constituent members of the response team (7). Many hospital also have a dedicated cardiac arrest team (CAT) to respond to IHCA (19). The management of patients during IHCA and after return of spontaneous circulation (ROSC) continues to evolve with a focus on mechanical cardiopulmonary resuscitation (CPR), extracorporeal membrane oxygenation CPR (ECPR), and early cardiac catheterization.
After recently demonstrating variation in RRS composition in five Northeastern U.S. states, our study group aimed to characterize RRS structure, composition, and function across the United States with an additional focus on the management of patients during and after IHCA.
MATERIALS AND METHODS
Survey Design and Distribution
The study was a prospective cross-sectional internet-based study. The study protocol was reviewed and approved by the New York University School of Medicine’s Office of Science and Research Institutional Review Board (i17-01584).
An 80-question survey was created by the investigators (Appendix 1, Supplemental Digital Content 1, http://links.lww.com/CCX/A74), all of whom collaborated in the creation of the survey. The survey was an expanded version of a 46-question survey used by members of our group in a previously published study (20). Although many of the questions were retained, the name of the hospital was added to avoid duplication of hospitals in the data and sections were added that focused on patient management during IHCA and after ROSC.
The study was conducted over a 7-month period from June 2018 to December 2018. In order to maximize the accuracy of the completed surveys as well as survey completion rates, the survey was sent only to clinicians involved in their hospital’s adult RRS who were willing to complete the survey. Once an appropriate subject was identified, a survey email was sent. After 2 weeks, a reminder email was sent if the survey had not been completed. Hospitals that had completed the initial version of the survey were included in this study only after consenting to and completing the updated version of the survey in its entirety. No data collected as part of the initial study were included. Study data were collected and managed using Research Electronic Data Capture, a secure, web-based application designed to support data capture for research studies (21). If duplicate entries were completed for a single hospital, the first to be completed was kept and other surveys were discarded.
To minimize the risk of duplicate surveys being completed, each survey contained a unique link, and the hospital name was included in the survey. All survey questions had to be completed in order to submit the survey. The number of inpatient beds at each hospital was found by study personnel on publicly available hospital or state Department of Health websites.
Statistical comparisons of categorical variables were performed using the chi-square test. Data analysis was completed using the Statistical Package for the Social Sciences (SPSS) Version 25 (IBM Corporation, Armonk, NY).
RESULTS
Characteristics of the Study Hospitals
One-hundred fourteen surveys were sent out over the study period. One-hundred nine surveys were completed (96%). All surveys were completed in their entirety. Six surveys represented duplicate responses for the same hospital and were discarded, leaving a total of 103 completed surveys, a response rate of 90%, from 30 states, with 31% of responses coming from New York, nine percent from California, and seven percent from Pennsylvania (Supplementary Table 1, Supplemental Digital Content 2, http://links.lww.com/CCX/A75).
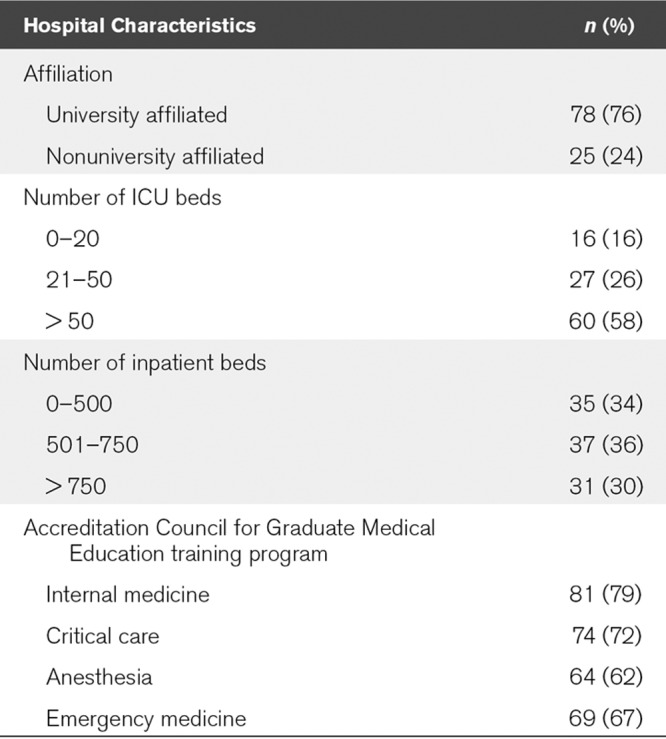
The majority of hospitals were university affiliated and had an Accreditation Council for Graduate Medical Education (ACGME) training program in either internal medicine (IM), emergency medicine (EM), critical care medicine (CCM), or anesthesia (83%). Large hospitals, defined as having more than 750 inpatient beds made up 30% of those studied and 58% had large ICUs, defined as ICUs with more than 50 ICU beds (Table 1).
TABLE 1.
Characteristics of Study Hospitals
Afferent Limb of RRS
Ninety-nine percent of the surveyed hospitals had a rapid response team (RRT), all but one of which were available 24 hours per day, 7 days per week. RRTs were most commonly dispatched by pager or overhead call (79% and 49%, respectively). The RRT could be activated for clinical concern in 96%, single vital sign abnormalities in 77%, and Early Warning Score (EWS) in 54%.
Efferent Limb of RRS
The composition of both the RRT (Fig. 1) and CAT (Fig. 2), as well as the type of clinician most frequently responsible for team leadership (Table 2) varied substantially between centers. The majority of RRTs contained a respiratory therapist (RT), critical care nurse, and a provider (physician, nurse practitioner, or physician assistant) (86%, 79%, and 74%, respectively). RRTs were led by registered nurses in 21%, and by a critical care physician (either attending or trainee) in 19%. In hospitals with ACGME training programs, 45% of RRTs were led by trainees (resident or fellow physicians) but had consistent attending presence only 31% of the time.
Figure 1.
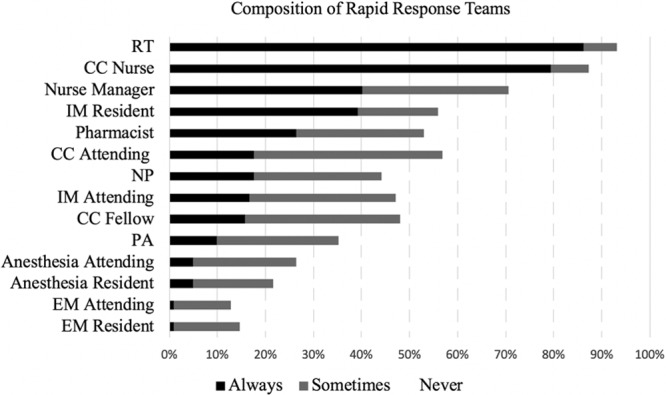
Composition of rapid response teams. CC = critical care, EM = emergency medicine, IM = internal medicine, NP = nurse practitioner, PA = physician assistant, RT = respiratory therapist.
Figure 2.
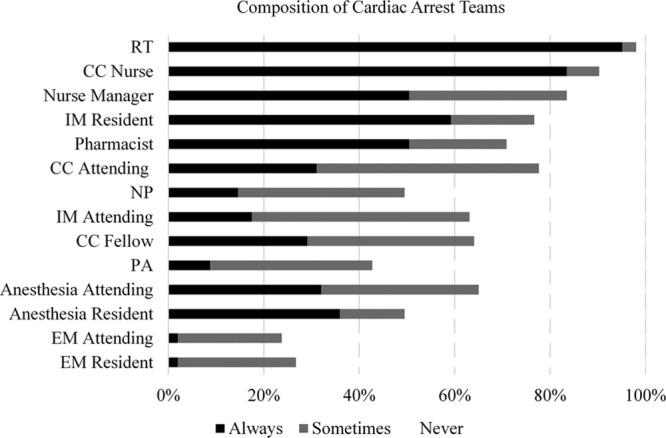
Composition of cardiac arrest teams. CC = critical care, EM = emergency medicine, IM = internal medicine, NP = nurse practitioner, PA = physician assistant, RT = respiratory therapist.
TABLE 2.
Efferent Team Leadership
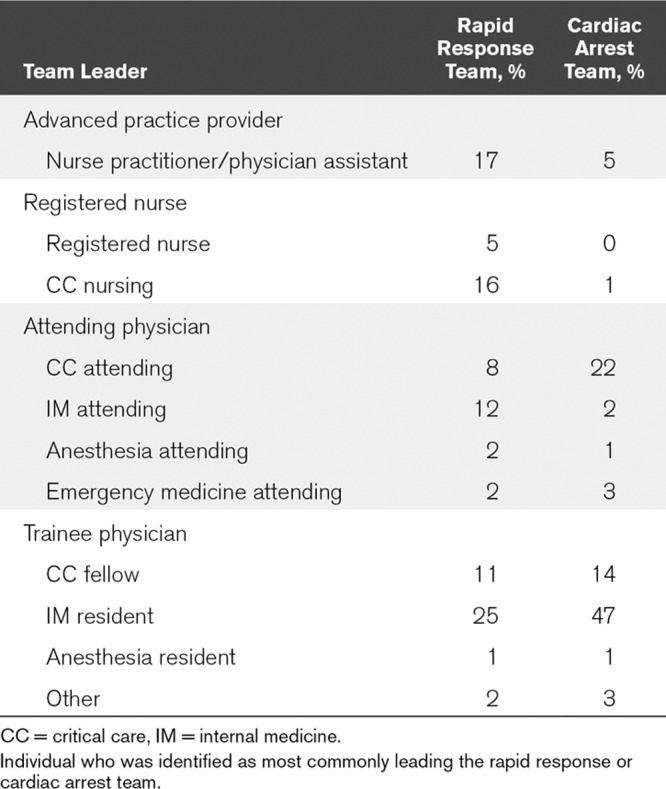
Ninety-four percent of hospitals had a dedicated CAT, 40% of which had an identical structure to their RRT. Seventy-five percent of CATs in hospitals with ACGME training programs were led by a trainee physician, with an attending consistently present in 62% of these CATs. Anesthesia providers were responsible for airway management in 66% of CATs (33% anesthesia residents, 29% anesthesia attending, and 4% certified registered nurse anesthetists). CCM (17%), RT (7%), and EM (5%) providers were responsible for the majority of the remainder of airway management. Thirty-five percent of hospitals reported not having consistent attending supervision of airway management at IHCAs.
Cardiopulmonary Arrest
Mechanical CPR devices were used in 16% of hospitals, most commonly for cases of refractory cardiopulmonary arrest (CPA) (50%). In 19% of hospitals with mechanical CPR, it was only available in certain areas of the hospital, for example, the Emergency Department or the ICU.
End-tidal CO2 (etco2) was used routinely during IHCA in 63% of hospitals and was more frequently used in large than smaller hospitals (≤ 750 beds) (84% vs 54%; p = 0.004), hospitals with large ICUs (75% vs 47%; p = 0.003), ECPR availability (76% vs 51%; p = 0.008), and hospitals that used EWS (73% vs 52%; p = 0.03).
Extracorporeal membrane oxygenation (ECMO) was available in 49% of hospitals, the majority (58%) of which was available as part of a structured 24 hour per day ECPR program. The remainder were only able to offer ECMO if resources were available during the day. Large hospitals and those with large ICUs were more likely to have ECPR (77% of large hospitals vs 36% of smaller hospitals; p < 0.001 and 62% of hospitals with large ICUs vs 30% of hospitals with smaller ICUs; p = 0.002). Hospitals that used EWS to trigger their RRT were also more likely to have ECPR (64% vs 31%; p = 0.001). Cardiothoracic surgery was responsible for ECMO cannulation in 88% of ECPR sites, with EM, CCM, cardiology, and trauma surgery responsible for the remaining 12%. Hospitals with ECPR were more likely to use etco2 monitoring (76% vs 51%; p = 0.008), and to place intraosseous lines (98% vs 81%; p = 0.006).
Post ROSC Care
Most hospitals (91%) used temperature targeted management (TTM) in patients with IHCA: Forty-four percent used TTM in all IHCA, regardless of initial cardiac rhythm; 10% used TTM for only cases where ventricular tachycardia (VT) or ventricular fibrillation (VF) was the initial rhythm; and 38% used TTM for all cases where VT or VF was the initial rhythm as well as select cases of pulseless electrical activity or asystole. The temperature target for hospitals using TTM was 33–34°C in 30%, 36°C in 29% and the remainder reported that the TTM target would be chosen based on the clinical situation.
Most institutions sent patients for post-IHCA cardiac catheterization (97%), but indications varied: 43% of institutions sent most or all patients with VF/VT IHCA for emergency cardiac catheterization regardless of whether ischemic changes were found on electrocardiogram (ECG); 33% sent only cases of VF (VF/VT) with ST elevations on the ECG after ROSC; 14% did not always perform coronary catheterization even on patients with ST elevations on the ECG after ROSC and the remaining institutions reported varying practice. Hospitals with large ICUs were more likely to take most or all patients with ROSC for cardiac catheterization (52% vs 30%; p = 0.03).
DISCUSSION
Our study demonstrated wide availability of both RRTs and CATs across the hospitals surveyed. We also found substantial variation in both the afferent and efferent limbs of the RRS.
Certain features of the afferent limb have been well studied, particularly the importance of early identification and intervention; however, the composition of response teams is less well studied (22–27). This study builds on a previous survey by our group which demonstrated variation in both RRT and CAT composition across five northeastern U.S. states, adding information from multiple states and highlighting the variability in management of patients during IHCA and after ROSC (20).
Delaying activation of the RRT leads to worse clinical outcomes, presumably due to continued patient deterioration resulting in failure-to-rescue (28–30). RRT dispatch, on the other hand, has been associated with improved outcomes but has also been associated with increased utilization of ICU resources (31, 32). EWS were designed to allow for prompt and accurate identification of critically ill inpatients and have evolved from simple vital sign based EWS, such as the National Health Service’s National EWS, to much more complex scores that integrate multiple variables such as laboratory results, patient location, and recent procedures. Despite this heterogeneity, EWS have been shown to be more accurate than the use of single vital sign abnormalities, have been validated across multiple specialties, and their introduction has been associated with a reduction in the rate of out-of-ICU IHCA (22–25, 27, 33). More recently, machine learning algorithms have allowed for the creation of EWS models that may be more accurate than traditional EWS (26). Given the relatively low uptake among hospitals in this study, a focus on broader adoption of EWS and continued efforts to develop predictive models with higher accuracy may lead to improved patient outcomes.
Once the RRT has been dispatched, its function is to assess the patient, intervene if possible, and to move the patient to a higher level of care if required. To do so, its members must have an appropriate balance of expertise to correctly diagnose, manage, and triage the patient. Neither the IHI nor other professional guidelines have clearly defined the individual members who should be present in the RRT, nor the required skill set of the RRT (34). Among hospitals included in our survey, this seems to have resulted in wide variation in RRT composition and leadership. Most RRTs contained at least an RT, critical care nurse, and a provider, with only a minority of teams containing a critical care physician and other members of the RRT varying widely (Fig. 1). This team makeup certainly brings a valuable skill set to the patient, especially in airway management and critical care nursing. However, some could argue that the lack of a standardized team composition and infrequent inclusion of critical care providers risks falling short of the IHI’s stated goal for the RRT to bring “critical care expertise to the bedside.” We also found considerable differences in RRT leadership, likely due largely to differences in local resources and requirements.
The interaction between individual RRT configurations on patient outcomes has not been well studied and merit further investigation. Until then, the composition of the RRS efferent team should focus on including providers who are qualified to treat the most common RRT calls and contain members with expertise in the diagnosis and management of critically ill patients (ig. 3).
Figure 3.
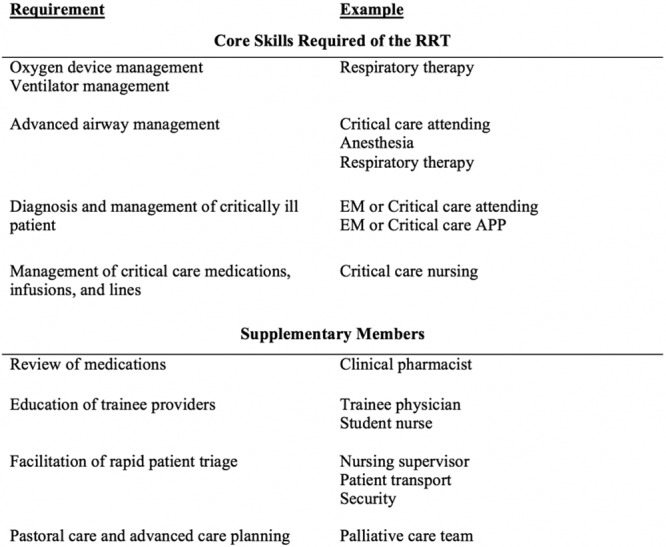
Skills required to treat common and acutely life-threatening inpatient emergencies. APP = advanced practice provider, EM = emergency medicine, RRT = rapid response team.
Aside from requiring Adult Cardiovascular Life Support (ACLS) certification from the team leader, professional guidelines make few recommendations about CAT composition (35–37). It is not clear whether the specialty of the leader of the CAT impacts performance, but it has been well established that leadership, communication skills and a focus on team dynamics are essential for maximizing performance during IHCA (38–40). CATs were almost always led by a physician, with trainee physicians frequently leading the team without attending physician supervision. This finding is consistent with the results of another recent study (19), and is of some concern as residents have low confidence about leading CATs, feel unprepared to do so, and have variable performance and experience in leading them (41, 42). Hospitals with the best outcomes from IHCA have a number of features in common, including the use of a dedicated and multidisciplinary CAT, and a focus on high-quality communication and ongoing training (43). The use of a dedicated CAT who regularly work together and who use simulation for team dynamic and leadership training is likely the most sensible approach to improve patient outcomes. Teams composed of a continuously rotating crew of IM, critical care, and anesthesia trainees that may never have worked together before are common, but probably not ideal (43).
Newer adjunctive tools for cardiac arrest care have become available, including physiologic monitoring with etco2, and therapeutic adjuncts such as intraosseous devices, and mechanical CPR (44). These all come with a cost for (re)training, upkeep, and device distribution and have not been associated with improved survival from CPA (45–47). Mechanical CPR and etco2 were more commonly used in our study than in a 2014 study of CPR practices, suggesting gradual uptake of national ACLS guidelines (19). The use of these CPR adjuncts was particularly common in large hospitals, hospitals with ECPR, and hospitals that used EWS. This association likely represents a more robust quality improvement effort, mature RRS, and increased resources at these hospitals.
A large number of hospitals reported using TTM for IHCA, an increase in its use compared with previous studies (19, 48). Although we found high overall uptake, there remains marked variation in practice in terms of indications for starting TTM, and further work could investigate variation in duration and dose of TTM (49).
Finally, we found a surprisingly high availability of ECPR in hospitals in our study. Although this is based on self-report, it may be a biased representation due to the size and academic nature of hospitals in our study, ECPR has become a feature of CATs across the country, likely due to encouraging results of ECPR for IHCA and inclusion in the American Heart Association ACLS guidelines (44, 50).
Our study has several weaknesses which will limit its generalizability. One substantial limitation is the small number of hospitals surveyed. In contrast to our original study, which was distributed broadly to a range of clinicians, administrative staff, and hospital leadership, our study group ensured that the survey was sent to a willing, identified clinician working in their hospital’s RRS. Although this led to a much-improved survey completion rate, this likely resulted in substantial selection bias, with a disproportionate number of large, academic medical centers that were mainly located on the east or west coasts of the United States. Smaller hospitals may have fewer resources, less frequent IHCAs, and more limited physician availability when compared with large hospitals, factors which might affect RRT and CAT design. It is not clear whether our findings can be extrapolated to smaller hospitals or to hospitals outside of the United States, nor whether the high proportion of hospitals located in New York and California biased our findings, but other U.S.-based studies and several studies in Europe and New Zealand have also demonstrated substantial variation in RRT and CATs (19, 51–53).
By the nature of the study design, the survey was only completed by a single individual at each hospital. Although we made substantial effort to ensure that the person completing the survey could do so accurately, we had no way of confirming that the survey was completed correctly. We collected descriptive data only and did not collect data on RRT call frequency, cardiac arrest rates, or patient complexity at each hospital, which may well impact RRT composition. Finally, we were unable to correlate our findings of the structure and function of these teams with hospital- or patient-level outcomes.
CONCLUSIONS
There is wide variation in the structure and function of RRT and CATs. The creation of evidence-based practice guidelines on the structure and function of RRT and CATs is critical to optimize outcomes from in hospital deterioration. Future research efforts should focus on the variability in outcomes with different RRT composition and establishing current practice in community hospitals, smaller hospitals, and settings with fewer resources.
ACKNOWLEDGMENTS
We thank Christopher Anderson, New York School of Medicine, Department of Internal Medicine, New York, NY.
Supplementary Material
Footnotes
New affiliation for Dr. Mitchell: Division of Pulmonary, Allergy, and Critical Care, Department of Medicine, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA.
Supplemental digital content is available for this article. Direct URL citations appear in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Dr. Horowitz received funding from Steering committee for Evaluating the Safety and Efficacy of the Indigo Aspiration System in Acute Pulmonary Embolism (EXTRACT-PE) Clinical Trial with the Penumbra Indigo Aspiration System. Dr. Friedman received funding from Bristol Myers Squibb speaker’s bureau. Dr. Nichol received funding from National Heart Lung Blood Institute, Bethesda, MD, Pragmatic Airway Resuscitation Trial (PART), UH2 HL125163-03, Co-I, Modest; National Heart Lung Blood Institute, Bethesda, MD, Intravenous Sodium Nitrite as Therapy for Cardiac Arrest Pilot Trial (SNOCAT) UH2 HL129722-02, Co-I, Modest; Department of Defense, Washington, DC. Translating Military Simulation-based Trauma Team Research into Outcomes: LEADing Effective Resuscitations (LEADER), Co-I, Modest; Abiomed, Danvers, MA. Program to Identify Cardiogenic Shock Early (PRIME). PI, Modest; GE Healthcare, Chicago, IL. Very Early Identification of Shock by Ultrasound Exam (VENUE) PI, Modest; ZOLL Circulation, Sunnyvale, CA. STEMI Cool Pilot Trial to Assess Cooling as an Adjunctive Therapy to PCI In Patients With Acute MI (STEMI Cool) U.S. Pilot Trial, PI, Modest; and ZOLL Medical, Chelmsford, MA. Cardiopulmonary resuscitation process versus outcome, PI, Modest. Dr. Evans consulting for the Healthcare Association of New York State on Sepsis Quality Improvement. Dr. Mukherjee consult for 2nd MD, an online physician consultation enterprise. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Peberdy MA, Ornato JP, Larkin GL, et al. ; National Registry of Cardiopulmonary Resuscitation Investigators Survival from in-hospital cardiac arrest during nights and weekends. JAMA 2008299785–792 [DOI] [PubMed] [Google Scholar]
- 2.Nolan JP, Soar J, Smith GB, et al. ; National Cardiac Arrest Audit Incidence and outcome of in-hospital cardiac arrest in the United Kingdom National Cardiac Arrest Audit. Resuscitation 201485987–992 [DOI] [PubMed] [Google Scholar]
- 3.Thompson LE, Chan PS, Tang F, et al. ; American Heart Association’s Get With the Guidelines-Resuscitation Investigators Long-term survival trends of medicare patients after in-hospital cardiac arrest: Insights from Get With The Guidelines-Resuscitation®. Resuscitation 201812358–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schein RM, Hazday N, Pena M, et al. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest 1990981388–1392 [DOI] [PubMed] [Google Scholar]
- 5.Smith AF, Wood J. Can some in-hospital cardio-respiratory arrests be prevented? A prospective survey. Resuscitation 199837133–137 [DOI] [PubMed] [Google Scholar]
- 6.Kause J, Smith G, Prytherch D, et al. ; Intensive Care Society (UK); Australian and New Zealand Intensive Care Society Clinical Trials Group A comparison of antecedents to cardiac arrests, deaths and emergency intensive care admissions in Australia and New Zealand, and the United Kingdom–the ACADEMIA study. Resuscitation 200462275–282 [DOI] [PubMed] [Google Scholar]
- 7.Calzavacca P, Licari E, Tee A, et al. The impact of rapid response system on delayed emergency team activation patient characteristics and outcomes–a follow-up study. Resuscitation 20108131–35 [DOI] [PubMed] [Google Scholar]
- 8.Jones DA, DeVita MA, Bellomo R. Rapid-response teams. N Engl J Med 2011365139–146 [DOI] [PubMed] [Google Scholar]
- 9.Devita MA, Bellomo R, Hillman K, et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med 2006342463–2478 [DOI] [PubMed] [Google Scholar]
- 10.Jones D, Rubulotta F, Welch J. Rapid response teams improve outcomes: Yes. Intensive Care Med 201642593–595 [DOI] [PubMed] [Google Scholar]
- 11.Institute for Healthcare Improvement. 5 Million Lives Campaign, 2006. Available at http://www.ihi.org/about/Documents/5MillionLivesCampaignCaseStatement.pdf. Accessed July 14, 2019.
- 12.National Quality Forum: Safe Practices for Better Healthcare - 2010 Update. 2010. Available at: https://www.qualityforum.org/Publications/2010/04/Safe_Practices_for_Better_Healthcare_%E2%80%93_2010_Update.aspx. Accessed July 14, 2019.
- 13.Bellomo R, Goldsmith D, Uchino S, et al. A prospective before-and-after trial of a medical emergency team. Med J Aust 2003179283–287 [DOI] [PubMed] [Google Scholar]
- 14.DeVita MA, Braithwaite RS, Mahidhara R, et al. ; Medical Emergency Response Improvement Team (MERIT) Use of medical emergency team responses to reduce hospital cardiopulmonary arrests. Qual Saf Health Care 200413251–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillman K, Chen J, Cretikos M, et al. ; MERIT study investigators Introduction of the medical emergency team (MET) system: A cluster-randomised controlled trial. Lancet 20053652091–2097 [DOI] [PubMed] [Google Scholar]
- 16.Buist M, Harrison J, Abaloz E, et al. Six year audit of cardiac arrests and medical emergency team calls in an Australian outer metropolitan teaching hospital. BMJ 20073351210–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maharaj R, Raffaele I, Wendon J. Rapid response systems: A systematic review and meta-analysis. Crit Care. 2015;19:254. doi: 10.1186/s13054-015-0973-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones D, Bellomo R, Bates S, et al. Long term effect of a medical emergency team on cardiac arrests in a teaching hospital. Crit Care 20059R808–R815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edelson DP, Yuen TC, Mancini ME, et al. Hospital cardiac arrest resuscitation practice in the United States: A nationally representative survey. J Hosp Med 20149353–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell OJL, Motschwiller CW, Horowitz JM, et al. Characterising variation in composition and activation criteria of rapid response and cardiac arrest teams: A survey of Medicare participating hospitals in five American states. BMJ Open. 2019;9:e024548. doi: 10.1136/bmjopen-2018-024548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 200942377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Subbe CP, Kruger M, Rutherford P, et al. Validation of a modified Early Warning Score in medical admissions. QJM 200194521–526 [DOI] [PubMed] [Google Scholar]
- 23.Gardner-Thorpe J, Love N, Wrightson J, et al. The value of Modified Early Warning Score (MEWS) in surgical in-patients: A prospective observational study. Ann R Coll Surg Engl 200688571–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith GB, Prytherch DR, Meredith P, et al. The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation 201384465–470 [DOI] [PubMed] [Google Scholar]
- 25.Churpek MM, Snyder A, Sokol S, et al. Investigating the impact of different suspicion of infection criteria on the accuracy of quick sepsis-related organ failure assessment, systemic inflammatory response syndrome, and early warning scores. Crit Care Med 2017451805–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Churpek MM, Yuen TC, Winslow C, et al. Multicenter comparison of machine learning methods and conventional regression for predicting clinical deterioration on the wards. Crit Care Med 201644368–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarvis S, Kovacs C, Briggs J, et al. Aggregate National Early Warning Score (NEWS) values are more important than high scores for a single vital signs parameter for discriminating the risk of adverse outcomes. Resuscitation 20158775–80 [DOI] [PubMed] [Google Scholar]
- 28.Barwise A, Thongprayoon C, Gajic O, et al. Delayed rapid response team activation is associated with increased hospital mortality, morbidity, and length of stay in a tertiary care institution. Crit Care Med 20164454–63 [DOI] [PubMed] [Google Scholar]
- 29.Boniatti MM, Azzolini N, Viana MV, et al. Delayed medical emergency team calls and associated outcomes. Crit Care Med 20144226–30 [DOI] [PubMed] [Google Scholar]
- 30.Reardon PM, Fernando SM, Murphy K, et al. Factors associated with delayed rapid response team activation. J Crit Care 20184673–78 [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Bellomo R, Flabouris A, et al. ; MERIT Study Investigators for the Simpson Centre; ANZICS Clinical Trials Group The relationship between early emergency team calls and serious adverse events. Crit Care Med 200937148–153 [DOI] [PubMed] [Google Scholar]
- 32.Karpman C, Keegan MT, Jensen JB, et al. The impact of rapid response team on outcome of patients transferred from the ward to the ICU: A single-center study. Crit Care Med 2013412284–2291 [DOI] [PubMed] [Google Scholar]
- 33.Moon A, Cosgrove JF, Lea D, et al. An eight year audit before and after the introduction of modified early warning score (MEWS) charts, of patients admitted to a tertiary referral intensive care unit after CPR. Resuscitation 201182150–154 [DOI] [PubMed] [Google Scholar]
- 34.Institute for Healthcare Improvement. Getting Started Kit: Rapid Response Teams In. 2008. Available at: http://www.ihi.org/IHI/Programs/Campaign. Accessed July 14, 2019.
- 35.Meaney PA, Bobrow BJ, Mancini ME, et al. ; CPR Quality Summit Investigators, the American Heart Association Emergency Cardiovascular Care Committee, and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation Cardiopulmonary resuscitation quality: [corrected] improving cardiac resuscitation outcomes both inside and outside the hospital: A consensus statement from the American Heart Association. Circulation 2013128417–435 [DOI] [PubMed] [Google Scholar]
- 36.Kronick SL, Kurz MC, Lin S, et al. Part 4: Systems of care and continuous quality improvement: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015132S397–S413 [DOI] [PubMed] [Google Scholar]
- 37.Soar J, Nolan JP, Böttiger BW, et al. ; Adult advanced life support section Collaborators European Resuscitation Council Guidelines for resuscitation 2015: Section 3. Adult advanced life support. Resuscitation 201595100–147 [DOI] [PubMed] [Google Scholar]
- 38.Andersen PO, Jensen MK, Lippert A, et al. Identifying non-technical skills and barriers for improvement of teamwork in cardiac arrest teams. Resuscitation 201081695–702 [DOI] [PubMed] [Google Scholar]
- 39.Marsch SC, Müller C, Marquardt K, et al. Human factors affect the quality of cardiopulmonary resuscitation in simulated cardiac arrests. Resuscitation 20046051–56 [DOI] [PubMed] [Google Scholar]
- 40.Hunziker S, Tschan F, Semmer NK, et al. Hands-on time during cardiopulmonary resuscitation is affected by the process of teambuilding: A prospective randomised simulator-based trial. BMC Emerg Med. 2009;9:3. doi: 10.1186/1471-227X-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayes CW, Rhee A, Detsky ME, et al. Residents feel unprepared and unsupervised as leaders of cardiac arrest teams in teaching hospitals: A survey of internal medicine residents. Crit Care Med 2007351668–1672 [DOI] [PubMed] [Google Scholar]
- 42.Wayne DB, Butter J, Siddall VJ, et al. Graduating internal medicine residents’ self-assessment and performance of advanced cardiac life support skills. Med Teach 200628365–369 [DOI] [PubMed] [Google Scholar]
- 43.Nallamothu BK, Guetterman TC, Harrod M, et al. How do resuscitation teams at top-performing hospitals for in-hospital cardiac arrest succeed? A qualitative study. Circulation 2018138154–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Link MS, Berkow LC, Kudenchuk PJ, et al. Part 7: Adult Advanced Cardiovascular Life Support: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015132S444–S464 [DOI] [PubMed] [Google Scholar]
- 45.Perkins GD, Lall R, Quinn T, et al. Mechanical versus manual chest compression for out-of-hospital cardiac arrest (PARAMEDIC): A pragmatic, cluster randomised control trial. J Intensive Care Soc 201516241–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheak KR, Wiebe DJ, Leary M, et al. Quantitative relationship between end-tidal carbon dioxide and CPR quality during both in-hospital and out-of-hospital cardiac arrest. Resuscitation 201589149–154 [DOI] [PubMed] [Google Scholar]
- 47.Reades R, Studnek JR, Vandeventer S, et al. Intraosseous versus intravenous vascular access during out-of-hospital cardiac arrest: A randomized controlled trial. Ann Emerg Med 201158509–516 [DOI] [PubMed] [Google Scholar]
- 48.Mikkelsen ME, Christie JD, Abella BS, et al. ; American Heart Association’s Get With the Guidelines-Resuscitation Investigators Use of therapeutic hypothermia after in-hospital cardiac arrest. Crit Care Med 2013411385–1395 [DOI] [PubMed] [Google Scholar]
- 49.Callaway CW, Donnino MW, Fink EL, et al. Part 8: Post-cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015132S465–S482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thiagarajan RR, Barbaro RP, Rycus PT, et al. ; ELSO member centers Extracorporeal life support organization registry international report 2016. ASAIO J 20176360–67 [DOI] [PubMed] [Google Scholar]
- 51.Psirides A, Hill J, Hurford S. A review of rapid response team activation parameters in New Zealand hospitals. Resuscitation 2013841040–1044 [DOI] [PubMed] [Google Scholar]
- 52.Tirkkonen J, Nurmi J, Olkkola KT, et al. Cardiac arrest teams and medical emergency teams in Finland: A nationwide cross-sectional postal survey. Acta Anaesthesiol Scand 201458420–427 [DOI] [PubMed] [Google Scholar]
- 53.Lauridsen KG, Schmidt AS, Adelborg K, et al. Organisation of in-hospital cardiac arrest teams - a nationwide study. Resuscitation 201589123–128 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


