Abstract
Background
Healthcare professionals frequently advise people to improve their health by stopping smoking. Such advice may be brief, or part of more intensive interventions.
Objectives
The aims of this review were to assess the effectiveness of advice from physicians in promoting smoking cessation; to compare minimal interventions by physicians with more intensive interventions; to assess the effectiveness of various aids to advice in promoting smoking cessation, and to determine the effect of anti‐smoking advice on disease‐specific and all‐cause mortality.
Search methods
We searched the Cochrane Tobacco Addiction Group trials register in January 2013 for trials of interventions involving physicians. We also searched Latin American databases through BVS (Virtual Library in Health) in February 2013.
Selection criteria
Randomised trials of smoking cessation advice from a medical practitioner in which abstinence was assessed at least six months after advice was first provided.
Data collection and analysis
We extracted data in duplicate on the setting in which advice was given, type of advice given (minimal or intensive), and whether aids to advice were used, the outcome measures, method of randomisation and completeness of follow‐up.
The main outcome measure was abstinence from smoking after at least six months follow‐up. We also considered the effect of advice on mortality where long‐term follow‐up data were available. We used the most rigorous definition of abstinence in each trial, and biochemically validated rates where available. People lost to follow‐up were counted as smokers. Effects were expressed as relative risks. Where possible, we performed meta‐analysis using a Mantel‐Haenszel fixed‐effect model.
Main results
We identified 42 trials, conducted between 1972 and 2012, including over 31,000 smokers. In some trials, participants were at risk of specified diseases (chest disease, diabetes, ischaemic heart disease), but most were from unselected populations. The most common setting for delivery of advice was primary care. Other settings included hospital wards and outpatient clinics, and industrial clinics.
Pooled data from 17 trials of brief advice versus no advice (or usual care) detected a significant increase in the rate of quitting (relative risk (RR) 1.66, 95% confidence interval (CI) 1.42 to 1.94). Amongst 11 trials where the intervention was judged to be more intensive the estimated effect was higher (RR 1.84, 95% CI 1.60 to 2.13) but there was no statistical difference between the intensive and minimal subgroups. Direct comparison of intensive versus minimal advice showed a small advantage of intensive advice (RR 1.37, 95% CI 1.20 to 1.56). Direct comparison also suggested a small benefit of follow‐up visits. Only one study determined the effect of smoking advice on mortality. This study found no statistically significant differences in death rates at 20 years follow‐up.
Authors' conclusions
Simple advice has a small effect on cessation rates. Assuming an unassisted quit rate of 2 to 3%, a brief advice intervention can increase quitting by a further 1 to 3%. Additional components appear to have only a small effect, though there is a small additional benefit of more intensive interventions compared to very brief interventions.
Plain language summary
Does advice from doctors encourage people who smoke to quit
Advice from doctors helps people who smoke to quit. Even when doctors provide brief simple advice about quitting smoking this increases the likelihood that someone who smokes will successfully quit and remain a nonsmoker 12 months later. More intensive advice may result in slightly higher rates of quitting. Providing follow‐up support after offering the advice may increase the quit rates slightly.
Background
The role of healthcare professionals in smoking cessation has been the subject of considerable debate (Chapman 1993). During the late 1980s there was evidence from some randomised trials to suggest that advice from motivated physicians to their smoking patients could be effective in facilitating smoking cessation (Kottke 1988). However, concern was expressed about the low detection rate of smokers by many physicians and the small proportion of smokers who routinely receive advice from their physicians to quit (Dickinson 1989).
From a public health perspective, even if the effectiveness of facilitating smoking cessation by physicians is small, provided large numbers of physicians offer advice the net effect on reducing smoking rates could still be substantial (Chapman 1993). Since that time, there have been numerous attempts to encourage physicians to routinely identify all people who smoke and to provide smoking cessation advice (Fiore 1996; Fiore 2008; Raw 1998; Taylor 1994; West 2000).
The first systematic review on this topic was published over two decades ago (Kottke 1988). Since then a number of further studies have examined the effectiveness of medical practitioners in facilitating smoking cessation. Much of this research has occurred amidst a culture in which medical practitioners are playing an increasing role in health education and health promotion, and have an increasing array of options to assist people who want to quit. Doctors now have access to pharmacotherapies that have been shown to increase the chances of success for people making quit attempts, including nicotine replacement therapy (Stead 2012), bupropion (Hughes 2007) and varenicline (Cahill 2012). In some healthcare settings they can also refer people to more intensive behavioural counselling and support, either face‐to‐face (Lancaster 2005a; Stead 2005) or via telephone quitline services (Stead 2006). Specific theory‐based interventions have also been tested, based on approaches including the Transtheoretical Model (Velicer 2000; Cahill 2010), and motivational interviewing (Rollnick 1997; Lai 2010).
Objectives
The primary objective of the review was to determine the effectiveness of advice from medical practitioners in promoting smoking cessation. A secondary objective (added in 1996) was to determine the effectiveness of advice from medical practitioners on reducing smoking‐related mortality and morbidity. Our a priori hypotheses were:
advice from a medical practitioner to stop smoking is more effective than not giving advice.
the effectiveness of advice from a medical practitioner is greater if the advice is more intensive and includes follow‐up.
the supplementation of advice with aids such as self help manuals is more effective than advice alone.
motivational advice is more effective than simple advice (added in 2001 update).
The review does not address the incremental effects of adding nicotine replacement therapy or other pharmacotherapies to advice, as these interventions are addressed in separate Cochrane reviews (Cahill 2012; Hughes 2007; Stead 2012). From 2008 it does not address the incremental effect of demonstrating the pathophysiological effect of smoking (e.g. spirometry, expired carbon monoxide), which is covered by a separate Cochrane review (Bize 2012).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials. Trials where allocation to treatment was by a quasi‐randomised method were also included, but appropriate sensitivity analysis was used to determine whether their inclusion altered the results. Studies which used historical controls were excluded.
Types of participants
Participants could be smokers of either gender recruited in any setting, the only exception being trials which only recruited pregnant women. These were excluded since they are reviewed elsewhere (Lumley 2009).
Types of interventions
We included trials if they compared physician advice to stop smoking versus no advice (or usual care), or compared differing levels of physician advice to stop smoking. We defined advice as verbal instructions from the physician with a 'stop smoking' message irrespective of whether or not information was provided about the harmful effects of smoking. We excluded studies in which participants were randomised to receive advice versus advice plus some form of nicotine replacement therapy, since these were primarily comparisons of the effectiveness of NRT rather than advice. We excluded studies where advice to stop smoking was included as part of multifactorial lifestyle counselling (e.g. including dietary and exercise advice).
Therapists were physicians, or physicians supported by another healthcare worker. Trials which randomised therapists rather than smokers were included except where the therapists were randomised to receive an educational intervention in smoking cessation advice, since this is the subject of another Cochrane review (Carson 2012).
We defined trials where advice was provided (with or without a leaflet) during a single consultation lasting less than 20 minutes plus up to one follow‐up visit as minimal intervention. We defined a trial as intensive when the intervention involved a greater time commitment at the initial consultation, the use of additional materials other than a leaflet, or more than one follow‐up visit. We considered adjunctive aids to advice as additional strategies other than simple leaflets (e.g. demonstration of expired carbon monoxide or pulmonary function tests, self help manuals).
Types of outcome measures
The principal outcome used in the review was smoking cessation rather than reduction in withdrawal symptoms, or reduction in amount of cigarettes smoked. Thus we excluded trials that did not provide data on smoking cessation rates. In each study we used the strictest available criteria to define abstinence . That is, we used rates of sustained cessation rather than point prevalence abstinence where possible. Where biochemical validation was used, we classified only those people meeting the biochemical criteria for cessation as abstainers; and where participants were lost to follow‐up, they were regarded as continuing smokers. We required a minimum follow‐up of at least six months for inclusion, and used the longest follow‐up reported. A secondary outcome was the effect of smoking advice on subsequent mortality and morbidity.
Search methods for identification of studies
We identified trials from the Cochrane Tobacco Addiction Group specialised register. This has been developed from electronic searching of MEDLINE, EMBASE and PsycINFO and the Cochrane Central Register of Controlled Trials (CENTRAL) together with handsearching of specialist journals, conference proceedings and reference lists of previous trials and overviews in smoking cessation. We used the following MeSH terms to identify reports of potentially relevant trials in the register: 'physician‐patient‐relations' or 'physicians' or 'family‐practice' or 'physician's‐role'. We also checked records with the terms 'general practice' or 'general practitioner' or 'GP' or 'physician*' in the title, abstract or any keyword field. At the time of the search in January 2013 the Register included the results of searches of the Cochrane Central Register of Controlled Trials (CENTRAL), issue 12, 2013; MEDLINE (via OVID) to update 20130104; EMBASE (via OVID) to week 201252; PsycINFO (via OVID) to update 20121231. See the Tobacco Addiction Group Module in The Cochrane Library for full search strategies and list of other resources searched, and Appendix 1 for the full search strategy used to identify reports of trials relevant to the topic.For this update we also searched in Latin American databases through BVS (Virtual Library in Health) which covered six databases (Lilacs, Biblioteca Cochrane, Wholis, Leyes, Scielo, Inbiomed) (Appendix 2). The most recent search of this was in February 2013.
Data collection and analysis
Data extraction:
In all versions of this review two people independently extracted data from the published reports. For this update, NP & DB reviewed potentially relevant reports and extracted data. Any disagreements were resolved by referral to a third author. For each trial, we documented the following aspects:
country of origin.
study population (including whether studies randomised only selected, motivated volunteers or all smokers, unselected by motivation to quit).
eligibility criteria.
nature of the intervention (including the nature, frequency and duration of advice, use of aids, and training of therapist).
details of study design (including method of allocation, blinding, study structure).
outcome measures.
validation of smoking status.
In trials where details of the methodology were unclear or where results were not expressed in a form that allowed extraction of the necessary key data, we wrote to the individual investigators to provide the required information. In trials where participants were lost to follow‐up they were regarded as being continuing smokers. Reports that only appeared in non‐English language journals were examined with the assistance of a translator.
Quality assessment:
For this update we assessed the methodological quality of the studies included in the review on three items covering two domains (Cochrane Handbook). We assessed the quality of sequence generation and allocation concealment as markers for the risk of selection bias, and we assessed the level and reporting of incomplete outcome data as a measure of attrition bias. JH‐B completed the Risk of Bias tables for studies already included in the review, and agreed bias assessments with LS.
Data Analysis:
We expressed results as the relative risk (intervention:control) of abstinence from smoking at a given point in time, or for mortality and/or morbidity, together with the 95% confidence intervals for the estimates. Early versions of this review used the odds ratio. but most clinicians find the relative risk more straightforward to interpret than the odds ratio.
We estimated pooled treatment effects using the Mantel‐Haenszel fixed‐effect method. We now use the I² statistic to investigate statistical heterogeneity, given by the formula [(Q ‐ df)/Q] x 100, where Q is the Chi² statistic and df is its degrees of freedom (Higgins 2003). This describes the percentage of the variability in effect estimates that is due to heterogeneity rather than to sampling error (chance). A value greater than 50% may be considered substantial heterogeneity.
Studies that used cluster randomisation (with the physician or practice as the unit of allocation) were included in the meta‐analyses using the patient‐level data, but we assessed the effect on the results of excluding them. Where reported, we have recorded the statistical methods used in studies to investigate or compensate for clustering.
Results
Description of studies
We include 42 trials, published between 1972 and 2012 and including more than 31,000 participants. Twenty‐six trials with 22,000 participants contributed to the primary comparison between advice and a no‐advice or usual care control.
Seventeen studies compared a minimal advice intervention with a control intervention in which advice was not routinely offered. Eleven studies compared an intervention that we classified as intensive with a control. Fourteen studies (thirteen of which did not have a non‐advice control group) compared an intensive with a minimal intervention, and one study compared two intensive interventions (Gilbert 1992). One study compared an intervention based on the 4As model (Ask, Advise, Assist, Arrange follow‐up), delivered in two different styles (Williams 2002). Two studies compared advice to computer‐tailored letters (Meyer 2008; Meyer 2012). Some studies tested variations in interventions and contributed to more than one comparison. These are described and the meta‐analyses to which they contribute are identified in the Table 'Characteristics of included studies'.
The definition of what constituted 'advice' varied considerably. In one study (Slama 1995) participants were asked whether they smoked, and were given a leaflet if they wanted to stop. The control group were not asked about their smoking status until follow‐up. In all other studies the advice included a verbal 'stop smoking' message. This verbal advice was supplemented by provision of some sort of printed 'stop smoking' material (27 studies), or additional advice from a support health worker or referral to a cessation clinic or both. Four studies described the physician intervention as behavioural counselling with a stop‐smoking aim. Butler 1999 compared motivational consulting (based on information from theoretical models) with simple advice. In two studies the smoker was encourage to make a signed contract to quit (BTS 1990a; BTS 1990b). Higashi 1995 provided an incentive (a telephone card) to those who successfully quit. Three studies included an intervention which involved a demonstration of the participant's pulmonary function (Li 1984; Richmond 1986; Segnan 1991), or expired air carbon monoxide (Jamrozik 1984). Severson 1997, using a cluster design, compared information and a letter alone to advice from a paediatrician to mothers of babies attending well‐baby clinics with a view to reducing exposure of the children to passive smoke. Unrod 2007 used a computer‐generated tailored report to assist with cessation, and Meyer 2008 compared brief advice to computer‐generated tailored letters and to no intervention. Meyer 2012 compared brief advice, tailored letters and the combination of both. It only contributed to the direct comparison of advice and tailored letters
In the analysis we aggregated groups allocated to brief advice alone with those allocated to brief advice plus brief printed material. We did this with the view that advice plus provision of printed material is a practical approach in the primary care setting. In the two studies which directly compared the additional benefit of offering printed material none was observed (Jamrozik 1984; Russell 1979). Studies which provided a smoking cessation 'manual' were classified as offering an intensive intervention, but there is only weak evidence that self‐help materials have a small benefit when combined with face‐to‐face support (Lancaster 2005b). The intensive intervention subgroup also included studies that offered additional visits.
As required by the inclusion criteria, all trials assessed smoking status at least six months after the start of the intervention. Thirty‐one of the 42 studies (74%) had a longer follow‐up period, typically one year, the longest being three years. Since the interventions generally did not require a quit date to be set, the definitions of cessation used are less strict than are typically found in trials of pharmacotherapies. About half the studies defined the cessation outcome as the point prevalence of abstinence at the longest follow up, and the other half reported sustained abstinence, which typically required abstinence at an intermediate follow‐up point as well. Validation of all self‐reported cessation by biochemical analysis of body fluids or measurement of expired carbon monoxide was reported in 11 studies (26%) (Ardron 1988; BTS 1990a; BTS 1990b; Gilbert 1992; Hilberink 2005; Li 1984; Marshall 1985; Segnan 1991; Slama 1990; Vetter 1990; Williams 2002), but only three of these contributed to the primary analysis. Validation in a sample of quitters was reported in four studies (Lang 2000; Russell 1979; Russell 1983; Unrod 2007). Haug 1994 used biochemical validation at 12 months but not at 18 month follow‐up, and Richmond 1986 used biochemical validation or confirmation by a relative/friend. Fagerström 1984 adjusted rates based on the deception rate found in a subsample where validation was performed. No biochemical validation was used in the remaining 24 studies (57%).
Risk of bias in included studies
Sequence generation & allocation concealment (selection bias)
Nine trials were cluster‐randomised, with practices, physicians or clinics allocated to deliver a single condition (Haug 1994; Hilberink 2005; Janz 1987; Lang 2000; Meyer 2012; Morgan 1996; Unrod 2007; Severson 1997; Wilson 1990). In a further eight trials allocation to treatment was by day or week of attendance, so physicians delivered different interventions at different times (Burt 1974; Jamrozik 1984; Meyer 2008; Nebot 1989; Page 1986; Richmond 1986; Russell 1979, Russell 1983). All these studies are at potential risk of selection bias because individual smokers were likely to have been recruited by people who were not blind to treatment condition. We judged that the risk of bias was low when a systematic procedure for recruitment was described (usually involving screening questionnaires given to all participants before contact with the doctor), and there were no important differences in baseline characteristics. We judged risk to be high where differences in baseline characteristics and number recruited suggested selection bias (Hilberink 2005; Meyer 2012; Richmond 1986; Unrod 2007), or where the doctors themselves decided who to enrol (Haug 1994). Meyer 2008 was judged to be at high risk because each practice provided each of the three treatment conditions for a week, in the same order with a gap between recruitment periods; frequent attenders were therefore more likely to be assigned to the earlier conditions. Two studies were judged to be at low risk (Lang 2000; Wilson 1990). In this group of 17 studies, 13 were judged to be at high risk for one or both of the items related to selection bias.
Some studies reported post‐randomisation drop‐outs of clinics or physicians. We note in the Characteristics of included studies table where authors had allowed for or ruled out an effect of clustering, and we used sensitivity analyses to test the contribution of the cluster‐randomised trials to the meta‐analysis.
Of the remaining 25 studies using individual randomisation, two used methods which prevented allocation concealment by using elements of the medical record (Demers 1990) or birth date (Fagerström 1984), and were judged to be at high risk for both sequence generation and allocation concealment. Slama 1995 and Porter 1972 were judged to be at high risk of bias due to lack of allocation concealment. Most of the others did not give sufficient information about methods of sequence generation or allocation concealment to be judged as being at low risk.
Incomplete outcome data (attrition bias)
Four studies (McDowell 1985; Meyer 2012; Richmond 1986; Slama 1995) were judged to be at high risk of attrition bias because it was unclear how many randomised participants had been lost to follow‐up or how they had been treated in the analyses, or because the loss to follow‐up was very different between groups.
Effects of interventions
Advice versus no advice
When all 17 trials of brief advice (as part of a minimal intervention) versus no advice (or usual care) were pooled, the results demonstrated a statistically significant increase in quit rates; relative risk (RR) 1.66, 95% confidence interval (CI) 1.42 to 1.94 (Figure 1, Analysis 1.1.1). Heterogeneity was low (I² = 31%). When trials compared a more intensive intervention to a no‐advice control, the point estimate was a little larger, with moderate heterogeneity between the trials (11 trials, RR 1.86, 95% CI 1.60 to 2.15, I² = 50%, Figure 1, Analysis 1.1.2). Although the estimate for the more intensive subgroup was higher, the confidence intervals overlapped and the division of the trials into two groups based on this classification of intensity did not explain any of the overall heterogeneity (I² = 39% across the 28 trials). The estimated effect combining both groups was RR 1.76 (95% CI 1.58 to 1.96). We classified a more intensive intervention as a longer consultation, additional visits, or a self‐help manual. There is only weak evidence that self‐help materials have a small additional benefit when combined with face‐to‐face support (Lancaster 2005b), and the absence of a difference between the subgroups may in part reflect the difficulty in categorising intensity. From this indirect comparison there was insufficient evidence to establish a significant difference in the effectiveness of physician advice according to the intensity of the intervention.
1.
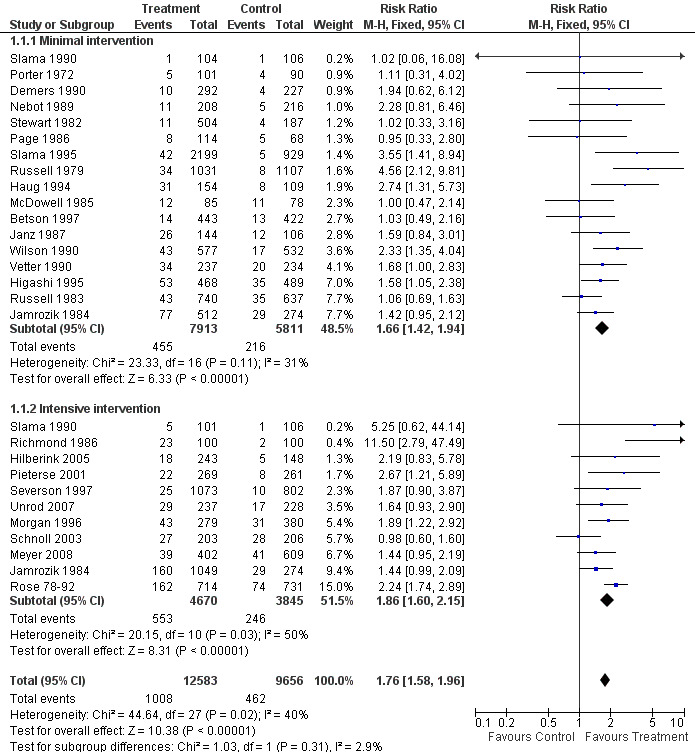
Effect of advice versus control (subgroups by intensity), Outcome: Smoking cessation at longest follow up
1.1. Analysis.
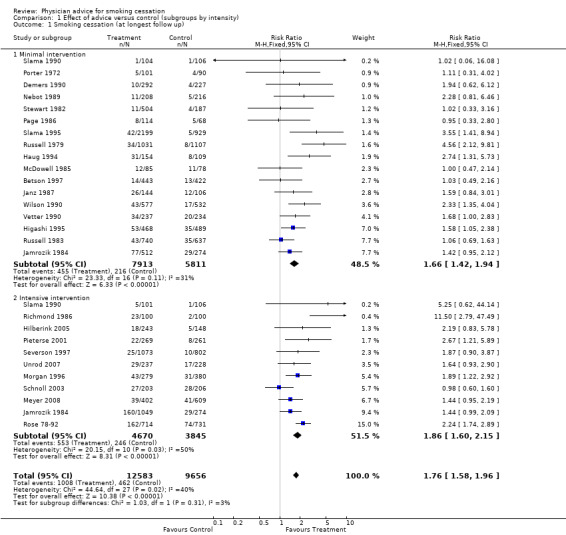
Comparison 1 Effect of advice versus control (subgroups by intensity), Outcome 1 Smoking cessation (at longest follow up).
More intensive versus minimal advice
The direct comparison between intensive and minimal advice in 15 trials (Analysis 2.1) suggested overall that there was a small but significant advantage of more intensive advice (RR 1.37, 95% CI 1.20 to 1.56), with little evidence of heterogeneity (I² = 32%). In the subgroup of 10 trials in populations of smokers not selected as having smoking‐related disease, the increased effect of the more intensive intervention was small and the confidence interval only narrowly excluded 1 (RR 1.20, 95% CI 1.02 to 1.43). No individual trials in this subgroup showed a significant benefit and there was no evidence of heterogeneity (I² = 0%). Statistical significance was lost if the trial that used cluster randomisation (Lang 2000) was removed. Amongst five trials in people with, or at high risk of, smoking‐related diseases the pooled estimate was larger, with little sign of heterogeneity, (RR 1.65, 95% CI 1.35 to 2.03, I² = 21%) and three of the trials showed significant effects. Since the confidence intervals overlapped this does not however provide strong evidence for a differential effect in these two populations.
2.1. Analysis.
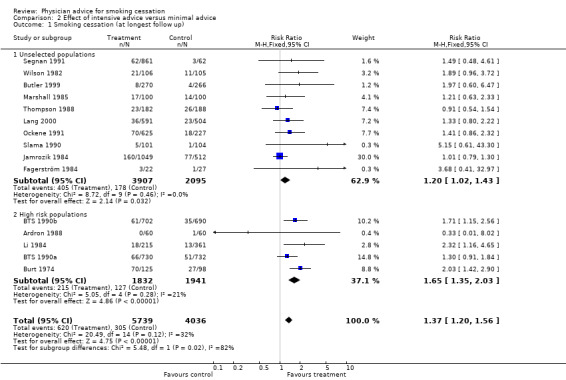
Comparison 2 Effect of intensive advice versus minimal advice, Outcome 1 Smoking cessation (at longest follow up).
Number of follow‐up visits
The direct comparison of the addition of further follow‐up to a minimal intervention showed a barely significant increase in quitting in the pooled analysis, although none of the five studies individually detected significant differences (RR 1.52, 95% CI 1.08 to 2.14, Analysis 3.1). This analysis did not include one study of the effect of follow‐up visits (Gilbert 1992), because the control group received more than minimal advice, including two visits to the doctor. In this study, there was no significant difference in biochemically validated cessation rates between the two‐visit group and a group offered a further four follow‐up visits.
3.1. Analysis.
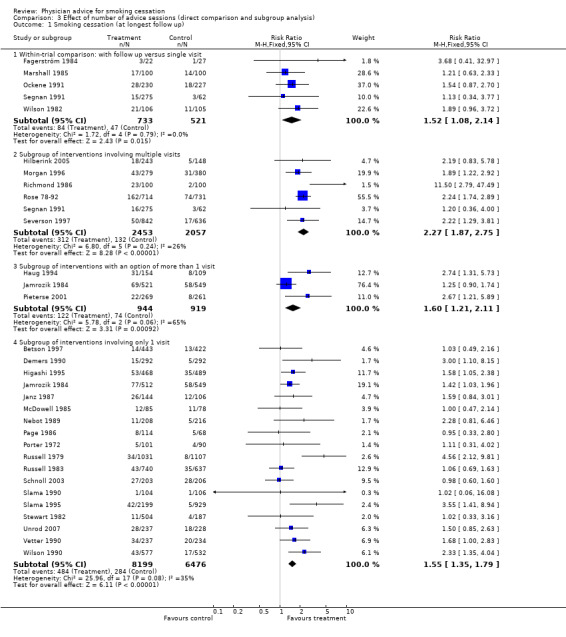
Comparison 3 Effect of number of advice sessions (direct comparison and subgroup analysis), Outcome 1 Smoking cessation (at longest follow up).
Indirect comparison between subgroups of studies suggested that an intervention including follow‐up visits had a slightly larger estimated effect compared to no advice than an intervention delivered at a single visit . The RR for cessation when follow‐up was provided was 2.27 (six studies, 95% CI 1.87 to 2.75, I² = 30%, Analysis 3.1.2), compared to 1.55 (18 studies, 95% CI 1.35 to 1.79, I² = 35%, Analysis 3.1.4) when it was not.
Use of additional aids
Indirect comparison between 10 studies in which the intervention incorporated additional aids such as demonstration of expired carbon monoxide levels or pulmonary function tests or provision of self‐help manuals and 17 where such aids were not used did not show important differences between subgroups (Analysis 4.1).
4.1. Analysis.
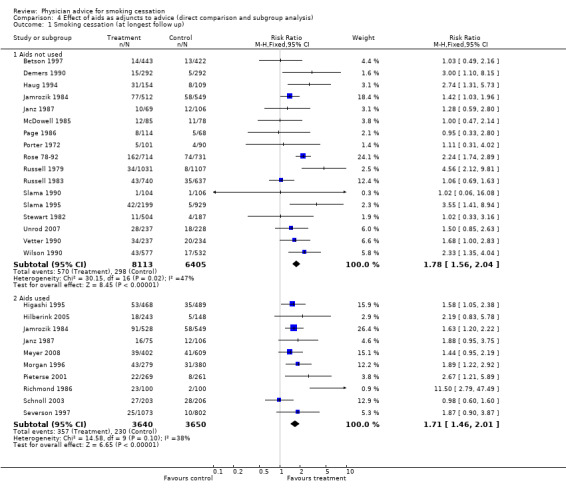
Comparison 4 Effect of aids as adjuncts to advice (direct comparison and subgroup analysis), Outcome 1 Smoking cessation (at longest follow up).
Comparisons between different types of advice
In a single trial of motivational counselling (approximately 10 minutes) compared to brief advice (2 minutes) a significant benefit was not detected, but the point estimate favoured the motivational approach and confidence intervals were wide (Butler 1999, RR 1.97, 95% CI 0.6 to 6.7, Analysis 5.1). Quit rates were low in both groups, but motivational advice appeared to increase the likelihood of making a quit attempt. This study also contributes to the comparison between intensive and minimal advice. Williams 2002, comparing brief advice using an autonomy‐supporting style to advice given in a controlling style, did not detect a significant difference. Quit rates were high in both groups and the point estimate favoured a controlling style (RR 0.51, 95% CI 0.19 to 1.32, Analysis 5.1). Both interventions took about 10 minutes and this trial does not contribute to the intensive versus minimal comparison.
5.1. Analysis.
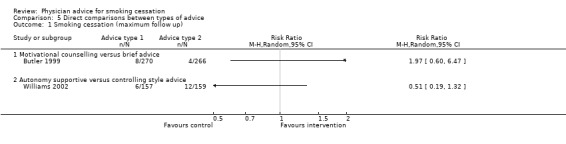
Comparison 5 Direct comparisons between types of advice, Outcome 1 Smoking cessation (maximum follow up).
Comparison between advice and tailored letters
Two studies by the same research group compared brief advice to computer‐generated tailored letters (Meyer 2008; Meyer 2012). The pooled estimate favoured the tailored letter condition but confidence intervals included 1 (RR 0.88; 95% CI 0.67 to 1.16). Meyer 2012 also tested a combination of advice and letters which was found to be more effective than either intervention alone, in both crude analyses and adjusting for baseline imbalance and missing data.
Effect of advice on mortality
Only one study (Rose 78‐92) has reported the health outcomes of anti‐smoking advice as a randomised single factor intervention. At 20‐year follow‐up, in the intervention compared to the control group, total mortality was 7% lower, fatal coronary disease was 13% lower and lung cancer (death plus registrations) was 11% lower. These differences were not statistically significant, reflecting low power and the diluting effects of incomplete compliance with the cessation advice in the intervention group, and a progressive reduction in smoking by men in the control group. After 33 years of follow‐up differences in rates for most causes of death were not significant but there was a significantly smaller number of deaths from respiratory conditions. The age‐adjusted hazard ratio was 0.72 (95% CI 0.54 to 0.96).
Sensitivity analyses
The results of the main meta‐analyses were not sensitive to exclusion of trials rated at high risk of bias on any single item, or to exclusion of all trials rated at high risk of bias for any item.
Discussion
The results of this review, first published in 1996 and updated in 2013 with one new study that did not contribute to the primary analyses, continue to confirm that brief advice from physicians is effective in promoting smoking cessation. Based on the results of a meta‐analysis incorporating 28 trials and over 20,000 participants, a brief advice intervention is likely to increase the quit rate by 1 to 3 percentage points. The quit rate in the control groups in the included studies was very variable, ranging from 1% to 14% across the trials in the primary comparison. However the relative effect of the intervention was much less variable, because trials with low control group quit rates generally had low rates with intervention, and vice versa. The general absence of substantial heterogeneity between trials when relative risks are compared makes for reliable estimates of relative effect. However it is more difficult to estimate the absolute effect on quitting, and the number needed to treat. Absolute quit rates will be influenced by motivation of the participants who are recruited or treated, the period of follow‐up, the way in which abstinence is defined, and whether biochemical confirmation of self‐reported abstinence is required. Many of the trials in this review were conducted in the 1970s and 1980s, and did not use the gold standard methods for assessing smoking abstinence that would now be recommended (West 2005). Only a minority of trials used biochemical measures to confirm self reports of abstinence, and although 12‐month follow‐up was common, many trials assessed smoking status at a single follow‐up point. This will tend to lead to higher quit rates overall than in trials with biochemical validation and requiring repeated abstinence at or between multiple assessments, but there is not strong evidence that it will lead to bias in the estimates of relative effect. There were too few trials in the primary analysis to test the effect size when including only trials with complete biochemical validation. We did not find that the control group quit rates were any less variable amongst studies with a longer period of follow‐up and with abstinence sustained at more than one assessment.
If an unassisted quit rate of 2% at 12 months in a population of primary care attenders is assumed, we can use the confidence intervals for the minimal intervention subgroup, 1.42 to 1.94, to estimate a number needed to treat for an additional beneficial outcome (NNTB) of 50 ‐ 120. If the background rate of quitting was expected to be 3%, then the same effect size estimate would translate to an NNTB of 35‐80. Using the pooled estimate from combining both intensity subgroups in the primary comparison would raise the lower confidence interval and reduce the upper estimate of the NNTBs.
Although the methodological quality of the trials was mixed, with a number using unclear or unsatisfactory methods of treatment allocation, our sensitivity analyses did not suggest that including these trials has led to any overestimate of treatment effects. Although we noted heterogeneity in some subgroups, overall the trials showed consistent relative effects. As noted above, the lack of biochemically validated cessation was the other possible methodological limitation.
Based on subgroup analyses there is little evidence about components that are important as part of an intervention, although direct comparison in a small number of trials suggest that providing a follow‐up appointment may increase the effect. Indirect comparisons indicate that various aids tested do not appear to enhance the effectiveness of physician advice. However, caution is required in interpreting such indirect comparisons since they do not take account of any inherent systematic biases in the different populations from which the study samples are drawn. Direct comparison of differing intensities of physician advice suggest a probable benefit from the more intensive interventions compared to a briefer intervention, although subgroup analyses suggest that this might be small or non‐existent in unselected smokers, but larger when provided to smokers in high risk groups. The effect of intensified advice in a population with established disease is however based on a small number of trials. If the marginal benefit of a more intensive advice‐based intervention is based on the pooled estimate combining unselected and high risk population subgroups (RR 1.37, 95% CI 1.20 to 1.56), and assuming that the minimal intervention alone could achieve a quit rate of 3.5%, an NNTB of 50 ‐ 140 would be estimated for the effect of providing more support. There was insufficient evidence to draw any conclusion about the effect of motivational as opposed to simple advice (Butler 1999), or between different advice‐giving styles (Williams 2002). Two recent studies compared physician advice to mailed computer‐generated letters, using baseline data to tailor advice based on the transtheoretical model (Velicer 2000).
If these results are to translate into a public health benefit, the important issue will be the proportion of physicians who actually offer advice. Although 80% of the general population visit a physician annually, reports of the proportion who receive any form of smoking cessation advice vary considerably. While many of those who are not offered smoking cessation advice will quit unaided, every smoker who does not receive advice represents a 'missed opportunity'. Provision of lifestyle advice within the medical consultation is now promoted as a matter of routine, but advice on smoking may still not be offered systematically (Denny 2003; McLeod 2000). Not all primary care physicians agree that advice should be given at every consultation (McEwen 2001), and some practitioners still consciously choose not to raise smoking cessation as an issue in order to preserve a positive doctor‐patient relationship (Coleman 2000), although some research indicates that satisfaction may be increased by provision of advice (Solberg 2001). Two recent studies (Meyer 2008; Meyer 2012) compared physician advice to computer‐generated letters that were tailored to stage of change. The pooled estimate did not show a significant difference between the interventions. Although this type of approach could help practitioners to eliminate barriers against giving smoking cessation advice due to lack of time, financial resources or skills, the confidence intervals were too wide to be sure that effects were equivalent.
Several strategies have been shown convincingly to enhance the effectiveness of advice from a medical practitioner, including provision of pharmacotherapy. Effective pharmacotherapies for smoking cessation include nicotine replacement therapy (Stead 2012) bupropion or nortriptyline (Hughes 2007) and varenicline or cytisine (Cahill 2012). Use of these forms of therapy increases quit rates 1.5‐ to 2.5‐fold, and is a potentially valuable adjunct to any advice provided. Both individual and group‐based counselling are also effective at increasing cessation rates amongst smokers prepared to accept more intensive intervention (Lancaster 2005a; Stead 2005). Telephone counselling can also be effective (Stead 2006). National clinical practice guidelines generally advise the use of a brief intervention in which asking about tobacco use is followed by advice to quit, and an assessment of the smoker's willingness to make a quit attempt. Those willing to make a quit attempt can then be offered specific assistance and follow‐up (Fiore 2008; Miller 2001; NHC 2002; West 2000).
Authors' conclusions
Implications for practice.
The results of this review indicate the potential benefit from brief simple advice given by physicians to their smoking patients. The challenge as to whether or not this benefit will be realised depends on the extent to which physicians are prepared to systematically identify their smoking patients and offer them advice as a matter of routine. Providing follow‐up, if possible, is likely to produce additional benefit. However, the marginal benefits of more intensive interventions, including use of aids, are small, and cannot be justified as a routine intervention in unselected smokers. They may, however, be of benefit for individual, motivated smokers.
Implications for research.
Further studies of interventions offered by physicians during routine clinical care are unlikely to yield new information about the role of advice. Work is now required to develop strategies to increase the frequency with which smokers are identified and offered advice and support.
Feedback
Intention to treat analyses
Summary
I wonder whether the studies included were based on intention to treat analysis? If they were not, I believe that a selection of more motivated subjects has taken place even in studies where unselected populations were invited. Smokers who are not motivated to quit, do not take the same interest in such on offer. Intention to treat principles should be applied if the size of the effect should apply to whole practice populations.
Reply
In extracting data from the studies, the denominators were derived from the number of participants stated to be randomised to each condition, and participants lost to follow‐up were assumed to be continuing smokers, an intention to treat analysis. Where unselected participants were recruited, the results should therefore reflect whole practice smoker populations. However, the exact way in which participants were recruited differed between trials. In some studies where the intention was to recruit unselected participants, it may be that those recruited were not typical of the practice populations.
Contributors
Ann Dorrit Guassora (commenter); Lindsay Stead (author)
What's new
| Date | Event | Description |
|---|---|---|
| 27 March 2013 | New citation required but conclusions have not changed | Updated and additional authors added, no change to conclusions |
| 27 March 2013 | New search has been performed | Searches updated including new searches of Latin American databases. One new included study. |
History
Protocol first published: Issue 2, 1996 Review first published: Issue 2, 1996
| Date | Event | Description |
|---|---|---|
| 14 February 2008 | New citation required but conclusions have not changed | Updated for issue 2, 2008. Three new included studies added, new author added, and metric changed from odds ratios to risk ratios. |
| 12 July 2004 | New search has been performed | Updated for issue 4, 2004. Five additional studies added, and statistical methods for meta‐analysis changed from Peto to Mantel‐Haenszel. There were no major changes to the conclusions of the review. |
Notes
Chris Silagy was first author at the time of his death in 2001. The authors for citation were changed when the review was updated in 2004.
Acknowledgements
Chris Silagy initiated the review and was first author until his death in 2001. Sarah Ketteridge developed the initial version of this review and was a co‐author of the review until 1999. Ruth Ashenden provided technical support in the preparation of the initial version of this review. Gillian Bergson was a co‐author for the 2008 update of this review. Dr Chris Hyde, Iain Chalmers and Helen Handoll have all provided constructive comments on previous versions of the review which have been taken account of in the updates. Martin Shipley provided 33‐year follow‐up data on the Whitehall study (Rose 78‐92) via Iain Chalmers. Rafael Perera gave statistical and translation support for the 2008 update. Gillian Bergson extracted data from trials for the 2008 update, and contributed to revisions of the text.
Appendices
Appendix 1. Register search Strategy (2013 update, via Cochrane Register of Studies)
#1 Physician's Role:MH,EMT,KW #2 Physician‐Patient Relations:MH,EMT,KW #3 Family Practice:MH,EMT,KW #4 physicians*:MH,EMT,KW #5 general practice:TI,AB,EMT,KW #6 GP:TI,AB,EMT,KW #7 physician*:TI, AB,EMT,KW #8 general practitioner:TI,AB,EMT,KW #9 general practice:TI,AB,EMT,KW #10 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9
Appendix 2. Latin American database search via BVS
Covering Lilacs, Biblioteca Cochrane, Wholis, Leyes, Scielo, Inbiomed
Cese del Uso de Tabaco
Visita a Consultorio Médico
Relaciones Médico‐Paciente
Rol del Médico
Cese del Tabaquismo
Ensayo Clínico
"Cese del Uso de Tabaco" OR "Cese del Tabaquismo" AND "Ensayo Clínico" OR "Ensayo Clínico Controlado" OR "Ensayo Clínico Controlado randomisado"
Data and analyses
Comparison 1. Effect of advice versus control (subgroups by intensity).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation (at longest follow up) | 26 | 22239 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.58, 1.96] |
| 1.1 Minimal intervention | 17 | 13724 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.42, 1.94] |
| 1.2 Intensive intervention | 11 | 8515 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.60, 2.15] |
Comparison 2. Effect of intensive advice versus minimal advice.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation (at longest follow up) | 15 | 9775 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.20, 1.56] |
| 1.1 Unselected populations | 10 | 6002 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.02, 1.43] |
| 1.2 High risk populations | 5 | 3773 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.35, 2.03] |
Comparison 3. Effect of number of advice sessions (direct comparison and subgroup analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation (at longest follow up) | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Within‐trial comparison: with follow up versus single visit | 5 | 1254 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.08, 2.14] |
| 1.2 Subgroup of interventions involving multiple visits | 6 | 4510 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.27 [1.87, 2.75] |
| 1.3 Subgroup of interventions with an option of more than 1 visit | 3 | 1863 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.21, 2.11] |
| 1.4 Subgroup of interventions involving only 1 visit | 18 | 14675 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.35, 1.79] |
Comparison 4. Effect of aids as adjuncts to advice (direct comparison and subgroup analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation (at longest follow up) | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Aids not used | 17 | 14518 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.56, 2.04] |
| 1.2 Aids used | 10 | 7290 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.46, 2.01] |
Comparison 5. Direct comparisons between types of advice.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation (maximum follow up) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Motivational counselling versus brief advice | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Autonomy supportive versus controlling style advice | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Advice versus tailored letters.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation (maximum follow up) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Brief advice versus tailored letters | 2 | 2915 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.67, 1.16] |
6.1. Analysis.
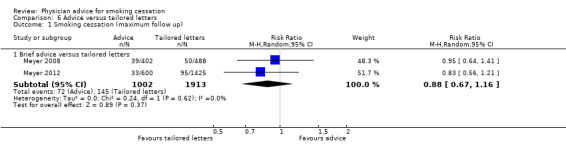
Comparison 6 Advice versus tailored letters, Outcome 1 Smoking cessation (maximum follow up).
Comparison 7. Effect of advice on mortality and morbidity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of events during 20 years follow up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Death or registration of lung cancer | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Death or registration of cancers other than lung | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Death from coronary heart disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Death from all causes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of events during 33 years follow up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Death from coronary heart disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Death from cardiovascular disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Death from respiratory disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Death from cancer | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Death from all causes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
7.1. Analysis.
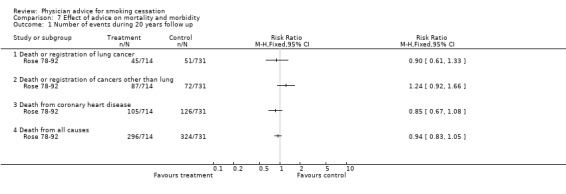
Comparison 7 Effect of advice on mortality and morbidity, Outcome 1 Number of events during 20 years follow up.
7.2. Analysis.
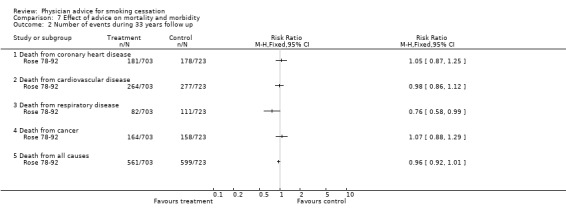
Comparison 7 Effect of advice on mortality and morbidity, Outcome 2 Number of events during 33 years follow up.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ardron 1988.
| Methods | Setting: Adult diabetic outpatient clinic in Liverpool, UK. Recruitment: volunteers who responded 'yes' to the question 'Do you want to give up smoking?' (selected by motivation). | |
| Participants | 60 clinically stable diabetic patients < 40 yrs, smoking > 5 cpd, motivated to stop. Therapists: medical registrar supported by health visitor. | |
| Interventions | 1. Routine advice (5 mins talk) 2. Intensive advice (longer talk, leaflet, and visit from heath visitor at home within two wks involving family, giving further advice and written materials). Intervention level: intensive (2) vs minimal (1). Aids used: none. Follow‐up visits: 1. | |
| Outcomes | Point prevalence at 6m. Validation: expired CO and urinary cotinine. | |
| Notes | Contributes data to intensive vs minimal comparison only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised"; details not provided. |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study. |
Betson 1997.
| Methods | Setting: government outpatient clinic, Hong Kong. Recruitment: older smokers, unselected. | |
| Participants | 865 smokers, aged > 65, 92% male, 49% smoking > 10 cpd | |
| Interventions | 1. No intervention. 2. Written materials (Chinese translation of American Cancer Society booklet). 3. Physician advice (1min, based on 4As). 4. Physician advice and booklet. Intervention level: minimal (3 & 4). Aids used: none; follow‐up visits: none. | |
| Outcomes | Abstinence at 1 yr (sustained from 3m). Validation: poor response to request for urine specimen so data based on self report. | |
| Notes | Groups 3 & 4 compared to 1 & 2 for minimal advice vs control; full paper provided by Professor Lam. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | "Every doctor was given a set of sealed envelopes with serial numbers."; unclear if envelopes were opaque |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 64% of participants provided data at 1 yr, breakdown by group not specified; participants with missing data were considered smoking. |
BTS 1990a.
| Methods | Setting: hospital or chest clinic in UK. Recruitment: volunteers selected by motivation. | |
| Participants | New patients with smoking‐related disease but not pregnant, terminally or psychiatrically ill. 1462 patients, smoking at least 1cpd, mean: 17cpd. Therapists: physicians. | |
| Interventions | 1. Advice. 2. Advice + signed agreement to stop, health visitor support, letters from physician. Intervention level: intensive vs minimal. Aids used: yes; Follow‐up visits: from health visitor, not doctor. | |
| Outcomes | Sustained at 12m (& 6m). Validation: expired CO. | |
| Notes | Contributes data to intensive vs minimal comparison only. (Two studies are reported in the same paper). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were allocated at random." Method of sequence generation not specified. |
| Allocation concealment (selection bias) | Unclear risk | Patients were randomised using "a sequence of sealed envelopes." Unclear if envelopes were opaque. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar numbers lost in both groups (control 138/732; intervention 144/730); participants with missing data were considered smoking. |
BTS 1990b.
| Methods | Setting: hospital or chest clinic in UK. Recruitment: Volunteers selected by motivation. | |
| Participants | New patients with smoking‐related disease but not pregnant, terminally or psychiatrically ill. 1392 patients, smoke at least 1cpd, mean 17cpd. Therapists: physicians. | |
| Interventions | 1. Advice. 2. Advice plus signed agreement. 3. Advice plus letters of support. 4. Advice plus letters plus signed agreement. Intervention level: intensive vs minimal. Aids used: yes. Follow‐up visits: none. | |
| Outcomes | Sustained at 12 m (& 6m). Validation: expired CO. | |
| Notes | The use of supportive letters was classified as intensive, so 3 & 4 compared to 1 & 2 in the intensive vs minimal comparison; (two studies are reported in the same paper). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were allocated at random."; method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | Patients were randomised using "a sequence of sealed envelopes."; it is unclear whether envelopes were opaque. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar numbers lost across all 4 groups. 1) 72/343; 2) 80/347; 3) 90/351; 4) 86/351); participants with missing data were considered smoking. |
Burt 1974.
| Methods | Setting : hospital cardiac unit and cardiac outpatient clinic in Scotland Recruitment: consecutive survivors of acute myocardial infarction identified as smokers (unselected) | |
| Participants | 210 survivors of acute myocardial infarction Ages not stated; pipe and cigarette smokers Number of cpd not stated Therapists: hospital consultants, reinforced by junior medical and nursing staff | |
| Interventions | 1. Repeated emphatic advice to quit as an inpatient with follow up in a special clinic 2. Normal inpatient care followed by discharge to care of the family doctor Intervention level: intensive vs minimal Aids used: none. Follow‐up visits: yes, number not stated | |
| Outcomes | PP abstinence at 12m. Validation: none. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Selection between the study and control groups was random, being determined by the day of admission." |
| Allocation concealment (selection bias) | Unclear risk | Method not specified. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants "were followed up for one year or longer." |
Butler 1999.
| Methods | Setting: general practices (registrars), UK. Recruitment: All smokers attending for consultation (except those with terminal illness) (unselected). | |
| Participants | 536 smokers (70% female) at various stages of change. | |
| Interventions | 1. Standardised brief advice (estimated time 2 minutes). 2. Structured motivational counselling (mean length 10 mins) (based on stage of readiness to change). | |
| Outcomes | PP at 6m (self‐reported abstinence in the previous month). Validation: none | |
| Notes | Contributes data to intensive vs minimal comparison only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not specified. |
| Allocation concealment (selection bias) | Unclear risk | Sealed "numbered envelopes were filed in a study pack and clinicians were instructed to open them in order." Unclear if envelopes were opaque. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar number lost to follow‐up in both groups (brief advice: 54/266, motivational counselling: 64/270). Subjects missing data at follow‐up considered smokers. |
Demers 1990.
| Methods | Setting: family practices in southeast Michigan, USA. Recruitment: patients attending the practices in a defined intake period identified as smokers by questionnaire (unselected). | |
| Participants | 519 adult smokers; Mean cpd 22. Therapists : Family practitioners. | |
| Interventions | 1. 3 ‐ 5 min smoking cessation counselling and written materials plus routine care. 2. Routine care. Intervention level: minimal Aids used: none. Follow‐up visits: no. | |
| Outcomes | Sustained at 12m (& 6m). Validation: none. | |
| Notes | Sustained replaced PP abstinence from 2008. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "based on the last digit of their medical chart." |
| Allocation concealment (selection bias) | High risk | By receptionist based on medical record number. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar percentages lost to follow‐up at 12m (38% intervention, 41% control). Participants missing data counted as smokers in final analysis. |
Fagerström 1984.
| Methods | Setting: Swedish general practices and industrial clinics. Recruitment: Smokers who were considered motivated to stop, accepted advice and agreed to follow‐up (selected). | |
| Participants | 145 adult smokers (49 in relevant arms), mean cpd: 19. Therapists: 10 Swedish GPs, 3 Swedish industrial physicians. | |
| Interventions | 1. Short follow‐up (advice plus 1 appointment). 2. Long follow‐up ( advice plus 2 appointments, phone call + letter). 3. Short follow‐up plus nicotine gum (not used in review). 4. Long follow‐up plus nicotine gum (not used in review). Intervention level: Intensive vs minimal. Aids used: yes. Follow‐up: 1 vs 2 visits. | |
| Outcomes | Sustained abstinence at 1, 6 and 12m. Validation: Results adjusted for 15% deception rate detected by expired CO measured in a random subset of claimed non‐smokers. | |
| Notes | Contributes data to intensive vs minimal comparison only. Adjusted rates used in analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Birthdate served as basis for randomisation". |
| Allocation concealment (selection bias) | High risk | Recruited by physicians and assigned by date of birth. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Results are based on the 145 patients who attended at least one follow‐up activity." Number of enrolled participants who did not attend any follow‐up activities not specified. |
Gilbert 1992.
| Methods | Setting: 41 family practices in Ontario, Canada. Recruitment: Patients volunteering for a smoking cessation programme in physician's office (selected). | |
| Participants | 647 smokers, mean cpd 22. Therapists: Family practitioners who had attended 4‐hr training session. | |
| Interventions | 1. Brief advice, self‐help booklet and 1 follow‐up visit including use of nicotine gum. 2. As group 1, plus 3 further follow‐up visits. | |
| Outcomes | Sustained at 12m (& 3m). Validation: salivary cotinine. | |
| Notes | Not included in any meta‐analysis table since the control group received more than a minimal intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computerized randomization program that balanced the number per group within each physician practice across each block of six patients". |
| Allocation concealment (selection bias) | Low risk | "The receptionist passed a sequence of numbered envelopes to the physician, containing the treatment randomization." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 98.4% participants followed up at one year. Participants missing data counted as smokers. |
Haug 1994.
| Methods | Setting: 187 general practices in Norway, Recruitment: opportunistically by the general practitioners (unselected), | |
| Participants | Reports separate trials in pregnant and non‐pregnant women: 274 non‐pregnant women age 18‐34: Smoking > 4 cpd, mean 13. Therapists: GPs. | |
| Interventions | 1. Advice + leaflet + invitation to attend 4 follow‐up visits. 2. Normal care controls. Intervention level: minimal. Aids used: none. Follow‐up visits: Offered. | |
| Outcomes | Abstinence at 18m. Validation: none (serum thiocyanate at 12m only). | |
| Notes | Cluster‐randomised, but 187 GPs so cluster size small. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocating GPs into subgroups," method not specified. |
| Allocation concealment (selection bias) | High risk | GPs recruited patients after they knew their group. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐outs not reported by group. Only completers included in analysis. |
Higashi 1995.
| Methods | Country: Japan. Recruitment: Primary Care (unclear whether selected). | |
| Participants | 957 adult smokers. | |
| Interventions | 1. Brief advice plus leaflet, encouragement card at 1m and telephone card at 6m. 2. No intervention. Intervention level: minimal. Aids used: yes. Follow‐up: no. | |
| Outcomes | PP abstinence at 12m. Validation: none. | |
| Notes | Information derived from English abstract. Full publication in Japanese and not translated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described. |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number lost to follow‐up not specified. |
Hilberink 2005.
| Methods | Setting: 43 general practices in the Netherlands. Recruitment: Patients with COPD identified from medical records in participating practices (?unselected). | |
| Participants | 392 patients with COPD (244 intervention, 148 control due to drop‐out of large control group practices), cpd not stated. Intervention had more patients in preparation (25.8% vs 17.6%) or contemplation stage (32.0% vs 28.4%) P = 0.059. | |
| Interventions | 1. Intensive advice ‐ Initial session to identify preparers and contemplators, further 3 visits and up to 3 follow‐up phone calls from nurse, plus booklet and video. Use of NRT recommended. 2. No intervention (usual care). Intervention level: Intensive. Aids used: yes. Follow‐up visits: yes (for motivated patients). | |
| Outcomes | PP abstinence at 12m (reported in Hilberink 2011; 6m outcomes in original paper). Validation: urine cotinine 50 ng/mL at 12m. | |
| Notes | Multi‐level analysis did not alter results. Twelve‐month outcome replaces 6m results from 2013. Third arm involving advice about bupropion not included in analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomised by practice, method not specified. |
| Allocation concealment (selection bias) | High risk | Patients identified from medical records. Difference in baseline stage of change between conditions suggests selection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar drop‐out and losses to follow‐up in both groups. 3/25 control practices and 2/23 intervention practices dropped out. Participants who dropped out considered to be smokers. |
Jamrozik 1984.
| Methods | Setting: general practices in Oxfordshire, UK. Recruitment: Identified smokers attending the practices during recruitment period (unselected). | |
| Participants | 2110 adult smokers, cpd not stated. Therapists: General practitioners who had in earlier studies indicated an interest in participating in smoking research. | |
| Interventions | 1. Normal care control group. 2. Brief advice to quit plus smoking cessation pamphlet. 3. Advice plus pamphlet plus a demonstration of the patient's level of exhaled CO (by research supervisor). 4. Advice plus pamphlet plus provision of a card offering follow‐up from health visitor. Intervention level: Minimal (groups 1 and 2), Intensive (groups 3 and 4). Aids used: Yes (groups 3 and 4). Follow‐up visits: Offered in group 4. | |
| Outcomes | PP abstinence at 12m. Validation: a sample of self‐reported quitters selected for urinary cotinine validation (up to 40% deception rate). Results not adjusted, and no evidence that deception rates differed in treatment and control groups. | |
| Notes | 2 compared to 1 for effects of minimal intervention, 3 & 4 compared to 1 for effects of intensive intervention. To avoid double counting group 1 when minimal and intensive pooled together, the control group is divided between the two categories. 3 & 4 compared to 2 for intensive vs minimal intervention, 4 compared to 1 for effects of intervention with offer of follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Intervention condition determined by day of attendance |
| Allocation concealment (selection bias) | Low risk | Patients were screened by non‐blind study staff and smokers filled in a questionnaire before and after their appointment. "Each doctor was provided with a small desktop card reminding him of the 'treatment' to be given to smokers seen on that day". Risk not judged to be high. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "A one year questionnaire was returned by 72% of the smokers and the response rate did not vary appreciably among the four groups....Non‐responders assumed not to have stopped smoking." |
Janz 1987.
| Methods | Setting: Outpatient medical clinic at midwestern US teaching hospital/ Recruitment: Consecutive attenders identified as smoking over 5 cpd and giving consent to participation (unselected). | |
| Participants | 250 adult smokers, mean cpd 24. Therapists : Intervention physicians and nurses given brief tutorial. Control physicians not informed of study. | |
| Interventions | 1. Normal care. 2. Brief advice from physician and brief consultation from nurse. 3. As 2 plus self‐help manual. Intervention level: Minimal. Aids used: group 3. Follow‐up visits: no. | |
| Outcomes | PP abstinence at 6m. Validation : none. | |
| Notes | Numbers quit estimated from graphs with unclear denominators ‐ original data sought but not obtainable. 2 & 3 compared to 1 for minimal advice vs no advice (Classifying 3 as intensive does not alter meta‐analysis findings). 3 vs 1 in advice plus aids subgroup, 2 vs 1 in advice without aids. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Each clinic site was divided into half‐day clinic units with each unit assigned to either experimental or control status." |
| Allocation concealment (selection bias) | Low risk | Procedure for systematically identifying and recruiting patients described, no important differences in baseline characteristics. Risk not judged to be high. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15.6% lost to follow‐up at 6m, "drop‐out rates did not vary significantly across study groups." Unclear if those lost to follow‐up counted as smokers. |
Lang 2000.
| Methods | Setting: Workplace Recruitment: smokers at annual check up (unselected) | |
| Participants | 1095 smokers (excludes losses to follow up due to company reorganization) 17% F, av cpd 14, >64% smoked >10 cpd | |
| Interventions | 1. Minimal advice; 5‐10 min from occupational physician. 2. Intensive intervention; contract with quit date, phone call 7 days post quit date, follow‐up visit | |
| Outcomes | Sustained abstinence (>= 6m) at 12m, assessed at annual check up. Validation: CO for a subsample. Unclear whether results reclassified | |
| Notes | Contributes data to intensive vs minimal comparison only. 28 physicians participated. Reported statistical analysis with physician as unit. Difference in 12m PP quit significant, not significant using sustained measure. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomised by occupational physician, method not specified. |
| Allocation concealment (selection bias) | Low risk | "Before randomisation, each... physician sent the scientific committee a list of their working units...then, one unit per physician was randomly selected among his or her units.." Comment: Participant selection centralised. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 physicians declined to participate after being allocated to minimal intervention. Participants lost to follow‐up for work‐related reasons excluded from analysis, all other participants lost to follow‐up counted as smokers. |
Li 1984.
| Methods | Setting: Worksite (naval shipyard) in the USA. Recruitment: Smokers identified at worksite screening (unselected). | |
| Participants | 871 asbestos‐exposed smokers; mean cpd: 24‐26. Therapists: Occupational physicians. | |
| Interventions | 1. Minimal warning, results of pulmonary function tests, leaflet. 2. As group 1 plus behavioural counselling. Intervention level: Minimal (1), Intensive (2). Aids used: yes. Follow‐up visits: no. | |
| Outcomes | Sustained abstinence at 11m. Validation: expired CO. | |
| Notes | Contributes data to intensive vs minimal comparison only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned," method not specified. |
| Allocation concealment (selection bias) | Unclear risk | Method not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 292/871 participants lost to follow‐up, breakdown by group not provided (loss due in part to change in follow‐up procedure early in study). |
Marshall 1985.
| Methods | Setting: Six general practices on the Isle of Wight , UK. Recruitment: Patients responding to a postcard from the GP (selected). | |
| Participants | 200 adult smokers, mean cpd 22. 21% had a smoking‐related disease. Therapists: 11 general practitioners with no specific training. | |
| Interventions | 1. Advice plus nicotine gum. 2. As 1 plus offer of 4 follow‐up visits over 3m. Intervention level: Intensive (2) vs Minimal (1). Aids used: yes. Follow up: 4 in group 2. | |
| Outcomes | Sustained at 12m (from 6m). Validation: CO. | |
| Notes | Contributes data to intensive vs minimal comparison only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not specified. |
| Allocation concealment (selection bias) | Low risk | "Participants were assigned randomly to the two groups on receipt of their postcard. The only constraint placed on allocation was that married couples were assigned to the same group." Comment: randomisation done centrally by study investigator. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 participants lost to follow‐up, all in minimal intervention group. Participants lost to follow‐up counted as smokers. |
McDowell 1985.
| Methods | Setting: Family practices in Canada. Recruitment: Volunteers for smoking cessation programme (selected). | |
| Participants | 366 adult cigarette smokers in 9 group family practices (153 relevant to review); mean cpd 25. Therapists: 56 family physicians. | |
| Interventions | 1. Brief physician advice. 2. Health education in groups for 8 wks (not used in review). 3. Cognitive behaviour modification in 8 group sessions (not used in review). 4. Control: self‐monitoring of smoking. Intervention level: Minimal. Aids used: none. Follow‐up visits: no. | |
| Outcomes | PP abstinence at 12m. Validation: none performed, although subjects threatened with salivary thiocyanate measurement. | |
| Notes | In this review only groups 1 (intervention) and 4 (control) are considered. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned," method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Method not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Differential drop‐out rates between groups. Participants missing outcome data at 6m excluded from final results (30). 6m data used for participants missing data at 12m who had provided at 6m (20 smokers). |
Meyer 2008.
| Methods | Setting: 34 general practices from a German region. Recruitment: smoking patients attending practices during 3 study wks (unselected). | |
| Participants | 1499 patients (1011 in relevant conditions) aged 18‐70 who reported daily cigarette smoking; 48% F, mean cpd 16. | |
| Interventions | 1. Control group (assessment only ‐ 22‐sided questionnaire administered in waiting room). 2. Computer‐generated tailored letters ‐ received 3 personalised letters tailored to the patient's stage of change and selected self‐help manuals (not included in meta‐analysis). 3. Brief advice from trained physician and selected self‐help manuals. Intervention level: Intensive. Aids used: yes. Follow‐up visits: no. | |
| Outcomes | Abstinence at 24m (sustained for 6m). Validation: none. | |
| Notes | 3 versus 1 contributes data to intensive versus control. Physicians trained between 2nd and 3rd study wks to avoid contamination of first two conditions. A stricter outcome reporting 6m abstinence at 12, 18 & 24m was also given. This gave a higher estimated effect with CIs that excluded 1. Advice compared to tailored letters in analysis 6.1, pooled with comparable arms in Meyer 2012. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "quasi‐randomisation based on the time of practice attendance." Fixed sequence of assessment‐only, tailored letters, advice. |
| Allocation concealment (selection bias) | High risk | "Patients attending the practice frequently had a lower probability of inclusion in the later study groups." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Additional analysis using different assumptions about losses to follow‐up did not substantially alter any results. ITT analysis treating participants lost to follow‐up as smokers provided. |
Meyer 2012.
| Methods | Setting: 151 practices from a North‐German region. Recruitment: smoking patients attending practices. |
|
| Participants | 3215 patients (113 excluded) age 18+ years who reported any tobacco smoking within the last six months; 44% F, cpd not stated. | |
| Interventions | 1. Brief Advice: The physician intervention was designed to last 10 minutes and incorporated elements of “health behavior change counseling”. Stage of change‐specific self‐help manuals. 2. Tailored letters: individually tailored computer‐generated letters. Manuals as for 1. 3. Combination: both interventions. Intervention level: Intensive (but does not contribute to analysis). Aids: Self‐help manuals. |
|
| Outcomes | Abstinence at 12m, self‐reported as prolonged for previous 6m. 7‐day PP ('not even a puff') also reported. Validation: none. |
|
| Notes | Does not contribute to primary analysis since since no control comparable to other studies. Studies comparing intensive versus minimal intervention had physician participation in both activites. In this study the physician only gave smoking cessation advice in the consultation. The tailored letters did not require physician action. Advice compared to tailored letters in analysis 6.1, pooled with equivalent comparison from Meyer 2008. Patients who revisited the practice within the study period could receive up to 2 follow‐up interventions. 21.4% of those followed up received 1 follow‐up intervention, 10.6% received 2. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Cluster‐randomised. Practices randomly assigned prior to recruitment. More practices had to be contacted to recruit 50 in the brief advice (75 contacted) and combined (77 contacted) than the tailored letters group (65 contacted). No significant differences between practice characteristics. Authors note "randomisation was seriously undermined by obviously different mechanisms of patient selection for each study condition". |
| Allocation concealment (selection bias) | High risk | Practices not blind to condition when patients recruited. Fewer patients recruited in practices assigned to Brief Advice and Combination conditions. More practices in Combination group recruited no patients (23.5%) than Brief Advice (18%) or Tailored (14%). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Uneven rate of drop‐out (30% Brief Advice group, 21% Tailored letters group, 29% Combination). Analysis 6.1 uses denominators from Table 2 which excludes ineligible participants recruited in error. "The analyses based on datasets including multiple imputed outcomes for patients lost to follow‐up revealed the same pattern of results found in the crude analyses." |
Morgan 1996.
| Methods | Setting: outpatient medical practices, USA. Recruitment: Practices volunteered, patients unselected by motivation. | |
| Participants | 659 smokers aged 50 ‐ 74. Therapists: Physicians with 45 ‐ 60 mins training. | |
| Interventions | 1. Physician advice, stage‐based, tailored self‐help guide. Follow‐up letter from physician and call from project staff. Smokers in contemplation given prescription and free 1 wk supply of gum. 2. Usual care (delayed intervention). Intervention level: intensive. Aids used: yes. Follow‐up visits: yes (phone call). | |
| Outcomes | Abstinence at 6m (assume PP). Validation: none. | |
| Notes | Results sensitive to use of a model allowing for correlation, but corrected OR not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomised by practice, method of sequence generation not specified. |
| Allocation concealment (selection bias) | Low risk | Recruitment procedures described and "these requirements and procedures were implemented equivalently across the two conditions". Statistically significant differences in some baseline characteristics but clinical significance small. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Some practices excluded post‐randomisation due to low recruitment (5 from each group). 13% of individual smokers lost to follow‐up, but breakdown by groups not provided. |
Nebot 1989.
| Methods | Setting: 7 primary care practices in Spain. Recruitment: smokers identified prior to seeing doctor (unselected). | |
| Participants | 424 adult smokers, 24% smoked > 20 cpd. Therapists: 25 doctors in 6 primary care centres. | |
| Interventions | 1. Brief physician advice, 3 ‐ 5 min, and self‐help leaflet, 2. Usual care, Intervention level: minimal, Aids used: none. Follow‐up visit: no, | |
| Outcomes | Abstinence at 12m (definition unclear), Validation: incomplete, | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised by week of attendance (over 6 wk period). |
| Allocation concealment (selection bias) | Low risk | Smokers identified and recruited before seeing doctor. Risk not judged to be high. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar number lost to follow‐up in both groups (intervention: 27/208, control: 18/216). |
Ockene 1991.
| Methods | Setting: US primary care residency programme (physicians in training). Recruitment: unselected. | |
| Participants | 1286 smoking patients not selected for motivation to quit. Therapist: 196 primary care physicians in training. | |
| Interventions | 1. Advice only. 2. Patient‐centred counselling, written materials, asked to schedule follow‐up visit, follow‐up letter. 3. Patient‐centred counselling and offer of prescription for nicotine gum (not used in review). Each group was further randomised to minimal (no calls) or intensive follow‐up by telephone (3 calls over 6m) from a health educator (HE). Intervention level: Minimal (1 without follow‐up counselling) vs Intensive (all other conditions). Aids used: yes. Follow‐up: with physician (2). | |
| Outcomes | PP abstinence at 6m (self‐reported). Validation: none. | |
| Notes | Contributes data to intensive vs minimal comparison only. Adjusted rates used in analysis. All physicians received training in minimal vs intensive interventions and delivered them according to random allocation of patient. Group 1 without HE follow‐up is considered minimal intervention and is compared to all the other arms as intensive intervention. 2 compared to 1 (both without HE follow‐up) for effect of physician follow‐up. 12m outcomes have been reported but do not give rates by HE follow‐up condition; no main effects or interactions were found. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned to the physician and follow‐up conditions". Method not specified. |
| Allocation concealment (selection bias) | Low risk | Physicians opened "a packet containing the intervention materials, which they received at the beginning of the clinic encounter". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses and drop‐outs were included as failures. 62 pts removed from denominator (4 deaths, 58 not contacted by study staff). |
Page 1986.
| Methods | Setting: Five family practitioners in Canada. Recruitment: all patients attending the practices identified as smokers during study period (unselected). | |
| Participants | 289 adult smokers, cpd not stated. Therapists: family practitioners in full time practice. | |
| Interventions | 1. No advice. 2. Advice to quit. 3. As 2 plus offer of nicotine chewing gum prescription (not used in review). Intervention level: minimal. Aids used: none. Follow‐up visits: no. | |
| Outcomes | PP abstinence at 6m. Validation: none. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomised by day of attendance. Post‐hoc tests of results by day of attendance showed no interaction. |
| Allocation concealment (selection bias) | Low risk | All smokers attending practices asked to participate. Risk not judged to be high. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 21% lost to follow‐up overall. Drop‐outs not reported by group, analyses include completers only. |
Pieterse 2001.
| Methods | Setting: 22 GPs in 18 Dutch family practices. Recruitment: proactive recruitment in waiting room (probably selected). | |
| Participants | 530 adult smokers (excludes 7 controls who received intervention). Cpd not stated, ˜15% smoked >= 25 cpd. Practitioners: 22 GPs and 19 assistants, 2 hrs training. | |
| Interventions | 1. Advice/counselling tailored to stage of change, self‐help manual, follow‐up visit if quit date set. Approx 10 mins. 2. Usual care. Intervention level: intensive. Aids used: yes. Follow‐up visits: offered. | |
| Outcomes | Sustained at 12m (from 6m). Validation: none. | |
| Notes | A logistic regression correcting for baseline differences gave a higher estimate of the effect on the odds of quitting (3.04, 95% CI 1.7 to 5.6). A RR derived from crude data is used in the meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "prestructured allocation list," method of sequence generation not specified. |
| Allocation concealment (selection bias) | Unclear risk | "After handing over the completed questionnaire, subjects were assigned to one of both groups by the office assistant." Further detail not provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 78% followed up at 12m, breakdown by group not provided. For patients with serious health problems related to tobacco use, GPs were permitted "to use the intervention for control patients." These patients were excluded from the analysis (7). |
Porter 1972.
| Methods | Setting: single London suburban general practice. Recruitment: opportunistic through direct question by the physician (unselected). | |
| Participants | 191 adult smokers, smoked 4+ cpd. Therapist: one general practitioner working in a group practice of three. | |
| Interventions | 1. No advice. 2. 5 mins of advice delivered 'with conviction and vigour' plus antismoking leaflet. Intervention level: minimal. Aids used: none. Follow ‐up visits: none. | |
| Outcomes | PP abstinence at 6m. Validation: by family report/neighbour report. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random numbers table". |
| Allocation concealment (selection bias) | High risk | Allocations listed in a book kept by the doctor, "so arranged that the doctor remained ignorant of the next identification." "An opportunist attitude was adopted throughout and a patient was admitted when circumstances were appropriate for counselling. It is conceded that this policy may have introduced physician bias into the selection of cases." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants followed‐up. |
Richmond 1986.
| Methods | Setting: single general practice in Australia. Recruitment: smokers who attended the practice ‐ no further methodology stated. | |
| Participants | 200 adult smokers; mean cpd 24. Therapists: 3 male GPs. | |
| Interventions | 1. Six visits to the GP over 6m, including advice, spirometry demonstration and serum cotinine and written materials. 2. Control group completed questionnaire and gave blood sample at single visit. Intervention level: intensive. Aids used: yes. Follow ‐up visits: 6. | |
| Outcomes | Sustained abstinence 3 yrs (assessed at 6m & 3 yrs). Validation: by serum carboxyhaemoglobin concentrations or by salivary cotinine measurement or confirmation by relatives and/or friends. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Alternate days were designated as either 'treatment' or 'control' days". |
| Allocation concealment (selection bias) | High risk | "Subjects were allocated to the appropriate group for the day of their attendance." More men in treatment group, suggesting risk of selection bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Fewer subjects were lost to follow up among those who completed the programme (3%) than among those who did not (21%). This discrepancy in follow up rates for the two groups introduces a possible source of bias in calculating abstention because subjects who were lost to follow up were classified as continuing smokers." |
Rose 78‐92.
| Methods | Setting: Whitehall research study of male civil servants. Recruitment: drawn from men who had undergone a cardiorespiratory screening examination in the Whitehall study, identified as smokers at entry to study, excluding those with major disease or receiving therapy for cardiovascular disease (unselected). | |
| Participants | 1445 male civil servants in London smoking > 5 cpd with high risk of cardiorespiratory disease. Therapists: doctors who were members of the research team. | |
| Interventions | 1. Controls: GPs were sent a record of their screening examination. 2. Intervention: received advice to stop, written materials and a follow‐up visit. Intervention level: Intensive. Aids used: no. Follow‐up visits: yes. | |
| Outcomes | PP of cessation at 1 and 3 yrs (Rose 1978), 20 yr follow‐up data on mortality (Rose 1992). Validation: none. | |
| Notes | Rose 1992 reports 20 yr follow‐up data on mortality from the intervention trial described in Rose 1978.
Unpublished 33 yr follow‐up provided by Martin Shipley and added to review from issue 1, 2007. PP at 3 yrs used in analyses. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "allocated randomly," method not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar rates of follow‐up in both groups (at 3 yrs, 64% intervention and 70% control). Analysis conducted treating those lost to follow‐up as smokers. |
Russell 1979.
| Methods | Setting: 5 group general practices in London. Recruitment: all identified cigarette smokers attending during the 4 wk entry period (unselected). | |
| Participants | 2138 adult smokers; cpd not stated. Therapists: 37 GPs. | |
| Interventions | 1. No intervention. 2. Questionnaire only. 3. Advice to stop smoking. 4. Advice to stop smoking plus leaflet plus a warning that the patient would be followed up. Intervention level: minimal. Aids used: no. Follow‐up visits: no, except group 4 (offer of 1 visit). | |
| Outcomes | Sustained abstinence at 12m (& 1m). Validation: small sample of those reporting cessation validated by salivary cotinine analysis (N = 23). | |
| Notes | (3 & 4) vs (1 & 2). Authors' discussion based on data after those lost to follow‐up excluded, not at randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Eligible patients were assigned according to their day of attendance." |
| Allocation concealment (selection bias) | Low risk | Non‐blind study staff systematically identified eligible patients. No differences in baseline characteristics related to smoking attitudes. Risk not judged to be high. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 73% followed up at 1 yr. "Most of the losses at one month were due to administrative and procedural difficulties...About two‐thirds of the additional losses at one year were due to changes of address." |
Russell 1983.
| Methods | Setting: 6 group general practices in England. Recruitment: Consecutive attenders admitting to being cigarette smokers and consenting to participate (unselected). | |
| Participants | 2106 adult smokers (1377 in relevant arms). Mean cpd 17.5. Therapists: GPs' level of training not stated. | |
| Interventions | 1. No intervention. 2. Advised to stop smoking plus provided with a 'give up smoking' booklet. 3. As group 2, plus offer of nicotine chewing gum prescription (not used in review). Intervention level: minimal. Aids used: none. Follow‐up visits: no. | |
| Outcomes | Sustained abstinence 12m (& 4m). Validation: 66% of those claiming to have quit validated with CO breath testing. | |
| Notes | Authors note that data could be pooled across practices without altering results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients assigned "according to their week of attendance". |
| Allocation concealment (selection bias) | Unclear risk | Non‐blind study staff systematically identified eligible patients. Reason for different numbers across groups unrelated to selection bias, no differences in measured baseline variables. Risk not judged to be high. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16 deaths and 152 who moved away were excluded from analyses. 327 with no or inadequate data at follow‐up were included as smokers. |
Schnoll 2003.
| Methods | Setting: 17 oncology centres in USA. Recruitment: cancer patients recruited by participating physicians (unselected). | |
| Participants | 432 smokers with cancer (406 surviving at 12m); 66% F, av cpd 20. | |
| Interventions | 1. Advice and self‐help manual, prescription of NRT if appropriate. Possible follow‐up. 2. Usual care. Intervention level: intensive. Aids used: yes. Follow ‐up visits: not routinely. | |
| Outcomes | PP abstinence at 12m. Validation: none. | |
| Notes | 34% of intervention and 19% of usual care group prescribed NRT. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Using permuted blocks with dynamic balancing within the recruitment sites." Method of sequence generation not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar number lost to follow‐up in both groups (41/215 in intervention, 37/217 in control). Apart from deaths, non‐respondents included in denominators. |
Segnan 1991.
| Methods | Setting: 44 general practices in Italy. Recruitment: Consecutive patients attending on study days (unselected). | |
| Participants | 923 smoking general practice attenders aged 20‐60. Therapists: GPs who had undergone a 3‐hr training session. | |
| Interventions | 1. Advice and leaflet. 2. Repeated counselling (follow‐up at 1,3,6,9m). 3. Repeated counselling plus nicotine gum (not used in review). 4. Repeated counselling plus spirometry (conducted by specialist centre). Intervention level : Minimal (1) Intensive (2 & 4). Aids used: yes (group 4). Follow‐up visits: yes. | |
| Outcomes | Sustained abstinence at 12m (sustained for 3m by self report). Validation: Urinary cotinine. | |
| Notes | Does not contribute to Comparison 1. 2 & 4 compared to 1 for effects of intensive vs minimal advice. 2 compared to 1 for effect of follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "blocked treatment‐allocation was based on a sequence of random numbers." |
| Allocation concealment (selection bias) | Low risk | "A package of closed, numbered envelopes... indistinguishable from the outside." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 87% (6% refused, 7% untraced) followed up at 12m, analyses included participants lost to follow‐up as smokers in final denominators. |
Severson 1997.
| Methods | Setting: 49 private paediatric practices. Recruitment: mothers attending for well baby visits (unselected). | |
| Participants | 1478 smoking mothers (intervention also given to recent quitters, data not used here); 25 intervention practices, 23 control. Therapists: paediatricians. | |
| Interventions | 1. Information pack including a letter from paediatrician on risks of passive smoking, provided by birth hospital. 2. As 1, and extended support (counselling plus follow‐up at 2, 4, and 5m visits) and materials (incl video tape, written materials, signs, magnets, bib). Intervention level: intensive. Aids used: yes. Follow‐up visits: yes. | |
| Outcomes | PP abstinence at 12m. Validation: none. | |
| Notes | Study design allowed for clustering in calculating sample size. Intraclass correlation proved to be low. Longer term follow‐up data with different denominators reported in Severson 1997 paper used from 2001. Logistic regression analysis controlling for baseline variance slightly reduced estimated effect (OR 1.78, 95% CI 0.84 to 3.74) but does not affect overall result so raw numbers used in meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Practices were block randomised after matching for location and number of practitioners," method of sequence generation not specified. |
| Allocation concealment (selection bias) | Low risk | Patient enrolment done by non‐blind practice staff, small differences in baseline characteristics. Risk not judged to be high. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Only one small practice dropped out of the study." 565/2901 participants lost to follow‐up at 6m, "no differences between minimal and extended conditions in terms of dropout rate." Participants lost to follow‐up included in final denominators and counted as smokers. |
Slama 1990.
| Methods | Setting: General practices in Newcastle, Australia. Recruitment: Practice attenders identified as smokers by questionnaire (unselected). | |
| Participants | 311 general practice attenders age 18 ‐ 64 years; mean cpd : not stated. Therapists: GPs who had received a 1‐hr training session. | |
| Interventions | 1. Advice plus leaflets (minimal intervention). 2. GP‐delivered, brief, behavioural change programme (intensive intervention). 3. Control. Intervention level : Minimal & Intensive. Aids used: none. Follow‐up visits: no. | |
| Outcomes | Sustained abstinence at 12m (& 1m & 6m). Validation: salivary cotinine. | |
| Notes | 1 compared to 3 for advice vs no advice, 2 compared to 3 for intensive intervention vs no advice, 2 compared to 1 for intensive vs minimal intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned," no further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Method not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 32 patients excluded from analysis because they could not be located for first follow‐up; breakdown by group not provided. Numbers followed up at further points not specified. |
Slama 1995.
| Methods | Setting: 373 general practices in France. Recruitment: Consecutive attenders aged > 15 yrs (unselected). | |
| Participants | 3128 adult smokers (a random sample of those randomised) followed up at 12m. Therapists: GPs, minimal training. | |
| Interventions | 1. Asking about smoking status and giving leaflet to smokers who wanted to stop. 2. Controls who were not asked about smoking status until follow‐up. Intervention level: minimal. Aids used: none. Follow‐up visits: no. | |
| Outcomes | Sustained abstinence at 12m (& 1m). Validation: none. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not specified. |
| Allocation concealment (selection bias) | High risk | At the end of the consultation, "the doctor randomised each patient according to the uppermost sheet of the patient information sheets... Yellow sheets indicated the intervention condition, white the control condition...". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Differential loss to follow‐up (32% intervention, 43% control). All subjects lost to follow‐up counted as smokers in final analyses, risks over‐estimating efficacy. |
Stewart 1982.
| Methods | Setting: Family practice in Ottawa, Canada. Recruitment: Consecutive attenders identified as smokers by questionnaire (unselected). | |
| Participants | 691 cigarette smokers age > 11 yrs; cpd not stated. Therapists: physicians ‐ level of training not stated. | |
| Interventions | 1. Advice to quit on 1 occasion. 2. Advice to quit on every visit for a 1 yr period. 3. Advice plus pamphlet. 4. Control. Groups 1 and 2 were subsequently merged as group 2 did not prove to be a practical intervention in this practice. Intervention level: minimal. Aids used: none. Follow‐up visits: no. | |
| Outcomes | Sustained abstinence at 12m (& 5m). Validation: none. | |
| Notes | 1 and 2 and 3 compared to 4 for advice vs no advice. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned," method of sequence generation not specified. |
| Allocation concealment (selection bias) | Unclear risk | Nurse "drew an envelope containing the assignment instructions for each patient," further details not provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | At 12m, similar rates of follow‐up in all groups (avg. 65%), "most of the nonrespondents had moved or were not in the city at the time for other reasons." Unclear if participants lost to follow‐up counted as smokers in final outcome data. |
Thompson 1988.
| Methods | Setting: Health Maintenance Organization, USA. Recruitment: consecutive attenders identified & recruited by study staff (unselected). | |
| Participants | 1039 adult smokers; mean cpd not stated, 68% smoked > 15 cpd. Therapists: 37 family physicians. | |
| Interventions | 1. Brief advice (internal control group).
2. Usual care (external control group).
3. Intervention group; The intervention group received, in a factorial design, 1 or 2 of the following interventions:
A. Structured physician advice (3 ‐ 5 min talk); B. National Cancer Institute self‐help materials; C. Referral to group therapy. Intervention level: minimal and intensive groups. Aids used: in some groups. Follow‐up visits: in some groups. |
|
| Outcomes | PP abstinence at 9m. Validation: none. | |
| Notes | Analyzed in a stratified fashion to take into account the fact the intervention being delivered by a number of different therapists. The internal control group received brief advice as defined by this review, so the study is excluded from all intervention vs control comparisons. In the comparison of intensive vs minimal advice, groups receiving Structured Advice and Referral to group therapy are compared to those receiving Structured Advice only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not specified. |
| Allocation concealment (selection bias) | Low risk | "The physician used a randomised folder, which had been placed in the patient chart at the start of the visit, to direct the intervention." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 86 participants (8%) lost to follow‐up (76 unreachable, 10 deleted because of incomplete information). Numbers not broken down by groups. Participants lost to follow‐up not included in final analysis. |
Unrod 2007.
| Methods | Setting: family physicians' offices in New York, USA. Recruitment: Physicians recruited & randomised. Patients recruited in waiting rooms by project staff, max 10 patients per physician. | |
| Participants | 518 adult smokers; mean cpd 14. | |
| Interventions | 1. Physician & patient given 1‐page tailored report based on computer‐based assessment in waiting room. Physician trained to provide 5As‐based brief counselling. Follow‐up appointment could be arranged. 2. No intervention (usual care). Intervention level: Intensive. Aids used: none. Follow ‐up visits: yes for some participants. | |
| Outcomes | PP abstinence at 6m. Validation: salivary cotinine < 25 ng/ml, in 35% subsample. | |
| Notes | Classified as intensive intervention based on provision of written materials and possibility of follow‐up appointment. Meta‐analysis results not sensitive to its classification. OR in paper derives from a generalized linear model allowing for clustering. Meta‐analysis data derived from percentage quit, sensitivity analysis does not affect summary estimate. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster‐randomised by physician; "A random number generator was used to assign physicians to intervention or usual care control." |
| Allocation concealment (selection bias) | High risk | Patients recruited in waiting rooms by non‐blind project staff; "All identified smokers were offered the opportunity to participate". However fewer patients recruited for control, and differences in length of smoking history so risk of bias judged as high. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar percentages lost to follow‐up in both groups (12% intervention, 8% control). Number randomised used as denominator in meta‐analysis. |
Vetter 1990.
| Methods | Setting: single group general practice in the UK. Recruitment: questionnaire sent by post to all patients registered with the practice > age 60 and those responding and identifying themselves as cigarette smokers included (unselected). | |
| Participants | 471 smokers aged > 60. cpd not stated. Therapists: GPs, backed up by practice nurse. No therapist training stated. | |
| Interventions | 1. Simple advice at a single visit with the GP, backed up by offer of advice on quitting strategies from practice nurse. 2. Non‐intervention control group. Intensity of intervention: minimal Aids used: none. Follow ‐up visits: no. | |
| Outcomes | PP abstinence at 6m. Validation: Expired CO. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "respondents were... randomised," method not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar number missing data in both groups: 10% intervention, 9% control. |
Williams 2002.
| Methods | Setting: 27 community physicians, USA. Recruitment: smokers willing to schedule a visit to discuss smoking, but not selected by motivation. | |
| Participants | 316 smokers unselected for motivation; mean cpd (6m completers): 22. Therapists: community physicians, trained on 4As model and 2 delivery styles. | |
| Interventions | 1. Advice using 4As model, using autonomy supportive style. NRT recommended for quit date setters. 2. Advice using 4As model, using controlling style. NRT prescribed for quit date setters without contraindications. Average session length 11 mins. In both conditions, patients setting a quit date were asked to schedule a follow‐up visit. | |
| Outcomes | Prolonged abstinence at 6, 12 & 30m. Validation: CO at 6 & 30m, 4 disconfirmations reclassified. | |
| Notes | Compares 2 types of advice, not included in main comparison. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not specified. |
| Allocation concealment (selection bias) | Unclear risk | "Just prior to a participant's meeting with a physician, the physician opened a sealed packet that contained a blank audiotape and a brief instruction sheet that indicated which style to use." No further information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 239/316 followed up at 6m. 77 drop‐outs at 6m not included in final outcome data. Of 239 followed up at 6m, 189 followed up at 30m. Participants followed up at 6m and not providing data at 12 and 30m counted as smokers. Drop‐out rates not differential at any follow‐up point. |
Wilson 1982.
| Methods | Setting: 2 university‐based family practices in Ontario, Canada. Recruitment: Consecutive attenders identified as smokers by questionnaire (unselected). | |
| Participants | 211 adult smokers; mean cpd: not stated. Therapists: family physicians not further categorised. | |
| Interventions | 1. Brief advice (5 min counselling). 2. Brief advice plus follow‐up appointments at 1, 3 and 6m. Intervention level: intensive vs minimal. Aids used: none. Follow‐up visits: 3. | |
| Outcomes | PP abstinence at 6m. Validation: none. | |
| Notes | Contributes data to intensive vs minimal comparison only. Effect of intervention greater in this than other follow‐up studies which have used biochemical validation. PP only given in study but Kozlowski 1987 notes that follow‐up was 6m post initial visit not 6m post intervention completion. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned," method not specified. |
| Allocation concealment (selection bias) | Unclear risk | Method not specified. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of patients lost to follow‐up at 6m not specified. Participants lost to follow‐up included in final denominator and treated as smokers. |
Wilson 1990.
| Methods | Setting: 31 general practices in Adelaide, Australia. Recruitment: consecutive attenders identified as smokers by questionnaire (unselected). | |
| Participants | 1238 adult smokers; cpd not stated. Therapists: GPs in the intervention group received a 2‐hr instruction seminar. | |
| Interventions | 1. Personalised advice plus leaflets and visual aids at single visit. 2. Normal care control group (advice given if clinically indicated). Intervention level: minimal. Aids used: none. Follow‐up visits: no. | |
| Outcomes | Sustained abstinence at 12m (& 6m). Validation: none. | |
| Notes | Doctors in the intervention group unaware of the results of the smoking survey, ie had to ascertain themselves if the patient was a smoker. Excluding this study on the basis that it tests the effect of training rather than advice does not materially affect any meta‐analysis findings. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomisation occurred by practice," method of sequence generation not specified. |
| Allocation concealment (selection bias) | Low risk | All practice attenders filled in a health questionnaire, those identified as smokers were followed up, no formal recruitment into a study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 66% followed up at 12m in both groups. Participants lost to follow‐up included as smokers in final analysis. |
CI: confidence interval. COPD: Chronic obstructive pulmonary disease. cpd: cigarettes per day. GP: general practitioner. m: month(s). NRT: nicotine replacement therapy. OR: Odds Ratio. PP: point prevalence.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahluwalia 1999 | Intervention tested was the use of a prompt to increase advice. |
| Aleixandre 1998 | Intervention provider unclear. |
| Andrews 2006 | Follow‐up less than 6m. |
| Angeles‐Silva 2005 | Follow‐up less than 6m. |
| Audrain‐McGovern 2011 | The intervention provider was unclear. |
| Bakkevig 2000 | General practice treatment was the control, compared with a behavioural programme. |
| Becker 2005 | A nurse practitioner‐delivered multicomponent intervention. |
| Bentz 2007 | Smoking cessation rates were not included as an outcome. |
| Borland 2008 | Intervention was referral; physician advice was the control. |
| BTS 1983 | All patients received advice from a physician with adjuncts of a booklet and/or nicotine gum; included in NRT and Self‐help reviews. |
| Buffels 2006 | The RCT did not include a comparison of advice versus placebo or usual care. All patients received advice and were then randomised to spirometry in addition or advice alone. |
| Cabezas 2009 | The intervention was a stepped smoking cessation based on the trans‐theoretical model of change |
| Carpenter 2004 | The intervention was delivered by psychologists rather than physicians. |
| Chahal 2005 | Insufficient data were available from the published abstract to allow analysis. |
| Cohen 1989a | This study principally assesses the effect of training doctors to provide smoking cessation interventions and contributes data to a separate Cochrane review (Carson 2012). |
| Cohen 1989b | This study principally assesses the effect of training doctors to provide smoking cessation interventions and contributes data to a separate Cochrane review (Carson 2012). |
| Colby 2005 | The intervention was delivered by research assistants rather than physicians. |
| Conger 1987 | Follow‐up < 6m. |
| Cummings 1989a | This study principally assesses the effect of training doctors to provide smoking cessation interventions and contributes data to a separate Cochrane review (Carson 2012). |
| Cummings 1989b | This study principally assesses the effect of training doctors to provide smoking cessation interventions and contributes data to a separate Cochrane review (Carson 2012). |
| Etter 2006 | This study prinicpally assesses the effect of training doctors to provide smoking cessation interventions. There are no data on quit rates, only on the number of physicians recommending a stop‐smoking programme. |
| Fang 2006 | Follow‐up < 6m. |
| Folsom 1987 | Follow‐up < 6m. |
| Graham 2011 | It was an internet program that didn't include a physician intervention. |
| Grandes 2000 | Not randomised ‐ 7 intervention and 3 control practices selected. |
| Grogan 2011 | The advice was provided by psychologists. |
| Haug 2010 | Data for this study were gathered from the Meyer 2008 study included in the 2008 update. |
| Haug 2012 | The intervention was provided by a computer programme. |
| Hjalmarson 2007 | The advice was delivered by a nurse and counsellors. |
| Hollis 1993 | Intervention delivered by a nurse. All intervention groups received brief advice from a physician. |
| Hurt 1994 | Evaluates the effect of physician advice and nicotine patch compared to advice alone. Contributes to Cochrane NRT review (Stead 2012). |
| Hymowitz 2006 | Assesses the effects of training doctors to provide smoking cessation. |
| Jackson 2004 | Intervention was delivered by research assistants rather than physicians. |
| Juarranz Sanz 1998 | Physician advice confounded with systematic use of nicotine gum. |
| Kadowaki 2000 | Intervention from an occupational physician included extended counselling. |
| Kalkhuis‐Beam 2011 | Advice provider unclear. |
| Knight 1989 | Follow‐up < 6m. |
| Kottke 1989 | This study principally assesses the effect of training doctors to provide smoking cessation interventions and contributes data to a separate Cochrane review (Carson 2012). |
| Kozlowski 1987 | Not a primary study; reanalyzes data from Wilson 1982. |
| Kreuter 2000 | Physician advice was not the intervention tested. |
| Loeb 1983 | Study population was pregnant women. |
| Loke 2005 | Non‐smoking pregnant women given advice by physicans to try to stop their husbands smoking. Follow‐up < 6m. |
| Lopez 2007 | A multicompenent intervention for cancer prevention in patients with a family member affected by of cancer. |
| Macarthur 1987 | Study population was pregnant women. |
| Manfredi 1999 | Multicomponent intervention; advice could be delivered by physician or nurse. Also included a motivational telephone counselling call, and practice‐based systems for identification of smokers. |
| Mayer 1990 | Study population was pregnant women, and advice was delivered by a health educator, not a physician. |
| McAfee 2005 | Examines recall of smoking advice delivered to patients rather than anaylsing quit rates. |
| McEwen 2002 | Intervention was intended to increase advice giving, not to test the impact of advice. Outcome was GP's behaviour. |
| McEwen 2006 | This study addressed the training of physicians to provide smoking cessation. |
| Messimer 1989 | Study population was pregnant women. |
| Miller 1997 | Intervention delivered by a nurse. All intervention groups received brief advice from a physician. It was a multicomponet intervention. |
| Murray 2008 | Intervention was written advice and referral to cessation services. |
| Ramos 2010 | The intervention included pharmacological treatment. |
| Regan 2011 | The intervention was delivered by nurses and social workers. |
| Richman 2000 | Only 3m follow‐up. |
| Risser 1990 | Provides interesting data on the effect of feedback from carbon monoxide and spirometry, but excluded here because advice was delivered by nurse‐practitioners. |
| Rodriguez 2003 | Intervention included NRT in addition to structured advice. |
| Rodriguez‐Alvarez 2011 | Intervention was spirometry; physician advice was control. |
| Russell 1987 | Randomised by practice and some practices unwilling to undertake the more intensive intervention. |
| Sanchez Beiza 1992 | Both treatment arms received a minimal intervention level of physician advice. |
| Sanz‐Pozo 2006 | Compares nurse‐led counselling to one‐off advice given by a GP; no control group. |
| Secades‐Villa 2009 | No suitable control. Arms received physician advice or a more intensive intervention. |
| Secker‐Walker 1998 | Advice given to pregnant women, included in Cochrane review 'Interventions for promoting smoking cessation during pregnancy' (Lumley 2009). |
| Sippel 1999 | Excluded since 2008; trial addresses the effect of spirometry and CO measurement as an adjunct to advice and was not relevant to the primary comparison. Now included in Cochrane review by Bize 2012 'Biomedical risk assessment as an aid for smoking cessation' which encompasses all trials of this approach. |
| Smith 2009 | The intervention was provided by nurses and included pharmacological treatment. |
| Soria 2006 | Advice given to control group. Intervention was multisession motivational interviewing, too intensive to compare with the intensive interventions in the review. |
| Stratelis 2006 | All patients received advice; compares the impact of repeated spirometry on smokers with and without COPD. |
| Strecher 1991 | This study principally assesses the effect of training doctors to provide smoking cessation interventions and contributes data to a separate Cochrane review (Carson 2012). |
| Tait 2007 | Compares telephone support and NRT to no intervention. |
| Takahashi 2006 | Not an RCT. Observational study of quit rates using the 5As approach. |
| Torrecilla 2001 | Potentially eligible only for intensive versus minimal comparison, borderline for a multiple session counselling intervention. |
| Twardella 2007 | Trial of training and financial incentives. |
| Van 2006 | A multicomponent intervention for prevention of cardiovascular disease. |
| Ward 2006 | Follow‐up < 6m. |
| Williams 2006 | Intervention delivered by counsellors rather than physicians; also attemped to modify serum cholesterol levels in those subjects with raised cholesterol. |
| Wilson 1988 | This study principally assesses the effect of training doctors to provide smoking cessation interventions and contributes data to a separate Cochrane review (Carson 2012). |
| Windsor 1985 | Advice delivered by a health educator, not a physician. |
| Young 2002 | Intervention tested strategies to increase and support advice giving, not the effect of advice. |
| Zhu 2012 | Intervention advice provider unclear. |
COPD: chronic obstructive pulmonary disease; NRT: nicotine replacement therapy
Characteristics of ongoing studies [ordered by study ID]
Martin‐Lujan 2011.
| Trial name or title | ESPITAP study. |
| Methods | RCT. |
| Participants | Current smokers attending one of 12 primary care centres in Spain. |
| Interventions | Usual advice to quit smoking by a general practitioner and a 20‐minute personalised visit to provide detailed information about spirometry results. |
| Outcomes | Smoking cessation at 12 months, validated by CO. |
| Starting date | 2011 |
| Contact information | |
| Notes |
Contributions of authors
Lindsay Stead has identified trials, extracted data and drafted updates since 1998. Tim Lancaster has checked data extraction and finalised updates since 2002. For the present update Diana Buitrago, Nataly Preciado and Guillermo Sanchez did additional searches and screened all search results, extracted data and contributed to drafting the text. Jamie Hartmann‐Boyce updated the Risk of Bias tables for all included studies and contributed to the text.
Sources of support
Internal sources
University of Oxford, Department of Primary Health Care, UK.
National School for Health Research School for Primary Care Research, UK.
External sources
NHS Research and Development Programme, UK.
Declarations of interest
None known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ardron 1988 {published data only}
- Ardron M, MacFarlane IA, Robinson C, Heyningen C, Calverley PM. Anti‐smoking advice for young diabetic smokers: is it a waste of breath?. Diabetic Medicine 1988;5:667‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Betson 1997 {published and unpublished data}
- Betson CL, Lam TH, Chung WH, Chung SF. A randomized controlled trial on smoking cessation in government out‐patient clinics in Hong Kong [Abstract]. Proceedings of the 10th World Conference on Tobacco or Health; 1997 Aug 24‐28; Beijing, China. 1997:113.
BTS 1990a {published data only}
- Research Committee of the British Thoracic Society. Smoking cessation in patients: two further studies by the British Thoracic Society. Thorax 1990;45:835‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
BTS 1990b {published data only}
- Research Committee of the British Thoracic Society. Smoking cessation in patients: two further studies by the British Thoracic Society. Thorax 1990;45:835‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burt 1974 {published data only}
- Burt A, Illingworth D, Shaw T, Thornley P, White P, Turner R. Stopping smoking after myocardial infarction. Lancet 1974;1(7852):304‐6. [DOI] [PubMed] [Google Scholar]
Butler 1999 {published data only}
- Butler CC, Rollnick S, Cohen D, Bachmann M, Russell I, Stott N. Motivational consulting versus brief advice for smokers in general practice: a randomized trial. British Journal of General Practice 1999;49(445):611‐6. [Google Scholar]
Demers 1990 {published data only}
- Demers RY, Neale AV, Adams R, Trembath C, Herman SC. The impact of physicians' brief smoking cessation counseling: a MIRNET study. Journal of Family Practice 1990;31:625‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Fagerström 1984 {published data only}
- Fagerström KO. Effects of nicotine chewing gum and follow‐up appointments in physician‐based smoking cessation. Preventive Medicine 1984;13:517‐27. [DOI] [PubMed] [Google Scholar]
Gilbert 1992 {published data only}
- Gilbert JR, Wilson DM, Singer J, Lindsay EA, Willms DG, Best JA, et al. A family physician smoking cessation program: an evaluation of the role of follow‐up visits. American Journal of Preventive Medicine 1992;8:91‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Haug 1994 {published data only}
- Haug K, Fugelli P, Aaro LE, Foss OP. Is smoking intervention in general practice more successful among pregnant than non‐pregnant women?. Family Practice 1994;11:111‐6. [DOI] [PubMed] [Google Scholar]
Higashi 1995 {published data only}
- Higashi A, Ozasa K, Watanabe Y, Hayashi K, Aoike A, Kawai K, et al. [Efficacy of smoking cessation instruction for general smokers at an annual physical examination] [Japanese]. Nippon Koshu Eisei Zasshi 1995;42:313‐21. [MEDLINE: ] [PubMed] [Google Scholar]
Hilberink 2005 {published data only}
- Hilberink SR, Jacobs JE, Bottema BJ, Vries H, Grol RP. Smoking cessation in patients with COPD in daily general practice (SMOCC): six months' results. Preventive Medicine 2005;41(5‐6):822‐7. [DOI] [PubMed] [Google Scholar]
- Hilberink SR, Jacobs JE, Breteler MHM, Vries H, Grol RPTM. General practice counseling for patients with chronic obstructive pulmonary disease to quit smoking: Impact after 1 year of two complex interventions. Patient Education & Counseling 2011;83(1):120‐4. [DOI] [PubMed] [Google Scholar]
Jamrozik 1984 {published data only}
- Jamrozik K, Vessey M, Fowler G, Wald N, Parker G, Vunakis H. Controlled trial of three different antismoking interventions in general practice. British Medical Journal 1984;288:1499‐503. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Janz 1987 {published data only}
- Janz NK, Becker MH, Kirscht JP, Eraker SA, Billi JE, Woolliscroft JO. Evaluation of a minimal‐contact smoking cessation intervention in an outpatient setting. American Journal of Public Health 1987;77:805‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirscht JP, Janz NK, Becker MH, Eraker SA, Billi JE, Woolliscroft JO. Beliefs about control of smoking and smoking behavior: a comparison of different measures in different groups. Addictive Behaviors 1987;12:205‐8. [DOI] [PubMed] [Google Scholar]
Lang 2000 {published data only}
- Lang T, Nicaud V, Slama K, Hirsch A, Imbernon E, Goldberg M, et al. Smoking cessation at the workplace. Results of a randomised controlled intervention study. Journal of Epidemiology and Community Health 2000;54:349‐54. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 1984 {published data only}
- Li VC, Kim YJ, Ewart CK, Terry PB, Cuthie JC, Wood J, et al. Effects of physician counseling on the smoking behaviour of asbestos‐exposed workers. Preventive Medicine 1984;13:462‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marshall 1985 {published data only}
- Marshall A, Raw M. Nicotine chewing gum in general practice: effect of follow up appointments. British Medical Journal 1985;290:1397‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDowell 1985 {published data only}
- McDowell I, Mothersill KJ, Rosser W, Hartman R. A randomized trial of three approaches to smoking cessation. Canadian Family Physician 1985;31:351‐5. [PMC free article] [PubMed] [Google Scholar]
- Mothersill KJ, McDowell I, Rosser WW. Subject characteristics and long term post‐program smoking cessation. Addictive Behaviors 1988;13(1):29‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rosser WW. The role of the family physician in smoking cessation. Canadian Family Physician 1984;30:160‐166. [PMC free article] [PubMed] [Google Scholar]
Meyer 2008 {published data only}
- Haug S, Meyer C, Ulbricht S, Gross B, Rumpf HJ, John U. Need for cognition as a predictor and a moderator of outcome in a tailored letters smoking cessation intervention. Health Psychology 2010;29(4):367‐73. [DOI] [PubMed] [Google Scholar]
- Haug S, Meyer C, Ulbricht S, Schorr G, Ruge J, Rumpf HJ, et al. Predictors and moderators of outcome in different brief interventions for smoking cessation in general medical practice. Patient Education & Counseling 2010;78(1):57‐64. [DOI] [PubMed] [Google Scholar]
- Klein G, Ulbricht S, Haug S, Gross B, Rumpf HJ, John U, et al. Effects of practitioner‐delivered brief counseling and computer‐generated tailored letters on cigarettes per day among smokers who do not quit‐‐a quasi‐randomized controlled trial. Drug & Alcohol Dependence 2010;112(1‐2):81‐9. [DOI] [PubMed] [Google Scholar]
- Meyer C, Ulbricht S, Baumeister SE, Schumann A, Ruge J, Bischof G, et al. Proactive interventions for smoking cessation in general medical practice: a quasi‐randomized controlled trial to examine the efficacy of computer‐tailored letters and physician‐delivered brief advice. Addiction 2008;103:294‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C, Ulbricht S, Schumann A, Hannover W, Hapke U, Rumpf HJ, et al. Interventions fostering the motivation to quit for smokers in general practice. Suchtmedizin in Forschung und Praxis 2003;5:134‐6. [Google Scholar]
- Ulbricht S, Baumeister SE, Meyer C, Schmidt CO, Schumann A, Rumpf HJ, et al. Does the smoking status of general practitioners affect the efficacy of smoking cessation counselling?. Patient Education & Counseling 2009;74(1):23‐8. [DOI] [PubMed] [Google Scholar]
- Ulbricht S, Klein G, Haug S, Gross B, Rumpf HJ, John U, et al. Smokers' expectations toward the engagement of their general practitioner in discussing lifestyle behaviors. Journal of Health Communication 2011;16(2):135‐47. [DOI] [PubMed] [Google Scholar]
Meyer 2012 {published data only}
- Meyer C, Ulbricht S, Gross B, Kastel L, Wittrien S, Klein G, et al. Adoption, reach and effectiveness of computer‐based, practitioner delivered and combined smoking interventions in general medical practices: a three‐arm cluster randomized trial. Drug and Alcohol Dependence 2012;121(1‐2):124‐32. [DOI] [PubMed] [Google Scholar]
Morgan 1996 {published data only}
- Morgan GD, Noll EL, Orleans CT, Rimer BK, Amfoh K, Bonney G. Reaching midlife and older smokers ‐ tailored interventions for routine medical care. Preventive Medicine 1996;25:346‐54. [DOI] [PubMed] [Google Scholar]
Nebot 1989 {published data only}
- Nebot‐Adell M, Soler‐Vila M, Martin‐Cantera C, Birules‐Pons M, Oller‐Colom M, Sala‐Carbonell E, et al. Effectiveness of the physician's advice to quit smoking: evaluation of the impact a year after the fact [Efectividad del consejo medico para dejar de fumar: evaluacion del impacto al ano de la intervencion]. Revista Clinica Española 1989;184:210‐5. [PubMed] [Google Scholar]
Ockene 1991 {published data only}
- Ockene JK, Kristeller J, Goldberg R, Amick TL, Pekow PS, Hosmer D, et al. Increasing the efficacy of physician‐delivered smoking interventions: a randomized clinical trial. Journal of General Internal Medicine 1991;6:1‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ockene JK, Kristeller J, Pbert L, Hebert JR, Luippold R, Goldberg RJ, et al. The physician‐delivered smoking intervention project: can short‐term interventions produce long‐term effects for a general outpatient population?. Health Psychology 1994;13:278‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Page 1986 {published data only}
- Page AR, Walters DJ, Schlegel RP, Best JA. Smoking cessation in family practice: the effects of advice and nicotine chewing gum prescription. Addictive Behaviors 1986;11:443‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pieterse 2001 {published data only}
- Pieterse ME, Boekema ER, Seydel ER, Wiegman O. Smoking cessation via the general practitioner: effects of a minimal contact intervention programme [abstract]. Patient Education and Counseling 1992;20:107. [Google Scholar]
- Pieterse ME, Seydel ER, Devries H, Mudde AN, Kok GJ. Effectiveness of a minimal contact smoking cessation program for Dutch general practitioners: A randomized controlled trial. Preventive Medicine 2001;32(2):182‐90. [DOI] [PubMed] [Google Scholar]
Porter 1972 {published data only}
- Porter AM, McCullough DM. Counselling against cigarette smoking. A controlled study from a general practice. Practitioner 1972;209:686‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Richmond 1986 {published data only}
- Richmond R, Webster I. Evaluation of general practitioner's use of a smoking intervention programme. International Journal of Epidemiology 1985;14:396‐401. [DOI] [PubMed] [Google Scholar]
- Richmond RL, Austin A, Webster IW. Three year evaluation of a programme by general practitioners to help patients to stop smoking. British Medical Journal 1986;292:803‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond RL, Webster IW. A smoking cessation programme for use in general practice. Medical Journal of Australia 1985;142:190‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Rose 78‐92 {published and unpublished data}
- Rose G, Colwell L. Randomised controlled trial of anti‐smoking advice: final (20 year) results. Journal of Epidemiology and Community Health 1992;46:75‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose G, Hamilton PJS. A randomised controlled trial of the effect on middle‐aged men of advice to stop smoking. Journal of Epidemiology and Community Health 1978;32:275‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley M. Personal communication to Iain Chalmers October 15 2004.
Russell 1979 {published data only}
- Russell MAH, Wilson C, Taylor C, Baker CD. Effect of general practitioners' advice against smoking. British Medical Journal 1979;2:231‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Russell 1983 {published data only}
- Russell MAH, Merriman R, Stapleton J, Taylor W. Effect of nicotine chewing gum as an adjunct to general practitioner's advice against smoking. British Medical Journal 1983;287:1782‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schnoll 2003 {published data only}
- Schnoll RA, Zhang B, Rue M, Krook JE, Spears WT, Marcus AC, et al. Brief physician‐initiated quit‐smoking strategies for clinical oncology settings: a trial coordinated by the Eastern Cooperative Oncology Group. Journal of Clinical Oncology 2003;21(2):355‐65. [DOI] [PubMed] [Google Scholar]
Segnan 1991 {published data only}
- Segnan N, Ponti A, Battista RN, Senore C, Rosso S, Shapiro SH, et al. A randomized trial of smoking cessation interventions in general practice in Italy. Cancer Causes and Control 1991;2:239‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Senore C, Battista RN, Ponti A, Segnan N, Shapiro SH, Rosso S, et al. Comparing participants and nonparticipants in a smoking cessation trial: Selection factors associated with general practitioner recruitment activity. Journal of Clinical Epidemiology 1999;52:83‐9. [DOI] [PubMed] [Google Scholar]
- Senore C, Battista RN, Shapiro SH, Segnan N, Ponti A, Rosso S, et al. Predictors of smoking cessation following physicians' counseling. Preventive Medicine 1998;27(3):412‐21. [DOI] [PubMed] [Google Scholar]
Severson 1997 {published data only}
- Severson HH, Andrews JA, Lichtenstein E, Wall M, Akers L. Reducing maternal smoking and relapse: Long‐term evaluation of a pediatric intervention. Preventive Medicine 1997;26:120‐30. [DOI] [PubMed] [Google Scholar]
- Wall MA, Severson HH, Andrews JA, Lichtenstein E, Zoref L. Pediatric office‐based smoking intervention ‐ impact on maternal smoking and relapse. Pediatrics 1995;96:622‐8. [PubMed] [Google Scholar]
Slama 1990 {published data only}
- Slama K, Redman S, Perkins J, Reid AL, Sanson‐Fisher RW. The effectiveness of two smoking cessation programmes for use in general practice: a randomised clinical trial. BMJ 1990;300:1707‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Slama 1995 {published data only}
- Slama K, Karsenty S, Hirsch A. Effectiveness of minimal intervention by general practitioners with their smoking patients: a randomised controlled trial in France. Tobacco Control 1995;4:162‐9. [Google Scholar]
Stewart 1982 {published data only}
- Stewart PJ, Rosser WW. The impact of routine advice on smoking cessation from family physicians. Canadian Medical Association Journal 1982;126:1051‐4. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Thompson 1988 {published data only}
- Thompson RS, Michnich ME, Friedlander L, Gilson B, Grothaus LC, Storer B. Effectiveness of smoking cessation interventions integrated into primary care practice. Medical Care 1988;26:62‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Unrod 2007 {published data only}
- Unrod M, Smith M, Spring B, DePue J, Redd W, Winkel G. Randomized controlled trial of a computer‐based, tailored intervention to increase smoking cessation counseling by primary care physicians. Journal of General Internal Medicine 2007;22:478‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vetter 1990 {published data only}
- Vetter NJ, Ford D. Smoking prevention among people aged 60 and over: a randomized controlled trial. Age and Ageing 1990;19:164‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Williams 2002 {published data only}
- Williams GC, Deci EL. Activating patients for smoking cessation through physician autonomy support. Medical Care 2001;39:813‐23. [DOI] [PubMed] [Google Scholar]
- Williams GC, Gagne M, Ryan RM, Deci EL. Facilitating autonomous motivation for smoking cessation. Health Psychology 2002;21(1):40‐50. [PubMed] [Google Scholar]
Wilson 1982 {published data only}
- Kozlowski LT, Page A. A second look at the effects of supportive follow‐up on smoking cessation. Canadian Medical Association Journal 1987;137:605‐7. [PMC free article] [PubMed] [Google Scholar]
- Wilson D, Wood G, Johnston N, Sicurella J. Randomized clinical trial of supportive follow‐up for cigarette smokers in a family practice. Canadian Medical Association Journal 1982;126:127‐9. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Wilson 1990 {published data only}
- Wilson DH, Wakefield MA, Steven ID, Rohrsheim RA, Esterman AJ, Graham NM. "Sick of Smoking": evaluation of a targeted minimal smoking cessation intervention in general practice. Medical Journal of Australia 1990;152:518‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahluwalia 1999 {published data only}
- Ahluwalia LS, Gibson CA, Kenney RE, Wallace DD, Resnicow K. Smoking status as a vital sign. Journal of General Internal Medicine 1999;14:402‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aleixandre 1998 {published data only}
- Aleixandre i Marti E, Casanova Matutano MA, Mitjans Lafont J, Sanchez Monfort J, Sanmartin Almenar A. [Clinical trial of 2 tobacco use cessation interventions in primary care] [Spanish]. Atencion Primaria 1998;22(7):424‐8. [PubMed] [Google Scholar]
Andrews 2006 {published data only}
- Andrews K, Bale P, Chu J, Cramer A, Aveyard P. A randomized controlled trial to assess the effectiveness of a letter from a consultant in causing smokers to stop smoking pre‐operatively. Public Health 2006;120(4):356‐8. [DOI] [PubMed] [Google Scholar]
Angeles‐Silva 2005 {published data only}
- Angeles‐Silva E, Castañeda E, Catari A, Daza G, Florez A, Morales A. Efficacy of brief intervention for tobacco smoking cessation [Eficacia de la intervención breve para la cesación del consumo de tabaco]. Medicina Interna (Caracas) 2005;21(1):33‐40. [Google Scholar]
Audrain‐McGovern 2011 {published data only}
- Audrain‐McGovern J, Stevens S, Murray PJ, Kinsman S, Zuckoff A, Pletcher J, et al. The efficacy of motivational interviewing versus brief advice for adolescent smoking behavior change. Pediatrics 2011;128(1):101‐11. [DOI] [PubMed] [Google Scholar]
Bakkevig 2000 {published data only}
- Bakkevig O, Steine S, Hafenbradl K, Laerum E. Smoking cessation ‐ A comparative, randomised study between management in general practice and the behavioural programme SmokEnders. Scandinavian Journal of Primary Health Care 2000;18(4):247‐51. [DOI] [PubMed] [Google Scholar]
Becker 2005 {published data only}
- Becker DM, Yanek LR, Johnson WR Jr, Garrett D, Moy TF, Reynolds SS, et al. Impact of a community‐based multiple risk factor intervention on cardiovascular risk in black families with a history of premature coronary disease. Circulation 2005;111(10):1298‐304. [DOI] [PubMed] [Google Scholar]
Bentz 2007 {published data only}
- Bentz CJ, Bayley KB, Bonin KE, Fleming L, Hollis JF, Hunt JS, et al. Provider feedback to improve 5A's tobacco cessation in primary care: a cluster randomized clinical trial. Nicotine and Tobacco Research 2007;9:341‐9. [DOI] [PubMed] [Google Scholar]
Borland 2008 {published data only}
- Borland R, Balmford J, Bishop N, Segan C, Piterman L, McKay‐Brown L, et al. In‐practice management versus quitline referral for enhancing smoking cessation in general practice: A cluster randomized trial. Family Practice 2008;25(5):382‐9. [DOI] [PubMed] [Google Scholar]
BTS 1983 {published data only}
- Research Committee of the British Thoracic Society. Comparison of four methods of smoking withdrawal in patients with smoking related diseases. Report by a subcommittee of the Research Committee of the British Thoracic Society. British Medical Journal 1983;286:595‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buffels 2006 {published data only}
- Buffels J, Degryse J, Decramer M, Heyrman J. Spirometry and smoking cessation advice in general practice: A randomised clinical trial. Respiratory Medicine 2006;100(11):2012‐7. [DOI] [PubMed] [Google Scholar]
Cabezas 2009 {published data only}
- Cabezas C, Martin C, Granollers S, Morera C, Ballve JL, Zarza E, et al. Effectiveness of a stepped primary care smoking cessation intervention (ISTAPS study): design of a cluster randomised trial. BMC Public Health 2009;106(9):1696‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabezas C, Martin C, Granollers S, Morera C, Ballve JL, Zarza E, et al. Effectiveness of a stepped primary care smoking cessation intervention: cluster randomized clinical trial (ISTAPS study). BMC Public Health 2009;9:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carpenter 2004 {published data only}
- Carpenter MJ, Hughes JR, Solomon LJ, Callas PW. Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. Journal of Consulting and Clinical Psychology 2004;72(3):371‐81. [DOI] [PubMed] [Google Scholar]
Chahal 2005 {published data only}
- Chahal AS, Kishan J, Dhillon A. A comparative study of the role of physician advice and nicotine replacement therapy in smoking cessation. Chest 2005;128(4):386S. [Google Scholar]
Cohen 1989a {published data only}
- Cohen SJ, Stooky GK, Katz BP, Drook CA, Smith DM. Encouraging primary care physicians to help smokers quit. A randomized, controlled trial. Annals of Internal Medicine 1989;110:648‐52. [DOI] [PubMed] [Google Scholar]
Cohen 1989b {published data only}
- Cohen SJ, Stookey GK, Katz BP, Drook CA, Christen AG. Helping smokers quit: a randomized controlled trial with private practice dentists. Journal of the American Dental Association 1989;118:41‐5. [DOI] [PubMed] [Google Scholar]
Colby 2005 {published data only}
- Colby SM, Monti PM, O'Leary‐Tevyaw T, Barnett NP, Spirito A, Rohsenow DJ. Brief motivational intervention for adolescent smokers in medical settings. Addictive Behaviors 2005;30:865‐74. [DOI] [PubMed] [Google Scholar]
Conger 1987 {published data only}
- Conger B, Nelson EC, Dietrich AJ, Blanchard C, McHugo GJ, Simmons JJ, et al. Effectiveness of physician antismoking advice. American Journal of Preventive Medicine 1987;3:223‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Cummings 1989a {published data only}
- Cummings SR, Richard RJ, Duncan CL, Hansen B, Vander Martin R, Gerbert B, et al. Training physicians about smoking cessation: a controlled trial in private practice. Journal of General Internal Medicine 1989;4:482‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cummings 1989b {published data only}
- Cummings SR, Coates TJ, Richard RJ, Hansen B, Zahnd EG, Vander Martin R, et al. Training physicians in counseling about smoking cessation. A randomized trial of the "Quit for Life" program. Annals of Internal Medicine 1989;110:640‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Etter 2006 {published data only}
- Etter JF. Impact of educational outreach visits on smoking cessation activities perfomed by specialist physicians: a randomized trial. Education for Health 2006;19:155‐65. [DOI] [PubMed] [Google Scholar]
Fang 2006 {published data only}
- Fang CY, Ma GX, Miller SM, Tan Y, Su X, Shive S. A brief smoking cessation intervention for Chinese and Korean American smokers. Preventive Medicine 2006;43(4):321‐4. [DOI] [PubMed] [Google Scholar]
Folsom 1987 {published data only}
- Folsom AR, Grimm RH. Stop smoking advice by physicians: a feasible approach?. American Journal of Public Health 1987;77:849‐50. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Graham 2011 {published data only}
- Graham AL, Cobb NK. A randomized trial of internet and telephone treatment for smoking cessation. Archives of Internal Medicine 2011;171(5):46‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grandes 2000 {published data only}
- Grandes G, Cortada JM, Arrazola A. An evidence‐based programme for smoking cessation: effectiveness in routine general practice. British Journal of General Practice 2000;50(459):803‐7. [PMC free article] [PubMed] [Google Scholar]
- Grandes G, Cortada JM, Arrazola A, Laka JP. Predictors of long‐term outcome of a smoking cessation programme in primary care. British Journal of General Practice 2003;53(487):101‐7. [PMC free article] [PubMed] [Google Scholar]
Grogan 2011 {published data only}
- Grogan S, Flett K, Clark‐Carter D, Conner M, Davey R, Richardson D, et al. A randomized controlled trial of an appearance‐related smoking intervention. Health Psychology 2011;30(6):805‐9. [PUBMED: 21767016] [DOI] [PubMed] [Google Scholar]
Haug 2010 {published data only}
- Haug S, Meyer C, Ulbricht S, Gross B, Rumpf HJ, John U. Need for cognition as a predictor and a moderator of outcome in a tailored letters smoking cessation intervention. Health Psychology 2010;29(4):367‐73. [PUBMED: 20658823] [DOI] [PubMed] [Google Scholar]
Haug 2012 {published data only}
- Haug S, Meyer C, Dymalski A, Lippke S, John U. Efficacy of a text messaging (SMS) based smoking cessation intervention for adolescents and young adults: study protocol of a cluster randomised controlled trial. BMC Public Health 2012;12:51. [PUBMED: 22260736] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hjalmarson 2007 {published data only}
- Hjalmarson A, Boëthius G. The effectiveness of brief advice and extended smoking cessation counseling programs when implemented routinely in hospitals.. Preventive Medicine 2007;45:202‐7. [DOI] [PubMed] [Google Scholar]
Hollis 1993 {published data only}
- Hollis JF, Lichtenstein E, Vogt TM, Stevens VJ, Biglan A. Nurse‐assisted counseling for smokers in primary care. Annals of Internal Medicine 1993;118:521‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hurt 1994 {published data only}
- Hurt RD, Dale LC, Fredrickson PA, Caldwell CC, Lee GA, Offord KP, et al. Nicotine patch therapy for smoking cessation combined with physician advice and nurse follow‐up: One‐year outcome and percentage of nicotine replacement. JAMA 1994;271:595‐600. [MEDLINE: ] [PubMed] [Google Scholar]
Hymowitz 2006 {published data only}
- Hymowitz N, Schwab J, Haddock CK, Pyle S, Meshberg S. The Pediatric Resident Training on Tobacco Project: Interim findings. Journal of the National Medical Association 2006;98:190‐203. [PMC free article] [PubMed] [Google Scholar]
- Hymowitz N, Schwab J, Keith‐Haddock C, Pyle S, Meshberg S. The pediatric residency training on tobacco project: baseline findings from the patient tobacco survey. Preventive Medicine 2005;41:159‐66. [DOI] [PubMed] [Google Scholar]
Jackson 2004 {published data only}
- Jackson AA, Manan WA, Gani AS, Eldridge S, Carter YH. Beliefs and behavior of deceivers in a randomized, controlled trial of anti‐smoking advice at a primary care clinic in Kelantan, Malaysia. The Southeast Asian Journal of Tropical Medicine and Public Health 2004;35(3):748‐55. [PubMed] [Google Scholar]
Juarranz Sanz 1998 {published data only}
- Juarranz Sanz M, Soriano Llora T, Reyes Martin G, Sanchez Sachez MI. Efficacy of treatment with nicotine replacement in smoking cessation through Primary Health Care [Spanish]. Revista de Medicina Familiar y Comunitaria 1998;8(6):376‐83. [Google Scholar]
Kadowaki 2000 {published data only}
- Kadowaki T, Watanabe M, Okayama A, Hishida K, Ueshima H. Effectiveness of smoking‐cessation intervention in all of the smokers at a worksite in Japan. Industrial Health 2000;38(4):396‐403. [DOI] [PubMed] [Google Scholar]
Kalkhuis‐Beam 2011 {published data only}
- Kalkhuis‐Beam S, Stevens SL, Baumritter A, Carlson EC, Pletcher JR, Rodriguez D, et al. Participant‐ and study‐related characteristics predicting treatment completion and study retention in an adolescent smoking cessation trial. The Journal of adolescent health : official publication of the Society for Adolescent Medicine 2011;49(4):371‐8. [PUBMED: 21939867] [DOI] [PubMed] [Google Scholar]
Knight 1989 {published data only}
- Knight RA, Hay DA. The relevance of the health belief model to Australian smokers. Social Science & Medicine 1989;28(12):1311‐4. [DOI] [PubMed] [Google Scholar]
Kottke 1989 {published data only}
- Kottke TE, Brekke ML, Solberg LI, Hughes JR. A randomized trial to increase smoking intervention by physicians. Doctors Helping Smokers, Round I. JAMA 1989;261:2101‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Kozlowski 1987 {published data only}
- Kozlowski LT, Page A. A second look at the effects of supportive follow‐up on smoking cessation. Canadian Medical Association Journal 1987;137:605‐7. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Kreuter 2000 {published data only}
- Kreuter MW, Chheda SG, Bull FC. How does physician advice influence patient behavior? Evidence for a priming effect. Archives of Family Medicine 2000;9:426‐33. [DOI] [PubMed] [Google Scholar]
Loeb 1983 {unpublished data only}
- Loeb BK, Waage G, Bailey J. Smoking intervention in pregnancy. Proceedings on the 5th World Conference on Smoking and Health. Ottawa: Canadian Council on Smoking and Health, 1983:389‐95.
Loke 2005 {published data only}
- Loke AY, Lam TH. A randomized controlled trial of the simple advice given by obstetricians in Guangzhou, China, to non‐smoking pregnant women to help their husbands quit smoking. Patient Education and Counseling 2005;59:31‐7. [DOI] [PubMed] [Google Scholar]
Lopez 2007 {published data only}
- Lopez ML, Iglesias JM, Valle MO, Comas A, Fernandez JM, Vries H, et al. Impact of a primary care intervention on smoking, drinking, diet, weight, sun exposure, and work risk in families with cancer experience. Cancer Causes & Control 2007;18(5):525‐35. [DOI] [PubMed] [Google Scholar]
Macarthur 1987 {published data only}
- MacArthur C, Newton JR, Knox EG. Effect of anti‐smoking health education on infant size at birth: a randomized controlled trial. British Journal of Obstetrics & Gynaecology 1987;94:295‐300. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Manfredi 1999 {published data only}
- Manfredi C, Crittenden KS, Cho YI, Gao S. Long‐term effects (up to 18 months) of a smoking cessation program among women smokers in public health clinics. Preventive Medicine 2004;38:10‐19. [DOI] [PubMed] [Google Scholar]
- Manfredi C, Crittenden KS, Warnecke R, Engler J, Cho YI, Shaligram C. Evaluation of a motivational smoking cessation intervention for women in public health clinics. Preventive Medicine 1999;28:51‐60. [DOI] [PubMed] [Google Scholar]
Mayer 1990 {published data only}
- Mayer JP, Hawkins B, Todd R. A randomized evaluation of smoking cessation interventions for pregnant women at a WIC clinic. American Journal of Public Health 1990;80(1):76‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McAfee 2005 {published data only}
- McAfee T, Ludman E, Grothaus L, Zbikowski SM, Bush T, Hollis J, et al. Physician tobacco advice to preteens in a smoking‐prevention randomized trial: steering clear. Journal of Pediatric Psychology 2005;30:371‐6. [DOI] [PubMed] [Google Scholar]
McEwen 2002 {published data only}
- McEwen A, Preston A, West R. Effect of a GP desktop resource on smoking cessation activities of general practitioners. Addiction 2002;97(5):595‐7. [DOI] [PubMed] [Google Scholar]
McEwen 2006 {published data only}
- McEwen A, West R, Preston A. Triggering anti‐smoking advice by GPs: mode of action of an intervention stimulating smoking cessation advice by GPs. Patient Education & Counseling 2006;62:89‐94. [DOI] [PubMed] [Google Scholar]
Messimer 1989 {published data only}
- Messimer SR, Hickner JM, Henry RC. A comparison of two antismoking interventions among pregnant women in eleven private primary care practices. Journal of Family Practice 1989;28:283‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Miller 1997 {published data only}
- Miller NH, Smith PM, DeBusk RF, Sobel DS, Taylor CB. Smoking cessation in hospitalized patients. Results of a randomized trial. Archives of internal medicine 1997;157(4):409‐15. [PUBMED: 9046892] [PubMed] [Google Scholar]
Murray 2008 {published data only}
- Murray RL, Coleman T, Antoniak M, Fergus A, Britton J, Lewis SA. The potential to improve ascertainment and intervention to reduce smoking in primary care: a cross sectional survey. Bmc Health Services Research 2008;8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RL, Coleman T, Antoniak M, Stocks J, Fergus A, Britton J, et al. The effect of proactively identifying smokers and offering smoking cessation support in primary care populations: A cluster‐randomized trial. Addiction 2008;103(6):998‐1006. [DOI] [PubMed] [Google Scholar]
Ramos 2010 {published data only}
- Ramos M, Ripoll J, Estrades T, Socias I, Fe A, Duro R, et al. Effectiveness of intensive group and individual interventions for smoking cessation in primary health care settings: a randomized trial. BMC public health 2010;10:89. [PUBMED: 20178617] [DOI] [PMC free article] [PubMed] [Google Scholar]
Regan 2011 {published data only}
- Regan S, Reyen M, Lockhart AC, Richards AE, Rigotti NA. An interactive voice response system to continue a hospital‐based smoking cessation intervention after discharge. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 2011;13(4):255‐60. [PUBMED: 21330278] [DOI] [PMC free article] [PubMed] [Google Scholar]
Richman 2000 {published data only}
- Richman PB, Dinowitz S, Nashed AH, Eskin B, Sylvan E, Allegra C, et al. The emergency department as a potential site for smoking cessation intervention: A randomized, controlled trial. Academic Emergency Medicine 2000;7(4):348‐53. [DOI] [PubMed] [Google Scholar]
Risser 1990 {published data only}
- Risser NL, Belcher DW. Adding spirometry, carbon monoxide, and pulmonary symptom results to smoking cessation counseling: a randomized trial. Journal of General Internal Medicine 1990;5:16‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rodriguez 2003 {published data only}
- Guallar‐Castillon P, Lafuente‐Urdinguio P, Garteizaurrekoa‐Dublang P, Sainz‐Martinez O, Diez‐Azcarate JI, Foj‐Aleman M, et al. [Probability of success in tobacco quitting during the course of two simple medical interventions] [Spanish] [Probabilidad de exito en el abandono del tabaco en el curso de dos intervenciones sencillas para dejar de fumar]. Revista Espanola de Salud Publica 2003;77(1):117‐24. [PubMed] [Google Scholar]
- Rodriguez‐Artalejo F, Lafuente‐Urdinguio P, Guallar‐Castillon P, Garteizaurrekoa‐Dublang P, Sainz‐Martinez O, Diez‐Azcarate JI, et al. One‐year effectiveness of an individualized smoking cessation intervention at the workplace: a randomized controlled trial. Occupational Environmental Medicine 2003;60(5):358‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodriguez‐Alvarez 2011 {published data only}
- Rodriguez‐Alvarez M, Toran‐Monserrat P, Munoz‐Ortiz L, Negrete‐Palma A, Montero‐Alia JJ, Jimenez‐Gonzalez M, et al. Effectiveness of regular reporting of spirometric results combined with a smoking cessation advice by a primary care physician on smoking quit rate in adult smokers: a randomized controlled trial. ESPIROTAB study. BMC Fam.Pract. 2011;12:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Russell 1987 {published data only}
- Russell MA, Stapleton JA, Hajek P, Jackson PH, Belcher M. District programme to reduce smoking: can sustained intervention by general practitioners affect prevalence?. Journal of Epidemiology and Community Health 1988;42:111‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell MAH, Stapleton JA, Jackson PH, Hajek P, Belcher M. District programme to reduce smoking: effect of clinic supported brief intervention by general practitioners. British Medical Journal 1987;295:1240‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanchez Beiza 1992 {published data only}
- Sanchez Beiza L, Martin Carrillo Dominguez P, Gil Serrano P, Gomez Gil E, Pascual de la Torre M, Valero Martinez A. [Preliminary study of smoking cessation at 6 months following medical counseling, pamphlet, and follow‐up] [Spanish] [Estudio preliminar del abandono del tabaco a los 6 meses tras consejo medico, folleto y seguimento]. Atencio Primaria 1992;10:738‐41. [MEDLINE: ] [PubMed] [Google Scholar]
Sanz‐Pozo 2006 {published data only}
- Sanz‐Pozo B, Miguel‐Diez J, Anegon‐Blanco M, Garcia‐Carballo M, Gomez‐Suarez E, Fernandez‐Dominguez JF. [Effectiveness of a programme of intensive tobacco counselling by nursing professionals] [Spanish] [Efectividad de un programa de consejo antitabaco intensivo realizado por profesionales de enfermeria]. Atencion Primaria 2006;37:266‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Secades‐Villa 2009 {published data only}
- Secades‐Villa R, Alonso‐Perez F, Garcia‐Rodriguez O, Fernandez‐Hermida JR. Effectiveness of three intensities of smoking cessation treatment in primary care. Psychological reports 2009;105(3 Pt 1):747‐58. [DOI] [PubMed] [Google Scholar]
Secker‐Walker 1998 {published data only}
- Secker‐Walker RH, Solomon LJ, Flynn BS, Skelly JM, Mead PB. Smoking relapse prevention during pregnancy. A trial of coordinated advice from physicians and individual counseling. American Journal of Preventive Medicine 1998;15:25‐31. [DOI] [PubMed] [Google Scholar]
Sippel 1999 {published data only}
- Sippel JM, Osborne ML, Bjornson W, Goldberg B, Buist AS. Smoking cessation in primary care clinics. Journal of General Internal Medicine 1999;14:670‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2009 {published data only}
- Smith PM, Burgess E. Smoking cessation initiated during hospital stay for patients with coronary artery disease: a randomized controlled trial. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne 2009;180(13):1297‐303. [PUBMED: 19546455] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soria 2006 {published data only}
- Aveyard P. Motivational interviewing for smokers. British Journal of General Practice 2007;57:67. [PMC free article] [PubMed] [Google Scholar]
- Soria R, Legido A, Escolano C, Yeste AL, Montoya J. A randomised controlled trial of motivational interviewing for smoking cessation. British Journal of General Practice 2006;56:768‐74. [PMC free article] [PubMed] [Google Scholar]
Stratelis 2006 {published data only}
- Stratelis G, Molstad S, Jakobsson P, Zetterstrom O. The impact of repeated spirometry and smoking cessation advice on smokers with mild COPD. Scandinavian Journal of Primary Health Care 2006;24:133‐9. [DOI] [PubMed] [Google Scholar]
Strecher 1991 {published data only}
- Strecher VJ, O'Malley MS, Villagra VG. Can residents be trained to counsel patients about quitting smoking? Results from a randomized trial. Journal of General Internal Medicine 1991;6:9‐17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tait 2007 {published data only}
- Tait RJ, Hulse GK, Waterreus A, Flicker L, Lautenschlager NT, Jamrozik K, et al. Effectiveness of a smoking cessation intervention in older adults. Addiction 2007;102:148‐55. [DOI] [PubMed] [Google Scholar]
Takahashi 2006 {published data only}
- Takahashi K, Saso H, Saka H, Saso H, Iwata M, Hashimoto I, et al. A pilot study on inducement of smoking cessation by a simple 5A (asking, advice, assess, assist, and arrange) approach at outpatient clinics. Asian Pacific Journal of Cancer Prevention 2006;7:131‐5. [PubMed] [Google Scholar]
Torrecilla 2001 {published data only}
- Torrecilla‐Garcia M, Barrueco‐Ferrero M, Maderuelo‐Fernandez J, Jimenez‐Ruiz C, Plaza‐Martin M, Hernandez‐Mezquita M. Tobacco detoxication at a primary care clinic: efficacy of medical counseling, minimal intervention and nicotine replacement therapy at the one‐year follow‐up. Atencion Primaria 2001;27(9):629‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Twardella 2007 {published data only}
- Breitling LP, Twardella D, Hoffmann MM, Witt SH, Treutlein J, Brenner H. Prospective association of dopamine‐related polymorphisms with smoking cessation in general care. Pharmacogenomics 2010;11(4):527‐36. [DOI] [PubMed] [Google Scholar]
- Salize HJ, Merkel S, Reinhard I, Twardella D, Mann K, Brenner H. Cost‐effective primary care‐based strategies to improve smoking cessation: more value for money. Archives of Internal Medicine 2009;169(3):230‐5. [DOI] [PubMed] [Google Scholar]
- Twardella D, Brenner H. Effects of practitioner education, practitioner payment and reimbursement of patients' drug costs on smoking cessation in primary care: a cluster randomised trial. Tobacco Control 2007;16:15‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twardella D, Brenner H. New strategies for the promotion of smoking cessation in general practice: a cluster‐randomised trial. Suchtmedizin in Forschung und Praxis 2003;5(2):142‐4. [Google Scholar]
- Twardella D, Brenner H. Strategies to promote smoking cessation in general practice: Results of a cluster‐randomized trial. American Journal of Epidemiology 2005;161(11):S105. [Google Scholar]
Van 2006 {published data only}
- Weel C, Bakx C, HH, Thien T, BW. Long‐term outcome of cardiovascular prevention: A Nijmegen Academic Family Practices Network study. Journal of the American Board of Family Medicine 2006;19(1):62‐8. [DOI] [PubMed] [Google Scholar]
Ward 2006 {published data only}
- Ward KD, Asfar T, Vander Weg MW, Hammal F, Eissenberg T, Maziak W. Outcomes from Syria's first smoking cessation trial (POS1‐74). Society for Research on Nicotine and Tobacco 12th Annual Meeting February 15‐18, Orlando, Florida. 2006.
Williams 2006 {published data only}
- Williams GC, McGregor H, Sharp D, Kouides RW, Levesque CS, Ryan RM, et al. A self‐determination multiple risk intervention trial to improve smokers' health. Journal of General Internal Medicine 2006;21(12):1288‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 1988 {published data only}
- Wilson DM, Taylor DW, Gilbert JR. A randomised trial of a family physician intervention for smoking cessation. JAMA 1988;260:1570‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Windsor 1985 {published data only}
- Windsor RA, Cutter G, Morris J, Reese Y, Manzella B, Bartlett EE, et al. The effectiveness of smoking cessation methods for smokers in public health maternity clinics: a randomized trial. American Journal of Public Health 1985;75:1389‐92. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2002 {published data only}
- Young JM, D'Este C, Ward JE. Improving family physicians use of evidence‐based smoking cessation strategies: a cluster randomization trial. Preventive Medicine 2002;35(6):572‐83. [DOI] [PubMed] [Google Scholar]
Zhu 2012 {published data only}
- Zhu SH, Cummins SE, Wong S, Gamst AC, Tedeschi GJ, Reyes‐Nocon J. The effects of a multilingual telephone quitline for Asian smokers: a randomized controlled trial. Journal of the National Cancer Institute 2012;104(4):299‐310. [PUBMED: 22282542] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Martin‐Lujan 2011 {published data only}
- Martin‐Lujan F, Piñol‐Moreso JL, Martin‐Vergara N, Basora‐Gallisa J, Pascual‐Palacios I, Sagarra‐Alamo R, et al. Effectiveness of a structured motivational intervention including smoking cessation advice and spirometry information in the primary care setting: the ESPITAP study . BMC Public Health 2011;11(1):859. [NCT01194596] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Bize 2012
- Bize R, Burnand B, Mueller Y, Rège‐Walther M, Camain J‐Y, Cornuz J. Biomedical risk assessment as an aid for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD004705.pub4] [DOI] [PubMed] [Google Scholar]
Cahill 2010
- Cahill K, Lancaster T, Green N. Stage‐based interventions for smoking cessation. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD004492.pub4] [DOI] [PubMed] [Google Scholar]
Cahill 2012
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD006103.pub6] [DOI] [PubMed] [Google Scholar]
Carson 2012
- Carson KV, Verbiest MEA, Crone MR, Brinn MP, Esterman AJ, Assendelft WJJ, Smith BJ. Training health professionals in smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD000214.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chapman 1993
- Chapman S. The role of doctors in promoting smoking cessation. BMJ 1993;307:518‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane Handbook
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Library. Available from www.cochrane‐handbook.org, [updated March 2011]. [Google Scholar]
Coleman 2000
- Coleman T, Murphy E, Cheater F. Factors influencing discussion of smoking between general practitioners and patients who smoke: a qualitative study. British Journal of General Practice 2000;50(452):207‐10. [PMC free article] [PubMed] [Google Scholar]
Denny 2003
- Denny CH, Serdula MK, Holtzman D, Nelson DE. Physician advice about smoking and drinking: are U.S. adults being informed?. American Journal of Preventive Medicine 2003;24(1):71‐4. [DOI] [PubMed] [Google Scholar]
Dickinson 1989
- Dickinson JA, Wiggers J, Leeder SR, Sanson Fisher RW. General practitioners' detection of patients' smoking status. Medical Journal of Australia 1989;150(8):420‐2,425‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fiore 1996
- Fiore MC, Bailey WC, Cohen SJ, et al. Smoking Cessation. Clinical Practice Guideline No 18. AHCPR Publication No. 96‐0692. Rockville (MD): U.S. Department of Health and Human Services, Agency for Health Care Policy and Research, April 1996. [Google Scholar]
Fiore 2008
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service, May 2008. [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 2007
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD000031.pub3] [DOI] [PubMed] [Google Scholar]
Kottke 1988
- Kottke TE, Battista RN, DeFriese GH, Brekke ML. Attributes of successful smoking cessation interventions in medical practice. A meta‐analysis of 39 controlled trials. JAMA 1988;259:2883‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lai 2010
- Lai DTC, Cahill K, Qin Y, Tang JL. Motivational interviewing for smoking cessation. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD006936.pub2] [DOI] [PubMed] [Google Scholar]
Lancaster 2005a
- Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001292.pub2] [DOI] [PubMed] [Google Scholar]
Lancaster 2005b
- Lancaster T, Stead LF. Self‐help interventions for smoking cessation. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD001118.pub2] [DOI] [PubMed] [Google Scholar]
Lumley 2009
- Lumley J, Chamberlain C, Dowswell T, Oliver S, Oakley L, Watson L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001055.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
McEwen 2001
- McEwen A, Akotia N, West R. General Practitioners' views on the English national smoking cessation guidelines. Addiction 2001;96(7):997‐1000. [DOI] [PubMed] [Google Scholar]
McLeod 2000
- McLeod D, Somasundaram R, Howden‐Chapman P, Dowell AC. Promotion of smoking cessation by New Zealand general practitioners: a description of current practice. New Zealand Medical Journal 2000;113(1122):480‐5. [PubMed] [Google Scholar]
Miller 2001
- Miller M, Wood L. Smoking cessation interventions: Review of evidence and implications for best practice in health care settings. Canberra, Australia: Commonwealth Department of Health and Ageing, 2001. [DOI] [PubMed] [Google Scholar]
NHC 2002
- National Health Committee. Guidelines for Smoking Cessation. Wellington, New Zealand: National Advisory Committee on Health and Disability, May 2002. [Google Scholar]
Raw 1998
- Raw M, McNeill A, West R. Smoking cessation guidelines for health professionals ‐ A guide to effective smoking cessation interventions for the health care system. Thorax 1998;53(Suppl 5 Part 1):S1‐S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rollnick 1997
- Rollnick S, Butler CC, Stott N. Helping smokers make decisions: the enhancement of brief intervention for general medical practice. Patient Education and Counseling 1997;31:191‐203. [DOI] [PubMed] [Google Scholar]
Solberg 2001
- Solberg LI, Boyle RG, Davidson G, Magnan SJ, Carlson CL. Patient satisfaction and discussion of smoking cessation during clinical visits. Mayo Clinic Proceedings 2001;76(2):138‐43. [DOI] [PubMed] [Google Scholar]
Stead 2005
- Stead LF, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001007.pub2] [DOI] [PubMed] [Google Scholar]
Stead 2006
- Stead LF, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD002850.pub2] [DOI] [PubMed] [Google Scholar]
Stead 2012
- Stead LF, Perera R, Bullen C, Mant D, Hartmann‐Boyce J, Cahill K, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD000146.pub4] [DOI] [PubMed] [Google Scholar]
Taylor 1994
- Taylor MC, Dingel JL. Prevention of Tobacco‐Caused Disease. Canadian Task Force on the Periodic Health Examination. Ottawa: Health Canada, 1994 Jan:500‐11. [Google Scholar]
Velicer 2000
- Velicer WF, Prochaska JO, Fava JL, Rossi JS, Redding CA, Laforge RG, Robbins ML. Using the transtheoretical model for population‐based approaches to health promotion and disease prevention. Homeostasis in Health and Disease 2000;40:174‐95. [Google Scholar]
West 2000
- West R, McNeill A, Raw M. Smoking cessation guidelines for health professionals: an update. Thorax 2000;55(12):987‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
West 2005
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100:299‐303. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lancaster 2004
- Lancaster T, Stead LF. Physician advice for smoking cessation. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD000165.pub2] [DOI] [PubMed] [Google Scholar]
Silagy 1996
- Silagy C, Ketteridge S. Physician advice for smoking cessation. Cochrane Database of Systematic Reviews 1996, Issue 1. [DOI: 10.1002/14651858.CD000165.pub2] [DOI] [PubMed] [Google Scholar]
Silagy 1997
- Silagy C, Ketteridge S. Physician advice for smoking cessation. Cochrane Database of Systematic Reviews 1997, Issue 4. [DOI: 10.1002/14651858.CD000165.pub2] [DOI] [PubMed] [Google Scholar]
Silagy 1999
- Silagy C, Ketteridge S. Physician advice for smoking cessation. Cochrane Database of Systematic Reviews 1999, Issue 1. [DOI: 10.1002/14651858.CD000165.pub2] [DOI] [PubMed] [Google Scholar]
Silagy 2001
- Silagy C, Stead L. Physician advice for smoking cessation. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD000165] [DOI] [PubMed] [Google Scholar]
Stead 2008a
- Stead LF, Bergson G, Lancaster T. Physician advice for smoking cessation. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD000165.pub3] [DOI] [PubMed] [Google Scholar]


