Abstract
Background
Treatment-resistant depression is among the most debilitating conditions in psychiatry. Recent studies have associated alterations in white matter microstructure measured with magnetic resonance imaging with poor antidepressant response. Therefore, the extent to which electroconvulsive therapy, the most effective therapeutic option for treatment-resistant depression, affects white matter microstructure warrants investigation.
Methods
A total 13 patients suffering from severe unipolar treatment-resistant depression underwent magnetic resonance imaging with a diffusion tensor imaging sequence before and after undergoing a series of right unilateral electroconvulsive therapy. Diffusivity metrics were compared voxel-wise using tract-based spatial statistics and repeated-measures ANOVA.
Results
A total 12 patients responded to electroconvulsive therapy and 9 were classified as remitters. An increase in axial diffusivity was observed in the posterior limb of the internal capsule of the right hemisphere (PFWE ≤ .05). The increase in this area was higher in the right compared with the left hemisphere (P < .05). No correlation of this effect with treatment response could be found.
Conclusions
The strong lateralization of effects to the hemisphere of electrical stimulation suggests an effect of electroconvulsive therapy on diffusivity metrics which is dependent of electrode placement. Investigation in controlled studies is necessary to reveal to what extent the effects of electroconvulsive therapy on white matter microstructure are related to clinical outcomes and electrode placement.
Keywords: electroconvulsive therapy, treatment-resistant depression, diffusion weighted imaging, magnetic resonance imaging
Significance Statement.
Alterations in white matter microstructure measured with magnetic resonance imaging (MRI) were associated with poor antidepressant response. In this trial, patients suffering from depression resistant to pharmacological treatment underwent a series of electroconvulsive therapy with right unilateral stimulation. Compared with baseline, an increase in axial diffusivity was observed in the right internal capsule using diffusion weighted magnetic resonance imaging after treatment. This effect was stronger in the right hemisphere, which suggests that the effect of electroconvulsive therapy on white matter microstructure depends on electrode placement. The relationship of changes in diffusivity metrics in motor pathways and clinical outcomes in treatment-resistant depression needs to be explored in controlled studies.
Introduction
Electroconvulsive therapy (ECT) constitutes one of the most effective treatment options for patients suffering from depression who do not respond to psychotropic medication or psychotherapy (Bauer et al., 2015). Alterations of white matter microstructure assessed using diffusion tensor imaging (DTI) have been reported in depression, and white matter structures have emerged as potential targets for deep brain stimulation in treatment-resistant cases (Bergfeld et al., 2017; Jiang et al., 2017). The most frequently investigated outcome measure, fractional anisotropy (FA), a marker for the strength of directionality of diffusion, is reduced in depressed patients, and more pronounced reductions were found to be related to an insufficient treatment response (De Diego-Adeliño et al., 2014; Grieve et al., 2016) and cognitive impairments (Liang et al., 2019). On the other hand, longitudinal studies following patients over the course of antidepressant treatment could show an increase in FA (Wang et al., 2013) in some studies to levels comparable with healthy control participants (Lyden et al., 2014). A handful of trials assessed the effects of ECT on white matter microstructure in depressive patients (Nobuhara et al., 2004; Lyden et al., 2014; Zeng et al., 2015; Nickl-Jockschat et al., 2016; Bai et al., 2019; Repple et al., 2019). The first investigation reported an increase in FA after ECT in frontal white matter of 8 patients diagnosed with late-life depression (Nobuhara et al., 2004). However, only 3 studies have conducted comprehensive analyses of changes in diffusivity metrics after ECT in the whole brain using tract-based spatial statistics (TBSS). One of these studies investigated FA and found no differences after ECT in 21 patients (Nickl-Jockschat et al., 2016). The other 2 studies included an extended analysis of directional diffusivity. In animal models, the corresponding metrics, axial (AD) and radial (RD) diffusivity, were shown to allow for the differentiation of alterations in axonal integrity (decreased AD) and myelin disruption (increased RD), respectively (Song et al., 2003). The first study reported increases in FA and decreases in RD in bilateral anterior cingulum, forceps minor, and the left superior longitudinal fasciculus after ECT in 20 patients. Moreover, a decrease in mean diffusivity (MD) was detected in the left anterior cingulum, anterior thalamic radiation, and forceps minor (Lyden et al., 2014). The second study found an increase in MD in the posterior limb of the internal capsule (PLIC) and the inferior longitudinal fasciculus among several other regions of the right hemisphere in 29 patients (Repple et al., 2019). Furthermore, the more recent study could not replicate associations between changes in diffusivity metrics and treatment response (Repple et al., 2019). As evident from these results, more information on the effect of ECT on white matter microstructure is desirable.
Aims of the Study
The aim of this study was to quantify changes in white matter microstructure after electroconvulsive therapy using whole-brain, tract-based spatial statistics analysis of diffusion weighted imaging data acquired in patients suffering from unipolar, treatment-resistant depression (TRD), a population that poses a serious challenge in clinical practice with a pressing need for improved therapeutic options. New insight on the lateralization of treatment effects was expected from the exclusively right-unilateral electrode placement employed.
METHODS
Participants and Study Design
In this interventional pre-post study, adult patients (18–60 years) suffering from a severe and treatment-resistant depressive episode (ICD-10: F32.2, F32.3, F33.2, F33.3) and a 17-item Hamilton Depression Rating Scale (HAM-D) score ≥23 were included. Recruitment was carried out at the inpatient clinic of the Department of Psychiatry and Psychotherapy at the Medical University of Vienna. Exclusion criteria were any contraindication to magnetic resonance imaging (MRI), metallic implants in the head, history of an ECT series, or diagnosis of bipolar affective disorder, schizoaffective disorder, or schizophrenia. Clinical diagnoses were supported by structured clinical interviews for DSM IV. Treatment resistance was defined by insufficient response to at least 2 adequate antidepressant treatment trials in the current episode. Adequate trials were defined as administration of approved antidepressants or augmentation therapies recommended in treatment guidelines for at least 2 weeks (Bauer et al., 2015). Ten days prior to MR scans, patients were maintained under stable medication, except benzodiazepines as rescue medication. Physical examination, thoracic X-ray, routine laboratory tests, electrocardiography, and urine screening tests for substance abuse and pregnancy were carried out to ascertain general health and obtain clearance for anesthesia. All patients provided written informed consent. All study procedures were carried out according to good clinical practice guidelines and the Declaration of Helsinki of 1975 as revised in 2008 and approved by the Ethics Committee of the Medical University of Vienna. The study was registered before start of recruitment at clinicaltrials.gov (NCT02379767). Changes in T1 weighted structural MRI outcomes in these patients were previously reported (Gryglewski et al., 2019).
Electroconvulsive Therapy
Each patient underwent an ECT series consisting of at least 8 sessions with right unilateral stimulations according to standard operating procedures established at the clinic. Post-ECT assessments were scheduled after 8 sessions. Additional ECT sessions were performed based on continuous clinical evaluation. Due to availability of MRI slots, 4 patients who continued ECT treatment received more than 8 sessions before undergoing post-ECT assessments. A maximum of 3 sessions per week were performed. The Thymatron System IV device (Somatics, LLC., Lake Bluff, IL) was used with right unilateral electrode placement and a brief pulse width (0.5 milliseconds). The stimulation dose was set to 50 mC (10%) during the first treatment and resulted in an adequate seizure in all patients, as evidenced by seizure duration, amplitude, ictal coherence, and post-ictal suppression. The second stimulation was performed at thrice the seizure threshold and further increased in case of inadequate seizure quality. Electrocardiography, electroencephalography, and electromyography of 1 forearm were performed during treatment. Methohexital and succinylcholine were applied for anesthesia and relaxation, respectively. Ongoing medication with putative effects on blood pressure or seizure quality was administered after ECT sessions on treatment days.
Diffusion Tensor Imaging
DTI scans were acquired on a 3T PRISMA MR Scanner (Siemens Medical, Erlangen, Germany) with a 64-channel head coil. Each patient received 2 scans in short succession (1–5 days) before ECT and 1 scan at least 1 day after the last ECT session (median: 1 day, maximum: 13 days). A single-shot, diffusion-weighted, echo planar imaging sequence was acquired (TE, 76 milliseconds; TR, 9400 milliseconds; flip angle, 90°; image resolution: 1.6 mm isotropic; b value, 800 s/mm2; 75 axial slices) in 64 diffusion-encoding directions and 5 nondiffusion b0 images. The Functional MRI of the Brain software library (FMRIB Software Library version 5.0.11; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) and TBSS (Smith et al., 2007) were used with default parameters for calculation of FA, MD, AD, and RD according to standard definitions, as done previously by our group (Kranz et al., 2014, 2017). AD was calculated as the eigenvalue of the primary eigenvector, RD as the average of the 2 smaller eigenvalues, and MD as the average of all 3 eigenvalues. FA was calculated as the square root of the ratio between the sum of squared differences between each eigenvalue and MD and twice the sum of each eigenvalue squared. Nonbrain tissue was removed with the brain extraction tool. Imaging data were corrected for eddy current artifacts, head movement between frames, and distortions using the eddy_cuda command (Andersson and Sotiropoulos, 2016) and outlier replacement (Andersson et al., 2016) as implemented in FSL. The tensor model was fitted using dtifit and the rotated b-vectors generated during the eddy current correction step. For registration of data to Montreal Neurological Institute (MNI) space, the mean FA image was used to create a skeleton of white matter tracts to which individual FA images were mapped. The same transformations were applied to individual AD, RD, and MD images. Raw data and the results of all processing steps were visually inspected to ensure high data quality.
Statistical Analysis
Analysis of longitudinal effects of ECT on the diffusivity metrics (FA, MD, AD, and RD) was performed voxel-wise using nonparametric permutation tests implemented in FSL. Paired t tests were calculated between pre-ECT and post-ECT data. To this end, data from the 2 MR scans acquired before ECT were averaged. To test for the asymmetry of the effect, clusters with significant changes in one of the diffusivity metrics were mirrored to the other hemisphere. As the white matter skeleton is not entirely symmetrical, each mirrored voxel was assigned to its nearest neighbor on the skeleton based on Euclidean distance. Subsequently, the change in the respective diffusivity metric between each significant cluster and its mirrored counterpart was compared by nonparametric permutation tests implemented in Matlab (500 permutations). Furthermore, the associations of changes in diffusivity metrics in significant clusters with changes in HAM-D scores, the interval between the last treatment and imaging procedures, and treatment parameters (number of sessions, average seizure duration, average dose) were assessed by calculation of Pearson correlation coefficients and inspection of scatterplots. The role of the effect of ECT on regional brain volumes reported in our previous work (Gryglewski et al., 2019) was assessed by correlating the changes in diffusivity metrics in significant clusters with changes in volumes in adjacent structures. Regional brain volumes were obtained using automated parcellation of T1 weighted data acquired during the same MRI sessions as diffusion weighted imaging using FreeSurfer 6.0 (Harvard Medical School, Boston, MA; http://surfer.nmr.mgh.harvard.edu/). Further information can be found in our previous publication (Gryglewski et al., 2019). For completeness, the correlation between diffusivity metrics at baseline and changes in HAM-D scores was assessed voxel-wise using nonparametric permutation tests. The statistical significance level was set at α ≤ 0.05 corrected for family wise error (FWE) using threshold-free cluster enhancement (Smith and Nichols, 2009). Significant clusters were matched with white matter tracts in the DTI-81 white matter atlas of the International Consortium for Human Brain Mapping (Mori et al., 2008). Trend-level effects with PFWE ≤ .1 were reported in the supplement.
RESULTS
Clinical Data
A total 16 patients suffering from TRD were enrolled in this trial. One patient had to be excluded from analysis due to acquisition of data on a different MRI scanner. Another patient dropped out because of a phobic reaction in the scanner. For 1 patient, no post-ECT MR data were available. Consequently, DTI data for longitudinal analysis of ECT effects on white matter microstructure was available from 13 patients (aged 48.9 ± 8.4 years, 10 female). Among the final 13 patients, 2 were diagnosed with psychotic symptoms and 12 were suffering from recurrent depressive disorder. Mean HAM-D was 25.4 ± 3.5 at inclusion and 6.8 ± 4.3 at completion. A total of 8.6 ± 1.0 (maximum 11) ECT sessions were applied before post-ECT scans, and a seizure duration of 42.5 ± 10.4 seconds was recorded on average. Nine patients were classified as remitters with a HAM-D ≤7, and only 1 patient did not respond (HAM-D reduction <50%). Each patient was receiving psychopharmacological treatment with a selective serotonin reuptake inhibitor (n = 10) or a serotonin-norepinephrine reuptake inhibitor (n = 5) at inclusion. Four patients had been prescribed noradrenergic and specific serotonergic antidepressants, and 1 patient was taking amitriptyline. Augmentation of antidepressant therapy had been performed with atypical antipsychotics in 10 patients and lamotrigine in 3 patients. A detailed tabulation of exact dosages, diagnoses, and ECT treatment parameters can be found in a previous paper on this patient sample published under an open-access license (Gryglewski et al., 2019).
Effects of ECT on Diffusivity Metrics
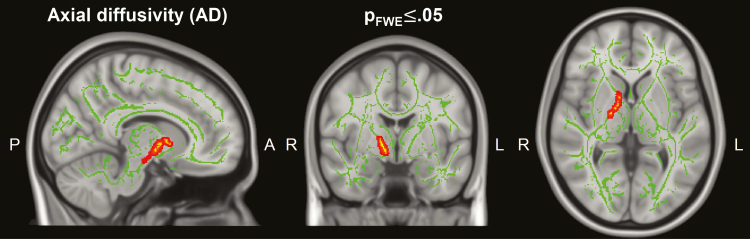
A significant increase in AD (PFWE ≤ .05, peak t value = 4.89, MNI-coordinates (xyz): 11|6|2) was observed in a cluster extending from the posterior limb of the right internal capsule to the posterior part of the anterior limb of the internal capsule (Figure 1). Changes in AD in this cluster were significantly higher than changes in AD in the corresponding areas in the left hemisphere (P < .05). No correlations of changes in AD in this cluster with changes in HAM-D scores, treatment parameters, or the interval between the last ECT session and MRI scans were found. No correlations between the changes in AD in this cluster with changes in the volumes of the adjacent gray matter structures (putamen, globus pallidus, thalamus, and caudate nucleus) were observed.
Figure 1.
Significant changes in axial diffusivity after electroconvulsive therapy (ECT). A cluster with increased axial diffusivity is overlaid on the mean fractional anisotropy (FA) skeleton and a T1 weighted standard brain (P family wise error [PFWE] < .05, MNI-coordinates (xyz): 11|-4|2).
Trend-level effects of ECT on AD, MD, and RD are reported in the supplement (PFWE ≤ .1; supplementary Figure 1). Briefly, widespread increases in AD and MD could be observed in white matter structures of both hemispheres, although marked asymmetry was present with lower changes in the left hemisphere. Trend-level increases in RD were observed exclusively in the right hemisphere. No significant or trend-level effects of ECT on FA could be found.
No correlations between diffusivity metrics at baseline and changes in HAM-D scores were found.
Discussion
We observed an increase in AD after ECT, which was predominantly located in the right PLIC. This white matter structure is composed to a large fraction of efferent fibers running in the pyramidal tract (Jang, 2009). Acute lesions identified using diffusion weighted imaging were associated with poor motor outcome and hemispatial neglect after stroke (Puig et al., 2011; Likitjaroen et al., 2012). The notion of alterations in motor pathways in major depression is further supported by a study which reported increased MD values in cortico-cortical connections of the supplementary- with the primary- and pre-motor cortices of depressed patients (Müller et al., 2012). A reduction in AD in the right PLIC was reported in a large sample of treatment-naïve patients suffering from a first episode of major depression (Jiang et al., 2018). Our results suggest that this alteration might be reversed with ECT. Alternatively, reduced AD in the right PLIC of depressed patients, and its increase with treatment might be a correlate of depressive symptoms, perhaps psychomotor retardation specifically. However, neither the observed change in AD nor diffusivity metrics at baseline were correlated with clinical response. While this might suggest that changes in diffusivity are not related to the antidepressant effects of ECT or that diffusion weighted imaging might not be useful in the prediction of the efficacy of ECT in individual patients, such conclusions can only be drawn with caution, as the lack of significant correlations could be a false negative result due to the limited sample size which only allows for the detection of strong correlations. Therefore, whether diffusivity metrics at baseline or changes after treatment are related to the clinical outcome of TRD patients needs to be explored in large controlled trials.
An indication that a part of the effect of ECT on DTI metrics may be attributed to direct electrical stimulation can be derived from the lateralization of significant changes to the side of electrode placement. Despite the lateralization of changes in gray matter structures to the right hemisphere reported previously in the same patients (Gryglewski et al., 2019), no associations of the change in AD in the right PLIC with changes in the volume of adjacent gray matter structures were observed in the current analysis. This suggests that the analysis of changes in white matter diffusivity, in addition to changes in regional brain volumes, provides complimentary information on the effects of ECT in individual patients. The lack of significant effects on AD in the literature may be due to the fact that previous studies investigating ECT effects on all diffusivity metrics were performed using MRI systems with lower peak gradient strength than the 80 mT/m of the 3T PRISMA MR Scanner or fewer diffusion directions (Lyden et al., 2014; Repple et al., 2019). Nevertheless, the significant increase in MD reported in the more recent study shows a high overlap with our results, especially concerning the right PLIC. Furthermore, the lack of significant effects on FA in the current data is in line with 2 of the 3 previous TBSS studies (Nickl-Jockschat et al., 2016; Repple et al., 2019). The fact that the observed increases in AD did not translate into an increase in FA could be attributed to the trend-level increase in RD in the right hemisphere. A possible interpretation of an increase in AD is strengthening of axonal structures, perhaps due to the engagement of tracts by electrical stimulation or seizure activity. However, the observed trend-level increases in MD and RD imply that the reduction in diffusion barriers after ECT might be omnidirectional. In temporal lobe epilepsy, increases in MD ipsilateral to the focus have been observed following seizures. These changes were negatively correlated with the interval between imaging and the last seizure and have been attributed to transient vasogenic edema (Concha et al., 2012). In the current study, no correlations between the increase in AD in the right PLIC and the interval between the last ECT session and imaging or any treatment or seizure parameters were found. Preclinical evidence for alterations in the permeability of the blood brain barrier due to experimentally induced seizures is available (Nitsch and Klatzo, 1983). While a role of the intra-ictal hypertensive surge for the disruption of the BBB has been suggested (Andrade and Bolwig, 2014), the lateralization of the changes in diffusivity in the present data implicates other mechanisms. Unfortunately, intra-ictal blood pressure was not systematically measured. The neurobiological process underlying the observed effect needs to be determined in postmortem tissue or animal models of ECT, and conclusions concerning the integrity of white matter cannot be drawn from the results of this study. Furthermore, a controlled study with different electrode placements needs to be performed before attributing the lateralized effects to unilateral stimulation.
Several limitations of the current study need to be mentioned. Firstly, the available sample size does not allow for the differentiation of small to medium effect sizes. The power for testing interesting between-patient effects is especially limited such that the chance for false negative results is high for correlation of imaging outcomes with clinical parameters. Based on the study design without a control group, no causal attribution of observed pre-post changes to the treatment can be stated (i.e., the effects may be due to natural tendencies or spontaneous remission). The included patients were taking psychopharmacological drugs, which may have affected the results. However, these were in steady-state before enrollment and many, if not most patients, who undergo ECT are taking medication.
In summary, we observed an increase in axial diffusivity in the PLIC in the right hemisphere of patients who underwent an ECT series with right unilateral stimulation for TRD. This effect was significantly stronger in the right hemisphere. The underlying neurobiological processes need to be scrutinized in postmortem tissue or animal models of ECT.
Supplementary Material
Acknowledgments
We thank D. Winkler, A. Komorowski, L. Silberbauer, G. M. Godbersen, M. Spies, A. Kautzky, M. Hienert, and C. Kraus for their medical assistance. We are grateful to J. Jungwirth, K. Einenkel, and E. Sittenberger for their administrative support. This scientific project was performed with the support of the Medical Imaging Cluster of the Medical University of Vienna. All authors read and approved the contents of the manuscript.
This work was supported by the Austrian Science Fund (FWF) (grant no. P27141 to R.L.).
Interest Statement
With relevance to this work there is no conflict of interest to declare. S. Kasper received grants/research support, consulting fees, and/or honoraria within the last 3 years from Angelini, AOP Orphan Pharmaceuticals AG, AstraZeneca, Celegne GmbH, Eli Lilly, Janssen-Cilag Pharma GmbH, KRKA-Pharma, Lundbeck A/S, Neuraxpharm, Pfizer, Pierre Fabre, Schwabe, and Servier. R. Lanzenberger received travel grants and/or conference speaker honoraria from Shire, AstraZeneca, Lundbeck A/S, Dr. Willmar Schwabe GmbH, Orphan Pharmaceuticals AG, Janssen-Cilag Pharma GmbH, and Roche Austria GmbH.
References
- Andersson JLR, Sotiropoulos SN (2016) An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125:1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JLR, Graham MS, Zsoldos E, Sotiropoulos SN (2016) Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage 141:556–572. [DOI] [PubMed] [Google Scholar]
- Andrade C, Bolwig TG (2014) Electroconvulsive therapy, hypertensive surge, blood-brain barrier breach, and amnesia: exploring the evidence for a connection. J ECT 30:160–164. [DOI] [PubMed] [Google Scholar]
- Bai T, Wei Q, Xie W, Wang A, Wang J, Ji GJ, Wang K, Tian Y (2019) Hippocampal-subregion functional alterations associated with antidepressant effects and cognitive impairments of electroconvulsive therapy. Psychol Med 49:1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Severus E, Köhler S, Whybrow PC, Angst J, Möller HJ; Wfsbp Task Force on Treatment Guidelines for Unipolar Depressive Disorders (2015) World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders. part 2: maintenance treatment of major depressive disorder-update 2015. World J Biol Psychiatry 16:76–95. [DOI] [PubMed] [Google Scholar]
- Bergfeld IO, Mantione M, Hoogendoorn MLC, Ruhé HG, Horst F, Notten P, van Laarhoven J, van den Munckhof P, Beute G, Schuurman PR, Denys D (2017) Impact of deep brain stimulation of the ventral anterior limb of the internal capsule on cognition in depression. Psychol Med 47:1647–1658. [DOI] [PubMed] [Google Scholar]
- Concha L, Kim H, Bernasconi A, Bernhardt BC, Bernasconi N (2012) Spatial patterns of water diffusion along white matter tracts in temporal lobe epilepsy. Neurology 79:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Diego-Adeliño J, Pires P, Gómez-Ansón B, Serra-Blasco M, Vives-Gilabert Y, Puigdemont D, Martín-Blanco A, Alvarez E, Pérez V, Portella MJ (2014) Microstructural white-matter abnormalities associated with treatment resistance, severity and duration of illness in major depression. Psychol Med 44:1171–1182. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Korgaonkar MS, Gordon E, Williams LM, Rush AJ (2016) Prediction of nonremission to antidepressant therapy using diffusion tensor imaging. J Clin Psychiatry 77:e436–e443. [DOI] [PubMed] [Google Scholar]
- Gryglewski G, Baldinger-Melich P, Seiger R, Godbersen GM, Michenthaler P, Klöbl M, Spurny B, Kautzky A, Vanicek T, Kasper S, Frey R, Lanzenberger R (2019) Structural changes in amygdala nuclei, hippocampal subfields and cortical thickness following electroconvulsive therapy in treatment-resistant depression: longitudinal analysis. Br J Psychiatry 214:159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SH. (2009) A review of corticospinal tract location at corona radiata and posterior limb of the internal capsule in human brain. Neurorehabilitation 24:279–283. [DOI] [PubMed] [Google Scholar]
- Jiang J, Zhao YJ, Hu XY, Du MY, Chen ZQ, Wu M, Li KM, Zhu HY, Kumar P, Gong QY (2017) Microstructural brain abnormalities in medication-free patients with major depressive disorder: a systematic review and meta-analysis of diffusion tensor imaging. J Psychiatry Neurosci 42:150–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Cheng Y, Jiang H, Xu J, Lu J, Shen Z, Lu Y, Liu F, Li L, Xu X (2018) Association between abnormal serum myelin-specific protein levels and white matter integrity in first-episode and drug-naïve patients with major depressive disorder. J Affect Disord 232:61–68. [DOI] [PubMed] [Google Scholar]
- Kranz GS, Hahn A, Kaufmann U, Küblböck M, Hummer A, Ganger S, Seiger R, Winkler D, Swaab DF, Windischberger C, Kasper S, Lanzenberger R (2014) White matter microstructure in transsexuals and controls investigated by diffusion tensor imaging. J Neurosci 34:15466–15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz GS, Seiger R, Kaufmann U, Hummer A, Hahn A, Ganger S, Tik M, Windischberger C, Kasper S, Lanzenberger R (2017) Effects of sex hormone treatment on white matter microstructure in individuals with gender dysphoria. Neuroimage 150:60–67. [DOI] [PubMed] [Google Scholar]
- Liang S, Wang Q, Kong X, Deng W, Yang X, Li X, Zhang Z, Zhang J, Zhang C, Li X min, Ma X, Shao J, Greenshaw AJ, Li T (2019) White matter abnormalities in major depression biotypes identified by diffusion tensor imaging. Neurosci Bull 35:867–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likitjaroen Y, Suwanwela NC, Mitchell AJ, Lerdlum S, Phanthumchinda K, Teipel SJ (2012) Isolated motor neglect following infarction of the posterior limb of the right internal capsule: a case study with diffusion tensor imaging-based tractography. J Neurol 259:100–105. [DOI] [PubMed] [Google Scholar]
- Lyden H, Espinoza RT, Pirnia T, Clark K, Joshi SH, Leaver AM, Woods RP, Narr KL (2014) Electroconvulsive therapy mediates neuroplasticity of white matter microstructure in major depression. Transl Psychiatry 4:e380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J (2008) Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40:570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller TJ, Wiest R, Walther S, Bracht T, Strik W, Höfle O, Dierks T, Horn H, Schnell S, Federspiel A (2012) Cortico-cortical white matter motor pathway microstructure is related to psychomotor retardation in major depressive disorder. PLoS One 7:e52238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickl-Jockschat T, Palomero Gallagher N, Kumar V, Hoffstaedter F, Brügmann E, Habel U, Eickhoff SB, Grözinger M (2016) Are morphological changes necessary to mediate the therapeutic effects of electroconvulsive therapy? Eur Arch Psychiatry Clin Neurosci 266:261–267. [DOI] [PubMed] [Google Scholar]
- Nitsch C, Klatzo I (1983) Regional patterns of blood-brain barrier breakdown during epileptiform seizures induced by various convulsive agents. J Neurol Sci 59:305–322. [DOI] [PubMed] [Google Scholar]
- Nobuhara K, Okugawa G, Minami T, Takase K, Yoshida T, Yagyu T, Tajika A, Sugimoto T, Tamagaki C, Ikeda K, Sawada S, Kinoshita T (2004) Effects of electroconvulsive therapy on frontal white matter in late-life depression: a diffusion tensor imaging study. Neuropsychobiology 50:48–53. [DOI] [PubMed] [Google Scholar]
- Puig J, Pedraza S, Blasco G, Daunis-I-Estadella J, Prados F, Remollo S, Prats-Galino A, Soria G, Boada I, Castellanos M, Serena J (2011) Acute damage to the posterior limb of the internal capsule on diffusion tensor tractography as an early imaging predictor of motor outcome after stroke. AJNR Am J Neuroradiol 32:857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repple J, et al. (2019) Influence of electroconvulsive therapy on white matter structure in a diffusion tensor imaging study. Psychol Med 23:1–8. [DOI] [PubMed] [Google Scholar]
- Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, Robson MD, Jones DK, Klein JC, Bartsch AJ, Behrens TE (2007) Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat Protoc 2:499–503. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE (2009) Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44:83–98. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH (2003) Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20:1714–1722. [DOI] [PubMed] [Google Scholar]
- Wang T, Huang X, Huang P, Li D, Lv F, Zhang Y, Zhou L, Yang D, Xie P (2013) Early-stage psychotherapy produces elevated frontal white matter integrity in adult major depressive disorder. PLoS One 8:e63081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J, Luo Q, Du L, Liao W, Li Y, Liu H, Liu D, Fu Y, Qiu H, Li X, Qiu T, Meng H (2015) Reorganization of anatomical connectome following electroconvulsive therapy in major depressive disorder. Neural Plast 2015:271674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.