Abstract
Purpose
Kras mutation and abnormal immune status are associated with pancreatic cancer development and progression. In this study, we evaluated the Kras mutation status in circulating tumor DNA and circulating T cell subsets in a cohort of advanced pancreatic cancer patients.
Methods
Samples were retrospectively obtained from a series of 210 pathological advanced pancreatic cancer patients between 2012 and 2014. The Kras mutation status was detected in cell‐free circulating tumor DNA (ctDNA) by ddPCR and circulating T cell subsets were analyzed by flow cytometry.
Results
Univariate analysis found that tumor node metastasis (TNM) stage, chemotherapy, circulating regulatory T cells, CA19‐9 levels, CA125 levels, and KrasG12D and KrasG12V mutations were significantly related to overall survival in advanced pancreatic cancer patients. Multivariate analysis identified that TNM stage (P = .03, HR:1.422), Tregs (P = .004, HR:1.522), CA19‐9 levels (P = .009, HR:1.488), KrasG12D mutation (P = .044, HR:1.353), and KrasG12V mutation (P = .001, HR:1.667) were independent prognostic markers. Furthermore, we found that KrasG12V mutation in ctDNA was correlated with high circulating proportion of Tregs, and patients with both KrasG12V mutation and high levels of Tregs were associated with extremely poor survival in advanced pancreatic cancer.
Conclusion
KrasG12V mutation was associated with high circulating regulatory T cell levels, and both of them predicted worse prognosis in advanced pancreatic cancer patients.
Keywords: Kras mutation, pancreatic cancer, prognosis, Tregs
KrasG12V mutation in ctDNA was correlated with high circulating proportion of Tregs. Patients with both KrasG12V mutation and high levels of Tregs were associated with extremely poor survival in advanced pancreatic cancer.
![]()
1. INTRODUCTION
Pancreatic cancer is one of the most lethal cancer with an extremely poor prognosis. It was supposed to be the second leading cause of cancer‐related deaths in the USA by the year 2020.1 About 80%‐85% of patients are diagnosed at advanced stage because of lacking specific symptoms, and lose the opportunity for radical surgery.2 Chemotherapy is the preferred option for these patients and there has been great progress in recent years.3, 4 However, 5‐year survival rate in advanced pancreatic cancer is still less than 5%. As it is invasive and uneasy to obtain enough tumor tissues in advanced pancreatic cancer, CA19‐9 is the most used noninvasive prognostic markers in these patients but with several limitations.5 It is necessary to identify other circulating prognostic biomarkers.
Cell‐free circulating tumor DNA (ctDNA), also known as liquid biopsy, is a noninvasive biomarker in various cancer.6 It was reported that specific gene mutations in ctDNA can be used as diagnostic and prognostic markers in pancreatic cancer.7 Kras is the most frequently reported oncogenic mutation in ctDNA of pancreatic cancer with the rate ranging from 65% to 85%.8 Several studies identified that Kras mutation in ctDNA plays a prognostic role in pancreatic cancer.9 However, Kras‐related target therapy or immune treatment almost failed to improve survival in clinical trials.10, 11
Immune disorder frequently occurred in pancreatic cancer and associated with the tumor progression and development. Abnormal distribution of T cell subsets such as high level of regulatory T cells (Tregs) and low level of cytotoxic T cells contributed to immunosuppressive environment in pancreatic cancer, and led to the escape of tumor cells from immune surveillance.12 It was reported that mutated Kras is associated with T cell differentiation and function in colorectal and lung cancers.13 Our previous study also found that Kras mutation correlates with Tregs infiltration in resectable pancreatic cancer tissues.14 However, the possible association of Kras mutation and T cell subsets distribution in circulating peripheral blood of pancreatic cancer has not been elaborated to date. Therefore, in this study, we focused on the potential correlation between the Kras mutation in ctDNA and circulating T cell subsets in a cohort of Chinese patients with advanced pancreatic cancer.
2. MATERIAL AND METHODS
2.1. Study population
This study included 210 advanced pancreatic cancer patients with pathologically confirmed adenocarcinoma in our center from 2012 to 2014. All the patients did not receive any anticancer treatments before the first hospitalization in our center. Tumor node metastasis (TNM) stage was defined by AJCC TNM staging of pancreatic cancer 2018, and patients with stage III and IV were included. Overall survival (OS) was measured by the date of diagnosis to the time of death, and the clinical parameters were obtained from electronic records. The final date of follow‐up was January 2019. Written informed consent was obtained from each patient. This study was approved by the Clinical Research Ethic Committee of Shanghai Cancer Center.
2.2. T cell subsets detected by flow cytometry
Peripheral blood samples were collected in heparinized tubes at admission, and processed for flow cytometry within 2 hours. To identify different T cell subsets, anti‐CD3, anti‐CD4, anti‐CD8, anti‐CD25, and anti‐CD127 from BD Bioscience were used. A minimum of 10,000 events gated on the population of interest were analyzed. The experimental steps for flow cytometry to identify different T cell subsets in peripheral blood sample have been described in detail previously.15
2.3. CTDNA mutation detected using droplet digital PCR (DDPCR)
Circulating DNA was isolated and collected from about 5 mL of plasma according to the QIAamp Circulating Acid Kit (Qiagen), and then processed to droplet digital PCR to detect the Kras mutation levels of circulating tumor DNA. Primers and probes for detection of KrasG12V and KrasG12D mutation were acquired following the experimental protocol (Bio‐Rad Laboratories). Kras‐G12V‐F (Forward primer): TGCTGAAAATGACTGAATATAAACTTGTG, Kras‐G12V‐R (Reverse primer): AGCTGTATCGTCAAGGCACTCTT and Kras‐G12V‐P (Probe): TTGGAGCTGTTGGC; Kras‐G12D‐F: TGCTGAAAATGACTGAATATAAACTTGTG, Kras‐G12V‐D: AGCTGTATCGTCAAGGCACTCTT and Kras‐G12D‐P: TGGAGCTGATGGCGT. Detailed steps for ddPCR were previously described.8 For the threshold of ddPCR determination, positive result was identified as PCR monodispersed droplets had a fluorescence signal, while none fluorescence signal represented none mutation (Figure S1 and S2).
2.4. Statistical analysis
The statistical analyses were conducted using SPSS version 19.0 software. Kaplan‐Meier method was used to plot the survival curve. The independent prognostic factors were identified through univariate and multivariate analyses using the Cox proportional hazard regression model. Continuous variable data between two groups were compared by the student's t test. Significant difference was defined as a P‐value < .05.
3. RESULTS
3.1. Patient characteristics
We retrospectively collected data from 210 advanced pancreatic cancer patients including 71 locally advanced and 139 metastatic cases. The basic features of these patients are listed in Table 1. The median age of this group patients was 63 years old (range from 33 to 79 years). At the time of last follow‐up, all the patients died. Among the 210 patients, 178 (84.8%) patients received gemcitabine‐based or 5‐FU‐based chemotherapy, and other 32 (15.2%) patients accepted only best supportive care. In addition, we also detected the Kras mutation status in ctDNA and circulating T cell subsets in this group patients; the KrasG12V mutation was detected in 61 (29%) cases and KrasG12D mutation in 93 (44.3%) cases. The mean values of CD3 + CD4+ T cells, CD3+ CD8+ T cells, and Tregs were 38.9%±9.0%, 22.7%±9.2%, and 9.1%±3.3%, respectively.
Table 1.
Clinicopathological parameters of patients with advanced pancreatic cancer (n = 210)
| Parameter | Category | No | % |
|---|---|---|---|
| Age | <65 | 139 | 66.2% |
| ≥65 | 71 | 33.8% | |
| Gender | Male | 132 | 62.9% |
| Female | 78 | 37.1% | |
| Stage | III | 71 | 33.8% |
| IV | 139 | 66.2% | |
| Chemotherapy | Yes | 178 | 84.8% |
| No | 32 | 15.2% | |
| CA19‐9 level | <1000 U/mL | 130 | 61.9% |
| ≥1000 U/mL | 80 | 38.1% | |
| CA125 level | <35 U/mL | 88 | 41.9% |
| ≥35 U/mL | 122 | 58.1% | |
| Kras G12V | Mutation | 61 | 29% |
| None G12V mutation | 149 | 71% | |
| Kras G12D | Mutation | 93 | 44.3% |
| None G12D mutation | 117 | 55.7% |
3.2. The prognostic role of KRAS mutation and circulating T cell subsets in patients with advanced pancreatic cancer via univariate and multivariate analyses
The cutoff for CA19‐9 and CA125 were 1000 U/mL and 35 U/mL according to our previous studies.16, 17 We chose the median value of CD3+ CD4+ T cells (38.99%), CD3+ CD8+ T cells (21.06%), and Tregs (8.66%) as cutoff, respectively. The association between various clinicopathological factors and OS is shown in Table 2. Overall survival curves are presented by Kaplan‐Meier analysis in Figure 1. Univariate analysis revealed that TNM stage, chemotherapy, Tregs, CA19‐9 levels, CA125 levels, and KrasG12V and KrasG12D mutations were significantly associated with OS, while age, gender, CD3+ CD4+ T cells, and CD3+ CD8+ T cells have no sense for prognosis. Furthermore, multivariate analysis identified stage IV (P = .03), high proportion of Tregs (P = .004), CA19‐9 ≥ 1000U/ml (P = .009), KrasG12V mutation (P = .001), and KrasG12D mutation (P = .044) as independent poor prognostic factors for OS in these advanced pancreatic cancer cases.
Table 2.
Univariate and multivariate analyses of clinicopathological parameters for the prediction of overall survival in patients with advanced pancreatic cancer (n = 210)
| Parameters | Univariate analyses | Multivariate analyses | ||
|---|---|---|---|---|
| P | HR (95%CI) | P | HR (95%CI) | |
| Age (years): <65 vs ≥65 | .145 | — | — | — |
| Gender: Male vs Female | .766 | — | — | — |
| TNM stage: IV vs III | .001 | 1.626 (1.212‐2.179) | .03 | 1.422 (1.034‐1.957) |
| Chemotherapy: Yes vs No | .018 | 0.632 (0.432‐0.924) | .066 | 0.698 (0.476‐1.025) |
| Tregs: High vs Low (Median:8.66%) | .007 | 1.458 (1.109‐1.912) | .004 | 1.522 (1.143‐2.028) |
| CD3+ CD4+ T cells: High vs Low (Median:38.99%) | .211 | 1.189 (0.906‐1.56) | — | — |
| CD3+ CD8+ T cells: High vs Low (Median:21.06%) | .494 | 0.909 (0.69‐1.196) | — | — |
| Kras G12V | .002 | 1.616 (1.192‐2.183) | .001 | 1.667 (1.217‐2.028) |
| Mutation vs None | ||||
| Kras G12D | .002 | 1.577 (1.188‐2.092) | .044 | 1.353 (1.009‐1.815) |
| Mutation vs None | ||||
| CA19‐9 level (U/mL) | <.001 | 1.822 (1.367‐2.429) | .009 | 1.488 (1.103‐2.008) |
| ≥1000 vs <1000 | ||||
| CA125 level (U/mL) | <.001 | 0.576 (0.434‐0.764) | .055 | 0.747 (0.555‐1.007) |
| <35 vs ≥35 | ||||
Abbreviation: 95%CI, 95% confidence interval; HR: hazard ratio.
Figure 1.
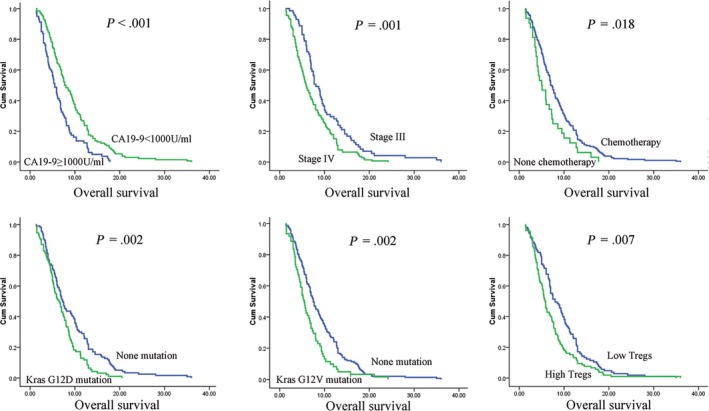
Kaplan‐Meier analyses of the overall survival difference in patients with advanced pancreatic cancer. Groups were compared by univariate analysis
3.3. The status of KRAS mutation correlates with circulating regulatory T cells to further stratify OS in patients with advanced pancreatic cancer patients
It was reported that Kras mutation was associated with Tregs infiltration in various tumor tissues.13, 14, 18 Therefore, we analyzed the potential correlation between Kras mutation status and Tregs distribution in peripheral blood samples in advanced pancreatic cancer. Interestingly, we found that KrasG12V mutation was notably associated with high levels of Tregs (P = .028), while KrasG12D had no relationship with Tregs (Figure 2). As both KrasG12V mutation and Tregs were independent prognostic factors in this study, patients were divided into three groups: 1. KrasG12V mutation (+) and high Tregs; 2. KrasG12V mutation (+), low Tregs or KrasG12V mutation (−), high Tregs; 3. KrasG12V mutation (−) and low Tregs. Kaplan‐Meier analysis with a log‐rank test found that patients with both KrasG12V mutation and high Tregs (n = 32) had the worst survival with a median OS of 4.5 m (95%CI: 3.53‐5.47 m), whereas those with none KrasG12V mutation and low Tregs (n = 76) had a median OS of 8.5 m (95%CI: 6.26‐10.73 m; P < .001), predicting a better prognosis (Figure 3 and Table 3).
Figure 2.
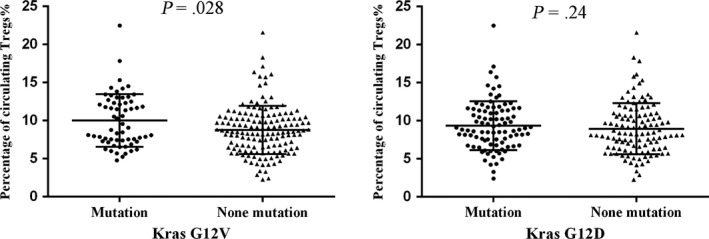
KrasG12V mutation was associated with a high proportion of Tregs, while KrasG12D mutation was not
Figure 3.
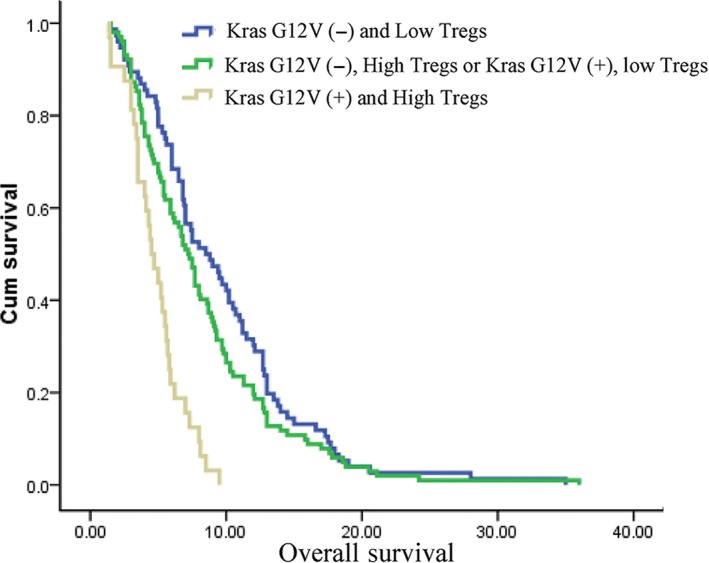
Combination of KrasG12V mutation and regulatory T cells further stratify prognosis in advanced pancreatic cancer patients
Table 3.
The overall survival stratified by combination of KrasG12V mutation and Tregs
| Group | Number | Median OS (mon) | 95% Confidence Interval |
|---|---|---|---|
| 1. KrasG12V (−) and Low Tregs | 76 | 8.5 | 6.26‐10.74 |
| 2. KrasG12V (−), High Tregs or KrasG12V (+), Low Tregs | 102 | 7.2 | 6.29‐8.11 |
| 3. KrasG12V (+) and High Tregs | 32 | 4.5 | 3.53‐5.47 |
P < .001.
4. DISCUSSION
The genetic landscape of pancreatic cancer is notable for activating Kras mutation and inactivation of smad4, TP53, and CDKN2A. Among these four driver genes, Kras is the most frequent mutated gene, and runs through the initiation, progression, and metastasis of pancreatic cancer.19 Scientists had already been aware of the importance of Kras mutation in pancreatic cancer, and inhibition of Kras activity in mice model of pancreatic cancer induced tumor regression.20 However, almost all treatments against Kras failed to improve prognosis in clinical trials. It was reported that Kras mutation activates several key pathways to allow tumor cells growth and metastasis.11 The prognostic role of Kras mutation in pancreatic cancer is still controversy and inconsistent.21 It was reported that Kras mutation detected in pancreatic cancer tissues associated with worse disease‐free survival and OS compared with Kras wild‐type tumors. In addition, subtype analysis revealed that patients with KrasG12D mutation had an extremely poor prognosis with a median OS of 15.3 months in resectable pancreatic cancer, while other studies showed different results.22, 23 Change of Kras mutation in ctDNA could also be used to monitor treatment response in metastatic pancreatic cancer and Kras mutation detected in ctDNA after surgery is associated with early recurrence and metastasis.8, 24 Two patterns (G12V and G12D) of Kras mutation account for about 90% of all mutations in pancreatic cancer and both mutation rates range from 30% to 50%. Therefore, in this study, we detected these two mutation sites in ctDNA of advanced pancreatic cancer, and found that both KrasG12D mutation and KrasG12V mutation were associated with poor prognosis, which was consistent with other studies.25
Pancreatic cancer is characteristically surrounded by abundant stroma, which caused a hypoxia status and abnormal immune environment.26 T cell subset abnormal distribution and dysfunction are important features of immunosuppresive status in pancreatic cancer.15 Tregs is a classic immune‐suppressive T cell subset, which secretes various cytokines to inhibit CD8+ T cell function and allows tumor cells escape from immune surveillance.27 It was reported that high Tregs infiltration in tumor tissues was associated with poor OS.12 In this study, we found that high proportion of Tregs in peripheral blood was an independent negative prognostic factor for advanced pancreatic cancer patients.
Increasing evidences revealed that there is a crosstalk between Kras mutation and T cell immune disorder in Kras mutation tumors.13, 28 Pancreatic cancer cells with oncogenic Kras mutation secrete various important molecules to affect components of the stroma, such as innate and adaptive immune cells.29, 30 These cells in turn promote and maintain tumor growth and metastasis. Several studies identified that KrasG12D or KrasG12V mutation contributes to T cell differentiation in colorectal and lung cancer cells.13, 31 Our previous studies also found that KrasG12D mutation is associated with high Tregs infiltration in resectable pancreatic cancer tissues.14 However, the potential correlation of Kras mutation and T cell subsets is still unclear in advanced pancreatic cancer. Endoscopic ultrasound‐guide fine‐needle aspiration (EUS‐FNA) is an invasive approach and often obtain insufficient tissues for infiltrating immune cell and Kras mutation detection, and therefore, we identified Kras mutation status in ctDNA and also detected T cell subsets proportion in peripheral blood samples. We found that KrasG12V mutation, not KrasG12D, was associated with high proportion of Tregs. In addition, KrasG12V mutation combined with a high proportion of Tregs correlated strongly with poor survival.
Palliative chemotherapy is the main and standard treatment for advanced pancreatic cancer, but the outcomes are diverse from suboptimal. Patients with adverse prognostic factors, such as Kras mutation and high Tregs, might benefit from more aggressive multiagent scheme. Moreover, understanding the detailed molecular events of patients with high‐risk negative prognostic factors in advanced pancreatic cancer may help guide the treatment strategy and improve OS.
There are several limitations in this study. Firstly, this is a retrospective study with relatively low evidence grade and lack of continuous samples after chemotherapy for monitoring treatment responses. Secondly, as tumor tissues or cells obtained by EUS‐FNA are few and mostly used for diagnosis, it was uneasy to detect the T cell infiltration in pancreatic cancer tissues. Therefore, we were unable to detect the correlation of Kras mutation and T cell infiltration in advanced pancreatic cancer. At last, the potential mechanism underlying this correlation is not elaborated in this clinical study.
5. CONCLUSION
In summary, this study identified potential circulating biomarkers to predict prognosis in advanced pancreatic cancer. We found that KrasG12V mutation in ctDNA was correlated with suppressive immune status marked with high proportion of Tregs in peripheral blood for the first time. Combining these two factors could further stratify advanced pancreatic cancer into different prognostic subgroups. Further studies should demonstrate the detailed mechanism about the relationship between Kras mutation and immune disorder.
Supporting information
ACKNOWLEDGMENT
This work was supported by the National Natural Science Foundation of China grants (81871940, 81902417) and The National Science Fund for Distinguished Young Scholars (81625016).
Cheng H, Luo G, Jin K, et al. Kras mutation correlating with circulating regulatory T cells predicts the prognosis of advanced pancreatic cancer patients. Cancer Med. 2020;9:2153–2159. 10.1002/cam4.2895
He Cheng and Guopei Luo contributed equally to this article.
Contributor Information
Xianjun Yu, Email: yuxianjun@fudanpci.org.
Chen Liu, Email: liuchen@fudanpci.org.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7‐30. [DOI] [PubMed] [Google Scholar]
- 2. Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388(10039):73‐85. [DOI] [PubMed] [Google Scholar]
- 3. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab‐paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691‐1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817‐1825. [DOI] [PubMed] [Google Scholar]
- 5. Luo G, Liu C, Guo M, et al. Potential biomarkers in Lewis negative patients with pancreatic cancer. Ann Surg. 2017;265(4):800‐805. [DOI] [PubMed] [Google Scholar]
- 6. Diaz LA Jr, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32(6):579‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kinugasa H, Nouso K, Miyahara K, et al. Detection of K‐ras gene mutation by liquid biopsy in patients with pancreatic cancer. Cancer. 2015;121(13):2271‐2280. [DOI] [PubMed] [Google Scholar]
- 8. Cheng HE, Liu C, Jiang J, et al. Analysis of ctDNA to predict prognosis and monitor treatment responses in metastatic pancreatic cancer patients. Int J Cancer. 2017;140(10):2344‐2350. [DOI] [PubMed] [Google Scholar]
- 9. Chen L, Zhang Y, Cheng Y, Zhang D, Zhu S, Ma X. Prognostic value of circulating cell‐free DNA in patients with pancreatic cancer: a systemic review and meta‐analysis. Gene. 2018;679:328‐334. [DOI] [PubMed] [Google Scholar]
- 10. Gjertsen MK, Bakka A, Breivik J, et al. Ex vivo ras peptide vaccination in patients with advanced pancreatic cancer: results of a phase I/II study. Int J Cancer. 1996;65(4):450‐453. [DOI] [PubMed] [Google Scholar]
- 11. Eser S, Schnieke A, Schneider G, Saur D. Oncogenic KRAS signalling in pancreatic cancer. Br J Cancer. 2014;111(5):817‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tang Y, Xu X, Guo S, et al. An increased abundance of tumor‐infiltrating regulatory T cells is correlated with the progression and prognosis of pancreatic ductal adenocarcinoma. PLoS ONE. 2014;9(3):e91551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zdanov S, Mandapathil M, Abu Eid R, et al. Mutant KRAS conversion of conventional T cells into regulatory T cells. Cancer Immunol Res. 2016;4(4):354‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheng HE, Fan K, Luo G, et al. KrasG12D mutation contributes to regulatory T cell conversion through activation of the MEK/ERK pathway in pancreatic cancer. Cancer Lett. 2019;446:103‐111. [DOI] [PubMed] [Google Scholar]
- 15. Xu Y‐F, Lu YU, Cheng HE, et al. Abnormal distribution of peripheral lymphocyte subsets induced by PDAC modulates overall survival. Pancreatology. 2014;14(4):295‐301. [DOI] [PubMed] [Google Scholar]
- 16. Yang C, Cheng H, Luo G, et al. The metastasis status and tumor burden‐associated CA125 level combined with the CD4/CD8 ratio predicts the prognosis of patients with advanced pancreatic cancer: a new scoring system. Eur J Surg Oncol. 2017;43(11):2112‐2118. [DOI] [PubMed] [Google Scholar]
- 17. Liu C, Cheng HE, Luo G, et al. Circulating regulatory T cell subsets predict overall survival of patients with unresectable pancreatic cancer. Int J Oncol. 2017;51(2):686‐694. [DOI] [PubMed] [Google Scholar]
- 18. Adeegbe DO, Liu S, Hattersley MM, et al. BET bromodomain inhibition cooperates with PD‐1 blockade to facilitate antitumor response in Kras‐mutant non‐small cell lung cancer. Cancer Immunol Res. 2018;6(10):1234‐1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Waddell N, Pajic M, Patch A‐M, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518(7540):495‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Collins MA, Bednar F, Zhang Y, et al. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest. 2012;122(2):639‐653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bournet B, Buscail C, Muscari F, Cordelier P, Buscail L. Targeting KRAS for diagnosis, prognosis, and treatment of pancreatic cancer: hopes and realities. Eur J Cancer. 2016;54:75‐83. [DOI] [PubMed] [Google Scholar]
- 22. Ako S, Nouso K, Kinugasa H, et al. Utility of serum DNA as a marker for KRAS mutations in pancreatic cancer tissue. Pancreatology. 2017;17(2):285‐290. [DOI] [PubMed] [Google Scholar]
- 23. Bournet B, Muscari F, Buscail C, et al. KRAS G12D mutation subtype is a prognostic factor for advanced pancreatic adenocarcinoma. Clin Transl Gastroenterol. 2016;7:e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakano Y, Kitago M, Matsuda S, et al. KRAS mutations in cell‐free DNA from preoperative and postoperative sera as a pancreatic cancer marker: a retrospective study. Br J Cancer. 2018;118(5):662‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Montagut C, Vidal J, Visa L. KRAS mutations in ctDNA: a promising new biomarker in advanced pancreatic cancer. Ann Oncol. 2018;29(12):2280‐2282. [DOI] [PubMed] [Google Scholar]
- 26. Wang WQ, Liu L, Xu JZ, Yu XJ. Reflections on depletion of tumor stroma in pancreatic cancer. Biochim Biophys Acta Rev Cancer. 2019;1871(2):267‐272. [DOI] [PubMed] [Google Scholar]
- 27. Banerjee A, Vasanthakumar A, Grigoriadis G. Modulating T regulatory cells in cancer: how close are we? Immunol Cell Biol. 2013;91(5):340‐349. [DOI] [PubMed] [Google Scholar]
- 28. Gjertsen MK, Breivik J, Saeterdal I, et al. Vaccination with mutant ras peptides and induction of T‐cell responsiveness in pancreatic carcinoma patients carrying the corresponding RAS mutation. Lancet. 1995;346(8987):1399‐1400. [DOI] [PubMed] [Google Scholar]
- 29. Chang DZ, Ma Y, Ji B, et al. Mast cells in tumor microenvironment promotes the in vivo growth of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2011;17(22):7015‐7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pylayeva‐Gupta Y, Lee KE, Hajdu CH, Miller G, Bar‐Sagi D. Oncogenic Kras‐induced GM‐CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21(6):836‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lal N, White BS, Goussous G, et al. KRAS mutation and consensus molecular subtypes 2 and 3 are independently associated with reduced immune infiltration and reactivity in colorectal cancer. Clin Cancer Res. 2018;24(1):224‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


