Abstract
Young man with acute onset nausea, vomiting, joint pain, abdominal pain, fever and weight loss was found to have gait ataxia and positive B rucella titres. He deteriorated despite appropriate antibiotics and developed confusion and disorientation. Lumbar puncture revealed lymphocytosis with high protein and low glucose. MRI showed diffuse demyelination. Pulse steroids resulted in rapid clinical, biochemical and radiological recovery.
Keywords: meningitis, medical management, infection (neurology), exposures, disease and health outcomes
Background
Brucellae are non-motile, facultative intracellular aerobic rods.1 2 On gram stain, they appear as gram-negative coccobacilli.3 Brucellosis can involve multiple organ systems with rare central nervous system (CNS) involvement. One of the least reported patterns is corpus callosum demyelination. The prognosis of neurobrucellosis depends on timely diagnosis and management, as well as the extent and pattern of brain insult. Treatment consists of ceftriaxone, rifampin and doxycycline for 3–6 months. The role of steroids is not well established. The diagnosis of neurobrucellosis is challenging. A detailed history, focussing on the occupation and other epidemiological facets, a comprehensive physical exam, blood and cerebrospinal fluid (CSF) cultures and titres with other relevant laboratory tests help.
We describe a case with rare presentation of brucellosis as meningitis with corpus callosum demyelinating changes, followed by a rapid response to steroid therapy.
Case presentation
A 37-year-old Bangladeshi man, presented with a history of progressive nausea and vomiting for 10 days. He also described intermittent dizziness and pain in both knee joints. He also had subjective fever, mostly nocturnal and abdominal pain with approximately 10 kg weight loss over the last 5 months. He did not seek medical help before and did not receive any antibiotics. He used over-the-counter acetaminophen for pain. Patient was working as a shepherd in a sheep farm for the past 2 years. He was a non-smoker, non-alcohol user with an unremarkable family history for any chronic medical conditions.
Examination showed a cachectic and lethargic man with unremarkable vital signs. Systemic examination disclosed mild gait ataxia and positive Romberg’s sign, with the rest of the exam unremarkable.
Investigations
Initial lab work depicted neutrophilic leucocytosis (table 1). Comprehensive metabolic panel (including liver and kidney function tests), chest X-ray and rapid malaria test were unremarkable. Blood cultures, along with Brucella serology, were sent on the day of admission. Cultures remained negative despite prolonged incubation, but the serology came positive for Brucella m elitensis and Brucella a bortus with a titre of 1:160 and 1:320, respectively. Brucella antibodies showed an IgG positive and a negative IgM. On the second day of admission, the patient received doxycycline, followed by rifampin and ceftriaxone on the subsequent day.
Table 1.
Comparison of lab’s work done initially and before discharge
| Investigation | Initial | Repeated (1 month) |
| CBC neutrophil count (n×103) | 20 | 9.8 |
| Brucella m elitensis titre | 1:160 | 1:160 |
|
Brucella a bortus titre |
1:320 | 1:160 |
| CSF lymphocyte percentage | 95 | 75 |
| CSF glucose level (mmol/L) |
1.8 | 3.1 |
| CSF protein count (g/L) | 0.6 | 6 |
CBC, complete blood count; CSF, cerebrospinal fluid.
Unanticipatedly, the patient’s condition deteriorated third-day postadmission with an increased frequency of vomiting, new-onset confusion, disorientation to time, place and person. His Glasgow Coma Scale (GCS) dropped to 13/15 (E5, V3, M5). A fundoscopic examination was unrevealing. CSF analysis from lumbar puncture showed yellow-coloured fluid, high white cell count, that is, 250/µL (95% lymphocytes), low glucose 1.8 mmol/L and elevated protein 0.63 g/L. CSF cultures were negative for tuberculosis (TB), fungal or bacterial growth. CSF serology for Brucella was not available. CSF showed oligoclonal bands.
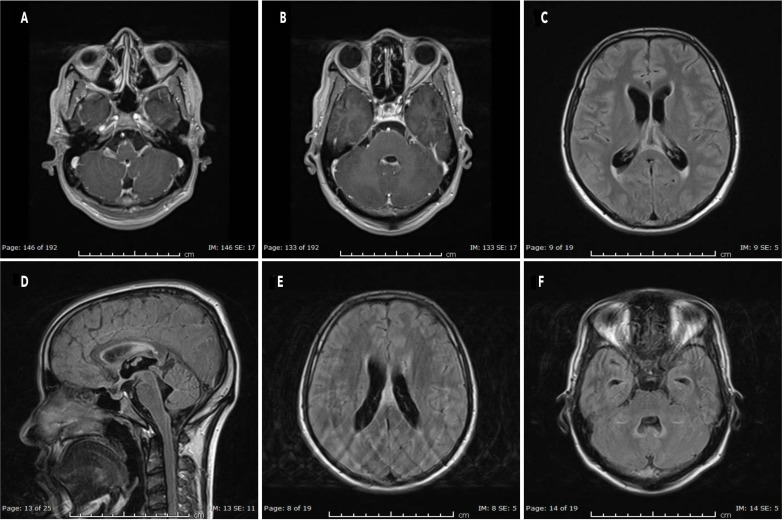
Contrast MRI of head and spine revealed meningeal enhancement, mainly around the brainstem (figure 1A), bilaterally along the fifth cranial nerve (figure 1B), seventh and eighth cranial, around lower cranial nerves and the cervical spinal cord. There was hyperintense signal along the inferior aspect of the corpus callosum, posterior parietal periventricular white matter (figure 1C) and anterior part of the splenium of the corpus callosum (figure 1D, E). Also, there was some dilatation in the ventricular system. Finally, there was hyperintense area noted along the lateral aspect of the fourth ventricle (figure 1F) in the cerebellar hemisphere and the periventricular area. All of these changes were likely due to demyelination.
Figure 1.
Pretreatment. (A) Brainstem enhancement. (B) Trigeminal enhancement. (C) Posterior parietal periventricular demyelination. (D, E) Splenium of corpus callosum demyelination. (F) Fourth ventricle demyelination.
Differential diagnosis
With the development of disorientation and working diagnosis of meningitis, the considerations were bacterial versus viral meningitis. TB with neurological involvement was a high possibility as the patient came from an area with high endemicity. Initially, serum interferon-γ release assay as well as acid fast bacilli (AFB) smear and polymerase chain reaction (PCR) from sputum and CSF were negative. CSF and sputum TB culture came out negative. These results effectively excluded TB from the differentials.
Due to chronicity of constitutional symptoms, history of weight loss and positive CSF findings, HIV was considered a significant differential, but was ruled out early during the course via HIV antigen/antibody combined assay.
Fungal meningitis was also a possibility; however, the fungal culture and India ink stain were negative.
Considering the high Brucella titres, efforts were made to explore the possibility of neurobrucellosis. This was eventually labelled as the final diagnosis based on two different criteria used in previous studies.4 5 The common points fulfilled by the patient in both criteria were presence of >1:320 B rucella titre in a patient in endemic area, CSF findings revealing chronic meningitis and signs/symptoms of neurological disease in absence of any other disease. Also, there was clear improvement in subsequent MRI scans, decreasing Brucella titres and a vivid clinical improvement in patient’s condition, after treatment.
Treatment
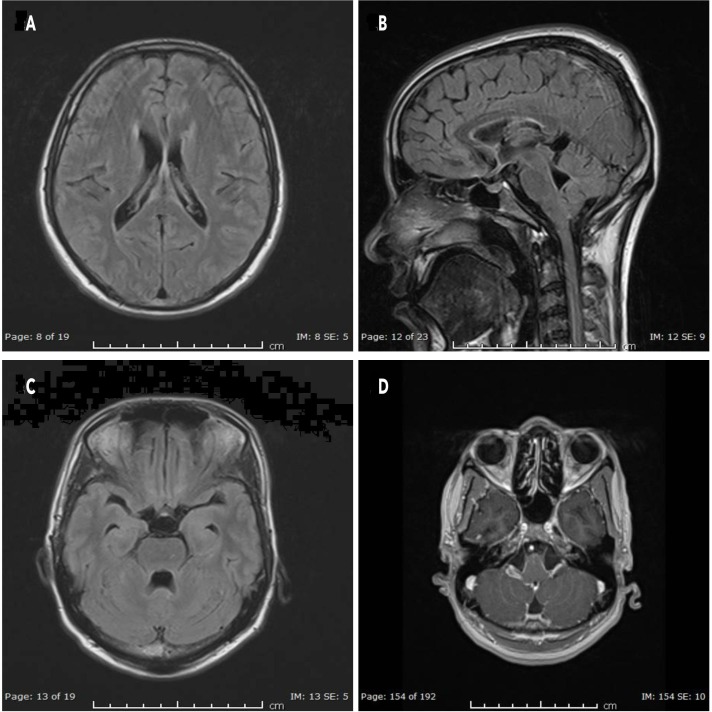
Patient received rifampin, doxycycline and ceftriaxone. Due to the extensive brain involvement and deteriorating patient condition, on the fourth day, steroids were initiated; methylprednisolone 500 mg for 3 days, followed by prednisolone 60 mg/day with a taper of 10 mg/week, in addition to continued antibiotic therapy. There was a marked improvement in the patient’s clinical condition. A repeat contrast MRI head, done 20 days after the initial imaging, showed persistent but reduced intensity of enhancement around previously noted areas including reduced size and intensity of T2-hyperintense signals along the inferior aspect as well as anterior part of the splenium of corpus callosum (figure 2A, B) and posterior parietal periventricular white matter. There was complete resolution of the enhancement along the frontal horns of both lateral ventricles, lateral aspect of the fourth ventricle (figure 2C) in the cerebellar hemisphere, the periventricular region and the brainstem (figure 2D).
Figure 2.
Post-treatment. (A, B) Splenium of corpus callosum. (C) Resolved fourth ventricle demyelination. (D) Brainstem.
Outcome and follow-up
One month after presentation, the patient’s symptoms including vomiting and arthralgia, improved. He was alert with a GCS of 15/15. Repeat lumbar puncture showed the CSF glucose improved from 1.8 to 3.1 mmol/L, with a reduction in CSF lymphocytosis (table 1). He had marked improvement in gait with some residual ataxia. Repeat serology of Brucella in serum showed a reduction in titres of B. abortus, that is, from 1:320 to 1:160, whereas B. m elitensis titre remained stable. The patient was discharged on ceftriaxone, rifampin, doxycycline and tapered dose of prednisolone with a follow-up appointment after 1 month. Over the phone to follow-up, he revealed that he was symptom-free and did not wish to attend the clinic. Shortly after that, he travelled to his home country.
Discussion
Brucellosis, a Mediterranean zoonotic infection, is caused by Brucella species, most commonly B. m elitensis. It can present either acutely or chronically. Route of transmission is through infected animal products. These include ingestion of contaminated food, unpasteurised dairy products, contact with infected animals having wounds or inhalation of aerosols.6 7 Typical clinical features include fever, night sweats, lethargy, abdominal pain, back pain, arthralgias, vomiting, anorexia and weight loss.
Common areas to encounter brucellosis include the Mediterranean, Middle East, Central Asia, China, the Indian subcontinent, sub-Saharan Africa, Mexico and South America.8 9
It is distinct from other bacterial infections in its difficult diagnosis, prolonged treatment and complications. Osteoarticular disease is the most common, with cardiac (4%) and neurologic involvement (up to 5%) being other treacherous sequelae.7
Neurobrucellosis has always been a diagnostic dilemma as it has no specific pattern of brain involvement. The spectrum ranges from the widely recognised presentations such as meningitis to the rarely encountered demyelinating diseases of the white matter. These manifestations include, but are not limited to, meningitis, encephalitis, brain abscess, myelitis, radiculitis and neuritis with involvement of cranial or peripheral nerves.10–12 Besides, it can vary in being acute or chronic, the first presentation of brucellosis or a sequel of the disease. Clinical features of neurobrucellosis can include headache, fever, hearing loss, nausea, vomiting, confusion, diplopia, seizures, facial paralysis, dizziness, nervousness, agitation, unconsciousness, insomnia, behavioural and psychological disorders.13
A thorough history, comprehensive physical examination and lab work such as complete blood count, inflammatory markers and liver function tests can hint towards the diagnosis. Once there is enough suspicion of neurobrucellosis, every effort should be made for a speedy diagnosis to initiate treatment, as a delay in recognition can result in extensive and irreversible brain injury. Although culture is the gold standard for diagnosis, Brucella is comparatively slow growing; up to 30%–90% cultures come out negative, and there is a risk of laboratory-acquired infection.14 Other than blood and CSF cultures, serology is widely used and is quicker as compared with blood cultures in guiding towards the diagnosis. Rose Bengal test is a cheaper test, with a sensitivity of 87.4% and specificity of 100%, however, it is not used in the local lab.15 WHO guidelines state that even if this test is positive, it needs further confirmation by other tests.16 Points of concern regarding use of Rose Bengal test by several authors include lower sensitivity and false negative test if the titre in serum is very high due to prozone phenomenon.15 17 Serum agglutinin test and enzyme-linked immune assay are the most commonly used methods. In general, serum agglutination test (SAT) titres can be labelled positive if >1:160 outside endemic regions and >1:320 within endemic areas.18 The sensitivity of serum agglutinin test is 95.6% and specificity is 100%.19 CSF analysis usually unveils a lymphocytic predominant leucocytosis, with increased protein and reduced glucose content. Gram stains are usually negative, and culture may be positive in less than one-third of the cases.20 CSF agglutinin test has a 25% sensitivity and 100% specificity, but it is not available locally.21
The treatment of neurobrucellosis beyond antibiotics is not well studied. Available literature is primarily limited to observational studies with no clinical trial to date. Current guidelines suggest the use of ceftriaxone (due to its excellent CNS penetration), in addition to rifampin and doxycycline, the former for the first 4–6 weeks and latter two for at least 12 weeks; the treatment can be extended up to 6 months. Another regimen is doxycycline, rifampin and trimethoprim/sulfamethoxazole (TMP-SMX) for 12 weeks.22
There is no study done to validate the role of steroids in neurobrucellosis. The evidence for the effectiveness of steroid therapy in brain involvement in patients with brucellosis comes from case reports, especially in demyelinating diseases.23
Patient’s perspective.
I was throwing up whatever I was eating, and this was causing much discomfort to me. I have never experienced this before. I was wondering why I was losing weight and did not understand that I was having some disease going on with me until the vomiting started. I am happy that it’s gone now. It’s good that I have a diagnosis. I feel useful to help other doctors understand my disease and help others.
Learning points.
Neurobrucellosis has a broad spectrum of presentation and should be kept in mind while seeing a patient with neurological signs and symptoms, coming from an endemic area or occupation, especially with background symptoms suggestive of a chronic illness.
Blood and cerebrospinal fluid cultures are not enough to diagnose neurobrucellosis as they may be unrevealing most of the times;20 hence, a combination of serology, cultures, lab work, imaging and lumbar puncture is required to narrow in the diagnosis.
Steroid therapy, which is not a part of guideline-based management, can be highly effective in demyelinating neurobrucellosis and can prevent permanent neurological damage if initiated timely.
Acknowledgments
The authors would like to acknowledge Dr Hussam Asad Almasri and Dr Riyadh Ali Mohammed Hammamy for the continuation of excellent patient care until discharge as mentioned by the patient on the follow-up phone call.
Footnotes
Twitter: @zohaibyousaf17
Contributors: FA: literature review, case writing, clinical follow-up; ZY: case write-up and discussion; MKS: case identification and literature review; AA: image selection for pretreatment and post-treatment images.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Godfroid J, Cloeckaert A, Liautard J-P, et al. From the discovery of the Malta fever's agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet Res 2005;36:313–26. 10.1051/vetres:2005003 [DOI] [PubMed] [Google Scholar]
- 2.Al Dahouk S, Nöckler K, Tomaso H, et al. Seroprevalence of brucellosis, tularemia, and yersiniosis in wild boars (Sus scrofa) from north-eastern Germany. J Vet Med B Infect Dis Vet Public Health 2005;52:444–55. 10.1111/j.1439-0450.2005.00898.x [DOI] [PubMed] [Google Scholar]
- 3.Murray P, Baron E. Manual of clinical microbiology. 9th edn Washington, D.C: ASM Press, 2007. [Google Scholar]
- 4.Guven T, Ugurlu K, Ergonul O, et al. Neurobrucellosis: clinical and diagnostic features. Clin Infect Dis 2013;56:1407–12. 10.1093/cid/cit072 [DOI] [PubMed] [Google Scholar]
- 5.Ceran N, Turkoglu R, Erdem I, et al. Neurobrucellosis: clinical, diagnostic, therapeutic features and outcome. unusual clinical presentations in an endemic region. Braz J Infect Dis 2011;15:52–9. 10.1016/S1413-8670(11)70140-4 [DOI] [PubMed] [Google Scholar]
- 6.Jiao L-D, Chu C-B, Kumar CJ, et al. Clinical and laboratory findings of nonacute neurobrucellosis. Chin Med J 2015;128:1831–3. 10.4103/0366-6999.159362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haji-Abdolbagi M, Rasooli-Nejad M, Jafari S, et al. Clinical and laboratory findings in neurobrucellosis: review of 31 cases. Arch Iran Med 2008;11:21–5.doi:08111/AIM.007 [PubMed] [Google Scholar]
- 8.Pappas G, Papadimitriou P, Akritidis N, et al. The new global map of human brucellosis. Lancet Infect Dis 2006;6:91–9. 10.1016/S1473-3099(06)70382-6 [DOI] [PubMed] [Google Scholar]
- 9.Seleem MN, Boyle SM, Sriranganathan N. Brucellosis: a re-emerging zoonosis. Vet Microbiol 2010;140:392–8. 10.1016/j.vetmic.2009.06.021 [DOI] [PubMed] [Google Scholar]
- 10.Araj GF. Update on laboratory diagnosis of human brucellosis. Int J Antimicrob Agents 2010;36:S12–17. 10.1016/j.ijantimicag.2010.06.014 [DOI] [PubMed] [Google Scholar]
- 11.Mantur BG, Amarnath SK, Shinde RS. Review of clinical and laboratory features of human brucellosis. Indian J Med Microbiol 2007;25:188–202. 10.4103/0255-0857.34758 [DOI] [PubMed] [Google Scholar]
- 12.Bosilkovski M, Krteva L, Dimzova M, et al. Brucellosis in 418 patients from the Balkan Peninsula: exposure-related differences in clinical manifestations, laboratory test results, and therapy outcome. Int J Infect Dis 2007;11:342–7. 10.1016/j.ijid.2006.10.002 [DOI] [PubMed] [Google Scholar]
- 13.Bodur H, Erbay A, Akinci E, et al. Neurobrucellosis in an endemic area of brucellosis. Scand J Infect Dis 2003;35:94–7. 10.1080/0036554021000027000 [DOI] [PubMed] [Google Scholar]
- 14.Murray P. Manual of clinical microbiology. 6th edn Washington, D.C: ASM Press, 1995. [Google Scholar]
- 15.Díaz R, Casanova A, Ariza J, et al. The rose Bengal test in human brucellosis: a neglected test for the diagnosis of a neglected disease. PLoS Negl Trop Dis 2011;5:e950 10.1371/journal.pntd.0000950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corbel M. Brucellosis in humans and animals, 2006. Available: https://www.who.int/csr/resources/publications/Brucellosis.pdf [Accessed 31 Jan 2020].
- 17.Muma JB, Lund A, Nielsen K, et al. Effectiveness of rose Bengal test and fluorescence polarization assay in the diagnosis of Brucella spp. infections in free range cattle reared in endemic areas in Zambia. Trop Anim Health Prod 2009;41:723–9. 10.1007/s11250-008-9244-0 [DOI] [PubMed] [Google Scholar]
- 18.Pappas G, Akritidis N, Bosilkovski M, et al. Brucellosis. N Engl J Med 2005;352:2325–36. 10.1056/NEJMra050570 [DOI] [PubMed] [Google Scholar]
- 19.Memish ZA, Almuneef M, Mah MW, et al. Comparison of the Brucella standard agglutination test with the ELISA IgG and IgM in patients with Brucella bacteremia. Diagn Microbiol Infect Dis 2002;44:129–32. 10.1016/S0732-8893(02)00426-1 [DOI] [PubMed] [Google Scholar]
- 20.Mandell G, Douglas R, Bennett J. Mandell, Douglas and Bennett's principles and practice of infectious diseases. 5th edn New York: Churchill Livingstone, 2000. [Google Scholar]
- 21.Araj GF, Lulu AR, Khateeb MI, et al. Elisa versus routine tests in the diagnosis of patients with systemic and neurobrucellosis. APMIS 1988;96:171–6. 10.1111/j.1699-0463.1988.tb05286.x [DOI] [PubMed] [Google Scholar]
- 22.Erdem H, Ulu-Kilic A, Kilic S, et al. Efficacy and tolerability of antibiotic combinations in neurobrucellosis: results of the Istanbul study. Antimicrob Agents Chemother 2012;56:1523–8. 10.1128/AAC.05974-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradley W. Neurology in clinical practice. 4th edn Boston: Butterworth-Heinemann, 2004. [Google Scholar]