Abstract
Introduction
Many bowel problems following low anterior resection (LAR) for rectal cancer considerably impair the quality of life (QoL) of patients. The LAR syndrome (LARS) scale is a self-report questionnaire to identify and assess bowel dysfunction after rectal cancer surgery. It has been translated and validated in several languages but not in French (metropolitan French). The primary objective is to adapt the LARS scale to the French language (called French-LARS score) and to assess its psychometric properties. Secondary objectives are to assess both the prevalence and severity of LARS and to measure their impact on QoL.
Methods and analysis
A French multicentre observational cohort study has been designed. The validation study will include translation of the LARS scale following the current international recommendations, assessment of its reliability, convergent and discriminant validities, sensitivity, internal consistency, internal validity and confirmatory analyses. One thousand patients will be enrolled for the analyses. The questionnaire will be initially administered to the first 100 patients to verify the adequacy and degree of comprehension of the questions. Then reproducibility will be investigated by a test–retest procedure in the following 400 patients.
An analysis will be conducted to determine the correlation between the LARS score and the Quality of Life Questionnaire (QLQ; European Organization for Treatment and Research of Cancer’s QLQ-C30, QLQ-CR29). Risk factors linked to QoL deterioration will be identified and their impact will be measured. This study will meet the need for a validated tool to improve patient care and QoL.
Ethics and dissemination
The institutional review board of the University Hospital of Caen and the ethics committee (CPP Nord Ouest I, 25 January 2019) approved the study.
Trial registration number
Keywords: bowel dysfunction, rectal cancer, low anterior resection syndrome, colorectal functional outcome, quality of life, validation
Strengths and limitations of this study.
The validation of the French version of the low anterior resection syndrome (LARS) score (the French-LARS score) will allow the use of a scientific instrument to assess both the prevalence and severity of LARS in French language.
The French-LARS study is a multicentre cohort study of patients with rectal cancer included from 34 units of colorectal surgery in France.
Limitations include the use of non-probability sampling, which is expected to impose selection bias.
Background
Rectal cancer management
In recent years, progress in the multimodal treatments of rectal cancer (RC) has improved local disease control and increased the survival rate (up to 50% survival at 5 years).1 2 At the same time, the evolution of surgical techniques and the achievement of a 1 cm distal margin below the tumour have pushed back the limits of sphincter-preserving surgery (SPS) without impairing oncological prognosis.3 4 Up to 80% of patients with RC undergo SPS.5 The assessment of RC outcome has traditionally focused on morbidity rate, tumour recurrence and survival, while functional sequelae (ie, bowel and/or genitourinary impairment) have long been regarded as inherent to the nature of RC treatments.6 7 However, with improved surgical outcomes, we and others have observed a rising number of RC survivors who live with numerous potential side effects and, eventually, an impaired quality of life (QoL).6–9 Therefore, bowel function, like QoL, has become an increasingly important focus of care.10
Bowel dysfunction following SPS
It is widely accepted that as many as 50%–90% of patients who undergo SPS will have a subsequent change in bowel habit.11 12 The wide spectrum of bowel symptoms after resection with SPS has been termed the ‘low anterior resection syndrome (LARS)’. The prevalence and severity of LARS remain difficult to assess. Several authors still consider faecal incontinence to be the foremost intestinal sequela, underscoring the impact of urgency and impaired evacuation.
LARS is defined as follows: frequent bowel movements (increased number of stools during the day and/or night); clustering (repeated passage of several stools over a few hours, sometimes requiring the patient to defecate four of five times in 1–2 hours); disorders of continence from minimal gas leaks or staining to debilitating faecal incontinence and faecal urgency and urgency (inability to prevent defecation for >15 min when the need arises).11 12 These symptoms usually appear immediately after surgery, become most pronounced during the first few months, improve somewhat thereafter and reach a steady state after approximately 1–2 years.11 12 Recently, a pragmatic definition of LARS has been proposed: ‘disordered bowel function after rectal resection, leading to a detriment in quality of life (QoL)’.13
Rationale for using the LARS score
Although many questionnaires or instruments have been used to assess the impact of LARS on QoL, a recent systematic review and meta-analysis observed that 65% of the studies included did not use a validated assessment instrument.14 Furthermore, there is a wide range of assessment tools, including single examinations and different scoring systems, such as the Memorial Sloan Kettering Cancer Center (MSKCC) score, the bowel function instrument (BFI) and the Wexner, St Marks and Female Sexual Function Index (FSFI) scores. Most of the instruments used to assess bowel function measure faecal incontinence but leave aside other symptoms that have been shown to have a more significant correlation with QoL, such as clustering and urgency.15 Since 2012, a group of Danish authors has developed and validated a five-item instrument for the evaluation of LARS: the LARS score.16 The items are incontinence for flatus or for liquid stool, frequency of bowel movements, clustering of stools and urgency. It allows the categorisation of patients into three groups: no LARS (0–20 points), minor LARS (21–29 points) and major LARS (30–42 points).16 To date, it is the best questionnaire for capturing anorectal postoperative function. When faecal incontinence is the major concern, the Wexner, St Marks or FSFI scores are adequate, the latter being the most sound from a methodological viewpoint. While the MSKCC-BFI is the best questionnaire for evaluating LARS, its use is complex. For this reason, the LARS score is currently used preferentially for first-line evaluation.15 Its ability to reflect the impact of bowel dysfunction on QoL was proven in its initial validation and subsequently through its association with the European Organization for Treatment and Research of Cancer (EORTC) QLQ-C30 scale.17 In clinical settings, its severity categories (no LARS, minor LARS and major LARS) can facilitate rapid identification of patients most in need of treatment. Patients with major LARS reported seriously compromised QoL and significantly worse QoL compared with those with no/minor LARS. Consequently, half of the patients restricted their diet and limited their social activity.17 In addition to the original Danish version, the LARS score has been translated into English, Dutch, Swedish, Spanish, German and Chinese and can potentially be used widely.18–20
Hypothesis and objectives of the investigations
Both the adoption of a uniform definition of LARS and the consistent use of the same questionnaire allow researchers to pool and compare the results of different studies and institutions. However, a validated French version of the LARS score is not yet available.
The main objective therefore will be to adapt and validate the LARS scale questionnaire to the French language (called French-LARS score) and assess its psychometric properties. Secondary objectives are to assess both the prevalence and severity of LARS and to measure their impact on QoL.
Methods
Study design
The French-LARS study is an observational, multicentre, cohort study of patients with rectal cancer who have undergone curative sphincter-preserving surgery with partial or total mesorectal excision. Patients are included from 34 units of colorectal surgery in France (see list of participating centres in the Acknowledgements section). The study has been approved by the scientific board of the French Research Group of Rectal Cancer Surgery (GRECCAR). This group was created by surgical teams in France who are involved in the management of rectal cancer, with the aim of conducting and publishing multicentre clinical trials on the subject in high-level journals and expanding this surgical specialty to various learned societies. Most of the participating teams in the study are affiliated with the GRECCAR. All investigators will proceed with this study in accordance with the Declaration of Helsinki.
Study population
All patients will provide written informed consent prior to their enrolment for study participation.
The inclusion criteria are as follows: aged between 18 and 80 years (octogenarians were excluded because they suffer from significant comorbidities that exclude them from the majority clinical trials, they experience worse physical functioning compared with younger patients and third very few data are available about bowel function in octogenarians following rectal resection with nerve-sparing); rectal cancer patients who have undergone curative sphincter-preserving surgery with partial or total mesorectal excision; surgery performed between January 2007 and January 2017, with reversal of the defunctioning stoma before January 2017; bowel continuity restored for at least 24 months (including the reversal of the temporary stoma) and voluntary participation in the study. The exclusion criteria are as follows: a palliative rectal cancer resection; presence of stoma; known disseminated or recurrent disease and cognition and/or language issues.
For patients lost to follow-up, an active search will be carried out with general practitioners and, if necessary, with the birth councils to know the vital status. Participants in the validation study will be identified through local databases by the investigators at each of the participating centres. They will be selected randomly from the pool of eligible subjects. Participants will be approached not earlier than 24 months after surgery to allow their bowel function to have regained stability.11 12 17
Data collected
Demographic and clinical information will be obtained from the databases. Patient characteristics will be collected on electronic case report forms (e-CRFs) and include age, sex, body mass index, tumour height (distance from anal verge on MRI or rigid sigmoidoscopy in centimetres), timing and type of neoadjuvant radiotherapy and chemotherapy, if recommended; time since surgery; type of surgery (partial mesorectal excision or total mesorectal excision); type of anastomosis; defunctioning stoma and postoperative mortality and morbidity such as pelvic abscess, anastomotic leakage and reoperation. Morbidity will be evaluated with the new classification of surgical complications by Dindo et al,21 which includes five grades. The usual data will be recorded: distal and circumferential margins, the number of resected and invaded nodes, tumorous differentiation, the presence of vascular emboli (venous or lymphatic, intramural or extramural), perineural invasion and the quality of the mesorectal excision. The resected specimen will be staged according to American Joint Committee on Cancer criteria (seventh version).
Data collection will be performed according to the following procedures: (1) the researchers will identify eligible participants by reviewing the medical records of rectal cancer patients; (2) Eligible patients will be contacted by postal invitation and will be informed about the purpose of the study and (3) the completed questionnaires will be carefully checked by the researchers for any missing information. Eligible patients will be contacted by postal invitation twice if they do not reply. Any unclear item of missing information will be reconfirmed through a phone call. If this is not possible, the questionnaire will be considered invalid.
Study endpoints
The primary objective is to validate a French-language version of the LARS score to adapt the LARS scale questionnaire to the French language (called French-LARS score) and to assess its psychometric properties and factor structure. The secondary objectives are to assess both the prevalence and severity of LARS and to measure their impact on QoL.
Detailed description of implemented techniques
The validation study of the French version of the LARS score is based on face and content validity as well as on the measurement of its psychometric properties, in compliance with the standards published by the American Educational Research Association et al.22
Validation study of French version
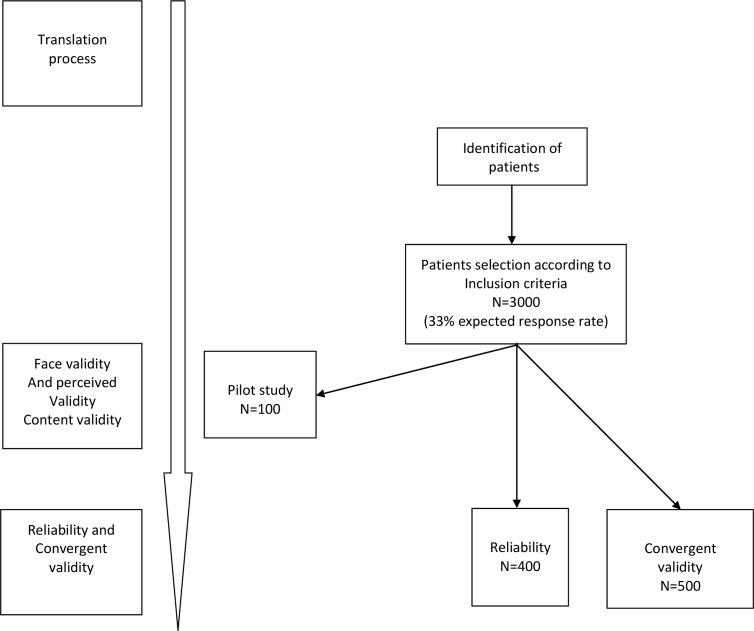
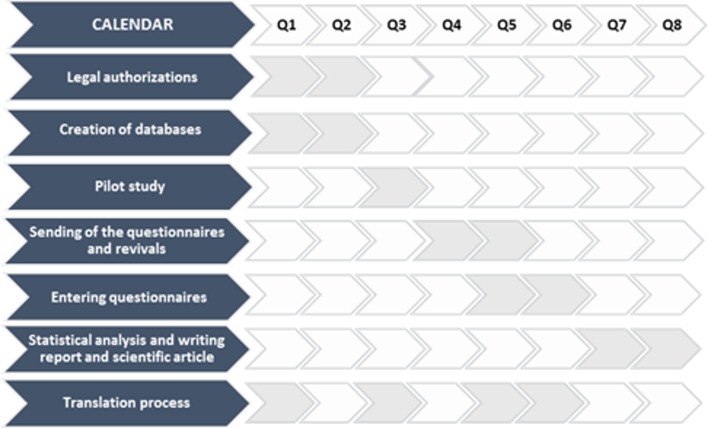
The different steps are detailed in figures 1 and 2.
Figure 1.
Consort diagram: flow of participants throughout study.
Figure 2.
Forecasting steps adapted to the study.
Translation process
After obtaining permission from the original authors,18 19 we will conduct the forward-translation and back-translation procedures in accordance with the translation guidelines provided by the authors. The French-LARS questionnaire will be developed by translating the questions into French, a task that will be performed by two independent translators who are native French speakers with a high level of fluency. The two translators will check and discuss the two translations only for inconsistency and will establish a single preliminary French version. Thereafter, the French version will be back translated into English by two independent professional translators; both are fluent in French and with English as their mother tongue, and both are unfamiliar with the background objectives of the study. Both versions of the backward translation will be compared with each other and with the initial version. After minor adjustments, a final French version will be agreed on. The final French version and the whole translation process mentioned above will be sent to the original authors for approval.
Content validity
Content validity will be assessed by a panel of experts during the process, which will lead to the final French version of the scale. Using a 3-point Likert scale (poor, average and good), each expert will judge independently whether the content from the original LARS score is conserved and adapted in the French language (see Acknowledgements).
Face validity and perceived validity
Then, a pilot study will be conducted. The French LARS score will be administered to 100 patients to verify the adequacy and degree of comprehension of the questions (figure 1). These patients will be chosen according to their representativeness based on a wide range of sociodemographic and clinical characteristics. Both male and female patients will complete the questionnaire. They will have primary education levels, secondary education levels and college or higher education levels and tumour stages I, II and III.
Each patient using the French-LARS score will be asked to review the questionnaire by precisely pointing out all the difficulties encountered when using the instrument, including the following: items that are ambiguous or poorly formulated; difficulties or confusion completing the scale. They will then be asked to indicate whether the questionnaire is acceptable and easy to understand.
Reliability
Reproducibility will be investigated by a test–retest procedure. A randomly selected subgroup of participants (n=400) will be sent the French-LARS score questionnaire twice (figure 1). The second test will be sent to the participants 1 or 2 weeks after completion of the first test. Patients will be asked if they have experienced any significant change in bowel function between the first and the second test. Those confirming a change in bowel function will be excluded from the test–retest analysis. Non-responders will be further contacted twice, either via postal invitation or by phone. The test–retest study will be performed by comparing the French-LARS scores obtained at the two time points. The test–retest reliability of the questionnaire will be assessed by Cohen’s Kappa coefficient (no LARS, minor LARS and major LARS scores) or by intraclass correlation coefficient (ICC; quantitative LARS score). Internal consistency will be estimated by the Cronbach’s alpha coefficient.
Convergent validity
Convergent validity will be determined by computing the correlations between the French-LARS score and the EORTC QLQ-C30 and QLQ-CR29 domains,23 which have been globally accepted and widely used as valid instruments for measuring QoL. Thus, eligible patients will receive a postal invitation to complete the EORTC QLQ-C30 and QLQ-CR29 along with the French-LARS scores. Furthermore, to study the convergent validity between qualitative measures of the LARS scores (no LARS, minor LARS and major LARS) and QoL, the patients will be asked a general question16 19: “Overall, how much does your bowel function affect your quality of life?” Four mutually exclusive responses, ‘not at all’, ‘very little’, ‘somewhat’ or ‘a lot’ will be proposed.
Discriminant validity
Regarding the scoring instructions for these two instruments, a high score represents a high QoL or a high level of functioning for the global QoL subscale and functional subscale. However, for a symptom subscale/item, the higher the score, the more severe the symptom. The ability to discriminate between patients with different clinical characteristics is necessary for an instrument to be considered valid. To test the tool’s discriminant validity, we will use known variables, including gender, age, neoadjuvant radiation therapy, distance of the tumour from the anal verge, the extent of mesorectal excision (partial vs total), prior temporary stoma, length of postoperative period (time since stoma-free rectal resection surgery or reversal surgery from temporary stoma) and postoperative septic complications such as pelvic abscess or anastomotic leakage. These variables are known to affect bowel function after SPS in patients with rectal cancer.11–13 24 The following numerical variables will be changed into binary variables: age, distance of the tumour from the anal verge and length of the postoperative period. The median value for each will serve as the cut-off point. Neoadjuvant radiation therapy, the extent of mesorectal excision (partial vs total) and prior temporary stoma will be treated as dichotomous variables: no treatment at all versus treatment. Moreover, interactions with neoadjuvant radiation therapy will be systematically tested. The EORTC will provide us with and authorise our use of the French version of the two questionnaires.
Sensitivity of the items
A systematic search for ceiling or floor effects will be performed.
Internal consistency
Internal consistency will be assessed using Cronbach’s alpha coefficient.
Internal validity
A factorial analysis will allow verification of the internal structure of the scale. The statistical method used is described below.
Confirmatory analysis
A confirmatory analysis will be conducted to evaluate the recognised structural validity of the scale with regard to its first edition.
Statistical analysis
Quantitative variables will be expressed as the mean±SD, and qualitative variables will be expressed as the number of patients and percentages. The experimental design of the study leads to the same patient being seen several times during their oncology follow-up. However, apart from the subgroup of patients who participate in the study of repeatability, each patient will complete only one questionnaire in the study. Comparisons between the mean scores of the three groups (no LARS, minor LARS or major LARS) will be carried out with the help of an analysis of variance (ANOVA) or a Kruskal-Wallis test, depending on whether the data follow the verified homoscedasticity hypothesis or not. Post hoc comparisons will be performed with the Bonferroni correction or the Nemenyi test.
Factor analysis will be performed with a principal component analysis. The selected factors will correspond to an eigenvalue ≥1.
The repeatability test (test–retest), in which 400 patients will be asked to complete the F-LARSF-LARSF-LARS twice within 15 days, will be Student’s t-test for repeated measurements, with the help of the ICC and its 95% CI, will use ANOVA for random effects models. After estimating the various components of the total variance, the ICC will be calculated in the usual manner. A Bland and Altman plot will be used to show the level of agreement of the repeatability test.
The sensitivity and specificity of the French-LARS score in predicting the impact on QoL will be assessed by receiver operating characteristic curves of the score versus groups reporting no/minor or some/major impact on QoL.
The correlation between the LARS validated score and the QLQ questionnaires (EORTC’s QLQ-C30, QLQ-CR29) will be estimated with the Pearson correlation coefficient as well as with the Spearman correlation coefficient and its 95% CI.
The inclusion of the data indicating the impact of LARS on QoL will be based on a univariate approach and then a multivariate approach using ad hoc models according to the nature of the dependent variable (binary or multinomial logistic regression or linear regression depending on whether the QoL score is considered qualitative or quantitative). Only variables whose level of significance in the univariate analysis is p<0.15 will be included in the multivariate model. This approach will enable the identification of the risk factors linked to a deterioration in QoL and an evaluation of their impact.
Confirmatory analysis will use structural equation models that enable the validation of the measurement structure of various concepts.
All the tests will be two sided with a level of significance (p) that equals 0.05. IBM-SPSS V.22.0 and AMOS for Windows software will be used.
Patient and public involvement
Patients were not involved in the design, the recruitment and conduct of the study. The results will be disseminated to study participants by email/paper and to the physicians who included them in the study.
Feasibility
Thirty-four colorectal cancer centres, including both university hospitals and cancer control centres, have given their consent to include between 50 and 100 patients who underwent SPS from 2007 to 2017 (see the list of participating centres in the Acknowledgements section). The availability of patients for study inclusion from each GRECCAR centre has been demonstrated in published randomised studies (25–28). We chose to include patients who underwent SPS between 2007 and 2017 for two reasons. First, the French recommendations for clinical practice and therapeutic choices for rectal cancer were published after 2007, which make the diagnoses and therapeutic strategies homogeneous.10 Second, participants were approached a minimum of 24 months after surgery to allow their bowel function to have regained stability.11 18 19 Finally, eligible participants are usually monitored in each centre at regular intervals to screen for local recurrence and/or distant metastasis. For all these reasons, approximately 3000 patients will be contacted to include more than 1000 patients, expecting a 33% response rate.
Registration
The data will be collected and registered in e-CRFs by a dedicated local technical research team using the Ennov Clinical software.
Study organisation
The lead partner will be the University Hospital of Caen, France. The study will receive financial support from the Program for Hospital Clinical Cancer Research ‘INCa-DGOS_12112’.
Duration and timeline
Patients will be included for 12 months. The approval protocol from the ethical committee, financial support and e-CRFs were developed in 2018 and 2019. Recruitment of the patients is planned to continue until the first semester of 2021. The database will be closed in 2021, after which data analysis, manuscript writing and submission for publication will follow (figure 2).
Ethics and dissemination
This study is supported by a grant from the French Ministry of Health (PHRC- K17-031). The institutional promoter is the University Hospital of Caen Department of Clinical Research and Innovation.
Results of this study will be disseminated by publication through peer-reviewed professional and scientific journals. Participant data will be kept confidential and will not be shared with the public. If there are requests for data sharing for appropriate research purposes, this will be considered on an individual basis after trial completion and after the publication of the primary manuscripts.
Discussion
Although the prevalence and severity of LARS remain difficult to assess, the LARS score, which has been developed and validated for 7 years, represents the best questionnaire to capture anorectal postoperative function to date.15 However, a validated French version of the LARS score is not yet available. This French-LARS score will allow for the development of future research and clinical practice in France. LARS remains a major problem, but it is not well understood among healthcare professionals, and it is frequently underestimated. Furthermore, there is considerable discrepancy between the clinician’s judgement of patient perception and the patient’s actual view or experience.25 26 For example, specialists tend to overestimate the impact of incontinence and frequent bowel movements, while they underestimate the impact or urgency and clustering.25 Therefore, knowledge of therapeutic options such as transanal irrigation, biofeedback or sacral nerve stimulation for patients with LARS is limited.27–29 These recent studies have indicated that there is a need for improved LARS education for clinicians.25 26 There is now evidence that both the distribution of patients within different LARS groups (minor and/or major) and the impact of LARS on QoL do not change over time.30 According to recent studies,31 nearly 50% of patients still experience major LARS 13 to 15 years after surgery. Interestingly, only major LARS has an impact on patients’ QoL.32
A 2019 survey highlights the notable functional consequences reported by RC survivors after SPS surgery.33 Based on validated instruments and recent studies, 40% of RC survivors suffer from major LARS symptoms at long-term follow-up that significantly impairs their QoL. More interestingly, bowel dysfunction was the only predictor of QoL for such patients after adjustment for age and various QoL components (urinary and sexual function).33
Clinicians will be able to use the validated French LARS score in daily clinical practice to identify patients with elevated LARS scores and to predict bowel dysfunction for prevention and rapid management. It will hopefully lead to improved clinician awareness to improve both the prevention and treatment of bowel dysfunction and the information given to patients. In the future, we will be able to develop a new patient-led follow-up programme based on symptom burden and health-related QoL. To this end, a recently published nomogram, ‘the POLARS score’, has been developed to predict bowel dysfunction severity prior to anterior resection.34 Theoretically, it allows clinicians to personalise care during multidisciplinary team meetings, to prepare patients for the consequences of treatment and to guide the treatment decision with patient consent. An alternative strategy for high-risk patients, called the ‘watch-and-wait’ policy, has been proposed in cases of complete clinical response following chemoradiation therapy. Although it leads to fewer functional problems than rectal resection, major LARS symptoms have been reported in up to one-third of these patients.35 However, there is, to date, insufficient evidence to draw firm conclusions about the oncological safety of this approach.
In summary, the validation of the French-LARS score will allow the use of a scientific instrument to assess both the prevalence and severity of LARS. Together with oncological data, it will also form a basis on which to discuss functional outcomes with patients.
Supplementary Material
Acknowledgments
The authors thank Cathy Gaillard from the Clinical Research Unit of Caen University Hospital for her practical help with the study. The authors also thank the following clinicians from the participating centres: Pr Berdah Stéphane, Dr Beyer-Berjot Laura, Hôpital Nord, Marseille. Pr Panis Yves, Dr Maggiori Leon, Chu Beaujon, Clichy. Dr Dumont Frédéric, Institut de Cancérologie de l’Ouest, Nantes. Dr Dubois Anne, Chu Estaing, Clermont-Ferrand. Pr Rullier Eric, Dr Denost Quentin, Chu Bordeaux Hôpital Saint André. Pr Tuech Jean-Jacques, Dr Bridoux Valérie, Chu de Rouen. Pr Karoui Mehdi, Dr Manceau Gilles, Chu Pitié-Salpétrière, Paris. Pr Piessen Guillaume, Chu Lille. Pr Cotte Eddy, Chu Lyon Sud. Dr Jafari Merdhad, Centre Oscar Lambret, Lille. Dr Denet Christine, Institut Mutualiste Montsouris, Paris. Dr Pol Bernard, Hôpital Saint Joseph, Marseille. Dr Chouillard Elie, CH Poissy et Saint-Germain-en-Laye. Pr Benoist Stéphane, Pr Brouquet Antoine, Chu de Bicêtre. Pr Parc Yann, Pr Lefevre Jérémie, Hôpital Saint-Antoine. Pr Faucheron Jean-Luc, Chu de Grenoble. Pr Rouanet Philippe, Institut Régional du Cancer, Montpellier. Dr Loriau Jérôme, CH Saint-Joseph, Paris. Pr Pocard Marc, Dr Eveno Clarisse, Hôpital Lariboisière, Paris. Dr Desolneux Grégoire Institut Bergonié, Bordeaux. Dr Lakkis Zaher, Chu de Besançon. Pr Prudhomme Michel, Chu de Nîmes. Pr Regimbeau Jean Marc, Pr Sabbagh Charles, Chu d’Amiens. Pr Ouaissi Mehdi, Chu de Tours. Pr Sielezneff Igor, Dr Mege Diane, Chu Timone, Marseille. Dr Badic Bogdan, Chu de Brest. Pr Bresler Laurent, Pr, Chu Brabois Nancy. Dr Benhaim Leonor, Institut Gustave Roussy, Villejuif. Pr Meunier Bernard, Chu Rennes. Pr Rohr Serge, Dr Romain Benoît, Chu Hautepierre Strasbourg. Dr Marchal Frédéric. Institut de cancérologie de Lorraine, Nancy. Dr Lelong Bernard, Dr de Chaisemartin Cécile, Institut Paoli Calmette, Marseille. Pr Berger Anne, Hôpital Georges Pompidou, Paris. Dr d’Angelis Nicolas, Hôpital Henri Mondor, Créteil. We would like to thank Frederic Bretagnol, Laurent Siproudhis, François Pigot and Amelie Anota for their help in producing the final French version of the scale.
Footnotes
Twitter: @Chaillot Fabien
Contributors: Study conception and design: AA, VB, RM, YE. Intervention design: RM, AA, VB, YE, JJD, FC, YC, JJD, BM and YE. Analysis of data will be done by YE, OD and RM. AA drafted the work, which was revised critically for intellectual content by TJ. All authors gave final approval of this version to be published.
Funding: The study received financial support from the Program for Hospital Clinical Cancer Research “INCa-DGOS_12112”. This study is supported by a grant from the French Ministry of Health (PHRC- K17-031). The institutional promoter is the University Hospital of Caen Department of Clinical Research and Innovation.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374–403. 10.1016/j.ejca.2012.12.027 [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177–93. 10.3322/caac.21395 [DOI] [PubMed] [Google Scholar]
- 3. Lakkis Z, Manceau G, Bridoux V, et al. Management of rectal cancer: the 2016 French guidelines. Colorectal Dis 2017;19:115–22. 10.1111/codi.13550 [DOI] [PubMed] [Google Scholar]
- 4. Rullier E, Laurent C, Bretagnol F, et al. Sphincter-saving resection for all rectal carcinomas: the end of the 2-cm distal rule. Ann Surg 2005;241:465–9. 10.1097/01.sla.0000154551.06768.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chau A, Maggiori L, Debove C, et al. Toward the end of abdominoperineal resection for rectal cancer? an 8-year experience in 189 consecutive patients with low rectal cancer. Ann Surg 2014;260:801–6. 10.1097/SLA.0000000000000979 [DOI] [PubMed] [Google Scholar]
- 6. Sarcher T, Dupont B, Alves A, et al. Anterior resection syndrome: what should we tell practitioners and patients in 2018? J Visc Surg 2018;155:383–91. 10.1016/j.jviscsurg.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 7. Abdelli A, Tillou X, Alves A, et al. Genito-urinary sequelae after carcinological rectal resection: what to tell patients in 2017. J Visc Surg 2017;154:93–104. 10.1016/j.jviscsurg.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 8. Wiltink LM, Marijnen CAM, Meershoek-Klein Kranenbarg E, et al. A comprehensive longitudinal overview of health-related quality of life and symptoms after treatment for rectal cancer in the Tme trial. Acta Oncol 2016;55:502–8. 10.3109/0284186X.2015.1088171 [DOI] [PubMed] [Google Scholar]
- 9. Krouse RS, Wendel CS, Garcia DO, et al. Physical activity, bowel function, and quality of life among rectal cancer survivors. Qual Life Res 2017;26:3131–42. 10.1007/s11136-017-1641-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alves A. [Recommendations for clinical practice. Therapeutic choices for rectal cancer. How can we reduce therapeutic sequelae and preserve quality of life?]. Gastroenterol Clin Biol 2007;31:1S52-62–1S95-7. [PubMed] [Google Scholar]
- 11. Bryant CLC, Lunniss PJ, Knowles CH, et al. Anterior resection syndrome. Lancet Oncol 2012;13:e403–8. 10.1016/S1470-2045(12)70236-X [DOI] [PubMed] [Google Scholar]
- 12. Ziv Y, Zbar A, Bar-Shavit Y, et al. Low anterior resection syndrome (LARS): cause and effect and reconstructive considerations. Tech Coloproctol 2013;17:151–62. 10.1007/s10151-012-0909-3 [DOI] [PubMed] [Google Scholar]
- 13. Keane C, Wells C, O'Grady G, et al. Defining low anterior resection syndrome: a systematic review of the literature. Colorectal Dis 2017;19:713–22. 10.1111/codi.13767 [DOI] [PubMed] [Google Scholar]
- 14. Scheer AS, Boushey RP, Liang S, et al. The long-term gastrointestinal functional outcomes following curative anterior resection in adults with rectal cancer: a systematic review and meta-analysis. Dis Colon Rectum 2011;54:1589–97. 10.1097/DCR.0b013e3182214f11 [DOI] [PubMed] [Google Scholar]
- 15. Chen TY-T, Emmertsen KJ, Laurberg S. What are the best questionnaires to capture anorectal function after surgery in rectal cancer? Curr Colorectal Cancer Rep 2015;11:37–43. 10.1007/s11888-014-0217-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Emmertsen KJ, Laurberg S. Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg 2012;255:922–8. 10.1097/SLA.0b013e31824f1c21 [DOI] [PubMed] [Google Scholar]
- 17. Emmertsen KJ, Laurberg S. Impact of bowel dysfunction on quality of life after sphincter-preserving resection for rectal cancer. Br J Surg 2013;100:1377–87. 10.1002/bjs.9223 [DOI] [PubMed] [Google Scholar]
- 18. Juul T, Ahlberg M, Biondo S, et al. International validation of the low anterior resection syndrome score. Ann Surg 2014;259:728–34. 10.1097/SLA.0b013e31828fac0b [DOI] [PubMed] [Google Scholar]
- 19. Juul T, Battersby NJ, Christensen P, et al. Validation of the English translation of the low anterior resection syndrome score. Colorectal Dis 2015;17:908–16. 10.1111/codi.12952 [DOI] [PubMed] [Google Scholar]
- 20. Hou X-ting, Pang D, Lu Q, et al. Validation of the Chinese version of the low anterior resection syndrome score for measuring bowel dysfunction after sphincter-preserving surgery among rectal cancer patients. Eur J Oncol Nurs 2015;19:495–501. 10.1016/j.ejon.2015.02.009 [DOI] [PubMed] [Google Scholar]
- 21. Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. American Educational Research Association, American Psychological Association, National Council on Measurement in Education . Joint Committee on Standards for Educational & Psychological Testing (US). Standards for educational and psychological testing. Washington, DC: American Educational Research Association, 2014. [Google Scholar]
- 23. Wong CKH, Chen J, Yu CLY, et al. Systematic review recommends the European organization for research and treatment of cancer colorectal cancer-specific module for measuring quality of life in colorectal cancer patients. J Clin Epidemiol 2015;68:266–78. 10.1016/j.jclinepi.2014.09.021 [DOI] [PubMed] [Google Scholar]
- 24. Hain E, Manceau G, Maggiori L, et al. Bowel dysfunction after anastomotic leakage in laparoscopic sphincter-saving operative intervention for rectal cancer: a case-matched study in 46 patients using the low anterior resection score. Surgery 2017;161:1028–39. 10.1016/j.surg.2016.09.037 [DOI] [PubMed] [Google Scholar]
- 25. Chen TY-T, Emmertsen KJ, Laurberg S. Bowel dysfunction after rectal cancer treatment: a study comparing the specialist's versus patient's perspective. BMJ Open 2014;4:e003374 10.1136/bmjopen-2013-003374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jimenez-Gomez LM, Espin-Basany E, Marti-Gallostra M, et al. Low anterior resection syndrome: a survey of the members of the American Society of colon and rectal surgeons (ASCRS), the Spanish association of surgeons (AEC), and the Spanish Society of Coloproctology (AECP). Int J Colorectal Dis 2016;31:813–23. 10.1007/s00384-016-2511-z [DOI] [PubMed] [Google Scholar]
- 27. Maris A, Devreese AM, D'Hoore A, et al. Treatment options to improve anorectal function following rectal resection: a systematic review. Colorectal Dis 2013;15:e67–78. 10.1111/codi.12036 [DOI] [PubMed] [Google Scholar]
- 28. Visser WS, Te Riele WW, Boerma D, et al. Pelvic floor rehabilitation to improve functional outcome after a low anterior resection: a systematic review. Ann Coloproctol 2014;30:109–14. 10.3393/ac.2014.30.3.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramage L, Qiu S, Kontovounisios C, et al. A systematic review of sacral nerve stimulation for low anterior resection syndrome. Colorectal Dis 2015;17:762–71. 10.1111/codi.12968 [DOI] [PubMed] [Google Scholar]
- 30. Pieniowski EHA, Palmer GJ, Juul T, et al. Low anterior resection syndrome and quality of life after sphincter-sparing rectal cancer surgery: a long-term longitudinal follow-up. Dis Colon Rectum 2019;62:14–20. 10.1097/DCR.0000000000001228 [DOI] [PubMed] [Google Scholar]
- 31. Chen TY-T, Wiltink LM, Nout RA, et al. Bowel function 14 years after preoperative short-course radiotherapy and total mesorectal excision for rectal cancer: report of a multicenter randomized trial. Clin Colorectal Cancer 2015;14:106–14. 10.1016/j.clcc.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 32. Juul T, Ahlberg M, Biondo S, et al. Low anterior resection syndrome and quality of life: an international multicenter study. Dis Colon Rectum 2014;57:585–91. 10.1097/DCR.0000000000000116 [DOI] [PubMed] [Google Scholar]
- 33. Eid Y, Bouvier V, Menahem B, et al. Digestive and genitourinary sequelae in rectal cancer survivors and their impact on health-related quality of life: Outcome of a high-resolution population-based study. Surgery 2019;166:327–35. 10.1016/j.surg.2019.04.007 [DOI] [PubMed] [Google Scholar]
- 34. Battersby NJ, Bouliotis G, Emmertsen KJ, et al. Development and external validation of a nomogram and online tool to predict bowel dysfunction following restorative rectal cancer resection: the POLARS score. Gut 2018;67:688–96. 10.1136/gutjnl-2016-312695 [DOI] [PubMed] [Google Scholar]
- 35. Hupkens BJP, Martens MH, Stoot JH, et al. Quality of life in rectal cancer patients after chemoradiation: watch-and-wait policy versus standard resection - a matched-controlled study. Dis Colon Rectum 2017;60:1032–40. 10.1097/DCR.0000000000000862 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.