Abstract
Background
Treatment of cancer is increasingly effective but is associated with short and long term side effects. Oral and gastrointestinal side effects, including oral candidiasis, remain a major source of illness despite the use of a variety of agents to treat them.
Objectives
To assess the effectiveness of interventions for the treatment of oral candidiasis for patients with cancer receiving chemotherapy or radiotherapy or both.
Search methods
Computerised searches of Cochrane Oral Health Group and PaPaS Trials Registers (to 1 June 2010), CENTRAL via the Cochrane Library (Issue 2, 2010, 1 June 2010), MEDLINE via OVID (1 June 2010), EMBASE via OVID (1 June 2010), CINAHL via EBSCO (1 June 2010), CANCERLIT via PubMed (1 June 2010), OpenSIGLE (1 June 2010) and LILACS via Virtual Health Library (1 June 2010) were undertaken. Reference lists from relevant articles were searched and the authors of eligible trials were contacted to identify trials and obtain additional information.
Selection criteria
All randomised controlled trials comparing agents prescribed to treat oral candidiasis in people receiving chemotherapy or radiotherapy for cancer. The outcomes were eradication of oral candidiasis, dysphagia, systemic infection, amount of analgesia, length of hospitalisation, cost and patient quality of life.
Data collection and analysis
Data were independently extracted, in duplicate, by two review authors. Trial authors were contacted for details of randomisation and withdrawals and a quality assessment was carried out. Risk ratios (RR) were calculated using fixed‐effect models.
Main results
Ten trials involving 940 patients, satisfied the inclusion criteria and are included in this review. Drugs absorbed from the gastrointestinal (GI) tract were beneficial in eradication of oral candidiasis compared with drugs not absorbed from the GI tract (three trials: RR = 1.29, 95% confidence interval (CI) 1.09 to 1.52), however there was significant heterogeneity. A drug absorbed from the GI tract, ketoconazole, was more beneficial than placebo in eradicating oral candidiasis (one trial: RR = 3.61, 95% CI 1.47 to 8.88). Clotrimazole, at a higher dose of 50 mg was more effective than a lower 10 mg dose in eradicating oral candidiasis, when assessed mycologically (one trial: RR = 2.00, 95% CI 1.11 to 3.60). Only one of the ten trials was assessed as at low risk of bias.
Authors' conclusions
There is insufficient evidence to claim or refute a benefit for any antifungal agent in treating candidiasis. Further well designed, placebo‐controlled trials assessing the effectiveness of old and new interventions for treating oral candidiasis are needed. Clinicians need to make a decision on whether to prevent or treat oral candidiasis in patients receiving treatment for cancer.
Plain language summary
Interventions for treating oral candidiasis for patients with cancer receiving treatment
Cancer treatment can lead to severe fungal infections (candidiasis, called thrush) in the mouth. This can cause pain, difficulties in eating and longer hospital stays. Infection can sometimes spread through the body and become life‐threatening. Different drugs are used to try and relieve candidiasis. There is insufficient evidence that any of the antifungal drugs may cure fungal infections in the mouth for people with cancer and more research is needed.
Background
Treatment of solid malignant tumours and the leukemias with cytotoxic chemotherapy or radiotherapy or both is becoming increasingly more effective but it is associated with short and long term side effects. Among the clinically important acute side effects is the disruption in the function and integrity of the oral mucosa. The consequences of this include severe ulceration (mucositis) and fungal infection of the mouth (oral candidiasis). These disease and treatment induced complications may also produce oral discomfort and pain, poor nutrition, delays in drug administration, increased hospital stays and costs and in some patients life threatening infection (septicaemia).
Patients with cancer are advised to maintain oral hygiene. Depending on the cancer centre, the patient's age and the expected toxicity of their treatment protocol, additional agents may be provided to prevent oral complications. Nevertheless, oral complications remain a major source of illness despite the use of a variety of agents to prevent them. A recent Cochrane review looked at the use of oral and topical prophylactic agents for the prevention of oral candidiasis in patients with cancer treated by chemotherapy (Clarkson 2007a). The review concluded that there is strong evidence, from randomised controlled trials, that drugs absorbed or partially absorbed from the gastrointestinal (GI) tract prevent oral candidiasis in patients receiving treatment for cancer. There is also evidence that these drugs are significantly better at preventing oral candidiasis than drugs not absorbed from the GI tract. This present review follows on from this and looks at the treatment of overt oral candidiasis in patients receiving treatment for cancer. This review is one in a series of four Cochrane reviews looking at the prevention and treatment of both oral candidiasis and oral mucositis (Clarkson 2007a; Clarkson 2007b; Worthington 2007).
Objectives
To assess the effectiveness of interventions (which may include placebo or no treatment) for the treatment of oral candidiasis for patients with cancer, receiving chemotherapy or radiotherapy or both.
The following primary null hypothesis was tested for comparisons between groups treated for oral candidiasis: There is no difference in the proportion of patients without oral candidiasis after treatment.
The primary outcomes were therefore:
Eradication of candidiasis
Improvement of candidiasis.
In this review we proposed to address the hypothesis of no difference between groups treated for oral candidiasis for the following secondary outcomes if data were available from studies which included a primary outcome:
Relief of pain
Amount of analgesia
Relief of dysphagia
Incidence of systemic infection
Days stay in hospital
Cost of oral care
Patient quality of life.
The following subgroup analyses were proposed:
Cancer type (leukaemia, solid cancer and mixed)
Cancer treatment type
Age group (children, adults, children and adults).
Methods
Criteria for considering studies for this review
Types of studies
Only randomised controlled trials were eligible for inclusion in this review.
Types of participants
Anyone with cancer who received chemotherapy or radiotherapy or both and had overt oral candidiasis.
Types of interventions
Active agents: any antifungal intervention for the treatment of oral candidiasis. Control: may be placebo or no treatment, or another active intervention.
Types of outcome measures
Primary outcome:
Oral candidiasis (absent or present)
Secondary outcomes:
Relief of pain
Amount of analgesia
Relief of dysphagia
Incidence of systemic infection
Days stay in hospital
Cost of oral care
Patient quality of life.
Search methods for identification of studies
This review is part of a series of four reviews on the prevention and treatment of oral candidiasis and oral mucositis in patients with cancer, and the same search strategies were used for all four reviews. The searches attempted to identify all relevant trials irrespective of language. Papers not in English were translated by members of The Cochrane Collaboration.
Electronic searches:
The following databases were searched: Cochrane Oral Health Group Trials Register (whole database, to 1 June 2010) (see Appendix 1) Cochrane Pain, Palliative and Supportive Care (PaPaS) Group Trials Register (whole database, to 1 June 2010) (see Appendix 1) Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 2; searches conducted 1 June 2010) (see Appendix 2) MEDLINE via OVID (1950 to 1 June 2010) (see Appendix 3) EMBASE via OVID (1980 to 1 June 2010) (see Appendix 4) CINAHL via EBSCO (1980 to 1 June 2010) (see Appendix 5) CANCERLIT via PubMed (1950 to 1 June 2010) (see Appendix 6) OpenSIGLE (1980 to 1 June 2010) (see Appendix 7) LILACS via the Virtual Health Library (1980 to 1 June 2010) (see Appendix 8)
Sensitive search strategies were developed for each database using a combination of free text and MeSH terms. The MEDLINE and CANCERLIT subject searches were conducted with the addition of the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomized trials in MEDLINE: sensitivity maximising version (2009 revision) as referenced in Chapter 6.4.11.1 and detailed in boxes 6.4.a and 6.4c of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (Higgins 2009). Filters developed by the Cochrane Oral Health Group for identifying randomised controlled trials were used for the searches of EMBASE and CINAHL. The LILACS subject search was linked to the Brazilian Cochrane Center search strategy for identifying randomised controlled trials in LILACs.
Searching other resources:
Only handsearching carried out by The Cochrane Collaboration is included in the search (see master list www.cochrane.org).
The controlled trials database (www.controlled‐trials.com) was also searched to identify ongoing and completed trials and to contact trialists for further information about these trials.
The reference list of related review articles and all articles obtained were checked for further trials. Authors of trial reports and specialists in the field known to the review authors were written to concerning further published and unpublished trials.
The review will be updated every 2 years using the Cochrane Oral Health Group Trials Register, CENTRAL, MEDLINE, EMBASE, CINAHL, CANCERLIT and LILACS. OpenSIGLE is no longer being updated and will not be searched for future updates of this review.
Data collection and analysis
Selection of studies
The titles and abstracts (when available) of all reports identified through the searches were scanned by two review authors (Jan Clarkson (JC) and Helen Worthington (HW)). Full reports were obtained for trials appearing to meet the inclusion criteria or for which there was insufficient information in the title and abstract to make a clear decision. The full reports obtained from all the electronic and other methods of searching were assessed independently, in duplicate, by two review authors to establish whether the trials met the inclusion criteria or not. Disagreements were resolved by discussion.
Data extraction and management
Data were extracted by two review authors independently using specially designed data extraction forms. The characteristics of the trial participants, interventions and outcomes for the included trials are presented in the study tables. Candidiasis was recorded as absent or present, and data for both clinical and mycological assessments were extracted. The duration of trials was recorded along with interim assessments and a decision made about which to use to maximise commonality. We also recorded the country where the trial was conducted, which year it was conducted and whether a dentist was involved in the investigation. Trial authors were contacted for clarification or for further information.
Assessment of risk of bias in included studies
The assessment of risk of bias for included trials was undertaken independently and in duplicate by two review authors. Studies were analysed for the following to assess validity as a threshold for inclusion of the studies, which is described as one of the options in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (Higgins 2009) on the following individual quality criteria:
Adequate sequence generation: Yes, No, Unclear
Allocation concealment: Yes, No, Unclear
Blinding of participants and carers: Yes, No, Unclear
Blinidng of outcome assessors: Yes, No, Unclear
Incomplete outcome data addressed: Yes, No, Unclear
Free of selective outcome reporting: Yes, No, Unclear
Free of other biases: Yes, No, Unclear
'Yes' indicates a low risk of bias, 'No' indicates high risk of bias and 'Unclear' indicates either lack of information or uncertainty over the potential for bias. A risk of bias table was completed for each included study. Results are presented graphically by study (see Figure 1) and by domain over all studies (Figure 2) .
1.
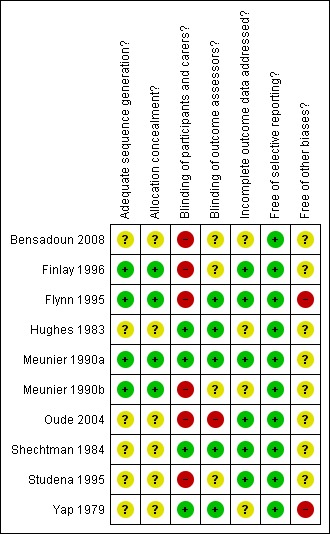
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
2.
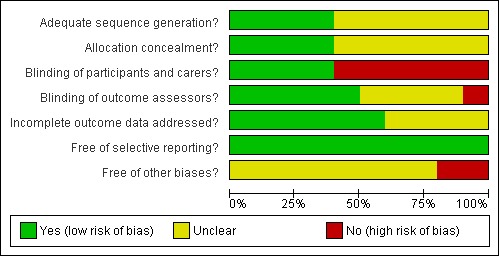
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Risk of bias was assessed for each study. Studies were considered to be at low risk of bias if there was adequate concealment of allocation, blinded outcome assessment and information on the reason for withdrawal provided by trial group. If one of these criteria was not met a study would be considered at moderate risk of bias, otherwise at high risk of bias.
Measure of treatment effect
For dichotomous outcomes, the estimates of effect of an intervention were expressed as risk ratios together with 95% confidence intervals. For continuous outcomes mean differences together with 95% confidence intervals were used.
Dealing with missing data
Intention‐to‐treat analysis was to be conducted where possible. Methods outlined in the handbook (Higgins 2009) were used to impute missing standard deviations if these could not be obtained from trial authors.
Assessment of heterogeneity
We planned to investigate clinical heterogeneity by examining the different cancer types and age groups, however there were insufficient trials looking at the same intervention to undertake this.
Assessment of reporting biases
We tabulated all the outcomes considered here.
Data synthesis
Meta‐analyses were done only with studies of similar comparisons. Risk ratios were combined for dichotomous data using random‐effects models (fixed‐effect models used if less than 3 studies in meta‐analysis).
Subgroup analysis and investigation of heterogeneity
It was planned to undertake a sensitivity analysis to examine the effect of concealed allocation and blind outcome assessment on the overall estimates of effect. However there were insufficient trials to undertake this.
We proposed a priori to conduct subgroup analyses for different cancer types (solid, leukaemia and mixed), different types of cancer treatment (chemotherapy, radiotherapy) and age groups (children, adults and mixed). There were insufficient trials by intervention type to undertake this.
The significance of any discrepancies in the estimates of the treatment effects from the different trials was assessed by means of Cochran's test for heterogeneity and quantified by I2 statistics.
Results
Description of studies
Results of the search
The search was conducted for the four similar reviews in this series (Clarkson 2007a; Clarkson 2007b; Worthington 2007) and has now been repeated seven times since 1999 for different updates. The most recent searches in October 2008, August 2009, January 2010 and June 2010 identified 1924, 621, 394 and 294 records respectively. Following screening of all three databases 125 potential trials were identified for the four reviews. There was only one further trial to be included in this review update (Bensadoun 2008) and one further study to be excluded (Yamaguchi 2006).
Included studies
SeeCharacteristics of included studies table for further details. One included study included episodes (n = 60) rather than patients (n = 56), but as these numbers were similar we decided to include the study.
Setting
Of the 10 included trials, four were conducted in USA (Flynn 1995; Hughes 1983; Shechtman 1984; Yap 1979) and six in Europe (Bensadoun 2008; Finlay 1996; Meunier 1990a; Meunier 1990b; Oude 2004; Studena 1995). Six of the trials received external funding, three obtained government funding and five acknowledged assistance from the pharmaceutical industry. The providers and assessors of the treatments were mainly medical staff although one of the trials involved a dentist (Finlay 1996). None of the trials involved the patients in the outcome measurement.
Participants
The results of the 10 trials included in the review are based on 940 patients. The range of patients was from 6 to 141 per treatment or control group.
Six of the 10 trials recruited only adult patients with cancer, one included both adults and children (Hughes 1983), one included only children (Flynn 1995) and in two trials the age of the patients was unclear (Meunier 1990b; Shechtman 1984). The type of cancer being treated was a combination of leukemias and solid tumours in seven trials (Flynn 1995; Hughes 1983; Meunier 1990a; Meunier 1990b; Oude 2004; Shechtman 1984; Studena 1995), head and neck cancer in two trials (Bensadoun 2008; Finlay 1996), and children with unspecified malignancies in the remaining trial (Flynn 1995). Little information was provided on the cancer treatment regimens received by patients in the trials. In one trial only radiotherapy was used (Finlay 1996), one trial used both cytotoxic chemotherapy and radiotherapy (Oude 2004) and for one trial information was provided for individual patients regarding the use of steroids and antibiotics in addition to chemotherapy (Shechtman 1984). The diagnosis of oral candidiasis at entry into the trial was usually a combination of both clinical and mycological diagnosis. However in two trials only clinical diagnosis was used (Finlay 1996; Studena 1995).
Interventions
All of the 10 trials provided a clear description of the interventions including the dose and method of administration for both the test and control groups. In only two trials was a comparison made with a placebo (Hughes 1983; Shechtman 1984). The majority of trials (six) compared different test agents with varying doses, frequency and duration of use. Two trials compared different doses of a test agent used at the same frequency and duration (Bensadoun 2008; Yap 1979).
The interventions for the 10 trials assessing the treatment of oral candidiasis were categorised according to the degree of absorption from the gastrointestinal (GI) tract.
Absorbed from the GI tract:
fluconazole (Finlay 1996; Flynn 1995; Meunier 1990a; Oude 2004; Studena 1995)
ketoconazole (Hughes 1983; Meunier 1990a; Meunier 1990b)
itraconazole (Oude 2004; Studena 1995).
Partially absorbed from the GI tract:
clotrimazole (Shechtman 1984; Yap 1979).
miconazole (Bensadoun 2008)
Not absorbed from the GI tract:
amphotericin B (Finlay 1996)
nystatin (Flynn 1995; Meunier 1990b).
Outcomes
There was variation between the trials in the assessment of oral candidiasis. All trials reported both a clinical and microbiological outcome of oral candidiasis. All trials used the dichotomous clinical outcome 'eradicated' verus 'not eradicated'. In addition two trials (Finlay 1996; Flynn 1995) compared the severity before and after treatment using a 4‐point scoring system. For three trials (Meunier 1990b; Studena 1995; Yap 1979) the method of assessment was not given. Mycological assessments were based on cultures rather than smears in all trials and the dichotomous classification of eradicated or not could be obtained from all the 10 trials. Only in three trials were outcome measures of pain or dysphagia collected (Bensadoun 2008; Flynn 1995; Shechtman 1984) and only three reported side effects (Bensadoun 2008; Flynn 1995; Oude 2004).
Excluded Studies
SeeCharacteristics of excluded studies table for further details.
Seventeen of the apparently eligible studies were excluded: four were not randomised controlled trials (Holst 1984; Jorgensen 2006; Urabe 1990; Walsh 2002); nine did not have just oral candidiasis for entry into the study (Anaissie 1996; Benhamou 1991; Bourhis 2004; Fleming 2001; Lake 1996; Lefebvre 2002; Subira 2004; Verweij 1994; Walsh 2004); in one study the data were presented in terms of episodes not patients (Kostiala 1982); two trials were excluded as the data were not presented in an accessible form (Conrad 1990; Domenge 1999); and one study conducted in Japan included patients who were not receiving treatment for cancer (Yamaguchi 2006).
Risk of bias in included studies
The kappa score between the two raters was one for each item assessed. Letters were sent to authors of the trials and only one replied (Finlay 1996), the information supplied changed the concealment of randomisation from unclear to adequate, and clarified the withdrawals.
One study was assessed as at low risk of bias (Meunier 1990a).The risk of bias assessment is summarised overall and for each trial in Figure 1 and Figure 2.
Adequate sequence generation
Adequate sequence generation was observed in four trials (40%), where a clear statement of the method of randomisation was reported. In the remainder of trials a judgment of 'unclear' was given as reporting lacked description with such statements as 'were randomised' or 'were stratified' appearing most commonly.
Allocation
Adequate allocation concealment was observed in the same four trials as above (40%). The remainder failed to indicate whether the generated randomisation sequence was concealed from individuals involved in the enrolment and assignment of participants.
Blinding
In four trials (40%) participants and carers were blinded to the allocated intervention. This was not done for the remaining six trials. Blinding of outcome assessors was adequate for five trials (50%), four being unclear and one not blinded.
Incomplete outcome data
In six trials (60%), incomplete outcome data was assessed as adequate. In the remaining four trials it was unclear which group the patients who were excluded for specific reasons belonged to.
Selective reporting
We consider all trials to be free of selective reporting as the primary outcomes were included in all.
Other potential sources of bias
This was unclear in eight trials and assessed as 'no' in two (Flynn 1995; Yap 1979) due to there being a unit of analysis problem with episodes rather than patients being used for the analysis. As the number of episodes was similar to the number of patients in both (60 and 56 in Flynn 1995; 186 and 180 in Yap 1979), episodes were used in the data analysis.
Effects of interventions
Comparison 1, Outcome 1.1 ‐ Clinical: eradication of oral candidiasis
One of the two placebo controlled trials found a significant benefit (risk ratio (RR) = 3.61, 95% confidence interval (CI) 1.47 to 8.88) for patients taking the absorbed drug ketoconazole (Hughes 1983). In the other placebo controlled trial on the partially absorbed drug, clotrimazole, no benefit was demonstrated (Shechtman 1984).
Three trials compared different types of absorbed drugs with each other and they failed to demonstrate a benefit of one drug against another: one trial compared fluconazole with ketoconazole (Meunier 1990a); two trials fluconazole versus itraconazole (Oude 2004; Studena 1995).
Three trials compared absorbed drugs (ketoconazole or fluconazole) with drugs not absorbed (nystatin or amphotericin B). Two of these trials demonstrated a significant clinical benefit of the absorbed drug fluconazole over the non‐absorbed drug nystatin (Finlay 1996; Flynn 1995), and the meta analysis found a benefit for the absorbed drugs over the non‐absorbed drugs (RR = 1.29, 95% CI fixed 1.09 to 1.52; Chi2 for heterogeneity P = 0.01). However there was substantial heterogeneity between the three trials with I2 = 78%.
One trial compared different doses of a partially absorbed drug, clotrimazole, and failed to find a significant difference (Yap 1979) (RR = 1.00, 95% CI 0.90 to 1.11).
A further trial compared the partially absorbed drug miconazole at different doses as a tablet and gel and found no statistically significant difference in eradication of candidiasis (Bensadoun 2008).
Comparison 1, Outcome 1.2 ‐ Mycological: eradication of oral candidiasis
There were some differences between the results for the mycological assessments compared with those from the clinical assessment. Despite a significant clinical improvement there was no statistically significant difference in mycological eradication between an absorbed drug ketoconazole and placebo (Hughes 1983). However, there was evidence of different eradication rates with different absorbed drugs and a statistically significant benefit was found for fluconazole over itraconazole (RR = 1.17, 95% CI 1.04 to 1.33; Chi2 for heterogeneity P = 0.30). In agreement with the clinical assessment there was a statistically significant difference in terms of a benefit for absorbed drugs compared to not absorbed drugs (RR = 1.82, 95% CI 1.28 to 2.57; Chi2 for heterogeneity P = 0.001). One further trial (Yap 1979) demonstrated that 50 mg of the partially absorbed drug clotrimazole eradicated more cases than the lower dose of 10 mg (RR = 2.00, 95% CI 1.11 to 3.60).
None of the studies reported: relief of pain, relief of dysphagia, incidence of systemic infection, amount of analgesia, days stay in hospital, cost of oral care, patient quality of life.
Discussion
Whilst we have been able to achieve our objective in evaluating the effectiveness of interventions to treat oral candidiasis, there were insufficient trials to make strong recommendations for patient care. The generalisability of the results is difficult to comment on as reporting of the types of cancer and details of treatment was unclear and few trials included children.
There were only two trials that compared the treatment of candidiasis using an active drug with a placebo. There was some evidence, based on one trial, that ketoconazole is effective, but there is a need for more trials that include a placebo group. The risk of hepatotoxicity with prolonged use of ketoconazole could influence treatment decisions and the UK Committee on Safety of Medicines has recommended that prescibers should weigh up the potential benefits against the risk of liver damage, and should carefully monitor patients both clinically and biochemically (BNF 2009).
There is evidence that absorbed drugs are more effective than drugs not absorbed from the gastrointestinal tract. There was no difference found in either trial comparing two absorbed drugs, and there was an indication that a higher dose of clotrimazole was more effective than a lower dose, although this was only found for the mycological assessment. There were no trials comparing partially absorbed drugs with either absorbed drugs or drugs not absorbed.
The findings from this review are disappointing as there were only 10 trials including 940 patients, 69 of whom were included in the two trials with placebo control groups. This is far fewer than the 28 trials with 4226 patients included in the prevention review (Clarkson 2007a).
There was limited consistency between trials on the clinical diagnosis of oral candidiasis and there was also little reported in terms of relief of pain, relief of dysphagia, incidence of systemic infection, amount of analgesia, days stay in hospital, cost of oral care and patient quality of life. It is therefore difficult to comment on the importance of these patient based outcomes, although they are frequently cited as the justification for conducting trials.
It is not possible to assess whether there was any evidence of publication bias however, with few trials and patients, this could be a major problem.
For patients being treated for cancer the clinical dilemma is whether to prevent or treat oral candidiasis. The findings from the prevention review would suggest that if the incidence of oral candidiasis for a patient subgroup is likely to be high then a drug absorbed or partially absorbed from the gastrointestinal tract should be prescribed at the start of cancer treatment. The incidence of oral candidiasis is variable and depends on the nature of the underlying disease and the intensity of treatment. For absorbed drugs in populations with an incidence of 20% (mid range of results in control groups), the number needed to treat (NNT) to prevent one extra case of oral candidiasis was 9 (95% confidence interval 7 to 13) (Clarkson 2007a).
The findings of this review should be considered in the context of the general medical management of patients with cancer. A review investigating the routine use of antifungal therapy in cancer patients did not find an effect on mortality and only a modest effect on systemic fungal invasion (Gotzsche 2002). The authors questioned the current widespread practice of prophylactic antifungal therapy and this finding should be considered when interpreting the results of this review where we are specifically looking at oral outcomes.
Authors' conclusions
Implications for practice.
Clinicians need to make a decision on whether to prevent or treat oral candidiasis in patients receiving treatment for cancer. The evidence on which drug should be prescribed is weak and unreliable.
Implications for research.
There is a need for more well designed trials that compare the effectiveness of drugs absorbed, partially absorbed or not absorbed from the gastrointestinal tract with a placebo control. These should be conducted before comparing specific agents with each other. The limited evidence of effectiveness of current therapies, combined with side‐effects profiles of those agents with proven efficacy suggest that new interventions for treating oral candidiasis are needed.
What's new
| Date | Event | Description |
|---|---|---|
| 9 June 2010 | New search has been performed | Substantive amendment. Updated search found 1 new included trial and 1 excluded study. New methodology. |
| 9 June 2010 | New citation required but conclusions have not changed | New authorship. |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 1, 2002
| Date | Event | Description |
|---|---|---|
| 5 February 2007 | New citation required but conclusions have not changed | Substantive amendment. An updated search in 2006 has found one more trial to include in this review, and seven more excluded studies. This update has updated references to other Cochrane reviews however the results and conclusions remain unchanged. |
Acknowledgements
Thanks go to Anne Littlewood, Trials Search Co‐ordinator for the Cochrane Oral Health Group for carrying out the searches for the review, Luisa Fernandez Mauleffinch (Managing Editor) and Phil Riley (Assistant Managing Editor) for their help with the administration of the review which included sending out letters to authors and in locating all the articles for the review. Thanks also go to Tim Eden who provided advice on cancer, its treatment and the interventions included in previous versions of the review. The review authors would like to thank Dr Patricia Finlay for responding to our letter requesting further information. Thanks also go to Dr Toru Naito for translating a potential study in Japanese.
Appendices
Appendix 1. Cochrane Oral Health Group Trials Register; Cochrane Pain, Palliative & Supportive Care Group Trials Register search strategy
((neoplasm* OR leukemia OR leukaemia OR leukaemia OR lymphoma* OR plasmacytoma OR "histiocytosis malignant" OR reticuloendotheliosis OR "sarcoma mast cell" OR "Letterer Siwe disease" OR "immunoproliferative small intestine disease" OR "Hodgkin disease" OR "histiocytosis malignant" OR "bone marrow transplant*" OR cancer* Or tumor* OR tumour* OR malignan* OR neutropeni* OR carcino* OR adenocarcinoma* OR radioth* OR radiat* OR radiochemo* OR irradiat* OR chemo*) AND (stomatitis OR "Stevens Johnson syndrome" OR "candidiasis oral" OR mucositis OR (oral AND (cand* OR mucos* OR fung*)) OR mycosis OR mycotic OR thrush))
Appendix 2. The Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
Search strategy for the Cochrane Library
Exp NEOPLASMS
Exp LEUKEMIA
Exp LYMPHOMA
Exp RADIOTHERAPY
Exp BONE MARROW TRANSPLANTATION
neoplasm* or cancer* or carcino* or malignan*
leukemi* or leukaemia*
tumour* or tumor*
neutropeni*
adenocarcinoma*
lymphoma*
(radioth* or radiat* or irradiat* or radiochemo*)
(bone next marrow next transplant*)
chemo* or radiochemo*
(#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14)
Exp STOMATITIS
MUCOSITIS
CANDIDIASIS ORAL
stomatitis
(stevens next johnson next syndrome)
mucositis
oral near cand*
mouth near cand*
oral and fung*
mouth and fung*
(mycosis or mycotic or thrush)
#16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26
#15 AND #27
Appendix 3. MEDLINE via OVID search strategy
1. exp NEOPLASMS/ 2. exp LEUKEMIA/ 3. exp LYMPHOMA/ 4. exp RADIOTHERAPY/ 5. Bone Marrow Transplantation/ 6. neoplasm$.mp. 7. cancer$.mp. 8. (leukaemi$ or leukemi$).mp. 9. (tumour$ or tumor$).mp. 10. malignan$.mp. 11. neutropeni$.mp. 12. carcino$.mp. 13. adenocarcinoma$.mp. 14. lymphoma$.mp. 15. (radioth$ or radiat$ or irradiat$).mp. 16. (bone adj marrow adj5 transplant$).mp. 17. chemo$.mp. 18. or/1‐17 19. exp STOMATITIS/ 20. Candidiasis, Oral/ 21. stomatitis.mp. 22. mucositis.mp. 23. (oral and cand$).mp. 24. (oral adj6 mucos$).mp. 25. (oral and fung$).mp. 26. (mycosis or mycotic).mp. 27. or/19‐26 28. 18 and 27
The above search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomized trials in MEDLINE: sensitivity maximising version (2009 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of The Cochrane Handbook for Systematic Reviews of Interventions, Version 5.0.2 [updated September 2009].
1. randomized controlled trial.pt.
2. controlled clinical trial.pt.
3. randomized.ab.
4. placebo.ab.
5. drug therapy.fs.
6. randomly.ab.
7. trial.ab.
8. groups.ab.
9. or/1‐8
10. exp animals/ not humans.sh.
11. 9 not 10
Appendix 4. EMBASE SS via OVID search strategy
1. exp NEOPLASM/ 2. exp LEUKEMIA/ 3. exp LYMPHOMA/ 4. exp RADIOTHERAPY/ 5. exp bone marrow transplantation/ 6. (neoplasm$ or cancer$ or leukemi$ or leukaemi$ or tumour$ or tumor$ or malignan$ or neutropeni$ or carcino$ or adenocarcinoma$ or lymphoma$).mp. 7. (radioth$ or radiat$ or irradiat$ or radiochemo$).mp. 8. (bone marrow adj3 transplant$).mp. 9. chemo$.mp. 10. or/1‐9 11. exp Stomatitis/ 12. Thrush/ 13. (stomatitis or mucositis or (oral and candid$) or (oral adj4 mucositis) or (oral and fung$) or mycosis or mycotic or thrush).mp. 14. or/11‐13 15. 10 and 14
The above search was linked to the Cochrane Oral Health Group filter for identifying randomized controlled trials in EMBASE:
1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/ 16. HUMAN/ 17. 16 and 15 18. 15 not 17 19. 14 not 18
Appendix 5. CINAHLvia EBSCO search strategy
S1 (MH "Neoplasms+")
S2 (MH "Leukemia+")
S3 (MH "Lymphoma+")
S4 (MH "Radiotherapy+")
S5 (MH "Bone Marrow Transplantation")
S6 neoplasm*
S7 cancer*
S8 (leukemi* or leukaemi*)
S9 (tumour* or tumor*)
S10 malignan*
S11 neutropeni*
S12 carcino*
S13 adenocarcinoma*
S14 lymphoma*
S15 (radioth* or radiat* or irradiat*)
S16 (bone N1 marrow N5 transplant*)
S17 chemo*
S18 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or
S12 or S13 or S14 or S15 or S16 or S17
S19 MH "Stomatitis+"
S20 MH "Candidiasis, Oral"
S21 stomatitis
S22 mucositis
S23 (oral and cand*)
S24 (oral N6 mucos*)
S25 (oral and fung*)
S26 (mycosis or mycotic)
S27 S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26
S28 S18 AND S27
The above search was linked to the Cochrane Oral Health Group search strategy for identifying randomized controlled trials in CINAHL:
S1 MH Random Assignment or MH Single‐blind Studies or MH Double‐blind Studies or MH Triple‐blind Studies or MH Crossover design or MH Factorial Design
S2 TI ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or AB ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or SU ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study")
S3 TI random* or AB random*
S4 AB "latin square" or TI "latin square"
S5 TI (crossover or cross‐over) or AB (crossover or cross‐over) or SU (crossover or cross‐over)
S6 MH Placebos
S7 AB (singl* or doubl* or trebl* or tripl*) or TI (singl* or doubl* or trebl* or tripl*)
S8 TI blind* or AB mask* or AB blind* or TI mask*
S9 S7 and S8
S10 TI Placebo* or AB Placebo* or SU Placebo*
S11 MH Clinical Trials
S12 TI (Clinical AND Trial) or AB (Clinical AND Trial) or SU (Clinical AND Trial)
S13 S1 or S2 or S3 or S4 or S5 or S6 or S9 or S10 or S11 or S12
Appendix 6. CANCERLIT (PubMed Cancer Subset) search strategy
((neoplasm* OR leukemia OR leukaemia OR leukaemia OR lymphoma* OR plasmacytoma OR "histiocytosis malignant" OR reticuloendotheliosis OR "sarcoma mast cell" OR "Letterer Siwe disease" OR "immunoproliferative small intestine disease" OR "Hodgkin disease" OR "histiocytosis malignant" OR "bone marrow transplant*" OR cancer* Or tumor* OR tumour* OR malignan* OR neutropeni* OR carcino* OR adenocarcinoma* OR radioth* OR radiat* OR radiochemo* OR irradiat* OR chemotherap*) AND (stomatitis OR "Stevens Johnson syndrome" OR "candidiasis oral" OR mucositis OR (oral AND (candid* OR mucos* OR fung*)) OR mycosis OR mycotic OR thrush))
The above search strategy was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomized trials in MEDLINE via PubMed: sensitivity maximising version (2009 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.a of The Cochrane Handbook for Systematic Reviews of Interventions, Version 5.0.2 [updated September 2009].
(randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw] OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw] )) OR (placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp]) NOT (animals [mh] NOT human [mh]))
Appendix 7. OpenSIGLE search strategy
N.B. SIGLE is now provided through OpenSIGLE:http://opensigle.inist.fr/
SIGLE no longer supports complex searching, so a series of keyword searches was performed as below:
cancer AND mucositis AND oral
leukemia AND mucositis AND oral
leukaemia AND mucositis AND oral
carcinoma AND mucositis AND oral
lymphoma AND mucositis AND oral
tumour AND mucositis AND oral
tumor AND mucositis AND oral
cancer AND candidiasis AND oral
leukemia AND candidiasis AND oral
leukaemia AND candidiasis AND oral
carcinoma AND candidiasis AND oral
lymphoma AND candidiasis AND oral
tumour AND candidiasis AND oral
tumor AND candidiasis AND oral
Appendix 8. LILACS via the Virtual Health Library search strategy
(www.bireme.org) Mh NEOPLASMS OR Tw neoplasm$ OR Tw cancer$ OR Tw carcinoma$ OR Tw tumour$ OR Tw tumor$ OR Tw malignan$ OR Tw carcino$ OR Tw nuetropeni$ OR Tw adenocarcinoma$ OR Mh leukemia OR Tw leukaemia$ OR Tw leukemi$ OR Tw lymphoma$ OR Tw "bone marrow transplantation" OR Tw "bone marrow transplant$" OR Tw radiotherapy OR Tw radioth$ OR Tw radiat$ OR Tw irradiat$ OR Tw radiochemo$ OR Tw chemo$ AND Mh stomatitis OR Tw stomatitis OR Mh Candidiasis‐Oral OR Tw "oral candidiasis" OR (Tw candida$ AND (Tw mouth OR Tw oral)) OR Tw mucositis OR ((Tw oral OR mouth) AND Tw fung$) OR (Tw oral AND Tw candidiasis$)
The above search was linked to the Brazilian Cochrane Center search strategy for identifying randomized controlled trials in LILACs:
((Pt randomized controlled trial OR Pt controlled clinical trial OR Mh randomized controlled trials OR Mh random allocation OR Mh double‐blind method OR Mh single‐blind method) AND NOT (Ct animals AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh research design) AND NOT (Ct animals AND NOT (Ct human and Ct animals)) OR (Ct comparative study OR Ex E05.337$ OR Mh follow‐up studies OR Mh prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animals AND NOT (Ct human and Ct animals)))
Data and analyses
Comparison 1. All studies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical: eradication of oral candidiasis | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Drug absorbed (ketoconazole) versus placebo | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.61 [1.47, 8.88] |
| 1.2 Drug partially absorbed (clotrimazole) versus placebo | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.43 [0.51, 22.94] |
| 1.3 Drug absorbed versus drug absorbed (fluconazole versus itraconazole) | 2 | 293 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.00, 1.30] |
| 1.4 Drug absorbed versus drug absorbed (fluconazole versus ketoconazole) | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.72, 1.42] |
| 1.5 Drug absorbed (fluconazole/ketoconazole) versus drug not absorbed (amphotericin/nystatin) | 3 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.09, 1.52] |
| 1.6 Drug partially absorbed versus drug partially absorbed (clotrimazole 50 mg versus 10 mg) | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.90, 1.11] |
| 1.7 Drug partially absorbed versus drug partially absorbed (miconazole 50 mg tablet versus 500mg gel) | 1 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.91, 1.47] |
| 2 Mycological: eradication of oral candidiasis | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Drug absorbed (ketoconazole) versus placebo | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.09 [0.73, 35.49] |
| 2.2 Drug partially absorbed (clotrimazole) versus placebo | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.13 [0.38, 99.14] |
| 2.3 Drug absorbed versus drug absorbed (fluconazole versus itraconazole) | 2 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.04, 1.33] |
| 2.4 Drug absorbed versus drug absorbed (fluconazole versus ketoconazole) | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.52, 1.72] |
| 2.5 Drug absorbed (fluconazole/ketoconazole) versus not absorbed (amphotericin/nystatin) | 3 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.28, 2.57] |
| 2.6 Drug partially absorbed versus partially absorbed (clotrimazole 50 mg versus 10 mg) | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [1.11, 3.60] |
1.1. Analysis.
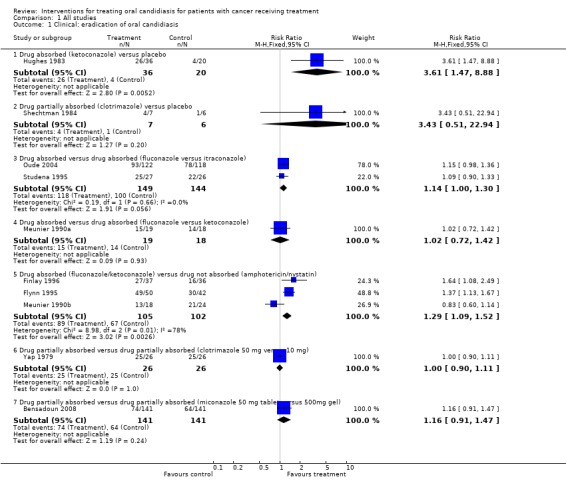
Comparison 1 All studies, Outcome 1 Clinical: eradication of oral candidiasis.
1.2. Analysis.
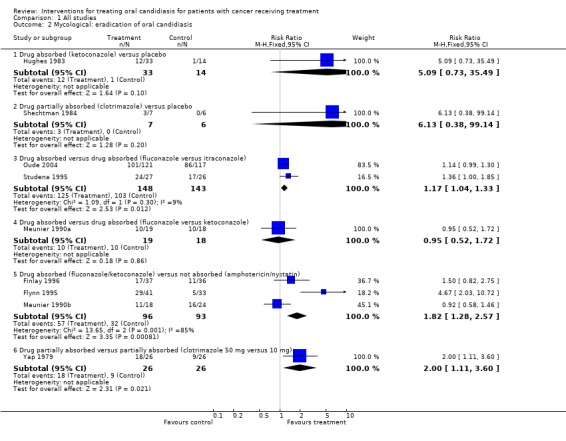
Comparison 1 All studies, Outcome 2 Mycological: eradication of oral candidiasis.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bensadoun 2008.
| Methods | Randomised, parallel group multicentre single blind study conducted in France, Tunsia and Morocco. Patients and carers not blinded. Primary outcome assessment made by blinded assessor. No evidence of funding apart from one collaborator is a consultant for pharmaceutical company who produced the tablets. Patients were recruited from May 2002 until June 2004. | |
| Participants | Adults with head and neck cancer. 306 patients randomised, 154 to miconazole tablet and 152 to miconazole gel. 6 patients in each group had no treatment, analysis conducted on 141 patient in each group. OP confirmed by direct mycological examination (culture). | |
| Interventions | 2 groups: miconazole tablet Lauriad 50 mg MBT (kept in mouth as long as possible) or 500 mg miconazole gel MOG (applied to gums) once daily for 14 days. | |
| Outcomes | Primary outcome success at day 14 (clinical eradication) and partial response was defined as improvement by 2 points on Murray Scoring Scale compared with score at baseline. Assessment made at 2, 6, 20 days, unclear which presented. Secondary endpoint was success at day 7. Improvement in clinical symptoms, mycological cure (culture), recurrence rate and safety also reported. |
|
| Notes | Modified intention‐to‐treat analysis ‐ all randomised patients who received at least 1 treatment dose and had efficacy evaluation after randomisation. Non‐inferiority statistical approach used. Authors contacted about assessor blinding. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "patients were randomised". |
| Allocation concealment? | Unclear risk | Not reported. |
| Blinding of participants and carers? | High risk | Comment: Tablet versus gel. |
| Blinding of outcome assessors? | Unclear risk | Quote: "An amendment introduced a blind assessment of the primary criterion performed in each investigational centre by an independent healthcare member who was unaware of the study drug allocated to each patient. It was implemented after the inclusion of 59 patients". Comment: lack of clarity about how this affected the results. |
| Incomplete outcome data addressed? | Unclear risk | Comment: Figure 1 provides clear description of patients for data analysis. Two patients were given the wrong intervention, 6 in each group did not receive treatment and 6 did not have an outcome assessment. Numbers do not add up and true intention to treat analysis was not undertaken. |
| Free of selective reporting? | Low risk | Comment: Both clinical and mycological assessment reported and other secondary outcomes. |
| Free of other biases? | Unclear risk | One author is consultant for pharmaceutical company who produced tablets for study. |
Finlay 1996.
| Methods | Randomised, parallel group study conducted in Scotland. The patients were not blinded. Information on withdrawals clarified by letter. No mention of funding but possible university funding. No dates for recruitment period. | |
| Participants | Adults with head and neck cancer. 77 enrolled, 73 completed. | |
| Interventions | 2 groups. Fluconazole 50 mg daily for 7 days. Amphotericin B 10 mg lozenge sucked for 14 days. | |
| Outcomes | Clinical and mycological eradication (culture). Assessment made at 2, 6, 20 days, unclear which presented. | |
| Notes | Communicating with authors changed randomisation assessment from Unclear to Yes (low risk of bias). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Comment: Changed after clarification by authors. |
| Allocation concealment? | Low risk | Comment: Changed after clarification by authors. |
| Blinding of participants and carers? | High risk | Comment: Tablet (7 days) versus lozenge (14 days). |
| Blinding of outcome assessors? | Unclear risk | Comment: Unclear for clinical assessment and mycological assessment. |
| Incomplete outcome data addressed? | Low risk | Comment: Clarified by authors. |
| Free of selective reporting? | Low risk | Comment: Clinical and mycological eradication. |
| Free of other biases? | Unclear risk | No information on funding. |
Flynn 1995.
| Methods | Randomised, multicentre, parallel group study conducted in USA. Patient, carer not blind, assessor blind. Clear information on withdrawals given. Pfizer provided the drugs but no funding mentioned. No dates for recruitment period. | |
| Participants | Children with malignancies and immunocompromised including HIV (data presented separately). 186 enrolled, 182 received drugs, 92 (cancer patients) completed. | |
| Interventions | 2 groups. Fluconazole 4 mg/kg suspension day 1 then 2 mg/day. Nystatin 4 ml USP suspension 4 times daily‐ swished in mouth and swallowed. Both for 14 days in total. | |
| Outcomes | Clinical and mycological eradication (culture). Assessment at 7 days or later. | |
| Notes | Study also included children with HIV, but data were presented separately. The dose of fluconazole was changed 1/4 way into study to 2 mg/kg day 1, then 3 mg/kg. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "....patients were randomly assigned to receive....A computer generated random number code was supplied to each centre by Pfizer Central Research". |
| Allocation concealment? | Low risk | Quote: "The randomisation code was held by the pharmacist; neither patient nor physician had knowledge of the category of assignment before enrolment". |
| Blinding of participants and carers? | High risk | Comment: Drugs given at different frequencies. |
| Blinding of outcome assessors? | Low risk | Quote: "All clinical assessments were performed by investigators unaware of the subjects treatment regime". |
| Incomplete outcome data addressed? | Low risk | Comment: Clear explanation of withdrawals by intervention but not for cancer patients as separate group. |
| Free of selective reporting? | Low risk | Comment: Clinical and mycological eradication (culture). |
| Free of other biases? | High risk | 6 patients were re‐enrolled and treated as new patients ‐ lack of independence of data. No reference to funding although Pfizer provided drug. |
Hughes 1983.
| Methods | Randomised, parallel group study conducted in USA. Patient, carer and assessor blind. Unclear information on withdrawals given. Pharmaceutical company provided the tablets but no other information about funding. No dates for recruitment period. | |
| Participants | Children and adults with mixed cancer. 64 enrolled, 56 completed. | |
| Interventions | 2 groups, placebo versus ketoconazole. 200 mg twice/day. 2 weeks duration. | |
| Outcomes | Clinical and mycological eradication (culture). Assessment made at day 14. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "Randomised in a double blind placebo controlled study". |
| Allocation concealment? | Unclear risk | Comment: Drug supplied by pharmaceutical company but concealment still unclear. |
| Blinding of participants and carers? | Low risk | Quote: "Randomised in a double blind placebo controlled study". |
| Blinding of outcome assessors? | Low risk | Quote: "Randomised in a double blind placebo controlled study". |
| Incomplete outcome data addressed? | Unclear risk | 8 patients (12%) withdrawn 5 for noncompliance and 3 by request, but unclear which group. |
| Free of selective reporting? | Low risk | Comment: Clinical and mycological eradication (culture). |
| Free of other biases? | Unclear risk | No information on funding except being given drug by pharmaceutical company. |
Meunier 1990a.
| Methods | Randomised, parallel group study conducted in Belgium. Patient, carer, assessor blind. No clear information on withdrawals given. No information on funding except all study drugs supplied by Pfizer. No dates for recruitment period. | |
| Participants | Adults with mixed cancer. 40 patients enrolled, 37 completed. | |
| Interventions | 2 groups. Ketoconazole 2 x 200 mg, once/day. Fluconazole 2 x 250 mg/day. Duration of therapy from 4 to 27 days, median 14 days. | |
| Outcomes | Clinical eradication, and improvement. Assessment made at days 4 to 27. Microbiological eradication of initial pathogen (culture). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "Randomisation chart". |
| Allocation concealment? | Low risk | Quote: "All study drugs were supplied by Pfizer and were administered in identical capsules". |
| Blinding of participants and carers? | Low risk | Quote: "All study drugs were supplied by Pfizer and were administered as identical capsules". |
| Blinding of outcome assessors? | Low risk | Quote: "....double‐blind". |
| Incomplete outcome data addressed? | Low risk | Quote: "Forty patients enrolled in the study, 3 were excluded (8%) before the code was opened". Comment: The reasons were given but not by group as the code was not broken. It is felt not to be a source of bias. |
| Free of selective reporting? | Low risk | Comment: Clinical and mycological eradication (culture). |
| Free of other biases? | Unclear risk | No information on funding except all study drugs supplied by Pfizer. |
Meunier 1990b.
| Methods | Randomised, parallel group study conducted in Belgium. Patient, carer not blind, unclear whether assessor blind. Unclear information on withdrawals. No information about funding. No dates for recruitment period. | |
| Participants | Patients with mixed cancer. 42 patients evaluated. | |
| Interventions | 2 groups. Ketoconazole tablets 200 mg every 8 hours. Nystatin 1000000 U suspension every 8 hours. Mean duration of ketoconazole was 13 days, nystatin 10 days, with maximum of 23 days for both groups. | |
| Outcomes | Clinical eradication of oropharyngeal candidiasis or oral thrush. Microbiological eradication of pathogen (culture). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "Patients were randomly allocated to one of the two arms of the study using a randomisation list". |
| Allocation concealment? | Low risk | Quote: "The allocations were placed in sealed envelopes numbered sequentially". Quote: "Randomisation was done by one of the investigators following the numerical order". |
| Blinding of participants and carers? | High risk | Comment: tablets and suspension. |
| Blinding of outcome assessors? | Unclear risk | No information. |
| Incomplete outcome data addressed? | Unclear risk | Comment: 2 ketoconazole patients had early discontinuation. All other patients were treated for at least 10 days. In nystatin group 3 patients died. It is unclear whether these patients were included in the 42 or not. |
| Free of selective reporting? | Low risk | Comment: Clinical and mycological eradication(culture) . |
| Free of other biases? | Unclear risk | No information about funding. |
Oude 2004.
| Methods | Randomised, parallel group, multicentre study conducted in Europe. Patients, carer and assessor not blind, but mycological assessment. No withdrawals. No information on funding. Recruitment between January 1992 and October 1997. | |
| Participants | Adults with mixed cancer. 279 randomised but only 252 eligible and evaluated. Of the 27 patients 23 were not eligible and 4 had no CRF. | |
| Interventions | 2 groups. Fluconazole capsules 100 mg per day for 10 days. Itraconazole capsules 200 mg per day for 15 days. | |
| Outcomes | Clinical and mycological eradication at day 15 (culture). Evaluated at days 3, 7, 10, 15 and post‐treatment assessment at day 42. | |
| Notes | It is surprising that the study was not published for 7 years. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "....patients were randomised....". |
| Allocation concealment? | Unclear risk | No information. |
| Blinding of participants and carers? | High risk | Quote: "An open multicentre comparative study....". |
| Blinding of outcome assessors? | High risk | Quote: "An open multicentre comparative study....". |
| Incomplete outcome data addressed? | Low risk | Comment: 4 patients had no CRF but unclear which group however we felt this was unlikely to cause bias. |
| Free of selective reporting? | Low risk | Comment: Clinical and mycological eradication (culture) . |
| Free of other biases? | Unclear risk | No information on funding. |
Shechtman 1984.
| Methods | Randomised, parallel group study conducted in USA. Patient, carer and assessor blind. Clear explanation of withdrawals. Funding from pharmaceutical company and charity. No dates for recruitment period. | |
| Participants | Adults with mixed cancer. 16 enrolled, 13 completed. | |
| Interventions | 2 groups, placebo versus clotrimazole 10 mg troche of clotrimazole 5 times/day (dissolving for 15 to 30 minutes). Duration 48 hours to 4 weeks. | |
| Outcomes | Clinical improvement with intention‐to‐treat analysis. Mycological not eradicated (culture). Unclear when assessment made. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "Eight patients were assigned by random allocation....". |
| Allocation concealment? | Unclear risk | No information given. |
| Blinding of participants and carers? | Low risk | Quote "....double blind clinical trial ....". Quote: "Neither the patient, microbiologist, physician or nurse know whether the patients were receiving placebo or clotrimazole". |
| Blinding of outcome assessors? | Low risk | Quote "....double blind clinical trial....". Quote: "Neither the patient, microbiologist, physician or nurse know whether the patients were receiving placebo or clotrimazole". |
| Incomplete outcome data addressed? | Low risk | Comment: 4 patients lost to follow‐up (25%), 2 in each group with known reasons. |
| Free of selective reporting? | Low risk | Comment: Clinical and mycological eradication (culture). |
| Free of other biases? | Unclear risk | Industry funding and charity grant. Miles pharmaceuticals provided "coded" drugs. |
Studena 1995.
| Methods | Randomised, parallel group study conducted in Slovac Republic. Patient, carer, not blind, unclear if assessor blind. Funding unclear. Recruitment 1.5.1992 until 1.5.1994. | |
| Participants | Adults with mixed cancer. 53 randomised and completed. | |
| Interventions | 2 groups. Fluconazole 10 days 100 mg OD or itraconazole 100 mg BID 15 days. | |
| Outcomes | Clinical and mycological eradication (culture) at 15 and 42 days. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "Patients were randomised....". |
| Allocation concealment? | Unclear risk | Comment: No information given. |
| Blinding of participants and carers? | High risk | Comment: Drugs taken over different periods. |
| Blinding of outcome assessors? | Unclear risk | Comment: No information given. |
| Incomplete outcome data addressed? | Low risk | Quote: "All cancer patients with neutrophil count more than 500 hospitalised at the National Cancer Centre clinical of the Post Graduate Medical School and Medical Faculty from 1.5.1992 to 1.5.1994 (53 patients) were randomised." Comment: Analysis on 53 patients, so no drop outs. |
| Free of selective reporting? | Low risk | Comment: Clinical and mycological eradication (culture). |
| Free of other biases? | Unclear risk | No information about funding. |
Yap 1979.
| Methods | Randomised, parallel group study conducted in USA. Patient, carer, assessor blind. No clear explanation of withdrawals. Pharaceutical and government funding. No recruitment dates given. | |
| Participants | Adults with mixed cancer. 56 patients, 60 episodes enrolled. 52 episodes, 48 patients completed. | |
| Interventions | 2 groups. 10 mg versus 50 mg troche clotrimazole, for 14 days. | |
| Outcomes | Clinical and mycological eradication (culture). Unclear when assessment made. | |
| Notes | As number of episodes 60 nearly same as number of patients so episodes used in analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "A randomised double blind trial....". Quote: "....a randomised double blind technique was used to divide the patients into two groups....". |
| Allocation concealment? | Unclear risk | No information given. |
| Blinding of participants and carers? | Low risk | Quote: "A randomised double blind trial....". |
| Blinding of outcome assessors? | Low risk | Quote: "A randomised double blind trial....". |
| Incomplete outcome data addressed? | Unclear risk | Quote: "56 cancer patients with 60 episodes of oropharyngeal candidiasis were entered into he study between September 1976 and September 1977". Quote: "Eight patients 8 episodes were considered inevaluable". Of the remaining 48 patients there were 52 episodes of infection. Comment: We don't know which group these patients were in. |
| Free of selective reporting? | Low risk | Comment: Clinical and mycological eradication (culture). |
| Free of other biases? | High risk | Quote: "If there was no clinical improvement after 5 days or the patients condition necessitated the start of systemic antifungal therapy, administration of the troches was discontinued". Possible bias due to episodes rather than patients and data not independent. Comment: Pharmaceutical and government funding. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anaissie 1996 | Patients with invasive candidiasis from 2 or more body sites were included (fluconazole versus amphotericin B). |
| Benhamou 1991 | Patients with and without fungal infection were included in study (ketoconazole versus placebo). |
| Bourhis 2004 | Empirical treatment of suspected fungal infections in neutropenic patients with fever. |
| Conrad 1990 | AIDS and malignancy patients. Data not presented separately (nystatin versus clotrimazole). |
| Domenge 1999 | Abstract, insufficient information (fluconazole versus amphotericin). |
| Fleming 2001 | Patients had 5 different conditions for entry including invasive fungal infection (amphotericin B versus AmBisome). |
| Holst 1984 | Not RCT (natamycin versus nystatin). |
| Jorgensen 2006 | Note on Walsh 2004, which is excluded (caspofungin versus amphotericin). |
| Kostiala 1982 | Episodes (85) not patients (53) (clotrimazole versus chlorhexidine). |
| Lake 1996 | Esophageal candidiasis present for entry into the study (fluconazole versus amphotericin B). |
| Lefebvre 2002 | Not all patients had oral candidiasis at the start of study (fluconazole versus amphotericin B). |
| Subira 2004 | All patients had to be hospitalised for neutropenic fever, but did not necessarily have oral candidiasis at entry to study (amphotericin B). |
| Urabe 1990 | Unclear if RCT (amphotericin B). |
| Verweij 1994 | Patients had histologically proved systemic mycosis for entry into the study (amphotericin B versus amphotericin B plus 5‐flucytosine). |
| Walsh 2002 | Not RCT (voriconazole). |
| Walsh 2004 | Empirical therapy only treating patients with infection (caspofungin versus amphotericin). |
| Yamaguchi 2006 | Patients who did not have cancer were included (translated from Japanese). |
RCT = randomised controlled trial
Contributions of authors
Jan Clarkson (JC) and Helen Worthington (HW) wrote the protocol and review. HW co‐ordinated the review and wrote the letters to authors. JC and HW independently and in duplicate assessed the eligibility of trials, extracted data and assessed the quality of the trials. HW conducted the statistical analysis which was interpreted by JC and HW. Tasneem Khalid provided advice on the interventions and Stefan Meyer and Martin McCabe provided input on the cancer treatments and the assessment of the candidiasis.
Sources of support
Internal sources
Scottish Executive Health Department, UK.
University of Dundee, UK.
University of Manchester, UK.
Scottish Council for Postgraduate Medical and Dental Education, UK.
Manchester Biomedical Research Centre, University of Manchester, UK.
Cancer Research UK, UK.
Teenage Cancer Trust, UK.
External sources
NIDCR grant ref 1 DE016950‐01, USA.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bensadoun 2008 {published data only}
- Bensadoun RJ, Daoud J, Gueddari B, Bastit L, Gourmet R, Rosikon A, et al. Comparison of the efficacy and safety of miconazole 50‐mg mucoadhesive buccal tablets with miconazole 500‐mg gel in the treatment of oropharyngeal candidiasis: a prospective, randomized, single‐blind, multicenter, comparative, phase III trial in patients treated with radiotherapy for head and neck cancer. Cancer 2008; Vol. 112, issue 1:204‐11. [DOI] [PubMed]
Finlay 1996 {published data only}
- Finlay PM, Richardson MD, Robertson AG. A comparative study of the efficacy of fluconazole and amphotericin B in the treatment of oropharyngeal candidosis in patients undergoing radiotherapy for head and neck tumours. British Journal of Oral & Maxillofacial Surgery 1996;34(1):23‐5. [DOI] [PubMed] [Google Scholar]
Flynn 1995 {published data only}
- Flynn PM, Cunningham CK, Kerkering T, San Jorge AR, Peters VB, Pitel PA, et al. Oropharyngeal candidiasis in immunocompromised children: a randomised, multicentre study of orally administered fluconazole suspension versus nystatin. Journal of Pediatrics 1995;127(2):322‐8. [DOI] [PubMed] [Google Scholar]
Hughes 1983 {published data only}
- Hughes WT, Bartley DL, Patterson GG, Tufenkeji H. Ketoconazole and candidiasis: a controlled study. Journal of Infectious Diseases 1983;147(6):1060‐3. [DOI] [PubMed] [Google Scholar]
Meunier 1990a {published data only}
- Meunier F, Aoun M, Gerard M. Therapy for oropharyngeal candidiasis in the immunocompromised host: a randomized double‐blind study of fluconazole vs. ketoconazole. Reviews of Infectious Diseases 1990;12(3):S364‐8. [DOI] [PubMed] [Google Scholar]
Meunier 1990b {published data only}
- Meunier F, Gerain J, Snoeck R. Oral treatment of oropharyngeal candidiasis with nystatin versus ketoconazole in cancer patients. Drug Investigation 1990;2(2):71‐5. [Google Scholar]
Oude 2004 {published data only}
- Oude Lashof AM, Bock R, Herbrecht R, Pauw BE, Krcmery V, Aoun M, et al. An open multicentre comparative study of the efficacy, safety and tolerance of fluconazole and itraconazole in the treatment of cancer patients with oropharyngeal candidiasis. European Journal of Cancer 2004;40(9):1314‐9. [DOI] [PubMed] [Google Scholar]
Shechtman 1984 {published data only}
- Shechtman LB, Funaro L, Robin T, Bottone EJ, Cuttner J. Clotrimazole treatment of oral candidiasis in patients with neoplastic disease. American Journal of Medicine 1984;76(1):91‐4. [DOI] [PubMed] [Google Scholar]
Studena 1995 {published data only}
- Studena V, Sycova Z, Helpianska L, Sorkovska D, Pichna P, Lacka J, et al. Fluconazole versus itraconazole in therapy of oropharyngeal candidiasis in cancer patients: a prospective comparative randomized trial. Journal of Chemotherapy 1995;7(4):204‐5. [PubMed] [Google Scholar]
Yap 1979 {published data only}
- Yap, B, Bodey GP. Oropharyngeal candidiasis treated with a troche form of clotrimazole. Archives of Internal Medicine 1979;139(6):656‐7. [PubMed] [Google Scholar]
References to studies excluded from this review
Anaissie 1996 {published data only}
- Anaissie EJ, Darouiche RO, Abi‐Said D, Uzun O, Mera J, Gentry LO, et al. Management of invasive candidal infections: results of a prospective, randomized, multicenter study of fluconazole versus amphotericin B and review of the literature. Clinical Infectious Diseases 1996;23(5):964‐72. [DOI] [PubMed] [Google Scholar]
Benhamou 1991 {published data only}
- Benhamou E, Hartmann O, Nogues C, Maraninchi D, Valteau D, Lemerle J. Does ketoconazole prevent fungal infection in children treated with high dose chemotherapy and bone marrow transplantation? Results of a randomized placebo‐controlled trial. Bone Marrow Transplantation 1991;7(2):127‐31. [PubMed] [Google Scholar]
Bourhis 2004 {published data only}
- Bourhis JH. Caspofungin in the empirical treatment of fungal infections. Journal de Mycologie Medicale 2004;14(4 II):240‐3. [Google Scholar]
Conrad 1990 {published data only}
- Conrad DA, Lentnek AL. Comparitive evaluation on nystatin pastille and clotrimazole troche for the treatment of candidal stomatitis in immunocompromised patients. Current Therapeutic Research, Clinical & Experimental 1990;47(4):627‐36. [Google Scholar]
Domenge 1999 {published data only}
- Domenge C, Wibauld P, Tancrede C, Sube B, Leridant AM, Marandas P, et al. Randomized study of Fluconozole (FCA) oral solution (OS) versus amphotericin B (AB) oral solution in oropharingeal candidiasis (OPC) in head and neck cancer patients (HNCP) after radiotherapy. Supportive Care in Cancer 1999;7(Suppl. Abstr. P‐180):210. [Google Scholar]
Fleming 2001 {published data only}
- Fleming RV, Kantarjian HM, Husni R, Rolston K, Lim J, Raad I, et al. Comparison of amphotericin B lipid complex (ABLC) vs. ambisome in the treatment of suspected or documented fungal infections in patients with leukemia. Leukemia and Lymphoma 2001;40(5‐6):511‐20. [DOI] [PubMed] [Google Scholar]
Holst 1984 {published data only}
- Holst E. Natamycin and nystatin for treatment of oral candidiasis during and after radiotherapy. The Journal of Prosthetic Dentistry 1984;51(2):226‐31. [DOI] [PubMed] [Google Scholar]
Jorgensen 2006 {published data only}
- Jorgensen KJ, Johansen HK, Gotzsche PC. Flaws in design, analysis and interpretation of Pfizer's antifungal trials of voriconazole and uncritical subsequent quotations. Trials 2006;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kostiala 1982 {published data only}
- Kostiala I, Kostiala AAI, Elonen E, Valtonen VV, Vuopio P. Comparison of clotrimazole and chlorhexidine in the topical treatment of acute fungal stomatitis in patients with hematological malignancies. Current Therapeutic Research 1982;31(5):752‐63. [Google Scholar]
Lake 1996 {published data only}
- Lake DE, Kunzweiler J, Beer M, Buell DN, Islam MZ. Fluconazole versus amphotericin B in the treatment of esophageal candidiasis in cancer patients. Chemotherapy 1996;42(4):308‐14. [DOI] [PubMed] [Google Scholar]
Lefebvre 2002 {published data only}
- Lefebvre JL, Domenge C. A comparative study of the efficacy and safety of fluconazole oral suspension and amphotericin B oral suspension in cancer patients with mucositis. Oral Oncology 2002;38(4):337‐42. [DOI] [PubMed] [Google Scholar]
Subira 2004 {published data only}
- Subira M, Martino R, Gomez L, Marti JM, Estany C, Sierra J. Low‐dose amphotericin b lipid complex vs. conventional amphotericin B for empirical antifungal therapy of neutropenic fever in patients with hematologic malignancies ‐ a randomized, controlled trial. European Journal of Haematology 2004;72(5):342‐7. [DOI] [PubMed] [Google Scholar]
Urabe 1990 {published data only}
- Urabe A, Takaku F, Mizoguchi H, Nomura T, Ogawa T, Maekawa T, et al. Prophylactic and therapeutic effects of oral administration of amphotericin B in mycosis associated with hematological diseases. The Japanese Journal of Antibiotics 1990;43(1):116‐30. [PubMed] [Google Scholar]
Verweij 1994 {published data only}
- Verweij PE, Donnelly JP, Kullberg BJ, Meis JF, Pauw BE. Amphotericin B versus amphotericin B plus 5‐flucytosine: poor results in the treatment of proven systemic mycoses in neutropenic patients. Infection 1994;22(2):81‐5. [DOI] [PubMed] [Google Scholar]
Walsh 2002 {published data only}
- Walsh TJ, Lutsar I, Driscoll T, Dupont B, Roden M, Ghahramani P, et al. Voriconazole in the treatment of aspergillosis, scedosporiosis and other invasive fungal infections in children. Pediatric Infectious Disease Journal 2002;21(3):240‐8. [DOI] [PubMed] [Google Scholar]
Walsh 2004 {published data only}
- Walsh TJ, Teppler H, Donowitz GR, Maertens JA, Baden LR, Dmoszynska A, et al. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. New England Journal of Medicine 2004;351(14):1391‐402. [DOI] [PubMed] [Google Scholar]
Yamaguchi 2006 {published data only}
- Yamaguchi H, Enomoto S, Kaku M, Sakamaki H, Tanaka K, Yoshida M. [An open randomized parallel‐comparison study of itraconazole oral solution versus itraconazole capsules in treatment of patients with oropharyngeal candidiasis]. Japanese Journal of Chemotherapy 2006; Vol. 54, issue Suppl 1:18‐31.
Additional references
BNF 2009
- Joint Formulary Committee. British National Formulary. 57. London: British Medical Association and Royal Pharmaceutical Society of Great Britain, 2009. [Google Scholar]
Clarkson 2007a
- Clarkson JE, Worthington HV, Eden OB. Interventions for preventing oral candidiasis for patients with cancer receiving treatment. Cochrane Database of Systematic Reviews 2007, Issue 1. [Art. No.: CD003807. DOI: 10.1002/14651858.CD003807.pub3] [DOI] [PubMed] [Google Scholar]
Clarkson 2007b
- Clarkson JE, Worthington HV, Eden OB. Interventions for treating oral mucositis for patients with cancer receiving treatment. Cochrane Database of Systematic Reviews 2007, Issue 2. [Art. No.: CD001973. DOI: 10.1002/14651858.CD001973.pub3] [DOI] [PubMed] [Google Scholar]
Gotzsche 2002
- Gøtzsche PC, Johansen HK. Routine versus selective antifungal administration for control of fungal infections in patients with cancer. Cochrane Database of Systematic Reviews 2002, Issue 2. [Art. No.: CD000026. DOI: 10.1002/14651858.CD000026] [DOI] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (updated September 2009). The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Worthington 2007
- Worthington HV, Clarkson JE, Eden OB. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database of Systematic Reviews 2007, Issue 4. [Art. No.: CD000978. DOI: 10.1002/14651858.CD000978.pub3] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Clarkson 2004
- Clarkson JE, Worthington HV, Eden OB. Interventions for treating oral candidiasis for patients with cancer receiving treatment. Cochrane Database of Systematic Reviews 2004, Issue 1. [Art. No.: CD001972. DOI: 10.1002/14651858.CD001972.pub2] [DOI] [PubMed] [Google Scholar]


