Abstract
Background
Incomplete miscarriage is a major problem that should be effectively managed with safe and appropriate procedures. Surgical evacuation of the uterus for management of incomplete miscarriage usually involves vacuum aspiration or sharp curettage.
Objectives
To compare the safety and effectiveness of surgical uterine evacuation methods for management of incomplete miscarriage.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (July 2010).
Selection criteria
Randomized trials where different surgical methods were used to manage incomplete miscarriage were eligible for inclusion.
Data collection and analysis
We extracted population characteristics, settings, and exclusion criteria, in addition to outcomes such as complications of the procedure, duration, need for re‐evacuation, blood transfusion, and analgesia/anesthesia.
Main results
Two trials (involving 550 women) were included. Vacuum aspiration was associated with statistically significantly decreased blood loss (mean difference (MD) ‐17.10 ml, 95% confidence interval (CI) ‐24.05 to ‐10.15 ml), less pain during the procedure (risk ratio (RR) 0.74, 95% CI 0.61 to 0.90), and shorter duration of the procedure (MD ‐1.20 minutes, 95% CI ‐1.53 to ‐0.87 minutes), than sharp metal curettage, in the single study that evaluated these outcomes in 357 women. Serious complications such as uterine perforation and other morbidity were rare and the sample sizes of the trials were not large enough to evaluate small or moderate differences.
Authors' conclusions
Although the review indicates that vacuum aspiration is safe, quick to perform, and less painful than sharp curettage, and should be recommended for use in the management of incomplete miscarriage, the results are based on data from only one study. Analgesia and sedation should be provided as necessary for the procedure.
Plain language summary
Surgical procedures to evacuate incomplete miscarriage
Vacuum aspiration is a safe and quick treatment for incomplete miscarriages.
Bleeding and infection generally result if the uterus is not emptied after incomplete miscarriage (where parts of the products of conception are left in the uterus). The review of two trials, involving 550 women, found that vacuum aspiration (a procedure that empties the uterus by using a vacuum source with or without electricity) was safe, quick and easy to perform. It was also less painful than dilatation and curettage, which is often done under general anesthesia in an operating room.
Background
Surgical evacuation of the uterus for management of incomplete miscarriage usually involves vacuum aspiration or sharp metal curettage (WHO 1995). Vacuum aspiration (also called suction curettage, menstrual regulation, endometrial aspiration, or mini‐suction) utilises a vacuum source for the evacuation of the uterus. It can be performed on an outpatient basis with local anesthesia or analgesics. Vacuum aspiration can be used without electricity with a hand‐held vacuum syringe (Manual Vacuum Aspiration). It can also be performed with an electric or foot‐operated mechanical pump. Sharp metal curettage (also called D & C or dilatation and curettage) is often performed in an operating room under regional or general anesthesia. In this method, a metal curette is used to evacuate the contents of the uterus. Sharp curettage is mostly performed without dilatation of the cervix, as the cervical canal is usually already open in incomplete miscarriage.
Many studies have documented the safety of vacuum aspiration (Greenslade 1993), and the World Health Organization includes it as an essential obstetric service at the first level of care (WHO 1991). In most developed countries, vacuum aspiration has replaced sharp metal curettage, but still in many developing countries, physicians continue to use sharp metal curettage because they are not trained in vacuum aspiration, they do not have the necessary equipment to perform the procedure, or in some cases they are not convinced of the effectiveness of the procedure. Medical management of incomplete miscarriage is becoming increasingly common and there are studies assessing the efficacy, safety and acceptability of this method as an alternative (Blum 2007; Zhang 2005). A recent Cochrane review has also evaluated the use of medical methods for incomplete miscarriage (Neilson 2010).
Incomplete miscarriage is a major problem that should be effectively managed with safe and appropriate procedures. This review will attempt to evaluate the surgical procedures for uterine evacuation with regard to the most effective and safe strategy for the management of incomplete miscarriage.
Objectives
To compare the safety and effectiveness of surgical uterine evacuation methods for management of incomplete miscarriage.
Methods
Criteria for considering studies for this review
Types of studies
Randomized trials with adequate allocation concealment, where different surgical methods were used to manage incomplete miscarriage, were eligible for inclusion. Trials with violations of allocated management, or exclusions after allocation not sufficient to materially affect outcomes were eligible.
Types of participants
All trials enrolling women with incomplete miscarriage were eligible, regardless of the cause of the incomplete miscarriage (i.e. spontaneous versus induced).
Types of interventions
Any type of vacuum aspiration versus dilatation and curettage or simple curettage (without dilatation).
Comparison of different types of vacuum aspiration including the use of different cannulas or different sources of vacuum pressure (manual/syringe, electric).
Exclusion criteria
Studies comparing different methods of induced miscarriages (i.e. elective termination of pregnancy).
Studies comparing different medical methods of termination of pregnancy.
Studies comparing surgical with medical methods for the management of incomplete miscarriage.
Comparisons of types of anesthesia/analgesia and hospital versus outpatient care are not evaluated in this review.
Types of outcome measures
Primary outcomes
Uterine perforation;
need for re‐evacuation/procedure failure;
duration of procedure;
post‐abortal infection/sepsis;
blood loss.
Secondary outcomes
Duration of bleeding/vaginal discharge after procedure;
side effects of procedure;
need for anesthesia/analgesia;
pain;
need for blood transfusion;
need for additional uterotonics;
length of hospital stay;
patient satisfaction.
Outcomes such as Ashermann Syndrome (uterine synechiae, adhesions of the uterine wall), infertility, incompetent cervix and ectopic pregnancy following surgical management of incomplete miscarriage are relevant and important outcomes. However, these are relatively infrequent, require long‐term follow up (years) and are not amenable to diagnosis unless the woman wants future pregnancies and the problems become apparent. It is therefore not easy (if not impossible) to evaluate these outcomes with the randomized controlled trial methodology.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (July 2010).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, seeAppendix 1.
For this update we used the following methods when assessing the trials identified previously awaiting classification (Caceres 1979; Caceres 1981a; de Holanda 2003; Edwards 2007).
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identify as a result of the search strategy. We resolved any disagreement through discussion and, when required, we consulted a third person.
Data extraction and management
We designed a form to extract data. For eligible studies, at least two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third person. Data were entered into Review Manager software (RevMan 2008) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
adequate (any truly random process, e.g. random number table; computer random number generator);
inadequate (any non random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence in sufficient detail and determined whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
adequate (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear.
(3) Blinding (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Studies were judged at low risk of bias if they were blinded, or if we judged that the lack of blinding could not have affected the results. Blinding was assessed separately for different outcomes or classes of outcomes.
We assessed the methods as:
adequate, inadequate or unclear for participants;
adequate, inadequate or unclear for personnel;
adequate, inadequate or unclear for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or can be supplied by the trial authors, we re‐included missing data in the analyses which we undertake. We assessed methods as:
adequate;
inadequate:
unclear.
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
adequate (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
inadequate (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear.
(6) Other sources of bias
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
yes;
no;
unclear.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2009). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We used the standardized mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomized trials
It is highly unlikely that there will be cluster randomized controlled trials suitable for this review. If found, the guidance given in the Handbook (Higgins 2009) will be followed.
Dealing with missing data
For included studies, levels of attrition was noted. For all outcomes analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomized to each group in the analyses. The denominator for each outcome in each trial was the number randomized minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
Due to the small number of trials, we did not perform heterogeneity assessment for this review. In future updates of this review, if more data become available, we will assess statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We will regard heterogeneity as substantial if I² is greater than 30% and either T² is greater than zero, or there is a low P‐value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
Where we suspect reporting bias (see ‘Selective reporting bias’ above), we attempted to contact study authors asking them to provide missing outcome data.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2008). We used fixed‐effect inverse variance meta‐analysis for combining data where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. Where we suspected clinical or methodological heterogeneity between studies sufficient to suggest that treatment effects may differ between trials we used random‐effects meta‐analysis.
Subgroup analysis and investigation of heterogeneity
Due to the small number of trials and patients, we did not perform subgroup analyses for this review. However, in future updates of this review, we will conduct these analyses if more data become available.
Sensitivity analysis
We did not undertake sensitivity analyses in this version due to the number of eligible studies. However, if more data become available, we will conduct these analyses in future updates to explore the risk of bias associated with an aspect of study quality (i.e. inadequate sequence generation, incomplete outcome data).
Results
Description of studies
Twenty‐nine trials were identified and considered for inclusion in this review. Of these, 27 were excluded (seeCharacteristics of excluded studies), and two were included. The included trials were conducted in Singapore (Tan 1969) and Zimbabwe (Verkuyl 1993) (seeCharacteristics of included studies).
The included trials were relatively small, with 193 women in the Tan 1969 study and 357 women in the Verkuyl 1993 study. Both of the trials examined vacuum aspiration versus sharp metal curettage. No trial compared different cannula types in vacuum aspiration, or different sources of suction pressure. Verkuyl 1993 used plastic cannulae with suction pressure generated via a syringe, and Tan 1969 used metal cannulae with electrical power source for suction.
Both procedures were performed in the same outpatient operating theatre in Verkuyl 1993, and all patients received intravenous pethidine and diazepam. Anesthesia use or the settings of the procedures were not specified in Tan 1969.
None of the trials noted the etiology of the incomplete miscarriage (e.g. spontaneous or induced).
Risk of bias in included studies
Allocation concealment was 'adequate' in Verkuyl 1993, which used sealed, opaque, envelopes. Tan 1969 did not make note of the method of allocation concealment.
Sequence generation was described in Tan 1969 as "being allocated to each group at random", and in Verkuyl 1993 sequences were generated using a "random number table". Given the nature of the intervention, it is not possible to blind the physicians performing the procedures to the method of uterine evacuation. It is, however, possible to blind the evaluator who assessed complications during the follow‐up visit. Verkuyl 1993 had blinding of the follow‐up evaluator, but Tan 1969 made no mention of blinding of any of the outcome assessments.
Tan 1969 did not note any losses to follow up. Verkuyl 1993 lost 22.9% in the vacuum aspiration group, and 25.8% in the sharp curettage group, to follow up.
The main limitations of these studies are their small sample sizes with regard to serious morbidity, and the large loss to follow‐up rate in the Verkuyl 1993 trial. Lack of blinding of outcome assessments is a limitation in Tan 1969.
Effects of interventions
The review includes data from two studies (involving 550 women) where vacuum aspiration was compared to sharp metal curettage. Uterine perforation and need for re‐evacuation were evaluated by both trials. The remaining outcomes (sepsis, pain, blood loss, postoperative hemoglobin levels, duration of procedure and duration of bleeding) were evaluated by only one trial (Verkuyl 1993).
Vacuum aspiration was associated with decreased blood loss (mean difference (MD) ‐17.10 ml, 95% confidence interval (CI) ‐24.05 to ‐10.15 ml; one study, 357 women), fewer women with blood loss greater than or equal to 100 ml, (risk ratio (RR) 0.28, 95% CI 0.10 to 0.73; one study 357 women), and fewer women with a post‐operative hemoglobin level less than 10 g/dl (RR 0.55, 95% CI 0.33 to 0.90; one study, 270 women). Fewer women undergoing vacuum aspiration reported moderate to severe pain during the procedure (RR 0.74, 95% CI 0.61 to 0.90; one study, 357 women), and the duration of the procedure was shorter for vacuum aspiration than for sharp metal curettage (MD ‐1.20 minutes, 95% CI ‐1.53 to ‐0.87 minutes; one study, 357 women).
The remaining findings were not statistically significant. For vacuum aspiration versus sharp curettage respectively, the results were as follows: uterine perforation 0/227 versus 1/221 (RR 0.32, 95% CI 0.01 to 7.76; two studies, 448 women); need for re‐evacuation 3/227 versus 2/236 (RR 1.50, 95% CI 0.29 to 7.83, two studies, 463 women); incidence of sepsis 2/138 versus 7/132 (RR 0.27, 95% CI 0.06 to 1.29; one study, 270 women). Duration of bleeding after the procedure (MD ‐0.30 days, 95% CI ‐1.30 to 0.70 days; one study, 270 women).
Discussion
This review evaluates vacuum aspiration versus sharp metal curettage in the management of incomplete miscarriage. Two trials (involving 550 women) are included.
The results indicate that vacuum aspiration is safe, quicker to perform, and less painful than sharp curettage, as evidenced by statistically significant findings of decreased blood loss, decreased perception of pain, and a shorter duration of the vacuum aspiration procedure. The conclusions of the review might be limited by the small number of trials evaluating these outcomes, and the large loss to follow‐up rate in the Verkuyl 1993 trial.
Uterine perforation is a serious complication of surgical evacuation procedures which is relatively rare with either of the approaches. Of the more than 200 patients included in each arm, perforation occurred in one case in the sharp curettage group, and none in the vacuum aspiration group. There were few cases that required re‐evacuation in either group of both trials. Given the rare occurrence of perforation and need for re‐evacuation with either approach, very large trials would be needed to evaluate any significant differences between vacuum aspiration and sharp curettage. When other advantages of vacuum aspiration are considered, such a trial may not be justifiable.
The need for re‐evacuation was slightly lower in the vacuum aspiration group in Tan 1969 (1/89 versus 2/104), but higher in Verkuyl 1993 (2/138 versus 0/132). Given the rare occurrence of perforation and need for re‐evacuation with either approach, very large trials would be needed to evaluate any significant differences between vacuum aspiration and sharp curettage. When other advantages of vacuum aspiration are considered, such a trial may not be justifiable.
Vacuum aspiration can be performed without the need for a fully equipped and staffed operating theatre as it can be done with or without electricity, under local anesthesia or sedation. A recent observational study has also concluded that manual vacuum aspiration could be routinely considered to treat incomplete miscarriage, thus avoiding the need for general anesthesia and access to operating theater (Milingos 2009). It can therefore be performed in settings with limited resources, saving time and money, and possibly minimizing complications. Eliminating the need for transport to a better equipped facility might decrease the severity of an infection, or decrease blood loss and the subsequent need for transfusions.
In conclusion, the results of this review suggest that vacuum aspiration is at least as effective as sharp curettage, if not more effective in the management of incomplete miscarriage. However, sharp curettage continues to be used widely in many parts of the world. Some clinicians argue that in experienced hands it is safe and effective and are therefore reluctant to change to suction curettage. While this may be true, vacuum curettage has other advantages that merit the change to this technology. It has been suggested that vacuum aspiration is more cost effective than sharp curettage (Greenslade 1993). Since the pain seems to be less and procedure time is shorter, efforts should be put into wider dissemination and use of the vacuum aspiration technology around the world.
Authors' conclusions
Implications for practice.
Although the review indicates that vacuum aspiration is safe, quick to perform, and less painful than sharp curettage, and should be recommended for use in the management of incomplete miscarriage, the results are based on data from only one study. Analgesia and sedation should be provided as necessary for the procedure.
Implications for research.
Different sources of vacuum pressure, cannula types, methods of analgesia, and duration of hospital stay have not been evaluated here and deserve to be reviewed and further researched if necessary.
What's new
| Date | Event | Description |
|---|---|---|
| 16 July 2010 | New citation required but conclusions have not changed | New authors updated the review. |
| 30 April 2010 | New search has been performed | Search updated. No new studies identified. Four studies previously awaiting classification have now been excluded (Caceres 1979; Caceres 1981a; de Holanda 2003; Edwards 2007). |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 1, 2001
| Date | Event | Description |
|---|---|---|
| 23 September 2009 | Amended | Search updated. Four new reports added to Studies awaiting classification (Caceres 1979; Caceres 1981a; de Holanda 2003; Edwards 2007). |
| 14 April 2008 | Amended | Converted to new review format. |
| 1 December 2002 | New search has been performed | Search updated. No new trials identified. |
Acknowledgements
The authors would like to thank Dr Fatu Forna for her participation in the previous version of this review.
Appendices
Appendix 1. Methods used to assess trials included in previous versions of this review
We used the following methods to assess Tan 1969; Verkuyl 1993.
All trials identified with this search strategy were considered for inclusion and listed in this review. Trials with objectives other than surgical uterine evacuation methods for management of incomplete miscarriage and where no evidence of random allocation was found were excluded without further evaluation.
Trials remaining after this stage were critically appraised for methodological quality. Quality score for allocation concealment was given as described in the Cochrane Reviewers' Handbook (Clarke 2000). Briefly, trials which use secure concealment methods such as central randomization, sealed, opaque, consecutively numbered envelopes, were given a quality score of (A). Trials with unknown or unclear methods of concealment were given a quality score of (B). Inadequately concealed trials, such as those that use open randomization methods were given a quality score of (C).
Data extraction: In addition to pre‐specified outcomes, the following characteristics of trials were extracted:
country;
settings (hospital/outpatient clinic);
exclusion criteria;
women excluded from analyses after randomization;
loss to follow up;
use of antibiotics.
Loss to follow‐up rate and reason for loss to follow up were scrutinized, and trials where there was a high likelihood of attrition bias (imbalance in the loss to follow‐up rates in study groups) were excluded.
Data extraction was performed by two review authors independently, and any disagreement was resolved by discussion.
Data and analyses
Comparison 1. Any type of vacuum aspiration versus sharp metal curettage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine perforation | 2 | 448 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.76] |
| 2 Need for re‐evacuation of uterus | 2 | 463 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.29, 7.83] |
| 3 Sepsis | 1 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.06, 1.29] |
| 4 Moderate to severe pain during procedure | 1 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.61, 0.90] |
| 5 Blood loss >= 100 ml | 1 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.10, 0.73] |
| 6 Blood loss (ml) | 1 | 357 | Mean Difference (IV, Fixed, 95% CI) | ‐17.10 [‐24.05, ‐10.15] |
| 7 Post‐op hemoglobin level < 10 g/dl | 1 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.33, 0.90] |
| 8 Duration of procedure (minutes) | 1 | 357 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐1.53, ‐0.87] |
| 9 Duration of bleeding (days) | 1 | 270 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.30, 0.70] |
| 10 Need for additional uterotonics | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Patient satisfaction | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Need for blood transfusion | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Length of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Need for anesthesia/analgesia | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
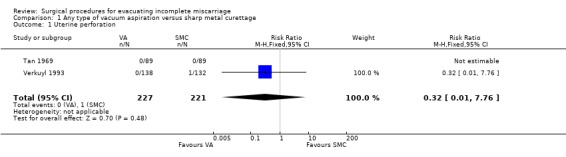
Comparison 1 Any type of vacuum aspiration versus sharp metal curettage, Outcome 1 Uterine perforation.
1.2. Analysis.
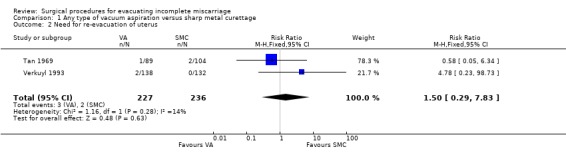
Comparison 1 Any type of vacuum aspiration versus sharp metal curettage, Outcome 2 Need for re‐evacuation of uterus.
1.3. Analysis.
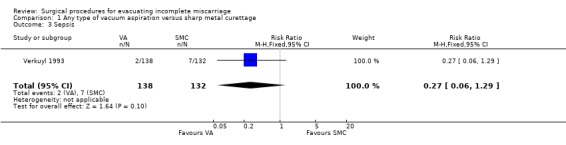
Comparison 1 Any type of vacuum aspiration versus sharp metal curettage, Outcome 3 Sepsis.
1.4. Analysis.
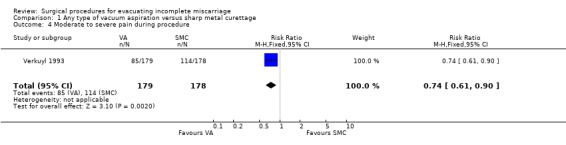
Comparison 1 Any type of vacuum aspiration versus sharp metal curettage, Outcome 4 Moderate to severe pain during procedure.
1.5. Analysis.
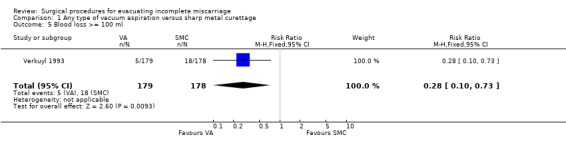
Comparison 1 Any type of vacuum aspiration versus sharp metal curettage, Outcome 5 Blood loss >= 100 ml.
1.6. Analysis.
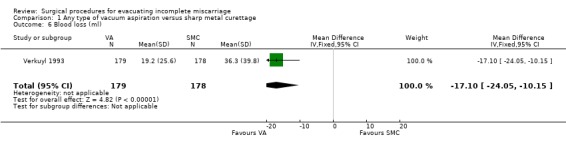
Comparison 1 Any type of vacuum aspiration versus sharp metal curettage, Outcome 6 Blood loss (ml).
1.7. Analysis.
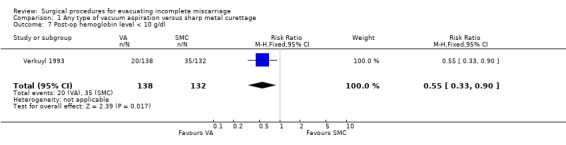
Comparison 1 Any type of vacuum aspiration versus sharp metal curettage, Outcome 7 Post‐op hemoglobin level < 10 g/dl.
1.8. Analysis.
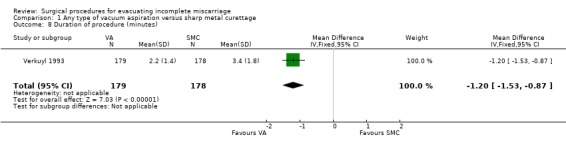
Comparison 1 Any type of vacuum aspiration versus sharp metal curettage, Outcome 8 Duration of procedure (minutes).
1.9. Analysis.
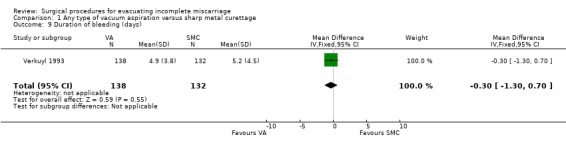
Comparison 1 Any type of vacuum aspiration versus sharp metal curettage, Outcome 9 Duration of bleeding (days).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Tan 1969.
| Methods | The method of allocation is not stated. No mention of blinding of outcome assessments. | |
| Participants | 193 women presenting with incomplete miscarriage in a hospital in Singapore. Exclusion criteria: women with missed miscarriage. | |
| Interventions | Treatment with electric pump vacuum aspiration using metal cannulae 9 mm to 16 mm in diameter, versus sharp metal curettage. | |
| Outcomes | Failure rate/need for re‐evacuation (macroscopic and histologic evidence of retained products), any complications e.g. perforated uterus. | |
| Notes | No mention was made of women excluded from analyses after randomization. No mention was made of prophylactic antibiotic use. No mention was made of anesthesia/analgesia use. Cases were followed up at 2‐week intervals on 2 or more occasions to evaluate for complications. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Reported as "being allocated to each group at random". No randomization method was described. |
| Allocation concealment? | Unclear risk | No allocation concealment method was described. |
| Blinding? All outcomes | Unclear risk | Participants were not blinded. No information was provided on the blinding of the assessors. |
| Incomplete outcome data addressed? All outcomes | Low risk | No loss to follow up occurred based on the numbers provided in the text. |
| Free of selective reporting? | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Free of other bias? | Unclear risk | There is insufficient information to assess whether an important risk of bias exists. |
Verkuyl 1993.
| Methods | Allocation was by means of a random number table, with group allocation sequentially placed in opaque, consecutively numbered envelopes. Follow‐up evaluator was unaware of patients study group. | |
| Participants | 357 women presenting with incomplete miscarriage in a hospital in Zimbabwe. Exclusion criteria: gestational age greater than 18 weeks, evidence of septicemia, peritonitis, severe hypovolemia requiring hospitalization. | |
| Interventions | Treatment with manual vacuum aspiration using plastic cannulae of 8 mm or 10 mm, versus sharp metal curettage, both in the theatre. | |
| Outcomes | Need for re‐evacuation, pain severity, possible uterine perforation, sepsis, mean blood loss, blood loss >= 100 ml, mean duration of procedure, duration >= 4 minutes, post‐op hemoglobin level, post‐op hemoglobin level <= 10 g/dl, hemoglobin level difference, mean duration of bleeding post‐evacuation. | |
| Notes | 41 (22.9%) women in the suction curettage group, and 46 (25.8%) in the sharp curettage group were lost to follow up and were excluded from some of the analyses. No mention was made of prophylactic antibiotic use. All patients received IV pethidine and diazepam. Ergometrine was also given routinely. Patients were followed up on post‐evacuation day 14. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Reported as "random number table". |
| Allocation concealment? | Low risk | Reported as using "opaque, consecutively numbered envelopes". |
| Blinding? All outcomes | Low risk | Participants were not blinded but "the follow‐up evaluator was unaware of the patient's study group". |
| Incomplete outcome data addressed? All outcomes | Low risk | "In the suction curettage group 138 (77%) patients attended for follow up compared with 132 (74%) in the conventional group." |
| Free of selective reporting? | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Free of other bias? | Low risk | |
IV = intravenous g/dl = grams per decilitre ml = millilitre mm =millimetre
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allen 1971 | No randomized or quasi‐randomized comparisons were made. The trial evaluated different forms of analgesia for curettage in incomplete miscarriage. |
| Antonovski 1975 | This randomized trial compared metal versus plastic cannulae for induced miscarriages. |
| Balogh 1982 | No randomized or quasi‐randomized comparisons were made. The trial evaluated vacuum aspiration in induced miscarriages. |
| Blumenthal 1994 | No randomized or quasi‐randomized comparisons were made. The trial involved a time and cost analysis for management of incomplete miscarriage with MVA. |
| Caceres 1979 | This study provides scarce data on the randomization procedures (i.e. sequence allocation, concealment of allocation) and excludes a significant portion of the study population post‐randomization. |
| Caceres 1981 | Multiple unsuccessful attempts were made to get this manuscript. |
| Caceres 1981a | This study provides scarce information on the randomization procedures and the characteristics of the patients in randomized groups show differences, increasing the risk of bias for the outcome analysis. |
| Cheng 1976 | This randomized trial compared inpatient versus outpatient management of induced miscarriage. |
| de Holanda 2003 | This study provides scarce data on the randomization procedures (i.e. sequence allocation, concealment of allocation) and excludes a significant portion of study population post‐randomization. |
| Edwards 2007 | This randomized trial compares manual vacuum aspiration with electric vacuum aspiration. |
| El Kabarity 1985 | Multiple unsuccessful attempts were made to get this manuscript. |
| Farell 1982 | No randomized or quasi‐randomized comparisons were made. The trial examined treatment of consecutive patients with suction curettage. |
| Filshie 1973 | No randomized or quasi‐randomized comparisons were made. The trial examined treatment of consecutive patients with suction curettage. |
| Fonseca 1997 | This randomized trial evaluated cost and duration of hospital stay. The data presented was not suitable for extraction. Unsuccessful attempts were made to get additional data. |
| Gruenberger 1979 | The trial evaluated different forms of analgesia for suction curettage. |
| Henderson Lewis 1979 | Multiple unsuccessful attempts were made to get this manuscript. |
| Hill 1971 | No randomized or quasi‐randomized comparisons were made. The trial compared consecutive patients undergoing sharp curettage to those undergoing vacuum curettage during another time period. |
| Johnson 1993 | No randomized or quasi‐randomized comparisons were made. This trial involved a cost analysis for treatment of incomplete miscarriage with MVA and sharp curettage. |
| Kizza 1990 | This trial was not randomized, as allocation was by alternation. Manual vacuum aspiration was compared to sharp metal curettage in women with incomplete miscarriage. |
| Lean 1976 | This randomized trial compared dilatation and curettage and vacuum aspiration for induced miscarriage. |
| Lukman 1996 | No randomized or quasi‐randomized comparisons were made. The trial evaluated vacuum aspiration and sharp curettage for management of incomplete miscarriage. |
| Magnelli 1992 | It was not clear whether this trial was randomized. Women with incomplete miscarriage, stillbirths, molar pregnancy, retained products, and anembryonic pregnancy were treated with either suction curettage or sharp curettage. |
| Magotti 1995 | This was a quasi‐randomized trial, but the data were not complete and suitable for extraction. Unsuccessful attempts were made to get additional data. |
| Mahomed 1994 | No randomized or quasi‐randomized comparisons were made. The trial evaluated vacuum aspiration and sharp curettage for management of incomplete miscarriage. |
| Rashid 1970 | No randomized or quasi‐randomized comparisons were made. The trial examined treatment of consecutive patients with suction curettage. |
| Ricalde 1997 | No randomized or quasi‐randomized comparisons were made. The trial examined vacuum aspiration and dilatation and curettage for incomplete miscarriage. |
| Suter 1970 | No randomized or quasi‐randomized comparisons were made. The trial examined treatment of consecutive patients with suction curettage. |
MVA = manual vacuum aspiration
Differences between protocol and review
The methods have been updated to reflect current Cochrane methodological guidelines and we have completed the risk of bias tables for the two included studies.
Contributions of authors
All three review authors contributed to the planning and preparation of the updated review.
Sources of support
Internal sources
HRP ‐ UNDP/UNFPA/WHO/World Bank Special Programme in Human Reproduction, Geneva, Switzerland.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Tan 1969 {published data only}
- Tan PM, Ratnam SS, Quek SP. Vacuum aspiration in the treatment of incomplete abortion. Journal of Obstetrics and Gynaecology of the British Commonwealth 1969;76:834‐6. [DOI] [PubMed] [Google Scholar]
Verkuyl 1993 {published data only}
- Verkuyl DAA, Crowther CA. Suction versus conventional curettage in incomplete abortion. A randomised controlled trial. South African Medical Journal 1993;83:13‐5. [PubMed] [Google Scholar]
References to studies excluded from this review
Allen 1971 {published data only}
- Allen A, Philpott RH. Management of incomplete abortion as an outpatient procedure. Central Afican Journal of Medicine 1971;17:91‐6. [PubMed] [Google Scholar]
Antonovski 1975 {published data only}
- Antonovski L, Ljatkova K, Sukarov L, Brenner W, Edelman D, Bernard R. A comparative study of metal and plastic (Karman) cannulae for first trimester abortion by suction curettage. International Journal of Gynecology & Obstetrics 1975;13:33‐8. [DOI] [PubMed] [Google Scholar]
Balogh 1982 {published data only}
- Balogh SA. Vacuum aspiration with the IPAS modified gynecologic syringe. Contraception 1983;27:63‐8. [DOI] [PubMed] [Google Scholar]
Blumenthal 1994 {published data only}
- Blumenthal PD, Remsburg RE. A time and cost analysis of the management of incomplete abortion with manual vacuum aspiration. International Journal of Gynecology & Obstetrics 1994;45:261‐7. [DOI] [PubMed] [Google Scholar]
Caceres 1979 {published data only}
- Caceres EM. Manangement of incomplete abortion: completion by vacuum aspiration and by sharp curettage. Peronal communication 1979.
Caceres 1981 {published data only}
- Caceres GH. Hospital management of incomplete abortion: comparative study of dilation and curettage versus vacuum aspiration [Manejo hospitalario del aborto incompleto: Estudio Comparitivo del curetaje uterino versus la aspiracion por vacio]. Monografias de la Corporacion Centro Regional del Poblacion 1981;16:45‐81. [Google Scholar]
Caceres 1981a {published data only}
- Caceres GH, Gamboa GR, Hernandez MA, Escobar GL. Hospital management of incomplete abortion: comparative study of dilation and curettage versus vacuum aspiration [Manejo hospitalario del aborto incompleto: Estudio Comparitivo del curetaje uterino versus la]. Personal communication 1981.
Cheng 1976 {published data only}
- Cheng MCE, Ng A, Seng KM, Ratnam SS. The safety of outpatient abortion, a controlled study. Annals of the Academy of Medicine, Singapore 1976;4:245‐8. [Google Scholar]
de Holanda 2003 {published data only}
- Holanda AAR, dos Santos HPFD, Barbosa MF, Barreto CFB, Felinto AS, Araujo IA. Treatment of miscarriage in the first trimester of pergnancy: curettage versus manual vacuum aspiration [Tratamento do abortamento do primeiro trimestre da gestacao: curetagem versus aspiracao manual a vacuo]. Revista Brasileira de Ginecologia e Obstetricia 2003;25(4):271‐6. [Google Scholar]
Edwards 2007 {published data only}
- Edwards S, Tureck R, Fredrick M, Huang X, Zhang J, Barnhart K. Patient acceptability of manual versus electric vacuum aspiration for early pregnancy loss. Journal of Women's Health 2007;16(10):1429‐36. [DOI] [PubMed] [Google Scholar]
El Kabarity 1985 {unpublished data only}
- Kabarity H, Louz SA, El‐Etribi A, Yeyha M, Ellian A. Suction abortion versus traditional evacuation in the management of incomplete inevitable abortions. International College of Surgeons, Fifth American Federation Congress; 1995 Nov 25‐28; Cairo. 1985.
Farell 1982 {published data only}
- Farell RG, Stonington DT, Ridgeway RA. Incomplete and inevitable abortion: treatment by suction in the emergency department. Annals of Emergency Medicine 1982;11:652‐8. [DOI] [PubMed] [Google Scholar]
Filshie 1973 {published data only}
- Filshie GM, Ahluwalia J, Beard RW. Portable Karman curette equipment in management of incomplete abortions. Lancet 1973;2:1114‐6. [DOI] [PubMed] [Google Scholar]
Fonseca 1997 {published data only}
- Fonseca W, Misago C, Fernandes L, Correia L, Silveira D. Adoption of manual vacuum aspiration for treatment of incomplete abortion reduces costs and duration of patient's hospital stay in an urban area of Northeastern Brazil. Revista de Saúde Pública 1997;31:472‐8. [DOI] [PubMed] [Google Scholar]
Gruenberger 1979 {published data only}
- Grunberger W, Mutz N, Reinold E, Ulm R. Experiences using the suction curettage system of Hamann and Pockrandt [Erfahrungen mit dem Saugkurettensystem nach Hamann und Pockrandt]. Zentralblatt fur Gynakologie 1979;101:320‐7. [PubMed] [Google Scholar]
Henderson Lewis 1979 {unpublished data only}
- Henderson Lewis JA. Management of incomplete abortion: completion by vacuum aspiration and by sharp curettage ‐ inpatient and outpatient procedures. Prepared for the Instituto Salvadoreno del Seguro Social 1979.
Hill 1971 {published data only}
- Hill DL. Management of incomplete abortion with vacuum curettage. Minnesota Medicine 1971;54(3):225‐8. [PubMed] [Google Scholar]
Johnson 1993 {published data only}
- Johnson BR, Benson J, Bradley J, Ordonez AR. Costs and resource utilization for the treatment of incomplete abortion in Kenya and Mexico. Social Science and Medicine 1993;36:1443‐53. [DOI] [PubMed] [Google Scholar]
Kizza 1990 {published data only}
- Kizza APM, Rogo KO. Assessment of the manual vacuum aspiration (MVA) equipment in the management of incomplete abortion. East African Medical Journal 1990;67:812‐22. [PubMed] [Google Scholar]
Lean 1976 {published data only}
- Lean TH, Vengadasalam D, Pachauri S, Miller ER. A comparison of D & C and vacuum aspiration for performing first trimester abortion. International Journal of Gynecology & Obstetrics 1976;14(6):481‐6. [DOI] [PubMed] [Google Scholar]
Lukman 1996 {published data only}
- Lukman HY, Pogharian D. Management of incomplete abortion with manual vacuum aspiration in comparison to sharp metallic curette in an Ethiopian setting. East African Medical Journal 1996;73:598‐603. [PubMed] [Google Scholar]
Magnelli 1992 {published data only}
- Magnelli A, Tellez A, Jiminez R, Azuaga A, Calderon F. Uterine aspiration with karman cannula [Aspiracion uterina con canula de Karman]. Revista de Obstetricia y Ginecologia de Venezuela 1992;52(1):43‐8. [Google Scholar]
Magotti 1995 {published data only}
- Magotti RF, Munjinja PGM, Lema RSM, Ngwalle EKW. Cost effectiveness of managing abortions: manual vacuum aspiration (MVA) compared to evacuation by curettage in Tanzania. East African Medical Journal 1995;72:248‐51. [PubMed] [Google Scholar]
Mahomed 1994 {published data only}
- Mahomed K, Healy J, Tandon S. A comparison of manual vacuum aspiration (MVA) and sharp curettage in the management of incomplete abortion. International Journal of Gynecology & Obstetrics 1994;46:27‐32. [DOI] [PubMed] [Google Scholar]
Rashid 1970 {published data only}
- Rashid S, Smith P. Suction evacuation of uterus for incomplete abortion. Journal of Obstetrics and Gynaecology of the British Commonwealth 1970;77:1047‐8. [DOI] [PubMed] [Google Scholar]
Ricalde 1997 {published data only}
- Ricalde RL, Ramirez AT, Barsse GC, Castro P. Manual intrauterine aspiration in the treatment of incomplete abortion [Aspiracion manual endouterina para el tratamiento del aborto incompleto]. Ginecologia y Obstetricia de Mexico 1997;65:101‐6. [PubMed] [Google Scholar]
Suter 1970 {published data only}
- Suter PEN, Chatfield WR, Kotonya AO. The use of suction curettage in incomplete abortion. Journal of Obstetrics and Gynaecology of the British Commonwealth 1970;77:464‐6. [DOI] [PubMed] [Google Scholar]
Additional references
Blum 2007
- Blum J, Winikoff B, Gemzell‐Danielsson K, Ho PC, Schiavon R, Weeks A. Treatment of incomplete abortion and miscarriage with misoprostol. International Journal of Gynecology & Obstetrics 2007;99:S186‐S189. [DOI] [PubMed] [Google Scholar]
Clarke 2000
- Clarke M, Oxman AD, editors. Cochrane Reviewers' Handbook 4.1 [updated June 2000]. In: Review Manager (RevMan) [Computer program]. Version 4.1. Oxford, England: The Cochrane Collaboration, 2000.
Greenslade 1993
- Greenslade FC, Leonard AL, Benson J, Wunkler J, Henderson VL. Manual vacuum aspiration: a summary of clinical and programmatic experience worldwide. IPAS, 1993. [Google Scholar]
Higgins 2009
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Milingos 2009
- Milingos DS, Mathur M, Smith NC, Ashok PW. Manual vacuum aspiration: a safe alternative for the surgical management of early pregnancy loss. British Journal of Obstetrics and Gynaecology 2009;116:1268‐71. [DOI] [PubMed] [Google Scholar]
Neilson 2010
- Neilson JP, Gyte GML, Hickey M, Vazquez JC, Dou L. Medical treatments for incomplete miscarriage (less than 24 weeks). Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007223.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
WHO 1991
- World Health Organization. Essential elements of obstetric care at first referral level. Geneva: WHO, 1991. [Google Scholar]
WHO 1995
- World Health Organization. Complications of abortion: technical and managerial guidelines for prevention and treatment. Geneva: WHO, 1995. [Google Scholar]
Zhang 2005
- Zhang J, Gilles JM, Barnhart K, Creinin MD, Westhoff C, Frederick CM. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. New England Journal of Medicine 2005;353:761‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Forna 2001
- Forna F, Gülmezoglu AM. Surgical procedures to evacuate incomplete miscarriage. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD001993] [DOI] [PubMed] [Google Scholar]


