Abstract
Background
Preclinical studies suggest that decreased levels of brain-derived neurotrophic factor in the amygdala play a role in anxiety and alcohol use disorder. The association between brain-derived neurotrophic factor levels and amygdala function in humans with alcohol use disorder is still unclear, although neuroimaging studies have also implicated the amygdala in alcohol use disorder and suggest that alcohol use disorder is associated with disrupted functional connectivity between the amygdala and prefrontal cortex during aversive states.
Methods
The current study investigated whether plasma brain-derived neurotrophic factor levels in individuals with and without alcohol use disorder (n = 57) were associated with individual differences in amygdala reactivity and amygdala-prefrontal cortex functional connectivity during 2 forms of aversive responding captured via functional magnetic resonance imaging: anxiety elicited by unpredictable threat of shock and fear elicited by predictable threat of shock. We also examined whether brain-derived neurotrophic factor and brain function were associated with binge drinking episodes and alcohol use disorder age of onset.
Results
During anxiety, but not fear, lower levels of plasma brain-derived neurotrophic factor were associated with less connectivity between the left amygdala and the medial prefrontal cortex and the inferior frontal gyrus. In addition, within individuals with alcohol use disorder (only), lower levels of brain-derived neurotrophic factor and amygdala-medial prefrontal cortex functional connectivity during anxiety were associated with more binge episodes within the past 60 days and a lower age of alcohol use disorder onset. There were no associations between brain-derived neurotrophic factor levels and focal amygdala task reactivity.
Conclusions
Together, the results indicate that plasma brain-derived neurotrophic factor levels are related to amygdala circuit functioning in humans, particularly during anxiety, and these individual differences may relate to drinking behaviors.
Keywords: plasma BDNF, amygdala, anxiety, alcohol use
Significance Statement.
Animal research indicates that decreased expression of brain-derived neurotrophic factor (BDNF) in the amygdala is involved in regulating anxiety and alcohol drinking behaviors. Within humans with alcohol use disorder (AUD), the relationship between BDNF levels and amygdala functioning has been unclear. The goal of the current study was to explore the links between peripheral BDNF levels, amygdala circuit function during anxiety, and drinking behaviors in humans. Results revealed that lower levels of plasma BDNF were associated with decreased functional connectivity between the amygdala and regulatory regions of the prefrontal cortex (PFC), captured via functional magnetic resonance imaging (fMRI). Within individuals with AUD, lower levels of BDNF and less amygdala-PFC connectivity during anxiety were associated with more recent binge episodes and lower age of AUD onset. The study provides evidence to suggest that plasma BDNF levels may serve as a biomarker in relation to amygdala circuit function in humans.
Alcohol use is the leading risk factor for global disease burden and has a substantial impact on physical and mental health (Griswold et al., 2018). In the United States, alcohol use disorder (AUD) is also highly prevalent with approximately 30% of individuals meeting criteria for the disorder at some point in their lifetime (Grant et al., 2015). Consequently, there is an urgent need to better understand the pathophysiology of AUD to aid in the development of novel prevention and intervention efforts. Translational, mechanistic research designed to bridge the gap between human and nonhuman findings are especially key to move towards greater understanding and improved treatment of AUD.
Preclinical models indicate that brain-derived neurotrophic factor (BDNF) is involved in the development of AUD. BDNF is a neurotrophin that mediates neuroplasticity and the formation, survival, and differentiation of neurons in the brain through interactions with the tropomyosin-related kinase B receptor and several signaling cascades (Thoenen, 2000; Moonat et al., 2010; Autry and Monteggia, 2012). In rodents, acute alcohol administration increases BDNF messenger RNA (mRNA) expression (McGough et al., 2004; Pandey et al., 2008), whereas prolonged excessive alcohol intake reduces BDNF, particularly in the amygdala, striatum, and medial prefrontal cortex (mPFC) (Pandey et al., 2008; Darcq et al., 2015; Logrip et al., 2015). Studies have also shown that haplodeficient BDNF and cyclic adenosine monophosphate (AMP) responsive-element binding protein mice showing about 50% lower BDNF levels have an increased preference for alcohol (Hensler et al., 2003; Pandey et al., 2004) and display exaggerated anxiety-like behaviors (Pandey et al., 2004). Inhibition of BDNF expression in the central nucleus of amygdala via administration of BDNF antisense oligodeoxynucleotides increases both alcohol consumption and anxiety, which can be rescued by subsequent BDNF co-infusion (Pandey et al., 2006). It has therefore been suggested that decreased BDNF levels in the amygdala are involved in regulating anxiety and alcohol consumption (Pandey, 2016; Berkel and Pandey, 2017).
With regard to clinical models, our recent study using human postmortem amygdala found that individuals with early onset-AUD (i.e., onset prior to age 21 years) but not late-onset AUD (i.e., onset after age 21 years) exhibited an upregulation of BDNF antisense and a decrease in amygdala BDNF expression relative to control participants (Bohnsack et al., 2019). No other studies to our knowledge have directly measured BDNF levels in the human amygdala, although fortunately, peripheral measures of BDNF have been shown to correlate with brain levels in rodents (Karege and Schwald, 2002); thus, it is purported that BDNF measured via serum or plasma reflects (to some extent) central BDNF activity. To date, numerous studies have tested differences in serum and/or plasma BDNF levels between individuals with and without AUD and have shown that AUD is associated with both increased BDNF (Chul et al., 2009; D’Sa et al., 2012) and decreased BDNF (Joe et al., 2007; Zanardini et al., 2011; Köhler et al., 2013) compared with controls. An even larger set of studies showed no association between BDNF and AUD diagnoses (Heberlein et al., 2010; Costa et al., 2011; Lhullier et al., 2015; Reynolds et al., 2015). These discrepant findings are likely related to the fact that AUD is a heterogeneous diagnosis that can include varying symptom combinations and patterns of drinking behavior. More robust and consistent associations with BDNF may therefore be found at the level of individual differences, particularly biologically based individual differences. As has been discussed extensively in the field of genetics (Meyer-Lindenberg and Weinberger, 2006), the identification of intermediate biological phenotypes that serve as a link between molecular variables and human behavior may be a way to reduce heterogeneity and improve our understanding of the role of BDNF in AUD.
BDNF promotes synaptic plasticity and connectivity in the brain (Koshimizu et al., 2009). Therefore, neural reactivity and functional connectivity measured via functional magnetic resonance imaging (fMRI) may be an important individual difference factor in humans that acts transiently and is associated with both BDNF levels and drinking behaviors. Indeed, a functional polymorphism in the BDNF gene, specifically, the valine-to-methionine substitution at codon 66 (Val66Met), is associated with alterations in functional connectivity within several large-scale neural networks during resting state (Thomason et al., 2009; Wang et al., 2014) and emotion processing (Mukherjee et al., 2011). More broadly, Val66Met is associated with reductions in gray matter in memory and emotion regions (Ho et al., 2006), exaggerated limbic activation to negative stimuli (Mukherjee et al., 2011), and deficits in cognitive function (Egan et al., 2003; Dempster et al., 2005). There has been only 1 prior study to our knowledge that has investigated the impact of peripheral BDNF, measured in blood plasma, on functional connectivity associating increased levels of plasma BDNF with increased resting-state connectivity between motor areas in healthy older adults (Mueller et al., 2016). Together these data highlight BDNF’s key role in neural function; however, no study to date has specifically examined the association between peripheral measures of BDNF and amygdalar functioning in humans despite the plethora of animal studies demonstrating the impact of BDNF levels in the amygdala on alcohol consumption and anxiety-like behaviors (Kyzar and Pandey, 2015).
The broader human neuroimaging literature clearly indicates that anxiety and alcohol abuse are associated with abnormalities in amygdala-based neural networks. Alcohol acutely dampens amygdala reactivity (Gilman et al., 2008; Sripada et al., 2011) and uncouples functional connections between the amygdala and regulatory prefrontal cortex (PFC) regions during threat perception (Gorka et al., 2013). When sober, individuals with AUD, and individuals at risk for AUD, show decreased amygdala reactivity to threatening stimuli (Glahn et al., 2007; Marinkovi et al., 2009). Decreased amygdala-PFC connectivity at rest has also been shown to longitudinally predict increases in alcohol use in adolescents (Peters et al., 2017). Taken together, the amygdala is implicated in alcohol dependence in both human and animal research, and the amygdala-PFC circuit, in particular, is a well-positioned target for the effects of varying BDNF levels.
The current study examined whether human plasma BDNF was related to individual differences in amygdala reactivity and amygdala-PFC functional connectivity during a task specifically designed to elicit anxiety and whether both BDNF levels and amygdala-circuit function were associated with real-world drinking behaviors. The sample included individuals with and without AUD in order to capture a full distribution of drinking behaviors, BDNF levels, and individual differences in amygdala reactivity and functional connectivity. Individuals completed a neuroimaging paradigm designed to probe both reactivity during anticipatory anxiety and fear using unpredictable (U-) and predictable (P-) threat of electric shock, respectively. Human and animal studies show that anticipatory anxiety and fear are separable aversive states that have overlapping, yet distinct, neural correlates (Davis et al., 2010; Alvarez et al., 2011). Notably, recent research found that the BDNF Val66Met polymorphism is associated with exaggerated anticipatory anxiety to an upcoming stressor (Colzato et al., 2011), and exaggerated anticipatory anxiety, but not fear reactivity, distinguished individuals with AUD from controls (Gorka et al., 2016a, 2016b). Here, we investigated changes in plasma BDNF levels and amygdala reactivity and amygdala-PFC functional connectivity, particularly during anticipatory anxiety (i.e., U-threat) and their association with greater real-world problem-drinking behaviors.
METHODS
Participants
Volunteers were recruited via advertisements posted in the Chicago community, local psychiatric clinics, and nearby college campuses. To be included in the study, individuals were required to be between 21 and 30 years old and able to provide written informed consent. Individuals also had to (1) have no personal or family history of AUD (i.e., controls) or (2) meet criteria for AUD within the past 2 years. Exclusion criteria included any serious medical condition, psychotropic medication use, deafness, contraindication for neuroimaging, pregnancy, lifetime moderate or severe substance use disorder (other than alcohol and nicotine), daily cigarette smoking, and psychosis. The protocol was approved by the university institutional review board and participants provided written informed consent. Individuals were instructed to abstain from drugs and alcohol 24 hours prior to the laboratory assessments, which was verified via breath alcohol and urine screens. Participants were monetarily compensated for their time. A total of 37 individuals with AUD and 24 controls enrolled in the study and completed all laboratory procedures (total n = 61). A total of 4 individuals had excessive motion during the fMRI scan (i.e., <2.5-mm displacement in any direction) and were excluded from analyses (3 AUD subjects and 1 control) for a final sample of 57 individuals. Please see Table 1 for participants’ demographics and clinical characteristics.
Table 1.
Demographics and clinical characteristics
| AUD (n = 33) | Controls (n = 24) | Combined (n = 57) | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 23.6 (2.8) | 24.0 (2.6) | 23.8 (2.7) |
| Sex (female) | 39.4% | 54.2% | 45.6% |
| Ethnicity (Hispanic) | 30.3% | 8.3% | 21.1% |
| Race | |||
| White | 66.7% | 45.8% | 56.1% |
| Black | 3.0% | 16.7% | 8.8% |
| Asian | 6.1% | 29.2% | 15.8% |
| Native Hawaiian | 3.0% | 0.0% | 1.8% |
| American Indian or Alaskan Native | 3.0% | 0.0% | 1.8% |
| Other | 18.2% | 8.3% | 15.7% |
| Clinical variables | |||
| Drinks per week | 10.0 (7.2) | 4.0 (5.8) | 7.5 (7.2) |
| Number of binge episodes | 8.3 (6.1) | 2.4 (4.6) | 5.2 (6.0) |
| AUD age of onset | 19.5 (2.8) | – | 19.5 (2.8) |
| Daily cigarette smoker | 0.0% | 0.0% | 0.0% |
| No. times used cannabis | 0.2 (0.6) | 0.2 (0.6) | 0.2 (0.6) |
| No. times used *other illicit drugs | 0.2 (1.3) | 0.04 (0.2) | 0.2 (0.9) |
| BDNF variable | |||
| Plasma BDNF (pg/μL) | 47.0 (11.2) | 40.4 (8.8) | 44.2 (10.7) |
| Diagnostic variables | |||
| Current major depressive disorder | 9.1% | 8.3% | 8.8% |
| Lifetime major depressive disorder | 24.2% | 16.7% | 21.1% |
| Current anxiety disorder | 12.1% | 8.3% | 10.5% |
| Lifetime anxiety disorder | 27.3% | 16.7% | 22.8% |
| Current posttraumatic stress disorder | 0.0% | 4.2% | 1.8% |
| Lifetime posttraumatic stress disorder | 0.0% | 4.2% | 1.8% |
All values are means, standard deviations, unless otherwise notes.
Abbreviation: BDNF, brain derived neurotrophic factor.
Clinical Assessments and Procedure
Participants completed 2 laboratory visits separated by 1–7 days. The first visit included a clinical interview, a battery of self-report questionnaires, a blood draw, and a set of psychophysiological tasks. The second visit involved fMRI data collection. At the initial session, lifetime psychopathology was assessed via the Structured Clinical Interview for DSM-5 Disorders (American Psychiatric Association, 2015), in-person, by trained assessors and supervised by a clinical psychologist. Age of onset of AUD was recorded during the Structured Clinical Interview for DSM-5 Disorders interview and examined in the current study given that lower age of onset is associated with worse AUD prognosis (Babor et al., 1992; Falk et al., 2008), and BDNF expression in the amygdala is decreased in individuals with early, but not late, AUD onset (Bohnsack et al., 2019). Participants also completed a detailed assessment of their recent alcohol use employing the Timeline Follow-Back technique (Sobell and Sobell, 1992). Participants were presented with a calendar of the past 60 days, marked with holidays and special events, and were asked to indicate on what days they drank and how many drinks they consumed on each occasion. Using the Timeline Follow-Back technique, we calculated average number of drinks consumed per week and total number of binge episodes over the past 60 days. Binge episodes were defined as consuming ≥5 standard drinks for men and ≥4 drinks for women in 1 sitting.
Blood Collection and BDNF Protein Measurement
Each participant provided 40 mL of whole blood, which was collected via a trained phlebotomist. All blood draws occurred between the hours of 10 am and 1 pm. Blood samples were collected in K2EDTA-coated tubes and centrifuged 700 rpm for 10 minutes at 4°C. Plasma supernatant was subsequently transferred to a clean tube and immediately frozen at −80°C until use. In preparation for protein measurement, plasma samples were thawed on ice, clarified via a 20-minute spin at 10 000 g at 4°C for complete platelet removal, and supernatant transferred to new tube. BDNF levels were ascertained using a quantitative in vitro enzyme-linked immunosorbent assay kit (BioVision, Milpitas, CA). Briefly, 100 µL of BDNF standards (0.066–16 pg/uL recombinant human BDNF) were applied in duplicate to 96-well plates precoated with an antibody specific for human BDNF. The samples were diluted (1:10) and 100 µL was added to the plate in duplicate. The plate was sealed and incubated overnight at 4°C. The next morning, the plate was rinsed with wash-buffer 4 times and incubated with biotinylated primary antiserum at room temperature with gentle shaking for 1 hour. Wells were washed again, incubated with a streptavidin-HRP complex for 45 minutes and developed in a solution of tetramethylbenzidine for 20 minutes in the dark prior to stopping the reaction with a 2N sulfuric acid stop solution. Samples and standards were run in duplicate and replicated across a minimum of 2 enzyme-linked immunosorbent assays. The optical density of each sample and standard was measured at 450 nm using the Spectra MR microplate reader (Dynex Technologies, Chantilly, VA), and the amount of BDNF in each sample was calculated against the BDNF standard curve and expressed as pg/uL of protein.
fMRI Threat Task
Prior to the task, electrodes were placed on participants’ left foot and a shock work-up was completed to identify the level of shock intensity each participant described as “highly annoying but not painful” (between 1 and 5 mA). The task was designed to be analogous to the widely used NPU-threat task described by Schmitz and Grillon (2012). There were 3 within-participant conditions: N, P shock, and U shock. During each condition, participants viewed a numeric countdown that ranged between 3 and 8 seconds (M = 5 seconds). Text at the bottom of the computer monitor informed participants of the current condition. During N, no shocks were delivered and the text read “no shock.” During P, participants received a shock only when the countdown reached “1” and the text read “shock at 1”. During U, participants received a shock at random, regardless of the number on the screen and the text read “shock at anytime.” Following each countdown, individuals saw a fixation cross for 5 to 7 seconds (M = 6 seconds). N, P, and U countdowns were presented in blocks of 6, and each condition/block was administered in a randomized order (counterbalanced) 6 times over the course of 2 runs. Notably, not all countdowns ran full-length or terminated with “1,” allowing us to match the 3 conditions on total number of data points (i.e., TRs/repetition times). Participants received 10 electric shocks during P and 10 electric shocks during U during each run. The rate of “shock at 1” during the P condition was 60%, consistent with the NPU version used by Grillon and colleagues (Schmitz and Grillon, 2012).
fMRI Data Collection and Processing
fMRI was performed on a 3.0 Tesla GE MR 750 scanner (General Electric Healthcare, Waukesha, WI) using an 8-channel phased-array radio frequency head coil. A standard T2-sensitive gradient-echo echoplanar imaging sequence was used (2 seconds TR; 22.2 milliseconds TE; 90° flip; 64 × 64 matrix; 22 cm FOV; 44 axial slices; 3.44 × 3.44 × 3.0 mm voxels; 336 volumes).
All fMRI data met criteria for high quality and scan stability with minimum motion correction (i.e., <2 mm displacement in any direction). Preprocessing of fMRI data was conducted using Statistical Parametric Mapping software (SPM12, Wellcome Department of Imaging Neuro-Science, London, UK), and, accordingly, we followed the standard preprocessing routines suggested by SPM. The first 4 volumes were discarded to allow for T1 equilibration effects. Images were slice time corrected with the reference slice based on the middle of each TR, realigned with the first volume to correct for head motion, co-registered to the participants’ T1-weighted image in Montreal Neurological Institute (MNI) space, re-sampled to 2-mm3 voxels, and smoothed using a 8-mm isotropic Gaussian kernel. The general linear model was applied to the time series, convolved with the canonical hemodynamic response function and with a 128-second high-pass filter. Condition effects for U, P, and N anticipation were separately estimated at each voxel for each participant. For each condition, the countdowns prior to the shock, or prior to trial termination in instances where there was no shock, were modeled. Of note, we conducted an additional set of analyses where the U- and P-shocks were included as regressors in the first level model. The results of the paper were consistent whether shocks were included or excluded from the model. Movement parameters obtained during realignment were included in the model as regressors of no interest to account for motion-related effects on BOLD. Individual contrast maps for U-threat > no-threat (i.e., anticipatory anxiety) and P-threat > no-threat (i.e., fear) were created for each participant.
For functional connectivity, we used a seed-based, generalized form of context-dependent psychophysiological interaction (PPI) analyses (http://brainmap.wisc.edu/PPI, McLaren et al, 2012), with left and right anatomical amygdala masks (created using AAL atlas) as the seeds of interest. The de-convolved time series from the amygdala masks were extracted for each participant to create the physiological variables. The condition onset times for U-threat, P-threat, and no-threat were separately convolved with the canonical hemodynamic response function (HRF) for each condition, creating the psychological regressors. The interaction terms (PPIs) were computed by multiplying the time series from the psychological regressors with the physiological variable. Activity within the amygdala was then regressed on a voxel-wise basis against the interaction, with the physiological and psychological variables serving as regressors of interest. Individual U-threat > no-threat and P-threat > no-threat PPI images were created for every participant.
Data Analysis Plan
To examine whether BDNF was associated with amygdala reactivity, we used a focused, region-of-interest analysis approach. We entered the U-threat > no-threat and P-threat > no-threat images into separate 1-sample t tests. Then we extracted mean task activation parameter estimates (β-weights; arbitrary unit) from separate left and right whole anatomical amygdala masks (created using AAL atlas) from each model (U-threat and P-threat). Using Pearson’s correlations, we assessed whether plasma BDNF levels were associated with focal left and right amygdala reactivity during U-threat and P-threat.
To examine the impact of BDNF on amygdala-PFC functional connectivity, the U-threat > no-threat and P-threat > no-threat images were entered into 2 separate 2nd-level 1-sample t tests with individual BDNF values as a regressor. To determine our fMRI significance threshold, we applied an anatomically derived (AAL atlas) partial brain mask of the entire PFC to our data (search volume = 451 840 mm3, encompassing 56 480 voxels). The search for significant results was restricted to the PFC given our strong a priori hypotheses regarding frontolimibic connectivity. Cluster-based significance thresholding was used to adjust for multiple comparisons within the search volume using Monte Carlo simulations (10 000 iterations) performed with the most up-to-date version of 3dClustSim, an adaptation of AlphaSim (https://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html) in AFNI (19.2.06). The mixed autocorrelation function was utilized to give an accurate estimation of non-Gaussian noise structure (Cox et al., 2017). A family wise error correction at α < 0.05 was achieved for voxel threshold of P < .005 with minimum cluster size of 233 contiguous voxels. Connectivity parameter estimates from 8-mm-radius spheres surrounding peak activations within the PFC associated with BDNF levels were then extracted for further analysis.
We next examined whether BDNF levels and the significant neural findings (reactivity and/or connectivity) identified above correlated with real-world drinking behaviors, particularly average number of drinks per week and total number of binge episodes in the past 60 days. We also examined associations with age of AUD onset within individuals with an AUD diagnosis. We conducted a series of Pearson’s correlations between plasma BDNF and peak significant parameter estimates and drinking behaviors. A total of 9 correlations were run. A P value correction for multiple comparisons was not applied in order to comprehensively test relationships with several drinking variables (i.e., drinks per week, binge episodes, and AUD age of onset).
Although the aims of the current study were dimensional, participants were recruited into 2 groups: individuals with AUD and controls. Therefore, post-hoc we tested whether group (AUD vs control) moderated any of the above associations. Similarly, the study included males and females and we therefore examined the moderating impact of biological sex. For each set of analyses (1: BDNF and amygdala reactivity; 2: BDNF and amygdala-PFC connectivity; and 3: correlations with drinking behaviors), we tested the impact of group and sex using hierarchical linear regression where group, sex, and the independent variable were entered in Step 1, and the 2-way interactions between group and the independent variable, and sex and the independent variable, were entered in Step 2. Significant interactions were followed-up using standard simple slopes approach (Aiken et al., 1991).
Lastly, we explored whether significant results were affected by 2 key variables/covariates: time (in days) between BDNF data collection and the fMRI session (for BDNF models only) and lifetime diagnosis of any major internalizing disorder (yes/no) defined as major depressive disorder, social anxiety disorder, panic disorder, specific phobia, agoraphobia, generalized anxiety disorder, and/or post-traumatic stress disorder. Correlations were run as partial correlations controlling for time and diagnosis. For linear regression analyses, covariates were entered in Step 1 of the model.
RESULTS
BDNF and Amygdala Reactivity
Whole-brain task activation patterns for individuals with and without AUD are reported in Gorka et al. (2019). In the current study, there were no significant associations between BDNF levels and amygdala reactivity to U-threat or P-threat (rs: −.07 to .07, P > .61). Group (AUD vs no AUD) and sex did not moderate any of the null associations between BDNF levels and amygdala reactivity (P > .26).
BDNF and Amygdala Functional Connectivity
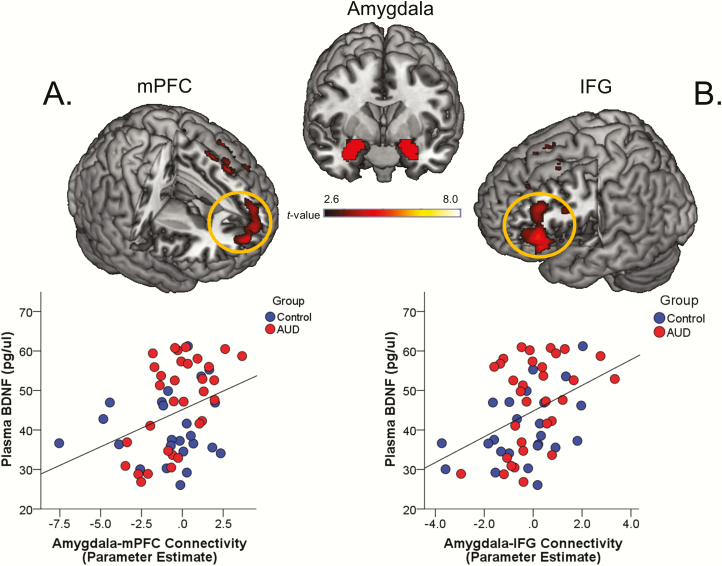
During U-threat, lower levels of plasma BDNF were associated with less functional connectivity between the left amygdala and the medial PFC (mPFC) (MNI peak [10, 62, 10], Z = 3.14, k = 2480 mm3, P = .001; Figure 1A) and the left amygdala and the left inferior frontal gyrus (IFG) (MNI peak [−42, 32, 0], Z = 3.47, k = 5104 mm3, P = .0001; Figure 1B). There were no other significant associations during U-threat or any significant BDNF and functional connectivity associations during P-threat. Group and sex did not moderate the association between BDNF and amygdala-mPFC connectivity (group: β = 0.23, t = 0.81, P = .42; sex: β = 0.06, t = 0.09, P = .93) or amygdala-IFG connectivity (group: β = −0.12, t = −0.44, P = .66; sex: β = 0.15, t = 0.23, P = .82) during U-threat.
Figure 1.
Top of the figure displays the left and right anatomical amygdala seed-regions-of-interest (SOIs). Top of panel (A) shows a statistical t-map on a canonical brain illustrating the significant correlation between plasma brain-derived neurotrophic factor (BDNF) levels and left amygdala and medial prefrontal cortex (mPFC) connectivity during unpredictable threat (U-threat). Bottom of panel (A) displays a scatter plot of the correlation (P < .05) between plasma BDNF levels and extracted parameter estimates of amygdala-mPFC functional connectivity. Top of panel (B) shows a statistical t-map on a canonical brain illustrating the significant correlation between plasma BDNF levels and left amygdala and left inferior frontal gyrus (IFG) connectivity during unpredictable threat (U-threat). Bottom of panel (B) displays a scatter plot of the correlation (P < .05) between plasma BDNF levels and extracted parameter estimates of amygdala-IFG functional connectivity. (n = 24 controls; 33 alcohol use disorder [AUD] participants).
Associations With Drinking Behaviors
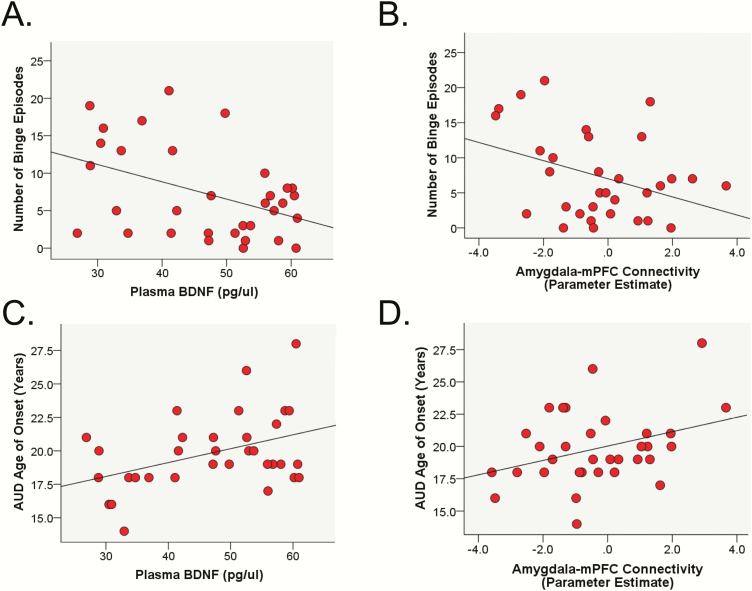
As a group, individuals with AUD had higher levels of plasma BDNF compared with controls (F[1, 56] = 5.60, P = .02). Across the entire sample, there were no associations between plasma BDNF levels and average number of drinks per week (r < 0.01, P = .98) or number binge episodes in the past 60 days (r = −0.08, P = .57). However, group (AUD vs controls) moderated the association between plasma BDNF levels and number of binge episodes (β = 0.68, t = 2.93, P = .01) such that within individuals with AUD, lower BDNF was associated with more binge episodes (β = −.51, t = −3.09, P = .02; Figure 2A), but within individuals without AUD there was no association between BDNF levels and binge episodes (β = .44, t = 1.33, P = .18). Group did not moderate the association between BDNF and drinks per week (β = −1.07, t = −1.66, P = .10). Sex did not moderate the association between BDNF and drinks per week (β = 0.12, t = 0.20, P = .84) or number of binge episodes (β = −0.07, t = −0.12, P = .91). Results within the AUD group only also revealed that lower BDNF was associated with lower age of AUD onset (r = .40, P = .02; Figure 2C).
Figure 2.
Scatter plots of the significant correlations. Top panel shows the association between number of binges in the past 60 days and plasma brain-derived neurotrophic factor (BDNF) levels (P = .01) (A) and amygdala-medial prefrontal cortex (mPFC) functional connectivity during unpredictable threat (U-threat) (P = .02) (B). Bottom panel shows the association between alcohol use disorder (AUD) age of onset and plasma BDNF levels (P = .02) (C) and amygdala-mPFC functional connectivity during U-threat (P = .03) (D). n = 33 AUD subjects.
Given the null amygdala reactivity findings, we only explored links between drinking behaviors and significant peak functional connectivity parameter estimates associated with BDNF levels (i.e., mPFC and IFG). Results revealed no associations across the entire sample between amygdala-mPFC connectivity and average number of drinks per week (r < 0.01, P = .98) or number of binge episodes (r = −0.05, P = .73). There were also no associations between amygdala-IFG connectivity and drinks per week (r = −0.02, P = .87) or number of binge episodes (r = −0.09, P = .50). However, group moderated the association between amygdala-mPFC connectivity and number of binge episodes (β = −0.40, t = −2.52, P = .02). Within individuals with AUD, less amygdala-mPFC connectivity was associated with more binges (β = −0.48, t = −2.55, P = .01; Figure 2B) and within controls, there was no association between amygdala-mPFC connectivity and binge episodes (β = 0.15, t = 1.25, P = .22). Group did not moderate the association between amygdala-mPFC connectivity and drinks per week or the association between amygdala-IFG connectivity and either drinking variable (P > .15). Sex also did not moderate any of the tested associations (P > .38). Results within the AUD group indicated that lower amygdala-mPFC connectivity was associated with younger age of AUD onset (r = .39, P = .03; Figure 2D).
Impact of Potential Covariates
All significant analyses were re-run controlling for time (in days) between BDNF data collection and the fMRI session and lifetime diagnosis of any major internalizing disorder (yes/no). The results indicated that neither covariate had a significant impact on the pattern of results, and the findings were identical in each covariate model.
Discussion
The primary aim of the study was to test whether plasma BDNF levels were associated with individual differences in amygdala reactivity and amygdala-PFC functional connectivity during 2 forms of aversive responding: anticipatory anxiety elicited by U-threat and fear elicited by P-threat. We also examined whether BDNF levels and our neural findings were associated with real-world drinking behaviors. Results obtained revealed that during U-threat, but not P-threat, lower levels of BDNF were associated with less functional connectivity between the left amygdala and both the mPFC and IFG. In addition, within individuals with AUD (only), lower levels of BDNF and amygdala-mPFC functional connectivity during U-threat were associated with more binge episodes within the past 60 days and a younger age of AUD onset. There were no associations with drinking behaviors in individuals without AUD. In addition, there were no associations between BDNF levels and amygdala task-based reactivity. Together, the results indicate that plasma BDNF levels are related to amygdala-PFC circuit functioning in humans, particularly during anticipatory anxiety, and these individual differences may contribute to drinking behaviors.
Across all participants, lower levels of BDNF were associated with decreased amygdala-mPFC and amygdala-IFG functional connectivity during anticipatory anxiety. These findings were observed in the absence of any direct associations between BDNF levels and amygdala reactivity, which is noteworthy in light of studies highlighting that measures of neural networks are more predictive than single regions of interest (e.g., Bolt et al., 2018). The amygdala networks identified in the current study include the mPFC and IFG, which are 2 key regions involved in emotion regulation and have been shown to exhibit regulatory influences on the amygdala during the inhibition of anxiety and negative affect (Banks et al., 2007; Ochsner et al., 2012). More specifically, prior studies have shown that healthy individuals exhibit greater functional coupling between the amygdala and the mPFC and IFG during threat (Prater et al., 2013) and that greater connectivity is associated with greater affect regulation efficiency (Banks et al., 2007). Meanwhile, individuals characterized by high levels of chronic anxiety (e.g., posttraumatic stress disorder, generalized anxiety disorder) evidence decreased functional connectivity between the amygdala and the mPFC/IFG (Shin et al., 2005; Dodhia et al., 2014). Together, these studies suggest that decreased amygdala-PFC connectivity reflects deficiencies in downregulating negative affect. Individuals with lower levels of BDNF may therefore have difficulty modulating anticipatory anxiety, particularly in the context of a U-threat. This emotion regulation deficit could be 1 potential mechanism that contributes to the link between BDNF levels and multiple forms of psychopathology (Andero et al. 2014; Pandey, 2016).
The mPFC also plays an essential role in assessing risk and determining the subjective value of uncertain outcomes (Xue et al., 2009) and has repeatedly been shown to be engaged during times of uncertainty (Hsu et al., 2005; Levy et al., 2010). Cross-talk between the amygdala and mPFC is necessary for effectively coding subjective value (Lin et al., 2015), and decreased functional connectivity between these regions could reflect abnormal salience processing of unpredictable threat cues. Thus, a complementary or potentially alternative hypothesis is that individuals with lower levels of BDNF exhibit impairments in appraising the relative danger vs safety of the U-threat conditions and determining threat salience.
This is the first study, to our knowledge, to examine associations between plasma BDNF levels and amygdala circuit function during fear and anxiety states in humans, although prior studies have examined whether differences in BDNF polymorphisms are associated with patterns of functional connectivity, especially during resting state. At rest, Val66Met carriers (who may be deficient in BDNF) show abnormalities across several large-scale circuits, including the default-mode network, executive control network, and salience network (Thomason et al., 2009; Jang et al., 2012). A few studies have also reported amygdala abnormalities including increased connectivity between the amygdala and insula (Wheeler et al., 2018). It is therefore possible that individual differences in BDNF expression have more widespread impact on brain function, and the nature of this impact depends on environmental and emotional context (e.g., resting-state vs U-threat).
We originally hypothesized that lower levels of BDNF and decreased amygdala-PFC functional connectivity would be associated with real-world problem drinking behaviors across all participants. However, within the AUD group, lower levels of BDNF and decreased amygdala-mPFC functional connectivity during U-threat were associated with more binge episodes in the past 60 days and a lower age of AUD onset, whereas there were no associations with drinking behaviors in controls. One factor that may have contributed to the moderating effect of group is the fact that the controls reported very low levels of alcohol consumption, resulting in a restricted range of drinking behaviors, whereas individuals with AUD reported substantial variability in their patterns of alcohol use. Although the purpose of including both controls and individuals with AUD in the sample was to capture a normal variable distribution to increase statistical power for dimensional analyses, in terms of the drinking behaviors, almost all of the variability was found in those with AUD. With that said, it is also worth highlighting that Kim et al. (2013) similarly found that plasma BDNF levels were negatively correlated with drinks consumed per day but only in individuals who exceeded 3 or more drinks per day. Thus, it is possible that specific associations between BDNF, neural function, and alcohol abuse are only observed in heavy drinkers.
Although BDNF and drinking variables were negatively associated with each other, we found that on average, individuals with AUD had higher levels of plasma BDNF compared with controls. This is consistent with a handful of other human studies (Chul et al., 2009; D’Sa et al., 2012) and highlights the heterogeneous nature of the AUD diagnosis. The current set of findings, as a whole, reinforces the utility of examining individual differences at the biological level to improve understanding of molecular, neural, and behavioral relationships.
The links between the biological and alcohol-related variables in individuals with AUD are therefore important. Binge drinking and earlier age of AUD onset are 2 indicators of AUD severity as both variables are associated with poorer AUD prognosis and a host of negative biopsychosocial outcomes (Carlson et al., 2010; Elsayed et al., 2018). Lower levels of plasma BDNF and decreased amygdala-PFC functional connectivity during U-threat may be 2 related individual difference factors that contribute to these specific aspects of AUD severity. Interestingly, separate studies have previously found associations between earlier age of AUD onset and lower levels of BDNF in amygdala (Bohnsack et al., 2019) and decreased amygdala-PFC functional connectivity (Peters et al., 2015). Increased alcohol consumption has also previously been associated with decreased amygdala-PFC functional connectivity (Hu et al., 2018) and lower peripheral BDNF levels (Kim et al., 2013), though the majority of human studies report no associations between BDNF levels and drinking behaviors (e.g., Joe et al., 2007; García-Marchena et al. 2017). The current study demonstrates for the first time, to our knowledge, associations across these variables within the same sample and suggests that within individuals with AUD, BDNF, and amygdala-mPFC functional connectivity during U-threat relate to more risky and chronic patterns of alcohol abuse.
Given the current pattern of results, targeting amygdala-mPFC functional connectivity, particularly during anxiety, via BDNF expression (or other strategies) may improve drinking outcomes. As briefly noted, decreased BDNF levels in the mPFC and amygdala have been implicated in excessive alcohol use (Logrip et al., 2015; Pandey et al., 2008; Moonat et al., 2011). In animals, infusion of BDNF or increasing BDNF via inhibition of miRNAs in the mPFC decreases alcohol consumption (Cui et al., 2015) and cocaine self-administration (Berglind et al., 2007), and BDNF infusion in the amygdala attenuated anxiety-like behaviors during ethanol withdrawal in rats (Pandey et al., 2008). The amygdala-mPFC circuit is therefore a potential key treatment target for novel AUD interventions.
The relationship between plasma BDNF levels and amygdala-PFC connectivity during U-threat, but not P-threat, is noteworthy. Anxiety and fear are separable aversive states (Davis et al., 2010), and accumulating research suggests that exaggerated anticipatory anxiety during U-threat is uniquely involved in the pathophysiology of AUD (Gorka et al., 2017; Moberg et al., 2017). We have repeatedly demonstrated that individuals with both current and remitted AUD display exaggerated behavioral and neural reactivity to U-threat, but not P-threat, compared with controls (Gorka and Shankman, 2017; Gorka et al., in press). It has also been shown in humans that acute alcohol administration selectively and preferentially dampens aversive reactivity to U-threat relative to P-threat (Moberg and Curtin, 2009; Bradford et al., 2013). Targeting neural dysfunction during U-threat, specifically, may therefore have the most robust impact on alcohol behaviors.
The current study had numerous strengths but also several limitations. First, blood samples for plasma BDNF extraction were collected 1–7 days prior to the fMRI session, and this time lag in data collection may have introduced unknown confounds in the association between BDNF levels and amygdala reactivity and connectivity. There were also unmeasured factors, known to influence BDNF expression, which could have contributed to BDNF levels on the day of extraction (e.g., sleep deprivation; Giese et al., 2014). To capture a more reliable estimate of BDNF levels, repeated measurements, spaced in time, would be beneficial. Second, we restricted our analyses to the amygdala and amygdala-PFC circuit given the robust animal literature implicating dysfunction in these areas; however, it is possible that other neural regions and circuits are also related to individual differences in plasma BDNF. Third, the total sample size was modest and subgroup cell sizes for AUD compared with controls was small. This issue limited statistical power and may have prevented the detection of additional associations. Lastly, the current study was correlational, and we are unable to make inferences about the directionality of the associations between BDNF, functional connectivity, and drinking behaviors. Related, to comprehensively test associations across study measures, corrections for multiple comparison were not applied to the correlations between biological variables and drinking behaviors. These results should therefore be interpreted with caution and require replication.
Results from the current study suggest that lower levels of plasma BDNF are associated with disruptions in amygdala-PFC functional connectivity during anticipatory anxiety and that these biological individual difference factors may contribute to risky patterns of alcohol use within individuals with AUD and may serve as a biomarker for AUD. Converging evidence across human and animal research suggests that the amygdala-PFC circuit is an important AUD treatment target, which may be modulated by BDNF expression.
Acknowledgments
We thank Kayla Kreutzer and Kelsey Petrey for assistance with data collection.
This work was supported by the National Institute of Alcohol Abuse and Alcoholism (NIAAA) at the National Institutes of Health (P50AA022538 and U01AA-019971 [Neurobiology of adolescent Drinking in Adulthood (NADIA)] project awarded to S.C.P. and K23AA025111 awarded to S.M.G.). S.C.P. is also supported by senior research career scientist award from the Department of Veterans Affairs.
1We conducted an additional set of analyses where the U- and P- shocks were included as regressors in the first level model. The results of the paper were consistent whether shocks were included or excluded from the model.
Interest Statement
None.
References
- Aiken LS, West SG, Reno RR (1991) Multiple regression: testing and interpreting interactions. Newbury Park, CA: Sage. [Google Scholar]
- Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C (2011) Phasic and sustained fear in humans elicits distinct patterns of brain activity. Neuroimage 55:389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andero R, Choi DC, Ressler KJ (2014) BDNF-TrkB receptor regulation of distributed adult neural plasticity, memory formation, and psychiatric disorders. Prog Mol Biol Transl Sci 122:169–192. [DOI] [PubMed] [Google Scholar]
- Autry AE, Monteggia LM (2012) Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev 64:238–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, Dolinsky ZS, Meyer RE, Hesselbrock M, Hofmann M, Tennen H (1992) Types of alcoholics: concurrent and predictive validity of some common classification schemes. Br J Addict 87:1415–1431. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL (2007) Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci 2:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW Jr, Miller SW, McGinty JF (2007) A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci 26:757–766. [DOI] [PubMed] [Google Scholar]
- Berkel TD, Pandey SC (2017) Emerging role of epigenetic mechanisms in alcohol addiction. Alcohol Clin Exp Res 41:666–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnsack JP, Teppen T, Kyzar EJ, Dzitoyeva S, Pandey SC (2019) The lncRNA BDNF-AS is an epigenetic regulator in the human amygdala in early onset alcohol use disorders. Transl Psychiatry 9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolt T, Prince EB, Nomi JS, Messinger D, Llabre MM, Uddin LQ (2018) Combining region- and network-level brain-behavior relationships in a structural equation model. Neuroimage 165:158–169. [DOI] [PubMed] [Google Scholar]
- Bradford DE, Shapiro BL, Curtin JJ (2013) How bad could it be? Alcohol dampens stress responses to threat of uncertain intensity. Psychol Sci 24:2541–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SR, Johnson SC, Jacobs PC (2010) Disinhibited characteristics and binge drinking among university student drinkers. Addict Behav 35:242–251. [DOI] [PubMed] [Google Scholar]
- Chul BL, Choi IG, Kim YK, Ham BJ, Yang BH, Roh S, Choi J, Lee JS, Oh DY, Chai YG (2009) Relation between plasma brain-derived neurotrophic factor and nerve growth factor in the male patients with alcohol dependence. Alcohol 43:265–269. [DOI] [PubMed] [Google Scholar]
- Colzato LS, Van der Does AJ, Kouwenhoven C, Elzinga BM, Hommel B (2011) BDNF Val66Met polymorphism is associated with higher anticipatory cortisol stress response, anxiety, and alcohol consumption in healthy adults. Psychoneuroendocrinology 36:1562–1569. [DOI] [PubMed] [Google Scholar]
- Costa MA, Girard M, Dalmay F, Malauzat D (2011) Brain-derived neurotrophic factor serum levels in alcohol-dependent subjects 6 months after alcohol withdrawal. Alcohol Clin Exp Res 35:1966–1973. [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA (2017) FMRI clustering in AFNI: false-positive rates redux. Brain Connect 7:152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, et al. (2015) Brain pathways to recovery from alcohol dependence. Alcohol 49:435–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcq E, Warnault V, Phamluong K, Besserer GM, Liu F, Ron D (2015) MicroRNA-30a-5p in the prefrontal cortex controls the transition from moderate to excessive alcohol consumption. Mol Psychiatry 20:1219–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C (2010) Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 35:105–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster E, Toulopoulou T, McDonald C, Bramon E, Walshe M, Filbey F, Wickham H, Sham PC, Murray RM, Collier DA (2005) Association between BDNF val66 met genotype and episodic memory. Am J Med Genet B Neuropsychiatr Genet 134B:73–75. [DOI] [PubMed] [Google Scholar]
- Dodhia S, Hosanagar A, Fitzgerald DA, Labuschagne I, Wood AG, Nathan PJ, Phan KL (2014) Modulation of resting-state amygdala-frontal functional connectivity by oxytocin in generalized social anxiety disorder. Neuropsychopharmacology 39:2061–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Sa C, Dileone RJ, Anderson GM, Sinha R (2012) Serum and plasma brain-derived neurotrophic factor (BDNF) in abstinent alcoholics and social drinkers. Alcohol 46:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR (2003) The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112:257–269. [DOI] [PubMed] [Google Scholar]
- Elsayed NM, Kim MJ, Fields KM, Olvera RL, Hariri AR, Williamson DE (2018) Trajectories of alcohol initiation and use during adolescence: the role of stress and amygdala reactivity. J Am Acad Child Adolesc Psychiatry 57:550–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk DE, Yi HY, Hilton ME (2008) Age of onset and temporal sequencing of lifetime DSM-IV alcohol use disorders relative to comorbid mood and anxiety disorders. Drug Alcohol Depend 94:234–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Marchena N, Silva-Peña D, Martín-Velasco AI, Villanúa MÁ, Araos P, Pedraz M, Maza-Quiroga R, Romero-Sanchiz P, Rubio G, Castilla-Ortega E, Suárez J, Rodríguez de Fonseca F, Serrano A, Pavón FJ (2017) Decreased plasma concentrations of BDNF and IGF-1 in abstinent patients with alcohol use disorders. Plos One 12:e0187634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese M, Beck J, Brand S, Muheim F, Hemmeter U, Hatzinger M, Holsboer-Trachsler E, Eckert A (2014) Fast BDNF serum level increase and diurnal BDNF oscillations are associated with therapeutic response after partial sleep deprivation. J Psychiatr Res 59:1–7. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Lovallo WR, Fox PT (2007) Reduced amygdala activation in young adults at high risk of alcoholism: studies from the Oklahoma Family Health Patterns Project. Biol Psychiatry 61:1306–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW (2008) Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci 28:4583–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Fitzgerald DA, King AC, Phan KL (2013) Alcohol attenuates amygdala-frontal connectivity during processing social signals in heavy social drinkers: a preliminary pharmaco-fMRI study. Psychopharmacology (Berl) 229:141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Hee D, Lieberman L, Mittal VA, Phan KL, Shankman SA (2016a) Reactivity to uncertain threat as a familial vulnerability factor for alcohol use disorder. Psychol Med 46:3349–3358. [DOI] [PubMed] [Google Scholar]
- Gorka SM, Lieberman L, Phan KL, Shankman SA (2016b) Association between problematic alcohol use and reactivity to uncertain threat in two independent samples. Drug Alcohol Depend 164:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Shankman SA (2017) Preliminary evidence that reactivity to uncertain threat is an endophenotype for alcohol use disorder. Drug Alcohol Depend 180:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Kreutzer KA, Petrey K, Radoman M, Phan KL (2019) Behavioral and neural sensitivity to uncertain threat in individuals with alcohol use disorder: associations with drinking behaviors and motives. Addict Biol. 7:e12774. doi: 10.1111/adb.12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS (2015) Epidemiology of DSM-5 alcohol use disorder: results from the national epidemiologic survey on alcohol and related conditions III. JAMA Psychiatry 72:757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MG, Fullman N, Hawley C, Arian N, Zimsen SR, Tymeson HD, Venkateswaran V, Tapp AD, Forouzanfar MH, Salama JS, Abate KH (2018) Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet 392:1015–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein A, Muschler M, Wilhelm J, Frieling H, Lenz B, Gröschl M, Kornhuber J, Bleich S, Hillemacher T (2010) BDNF and GDNF serum levels in alcohol-dependent patients during withdrawal. Prog Neuropsychopharmacol Biol Psychiatry 34:1060–1064. [DOI] [PubMed] [Google Scholar]
- Hensler JG, Ladenheim EE, Lyons WE (2003) Ethanol consumption and serotonin-1A (5-HT1A) receptor function in heterozygous BDNF (+/-) mice. J Neurochem 85:1139–1147. [DOI] [PubMed] [Google Scholar]
- Ho BC, Milev P, O’Leary DS, Librant A, Andreasen NC, Wassink TH (2006) Cognitive and magnetic resonance imaging brain morphometric correlates of brain-derived neurotrophic factor Val66Met gene polymorphism in patients with schizophrenia and healthy volunteers. Arch Gen Psychiatry 63:731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Ide JS, Chao HH, Zhornitsky S, Fischer KA, Wang W, Zhang S, Li CR (2018) Resting state functional connectivity of the amygdala and problem drinking in non-dependent alcohol drinkers. Drug Alcohol Depend 185:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF (2005) Neural systems responding to degrees of uncertainty in human decision-making. Science 310:1680–1683. [DOI] [PubMed] [Google Scholar]
- Jang JH, Yun JY, Jung WH, Shim G, Byun MS, Hwang JY, Kim SN, Choi CH, Kwon JS (2012) The impact of genetic variation in comt and bdnf on resting‐state functional connectivity. Int J Imag Syst Tech 22:97–102. [Google Scholar]
- Joe KH, Kim YK, Kim TS, Roh SW, Choi SW, Kim YB, Lee HJ, Kim DJ (2007) Decreased plasma brain-derived neurotrophic factor levels in patients with alcohol dependence. Alcohol Clin Exp Res 31:1833–1838. [DOI] [PubMed] [Google Scholar]
- Karege F, Schwald M, Cisse M (2002) Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett 328:261–264. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim SJ, Lee WY, Cheon YH, Lee SS, Ju A, K M, Kim DJ (2013) The effects of alcohol abstinence on BDNF, ghrelin, and leptin secretions in alcohol-dependent patients with glucose intolerance. Alcohol Clin Exp Res 37:E52–E58. [DOI] [PubMed] [Google Scholar]
- Köhler S, Klimke S, Hellweg R, Lang UE (2013) Serum brain-derived neurotrophic factor and nerve growth factor concentrations change after alcohol withdrawal: preliminary data of a case-control comparison. Eur Addict Res 19:98–104. [DOI] [PubMed] [Google Scholar]
- Koshimizu H, Kiyosue K, Hara T, Hazama S, Suzuki S, Uegaki K, Nagappan G, Zaitsev E, Hirokawa T, Tatsu Y, Ogura A, Lu B, Kojima M (2009) Multiple functions of precursor BDNF to CNS neurons: negative regulation of neurite growth, spine formation and cell survival. Mol Brain 2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyzar EJ, Pandey SC (2015) Molecular mechanisms of synaptic remodeling in alcoholism. Neurosci Lett 601:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy I, Snell J, Nelson AJ, Rustichini A, Glimcher PW (2010) Neural representation of subjective value under risk and ambiguity. J Neurophysiol 103:1036–1047. [DOI] [PubMed] [Google Scholar]
- Lhullier AC, Moreira FP, da Silva RA, Marques MB, Bittencourt G, Pinheiro RT, Souza LD, Portela LV, Lara DR, Jansen K, Wiener CD, Oses JP (2015) Increased serum neurotrophin levels related to alcohol use disorder in a young population sample. Alcohol Clin Exp Res 39:30–33. [DOI] [PubMed] [Google Scholar]
- Lin WJ, Horner AJ, Bisby JA, Burgess N (2015) Medial prefrontal cortex: adding value to imagined scenarios. J Cogn Neurosci 27:1957–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Barak S, Warnault V, Ron D (2015) Corticostriatal BDNF and alcohol addiction. Brain Res 1628:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGough NN, He DY, Logrip ML, Jeanblanc J, Phamluong K, Luong K, Kharazia V, Janak PH, Ron D (2004) RACK1 and brain-derived neurotrophic factor: a homeostatic pathway that regulates alcohol addiction. J Neurosci 24:10542–10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC (2012) A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage 61:1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR (2006) Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci 7:818–827. [DOI] [PubMed] [Google Scholar]
- Moberg CA, Bradford DE, Kaye JT, Curtin JJ (2017) Increased startle potentiation to unpredictable stressors in alcohol dependence: possible stress neuroadaptation in humans. J Abnorm Psychol 126:441–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonat S, Starkman BG, Sakharkar A, Pandey SC (2010) Neuroscience of alcoholism: molecular and cellular mechanisms. Cell Mol Life Sci 67:73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonat S, Sakharkar AJ, Zhang H, Pandey SC (2011) The role of amygdaloid brain‐derived neurotrophic factor, activity‐regulated cytoskeleton‐associated protein and dendritic spines in anxiety and alcoholism. Addict Biol 16:238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg CA, Curtin JJ (2009) Alcohol selectively reduces anxiety but not fear: startle response during unpredictable versus predictable threat. J Abnorm Psychol 118:335–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller K, Arelin K, Möller HE, Sacher J, Kratzsch J, Luck T, Riedel-Heller S, Villringer A, Schroeter ML (2016) Serum BDNF correlates with connectivity in the (pre)motor hub in the aging human brain–a resting-state fMRI pilot study. Neurobiol Aging 38:181–187. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Whalley HC, McKirdy JW, McIntosh AM, Johnstone EC, Lawrie SM, Hall J (2011) Effects of the BDNF Val66Met polymorphism on neural responses to facial emotion. Psychiatry Res 191:182–188. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT (2012) Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci 1251:E1–E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC. (2016) A critical role of brain-derived neurotrophic factor in alcohol consumption. Biol Psychiatry 79:427–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Zhang H, Xu T (2004) Partial deletion of the cAMP response element-binding protein gene promotes alcohol-drinking behaviors. J Neurosci 24:5022–5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Misra K (2006) Central and medial amygdaloid brain-derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxiety-like behaviors. J Neurosci 26:8320–8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Ugale R, Prakash A, Xu T, Misra K (2008) Effector immediate-early gene arc in the amygdala plays a critical role in alcoholism. J Neurosci 28:2589–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Jolles DJ, Van Duijvenvoorde AC, Crone EA, Peper JS (2015) The link between testosterone and amygdala-orbitofrontal cortex connectivity in adolescent alcohol use. Psychoneuroendocrinology 53:117–126. [DOI] [PubMed] [Google Scholar]
- Peters S, Peper JS, Van Duijvenvoorde AC, Braams BR, Crone EA (2017) Amygdala–orbitofrontal connectivity predicts alcohol use two years later: a longitudinal neuroimaging study on alcohol use in adolescence. Developmental Sci 20:e12448. [DOI] [PubMed] [Google Scholar]
- Prater KE, Hosanagar A, Klumpp H, Angstadt M, Phan KL (2013) Aberrant amygdala-frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depress Anxiety 30:234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds PM, Mueller SW, MacLaren R (2015) A comparison of dexmedetomidine and placebo on the plasma concentrations of NGF, BDNF, GDNF, and epinephrine during severe alcohol withdrawal. Alcohol 49:15–19. [DOI] [PubMed] [Google Scholar]
- Schmitz A, Grillon C (2012) Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test). Nat Protoc 7:527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL (2005) A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry 62:273–281. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB (1992) Timeline follow-back. In: Measuring alcohol consumption: psychosocial and biochemical methods (Litten RZ, Allen JP eds), pp 41–72. New York: Humana Press. [Google Scholar]
- Sripada CS, Angstadt M, McNamara P, King AC, Phan KL (2011) Effects of alcohol on brain responses to social signals of threat in humans. Neuroimage 55:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H. (2000) Neurotrophins and activity-dependent plasticity. Prog Brain Res 128:183–191. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Yoo DJ, Glover GH, Gotlib IH (2009) BDNF genotype modulates resting functional connectivity in children. Front Hum Neurosci 3:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zhang Y, Liu B, Long H, Yu C, Jiang T (2014) Dosage effects of BDNF Val66Met polymorphism on cortical surface area and functional connectivity. J Neurosci 34:2645–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler AL, Felsky D, Viviano JD, Stojanovski S, Ameis SH, Szatmari P, Lerch JP, Chakravarty MM, Voineskos AN (2018) BDNF-dependent effects on amygdala-cortical circuitry and depression risk in children and youth. Cereb Cortex 28:1760–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Lu Z, Levin IP, Weller JA, Li X, Bechara A (2009) Functional dissociations of risk and reward processing in the medial prefrontal cortex. Cereb Cortex 19:1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanardini R, Fontana A, Pagano R, Mazzaro E, Bergamasco F, Romagnosi G, Gennarelli M, Bocchio‐Chiavetto L (2011). Alterations of brain‐derived neurotrophic factor serum levels in patients with alcohol dependence. Alcohol Clin Exp Res 35:1529–1533. [DOI] [PubMed] [Google Scholar]