Abstract
Background
Monoclonal antibodies of anti-epidermal growth factor receptor (EGFR) have been recommended as first-line therapy for patients with left-sided metastatic colorectal cancer (mCRC) with wild-type RAS. The effect of tumour laterality on antivascular endothelial growth factor antibody and how to optimise targeted therapies for the right-sided cases remain controversial.
Patients and methods
A comprehensive meta-analysis enrolling 16 first-line clinical trials was performed to evaluate the efficacy of chemotherapy alone and chemotherapy plus targeted therapies for patients with mCRC with right primary tumour site, and we validated the results in metastatic setting (14 trials containing 4306 patients with unresectable mCRC).
Results
Here, we found that progression-free survival (PFS) (combined HR 1.30, 95% CI 1.17 to 1.44) and overall survival (OS) (combined HR 1.46, 95% CI 1.32 to 1.62) of the right-sided patients were significantly inferior to the left-sided individuals receiving chemotherapy alone in overall population, regardless of race. Similar results were also observed in metastatic setting. OS of patients with left-sided mCRC receiving chemotherapy plus bevacizumab was superior to the right-sided individuals (combined median survival ratio (MSR)=1.23, 95% CI 1.08 to 1.39 for overall population; combined MSR=1.23, 95% CI 1.05 to 1.45 for metastatic setting), especially for wild-type RAS and mixed population. Moreover, the right-sided patients benefited more from chemotherapy plus bevacizumab comparing with chemotherapy alone in both overall population and metastatic setting. Importantly, the RAS-wild right-sided patients achieved longer PFS (combined HR 0.67, 95% CI 0.52 to 0.88) and OS (combined HR 0.74, 95% CI 0.56 to 0.98) from chemotherapy plus bevacizumab comparing with chemotherapy associated with anti-EGFR agents.
Conclusions
Patients with right-sided mCRC show impaired chemosensitivity, and chemotherapy plus bevacizumab can be an optimal first-line therapeutic regimen for the RAS-wild patients with right-sided mCRC.
Keywords: primary tumor location, bevacizumab, prognosis, colorectal cancer
key questions.
What is already known about this subject?
Patients with left-sided metastatic colorectal cancer (mCRC) have superior survival than right-sided cases and the targeted drugs such as cetuximab and panitumumab have been proposed as first-line therapeutic defenses for the wild-type RAS patients with left-sided disease. But how to optimize targeted therapies for the right-sided cases remain unclear.
What does this study add?
Here, we present results of the meta-analysis about the efficacy of chemotherapy alone and chemotherapy plus targeted therapies for mCRC patients with right-sidedness based on 16 first-line clinical trials. We found that overall survival of the right-sided patients was significantly inferior to the left-sided individuals receiving chemotherapy alone or chemotherapy plus bevacizumab. Importantly, The right-sided patients benefited more from chemotherapy plus bevacizumab comparing with chemotherapy alone or chemotherapy combined with anti-EGFR agents.
How might this impact on clinical practice?
The results provide new evidence for clinical practice to precisely select optimal targeted therapeutic regimens for the patients with right-sided mCRC, and help to reduce medical costs and prolong the survival of those patients.
Introduction
Metastatic colorectal cancer (mCRC) is a refractory malignancy with remarkable heterogeneity,1 and it accounts for approximately 40% of the newly diagnosed disease in clinic settings.2 Although patients with the early-stage disease can receive radical resection and adjuvant chemotherapy, the majority of them frequently experience recurrence or distal metastasis after surgery. In regard to mCRC, palliative resection, radiochemotherapy, targeted therapy and immune checkpoint therapy are some of the clinical managements for these patients.3 4 However, responses of the patients to these treatments are variable. Moreover, inconsistent clinical benefits are also frequently dictated by their primary tumour sidedness.4–6
Studies suggest that patients with left-sided mCRC can benefit more from anti-epithelial growth factor receptor (EGFR) monocolonal antibodies (mAbs) compared with the right-sided cases.7 Consequently, the targeted drugs such as cetuximab and panitumumab have been proposed as first-line therapeutic defenses for the wild-type RAS patients with left-sided disease.8 9 Meanwhile, several clinical trials investigated the prognostic role of bevacizumab, the most commonly used antivascular endothelial growth factor (VEGF) mAb, in the treatment of patients with right-sided and left-sided mCRC.10 11 Specifically, AGITG MAX and CALGB 80405 trials revealed no effect of tumour laterality on prognosis of the patients undergoing first-line chemotherapy plus bevacizumab.7 12 In contrast, PROVETTA, AVF2107g and NO16966 trials identified improved outcome within bevacizumab-treated patients with left-sided mCRC compared with the right-sided cases.5 13 Compared with the left-sided patients, favourable efficacy and prognosis were also observed in the right-sided patients with the treatment of first-line chemotherapy plus bevacizumab as reported in ITACa trial.13 Overall, these trials highlighted an undergoing controversy regarding the efficacy and precise use of bevacizumab combined with chemotherapy. Importantly, there is no meta-analysis reported yet to evaluate the prognostic difference in patients with right-sided mCRC with first-line chemotherapy plus anti-EGFR mAbs or bevacizumab-based treatment.
Hence, a comprehensive meta-analysis with 16 first-line clinical trials was performed to investigate the effect of chemotherapy alone and chemotherapy plus either anti-EGFR mAbs or bevacizumab on prognosis of patients with right-sided mCRC, and to define which was more suitable as a first-line regimen for the patients.
Patients and methods
In the present study, we comprehensively screened and identified eligible studies to perform this meta-analysis in accordance with PRISMA guideline.14 First of all, medical subject heading terms including “rectal, colon, colorectal”; “cancer, tumour, neoplasms or carcinoma”; “sided, sidedness, side, location, localization, site, right and left-side, laterality”; “prognosis, survival, outcome”; and “bevacizumab, cetuximab, panitumumab, EGFR, VEGF, anti-VEGF or EGFR” were selected to identify candidate articles by two independent investigators (X-HY and Y-HJ). The retrieval was conducted in the following databases: PubMed, Embase, Cochrane and ASCO meeting library as well as CNKI database (as of 15 March 2019). The actual retrieval strategy is described in online supplementary materials. Meanwhile, additional studies were also discovered by screening references of the relevant articles. Second, we identified relevant articles by reading the title of the candidate article, and those unrelated to any of the terms were excluded from the present study. Third, eligible studies were identified by careful examination of the abstract or the full text according to the following inclusion criteria: (1) clinical trial reported association between primary tumour location and survival of palliative patients with resected or unresectable mCRC with treatment of first-line chemotherapy or chemotherapy plus targeted agents; (2) the cancer arising from the appendix, caecum, ascending colon, hepatic flexure or transverse colon was classified as the right-sided disease, and the disease originating in splenic flexure, descending colon, sigmoid colon and rectum was defined as left-sided CRC; (3) each eligible study provided clinical baseline characteristics and outcome.
esmoopen-2019-000605supp001.pdf (210KB, pdf)
Two independent investigators (X-HY and ZF) extracted clinical baseline characteristics (name of clinical trial or the first author, study design, phase, country, race, recruitment time, RAS status, number of included patients with mCRC, palliative resection, therapeutic regimen and outcome), median progression-free survival (PFS) and overall survival (OS) or HR and 95% CI from each eligible study. All the relevant data were thoroughly checked by the third investigator (FS) who reread the full text.
Median survival ratio (MSR), HR and 95% CI were selected as the common measurements to assess the robust strength between tumour laterality and prognosis of patients with mCRC. Heterogeneity within the included studies was evaluated by Q test and estimated I2, ph <0.1 or I2 >50% was recognised as indicative of substantial heterogeneity. Z test in fixed (ph>0.1) or random (ph<0.1) model was selected to investigate the combined effect. Sensitivity analysis was carried out to detect the robust result by stratified analysis and different pooled model. Publication bias within the included studies was evaluated by Egger’s and Begg’s test.15 16 SPSS V.17.0 and Stata V.11.0 (Stata, College Station, TX, USA) software were used in all statistical analyses and p value <0.05 was considered as statistically significant.
Results
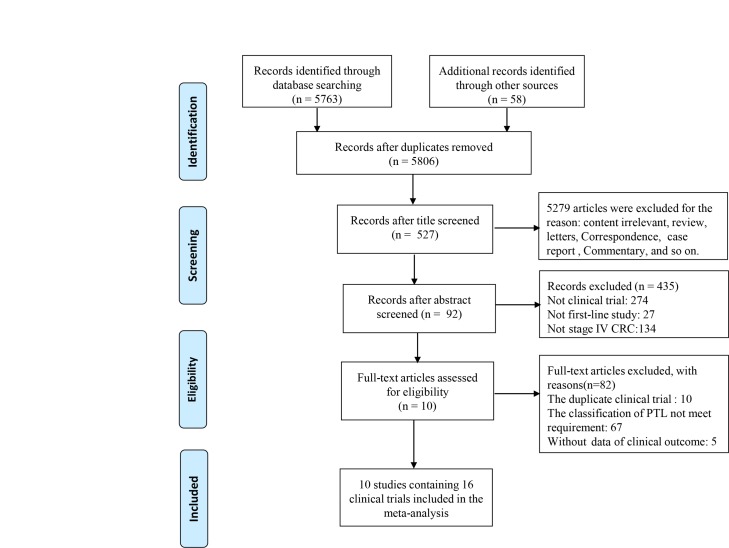
The detailed search and selection procedure are depicted in figure 1. A total of 16 first-line trials,5 7 17–24 including 4574 patients with mCRC, were ultimately fulfilled the inclusion criteria. The baseline characteristics within each eligible study are summarised in table 1. As shown in table 1, 4306 patients within 14 included trials were confirmed as unresectable mCRC cases, which composed the metastatic setting in our study. Eight trials with 3154 patients with mCRC5 7 18 19 23 24 and 10 trials including 3247 patients with mCRC5 7 17 20 22 25 reported the survival difference between the right-sided and left-sided patients receiving first-line chemotherapy alone and chemotherapy plus bevacizumab, respectively. Effects of bevacizumab within the left-sided and right-sided patients were examined in three trials.5 17 Moreover, we also evaluated data of 273 patients with mCRC within three clinical trials7 to better understand the type of biological antibody that is more suitable for treatment of the right-sided RAS-wild patients.
Figure 1.
Selection procedure of eligible study in accordance with PRISMA guidelines. CRC, colorectal cancer; PTL, primary tumor location.
Table 1.
Characteristics of 16 eligible first-line trials included in the meta-analysis
| Clinical trials | Design | Phase | Race | Recruitment time | RAS status | Palliative resection | Therapeutic regimen | Total | Left | Right | Outcome |
| Negri et al24 | Prospective RCT | NA | Caucasian | 1992–1998 | NA | No | 5-FU*† | 135 | 96 | 39 | OS |
| FFCD23 | Prospective RCT | III | Caucasian | 1997–2001 | NA | No | LV5FU2*† | 172 | 110 | 62 | OS, PFS |
| ITACa17 | Prospective RCT | III | Caucasian | 2007–2013 | NA | No | FOLFOX4, FOLFIRI+BEV †‡§ | 122 | 71 | 51 | OS, PFS |
| PROVETTA5 | Prospective RCT | NA | Mixed | NA | NA | No | CT+BEV‡ | 200 | 144 | 56 | OS, PFS |
| AVF2107g5 | Retrospective RCT | III | Mixed | 2000–2002 | NA | No | CT, CT+BEV* | 559 | 353 | 206 | OS, PFS |
| FIRE118 | Retrospective RCT | III | Caucasian | 2000–2004 | NA | No | FuFIRI/mIROX*† | 423 | 341 | 82 | OS, PFS |
| NO169665 | Retrospective RCT | III | Mixed | 2004–2005 | NA | No | CT, CT+BEV* | 1268 | 935 | 333 | OS, PFS |
| CRYSTAL7 | Retrospective RCT | III | Caucasian | 2004–2005 | RAS WT | No | FOLFIRI*† | 189 | 138 | 51 | OS, PFS |
| PRIME7 | Retrospective RCT | III | Mixed | 2006–2008 | RAS WT | No | FOLFOX4*† | 208 | 159 | 49 | OS, PFS |
| PEAK7 | Retrospective RCT | II | Caucasian | 2009–2011 | RAS WT | NA | FOLFOX6+BEV, FOLFOX6+Pani‡¶ |
68 | 54 | 14 | OS, PFS |
| FIRE 37 | Retrospective RCT | III | Caucasian | 2007–2012 | RAS WT | No | FOLFIRI+BEV, FOLFIRI+CET‡ |
199 | 149 | 50 | OS, PFS |
| CALGB 804057 | Retrospective RCT | III | Mixed | 2005–2012 | RAS WT | No | FOLFIRI/FOLFOX6+BEV, FOLFIRI/FOLFOX6+CET†‡¶ | 230 | 152 | 78 | OS, PFS |
| DREAM20 | Retrospective RCT | III | Caucasian | 2005–2012 | RAS WT mutation | No | CT+BEV†‡ | 172 | 124 | 48 | OS |
| MAVERICC21 | Retrospective RCT | II | Mixed | 2011–2015 | NA | No | mFOLFOX6/FOLFIRI+BEV†‡ | 376 | 212 | 154 | OS, PFS |
| NCT0131105022 | Prospective trial | I–II | Asian | 2009–2011 | NA | No | XELOXIRI+BEV‡ | 53 | 42 | 11 | OS, PFS |
| NCT0128265819 | Prospective trial | NA | Asian | 2010–2014 | NA | NA | FOLFIRI* | 200 | NA | NA | OS |
*Enrolled into the subgroup analysis (right-sided vs left-sided) in patients with mCRC with only chemotherapy treatment.
†Enrolled into the metastatic setting.
‡Enrolled into the subgroup analysis (right-sided vs left-sided) in patients with mCRC with chemotherapy plus bevacizumab treatment.
§Enrolled into the subgroup analysis (CT+BEV vs CT) in patients with right-sided mCRC.
¶Enrolled into the subgroup analysis (CT+BEV vs CT+anti-EGFR) in patients with right-sided mCRC.
BEV, bevacizumab; CET, cetuximab; CT, chemotherapy; FOLFIRI/FuFIRI, fluorouracil, leucovorin and irinotecan; FOLFOX, fluorouracil, leucovorin and oxaliplatin; FU, fluorouracil; LV, leucovorin;mCRC, metastatic colorectal cancer; mIROX, irinotecan and oxaliplatin; NA, not available; OS, overall survival; Pani, panitumumab; PFS, progression-free survival;RCT, randomised controlled trial; RAS/BRAF WT, RAS/BRAF wild-type; XELOXIRI, capecitabine, oxaliplatin and irinotecan.
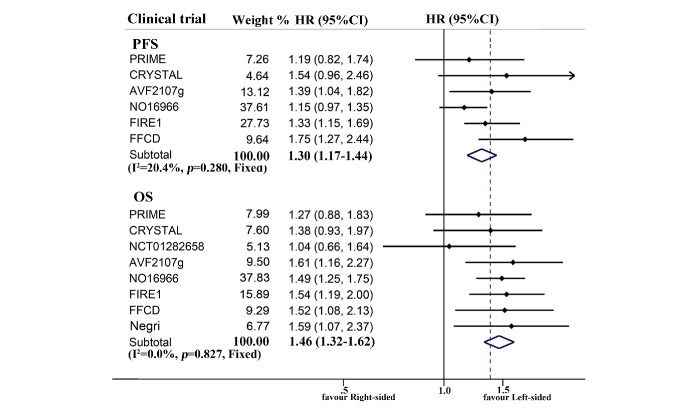
The combined survival of patients with mCRC receiving first-line chemotherapy is described in figure 2 and online supplementary table 1. Prognosis of chemotherapy-treated right-sided patients was significantly worse than the left-sided cases (ph=0.280, combined HR 1.30, 95% CI 1.17 to 1.44 for PFS; ph=0.827, combined HR 1.46, 95% CI 1.32 to 1.62 for OS), regardless of race. A similar result was also observed in metastatic setting (ph=0.567, combined HR 1.40, 95% CI 1.23 to 1.59 for PFS; ph=0.661, combined HR 1.43, 95% CI 1.24 to 1.64 for OS). Stratifying according to RAS status, the right tumour origin was only significantly associated with poor OS (ph=0.756, combined HR 1.32, 95% CI 1.02 to 1.72) in wild-type RAS subgroup.
Figure 2.
Forest plots of survival comparison between individuals with right-sided and left-sided metastatic colorectal cancer receiving first-line chemotherapy (right vs left). OS, overall survival; PFS, progression-free survival.
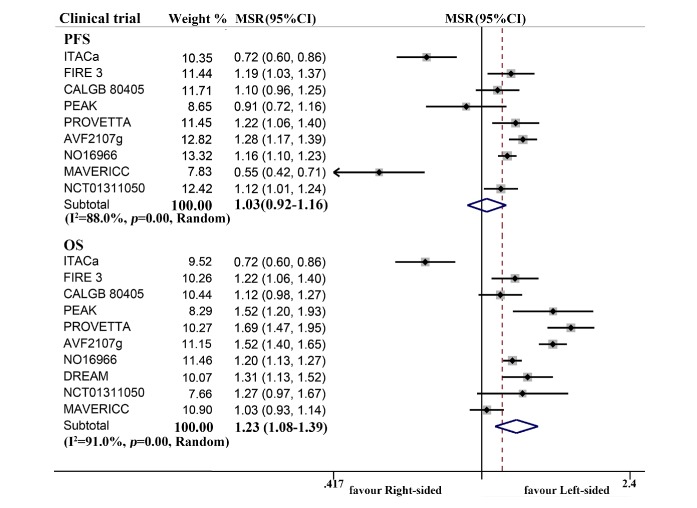
In analysis of patients with mCRC treated with first-line chemotherapy plus bevacizumab, combined OS (ph<0.001, combined MSR=1.23, 95% CI 1.08 to 1.39 for overall population; ph<0.001, combined MSR=1.23, 95% CI 1.05 to 1.45 for metastatic setting) of the left-sided patients was obviously longer than the right-sided cases (figure 3), particularly in the RAS-wild individuals (ph=0.169, combined MSR=1.11, 95% CI 1.01 to 1.21 for PFS; ph=0.045, combined MSR=1.29, 95% CI 1.12 to 1.48 for OS) and mixed population (ph=0.189, combined MSR=1.18, 95% CI 1.13 to 1.22 for PFS; ph<0.001, combined MSR=1.29, 95% CI 1.10 to 1.51 for OS) (online supplementary table 2).
Figure 3.
Forest plots of survival comparison between individuals with right-sided and left-sided metastatic colorectal cancer receiving first-line chemotherapy plus bevacizumab (left vs right). MSR, median survival ratio; OS, overall survival; PFS, progression-free survival.
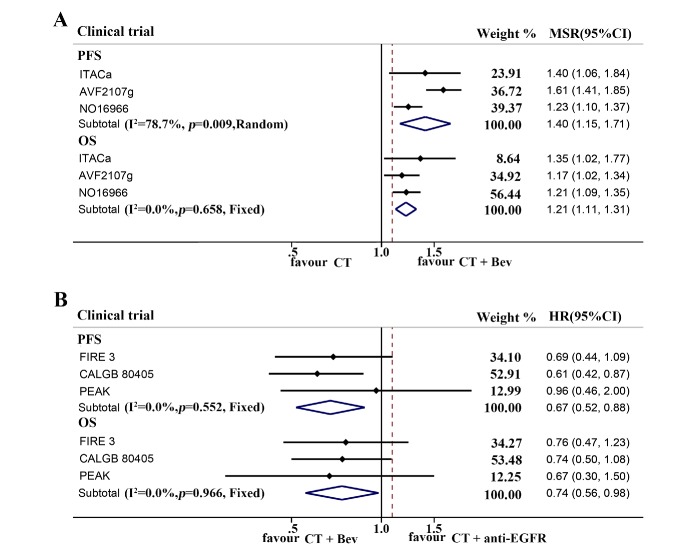
Next, we investigated the efficacy of the addition of bevacizumab to chemotherapy as compared with chemotherapy only or chemotherapy treatment plus anti-EGFR mAbs in patients with right-sided mCRC. In first-line chemotherapy plus bevacizumab-treated subgroup, PFS within the right-sided patients was obviously longer than those undergoing chemotherapy only (ph=0.009, combined MSR=1.40, 95% CI 1.15 to 1.71 for overall population; ph=0.369, combined MSR=1.57, 95% CI 1.39 to 1.77 for metastatic setting). Moreover, significantly improved OS was also observed in the right-sided patients (ph=0.658, combined MSR=1.21, 95% CI 1.11 to 1.31 for overall population; ph=0.363, combined MSR=1.20, 95% CI 1.06 to 1.36 for metastatic setting) (figure 4A and online supplementary table 3). Interestingly, the prognosis of RAS-wild right-sided patients receiving first-line chemotherapy plus bevacizumab was obviously superior to the patients undergoing chemotherapy plus anti-EGFR mAbs (ph=0.552, combined HR 0.67, 95% CI 0.52 to 0.88 for PFS; ph=0.966, combined HR 0.74, 95% CI 0.56 to 0.98 for OS) (figure 4B and online supplementary table 4).
Figure 4.
Forest plots of survival comparison in patients with right-sided metastatic colorectal cancer receiving first-line chemotherapy or chemotherapy plus targeted mAbs. (A) Chemotherapy (CT) vs chemotherapy plus bevacizumab (CT+Bev) in the right-sided patients. (B) Adjuvant chemotherapy plus bevacizumab (CT+Bev) vs adjuvant chemotherapy plus anti-EGFR antibody (CT+anti EGFR) in RAS-wild right-sided patients. EGFR, epithelial growth factor receptor; mAbs, monoclonal antibodies; MSR, median survival ratio; OS, overall survival; PFS, progression-free survival.
In our study, the relative symmetric funnel plots were observed in prognostic comparisons of the right-sided and left-sided patients receiving chemotherapy or chemotherapy combined with bevacizumab; p values of Egger’s and Begg’s tests were greater than 0.05 in each comparison (online supplementary figure 1).
Discussion
Studies demonstrate a lack of consensus regarding to which kind of biological antibody is more effective to improve prognosis of patients with right-sided mCRC.22 26 In the present study, we specifically observed that survival of the right-sided patients was inferior to the left-sided individuals with first-line chemotherapy alone or chemotherapy plus bevacizumab, respectively. Whereas, the right-sided patients could benefit significantly from first-line chemotherapy plus bevacizumab, and also achieved strikingly improved prognosis from first-line chemotherapy plus bevacizumab in comparison with combined therapeutic regimen of chemotherapy and anti-EGFR mAbs.
Over the recent decade, targeted therapy has been emerging as an optimal therapeutic option for the treatment of patients with refractory mCRC.27 28 Notably, clinical responses to treatments with anti-EGFR and VEGF mAbs are inconsistent across patients with different primary tumour locations.29 30 In the current study, we found that the outcome of patients with left-sided mCRC was superior to the right-sided patients who received first-line chemotherapy with or without bevacizumab. The results revealed that primary tumour sidedness was linked to the efficacy of chemotherapy. Right-sided mCRC might induce impaired sensitivity to common chemotherapy, leading to different benefits from first-line chemotherapy between the right-sided and left-sided cases. The finding was consistent with our previous study.31 A recent study by Loupakis and his coworkers reported that the right-sided and left-sided patients could significantly benefit from the treatment of chemotherapy plus bevacizumab, especially in the left-sided cases.13 In our study, remarkable PFS and OS improvements were also observed in the right-sided patients with treatment of first-line chemotherapy plus bevacizumab comparing with chemotherapy only. Moreover, we found that prognosis of patients with left-sided mCRC was superior to the right-sided patients receiving chemotherapy plus bevacizumab. These results suggest that bevacizumab improves the prognosis of patients with mCRC; however, impaired chemosensitivity restricts the survival benefit from bevacizumab plus chemotherapy, resulting in poor prognosis in the right-sided mCRC cases. The latest meta-analysis performed by Holch and his coworkers identified significant survival benefit from anti-EGFR mAbs compared with bevacizumab when added to standard chemotherapy in RAS-wild patients with left-sided mCRC.32 Interestingly, the drastically improved prognosis was examined in patients with right-sided mCRC receiving first-line chemotherapy plus bevacizumab comparing with the patients undergoing chemotherapy plus anti-EGFR mAbs in our study. It indicates that first-line chemotherapy combined with bevacizumab is an optimal clinical treatment of patients with right-sided mCRC to achieve a satisfactory prognosis.
Tumour laterality is one of the most debated topics in treatment of CRC.6 33 34 There is significant heterogeneity in genetic alteration and tumour microecology in right-sided and left-sided cancer.35 36 High CpG island methylator phenotype and microsatellite instability as well as hypermutation within DNA mismatch repair (MMR), MAPK, TGF-β and insulin signalling pathways are prevalent in the right-sided disease compared with its counterpart.22 37–39 The MMR-deficient status impairs genomic stability, leading to carcinogenesis, chemoresistance and progression of the disease.40 41 Meanwhile, chromosome instability, mutations of APC, SMAD4 and P53 as well as EGFR amplification are frequently detected within the left-sided CRC,42 43 while the low instability of genome-wide copy number alterations within right-sided mCRC confers no additional benefit from bevacizumab, resulting in drug resistance.44 Moreover, relatively abundant Prevotella, Pyramido-bacterium, Selenomonas and Peptostreptococcus with low infiltration of activated CD8+ T cell and T helper type 1 cell as well as high infiltration of neutrophils and regulatory T cells are commonly observed in the right-sided disease.45 46 Combination of the environmental factors cross-talk with the cancer cell to release various cytokines such as IL-6, CXCL8 and MIP-1α, creating excessive inflammatory microenvironment in the right-sided disease.47 Our previous study also identified severe inflammation in the right-sided mCRC, and severe inflammation was also linked with resistance to chemotherapy, leading to poor clinical response and prognosis.31 In addition, VEGF expression is relatively high in the left-sided cancer comparing with the right-sided disease.48 49 The right-sided patients often present with inactive EGFR pathway and low expressions of EGFR endogenous ligands such as epiregulin and amphiregulin,42 resulting in resistance to EGFR inhibition in these patients.50 These differences can likely explain the survival differences between the right-sided and left-sided patient receiving the same therapeutic regimen. Specifically, we come closer to understanding why the prognosis of the bevacizumab-treated right-sided patients is superior to the patients receiving anti-EGFR mAbs-based therapy.
This work, to the best of our knowledge, is the first comprehensively designed study examining clinical responses and survival differences in the right-sided patients treated with chemotherapy or chemotherapy plus biological antibodies. Moreover, our work first provides the evidence illustrating first-line bevacizumab-based treatment, instead of chemotherapy plus anti-EGFR mAbs, is likely more suitable for patients with right-sided mCRC. Only the first-line clinical trials were examined in our study, so as to arrive at accurate and robust conclusions.
The following limitations should be addressed to fully understand the findings in our study. The sample size of enrolled studies relating to comparison of the two kinds of biological therapies was small; our findings should be validated by large sample size and multicentre clinical trials. It is also important to emphasise that the majority of examined studies are from Caucasian population. and we do not know the role of primary tumour sidedness in Asian population, especially in Chinese. Finally, there was only one eligible study concerning RAS-mutated population, so we could not specifically examine the prognostic difference in RAS-mutated patients with right-sided and left-sided mCRC.
In summary, right tumour sidedness confers impaired sensitivity to chemotherapy, and chemotherapy plus bevacizumab can be selected as an optimal first-line therapeutic regimen for the treatment of RAS-wild patients with right-sided mCRC. The results provide new evidence for clinical practice to precisely select optimal targeted therapeutic regimens for patients with right-sided mCRC and also help to reduce medical costs and prolong the survival of those patients. Further studies are warranted to validate the findings in Asian population and to explore effective biomarkers to predict the prognosis of the patients.
Footnotes
Contributors: X-HY screened and selected the eligible study in the meta-analysis, and performed all the statistics. Y-HJ and ZF contributed to select and identify the eligible study, and extract the data of enrolled studies. FS, YL and Z-JX contributed to data extraction. X-ZW contributed to examining the data. H-QY provided the idea, established the study design, and revised and approved the manuscript.
Funding: This report was supported by the National Natural Science Foundation of China (grant no. 81702090), the Natural Science Youth Foundation of Jiangxi Province (grant no. 20171BAB215054) and the Key Technology Research and Development Program of Jiangxi Province (grant no. 20171BBG70049).
Patient consent for publication: Not required.
Ethics approval: The present study was approved by the Medical Ethics Committee of the Second Affiliated Hospital of Nanchang University (Nanchang, Jiangxi, China).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Van den Eynde M, Mlecnik B, Bindea G, et al. The link between the multiverse of immune microenvironments in metastases and the survival of colorectal cancer patients. Cancer Cell 2018;34:1012–26. 10.1016/j.ccell.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 2.Luo HY, Li YH, Wang W, et al. Single-agent capecitabine as maintenance therapy after induction of XELOX (or FOLFOX) in first-line treatment of metastatic colorectal cancer: randomized clinical trial of efficacy and safety. Ann Oncol 2016;27:1074–81. 10.1093/annonc/mdw101 [DOI] [PubMed] [Google Scholar]
- 3.Ilson DH. Adjuvant therapy in colon cancer: less is more. Lancet Oncol 2018;19:442–3. 10.1016/S1470-2045(18)30127-X [DOI] [PubMed] [Google Scholar]
- 4.Cohen R, Hain E, Buhard O, et al. Association of primary resistance to immune checkpoint inhibitors in metastatic colorectal cancer with misdiagnosis of microsatellite instability or mismatch repair deficiency status. JAMA Oncol 2019;5:551 10.1001/jamaoncol.2018.4942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loupakis F, Yang D, Yau L, et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst 2015;107 10.1093/jnci/dju427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barton MK. Primary tumor location found to impact prognosis and response to therapy in patients with metastatic colorectal cancer. CA Cancer J Clin 2017;67:259–60. 10.3322/caac.21372 [DOI] [PubMed] [Google Scholar]
- 7.Arnold D, Lueza B, Douillard J-Y, et al. Prognostic and predictive value of primary tumour side in patients with Ras wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol 2017;28:1713–29. 10.1093/annonc/mdx175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen G. [Interpretation of the updates of NCCN 2017 version 1.0 guideline for colorectal cancer]. Zhonghua Wei Chang Wai Ke Za Zhi 2017;20:28–33. [PubMed] [Google Scholar]
- 9.Benson AB, Venook AP, Al-Hawary MM, et al. NCCN guidelines insights: colon cancer, version 2.2018. J Natl Compr Canc Netw 2018;16:359–69. 10.6004/jnccn.2018.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cremolini C, Antoniotti C, Lonardi S, et al. Primary tumor sidedness and benefit from FOLFOXIRI plus bevacizumab as initial therapy for metastatic colorectal cancer. Retrospective analysis of the tribe trial by GONO. Ann Oncol 2018;113 10.1093/annonc/mdy140 [DOI] [PubMed] [Google Scholar]
- 11.Aljehani MA, Morgan JW, Guthrie LA, et al. Association of primary tumor site with mortality in patients receiving bevacizumab and cetuximab for metastatic colorectal cancer. JAMA Surg 2018;153:60–7. 10.1001/jamasurg.2017.3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tapia Rico G, Price T, Tebbutt N, et al. Right or left primary site of colorectal cancer: outcomes from the molecular analysis of the AGITG max trial. Clin Colorectal Cancer 2019;18:141–8. 10.1016/j.clcc.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 13.Loupakis F, Hurwitz HI, Saltz L, et al. Impact of primary tumour location on efficacy of bevacizumab plus chemotherapy in metastatic colorectal cancer. Br J Cancer 2018;119:1451–5. 10.1038/s41416-018-0304-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 16.Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ulivi P, Scarpi E, Chiadini E, et al. Right- vs. left-sided metastatic colorectal cancer: differences in tumor biology and bevacizumab efficacy. Int J Mol Sci 2017;18:1240 10.3390/ijms18061240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Modest DP, Schulz C, von Weikersthal LF, et al. Outcome of patients with metastatic colorectal cancer depends on the primary tumor site (midgut vs. hindgut): analysis of the FIRE1-trial (FuFIRI or mIROX as first-line treatment). Anticancer Drugs 2014;25:212–8. 10.1097/CAD.0000000000000041 [DOI] [PubMed] [Google Scholar]
- 19.Yu Q, Qiu H, Zhang M, et al. Relationship between primary tumor location and prognosis in metastatic colorectal cancer patients treated with irinotecan/5-FU/leucovorin (FOLFIRI). J Clin Oncol 2017;35 10.1200/JCO.2017.35.4_suppl.557 [DOI] [Google Scholar]
- 20.Chibaudel B, Andre T, Samson B, et al. Impact of primary tumor sidedness on erlotinib efficacy in patients with metastatic colorectal cancer treated with bevacizumab maintenance: results from the DREAM phase III trial. J Clin Oncol 2018;36 10.1200/JCO.2018.36.4_suppl.737 [DOI] [Google Scholar]
- 21.Lenz H-J, Lee F-C, Yau L, et al. MAVERICC, a phase II study of mFOLFOX6-bevacizumab (bv) vs FOLFIRI-BV as first-line (1L) chemotherapy (CT) in patients (PTS) with metastatic colorectal cancer (mCRC): outcomes by tumor location and KRAS status. J Clin Oncol 2016;34 10.1200/JCO.2016.34.15_suppl.3515 [DOI] [Google Scholar]
- 22.Bazarbashi S, Omar A, Aljubran AH, et al. Response rate and survival for patients with metastatic colorectal cancer from right-sided versus left-sided tumors, treated with first-line triplet chemotherapy with bevacizumab. J Clin Oncol 2017;35 10.1200/JCO.2017.35.4_suppl.801 [DOI] [Google Scholar]
- 23.Ferrand F, Malka D, Bourredjem A, et al. Impact of primary tumour resection on survival of patients with colorectal cancer and synchronous metastases treated by chemotherapy: results from the multicenter, randomised trial Fédération Francophone de Cancérologie digestive 9601. Eur J Cancer 2013;49:90–7. 10.1016/j.ejca.2012.07.006 [DOI] [PubMed] [Google Scholar]
- 24.Negri FV, Wotherspoon A, Cunningham D, et al. Mucinous histology predicts for reduced fluorouracil responsiveness and survival in advanced colorectal cancer. Ann Oncol 2005;16:1305–10. 10.1093/annonc/mdi244 [DOI] [PubMed] [Google Scholar]
- 25.Tejpar S, Stintzing S, Ciardiello F, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol 2017;3:194–201. 10.1001/jamaoncol.2016.3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallois C, Pernot S, Zaanan A, et al. Colorectal cancer: why does side matter? Drugs 2018;78:789–98. 10.1007/s40265-018-0921-7 [DOI] [PubMed] [Google Scholar]
- 27.Heinemann V, Douillard JY, Ducreux M, et al. Targeted therapy in metastatic colorectal cancer—an example of personalised medicine in action. Cancer Treat Rev 2013;39:592–601. 10.1016/j.ctrv.2012.12.011 [DOI] [PubMed] [Google Scholar]
- 28.Loree JM, Kopetz S. Recent developments in the treatment of metastatic colorectal cancer. Ther Adv Med Oncol 2017;9:551–64. 10.1177/1758834017714997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frentzas S, Simoneau E, Bridgeman VL, et al. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat Med 2016;22:1294–302. 10.1038/nm.4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Normanno N, Tejpar S, Morgillo F, et al. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol 2009;6:519–27. 10.1038/nrclinonc.2009.111 [DOI] [PubMed] [Google Scholar]
- 31.Chen Q-G, Zhang L, Sun F, et al. Elevated FPR confers to radiochemoresistance and predicts clinical efficacy and outcome of metastatic colorectal cancer patients. Aging 2019;11:1716–32. 10.18632/aging.101864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holch JW, Ricard I, Stintzing S, et al. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer 2017;70:87–98. 10.1016/j.ejca.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 33.Ciombor KK, Goldberg RM. Primary tumor sidedness as prognostic and predictive biomarker in metastatic colorectal cancer: further validation of a potentially practice-changing variable. JAMA Oncol 2017;3:165–6. 10.1001/jamaoncol.2016.3777 [DOI] [PubMed] [Google Scholar]
- 34.Cremolini C, Antoniotti C, Moretto R, et al. First-line therapy for mCRC—the influence of primary tumour location on the therapeutic algorithm. Nat Rev Clin Oncol 2017;14:113 10.1038/nrclinonc.2016.219 [DOI] [PubMed] [Google Scholar]
- 35.Stintzing S, Tejpar S, Gibbs P, et al. Understanding the role of primary tumour localisation in colorectal cancer treatment and outcomes. Eur J Cancer 2017;84:69–80. 10.1016/j.ejca.2017.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boeckx N, Janssens K, Van Camp G, et al. The predictive value of primary tumor location in patients with metastatic colorectal cancer: a systematic review. Crit Rev Oncol Hematol 2018;121:1–10. 10.1016/j.critrevonc.2017.11.003 [DOI] [PubMed] [Google Scholar]
- 37.Yamauchi M, Morikawa T, Kuchiba A, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut 2012;61:847–54. 10.1136/gutjnl-2011-300865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cancer Genome Atlas Network Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330–7. 10.1038/nature11252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lan Y-T, Jen-Kou L, Lin C-H, et al. Mutations in the Ras and PI3K pathways are associated with metastatic location in colorectal cancers. J Surg Oncol 2015;111:905–10. 10.1002/jso.23895 [DOI] [PubMed] [Google Scholar]
- 40.Li SKH, Martin A. Mismatch repair and colon cancer: mechanisms and therapies explored. Trends Mol Med 2016;22:274–89. 10.1016/j.molmed.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 41.Alex AK, Siqueira S, Coudry R, et al. Response to chemotherapy and prognosis in metastatic colorectal cancer with DNA deficient mismatch repair. Clin Colorectal Cancer 2017;16:228–39. 10.1016/j.clcc.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 42.Missiaglia E, Jacobs B, D'Ario G, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol 2014;25:1995–2001. 10.1093/annonc/mdu275 [DOI] [PubMed] [Google Scholar]
- 43.Takahashi Y, Sugai T, Habano W, et al. Molecular differences in the microsatellite stable phenotype between left-sided and right-sided colorectal cancer. Int J Cancer 2016;139:2493–501. 10.1002/ijc.30377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smeets D, Miller IS, O'Connor DP, et al. Copy number load predicts outcome of metastatic colorectal cancer patients receiving bevacizumab combination therapy. Nat Commun 2018;9:4112 10.1038/s41467-018-06567-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang L, Zhao Y, Dai Y, et al. Immune landscape of colorectal cancer tumor microenvironment from different primary tumor location. Front Immunol 2018;9:1578 10.3389/fimmu.2018.01578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao R, Kong C, Huang L, et al. Mucosa-associated microbiota signature in colorectal cancer. Eur J Clin Microbiol Infect Dis 2017;36:2073–83. 10.1007/s10096-017-3026-4 [DOI] [PubMed] [Google Scholar]
- 47.Krzystek-Korpacka M, Zawadzki M, Kapturkiewicz B, et al. Subsite heterogeneity in the profiles of circulating cytokines in colorectal cancer. Cytokine 2018;110:435–41. 10.1016/j.cyto.2018.05.015 [DOI] [PubMed] [Google Scholar]
- 48.Hutajulu SH, Paramita DK, Santoso J, et al. Correlation between vascular endothelial growth factor-A expression and tumor location and invasion in patients with colorectal cancer. J Gastrointest Oncol 2018;9:1099–108. 10.21037/jgo.2018.07.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bendardaf R, Buhmeida A, Hilska M, et al. VEGF-1 expression in colorectal cancer is associated with disease localization, stage, and long-term disease-specific survival. Anticancer Res 2008;28:3865–70. [PubMed] [Google Scholar]
- 50.Cremolini C, Loupakis F, Ruzzo A, et al. Predictors of benefit in colorectal cancer treated with cetuximab: are we getting “Lost in TranslationAL”? J Clin Oncol 2010;28:e173–4. 10.1200/JCO.2009.26.6148 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2019-000605supp001.pdf (210KB, pdf)