Abstract
Studies conducted over the past eight years in Latin America (LA) have continued to produce new knowledge regarding health impacts of arsenic (As) in drinking water. We conducted a systematic review of 92 peer-reviewed English articles published between 2011 and 2018 to expand our understanding on these health effects. Majority of the LA studies on As have been conducted in Chile and Mexico. Additional data have emerged from As-exposed populations in Argentina, Bolivia, Brazil, Colombia, Ecuador, and Uruguay. The present review has documented recent data on the biomarkers of As exposure, genetic susceptibility and genotoxicity, and risk assessment to further characterize the health effects and exposed populations. Some recent findings on the associations of As with bladder and lung cancers, reproductive outcomes, and declined cognitive performance have been consistent with what we reported in our previous systematic review article. We have found highly convincing evidence of in utero As exposure as a significant risk factor for several health outcomes, particularly for bladder cancer, even at moderate level. New data have emerged regarding the associations of As with breast and laryngeal cancers as well as type 2 diabetes. We observed early life As exposure to be associated with kidney injury, carotid intima-media thickness, and various pulmonary outcomes in children. Other childhood effects such as low birth weight, low gestational age, anemia, increased apoptosis, and decreased cognitive functions were also reported. Studies identified genetic variants of As methyltransferase that could determine susceptibility to As related health outcomes. Arsenic-induced DNA damage and alteration of gene and protein expression have also been reported. While the scope of research is still vast, the substantial work done on As exposure and its health effects in LA will help direct further large-scale studies for more comprehensive knowledge and plan appropriate mitigation strategies.
Keywords: water arsenic, Latin America, cancers, cardiopulmonary outcomes, in utero and early life effects, genetic susceptibility
1. Introduction
Millions of people from Latin America (LA) continue to be exposed to high levels of arsenic (As) from natural and anthropogenic sources for many centuries (Bundschuh et al., 2009; George et al., 2014; Litter et al., 2014; McClintock et al., 2012). In a number of LA countries, predominantly volcanic rocks and their weathering products together with geothermal fluids (water, gases) and volcanic exhalations, contaminate groundwater and soils with moderate to high levels of As. Volcanic ashes are also identified as the primary source of As over extended geographical areas in different countries in LA (Bhattacharya et al., 2006; Bundschuh et al., 2004; Ramirez-Aldaba et al., 2016). Anthropogenic As contamination occurs from mining operations and electric reefing industries in Brazil and from As-containing pesticides in Mexico (Castro de Esparza, 2009; Matschullat et al., 2007; Matschullat et al., 2000). To a lesser extent, As based pesticides and wood preservation agents also contribute to LA’s water contamination (Bhattacharya et al., 2006; Bundschuh et al., 2004). Based on common geographical and contamination routes of As in LA, deposition and distribution of As can be classified into three main distinct regions - the Chaco-Pampean plain, Andean range (including its Pacific coastal stripe), Central America, and Mexico. While each of these areas has their own defining characteristics, they all were affected by inorganic As transport into drinking water sources due to natural or anthropogenic sources (McClintock et al., 2012). Extremely high As levels were detected in nearly all possible drinking water sources such as springs, lakes, rivers and groundwater in many LA countries (Argentina, Bolivia, Brazil, Chile, Colombia, Cuba, Ecuador, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Peru, and Uruguay). Although widespread As contamination from both natural and anthropogenic sources has long been a threat to human health in LA over centuries, relatively little is known on occurrence, distribution, and exposed population other than Argentina, Brazil, Chile, and Mexico. It is estimated that at least 4.5 million people in LA are currently drinking As contaminated water (>50μg/L), with water observed to have As concentrations as high as 2000 μg/L - roughly 200 times higher than the current World Health Organization (WHO) standard (10 μg/L) for drinking water (Farías et al., 2008; WHO, 2003).
Numerous studies from LA have reported an array of adverse health effects of As including cancer, cardiovascular, lung diseases, reproductive outcome, and cognitive impairment in adults as well as children. Earlier epidemiological investigations from As endemic areas of Antofagasta in Chile and Córdoba province in Argentina have consistently reported elevated risk of bladder and lung cancer mortality in population chronically exposed to As (Bates et al., 2004; Hopenhayn-Rich et al., 1996; Marshall et al., 2007; Smith et al., 2006). A few studies from the same region have also documented As-induced kidney, liver and skin cancer (Hopenhayn-Rich et al., 1998; Yuan et al., 2010). The trend continued in the past decades with significantly increased risk of mortality and morbidity from cancers of the bladder, lung, and kidney. Males were also found to be at increased risk. In particular, these studies found several fold increased risk associated with in utero and early life As exposure even when the exposure levels were low to moderate. A small number of studies also reported increased risk of diabetes mellitus, cardiovascular and pulmonary diseases associated in As-exposed individuals from Chile and Mexico.
Over the past decade, a sizeable portion of research was devoted to understand As-associated genotoxicity and genetic susceptibility, particularly in relation to As methylation capacity. For instance, a number of studies from Argentina, Chile, Colombia, and Mexico have analyzed genetic polymorphisms in arsenic methyltransferase (AS3MT) and other As metabolism associated genes and linked them with various diseases (Ameer et al., 2017; Gamboa-Loira et al., 2017; Gamboa-Loira et al., 2018; Gomez-Rubio et al., 2011; Gonzalez-Martinez et al., 2018; Xu et al., 2016). However, most of these findings were not linked to health outcomes. Additionally, a number of studies from LA countries identified that smoking, male gender, long latency period, considerably increased risk of many health outcomes including cancer, cardiovascular and lung diseases (Steinmaus et al., 2010). About two decades ago, a study among Andean women in Argentina revealed for the first time that As crosses into placenta (Concha et al., 1998a; Concha et al., 1998b). Subsequent research showed low birth weight, and neonatal mortality from As exposure (Hopenhayn-Rich et al., 2000). Evidence of early life and childhood anemia, kidney injury, subclinical atherosclerosis, and poor lung function in As-exposed children were found in multiple recent studies, which have not been reviewed yet.
A majority of the studies from LA were based on ecological, cross-sectional, case-control or retrospective design using past exposure assessment with relatively small sample size and limited data on individual exposure. In many cases, particularly on the mortality studies, outcomes were ascertained by death records. In spite of these limitations, the studies have shown a consistent link with As exposure particularly in cancers. Studies have also shown a long latency period from 10 years to as long as 50 years following high exposure. A previous study conducted an in-depth review of the health effects, biomarkers, genetic susceptibility, and mechanistic studies from LA countries using research papers published until late 2010 (McClintock et al., 2012). We have extended this effort to provide an update of the findings on As-induced health effects in exposed LA populations. Our goal was to assemble new evidence from studies conducted between 2011 and 2018.
2. Methods
An extensive search of the electronic database from United States was carried out to identify peer-reviewed articles from indexed journals published in English on As and health related issues in LA countries. In our previous study, we reviewed papers from 1949 to 2010. For the current review, we included articles published between January 2011 and December 2018 (either online or printed version of the journal) from the following databases: PubMed, TOXLINE, Biological Abstracts, the Cochrane Library and SCOPUS. We excluded studies that were written in Spanish and Portuguese and others not written in English, not indexed and available online, non peer-reviewed manuscripts and reports, articles in conference proceedings, doctoral dissertations. A systematic search of each database with the following terms were conducted (matching terms in title or abstract fields or in “ all fields” or similar): ‘arsenic’ and ‘name of the country’ (Argentina, Bolivia, Brazil, Chile, Colombia, Costa Rica, Ecuador, El Salvador, French Guiana, Guyana, Guatemala, Honduras, Mexico, Nicaragua, Panama, Paraguay, Peru, Suriname, Uruguay, Venezuela, Latin America, South America, or Central America). If the search yielded several items unassociated with health effects, additional terms such as ‘health effect’, ‘cancer’, ‘lung disease’, ‘cardiovascular disease’, ‘biomarker’, and ‘metabolites’ were included (with AND) to restrict search results. The additional terms in conjunction with the term ‘arsenic’ improved the relevancy of the articles in the search results.
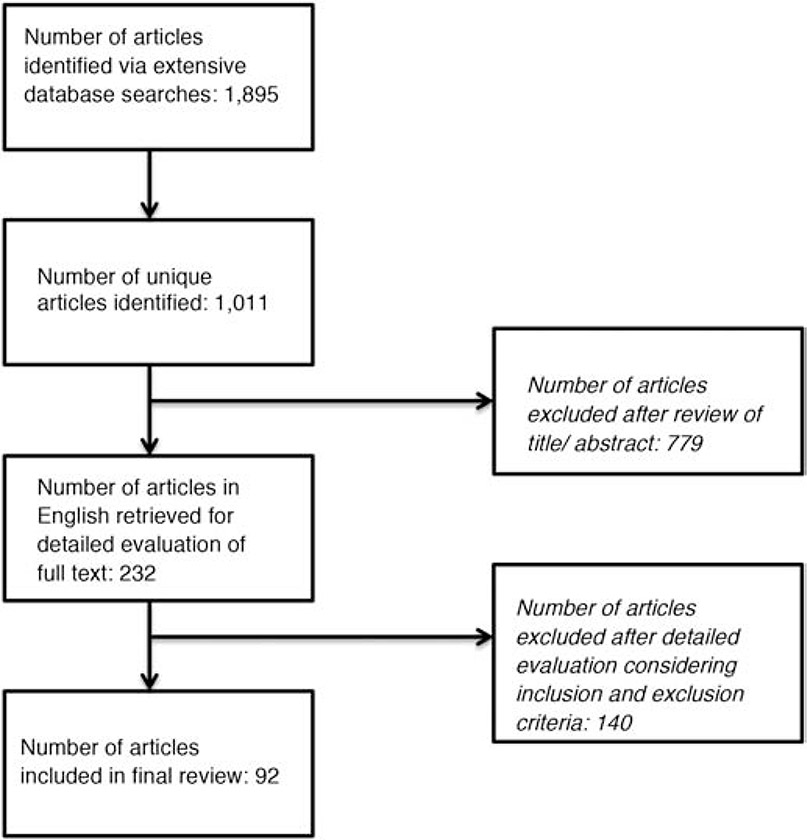
In case of PubMed database, we included several vocabulary items from PubMed’s Medical Subject Headings (MeSH) such as Arsenic Poisoning, South America, Latin America, and Central America. All searched articles were screened based on whether it was an original research, targeted LA human populations, entailed drinking water As exposure, and related to health outcomes, disease processes or biomarkers. The titles and abstracts of all short listed articles were reviewed to judge the relevance of the studies. We excluded 779 articles that showed evidence of one or more of the exclusion criteria. The citations for all identified potentially relevant articles were collected and the full text for each study was retrieved, if available. The process for the selection of studies is illustrated in Figure 1. Eventually, of the 1,895 items reviewed, a total of 92 peer-reviewed and published articles were included in this study.
Figure 1:
The Study Selection Process
Note: This is a single column fitting image
3. Biological markers of exposure, metabolism, toxicity, susceptibility, and risk assessment
3.1. Biomarkers of exposure
Studies from LA countries examined association of As with number of biomarkers based on availability of the types of samples and suitable for the study population. Overall, urinary As (UAs) has been consistently used as the most common biomarker of exposure in various types of LA studies to investigate As exposure from water, air pollution and mining operations. In a population exposed to drinking water As (WAs) at various locations of Chaco and Santiago del Estero provinces of Argentina, more than 90% of the population had UAs levels above 100 μg/g creatinine where WAs levels ranged between non-detectable to 2,000 μg/L (Navoni et al., 2014). How food and water contributed to UAs was explored in an Antofagasta (Chile) study. This study examined 20 WAs-exposed adults, who were between the age of 31 and 68 years with 3 controls and found that exposed group had markedly higher UAs (mean 219 μg/L) than the controls (mean 24 μg/L). All participants of the study exceeded the reference As intake of 149.8 μg/day as suggested by FAO/WHO (Diaz et al., 2015). In a Mexico study of 75 adults, total As in urine ranged from 1.3 to 398.7 μg/L, indicating 33 % of the inhabitants with elevated biological exposition index (BEI = 35 μg/L), the permissible limit for occupational exposure (Colin-Torres et al., 2014). High UAs has also been observed in workers due to their occupations. A study on workers in wood impregnation facilities that use chromated copper arsenate (CCA) revealed that 34% of the participants had a high exposure risk of As (>35 μg/L) due to their work tasks whereas 29% demonstrated moderate risk. In addition, UAs was significantly correlated with several work-related risk factors (Buhl et al., 2017).
Additionally, whole blood or serum As were reported as a biomarker of exposure in several countries including Bolivia, Brazil, and Mexico. Cord blood (n=240) along with maternal blood (n=419) were measured in a Mining community at Oruro, Bolivia. Modest percentages of maternal (17.9%) and cord blood (34.6%) demonstrated blood As (BAs) above the detection limit of 3.30 μg/L (Barbieri et al., 2016). Two studies conducted in Brazil compared BAs between male and female participants in populations from Metropolitan areas. Samples used in these studies were not directly exposed to known source of As such as WAs. A sample of 18–74 year-old participants (n=240) from Maringa demonstrated no significant difference in serum As between males (GM = 1.15 μg/L) and females (GM = 1.19 μg/L) although serum As was negatively correlated with age in both males (r= −0.22) and females (r = −0.30) (Rocha et al., 2016). In another study of 374 adults (18–65 years) conducted in Sao Paulo area, slightly higher exposure was reported even though the difference between BAs in males (GM= 3.5 μg/L) and females (GM= 3.7 μg/L) was non-significant (Takeda et al., 2017). BAs was also used to determine reference values and relationship with sociodemographic characteristics in another urban study in Brazil. This study used a large number of blood donors visiting Central Hemotherapic Unit of Rio Branco and found a reference value 9.87 μg/L for BAs, which was higher in smokers (10.86 μg/L) but did not differ significantly between males and females (Freire et al., 2015). BAs was also used in investigating the role of As exposure in developing anemic condition. In a study of 6–12 year-old children (n=296) in Hidalgo province of Mexico including both anemic and non-anemic children, As was higher in anemic than non-anemic children’s blood (0.041 ± 0.11 wt% vs 0.014 ± 0.05 wt%, p < 0.05) and BAs was negatively correlated with hemoglobin (r = −0.441, p < 0.01) indicating link of As contamination with anemia (Lopez-Rodriguez et al., 2017).
A few studies used both UAs and BAs. For example, two children studies in Mexico used both BAs and UAs to determine correlations of these biomarkers with WAs. In one study, 86% of children from Yucatan demonstrated detectable levels of BAs and UAs with two children exceeding safe levels of BAs of 10 μg/L. However, this study did not find significant correlations of these biomarkers with WAs (Arcega-Cabrera et al., 2017). In a separate study by the same group of researchers on 32 children, BAs above 10 μg/L was reported in 37% of the samples who were exposed to organoarsenic compounds used in the poultry (Arcega-Cabrera et al., 2018).
Two urban children studies have used UAs to assess exposure linked to air pollution and mining activities. In San Luis Potosi, Mexico, a study of 6–12 year-old schoolchildren identified moderate to high As in urine samples (mean UAs: 45.0 ± 15.0 μg/g creatinine) (Perez-Maldonado et al., 2017). A study of 328 children between the ages of 5–8 years in Montevideo, Uruguay, explored the relationships of dietary exposure with UAs and did not find household water (WAs: median 0.45 μg/L ) a major contributor to exposure (Kordas et al., 2016). Rice and meat consumption were related to higher UAs and higher %DMA (dimethylarsinic acid) in this child population (Kordas et al., 2016).
To a lesser extent, hair As has been utilized as a marker of As exposure. Hair has been used to study exposure in a community situated in close proximity to mining and smelting operations in Altiplano highlands, Bolivia. Hair samples from 123 children in Oruro non-industrial urban areas and 26 living in smelter vicinity were collected. The urban center children had much higher hair As than the smelter children (p<0.001) (Goix et al., 2011b). One study utilized hair and breast milk as well. In a multi-country study on metal exposure 21 non-smoking women from Argentina provided breast milk samples using standard mid-feed collection procedure. Mean As in breast milk in this small sample was 4.51 μg/L with the minimum and maximum reported to be 2.54 and 9.08 μg/L respectively (Klein et al., 2017). This mean was higher than a sample drawn from the US (p<0.001) but lower than Namibian women (p=0.02) (Klein et al., 2017).
3.2. As methylation capacity
Methylation of As is considered as the major detoxification pathway as it transforms iAs into monomethylarsonic acid (MMA) and then to less toxic DMA. Interests regarding methylation capacity in As-exposed individuals has grown considerably among the LA public health researchers in Argentina, Chile, Colombia and Mexico (Table 1). A study of adults and children living in Atacama desert with WAs exposure up to 1250 μg/L in Chile characterized As species in urine and found 93% of the As as methylated species with very low (0.06) reported primary [iAs/methylated As] and secondary methylation [MMA/DMA] indexes indicating a high biological converting capability (Yanez et al., 2015). Two studies that collected cord serum and urine samples from participants of Biomarkers of Exposure to ARsenic (BEAR) pregnancy cohort in Gomez Palacio, Mexico, demonstrated significant associations of cord serum metabolites with maternal total urinary iAs and/or iAs metabolites in regression models (Laine et al., 2017; Laine et al., 2015). A total of 17 cord serum metabolites, many of which are important indicators of biochemical pathways (e.g. vitamin and amino acid metabolisms and the citric acid (TCA) cycle), were found to be associated with total iAs and/or iAs metabolites in neonates cord serum. Therefore, these data have highlighted utility of important metabolites as effect biomarkers of in utero As exposure.
Table 1:
Studies from Latin American countries describing methylation and metabolism of arsenic
| Reference | Country | Design | Characteristics of subjects | Results/ Main Findings |
|---|---|---|---|---|
| Gomez-Rubio et al. (2011) | Mexico | Cross-sectional | 624 adult women exposed to As in drinking water | Higher BMI, AS3MT genetic variant 7388, and higher total UAs were significantly associated with low %uMMA or high uDMA/uMMA. AS3MT genetic variant M287T was associated with high %uMMA and low uDMA/uMMA |
| Gomez-Rubio et al. (2012) | Mexico | Cross-sectional | 240 men and 506 women | Adjusted multiple regression model showed higher indigenous Americans (AME) ancestry associated with lower urinary %MMA excretion (p <0.01). Negative association between BMI and urinary %MMA in women was stronger than men (p <0.01) |
| Laine et al. (2015) | Mexico | Cross-sectional | Pregnant women (n=200) from the Biomarkers of Exposure to ARsenic (BEAR) prospective pregnancy cohort in Gómez Palacio, Mexico | Drinking water iAs showed significant association with the sum of the urinary arsenicals. Maternal urinary %MMA showed significant negative associations with newborn birth weight and gestational age. Maternal urinary iAs was negatively and significantly associated with mean gestational age and newborn length |
| Yanez et al. (2015) | Chile | Cross-sectional | 22 children and 27 adults | In urine, 93% of the As was found as MMA(V) and DMA(V). As(V) represent <1% of the total UAs. Primary and secondary methylation indexes were 0.06 |
| Alegria-Torres et al. (2016) | Mexico | Cross-sectional | 84 children | UAs and the mean percentage of methylated cytosines in Alu sequences were positively correlated (Spearman r= 0.53, P < 0.001), and a trend of LINE-1 hypomethylation was found (Spearman r=−0.232, p=0.038) after adjustment. |
| López-Carrillo et al. (2016) | Mexico | Cross-sectional | 1027 healthy Mexican women of at least 20 years | After adjusting for covariates, methionine, choline, folate, vitamin B12, Zn, Se and vitamin C were found to favor elimination of iAs by decreasing the %MMA and/or increasing %DMA in urine |
| Torres-Sánchez et al. (2016) | Mexico | Cross-sectional | 591 children | Total As was negatively associated with PMI (β= −0.039; p=0.18) and SMI (β= −0.08; p=0.002) with significant sex differences; PMI reduction was significant in boys (β= −0.09; p=0.02 p for interaction=0.06. SMI reduction was significantly more in girls |
| Xu et al. (2016) | Mexico | Cross-sectional | 520 females and 252 males | Referent genotypes were associated with significant increases in the DMAs% and DMAs/MMAs, and significant reductions in MMAs% and iAs%. For 3 variants, associations between genotypes and iAs metabolism were significantly stronger among subjects exposed to water As >50 μg/L vs <50 μg/L (P < .05) |
| Ameer et al. (2017) | Argentina | Cross-sectional | 80 women | UAs concentrations were associated with decreased gene expression and hypermethylation. 64% of the top 1000 differentially expressed genes were down regulated and 87% of the top 1000 CpGs was hypermethylated with UAs increase |
| Gamboa-Loira et al. (2017) | Mexico | Case-control | 1016 breast cancer cases and 1028 age matched controls | A significant interaction (p = 0.002) was observed between MTR c.2756A > G polymorphism and %DMA on BC; AG + GG carriers had lower BC risk related with %DMA than AA carriers |
| Kordas et al. (2017) | Mexico | Double-blind randomized trial | 602 children in four treatment group: ferrous fumarate, zinc oxide, iron and zinc together or placebo | Children in the highest tertile of serum ferritin concentration at baseline had higher DMA excretion: 1.93 ± 0.86%; P < .05, but lower MMA excretion: −0.91 ± 0.39%; P < .05, than children in the lowest tertile. In an intention-to-treat analysis, children receiving zinc had lower %DMA in urine (−1.7 ± 0.8; P < .05) |
| Laine et al. (2017) | Mexico | Cross-sectional | Cord serum samples collected from participants from the Biomarkers of Exposure to ARsenic (BEAR) pregnancy cohort. | Ten cord serum metabolites were identified to be significantly related to U-%iAs, U-%MMAs and U-%DMAs (p ≤ 0.05, q < 0.2) |
| Gamboa-Loira et al. (2018) | Mexico | Cross-sectional | 1027 women exposed to iAs | Significant interactions for iAs metabolism were found with FOLH1 c.223 T > C polymorphism and vitamin B12 intake; CT and CC genotype carriers had significantly lower %iAs, and higher DMA/iAs with increased vitamin B12 intake than carriers of wild-type TT |
| Gonzalez-Martínez et al. (2018) | Columbia | Cross-sectional | 101 adults | Groundwater As was correlated with total UAs (r = 0.59; p < 0.001). Urinary %iAs was associated with GSTP1, LADD, GSTP1*Age, GSTP1*alcohol consumption (r2 = 0.43; likelihood-ratio test, p <0.001). GSTP1 (AG + GG) homozygotes/heterozygotes could increase urinary %iAs and decrease the PMI ratio in people exposed to As from drinking groundwater |
| Quiller et al. (2018) | Mexico | Cross-sectional | 1,027 women participating in a study investigating As exposure and breast cancer risk from 2007–2009 | Positive associations were found between DMA and flava-3-ols (β= 0.0112), flavones (β= 0.0144), as well as apigenin (β= 0.0115), luteolin (β= 0.0138), and eriodictyol (β= 0.0026) |
| De Loma et al. (2018) | Bolivia | Cross-sectional | 201 indigenous women | UAs ranged from 12– 407 μg/L. Efficient As metabolism was observed with low % uMMA (median 7.7%) and high % uDMA (median 80%). Uru community women had significantly lower UAs and lower % uMMA and higher % uDMA than Aymara-Quecha community women. |
In addition to simply measuring As metabolism, several studies from LA investigated roles of gene expression or genetic polymorphisms on As-methylation. For instance, within the broad range of UAs exposure (10–1251 μg/L), the expression of six genes involved in the methylation pathway and six individual CpG sites were significantly associated with increased UAs concentration in a women study (n=80) in Andes, Argentina (Ameer et al., 2017). On the other hand, a study conducted on Northern Mexican women (n=1027) examined the associations between polymorphisms in five one-carbon metabolism genes (FOLH1 c.223 T > C, MTHFD1 c.1958 G > A, MTHFR c.665 C > T, MTR c.2756 A > G, and MTRR c.66 A > G), nutrient intake and iAs methylation capacity (Gamboa-Loira et al., 2018). Significant interactions of iAs metabolism were only found with FOLH1 c.223 T > C polymorphism and vitamin B12 intake, so that CT and CC genotype carriers had significantly lower %iAs, and higher DMA/iAs (Gamboa-Loira et al., 2018).
Furthermore, a study from Colombia specifically looked at the polymorphic variants of glutathione-S-transferase and metallothioneins among 101 As-exposed adults in a wide age range (i.e. 18–75 years) to investigate influence of sociodemographic covariates on metabolism (Gonzalez-Martinez et al., 2018). The study revealed urinary %iAs to be associated with genes such as GSTP1 and LADD. Furthermore, interaction of GSTP1 gene with higher age and alcohol consumption demonstrated associations with %iAs. Primary Methylation Index (PMI) was associated with sex (r2 = 0.20; likelihood-ratio test, p = 0.007). GSTP1 (AG + GG) homozygotes/heterozygotes were associated with increased %iAs and reduced PMI ratio (Gonzalez-Martinez et al., 2018). In the same study, males appeared to have higher As methylation capacity than the females, whereas higher indigenous Americans (AME) ancestry was associated with lower urinary %MMA (p <0.01) in a subset of the participants from Sonora, Mexico. Another study in Colombia also demonstrated a significant positive association of BMI with urinary %MMA, although unlike the Sonora population, the association was stronger in women than men (p <0.01) (Gomez-Rubio et al., 2012). In a study of 591 children between the age of 6 and 8 years in Coahuila, North Central Mexico, primary (PMI: MMA/iAs) and secondary (SMI: DMA/MMA) methylation capacity indexes were assessed. Total As was associated with SMI (β= −0.08; p=0.002) and significantly associated in boys (β= −0.09; p=0.02) but not in girls (Torres-Sanchez et al., 2016). A study of elementary school children in Chihuahua, Mexico examined 3 variants of AS3MT and revealed that associations between genotypes and iAs metabolism were significantly stronger among subjects exposed to WAs >50 versus ≤50 μg/L (WAs X genotype interaction P < 0.05). In contrast, for 1 variant (rs17881215), associations were significantly stronger at exposures >50 μg/L (Xu et al., 2016). Methylation status of Alu and long interspersed nucleotide elements (LINE-1) were also assessed in Mexican children who were living in historic mining areas (Alegria-Torres et al., 2016). Associations of log-normalized UAs with Alu (β = 1.05, 95% CI: 0.67, 1.43) as well as with LINE-1 (β=−0.703, 95% CI: −1.36, −0.38) were reported providing evidence of subtle epigenetic imbalance measured as DNA methylation although no significant difference in As methylation pattern by sex was observed (Alegria-Torres et al., 2016).
The effects of nutritional parameters on As methylation were examined in multiple Mexico studies. In a study of Mexico and US adult participants, higher BMI, AS3MT genetic variant 7388, and higher UAs were significantly associated with urinary %MMA or high ratio between urinary DMA/MMA, whereas AS3MT genetic variant M287T was associated with high %MMA and low DMA/MMA in urine (Gomez-Rubio et al., 2011). In a double-blind randomized trial of 602 children in four treatment groups with UAs ranged from 3.2 to 215.9 μg/L, no effects of zinc or iron on methylation efficiency were detected (Kordas et al., 2017). In a cross-sectional study of 1027 healthy Mexican women aged over 20 years, the authors identified several other dietary factors including methionine, choline, folate, vitamin B12, Zn, Se and vitamin C demonstrated elimination of iAs mainly by reducing %MMA and/or increasing %DMA in urine (Lopez-Carrillo et al., 2016). Several flavonoids were also associated with increased methylation capacity as flava-3-ols (β= 0.0112) and flavones (β= 0.0144), as well as the individual intake of apigenin (β= 0.0115), luteolin (β= 0.0138), and eriodictyol (β= 0.0026) demonstrated positive association with urinary DMA (Quiller et al., 2018). The role of ethnicity in As methylation capacity was also studied on 201 indigenous women from Uru and Aymara-Quechua communities living near Lake Poopó in Bolivia. The UAs ranged from 12 to 407 μg/L while WAs varied from 3.3 to 571 μg/L (De Loma et al., 2019). The Aymara-Quecha women showed significantly higher UAs as well as urinary %MMA and lower %DMA than the Uru women indicating better arsenic metabolism efficiency in Uru women (De Loma et al., 2019).
Overall, several studies found associations between As exposure and altered expression of As methylation related genes. Genetic variants of AS3MT and glutathione-S-transferase were studied which revealed significant associations with As methylation. However, the findings on the role of sex in As methylation capacity have been inconsistent in LA. Some studies also found BMI to be positively associated with iAs metabolites.
3.3. Genetic susceptibility
Three studies in Chile and two in Mexico investigated how genetic variations could lead to As-induced health outcomes (Table 2). For instance, a study of 1016 breast cancer cases and 1028 controls from Northern Mexico reported significant interaction (p = 0.002) between MTR c.2756A > G polymorphism and %DMA on breast cancer in addition to an observation of lower risk related with %DMA in AG + GG carriers compared to AA carriers (Gamboa-Loira et al., 2017). Genetic polymorphisms in AS3MT were also examined with respect to other forms of cancers in a case-control study (n=722) in Camarones, Atacama Desert, Chile. This study identified individuals carrying minor alleles in AS3MT rs3740393, with lower risk for bladder (OR=0.3; 95% CI: 0.1, 0.6) and lung cancer (OR=0.6; 95% CI: 0.2, 1.1) when compared with the wild types (de la Rosa et al., 2017). In a study of 142 adults from both Northern and Southern regions of Chile, the authors conducted haplotype estimation for four protective variants of AS3MT genes and subsequently identified the combination of protective variants of CTTA to be more frequent in As-exposed Camarones (68%) and Azapa (48%) participants when compared with a sample from San Juan de la Costa (8%) (Apata et al., 2017). The relationship between AS3MT polymorphism and diabetes was investigated in a sample of 255 adults and children using six polymorphic sites of this gene (Drobna et al., 2013). Individuals with M287T and G4965C polymorphisms demonstrated more frequent diabetes and higher urinary DMAsIII compared to the wild–type with ORs of 11.4 (95% CI: 2.2, 58.8) and 8.8 (95% CI: 1.6, 47.3) respectively (Drobna et al., 2013).
Table 2:
Studies from Latin American countries related to arsenic induced genotoxicity, genetic susceptibility and risk
| Reference | Country | Design | Characteristics of subjects | Results/ Main Findings |
|---|---|---|---|---|
| Goix et al. (2011) | Bolivia | Cross-sectional | 123 hair samples were collected from children living in non-industrial urban areas and 26 living in smelter vicinity | Higher Z-scores were seen for As in both children’s hair and aerosols in the smelter district. Significant differences (p<0.001) in As in children’s hair were observed between the smelter and downtown area. |
| Rocha-Amador et al. (2011) | Mexico | Cross-sectional | 20 children from low As region and 20 children from high As region | None of the water samples from the low As region exceeded the Mexican guidelines. Urinary As, F level and apoptosis level in children from high As region were significantly higher |
| Bailey et al. (2013) | Mexico | Cross-sectional | 16 adults exposed to As | Total UAs had 5.3%– 21.4% iAs, 10.3%–28.9% MMAs, and 49.3%– 84.9% DMAs. 8 subjects had positive indicators of diabetes (HbA1c ≥ 6.5%) and 7 had positive indicators of pre-diabetes (HbA1c = 5.7%–6.4%). Methylation patterns of genes associated with diabetes mellitus were associated with urinary concentrations of iAs |
| Drobna et al. (2013) | Mexico | Cross-sectional | 255 individuals | Subjects with M287T and G4965C polymorphisms had higher levels of urinary DMA and were more often diabetic than the respective wild-type carriers, (not statistically significant). OR=11.4 (95% CI: 2.2, 58.8) and 8.8 (95% CI 1.6, 47.3) for the combined effects of As exposure in >75th percentile for 287T and 4965C genotypes, respectively |
| Gamino-Gutierrez et al. (2013) | Mexico | Cross-sectional | 98 children living in the study area for at least two years and 42 children from a non exposure site | Exposed children had a higher level of UAs (mean 40.3 μg/g Cr) than the control children (mean 16.3 μg/g Cr). Micronucleated exfoliated cells (MEC) assay confirmed genotoxic damage in As exposed children (Mean MEC frequency/1000 cells: 2.2 vs 1.06) |
| Bailey et al. (2014) | Mexico | Cross-sectional | 50 newborns and their mothers | Proteins (N=111) in newborn cord blood were significantly associated with maternal U-tAs. 30 “activator” newborns had a positive association between protein expression and maternal U-tAs while 20 “repressor” newborns showed a negative relationship between protein expression level and maternal U-tAs |
| Estrada-Capetillo et al. (2014) | Mexico | Cross-sectional | 72 children chronically exposed to As (154.2 g/L) and F (5.3 mg/L) from drinking water and from food cooked with the same water. | UAs concentrations were positively correlated with the UF concentrations (r2 = 0.413, p < 0.0001). The CD25 gene expression levels and UAs and UF concentrations were negatively correlated. CD40 expression levels were negatively correlated with UAs concentration. |
| Hernandez et al. (2014) | Chile | Cross-sectional | 207 Chilean men working in the copper industry | MN frequencies showed poor correlation with total UAs. MN values were significantly higher for those carrying the variant allele of AS3MT Met287Thr genotypes (OR=3.4, 95% CI: 1.6, 5.2) |
| Navoni et al. (2014) | Argentina | Cross-sectional | 650 individuals | 68% of the study locations had a Hazard Quotient of more than 1, and CR between 5·10−5 and 2·10−2. 0–14 and 0–40 year old groups had 1.39 and 1.53 folds increase respectively in the relative risk of cancer |
| Rager et al. (2014) | Mexico | Cross-sectional | A subset of 40 newborns from the Biomarkers of Exposure to ARsenic (BEAR) pregnancy cohort | Total UAs was associated with 12 miRNAs with increasing expression |
| Zamoiski et al. (2014) | Mexico | Cross-sectional | 512 adolescents with close residential proximity to a lead smelter | Urinary As was positively associated with serum 1,25(OH)2D (β=3.4) (95% CI: 0.9, 5.9) |
| Gaxiola-Roble et al. (2015) | Mexico | Cross-sectional | 108 Mexican women were recruited but 52 breast milk samples were analyzed | Glutathione peroxidase and glutathione reductase activities were significantly (p<0.01) associated with purine nucleotide phosphorylase activity in the adjusted model |
| Rojas et al. (2015) | Mexico | Cross-sectional | A subset of 38 mother-newborn pairs exposed to varying levels of As from the larger BEAR cohort | CpG islands within the first exon, the 5′UTR and 200 bp upstream of the Transcription Start Site showed DNA methylation changes most predictive of As related changes in gene expression. 16 genes correlated with iAs-associated changes in DNA methylation and mRNA expression were recognized; 7 of which showed DNA methylation levels associated with differences in birth outcomes |
| McEwen et al. (2016) | Bolivia | Environmental sample analysis | 49 samples from Potosí and 5 from Sucre. | Majority of the sampled households contained bioaccessible As concentrations. Moderate but positive correlation was observed between adobe brick and dirt floor samples for total As (rho = 0.572) |
| Apata et al. (2017) | Chile | Cross-sectional | 142 adults from three study areas. | Camarones had a higher frequency of the protective gene variants than other study areas. Combination of protective variants of CTTA was more frequent in Camarones (68%) and Azapa (48%) than San Juan de la Costa (8%). The C-variant related to toxicity risks in the SNP Met287Thr had a lower frequency in Camarones (1%) than the others |
| de la Rosa et al. (2017) | Chile | Cross-sectional analysis using case-control study data | 722 adults recruited for As cancer case control study | Subjects with minor alleles in AS3MT rs3740393 had lower %MMA (mean difference = −1.9%, 95% CI: −3.3, −0.4), higher %DMA (mean difference = 4.0%, 95% CI: 1.5, 6.5), and lower odds ratios for bladder (OR=0.3; 95% CI: 0.1,0.6) and lung cancer (OR=0.6; 95% CI: 0.2,1.1) than wild-type carriers |
| Garrido et al. (2017) | Bolivia | Environmental sample analysis | Not applicable | Agricultural soils had a total As concentration that exceeded agricultural soil guidelines by 22-fold. Potato tubers in mining-impacted sites had maximum As concentrations that exceeded the limits in commercially sold vegetables by 9-fold. Using conservative assumptions, HQ for potatoes were elevated for As for children (HQ range 9.1–71.8) as well as for adults (HQ range 7.1–34.2) |
| Gonzalez-Cortes et al. (2017) | Mexico | Cross-sectional | 50 children exposed to As from tap water | As levels were positively associated with DNA methylation of ECM remodeling genes. High exposed showed hypermethylation in MMP9 promoter region. Positive associations between MMP9 DNA methylation with toenail As concentrations, RAGE DNA methylation with both iAs and %MMA, and TIMP1 DNA methylation with the first As methylation was found |
| Jiménez-Villarreal et al. (2017) | Mexico | Cross-sectional | 76 As-exposed individuals and 112 controls. | The exposed population had low nutritional consumption than the control group (P < 0.05). The frequency of double strand break fragmentation was significantly higher in the exposed population that the control group (p<0.05) |
| Nunes et al. (2017) | Ecuador | Environmental sample analysis | 26 rice plant (with grain) samples were collected from rice paddies | Estimated daily intake (EDI) of As for urban infants of Ecuador was about four times higher than those of Europe. EDI for the whole population was nearly twice that of Europe, but between a half and a third of that of Brazil, Bangladesh, and India. Estimated excess lifetime risk for adults is 3 per 10,000; for infants, it varies between 10 per 10,000 in rural areas and 20 per 10,000 in urban areas |
| Pavilonis et al. (2017) | Bolivia | Environmental sample analysis | 74 soil measurements were collected at mine processing sites Yani (n=35), Dorado (n=23), and Ingenio (n=16) | Non-cancerous health effect risks were primarily influenced by exposure to As. There was an increased probability of individuals developing cancer on exposure, with adult miners having a probability of 1.3 out of 100 |
| Smeester et al. (2017) | Mexico | Cross-sectional | 40 children | Enrichment of Tumor Necrosis Factor (TNF)-regulated immune and inflammatory response proteins that showed decreased expression levels with increased U-tAs. The strongest response was observed in relation to the monomethylated arsenicals |
| Beck et al. (2018) | Mexico | Cross-sectional | 109 healthy residents | Six circulating miRNAs (miRs-423-5p, -142-5p −2, -423-5p +1, -320c-1, -320c-2, and -454-5p) were significantly correlated with plasma monomethyl-As (MMA). miRs-423-5p, -454-5p were previously linked to cardiovascular disease and diabetes |
| Kordas et al. (2018) | Uruguay | Cross-sectional | 143 children | Log-transformed U-As showed positive association with 8-OHdG (β=10.90 [95% CI: 3.82, 17.97]) after accounting for Cd and Pb exposure. In regression models, a mixture index (for all metals including As) showed association with higher 8-OHdG (β=8.71 [95% CI: 1.12, 16.3] for each 25% increase in index value, primarily driven by As exposure |
| Villarreal et al. (2019) | Mexico | Cross-sectional | 76 exposed males (WAs above 10 μg/L) and 112 control males | The exposed group showed an increase in telomere length with a T/S ratio = 0.74 ± 0.07 while the control group had = 0.54 ± 0.01. A linear relationship was seen between U-As and the telomere length in the exposed group (r2 = 0.47; P = 0.039), but not in the control (r2 = 0.09; P = 0.062) |
In high As-exposed and most frequently studied Antofagasta population, the authors examined a group of men working in the copper industry and reported significantly higher risk of cytogenetic damage (i.e. higher micronucleus frequency) for those carrying the variant allele of AS3MT Met287Thr genotypes (OR=3.4, 95% CI: 1.6, 5.2) (Hernandez et al., 2014). Overall, a small number of LA studies have presented the evidence of associations between genetic variants of AS3MT and several health outcomes including cancers. The limited number of studies investigating the influence of AS3MT genetic variants on cancer, diabetes as well as cytotoxicity indicate the need for further studies to better understand the role of genetic polymorphisms and As-induced health outcomes.
3.4. Genotoxicity, cytotoxicity and other biochemical effects
A number of studies in Mexico and one study in Uruguay investigated cellular, molecular and genetic alterations produced by As exposure via cross-sectional studies (Table 2). These changes observed in both adult and child studies presented evidences of As-induced DNA methylation, altered gene expressions, changes in micro RNA levels, altered protein levels and enzyme activities, apoptosis, DNA damage such as double strand break and change of the length of telomere, and modifications of bacterial flora and vitamin metabolism.
As-induced epigenomic changes were observed in multiple studies. A pilot study in 16 adults with either diabetes or pre-diabetes mellitus assessed relationships of UAs with genome-wide, gene-specific promoter (Bailey et al., 2013). The study identified 88.6% of 556 genes decreasing promoter DNA methylation levels with increasing urinary concentrations of MMAs (Bailey et al., 2013). The patterns of methylation in genes with known associations with diabetes mellitus were associated with urinary concentrations of MMAs and other As metabolites. A study of 6–12 year-old children from North Central Mexico found positive effect of As on DNA methylation of extracellular matrix remodeling genes, which could eventually be involved in biological processes associated with lung diseases (Gonzalez-Cortes et al., 2017). This study reported hypermethylation in matrix metalloproteinase-9 (MMP-9) promotor region in high As-exposed children. Additionally, positive associations of MMP-9 DNA methylation with toenail As, glycation end products (RAGE) DNA methylation with iAs as well as %DMA, and also positive association between tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) DNA methylation and primary As methylation were found (Gonzalez-Cortes et al., 2017). Cord blood samples (n=38) obtained from mother-newborn pairs of BEAR cohort participants in Gomez Palacio, Mexico, examined changes in DNA 5-methylcytosine methylation and detected 16 genes correlated with iAs-related changes in DNA methylation and mRNA expression (Rojas et al., 2015). Moreover, DNA methylation levels of 7 genes were associated differences in birth outcomes such as gestational age and head circumference (Rojas et al., 2015).
Genotoxicity of As was investigated in several cross-sectional studies. One study used micronucleated exfoliated cells (MEC) assay in mining community children between the ages of 4 to 10 years via a cross-sectional study. As-exposed children (mean UAs 40.3 μg/g Cr) demonstrated significantly higher MEC frequency per 1000 cells (2.20 vs 1.06) than the control (UAs 16.3 μg/g Cr) children in this study (Gamino-Gutierrez et al., 2013). Another study examining the children between the age of 6–12 years, who were chronically exposed to high levels of both As and fluoride in municipality area, showed alteration of expression patterns of CD25 and CD40 genes, an indication of reduced immune responses (Estrada-Capetillo et al., 2014). Effect of As exposure on the telomere length was studied among As-exposed male participants (n=76) who had WAs exposure above 10 μg/L with the controls who had low As exposure below 10 μg/L (n=112) living in Coahuila (Villarreal et al., 2019). The exposed group had a higher T/S ratio of 0.74 than the control group (0.54) with a reported linear relationship between UAs and the telomere length only in the exposed group (p = 0.04) (Villarreal et al., 2019). In the same area, another study found frequency of double strand break (DSB) fragmentation to be significantly higher in the population exposed to higher levels of WAs (mean 14.3 μg/L) compared to that of the control group (mean WAs 7.7 μg/L) (p<0.05) (Jimenez-Villarreal et al., 2017).
Alterations of microRNA (miRNA), proteins, and enzymes due to As exposure were investigated in several studies. In a subset (n=40) of newborn from the Biomarkers of Exposure to ARsenic (BEAR) pregnancy cohort, genome-wide miRNA expression in cord blood samples showed positive associations between total UAs and increased 12 miRNAs expressions (Rager et al., 2014). Additionally, UAs was associated with decreased expression levels of immune response-related mRNAs and this relationship was predicted to be mediated, in part, by the As-responsive miRNAs (Rager et al., 2014). This mediation effect highlighted miRNAs’ critical effects on regulating gene expression induced by As. An adult study involving residents of Zimapan and Legunera. (n=109) demonstrated significant correlations of plasma MMA with six circulating miRNAs including two linked to cardiovascular disease and diabetes (i.e. miRs-423–5p, −454–5p) although plasma iAs or DMA did not show any association (Beck et al., 2018). A study of 6–12 year old children in North Central Mexico indicated associations of total UAs and monomethylated arsenicals with decreased expression levels of Tumor Necrosis Factor-(TNF)-regulated immune and inflammatory response proteins (Smeester et al., 2017). Furthermore, proteomic shift associated with As was investigated in a study of 50 newborn and their mothers who provided urine samples in Gomez Palacio (Bailey et al., 2014). Significant associations between 111 protein levels in cord blood, including many regulated by TNF and related to immune and inflammatory response and maternal total UAs were reported. Additionally, inter-individual differences in proteomic response were found in this sample (Bailey et al., 2014). Breast milk samples from 52 Mexican women suggested enhanced Purine Nucleotide Phosphorylase (PNP) activity in breast milk associated with As exposure (p<0.01), presenting an evidence of possible detoxification mechanism (Gaxiola-Robles et al., 2015).
Oxidative stress, apoptosis, and markers of vitamin D metabolism were the three important physiological changes examined in children and adolescents of Uruguay and Mexico. In Montevideo, Uruguay, urine samples collected from 143 child participants between the age of 6–8 years exposed to low As revealed a positive association between log-transformed UAs and 8-hydroxy-2-deoxy-guanosine (8-OHdG) (β=10.90, 95% CI: 3.82, 17.97) (Kordas et al., 2018). Induction of apoptosis due to As and fluoride exposure was investigated in 40 children with equal numbers recruited from a high and a low As areas (Rocha-Amador et al., 2011a). A higher level of apoptosis was found in children from high As-exposed community than those from the low exposure group (Rocha-Amador et al., 2011a). Associations of As with two circulating vitamin D metabolites, [25(OH)D and 1,25(OH)2D] were examined in 512 adolescents living in close proximity to a smelter (Zamoiski et al., 2014). The study detected 1,25(OH)2D to be positively associated with UAs (β=3.4, 95% CI: 0.9, 5.9), even after accounting for serum 25(OH)D (Zamoiski et al., 2014). Even though this finding did not indicate a negative effect of As on vitamin D metabolites, further studies are necessary to comprehensively understand the underlying mechanisms. A possible reason maybe that As exposure induced renal proximal tubule damage, which increased urinary excretion that lead to increased 1,25(OH)2D concentration.
3.5. Risk assessment
Risk assessment using environmental, biomarker and dietary samples is an emerging area of research in LA region. In recent years, several studies have estimated health risks for populations in Argentina, Bolivia, and Ecuador (Table 2). In Chaco and Santiago del Estero provinces of Argentina, spatial analytical techniques were used to estimate average daily dose (ADD), hazard quotient (HQ), and carcinogenic risk (CR) for a wide age range of individuals (1 to 96 years) exposed to drinking WAs up to a concentration of 2000 μg/L (Navoni et al., 2014). Data from this study found similar health risks for various exposed groups of individuals such as 0–14 and 0–40. In 68% of the study area, a HQ of > 1, and CR between 5×10(−5) and 2.1×10(−2) was found. They also observed an increase in the relative risk of cancer by 1.39 fold in the 0–14 age group and by 1.53 fold in the 0–40 age group. (Navoni et al., 2014). In Bolivia, As exposure in mining community is a pressing public health issue as evident in two children studies. Hair As was used as a biomarker of As-exposed children living in mining and smelting communities in Bolivia (Goix et al., 2011a). Using 123 and 26 hair samples collected from children from non-industrial urban and smelter areas respectively, the authors found higher risk of exposure for smelter community children indicated by higher Z score (p<0.001) (Goix et al., 2011a). In Potosi, another study sampled adobe brick, dirt floor, and dust from mining and control sites showing positive correlations between adobe brick and dirt floor samples for total As (r = 0.57) (McEwen et al., 2016). Results also suggested an ingestion rate at or above 200 mg/day of combined soil-dust particles containing As in 1 and 6 year old children to be associated with potential health risk in mining communities (McEwen et al., 2016).
Soil samples (n=74) were assessed for As content from three mine processing sites in Bolivia and subsequently estimated probability of cancer development among adult miners to be 1.3 out of 100, whereas non-cancerous health effects associated with soil As were also predicted (Pavilonis et al., 2017). Another Bolivian mining community study used soil, irrigated water and potato crops and estimated high HQ for children (HQ range 9.1–71.8) as well as for adults (HQ range 7.1–34.2) (Garrido et al., 2017). In this study As in soil exceeded the agricultural guideline by 22-fold (Garrido et al., 2017). In Quito, Ecuador, rice plant samples were used to compute estimated daily intake (EDI) for infants and adults in rural and urban areas (Nunes and Otero, 2017). EDI for the whole Ecuadorian population was reported to be two times higher than Europe, but between a half and a third of Brazil, Bangladesh, and India. Estimated excess lifetime risk (ELTR) for infants in urban areas (20/10,000) was higher than rural infants (10/10,000) and adults (3/10,000) (Nunes and Otero, 2017).
4. Health effects of arsenic exposure in Latin America
Numerous studies from LA countries continue to demonstrate As to be an important risk factor for many health outcomes. Several studies published since 2012, showed that health effects of As could be related to low doses of As. More importantly, these studies found increased risk of cancer even after As dose reduced to a significant level.
4.1. Bladder cancer
Majority of the studies presenting risk of bladder cancer as a result of chronic As exposure in LA were conducted in As endemic areas in of Chile (Table 3). Evidence of bladder cancer mortality were reported in three ecological studies from Antofagasta, Chile. These studies clearly demonstrate early life exposure, in particular in utero is an important window of susceptibility for bladder cancer. Additionally, bladder cancer related genes were found to be associated with As methylation capacity.
Table 3:
Studies from Latin American countries showing association between arsenic exposure and bladder and lung cancer
| Reference | Country | Design | Characteristics of subjects | Results/ Main Findings |
|---|---|---|---|---|
| Smith et al. (2012) | Chile | Ecological | Mortality data for adults between 30–49 years who were in utero or ≤18 years of age during the high WAs exposure between 1989–2000 | Increased SMRs for bladder cancer was observed for exposures starting at all ages, but the highest SMR was for exposures beginning at birth [SMR = 18.1 (95% CI: 11.3, 27.4)] |
| Ferreccio et al. (2013b) | Northern Chile | Case-control | 232 bladder cancer and 306 lung cancer cases and 640 controls | Very high bladder and lung cancer ORs and greater than additive effects were observed in tobacco smokers exposed to As concentrations >335 μg/L. [bladder cancer OR = 23 (95% CI: 8.2, 66), Synergy Index = 2.0 (0.92–4.5); lung cancer OR = 16, 95% CI: 6.5, 40; Synergy Index = 4.0 (1.7–9.4)] |
| Steinmaus et al. (2013) | Northern Chile | Case-control | 232 bladder cancer and 306 lung cancer cases and 640 age and gender matched controls | Bladder cancer ORs for quartiles of average water As before 1971: <11, 11–90, 91–335, and >335 μg/L were 1.00, 1.36 (95%CI: 0.78,2.37), 3.87 (2.25,6.64), and 6.50 (3.69,11.43), respectively and lung cancer ORs were 1.00, 1.27 (95% CI: 0.81,1.98), 2.00 (1.24,3.24), and 4.32 (2.60,7.17) respectively |
| Melak et al. (2014) | Northern Chile | Case-control | 117 bladder cancer and 94 lung cancer cases and 347 controls | For increasing tertiles of %MMA, bladder cancer ORs were 1.00, 1.81 (95% CI: 1.06,3.11), and 2.02 (95% CI: 1.15, 3.54) (p trend <0.001) and lung cancer ORs were 1.00, 1.91 (95% CI: 0.99, 3.67), and 3.26 (1.76,6.04) (p-trend <0.001) |
| Steinmaus et al. (2014a) | Northern Chile | Case-control | 160 bladder cancer and 221 lung cancer cases and 508 age and gender matched controls | Adjusted bladder cancer ORs for those with only early life exposure to water As <110, 110 to 800, and >800 μg/L were 1.00, 2.94 (95% CI: 1.29,6.70), and 8.11 (95%: 4.31, 15.25; p-trend < 0.001) respectively and lung cancer ORs were 1.00, 1.88 (95% CI: 0.96, 3.71, and 5.24 (3.05, 9.00; p for trend< 0.001) respectively |
| Steinmaus et. al (2014b) | Northern Chile | Case-control | 92 lung cancer cases and 288 controls | Lung cancer ORs= 1.00, 1.43 (90% CI: 0.82, 2.52), and 2.01 (90% CI: 1.14, 3.52) for increasing tertiles of As exposure, respectively (P for trend = 0.02). For subjects <65 years, the corresponding ORs were 1.00, 1.62 (90% CI: 0.67, 3.90), and 3.41 (90% CI: 1.51, 7.70) |
| Rager et al. (2015) | Mexico | Cross-sectional | A subset of 46 Hispanic females participating in a larger study in Chihuahua, Mexico | As exposure and specific As metabolites present in EUCs were associated with altered promoter methylation of genes involved in bladder cancer and metabolic disease. 22 of 49 As-associated genes identified were related to differential methylation in bladder cancer tissues. |
| Recio-vega et al. (2015a) | Mexico | Case-control | 34 lung cancer cases and 68 controls | MRP1 expression showed significant decrease in those with urinary As levels >50 μg/L when compared with the controls (p=0.02). Chronic As exposure was negatively correlated with MRP1 expression in bronchoalveolar lavage cells in cases. |
| Steinmaus et al. (2015) | Northern Chile | Case-control | 289 bladder and 370 lung cancer cases and 872 controls | In early adulthood, subjects with BMIs <90th percentile had OR for lung and bladder cancer combined for As concentrations of <100, 100–800 and >800 μg/L were 1.00, 1.64 (95% CI, 1.19, 2.27), and 3.12 (2.30, 4.22). In subjects with BMIs ≥90th percentile, the corresponding ORs were: 1.00, 1.84 (0.75, 4.52), and 9.37 (2.88, 30.53), respectively |
| Roh et al. (2018) | Chile | Ecological | Mortality data for all regions of Chile for the years 2001–2010 | Increased SMRs for bladder and lung cancer was observed for exposures starting at all ages, but the highest SMRs were for exposures beginning at birth (bladder cancer SMR = 16.0, 95% CI: 10.3, 23.8 and lung cancer SMR = 3.8, 95% CI: 2.9, 4.9) |
| Smith et al. (2018) | Chile | Ecological | Region II compared with all the rest of Chile from 2001 to 2010, and with unexposed Region V from 1950 to 2010 | From 2001 to 2010, comparing Region II with the rest of Chile, bladder cancer mortality RR = 4.79, 95% CI = 4.20, 5.46, p < .001 in men; RR= 6.43, 95% CI = 5.49, 7.54, p< .001 in women and lung cancer mortality RR = 3.38, (95% CI: 3.19, 3.58), p < .001 in men and RR= 2.41, (95% CI: 2.20, 2.64), p < .001 in women |
Studies in Chile discovered that timing of exposure and As metabolism are important predictors of bladder cancer mortality. One study examined importance of early life (i.e. in utero, early and late childhood) As exposure on developing bladder cancer among middle-aged adults (Smith et al., 2012). This ecological study compared mortality data for the period of 1989–2000 between As-exposed populations in Antofagasta compared to the rest of Chile. The study found a very high mortality rate in bladder cancer [standardized mortality ratio (SMRs) of 18.1 (95% CI: 11.3, 27.4)] (Smith et al., 2012). Extremely high mortality rate was observed among those born during the high As exposure period of 1958–1970 and exposed in utero, particularly for males as compared to females (65.7 vs 43.0) (Smith et al., 2012). A study from Chile showed that As remained to be a significant risk for bladder cancer mortality even after the exposure was drastically reduced in 1970. An investigation using 2000–2010 mortality data, showed death from bladder cancer to be significantly higher for region II, a larger area along with Antofagasta compared to the rest of Chile. The rate ratio (RR) were: men (4.79; 95% CI: 4.20, 5.46) and women (6.43; 95% CI: 5.49, 7.54) (Smith et al., 2018). Similar to the early findings, risk of death was much higher among those exposed to in utero. Using same dataset (i.e. 2001–2010), additional analysis found individuals exposed in utero were at higher risk [men (SMR=16.8; 95% CI: 9.8, 27.0) and women (SMR=13.6; 95% CI: 5.5, 27.9)] (Roh et al., 2018). In a case-control study conducted in Northern Chile, in utero and early life As exposure of 110–800, and >800 μg/L were associated with incidence of bladder cancer [odds ratio (OR)=2.94; 95% CI: 1.29, 6.70)], and (OR=8.11; 95% CI: 4.31, 15.25) compared with <110 μg/L As after accounting for age, sex, and smoking (Steinmaus et al., 2014a). The same group of researchers reported high risk of bladder cancer for lower As exposure groups using <11 μg/L early life As exposure as the reference group, i.e., OR= 3.87 (95% CI: 2.25, 6.64), and OR=6.50 (95% CI: 3.69, 11.43), for As concentrations of 91–335, and >335 μg/L respectively (Steinmaus et al., 2013).
Other risk factors such as active and passive tobacco smoking, asbestos, silica, and wood dust exposures as well as higher BMI (≥90th percentile) were found to increase risk of early adulthood on bladder cancer in As exposed population in two case-control studies (Ferreccio et al., 2013b; Steinmaus et al., 2015). Additionally, bladder cancer related genes were found to be associated with As methylation capacity. A case-control study in Northern Chile found significant risks of bladder cancer for subjects in the upper tertile of %MMA compared to subjects in the lower two tertiles (OR=2.37; 95% CI: 1.01, 5.57) (Melak et al., 2014). A cross-sectional study of 46 female participants over the age of 18 who were residing in Chihuahua, Mexico for at least 5 years took a step forward to assess role of As methylation on bladder cancer. The study examined promoter methylation profiles in bladder cancer-associated DNA and the intracellular concentrations of total As and As species in exfoliated urothelial cells (EUC) (Rager et al., 2015). The study revealed 49 differentially methylated genes with increased promoter methylation associated with MMA. Neither of these genes were associated with DMA, confirming a role of MMA of As associated health outcomes (Rager et al., 2015). Twenty-two of these 49 As-associated genes were known for differential methylation in bladder cancer tissues.
4.2. Lung cancer
Evidence of As-induced lung cancer in LA continued to emerge from studies conducted in Region II of Chile, where water from mining was the main source of As exposure. Studies from Chile reported higher mortality and lung cancer cases in those exposed to high as well as moderate dose of As, particularly during early life. Most of the studies were case control studies while some were ecological. Several of them had large sample sizes of around 370 but some had as low as 34 subjects. While case control and ecological studies have their own methodological challenges in establishing causation, the studies yielded consistent findings, linking As exposure with increased lung cancer risk (Table 3).
Two recent ecological studies investigated mortality of lung cancer during 2001–2010 in Antofagasta where WAs was as high as 860 μg/L during the period of 1958–1970. Lung cancer mortality was high among men (RR = 3.38, 95% CI: 3.19, 3.58; p < 0.001) as well as women (RR= 2.41, 95% CI: 2.20, 2.64; p < 0.001), even after several decades of reduction in As exposure(Smith et al., 2018). The other study examined age of first peak As exposure and found in utero As exposure had the highest risk of mortality from lung cancer (SMR=3.8; 95% CI: 2.9, 4.9) (Roh et al., 2018). Additionally, several case-control studies in the Northern part of Chile reported risks of developing lung cancer due to As exposure, even at relatively lower doses. One study found increased risk of lung cancer in moderate As exposed individuals, less than 65 year-old, in the highest tertile of As (mean exposure 58.6 μg/L) compared to the lowest tertile group (mean exposure 6.5 μg/L) (OR=3.41, 90% CI: 1.51, 7.70) (Steinmaus et al., 2014b). In an earlier study, the same group of researchers reported dose-response association for developing lung cancer risk with increasing WAs exposure (OR=2.00; 95% CI: 1.24, 3.24, and OR=4.32; 95% CI: 2.60, 7.17 for individuals exposed to 91–335 and >335 μg/L of WAs respectively) (Steinmaus et al., 2013). In utero exposure to As was found to be a critical period of susceptibility. A case control study reported an OR of 5.24 (95% CI: 3.05, 9.00) for lung cancer in those with early life exposure to very high concentration of As (>800 μg/L) (Steinmaus et al., 2014a). Cumulative effects of As and other risk factors on lung cancer were also evident in multiple studies. One study reported exposure to high As (800 μg/L) along with high BMI increased lung cancer risk (OR= 9.37, 95% CI: 2.88, 30.53) (Steinmaus et al., 2015). Another study demonstrated additive effect of As>335 μg/L and tobacco smoking on lung cancer (OR = 16.0, 95% CI: 6.5, 40.0) (Ferreccio et al., 2013b). The importance of As methylation capacity in As-induced lung cancer was demonstrated in another case-control study from Northern Chile among subjects with <200 μg/L As exposure. In this study, participants in the upper tertile of %MMA showed higher risk of lung cancer compared to subjects in the lowest tertile (OR=2.48, 95% CI: 1.08, 5.68) (Melak et al., 2014). In Mexico, a case-control study investigated whether As could influence multidrug resistance-associated protein-1 (MRP1) expression, which has a protective role against arsenic. This study demonstrated significant reduction of MRP1 expression in bronchoalveolar lavage (BAL) cells in lung cancer patients with levels of >50 μg/L when compared with the controls with similar As levels (p=0.02) (Recio-Vega et al., 2015a). This may increase intracellular toxic compounds in lung cells, increasing lung cancer risk.
4.3. Kidney, breast and laryngeal cancers
Over the last eight years, new evidence of associations of As exposure with kidney cancer have emerged. Furthermore, two new cancers (i.e. breast and laryngeal) were also reported to be associated with As exposure in LA (Table 4). An ecological study in Antofagasta, Chile reported associations of As with kidney cancer. The risk was slightly higher among women (RR=2.09, 95% CI: 1.69, 2.57) compared to men (RR = 1.75, 95% CI: 1.49, 2.05) (Smith et al., 2018). In the same geographic region, a case-control study investigated risk of specific categories of kidney cancer. The study revealed that risk of combined renal pelvis and ureter cancers were significantly high (OR=5.71, 95% CI: 1.65, 19.82) among those consuming water 400–1000 μg/day. The risk doubled at a level above 1000 μg/day (OR=11.09, 95% CI: 3.60, 34.16) (Ferreccio et al., 2013a) showing a clear dose response relationship. However, no association for renal cell cancer was reported.
Table 4:
Studies from Latin American countries showing association between arsenic exposure and kidney, breast and laryngeal cancer
| Reference | Country | Design | Characteristics of subjects | Results/ Main Findings |
|---|---|---|---|---|
| Ferreccio et al. (2013a) | Northern Chile | Case-control | 122 kidney cancer cases and 640 controls | The adjusted ORs in average As exposures of <400, 400–1,000, and >1,000 μg/day were 1.00, 5.71 (95% CI: 1.65, 19.82), and 11.09 (95% CI: 3.60, 34.16) (P-trend < 0.001), respectively for renal pelvis and ureter cancers |
| López-Carrillo et al. (2014) | Mexico | Case-control | 1016 breast cancer cases and 1028 control women | Increased risk of breast cancer (BC) was seen in women with higher %MMA and primary methylation index (PMI) at the highest pentile (%MMA OR Q5vs.Q1 = 2.63; 95% CI: 1.89, 3.6, p for trend <0.001; PMI OR Q5vs.Q1 = 1.90; 95%CI: 1.39,2.59, p for trend <0.001) |
| Smith et al. (2014) | Chile | Ecological | For 1950–1970 years, death certificates for Region II and Region V were obtained. After 1970, computerized mortality data were obtained | Breast cancer mortality rates during 1958–1970 in Region II were half of those in Region V (RR = 0.51, 95% CI 0.40, 0.66; p <0.0001). Women less than 60 years had a 70% reduction in breast cancer mortality during 1965–1970 (RR = 0.30, 0.17, 0.54; p < 0.0001) |
| Michel-Ramirez et al. (2017) | Mexico | Cross-sectional | 120 breast biopsies (76 cases and 44 control biopsies negative for malignancy) | Cases showed a significantly lower percentage of cytoplasm YAP expression. YAP was found to act as a tumor suppressor protein. Urinary %MMA was found significantly higher in the controls whereas, the secondary methylation values were greater in the cases (P < 0.05) |
| Roh et al. (2018) | Chile | Ecological | Mortality data for all regions of Chile for the years 2001–2010 | Increased SMRs for laryngeal cancer was observed for exposures starting at all ages, but the highest SMR was for exposures beginning at birth (SMR = 6.8 (95% CI: 2.2, 15.8) |
| Smith et al. (2018) | Chile | Ecological | Region II compared with all the rest of Chile from 2001 to 2010, and with unexposed Region V from 1950 to 2010 | Comparing Region II with the rest of Chile, kidney cancer mortality during 2001– 2010 was found elevated (RR = 1.75, 95% CI= 1.49, 2.05, P < .001 for men; RR = 2.09, 95% CI = 1.69, 2.57, P < .001 for women) |
In recent years, As exposure were linked with breast cancer with contradictory findings. Two studies from Mexico and one from Chile reported increased risk of breast cancer. In a cross-sectional study of 120 As-exposed residents (76 breast cancer cases and 44 controls) of Comarca Lagunera, Mexico, the expression of yes-associated protein (YAP), a tumor suppressor protein and apoptosis inhibitor were measured. Results demonstrated a significantly lower percentage of YAP expression in cases indicating abnormal expression of YAP in As-exposed breast cancer patients (Michel-Ramirez et al., 2017). Unlike bladder and lung cancer, decreased risk of breast cancer was reported from Chile. In an ecological study that compared death rates of populations of high As-exposed region II with a low exposure population in Region V following installation of As removal plants in the 1970s in different cities (Smith et al., 2014), breast cancer mortality rate in As-exposed region II was half (RR =0.51, 95% CI: 0.40, 0.66) of that in region V in 1958–1970. This study also reported a 70% reduction in this cancer mortality (RR =0.30, 95% CI: 0.17, 0.54) particularly among women under the age of 60 years (Smith et al., 2014). On the other hand, an inverse effect of As exposure on breast cancer was found among women in Mexico, particularly in relation to incomplete As methylation. In a case-control study among 1016 cases and 1028 controls from Northern Mexico, women at the highest %MMA pentile were found to be significantly at risk of developing breast cancer when compared with the lowest pentile (OR=2.63, 95%CI: 1.89, 3.66). In contrast, women at highest pentile of %DMA demonstrated a significantly reduced risk (OR=0.63, 95%CI: 0.45, 0.87) (Lopez-Carrillo et al., 2014).
While attempting to investigate the risks of multiple cancers in As-exposed populations, Roh and associates found significant relationship between early life As exposure and laryngeal cancer mortality (Roh et al., 2018). Mortality rate for laryngeal cancer was the highest for the sub-population that received high As exposure right from the birth (SMR=6.8, 95% CI: 2.2, 15.8) (Roh et al., 2018).
4.4. Other chronic health effects
Non-malignant chronic health effects of As were reported in both adults and child populations in Chile and Mexico. Major health effects examined in adults in these studies included respiratory and cardiovascular, diabetes and pregnancy outcomes (Table 5). On the other hand, children and early life studies examined respiratory, birth, nutritional, renal and neurocognitive outcomes (Table 6).
Table 5:
Studies from Latin American countries showing association between arsenic exposure and chronic diseases (cardiopulmonary health outcomes and diabetes)
| Reference | Country | Design | Characteristics of subjects | Results/ Main Findings |
|---|---|---|---|---|
| Dauphine et al. (2011) | Chile | Cross-sectional | 32 adults exposed to high As (As) (>800 μg/L before age 10) and 65 adults without high early-life exposure | Exposure to early-life As showed significant associations with 11.5% lower FEV1 (p= 0.04), 12.2% lower FVC (p = 0.04), and increased breathlessness (prevalence odds ratio (OR) = 5.94, 95% CI: 1.36, 26.0) |
| Del Razo et al. (2011) | Mexico | Cross-sectional | 147 subjects from Zimapán and 111 from Lagunera of both sexes and ≥ 5 years were recruited. | Positive association between diabetes and iAs in drinking water (OR 1.13 per 10 μg/L, p < 0.01) and with DMA concentration in urine (OR 1.24 per inter-quartile range, p = 0.05) was observed |
| Roman et al. (2011) | Chile | Cross-sectional | 215 As-exposed patients of coronary heart diseases (CHD) and 25 As-unexposed patients of CHD | Significant difference between As-exposed and unexposed patients for As concentrations in auricle and mammary artery tissues |
| Smith et al. (2011) | Chile | Ecological | Mortality data for the two regions were obtained for 1958–2000. | The peak 5-year TB mortality rate ratio (RR) in men during 1982–1986 was RR = 2.1, 95% CI: 1.7, 2.6; p < 0.001, followed by a decline. Mortality rates in women showed less excess pulmonary TB deaths |
| Burgess et al. (2013) | Mexico | Cross-sectional | 215 adult residents from Arizona, USA and 163 from Sonora, Mexico | Drinking water As concentration and intake showed positive association with MMP-9, in crude analysis and after adjustment. On multivariable analysis, urinary As sum of species showed positive association with MMP-9 |
| Diaz-Villasenor et al. (2013) | Mexico | Cross-sectional | 40 Type 2 diabetic subjects and 32 non-diabetic matched subjects | Subjects with Type 2 diabetes mellitus (T2DM) had significantly lower beta cell function and insulin sensitivity. Beta cell function was inversely associated (pronounced in T2DM subjects) with iAs exposure. This association was enhanced in the presence of SNP-43 in CAPN-10 |
| Currier et al. (2014) | Mexico | Cross-sectional | 374 adults with ≥ 5 years of uninterrupted residency in the study area | Interquartile range increase in trivalent As species in EUC had positive and significant association with diabetes, (ORs=1.57; 95% CI: 1.19, 2.07 for iAsIII, OR=1.63; 95% CI; 1.24, 2.15 for MAsIII, and OR=1.31; 95% CI: 0.96, 1.84 for DMAsIII |
| Martin et al. (2015) | Mexico | Cross-sectional | 90 diabetic and 86 nondiabetic matched individuals enrolled in Chihuahua As cohort | iAs exposed diabetic subjects had 59 altered metabolites including those related to tricarboxylic acid cycle and amino acid metabolism |
| Mendez et al. (2016) | Mexico | Cross-sectional | 1,160 adults with a minimum 5-year uninterrupted residency in the study area | Second quartile of water As (25.5 to < 47.9 μg/L) and total speciated urinary As (< 55.8 μg/L) below the median were significantly associated with elevated triglycerides, high total cholesterol, and diabetes |
| Sandoval-Carrillo et al. (2016) | Mexico | Case-control | 104 preeclamptic cases and 202 healthy pregnant women as controls | No significant difference in As exposure between cases and controls. |
| Steinmaus et al. (2016) | Chile | Cross-sectional | 204, 208, and 383 people exposed to water As <11 μg/L, 11–200 μg/L and >200 μg/L respectively | Adults born in the high exposure period had elevated OR for shortness of breath = 5.56, 90% CI: 2.68, 11.5, and decreases in pulmonary function (224 mL decrease in FVC in nonsmokers, 90% CI: 97, 351 mL) than adults never exposed to >10 μg/L |
| Hall et al. (2017) | Chile | Case-control | 665 newly diagnosed cancer cases and 640 cancer free controls | Subjects in the 60–623μg/L and >623μg/L exposure categories had adjusted hypertension ORs of 1.49 (95% CI: 1.09, 2.05) and 1.65 (95% CI: 1.18, 2.32) respectively when compared with the lowest category (<60μg/L) |
| Nardone et al. (2017) | Chile | Cross-sectional | 751 adults | Adults with BMI > 90th percentile (> 33.9 kg/m2) and water As concentrations ≥11 μg/L had ORs for cough (OR = 10.7, 95% CI: 3.03, 50.1), shortness of breath (OR = 14.2, 95% CI: 4.79, 52.4), wheeze (OR = 14.4, 95% CI: 4.80, 53.7), and any respiratory symptom (OR = 9.82, 95% CI: 4.22, 24.5) |
| Castriota et al. (2018) | Chile | Case-control | 234 diabetic patients and 819 subjects without known diabetes | Cumulative As exposure group 610–5279 and ≥ 5280 μg/L-years had OR=0.97 (95% CI: 0.66, 1.43) and 1.53 (95% CI: 1.05, 2.23) respectively for T2D. The OR for ≥ 5280 μg/L-years =1.45 (95% CI: 0.74, 2.84) in participants with BMIs < 25 kg/m2 and OR =2.64 (95% CI: 1.14, 6.11) for BMIs ≥ 30 kg/m2 (synergy index = 2.49, 95% CI: 0.87, 7.09) |
| Munoz et al. (2018) | Chile | Cross-sectional | 244 pregnant women | No significant associations were found between gestational diabetes and iAs exposure tertiles. Tertile 2:OR= 2.98, 95% CI: 0.87, 10.18, Tertile 3:OR=1.07, 95% CI: 0.26, 4.33 |
Table 6:
Studies from Latin American countries showing association between As exposure and early life health effects
| Reference | Country | Design | Characteristics of subjects | Results/ Main Findings |
|---|---|---|---|---|
| Rocha – Amador et al. (2011) | Mexico | Cross-sectional | 20 from high As exposure and 20 from low As exposure areas since birth | Apoptosis level in PBMC showed positive correlation with urinary As (r=0.40; p=0.01) and was significantly higher in children exposed to high As |
| Roy et al. (2011) | Mexico | Cross-sectional | 526 children living near a metal foundry | UAs and urinary DMA had positive associations with scores on the Oppositional, Cognitive Problems and ADHD subscales of the teacher ratings. On adjusting for the Peabody Picture Vocabulary Test scores, the associations between UAs and behavior became non-significant |
| Osorio-Yanez et al. (2013) | Mexico | Cross-sectional | 199 children | Total speciated As (tAs) categories showed positive association with increased cIMT. cIMT diameter was greater in 35 to 70ng/mL and > 70ng/mL groups (0.035 mm and 0.058 mm per 1-ng/mL increase in urinary tAs, respectively), compared with the < 35-ng/mL group |
| Laine et al. (2015) | Mexico | Cross-sectional | Pregnant women (n=200) from the Biomarkers of Exposure to ARsenic (BEAR) prospective pregnancy cohort in Gómez Palacio, Mexico | Maternal urinary %MMA showed significant negative associations with newborn birth weight and gestational age. Maternal urinary iAs was negatively and significantly associated with mean gestational age and newborn length |
| Olivas-Calderon et al. (2015) | Mexico | Cross-sectional | 275 children exposed to drinking water As between 104–360 μg/L | 58% had restrictive spirometric pattern. Two highest exposed groups had significantly lower sRAGE sputum level and higher MMP-9 concentrations. Negative associations were seen between DMA, %MMA and %DMA with sRAGE |
| Recio-Vega et al. (2015) | Mexico | Cross-sectional | 358 boys and girls exposed to water As between 104–360 μg/L | FVC was significantly and negatively associated with %iAs. The urinary As level was higher in children with restrictive lung patterns than those with normal spirometric patterns. |
| Cardenas-Gonzalez et al. (2016) | Mexico | Cross-sectional | 83 children | Arsenic upper tertile was positively associated with urinary kidney injury molecule-1 (372 pg/mL) |
| Laine et al. (2017) | Mexico | Cross-sectional | 50 cord serum samples collected from participants from the Biomarkers of Exposure to ARsenic (BEAR) pregnancy cohort | In multivariable regression analyses, 10 cord serum metabolites were identified that were significantly (p ≤ 0.05, q < 0.2) related to U-tAs and iAs metabolism efficiency indicators (U-%iAs, U-%MMAs, U-%DMAs). |
| Lopez-Rodriguez et al. (2017) | Mexico | Cross-sectional | 88 samples from anemic children and 208 non-anemic children | Anemic children had a higher average As level than non-anemic children (0.041 ± 0.11 wt% vs 0.014 ± 0.05 wt%, p < 0.05). Average As level was correlated with hemoglobin concentration (r = −0.441, p < 0.01) |
4.4.1. Pulmonary effects
Respiratory outcomes investigated in Chile and Mexico in adult populations were lung function, respiratory symptoms, lung inflammation, and bacterial diversity in the respiratory tract. A cross-sectional study of 751 adults aged 39–60 years from Northern Chile reported high BMI (above 90th percentile or >33.9 kg/m2) and high WAs above 11 μg/L to be risk factors for respiratory symptoms. The study revealed an increased risk of respiratory symptoms such as cough (OR=10.7, 95% CI: 3.03, 50.1), shortness of breath (OR=14.2, 95% CI: 4.79, 52.4), and wheeze (OR=14.4, 95% CI: 4.80, 53.7) (Nardone et al., 2017) associated with high BMI and As exposure. Observations were similar in the same geographic region among 765 adults (>40 years), who spent 80% of their lives in three cities including Antofagasta. Adults who were born in Antofagasta during the high exposure period had higher risk of shortness of breath (OR=5.56, 90% CI: 2.68, 11.5), and 224 mL reduction in forced vital capacity (FVC) even among nonsmokers, compared to those in Arica who were never exposed to over 10 μg/L of As in drinking water (Steinmaus et al., 2016). A pilot study of 32 high As-exposed adults during their childhood (i.e. water As >800 μg/L before age 10) and 65 non-exposed adults revealed that early life As exposure was associated with 11.5% lower forced expiratory volume in 1 sec (FEV1) (p=0.04) and 12.2% lower FVC (p=0.04) (Dauphine et al., 2011).
In an ecological study, pulmonary tuberculosis mortality RRs for high As-exposed populations in Region II of Chile (i.e. Antofagasta and nearby Mejillones) were compared with low As-exposed populations in Region V using data collected from 1958–2000. The five year mortality RR among men reached the peak during 1982–1986 (RR=2.1, 95% CI: 1.7, 2.6; p< 0.001), which gradually declined after this peak period (Smith et al., 2011). In women, similar trend was observed although the excess pulmonary tuberculosis death in women was lower than in men (Smith et al., 2011).
4.4.2. Cardiovascular effects
Evidences of As-induced cardiovascular (CVD) outcomes in LA were observed in a case-control and multiple cross-sectional studies conducted in Chile and Mexico. In a case-control study in Northern Chile, a dose-response increase of hypertension was observed with increasing levels of As as the ORs for 60–623 μg/L and >623 μg/L of WAs were 1.49 (95% CI: 1.09, 2.05) and 1.65 (95% CI: 1.18, 2.32) respectively when compared with a reference range of <60 μg/L of WAs (Hall et al., 2017).
Three cross-sectional studies used biomarkers of exposure or CVD outcomes to examine health effects. A pilot study conducted in Antofagasta, Chile observed the fate of As in cardiovascular tissues of patients with coronary heart disease and found significantly higher As in auricle (AU) and mammary artery (MAM) tissues in As-exposed patients compared to the unexposed patients (Roman et al., 2011). Subjects exposed to drinking water As as high as 1004 μg/L were recruited from Sonora, Mexico who demonstrated both water and urinary As to be positively associated with serum MMP-9, a biomarker of CVD in the adjusted models (Burgess et al., 2013). On the other hand, another study in Chihuahua reported positive associations of moderate water and urinary As levels with triglycerides and high total cholesterol indicating risks when the WAs concentration exceeded 25 μg/L (Mendez et al., 2016).
4.4.3. Diabetes
Studies conducted in Northern Chile and Mexico have presented findings regarding the associations between As exposure and outcomes of diabetes such as type 2 and gestational diabetes. In addition, urinary markers of diabetes, synergistic effects of As and BMI, genetic polymorphism and effect of As metabolism on diabetes were also examined.
A case-control study in Chile reported an interaction between lifetime WAs exposure and BMI for developing type 2 diabetes. Results of this study showed that participants exposed to high cumulative As (≥ 5280 μg/L-years) over 40 years and had BMI ≥ 30 kg/m2 had greater risk of type 2 diabetes (OR=2.64;95% CI: 1.14, 6.11) (Castriota et al., 2018). Similar findings were observed in a Mexico study demonstrating WAs to be associated with diabetes confirmed by fasting blood glucose (FBG), and two–hour fasting blood glucose (2HBG). The study found a 13% increase of risk of diabetes mellitus per 10 μg/L increase of iAs (95% CI:1.05, 1.22) (Del Razo et al., 2011). Association was also significant for DMAsIII in urine (OR=1.24 per inter-quartile range, p = 0.05) in this study (Del Razo et al., 2011). However, a study in Chile of 244 pregnant women did not find association between As exposure and gestational diabetes assessed by glucose tolerance test (OR=2.98, 95% CI: 0.87, 10.18) (Munoz and Valdes, 2018).
Using As species in exfoliated urothelial cells (EUC) as an exposure marker, a Mexican study of Chihuahua Cohort suggested that trivalent As species in EUC could be more important for As toxicity. It found positive and significant associations of diabetes with iAsIII (OR=1.57, 95% CI: 1.19, 2.07) and more toxic methylated species of MMAIII (OR=1.63, 95% CI: 1.24, 2.15) (Currier et al., 2014). On the other hand, both DMAs/MMAs and DMAs/iAs ratios demonstrated negative relationships with diabetes (OR = 0.62; 95% CI: 0.47, 0.83 and OR = 0.72; 95% CI: 0.55, 0.96, respectively) highlighting possible reduction of toxicity due to increased methylation capacity (Currier et al., 2014).
A study conducted among 40 type 2 diabetic and 32 non-diabetic adults, living in selected municipalities in Mexico, reported inverse association between iAs exposure and beta-cell function (p=0.05). Beta cells primarily produce and secrete insulin. Therefore a decrease in beta cell function would lower insulin secretion, suggesting a possible mechanism of As-induced type 2 diabetes (Diaz-Villasenor et al., 2013). The study also found stronger evidence in genotypes SNP-43 of calpain-10 gene (CAPN-10), which encodes a protein associated with the action of insulin (Diaz-Villasenor et al., 2013). Diabetic individuals exposed to iAs also demonstrated impact of As on human metabolome by having 59 altered metabolites including those involved in tricarboxylic acid cycle and amino acid metabolism when compared with the non-diabetic As-exposed subjects (Martin et al., 2015).
4.5. In utero and early childhood effects
Effects of As exposure during pregnancy and early life was demonstrated in studies conducted only in Mexico reporting evidence of As-induced birth outcomes, kidney injury, anemia, apoptosis in peripheral blood mononuclear cells, cognitive decline, reduced lung function and lung inflammation, as well as cardiovascular risks. Apart from one study, all other studies were cross-sectional in design.
A cross-sectional study of 200 mother-newborn pairs in Gómez Palacio, Mexico, where WAs ranged from <0.5 to 236 μg/L found maternal urinary MMAs to be negatively and significantly associated with newborn birth weight (β=–24.4, 95% CI: –46.8, –2.0) and gestational age (β=–0.07;95% CI: –0.13, –0.009) (Laine et al., 2015). Total UAs in mothers was also negatively associated with the length of the newborn in the same study (β=–0.16; 95% CI: –0.31, –0.02) (Laine et al., 2015). Although effects of As on newborns were reported, a case-control study where the mean WAs exposure among cases and controls were very similar (i.e. 39.58 μg/L and 40.49 μg/L) found no association of As with preeclampsia (Sandoval-Carrillo et al., 2016).
Several health outcomes were investigated in middle and late childhood and early adolescence in a number of studies in Mexico. In a cross-sectional study of 83 children between 5 to 12 years of age, living in San Luis Potosi (SLP), Mexico, where more than 80% of the participants were exposed to As above 10 μg/L, UAs in the upper tertile was significantly associated with kidney injury molecule-1 (KIM-1) after adjusting for potential covariates (β=362.68, 95% CI: 3.12, 722.25) (Cardenas-Gonzalez et al., 2016). In a study conducted in the state of Hidalgo where WAs contamination was common, dry BAs was used as an exposure biomarker that demonstrated negative correlation with hemoglobin in blood of 5–12 year-old children (r = –0.44, p<0.01) indicating that As could be a risk factor of anemia (Lopez-Rodriguez et al., 2017). In a pilot study of 40 children from two areas of Mexico with different As exposure characteristics (low vs high), the level of apoptosis in peripheral blood mononuclear cells (PBMC) was found to be positively correlated with urinary As (r=0.40; p=0.01) (Rocha-Amador et al., 2011b). In order to study As-induced neurotoxicity in children, 526 children aged 5–7 years were recruited from Torreon, where a metal foundry was located and urinary metabolites and parent and teacher-reported cognitive functions were assessed (Roy et al., 2011). The study observed modest associations of UAs with the oppositional, cognitive problems and ADHD subscales for teacher-reported Conners Behavior Rating Scales although dose-response associations were not evident in this study (Roy et al., 2011). In a study of Zimapan children who were between 3–14 years of age and were exposed to WAs within the range of 3–135 μg/L, total UAs was positively associated with carotid intima-media thickness (cIMT), an indicator of subclinical atherosclerotic burden (Osorio-Yanez et al., 2013). Furthermore, cIMT and percent iAs in urine, and plasma soluble intercellular cell adhesion molecule-1 (sVCAM-1) were identified as significant predictors of plasma asymmetric dimethylarginine (ADMA) levels in adjusted models (Osorio-Yanez et al., 2013).
Two studies on 5–12 year-old rural Mexican children conducted by the same group of researchers in an area with WAs concentration between 104–360 μg/L demonstrated various pulmonary outcomes with respect to As exposure. The first study found negative association between in utero and early life As exposure (measured by total UAs) and FVC (β = – 0.003, 95% CI: – 0.005, – 0.008) (Recio-Vega et al., 2015b). Additionally, over 57% of these child participants demonstrated restrictive spirometry pattern (Recio-Vega et al., 2015b). A parallel study on the same age group of children with same levels of As exposure examined lung function biomarkers and reported UAs metabolites DMA, %MMA and %DMA to be negatively correlated with soluble receptor for advanced glycation end products’ (sRAGE) sputum level (p<0.01 in all associations) (Olivas-Calderon et al., 2015). Positive associations of %DMA with MMP-9 (β = 0.21, 95% CI: 0.06, 0.35) and MMP-9/ TIMP-1 ratio (β = 0.24, 95% CI: 0.09, 0.38) were also observed (Olivas-Calderon et al., 2015).
5. Discussion
In the past 8 years, considerable work has been done to understand adverse health effects of As exposure in LA countries. Bladder and lung cancer mortality remained the focus of many studies (Table 3). Along the same line, a number of genes were identified which were associated with As metabolites and simultaneously related to bladder cancer (Rager et al., 2015). Additionally, two new cancers were reported to be associated with As exposure (Table 4). For example, several studies have linked As with breast cancer (Lopez-Carrillo et al., 2014; Michel-Ramirez et al., 2017; Smith et al., 2014), while one ecological study in Chile found As exposure in early life to be associated with laryngeal cancer (Roh et al., 2018). Compared to the previous review, remarkable progress has been made to understand As-related cardiopulmonary outcomes and diabetes (Table 5). These studies found the relationship of As with hypertension, high cholesterol (Hall et al., 2017; Mendez et al., 2016) and reported presence of As in auricle and mammary artery tissues of As exposed CHD patients (Roman et al., 2011). Research also showed associations between As and decreased lung function and elevated respiratory symptoms (Dauphine et al., 2011; Nardone et al., 2017; Steinmaus et al., 2016). Multiple studies found negative influence of As on diabetes outcomes such as type 2 diabetes (Castriota et al., 2018; Del Razo et al., 2011) although no association was observed between As exposure and gestational diabetes (Munoz and Valdes, 2018).
Studies that examined effects of As exposure during developmental stages (e.g. in utero and childhood) was substantially found over the past eight years (Table 6). Several studies reported associations of As with kidney injury, carotid intima-media thickness, and various pulmonary outcomes in children (Cardenas-Gonzalez et al., 2016; Osorio-Yanez et al., 2013; Recio-Vega et al., 2015b). Additionally, As has demonstrated detrimental effects on birth weight and gestational age, and was found to be associated with anemia, increased apoptosis in PBMC, and decreased cognitive functions (Laine et al., 2015; Lopez-Rodriguez et al., 2017; Rocha-Amador et al., 2011b; Roy et al., 2011). The new findings along with the common findings reported in the current review (2011– 2018) and our previous review articles (1949–2010) are summarized in Figure 2.
Figure 2:
Key findings from the previous and current review articles on health effects of arsenic in Latin America
(↑) – Positive association with As; (↓) – Negative association with As; As- Arsenic; AS3MT- Arsenite methyltransferase; cIMT- Carotid intima media thickness; CVD- Cardiovascular disease; miRNA- microRNA; PBMC- Peripheral blood mononuclear cells; T2DM- Type 2 Diabetes Mellitus; 8-OHdG- 8-hydroxydeoxyguanosine
Note: This is a 2 column fitting image
We acknowledge several methodological weaknesses of this review. Our inclusion criteria allowed selection of indexed, online and printed peer-reviewed articles published in English only. Articles not fitting the inclusion criteria (e.g articles in Spanish and Portuguese) were excluded, which may have considerably narrowed the scope of this literature review. Since this is a follow-up of our previous review article (McClintock et al., 2012), which considered only articles published in English, we maintained the same selection criterion. Nevertheless, out of the 1,895 articles searched, 92 articles were thoroughly reviewed. Several potential limitations of these included articles were identified. A large number of studies were cross-sectional and heavily relied on retrospective data. Cross-sectional studies do not establish temporality, however, it is unlikely that As-induced health outcomes occurred before As exposure. In some studies, retrospective data were used that might have produced non-differential exposure misclassification as well. Several studies also had small sample sizes, which could affect the statistical power to detect the differences between As-exposed and unexposed populations in LA. Even though a number of new diseases were reported since our previous review, there is need for more prospective studies with larger sample sizes to establish causation between As exposure and the health outcomes. Finally, since this study was limited to LA countries, certain health outcomes may not be generalizable to other countries owing to differences in demographic and socioeconomic characteristics, genetic compositions, and lifestyle factors.
We propose that future studies on As-exposed LA populations may concentrate more on investigating breast and laryngeal cancer with respect to As exposure since these are relatively new As-induced health outcomes reported in the recent past. Effects of As in utero and in early childhood continue to be an important research area as well. Early life As exposure was found to have the strongest association with cancer risk. A study has also reported increased apoptosis of PBMC in children (Rocha-Amador et al., 2011b). However, more robust prospective studies, with larger samples are needed to yield more conclusive results regarding these health outcomes. Only one study investigated neurotoxic effects of As exposure in children in this specific part of the world (Roy et al., 2011). Increased research on this topic may provide crucial information on the possible mechanisms of early life As exposure and consequent neurotoxicity in later life.. Interestingly, some studies have observed changes in gene expression and protein expression in cord blood samples from As exposed populations. This area can be further explored as changes in gene and protein expressions can lead to a myriad of health effects in late childhood and several of the affected genes were found related to birth outcomes. Studies on these topics can significantly contribute to the understanding of early life health impacts of As and developing mitigation measures for preventing these outcomes in various developmental stages in childhood.
The studies discussed in our current review reflect the implications of the progress made in the field of As-associated health effects. Several new biomarkers have been identified that are associated with As-induced health outcomes. Biomarkers have been helpful in developing efficient and better screening techniques for earlier detection of health effects and control measures related to As exposure. We have also observed the important roles of nutrition and BMI in developing As-induced health effects (Gomez-Rubio et al., 2011; Quiller et al., 2018). Additionally, evidence of associations of obesity with higher risk of lung and bladder cancer (Steinmaus et al., 2015)and reduced pulmonary function (Nardone et al., 2017) will help us target the high BMI sub-populations while designing the preventive measures. Similarly, the protective effect of nutrient intake on As metabolism capacities will help public health policy makers and clinicians develop therapeutic interventions (Jimenez-Villarreal et al., 2017; Lopez-Carrillo et al., 2016). Additionally, novel reporting of As-induced breast and laryngeal cancers have significant implications and warrants more extensive studies for a comprehensive understanding of the mechanisms of As-induced carcinogenicity. We have also observed the importance of in utero As exposure to produce cognitive impairment (Roy et al., 2011), increased cancer risk (Steinmaus et al., 2014a), and changes in gene and protein expressions (Bailey et al., 2014) in later stages of life. These findings contribute to the development of stricter policies and As mitigation measures to prevent early life As exposure in LA countries.
Since As is a global public health concern, it may be possible to replicate these studies in other parts of the world to evaluate generalizability and consistency. Additionally, new knowledge regarding similarities or differences in genetic susceptibilities or As methylation capacities across various populations may be discovered. Therefore, we conclude that there was substantial research productivity in the last 8 years on As exposure and various related health outcomes in LA countries. New knowledge on several new health outcomes was discovered. Early life exposures were found to be significantly associated with health risks over the life course. Genetic toxicities following As exposure have also been studied, which may explain the underlying mechanisms of health outcomes and susceptibilities allowing us to derive appropriate preventive measures. Despite the progress made in discovering new information on As-related health effects, the scope of further research is immense. Scientific evidence of the adverse health effects of As exposure would eventually help drive public health awareness and intervention programs and frame much needed environmental policies in Latin America.
Highlights.
Arsenic (As) induced breast & laryngeal cancers - first reported in Latin America.
Associations found between As exposure and cardiopulmonary outcomes and diabetes.
Genetic susceptibility and polymorphisms were found crucial in As related effects.
New evidence on bladder and lung cancers in As- exposed populations has emerged.
6. Acknowledgments
Faruque Parvez was supported by US NIH grants: R01 ES029974, R01 ES023888 and P42 ES10349. Additional support came from an internal grant received by Khalid M. Khan from the School of Public Health, Indiana University-Bloomington..
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Disclosure Summary
The authors have nothing to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Alegria-Torres JA, Carrizales-Yanez L, Diaz-Barriga F, Rosso-Camacho F, Motta V, Tarantini L, et al. DNA methylation changes in Mexican children exposed to arsenic from two historic mining areas in San Luis potosi. Environ Mol Mutagen 2016; 57: 717–723. [DOI] [PubMed] [Google Scholar]
- Ameer SS, Engstrom K, Hossain MB, Concha G, Vahter M, Broberg K. Arsenic exposure from drinking water is associated with decreased gene expression and increased DNA methylation in peripheral blood. Toxicol Appl Pharmacol 2017; 321: 57–66. [DOI] [PubMed] [Google Scholar]
- Apata M, Arriaza B, Llop E, Moraga M. Human adaptation to arsenic in Andean populations of the Atacama Desert. American Journal of Physical Anthropology 2017; 163: 192–199. [DOI] [PubMed] [Google Scholar]
- Arcega-Cabrera F, Fargher L, Quesadas-Rojas M, Moo-Puc R, Oceguera-Vargas I, Norena-Barroso E, et al. Environmental Exposure of Children to Toxic Trace Elements (Hg, Cr, As) in an Urban Area of Yucatan, Mexico: Water, Blood, and Urine Levels. Bull Environ Contam Toxicol 2018; 100: 620–626. [DOI] [PubMed] [Google Scholar]
- Arcega-Cabrera F, Fargher LF, Oceguera-Vargas I, Norena-Barroso E, Yanez-Estrada L, Alvarado J, et al. Water Consumption as Source of Arsenic, Chromium, and Mercury in Children Living in Rural Yucatan, Mexico: Blood and Urine Levels. Bull Environ Contam Toxicol 2017; 99: 452–459. [DOI] [PubMed] [Google Scholar]
- Bailey KA, Laine J, Rager JE, Sebastian E, Olshan A, Smeester L, et al. Prenatal Arsenic Exposure and Shifts in the Newborn Proteome: Interindividual Differences in Tumor Necrosis Factor (TNF)-Responsive Signaling. Toxicological Sciences 2014; 139: 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KA, Wu MC, Ward WO, Smeester L, Rager JE, Garcia-Vargas G, et al. Arsenic and the Epigenome: Interindividual Differences in Arsenic Metabolism Related to Distinct Patterns of DNA Methylation. Journal of Biochemical and Molecular Toxicology 2013; 27: 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri FL, Gardon J, Ruiz-Castell M, Paco VP, Muckelbauer R, Casiot C, et al. Toxic trace elements in maternal and cord blood and social determinants in a Bolivian mining city. Int J Environ Health Res 2016; 26: 158–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates MN, Rey OA, Biggs ML, Hopenhayn C, Moore LE, Kalman D, et al. Case-control study of bladder cancer and exposure to arsenic in Argentina. Am J Epidemiol 2004; 159: 381–9. [DOI] [PubMed] [Google Scholar]
- Beck R, Bommarito P, Douillet C, Kanke M, Del Razo LM, Garcia-Vargas G, et al. Circulating miRNAs Associated with Arsenic Exposure. Environmental Science & Technology 2018; 52: 14487–14495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya P, Claesson M, Bundschuh J, Sracek O, Fagerberg J, Jacks G, et al. Distribution and mobility of arsenic in the Rio Dulce alluvial aquifers in Santiago del Estero Province, Argentina. Science of the Total Environment 2006; 358: 97–120. [DOI] [PubMed] [Google Scholar]
- Buhl V, Alvarez MC, Torre MH, Piston M, Manay N. Biomonitoring of arsenic in woodworkers exposed to CCA and evaluation of other non-occupational sources in Uruguay. Int J Occup Environ Health 2017; 23: 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundschuh J, Armienta M, P B, Bhattacharya P, Matschullat J, Mukherjee A. Natural arsenic in groundwaters of Latin America ― Occurrence, health impact and remediation. Vol 1: CRC Press/Taylor and Francis; 2009. [Google Scholar]
- Bundschuh J, Farias B, Martin R, Storniolo A, Bhattacharya P, Cortes J, et al. Groundwater arsenic in the Chaco-Pampean Plain, Argentina: Case study from Robles County, Santiago del Estero Province. Applied Geochemistry 2004; 19: 231–243. [Google Scholar]
- Burgess JL, Kurzius-Spencer M, O’Rourke MK, Littau SR, Roberge J, Meza-Montenegro MM, et al. Environmental arsenic exposure and serum matrix metalloproteinase-9. J Expo Sci Environ Epidemiol 2013; 23: 163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas-Gonzalez M, Osorio-Yanez C, Gaspar-Ramirez O, Pavkovic M, Ochoa-Martinez A, Lopez-Ventura D, et al. Environmental exposure to arsenic and chromium in children is associated with kidney injury molecule-1. Environ Res 2016; 150: 653–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castriota F, Acevedo J, Ferreccio C, Smith AH, Liaw J, Smith MT, et al. Obesity and increased susceptibility to arsenic-related type 2 diabetes in Northern Chile. Environ Res 2018; 167: 248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro de Esparza M he presence of arsenic in drinking water in Latin America and its effect on public health. Serise, 2009. [Google Scholar]
- Colin-Torres CG, Murillo-Jimenez JM, Del Razo LM, Sanchez-Pena LC, Becerra-Rueda OF, Marmolejo-Rodriguez AJ. Urinary arsenic levels influenced by abandoned mine tailings in the Southernmost Baja California Peninsula, Mexico. Environ Geochem Health 2014; 36: 845–54. [DOI] [PubMed] [Google Scholar]
- Concha G, Nermell B, Vahter MV. Metabolism of inorganic arsenic in children with chronic high arsenic exposure in northern Argentina. Environ Health Perspect 1998a; 106: 355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha G, Vogler G, Lezcano D, Nermell B, Vahter M. Exposure to inorganic arsenic metabolites during early human development. Toxicol Sci 1998b; 44: 185–90. [DOI] [PubMed] [Google Scholar]
- Currier JM, Ishida MC, Gonzalez-Horta C, Sanchez-Ramirez B, Ballinas-Casarrubias L, Gutierrez-Torres DS, et al. Associations between arsenic species in exfoliated urothelial cells and prevalence of diabetes among residents of Chihuahua, Mexico. Environ Health Perspect 2014; 122: 1088–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauphine DC, Ferreccio C, Guntur S, Yuan Y, Hammond SK, Balmes J, et al. Lung function in adults following in utero and childhood exposure to arsenic in drinking water: preliminary findings. Int Arch Occup Environ Health 2011; 84: 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Rosa R, Steinmaus C, Akers NK, Conde L, Ferreccio C, Kalman D, et al. Associations between arsenic (+3 oxidation state) methyltransferase (AS3MT) and N-6 adenine-specific DNA methyltransferase 1 (N6AMT1) polymorphisms, arsenic metabolism, and cancer risk in a chilean population. Environmental and Molecular Mutagenesis 2017; 58: 411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Loma J, Tirado N, Ascui F, Levi M, Vahter M, Broberg K, et al. Elevated arsenic exposure and efficient arsenic metabolism in indigenous women around Lake Poopo Bolivia. Science of the Total Environment 2019; 657: 179–186. [DOI] [PubMed] [Google Scholar]
- Del Razo LM, Garcia-Vargas GG, Valenzuela OL, Castellanos EH, Sanchez-Pena LC, Currier JM, et al. Exposure to arsenic in drinking water is associated with increased prevalence of diabetes: a cross-sectional study in the Zimapan and Lagunera regions in Mexico. Environ Health 2011; 10: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Villasenor A, Cruz L, Cebrian A, Hernandez-Ramirez RU, Hiriart M, Garcia-Vargas G, et al. Arsenic exposure and calpain-10 polymorphisms impair the function of pancreatic beta-cells in humans: a pilot study of risk factors for T2DM. PLoS One 2013; 8: e51642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz OP, Arcos R, Tapia Y, Pastene R, Velez D, Devesa V, et al. Estimation of arsenic intake from drinking water and food (raw and cooked) in a rural village of northern Chile. Urine as a biomarker of recent exposure. Int J Environ Res Public Health 2015; 12: 5614–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobna Z, Del Razo LM, Garcia-Vargas GG, Sanchez-Pena LC, Barrera-Hernandez A, Styblo M, et al. Environmental exposure to arsenic, AS3MT polymorphism and prevalence of diabetes in Mexico. J Expo Sci Environ Epidemiol 2013; 23: 151–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Capetillo BL, Ortiz-Perez MD, Salgado-Bustamante M, Calderon-Aranda E, Rodriguez-Pinal CJ, Reynaga-Hernandez E, et al. Arsenic and fluoride co-exposure affects the expression of apoptotic and inflammatory genes and proteins in mononuclear cells from children. Mutation Research-Genetic Toxicology and Environmental Mutagenesis 2014; 761: 27–34. [DOI] [PubMed] [Google Scholar]
- Farías S, Bianco de Salas G, Servant R, Mitre B, Escalante J, Ponce R. Survey of arsenic in drinking water and assessment of the intake of arsenic from water in Argentinie Pune, 2008.
- Ferreccio C, Smith AH, Duran V, Barlaro T, Benitez H, Valdes R, et al. Case-control study of arsenic in drinking water and kidney cancer in uniquely exposed Northern Chile. Am J Epidemiol 2013a; 178: 813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreccio C, Yuan Y, Calle J, Benitez H, Parra RL, Acevedo J, et al. Arsenic, tobacco smoke, and occupation: associations of multiple agents with lung and bladder cancer. Epidemiology 2013b; 24: 898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire C, Koifman RJ, Fujimoto D, de Oliveira Souza VC, Barbosa F Jr., Koifman S. Reference values of cadmium, arsenic and manganese in blood and factors associated with exposure levels among adult population of Rio Branco, Acre, Brazil. Chemosphere 2015; 128: 70–8. [DOI] [PubMed] [Google Scholar]
- Gamboa-Loira B, Cebrian ME, Salinas-Rodriguez A, Lopez-Carrillo L. Genetic susceptibility to breast cancer risk associated with inorganic arsenic exposure. Environ Toxicol Pharmacol 2017; 56: 106–113. [DOI] [PubMed] [Google Scholar]
- Gamboa-Loira B, Hernandez-Alcaraz C, Gandolfi AJ, Cebrian ME, Burguete-Garcia A, Garcia-Martinez A, et al. Arsenic methylation capacity in relation to nutrient intake and genetic polymorphisms in one-carbon metabolism. Environ Res 2018; 164: 18–23. [DOI] [PubMed] [Google Scholar]
- Gamino-Gutierrez SP, Gonzalez-Perez CI, Gonsebatt ME, Monroy-Fernandez MG. Arsenic and lead contamination in urban soils of Villa de la Paz (Mexico) affected by historical mine wastes and its effect on children’s health studied by micronucleated exfoliated cells assay. Environmental Geochemistry and Health 2013; 35: 37–51. [DOI] [PubMed] [Google Scholar]
- Garrido AE, Strosnider WHJ, Wilson RT, Condori J, Nairn RW. Metal-contaminated potato crops and potential human health risk in Bolivian mining highlands. Environmental Geochemistry and Health 2017; 39: 681–700. [DOI] [PubMed] [Google Scholar]
- Gaxiola-Robles R, Labrada-Martagon V, Bitzer-Quintero OK, Zenteno-Savin T, Mendez-Rodriguez LC. Purine nucleoside phosphorylase and the enzymatic antioxidant defense system in breast milk from women with different levels of arsenic exposure. Nutricion Hospitalaria 2015; 31: 2289–2296. [DOI] [PubMed] [Google Scholar]
- George CM, Sima L, Arias MH, Mihalic J, Cabrera LZ, Danz D, et al. Arsenic exposure in drinking water: an unrecognized health threat in Peru. Bull World Health Organ 2014; 92: 565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goix S, Point D, Oliva P, Polve M, Duprey JL, Mazurek H, et al. Influence of source distribution and geochemical composition of aerosols on children exposure in the large polymetallic mining region of the Bolivian Altiplano. Science of the Total Environment 2011a; 412: 170–184. [DOI] [PubMed] [Google Scholar]
- Goix S, Point D, Oliva P, Polve M, Duprey JL, Mazurek H, et al. Influence of source distribution and geochemical composition of aerosols on children exposure in the large polymetallic mining region of the Bolivian Altiplano. Sci Total Environ 2011b; 412–413: 170–84. [DOI] [PubMed] [Google Scholar]
- Gomez-Rubio P, Klimentidis YC, Cantu-Soto E, Meza-Montenegro MM, Billheimer D, Lu Z, et al. Indigenous American ancestry is associated with arsenic methylation efficiency in an admixed population of northwest Mexico. J Toxicol Environ Health A 2012; 75: 36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Rubio P, Roberge J, Arendell L, Harris RB, O’Rourke MK, Chen Z, et al. Association between body mass index and arsenic methylation efficiency in adult women from southwest U.S. and northwest Mexico. Toxicol Appl Pharmacol 2011; 252: 176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cortes T, Recio-Vega R, Lantz RC, Chau BT. DNA methylation of extracellular matrix remodeling genes in children exposed to arsenic. Toxicology and Applied Pharmacology 2017; 329: 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Martinez F, Sanchez-Rodas D, Caceres DD, Martinez MF, Quinones LA, Johnson-Restrepo B. Arsenic exposure, profiles of urinary arsenic species, and polymorphism effects of glutathione-s-transferase and metallothioneins. Chemosphere 2018; 212: 927–936. [DOI] [PubMed] [Google Scholar]
- Hall EM, Acevedo J, Lopez FG, Cortes S, Ferreccio C, Smith AH, et al. Hypertension among adults exposed to drinking water arsenic in Northern Chile. Environ Res 2017; 153: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A, Paiva L, Creus A, Quinteros D, Marcos R. Micronucleus frequency in copper-mine workers exposed to arsenic is modulated by the AS3MT Met287Thr polymorphism. Mutation Research-Genetic Toxicology and Environmental Mutagenesis 2014; 759: 51–55. [DOI] [PubMed] [Google Scholar]
- Hopenhayn-Rich C, Biggs ML, Fuchs A, Bergoglio R, Tello EE, Nicolli H, et al. Bladder cancer mortality associated with arsenic in drinking water in Argentina. Epidemiology 1996; 7: 117–24. [DOI] [PubMed] [Google Scholar]
- Hopenhayn-Rich C, Biggs ML, Smith AH. Lung and kidney cancer mortality associated with arsenic in drinking water in Cordoba, Argentina. Int J Epidemiol 1998; 27: 561–9. [DOI] [PubMed] [Google Scholar]
- Hopenhayn-Rich C, Browning SR, Hertz-Picciotto I, Ferreccio C, Peralta C, Gibb H. Chronic arsenic exposure and risk of infant mortality in two areas of Chile. Environ Health Perspect 2000; 108: 667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Villarreal J, Rivas-Armendariz DI, Pineda-Belmontes CP, Betancourt-Martinez ND, Macias-Corral MA, Guerra-Alanis AJ, et al. Detection of damage on single- or double-stranded DNA in a population exposed to arsenic in drinking water. Genet Mol Res 2017; 16. [DOI] [PubMed] [Google Scholar]
- Klein LD, Breakey AA, Scelza B, Valeggia C, Jasienska G, Hinde K. Concentrations of trace elements in human milk: Comparisons among women in Argentina, Namibia, Poland, and the United States. PLoS One 2017; 12: e0183367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordas K, Queirolo EI, Manay N, Peregalli F, Hsiao PY, Lu Y, et al. Low-level arsenic exposure: Nutritional and dietary predictors in first-grade Uruguayan children. Environ Res 2016; 147: 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordas K, Roy A, Lopez P, Garcia-Vargas G, Cebrian ME, Vera-Aguilar E, et al. Iron and Zinc Supplementation Does Not Impact Urinary Arsenic Excretion in Mexican School Children. J Pediatr 2017; 185: 205–210 e1. [DOI] [PubMed] [Google Scholar]
- Kordas K, Roy A, Vahter M, Ravenscroft J, Manay N, Peregalli F, et al. Multiple-metal exposure, diet, and oxidative stress in Uruguayan school children. Environmental Research 2018; 166: 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine JE, Bailey KA, Olshan AF, Smeester L, Drobna Z, Styblo M, et al. Neonatal Metabolomic Profiles Related to Prenatal Arsenic Exposure. Environ Sci Technol 2017; 51: 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine JE, Bailey KA, Rubio-Andrade M, Olshan AF, Smeester L, Drobna Z, et al. Maternal arsenic exposure, arsenic methylation efficiency, and birth outcomes in the Biomarkers of Exposure to ARsenic (BEAR) pregnancy cohort in Mexico. Environ Health Perspect 2015; 123: 186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litter M, Nicolli H, Meichtry M, Quici N, Bundschuh J, Bhattacharya P, et al. One Century of the Discovery of Arsenicosis in Latin America (1914–2014): CRC Press/Taylor and Francis, 2014. [Google Scholar]
- Lopez-Carrillo L, Gamboa-Loira B, Becerra W, Hernandez-Alcaraz C, Hernandez-Ramirez RU, Gandolfi AJ, et al. Dietary micronutrient intake and its relationship with arsenic metabolism in Mexican women. Environ Res 2016; 151: 445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Carrillo L, Hernandez-Ramirez RU, Gandolfi AJ, Ornelas-Aguirre JM, Torres-Sanchez L, Cebrian ME. Arsenic methylation capacity is associated with breast cancer in northern Mexico. Toxicol Appl Pharmacol 2014; 280: 53–9. [DOI] [PubMed] [Google Scholar]
- Lopez-Rodriguez G, Galvan M, Gonzalez-Unzaga M, Hernandez Avila J, Perez-Labra M. Blood toxic metals and hemoglobin levels in Mexican children. Environ Monit Assess 2017; 189: 179. [DOI] [PubMed] [Google Scholar]
- Marshall G, Ferreccio C, Yuan Y, Bates MN, Steinmaus C, Selvin S, et al. Fifty-year study of lung and bladder cancer mortality in Chile related to arsenic in drinking water. J Natl Cancer Inst 2007; 99: 920–8. [DOI] [PubMed] [Google Scholar]
- Martin E, Gonzalez-Horta C, Rager J, Bailey KA, Sanchez-Ramirez B, Ballinas-Casarrubias L, et al. Metabolomic characteristics of arsenic-associated diabetes in a prospective cohort in Chihuahua, Mexico. Toxicol Sci 2015; 144: 338–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschullat J, Birmann K, Borba RP, Ciminelli V, Deschamps EM, Figueiredo BR, et al. Long-term environmental impact of arsenic-dispersion in Minas Gerais, Brazil. Arsenic in Soil and Groundwater Environment: Biogeochemical Interactions, Health Effects and Remediation 2007; 9: 365–382. [Google Scholar]
- Matschullat J, Borba RP, Deschamps E, Figueiredo BR, Gabrio T, Schwenk M. Human and environmental contamination in the Iron Quadrangle, Brazil. Applied Geochemistry 2000; 15: 181–190. [Google Scholar]
- McClintock TR, Chen Y, Bundschuh J, Oliver JT, Navoni J, Olmos V, et al. Arsenic exposure in Latin America: biomarkers, risk assessments and related health effects. Sci Total Environ 2012; 429: 76–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen AR, Hsu-Kim H, Robins NA, Hagan NA, Halabi S, Barras O, et al. Residential metal contamination and potential health risks of exposure in adobe brick houses in Potosi, Bolivia. Science of the Total Environment 2016; 562: 237–246. [DOI] [PubMed] [Google Scholar]
- Melak D, Ferreccio C, Kalman D, Parra R, Acevedo J, Perez L, et al. Arsenic methylation and lung and bladder cancer in a case-control study in northern Chile. Toxicol Appl Pharmacol 2014; 274: 225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MA, Gonzalez-Horta C, Sanchez-Ramirez B, Ballinas-Casarrubias L, Ceron RH, Morales DV, et al. Chronic Exposure to Arsenic and Markers of Cardiometabolic Risk: A Cross-Sectional Study in Chihuahua, Mexico. Environ Health Perspect 2016; 124: 104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel-Ramirez G, Recio-Vega R, Ocampo-Gomez G, Palacios-Sanchez E, Delgado-Macias M, Delgado-Gaona M, et al. Association between YAP expression in neoplastic and non-neoplastic breast tissue with arsenic urinary levels. J Appl Toxicol 2017; 37: 1195–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz MP, Valdes M. Urinary Inorganic Arsenic Concentration and Gestational Diabetes Mellitus in Pregnant Women from Arica, Chile. 2018; 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone A, Ferreccio C, Acevedo J, Enanoria W, Blair A, Smith AH, et al. The impact of BMI on non-malignant respiratory symptoms and lung function in arsenic exposed adults of Northern Chile. Environ Res 2017; 158: 710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navoni JA, De Pietri D, Olmos V, Gimenez C, Bovi Mitre G, de Titto E, et al. Human health risk assessment with spatial analysis: study of a population chronically exposed to arsenic through drinking water from Argentina. Sci Total Environ 2014; 499: 166–74. [DOI] [PubMed] [Google Scholar]
- Nunes LM, Otero X. Quantification of health risks in Ecuadorian population due to dietary ingestion of arsenic in rice. Environmental Science and Pollution Research 2017; 24: 27457–27468. [DOI] [PubMed] [Google Scholar]
- Olivas-Calderon E, Recio-Vega R, Gandolfi AJ, Lantz RC, Gonzalez-Cortes T, Gonzalez-De Alba C, et al. Lung inflammation biomarkers and lung function in children chronically exposed to arsenic. Toxicol Appl Pharmacol 2015; 287: 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio-Yanez C, Ayllon-Vergara JC, Aguilar-Madrid G, Arreola-Mendoza L, Hernandez-Castellanos E, Barrera-Hernandez A, et al. Carotid intima-media thickness and plasma asymmetric dimethylarginine in Mexican children exposed to inorganic arsenic. Environ Health Perspect 2013; 121: 1090–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavilonis B, Grassman J, Johnson G, Diaz Y, Caravanos J. Characterization and risk of exposure to elements from artisanal gold mining operations in the Bolivian Andes. Environmental Research 2017; 154: 1–9. [DOI] [PubMed] [Google Scholar]
- Perez-Maldonado IN, Ochoa-Martinez AC, Orta-Garcia ST, Ruiz-Vera T, Varela-Silva JA. Concentrations of Environmental Chemicals in Urine and Blood Samples of Children from San Luis Potosi, Mexico. Bull Environ Contam Toxicol 2017; 99: 258–263. [DOI] [PubMed] [Google Scholar]
- Quiller G, Merida-Ortega A, Rothenberg SJ, Cebrian ME, Gandolfi AJ, Franco-Marina F, et al. Dietary flavonoids improve urinary arsenic elimination among Mexican women. Nutr Res 2018; 55: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rager JE, Bailey KA, Smeester L, Miller SK, Parker JS, Laine JE, et al. Prenatal arsenic exposure and the epigenome: Altered microRNAs associated with innate and adaptive immune signaling in newborn cord blood. Environmental and Molecular Mutagenesis 2014; 55: 196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rager JE, Tilley SK, Tulenko SE, Smeester L, Ray PD, Yosim A, et al. Identification of novel gene targets and putative regulators of arsenic-associated DNA methylation in human urothelial cells and bladder cancer. Chem Res Toxicol 2015; 28: 1144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Aldaba H, Valles OP, Vazquez-Arenas J, Rojas-Contreras JA, Valdez-Perez D, Ruiz-Baca E, et al. Chemical and surface analysis during evolution of arsenopyrite oxidation by Acidithiobacillus thiooxidans in the presence and absence of supplementary arsenic. Science of the Total Environment 2016; 566: 1106–1119. [DOI] [PubMed] [Google Scholar]
- Recio-Vega R, Dena-Cazares JA, Ramirez-de la Pena JL, Jacobo-Avila A, Portales-Castanedo A, Gallegos-Arreola MP, et al. MRP1 expression in bronchoalveolar lavage cells in subjects with lung cancer who were chronically exposed to arsenic. Environ Mol Mutagen 2015a; 56: 759–66. [DOI] [PubMed] [Google Scholar]
- Recio-Vega R, Gonzalez-Cortes T, Olivas-Calderon E, Lantz RC, Gandolfi AJ, Gonzalez-De Alba C. In utero and early childhood exposure to arsenic decreases lung function in children. J Appl Toxicol 2015b; 35: 358–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Amador DO, Calderon J, Carrizales L, Costilla-Salazar R, Perez-Maldonado IN. Apoptosis of peripheral blood mononuclear cells in children exposed to arsenic and fluoride. Environmental Toxicology and Pharmacology 2011a; 32: 399–405. [DOI] [PubMed] [Google Scholar]
- Rocha-Amador DO, Calderon J, Carrizales L, Costilla-Salazar R, Perez-Maldonado IN. Apoptosis of peripheral blood mononuclear cells in children exposed to arsenic and fluoride. Environ Toxicol Pharmacol 2011b; 32: 399–405. [DOI] [PubMed] [Google Scholar]
- Rocha GH, Steinbach C, Munhoz JR, Madia MA, Faria JK, Hoeltgebaum D, et al. Trace metal levels in serum and urine of a population in southern Brazil. J Trace Elem Med Biol 2016; 35: 61–5. [DOI] [PubMed] [Google Scholar]
- Roh T, Steinmaus C, Marshall G, Ferreccio C, Liaw J, Smith AH. Age at Exposure to Arsenic in Water and Mortality 30–40 Years After Exposure Cessation. Am J Epidemiol 2018; 187: 2297–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas D, Rager JE, Smeester L, Bailey KA, Drobna Z, Rubio-Andrade M, et al. Prenatal Arsenic Exposure and the Epigenome: Identifying Sites of 5-methylcytosine Alterations that Predict Functional Changes in Gene Expression in Newborn Cord Blood and Subsequent Birth Outcomes. Toxicological Sciences 2015; 143: 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman DA, Pizarro I, Rivera L, Camara C, Palacios MA, Gomez MM, et al. An approach to the arsenic status in cardiovascular tissues of patients with coronary heart disease. Hum Exp Toxicol 2011; 30: 1150–64. [DOI] [PubMed] [Google Scholar]
- Roy A, Kordas K, Lopez P, Rosado JL, Cebrian ME, Vargas GG, et al. Association between arsenic exposure and behavior among first-graders from Torreon, Mexico. Environ Res 2011; 111: 670–6. [DOI] [PubMed] [Google Scholar]
- Sandoval-Carrillo A, Mendez-Hernandez EM, Antuna-Salcido EI, Salas-Pacheco SM, Vazquez-Alaniz F, Tellez-Valencia A, et al. Arsenic exposure and risk of preeclampsia in a Mexican mestizo population. BMC Pregnancy Childbirth 2016; 16: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeester L, Bommarito PA, Martin EM, Recio-Vega R, Gonzalez-Cortes T, Olivas-Calderon E, et al. Chronic early childhood exposure to arsenic is associated with a TNF-mediated proteomic signaling response. Environmental Toxicology and Pharmacology 2017; 52: 183–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Marshall G, Liaw J, Yuan Y, Ferreccio C, Steinmaus C. Mortality in young adults following in utero and childhood exposure to arsenic in drinking water. Environ Health Perspect 2012; 120: 1527–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Marshall G, Roh T, Ferreccio C, Liaw J, Steinmaus C. Lung, Bladder, and Kidney Cancer Mortality 40 Years After Arsenic Exposure Reduction. J Natl Cancer Inst 2018; 110: 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Marshall G, Yuan Y, Ferreccio C, Liaw J, von Ehrenstein O, et al. Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environ Health Perspect 2006; 114: 1293–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Marshall G, Yuan Y, Liaw J, Ferreccio C, Steinmaus C. Evidence from Chile that arsenic in drinking water may increase mortality from pulmonary tuberculosis. Am J Epidemiol 2011; 173: 414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Marshall G, Yuan Y, Steinmaus C, Liaw J, Smith MT, et al. Rapid reduction in breast cancer mortality with inorganic arsenic in drinking water. EBioMedicine 2014; 1: 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, Castriota F, Ferreccio C, Smith AH, Yuan Y, Liaw J, et al. Obesity and excess weight in early adulthood and high risks of arsenic-related cancer in later life. Environ Res 2015; 142: 594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, Ferreccio C, Acevedo J, Balmes JR, Liaw J, Troncoso P, et al. High risks of lung disease associated with early-life and moderate lifetime arsenic exposure in northern Chile. Toxicol Appl Pharmacol 2016; 313: 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, Ferreccio C, Acevedo J, Yuan Y, Liaw J, Duran V, et al. Increased lung and bladder cancer incidence in adults after in utero and early-life arsenic exposure. Cancer Epidemiol Biomarkers Prev 2014a; 23: 1529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, Ferreccio C, Yuan Y, Acevedo J, Gonzalez F, Perez L, et al. Elevated lung cancer in younger adults and low concentrations of arsenic in water. Am J Epidemiol 2014b; 180: 1082–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, Yuan Y, Kalman D, Rey OA, Skibola CF, Dauphine D, et al. Individual differences in arsenic metabolism and lung cancer in a case-control study in Cordoba, Argentina. Toxicol Appl Pharmacol 2010; 247: 138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus CM, Ferreccio C, Romo JA, Yuan Y, Cortes S, Marshall G, et al. Drinking water arsenic in northern chile: high cancer risks 40 years after exposure cessation. Cancer Epidemiol Biomarkers Prev 2013; 22: 623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda SHK, Kuno R, Barbosa F Jr., Gouveia N. Trace element levels in blood and associated factors in adults living in the metropolitan area of Sao Paulo, Brazil. J Trace Elem Med Biol 2017; 44: 307–314. [DOI] [PubMed] [Google Scholar]
- Torres-Sanchez L, Lopez-Carrillo L, Rosado JL, Rodriguez VM, Vera-Aguilar E, Kordas K, et al. Sex differences in the reduction of arsenic methylation capacity as a function of urinary total and inorganic arsenic in Mexican children. Environ Res 2016; 151: 38–43. [DOI] [PubMed] [Google Scholar]
- Villarreal JJ, Ortiz BM, Garza SM, Armendariz DIR, Villa VDB, Rosales PC, et al. Telomere length analysis in residents of a community exposed to arsenic. Journal of Biochemical and Molecular Toxicology 2019; 33. [DOI] [PubMed] [Google Scholar]
- WHO. Background document for preparation of WHO Guidelines for drinking-water quality. Geneva: World Health Organization (WHO/SDE/WSH/03.04/75) Arsenic in drinking-water. 2003. [Google Scholar]
- Xu X, Drobna Z, Voruganti VS, Barron K, Gonzalez-Horta C, Sanchez-Ramirez B, et al. Association Between Variants in Arsenic (+3 Oxidation State) Methyltranserase (AS3MT) and Urinary Metabolites of Inorganic Arsenic: Role of Exposure Level. Toxicol Sci 2016; 153: 112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez J, Mansilla HD, Santander IP, Fierro V, Cornejo L, Barnes RM, et al. Urinary arsenic speciation profile in ethnic group of the Atacama desert (Chile) exposed to variable arsenic levels in drinking water. J Environ Sci Health A Tox Hazard Subst Environ Eng 2015; 50: 1–8. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Marshall G, Ferreccio C, Steinmaus C, Liaw J, Bates M, et al. Kidney cancer mortality: fifty-year latency patterns related to arsenic exposure. Epidemiology 2010; 21: 103–8. [DOI] [PubMed] [Google Scholar]
- Zamoiski RD, Guallar E, Garcia-Vargas GG, Rothenberg SJ, Resnick C, Andrade MR, et al. Association of arsenic and metals with concentrations of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D among adolescents in Torreon, Mexico. Environ Health Perspect 2014; 122: 1233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]