Abstract
The use of indwelling pleural catheters (IPC) is well established in the treatment of malignant pleural effusions. They allow symptom management with intermittent drainage without requiring overnight admission to hospital. However, little is known about their effectiveness in the treatment of pleural infections. Here, we present a case where an IPC is used in the therapeutic management of tuberculous empyema. The IPC enabled outpatient treatment, allowed the patient to return to work and reduced the cost of treatment and the risk of hospital-acquired complications.
Keywords: pleural infection, tuberculosis
Background
Several studies demonstrate the effectiveness of combined antituberculous treatment with early pleural drainage in the treatment of tuberculous empyema to improve symptoms and reduce the risk of complications like pleural thickening. However, there is very limited data (we could only find one case report) about use of indwelling pleural catheter (IPC) for management of tuberculous empyema.1 Tuberculous empyema is typically a chronic condition requiring long-term intermittent drainage and potentially a prolonged hospital stay, resulting in significant healthcare costs, risks of iatrogenic complications and inconvenience to patients.
Case presentation
A 26-year-old man presented to acute medical admissions with a 10-day history of chest pain, shortness of breath and haemoptysis. He had been suffering with night sweats for the past month. He had no medical history. He worked as a hotel porter and was a tobacco and cannabis smoker. He was born in Vietnam and migrated to the UK 12 years previously with no foreign travel since. He had received Bacillus Calmette–Guérin (BCG) vaccination on arrival to the UK. His uncle and cousin were treated for pulmonary TB in Scotland 2 years prior to presentation, however, he had no recent contact with them for 4 years.
On admission, he was feverish (39.6°C) though all other observations were within normal ranges. He had reduced air entry to his left lung, but the examination was otherwise unremarkable.
Investigations
Admission blood tests showed a raised white cell count of 28.0×109/L and C reactive protein of 200. An erect chest X-ray showed left perihilar consolidation and a left-sided pneumothorax (figure 1). Sputum smear for acid fast bacilli was positive, with Mycobacterium tuberculosis (TB) confirmed on PCR. Blood cultures, HIV and hepatitis screens were all negative.
Figure 1.
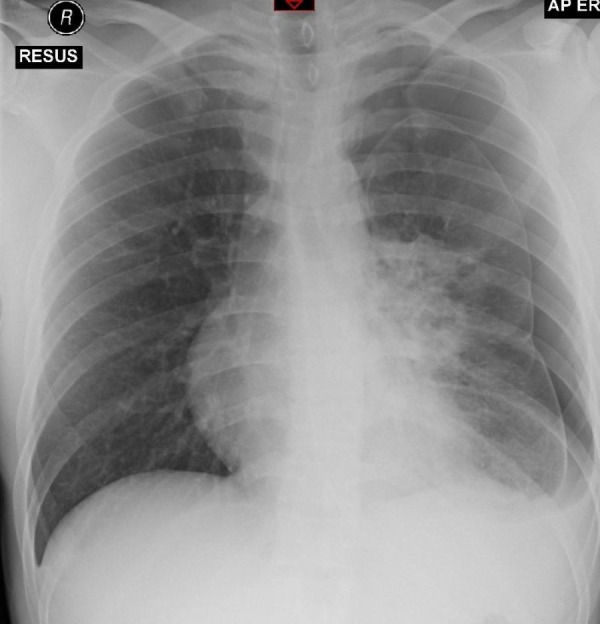
Admission chest X-ray showing left-sided pneumothorax and left perihilar consolidation. AP, anteroposterior view; ER, emergency room; RESUS, resuscitation room in Emergency Department.
Treatment
The patient was treated with a therapeutic chest drain and his pneumothorax resolved (confirmed on chest X-ray day 10 of admission). Two days after admission, he was started on rifampicin, isoniazid, pyridoxine, ethambutol and moxifloxacin empirically, moxifloxacin was stopped once antimicrobial sensitivities were known. Following removal of his chest drain on day 10, a new effusion developed (see figure 2) in the form of TB empyema (confirmed on CT (figure 3) and pleural aspirate on day 29 of his admission), and a second therapeutic chest drain was inserted. Analysis of the fluid revealed fluid glucose of 0.1 mmol/L, protein 67 g/L and lactate dehydrogenase (LDH) 7801 U/L and M. tuberculosis was grown repeatedly from pleural fluid samples. On day 52 of his admission, he underwent a repeat CT scan showing pleural thickening and a failure of his lung to fully expand. His case was discussed with the local cardiothoracic surgical centre on multiple occasions, who felt that he was not a candidate for surgical management of the empyema due to the ongoing infection risk. Despite uptitration of his rifampicin dose to therapeutic levels, his sputum cultures continued to grow M. tuberculosis until day 72 of admission, after which he persistently smear and culture negative. He remained an inpatient for a total of 80 until his chest drain was removed and he was discharged to be followed up 10 days later.
Figure 2.
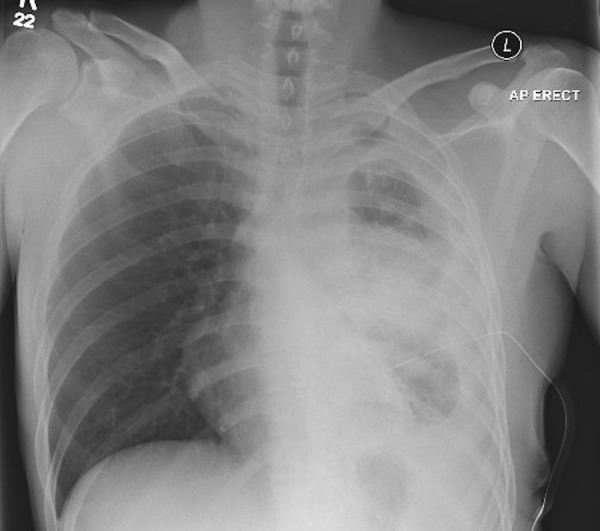
Left pleural effusion and consolidation. AP, anteroposterior view; ERECT, erect position.
Figure 3.
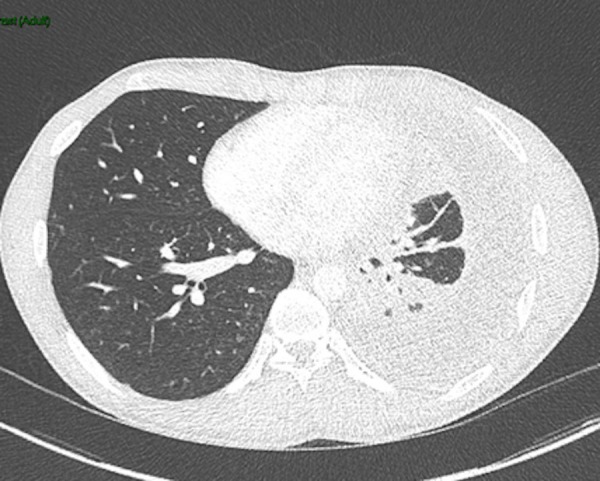
Left, moderately large pleural fluid collection highly suspicious of empyema. There is also a tiny locule of air in its posterior aspect/bronchopleural fistula related to chest drain insertion.
Outcome and follow-up
After discharge home, his effusion reaccumulated with return of his productive cough with thick white sputum, though he remained acid fast bacilli (AFB) smear and culture negative. Given his reluctance for readmission, an IPC was inserted in the outpatient setting. His IPC was drained twice weekly, initial draining approximately 500 mL at a time, but gradually reducing to less than 100 mL a week over a 4-month period. Frequent bedside chest ultrasounds were done showing pleural thickening but no residual locules were identified that could be drained. During this period, he was able to return to normal activities and even go back to work. He was assessed by cardiothoracic surgeons 5 months postdischarge, given his radiological appearances continued to improve the opinion was that surgical intervention for reinflation/drainage of pleural fluid was not indicated. Seven months postdischarge, his biweekly drainage was 80 mL and it was removed without complication. The patient finished anti-TB treatment 8 months postdischarge with no symptoms and no pleural fluid reaccumulation, but some residual pleural thickening (figure 4). Lung spirometry showed a mild restrictive pattern (forced expiratory volume in 1 s 2.55 (69%), forced vital capacity 3.35 (79%)), however, he was able to work, to resume regular exercise and normal activity without symptoms. He remains sputum smear and culture negative, confirming effective treatment of his TB.
Figure 4.
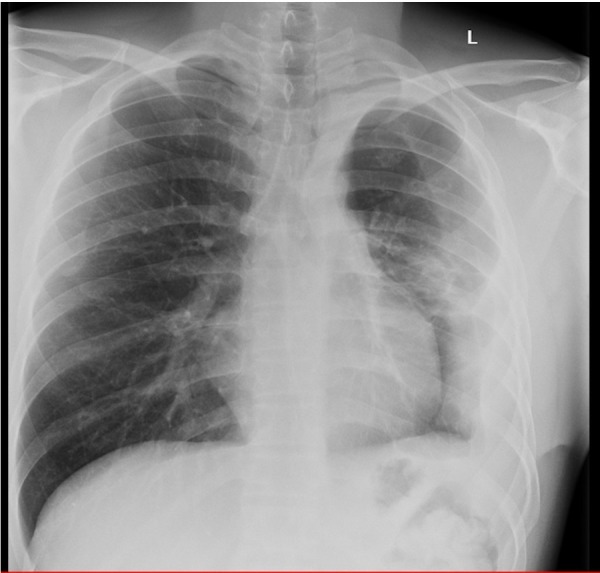
X-ray after treatment showing greatly improved appearances but some residual pleural thickening.
Discussion
Tuberculous empyema refers to chronic, grossly purulent infection of pleural space.2 It's rare as compared with tuberculous pleural effusion and contains large number of mycobacteria.2 It may diagnosed on chest radiograph and confirmed by sampling pleural fluid, or after developing bronchopleural fistula or empyema necessitatis.2
Pneumothorax in the context of TB is a sign of extensive pulmonary involvement by the infection and bronchopulmonary fistula.3 As was seen in our case, bronchopleural fistula can develop due to an open connection between bronchus and pleura secondary to damage caused by tuberculous infection.3
Severe inflammatory reaction secondary to TB results in residual pleural thickening. Previous several studies have demonstrated use of combined antituberculous treatment with pleural drainage in early stages to relieve dyspnoea and reduce residual pleural thickening.4–7 One other study reported use of intercostal pleural drainage as an inpatient for median 57 days to successfully treat tuberculous empyema.8
However, there is only one reported case of tuberculous empyema treated with IPC and repeated drainages over months as an outpatient.1
Our patient with tuberculous empyema was successfully managed with IPC with intermittent drainage for 7 months as an outpatient. Intervals between drainages was increased or reduced based on fluid drained, therefore, we do not think more frequent drainages could have prevented consequential mild lung restriction in this case.
This treatment option needs further testing, but should be considered in order to prevent significant pleural thickening and possibly surgical treatment. It also allows early discharge and outpatient management with improved quality of life. This is particularly important in low-income and middle-income countries where disease burden is very high and fewer patients can access surgical intervention. The economic impacts of TB infection to patients and their households are well described and can be catastrophic, resulting in a high risk of impoverishment.9
Introduction of IPC earlier in the course of disease may result in better outcomes, however, needs further studies in larger groups
Patient’s perspective.
During treatment, I was deeply frustrated at the need for prolonged hospital stay. I felt I won’t be able to get on with my life and was concerned about losing my job due to prolonged absence. Although, I was made aware that IPC has rarely been used in the treatment of Tuberculous empyema, I was keen to pursue this option when I was told it would enable outpatient treatment. I was able to return to work with IPC in situ and it made my day to day life much easier.
Learning points.
Tuberculous empyema requires long-term intermittent drainage.
Early drainage can improve long-term outcomes.
Indwelling pleural catheters should be considered to facilitate early discharge and reduce the risk of complications like pleural thickening and surgical interventions.
Where possible, outpatient management may reduce the economic impact of tuberculosis infection on the patient.
Footnotes
Contributors: AIM contributed to planning, writing, literature review and editing of this case report and have agreed to final version of this manuscript. JG contributed to acquiring data, writing and editing of this case report and have agreed to final version of this manuscript. AW contributed to conception, planning, literature review, editing and finalising of this case report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Davies HE, Rahman NM, Parker RJ, et al. Use of indwelling pleural catheters for chronic pleural infection. Chest 2008;133:546–9. 10.1378/chest.07-1742 [DOI] [PubMed] [Google Scholar]
- 2.Sahn SA, Iseman MD. Tuberculous empyema. Semin Respir Infect 1999;14:82–7. [PubMed] [Google Scholar]
- 3.Kim HY, Song K-S, Goo JM, et al. Thoracic sequelae and complications of tuberculosis. Radiographics 2001;21:839–58. 10.1148/radiographics.21.4.g01jl06839 [DOI] [PubMed] [Google Scholar]
- 4.Cases Viedma E, Lorenzo Dus MJ, González-Molina A, et al. A study of loculated tuberculous pleural effusions treated with intrapleural urokinase. Respir Med 2006;100:2037–42. 10.1016/j.rmed.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 5.Agha MA, El-Habashy MM, Helwa MA, et al. Role of thoracentesis in the management of tuberculous pleural effusion. Egypt J Chest Dis Tuberc 2015;64:97–102. 10.1016/j.ejcdt.2014.10.001 [DOI] [Google Scholar]
- 6.Lai Y-F, Chao T-Y, Wang Y-H, et al. Pigtail drainage in the treatment of tuberculous pleural effusions: a randomised study. Thorax 2003;58:149–51. 10.1136/thorax.58.2.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhuniya S, Arunabha DC, Choudhury S, et al. Role of therapeutic thoracentesis in tuberculous pleural effusion. Ann Thorac Med 2012;7:215–9. 10.4103/1817-1737.102176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khare P, Bhatnagar AK. “Tuberculous Empyema Thoracis Clinical, Bacteriological Features, and Its Medical Management.” 2015.
- 9.Tanimura T, Jaramillo E, Weil D, et al. Financial burden for tuberculosis patients in low- and middle-income countries: a systematic review. Eur Respir J 2014;43:1763–75. 10.1183/09031936.00193413 [DOI] [PMC free article] [PubMed] [Google Scholar]


