Abstract
Background
Repair of thoracoabdominal aortic aneurysm (TAAA) is high risk and resource intensive prophylactic operation. A benefit from the operation is realized only by those patients who both survive the procedure and remain free from permanently disabling complications. The majority of literature describing treatment for TAAA consist of operative series reported by national centers of excellence. These studies are limited by referral and selection bias, and they exclude patients not suited for the reported modality of repair. Little is known about the characteristics and survival of those patients who are not referred or who are turned down for repair. For those undergoing an intervention, outcomes such as functional status after recovery from surgery are rarely reported. This study attempts to address these gaps by reporting both survival and a patient-centered “good” outcome in an inclusive cohort of patients with TAAA, including all nonoperative and operative patients irrespective of treatment modality.
Methods
A single institution database was screened by diagnosis codes for TAAA from 2009–2017 using the International Classification of Diseases (ICD) versions 9 and 10. Diagnosis was confirmed by retrospective chart review and the CT finding of aneurysmal degeneration > 3.2 cm of the paravisceral aorta in continuity with aneurysmal aorta meeting standard criteria for repair. Patients under age 18 and those with mycotic aneurysm were excluded. Patients were either managed nonoperatively or by means of one of four categories of repair: (1) open, (2) endovascular with branched endografts, (3) hybrid, defined as ilio-visceral debranching followed by endovascular repair with standard devices, and (4) partial repair in which the paravisceral segment was intentionally left unaddressed. The primary outcomes were (1) survival and (2) a composite “good” outcome defined as successful aneurysm exclusion, freedom from permanent loss of organ system function, and return to preoperative functional status after recovery from surgery (6–18 months).
Results
After review of CT imaging, 432 of 718 patients initially identified by ICD codes met inclusion criteria. Advanced medical comorbidities were seen in 33% of the entire cohort. Nonoperative management was utilized for 48% of the cohort with a 1-year survival of 65%. A survival benefit was seen in the open, endovascular and partial but not hybrid operative groups over the nonoperative group over a three-year period. Overall 1-year survival was 81%, but only 68% had a “good” outcome (p=0.0016).
Conclusions
Close to half of the patients in this study did not undergo repair despite access to a variety of operative techniques, and many likely died in the short-term due to nonaneurysm-related causes. Among those who did undergo an operation, survival alone did not accurately represent the outcomes of operative intervention given the wide difference between survival and good outcome even with minimally invasive techniques. Operation appears to confer a survival advantage among appropriately selected patients, but a large proportion of patients with TAAA are unlikely to benefit from operative repair due to limited baseline survival and low probability of good outcome.
BACKGROUND
Decision for operative intervention for aneurysmal disease is often discussed in terms of balancing the risk of operative mortality against the risk of premature death due to rupture.1 Patients presenting with thoracoabdominal aortic aneurysms (TAAA) present a particular challenge as the risks of operation are high, and many patients are elderly with multiple comorbidities. There are limited data that suggest that many patients who are deemed high risk for TAAA repair die after a diagnosis of TAAA from causes other than aneurysm rupture.2,3 For those in whom an operation is undertaken, the relatively high rates of perioperative mortality and life-altering complication such as spinal cord injury and permanent renal failure often lead to severely reduced quality of life.4 Therefore, the goal of TAAA repair cannot merely be the prevention of rupture but must strike an appropriate balance between preserving both quantity and quality life.
Although the current literature contains a wealth of data describing operative outcomes after TAAA repair, both referral and selection bias are intrinsic to operative case series reported by these national centers of excellence.5,6,7,8 Since each center of excellence reports outcomes for a single technique of repair (e.g., traditional open or endovascular repair with branched grafts), this bias also results in the exclusion of patients with characteristics that are unsuitable for the reported modality of repair. Furthermore, little is known about the baseline characteristics and survival of those patients who are not referred to such centers, or those turned down for repair. Finally, functional status after recovery from surgery is rarely reported. In one series of 101 consecutive patients undergoing open surgical repair for TAAA, only 52% were living at home and ambulatory after one year.4 Such an outcome may conflict with patient goals for an operation.9
This study attempts to address these gaps by describing two primary endpoints: (1) survival at one year in all patients presenting with TAAA to a regional referral center including both operative and nonoperative patients, and (2) a patient-centered “good” outcome, which includes return to preoperative functional status for all patients receiving an operative intervention by all modalities performed at our institution. Patients were not excluded from the study on the basis of modality of repair.
METHODS
This study was approved by the University of Washington Institutional Review Board. Informed consent was waived for this retrospective review.
Patients
A retrospective review of patients with TAAA seen at an academic, multi-hospital institution was conducted. This institution is the primary referral center for a five-state region within the United States and serves a population of approximately 11 million people. Patients were initially identified using diagnosis codes for TAAA according to the International Classification of Diseases (ICD) versions 9 and 10 between January 2009 and April 2017 (ICD9: 441.6 and 441.7; ICD10: I71.5 and I71.6). This initial screening resulted in identification of 718 patient records. The diagnosis of TAAA was then confirmed or rejected after review of computed tomography imaging. This strategy therefore captured patients with the diagnosis who had not been referred for surgical evaluation. Patients were included in the study based on the finding of aneurysmal degeneration ≥ 3.2 cm of the paravisceral aorta in continuity with aneurysmal aorta meeting standard criteria for repair (i.e., maximal diameter ≥ 5.5 cm, aortic size index (ASI) ≥ 2.75, growth ≥ 5 mm over 6 months, or symptoms/rupture). A paravisceral diameter of ≥ 3.2 cm is considered to be ectatic and reflective of aneurysmal degeneration involving this segment.10,11,12 All operative and nonoperative patients who met these criteria were included in the study. Those under age 18, mycotic aneurysms, juxtarenal aneurysms (healthy aorta < 3.2 cm at the lowest lying renal level with any infra-renal aortic neck > 1 mm), and isolated aneurysms of the ascending, transverse arch, and descending thoracic aorta (i.e., any aneurysm that did not meet the ≥ 3.2 cm criterion at the paravisceral segment) were excluded. Data regarding baseline medical history, procedures, and outcomes were collected from the electronic medical record. Mortality data was obtained from follow-up records and a WA state death index.
Patients with one or more of the following conditions were considered to have a significant comorbidity: functionally dependent on others to perform activities of daily living, untreated cancer, COPD on home oxygen, dialysis-dependent renal failure, active substance abuse, unintentional weight loss greater than 10% of body weight, dementia, or BMI < 18.5 or > 40. This category was intended to serve as a composite variable for any preoperative major medical comorbidity that would generally preclude major prophylactic surgery.
Procedures
Patients were grouped into five broad categories, one nonoperative group and four operative groups. The operative groups were defined by how the paravisceral aorta was treated. Open repair was defined as traditional, in-line open surgery performed via clamp-and-sew technique or with distal perfusion using one of the following techniques: extra-anatomic bypass, Gott shunt, left-heart or femoral-femoral cardiopulmonary bypass. Some patients in this group received a staged procedure in which a thoracic endograft was placed followed by open repair of the paravisceral aorta. Although this combines both endovascular and open surgical techniques, patients in this group were subjected to the physiologic stress of a thoracophrenolaparotomy with cardiopulmonary bypass, so they were included in this group. Endovascular repair was defined as repair of the paravisceral segment with endovascular means. Fifty-eight patients were treated under a physician sponsored investigational device exemption (PS-IDE) clinical trial ( NCT01874197) using either physician modified devices or Cook t-Branch and Custom Made Devices with branches or fenestrated-branched constructs.13 Ten other patients received endovascular repair with various other endovascular techniques prior to initiation of the PS-IDE study. Hybrid repair was defined as visceral debranching with retrograde ilio-mesenteric and renal bypass followed by aortic endograft placement with coverage of the mesenteric and renal arteries. Partial repair was defined as any repair that by intention did not fully incorporate the segment of aorta from which the celiac, superior mesenteric, and renal arteries arise. The majority of these comprised of TEVAR of the descending thoracic aorta, but open and endovascular repairs of the infrarenal portion of the aortic aneurysm were also included in this group. A group of fifteen surgeons from the vascular and cardiothoracic surgical divisions at two major urban academic medical centers (University of Washington Medical Center and Harborview Medical Center) performed the above procedures.
Primary end points
The primary endpoints were Kaplan-Meier estimated survival and a composite patient-centered “good” outcome. Good outcome was defined as successful aneurysm exclusion, freedom from permanent loss of organ function (e.g., SCI and renal failure requiring dialysis), and return to preoperative functional status after recovery from surgery (6–18 months). Permanent spinal cord injury (SCI) was strictly defined as any bilateral lower extremity deficit, however mild, that resulted in either permanent weakness (paraparesis) or complete paralysis (paraplegia). Patients were categorized as having permanent SCI if they had any permanent impairment of lower extremity function even if they were able to recover to the point of independent ambulation with use of a walker/assist device. Patients with a “partial” repair (see above) did not by definition have complete aneurysm exclusion, and thus this criterion was not required for a good outcome for patients receiving a partial TAAA repair.
Statistical analysis
Baseline characteristics are presented as proportions for categorical variables, means with standard deviation for normally distributed continuous variables, and median with interquartile range for non-normally distributed variables. To determine the level of significance, categorical variables were compared using Pearson’s chi-squared test, normally distributed variables were compared using ANOVA, and non-normally distributed variables were compared using the Kruskal-Wallis test (given that there were >2 groups). The log-rank test was used to compare survival estimates. Post-operative clinical outcomes including the composite, patient-centered “good” outcome, were calculated for all operative groups but not for the nonoperative group; survival using the Kaplan-Meier estimator was calculated for all groups. To compare the difference between 1-year survival and good outcome, a z-test was used to compare the two proportions. Outcomes are presented by treatment type (e.g. “open”, “endovascular”, “hybrid”, etc.) and also by the presence of symptoms at presentation. All statistical analysis was performed using Stata 14MP (StataCorp, College Station, TX).
RESULTS
Cohort demographics
Screening by ICD codes yielded 718 patients. Review of CT images confirmed the diagnosis in 432 patients (60%). Cohort demographics are reported in Table I. The median age for the entire cohort was 72 with 60% being men. Thirty-three percent of the overall cohort had at least one significant comorbidity as defined above. The mean follow-up times was 17.5 months (IQR 3.9–38.1) and 45 patients (10%) had a single point of contact without any subsequent follow up available.
Table I.
Baseline characteristics for all patients with TAAA
| Non-operative | Open | Endovascular | Hybrid | Partial | Total | P | |
|---|---|---|---|---|---|---|---|
| N | 205 | 54 | 68 | 24 | 81 | 432 | |
| Age, median, IQR | 73.6 (67.2, 79.9) | 62.0 (41.5, 69.1) | 74.9 (68.2, 78.5) | 63.8 (55.2, 74.2) | 71.1 (61.6, 77.3) | 72.0 (63.8, 78.6) | <0.001 |
| Sex | <0.001 | ||||||
| Male | 49.8% | 66.7% | 75.0% | 62.5% | 69.1% | 60.2% | |
| Female | 50.2% | 33.3% | 25.0% | 37.5% | 30.9% | 39.8% | |
| Diameter, mean (cm) | 6.3 (1.3) | 6.8 (1.6) | 6.7 (1.2) | 6.8 (0.9) | 6.4 (1.1) | 6.5 (1.3) | 0.018 |
| Presentation | 0.18 | ||||||
| Asymptomatic | 77.6% | 70.4% | 82.4% | 79.2% | 64.2% | 74.9% | |
| Symptomatic | 15.8% | 25.9% | 17.6% | 16.7% | 23.5% | 18.2% | |
| Ruptured | 6.6% | 3.7% | 0.0% | 4.2% | 12.3% | 6.1% | |
| Dissection | 25.0% | 37.0% | 4.4% | 34.8% | 51.9% | 28.8% | <0.001 |
| Crawford classification | <0.001 | ||||||
| Extent I | 13.0% | 7.4% | 14.7% | 8.3% | 37.0% | 16.9% | |
| Extent II | 40.0% | 33.3% | 22.1% | 66.7% | 50.6% | 39.8% | |
| Extent III | 11.0% | 14.8% | 19.1% | 4.2% | 7.4% | 11.7% | |
| Extent IV | 33.5% | 40.7% | 42.6% | 16.7% | 3.7% | 29.3% | |
| Extent V | 2.5% | 3.7% | 1.5% | 4.2% | 1.2% | 2.3% | |
| CTD | 2.0% | 22.2% | 0.0% | 25.0% | 4.9% | 6.0% | <0.001 |
| Previous aortic surgery | 44.9% | 38.9% | 44.1% | 45.8% | 40.7% | 43.3% | 0.92 |
| Significant comorbidity | 50.5% | 7.5% | 17.7% | 13.0% | 23.1% | 32.7% | <0.001 |
CTD, connective tissue disorder
Significant heterogeneity in baseline characteristics was observed between the management groups. Nonoperative patients comprised 47% of the entire cohort and had a significantly higher incidence of significant comorbidity (50.3%, p<0.001). The open and hybrid patients were 10 years younger compared to those in the other groups and were more likely to have connective tissue disease. Those undergoing a partial repair were the most likely to have dissection as the etiology of TAAA (52% of patients in this group). Women were significantly less likely to undergo repair than men at 41% vs. 61%, p<0.01.
Survival versus good outcome
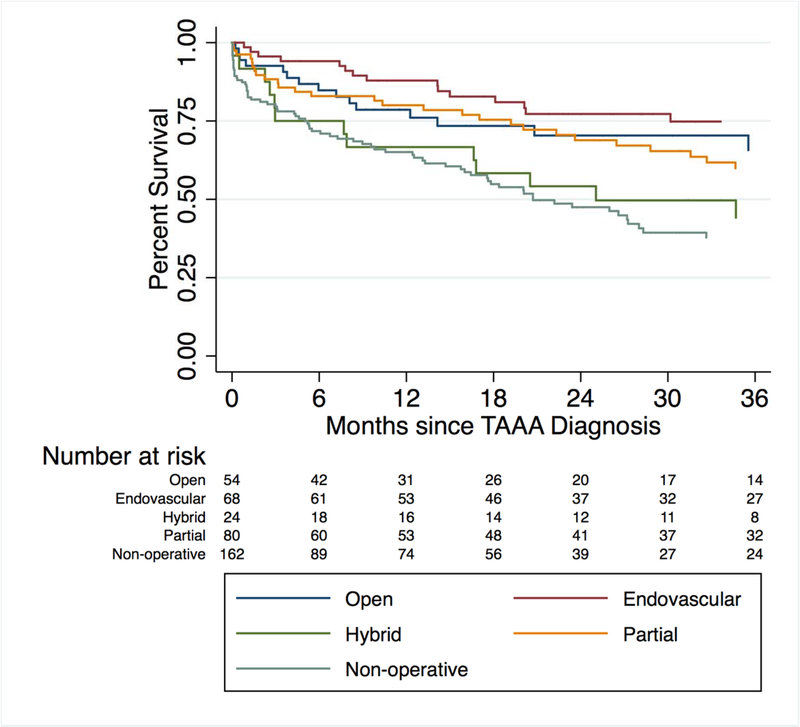
Kaplan-Meier estimated survival for all groups is shown in Figure 1. For nonoperative patients, 1-year survival and median survival were 65% and 21 months, respectively (Table II). Among operative patients, 1-year survival for the open, endovascular, hybrid, and partial repair groups was 79%, 88%, 67%, and 80%. Greater survival was observed in the open, endovascular and partial operative groups in comparison to the nonoperative group. A notable exception is the hybrid group, which did not have greater survival compared to the nonoperative group over a period of three years.
Figure 1.
Kaplan-Meier survival for all patients with TAAA
Table II.
Outcomes for all patients with TAAA
| Nonoperative | Open | Endovascular | Hybrid | Partial | All operative | P | |
|---|---|---|---|---|---|---|---|
| N | 205 | 54 | 68 | 24 | 81 | 227 | |
| Index hospitalization | |||||||
| Mortality | - | 16.7% | 4.0% | 17.0% | 9.0% | 10.1% | 0.080 |
| Permanent SCI | - | 18.5% | 11.8% | 4.2% | 4.9% | 10.1% | <0.001 |
| Permanent RF | - | 7.4% | 1.5% | 8.3% | 3.7% | 4.4% | <0.001 |
| Discharged Home | - | 55.0% | 75.0% | 54.0% | 70.0% | 66.0% | 0.033 |
| Follow-up | |||||||
| Median in months, IQR | 3 (0.1, 20) | 16 (7.2, 39) | 28 (14, 43) | 23 (5.3, 54) | 25 (5.8, 46) | 23.3 (8.5, 43.3) | <0.001 |
| Survival at one year | 65% | 79% | 88% | 67% | 80% | 81% | <0.001 |
| Good outcome (%) | - | 30/48 (62.5%) | 47/61 (77.0%) | 14/23 (60.9%) | 33/58 (56.9%) | 124/190 (65.3%) | 0.11 |
SCI, spinal cord injury; RF, renal failure necessitating dialysis
Rates of good outcome were significantly lower than rates of survival for all groups as reported in Table II. Overall, 81% of operative patients survived to one year, but only 68% of patients had a good outcome.
Differences based on presentation
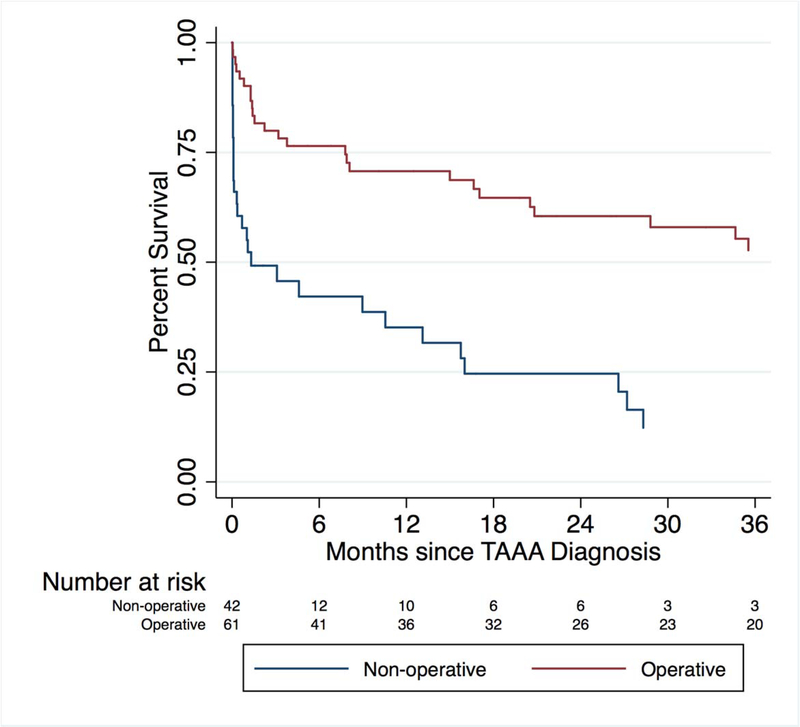
Interestingly, the clinical diagnosis of symptomatic aneurysm reflected poor prediction of impending rupture (Figure 2). Fifty percent of patients with what was clinically determined to be a symptomatic aneurysm who did not undergo an operation were alive three months after that event. Rates of perioperative mortality, major complication, survival, and good outcome were lower in both the symptomatic and especially the ruptured group in comparison to the asymptomatic group (Table III, Figure 2). Outcomes among symptomatic patients were inferior to those treated in an elective setting. (Table III) Among the 26 patients who presented to the hospital with rupture, only 38.5% survived to discharge and a “good” outcome was achieved in only 4/11 patients (36.4%) (Table III). Of note, this study does not include those patients who died prior to arrival, and as such, is an underestimate of the overall mortality following presentation with rupture.
Figure 2.
Kaplan-Meier survival for operative versus nonoperative patients presenting with symptomatic TAAA
Table III.
Outcomes for TAAA patients by presence of symptoms or rupture
| Nonoperative | Operative | ||||||
|---|---|---|---|---|---|---|---|
| Asymptomatic | Symptomatic | Ruptured | Asymptomatic | Symptomatic | Ruptured | P | |
| N | 152 | 31 | 13 | 165 | 49 | 13 | |
| Index hospitalization | |||||||
| Mortality | - | - | - | 7.3% | 12.2% | 38.5% | 0.005 |
| Permanent SCI | - | - | - | 8.5% | 18.4% | 0% | 0.081 |
| Permanent RF | - | - | - | 3.6% | 6.1% | 7.7% | 0.39 |
| Discharged home | - | - | - | 70.9% | 54.2% | 53.8% | 0.12 |
| Follow-up | |||||||
| Months, median (IQR) | 6.3 (0.2, 22.7) | 0.3 (0.1, 9.0) | 0.0 (0.0, 0.4) | 25 (10.5, 42.9) | 21 (5.2, 45) | 2.2 (0.3, 45) | 0.091 |
| 1-year survival | 75% | 39% | 11% | 84% | 76% | 47% | 0.176 |
| Good outcome (%) | - | - | 94/138 (68.1) | 26/41 (63.4) | 4/11 (36.4) | 0.10 | |
SCI, spinal cord injury; RF, renal failure necessitating dialysis
DISCUSSION
In this inclusive cohort of TAAA patients presenting to a regional tertiary referral center, half did not undergo an operative intervention despite access to a variety of treatment strategies. Many of these patients were deemed to be poor surgical candidates either due to advanced age with low functional reserve or due to the presence of one or more major comorbid conditions. Included in this group were a number of patients who declined operative repair after a thorough discussion of the peri-operative risk. Survival among this group was poor with a median survival of less than 2 years. Given the retrospective nature of this study, an accurate cause of death was not available for all patients. This raises the concern that a potentially high number of patients in the nonoperative group may have died from aneurysm rupture and may have stood to benefit from TAAA repair.
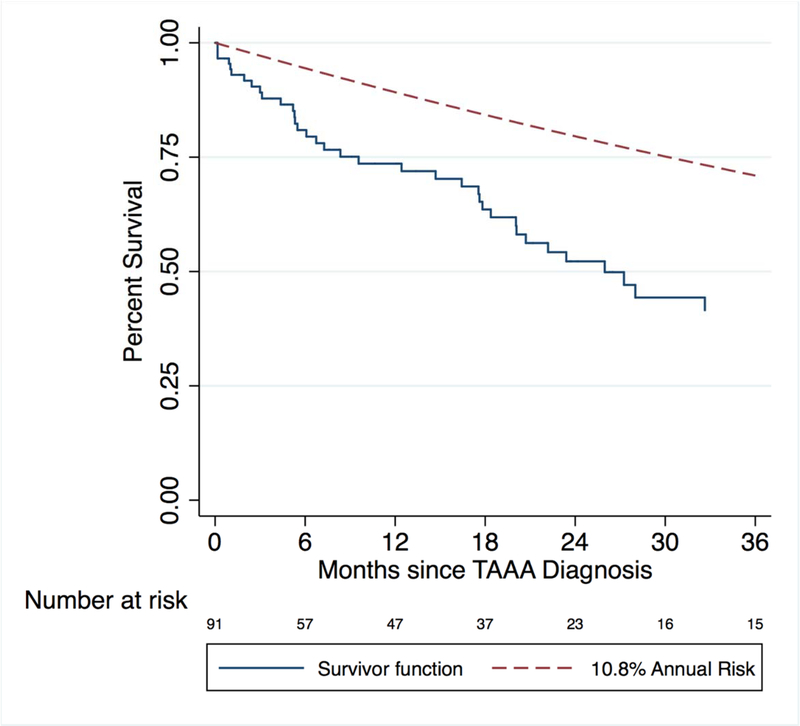
There are two main counterpoints to this argument. First, as many as half of patients deemed unfit for operation likely die due to nonaneurysm-related causes over a period of 1–2 years.14,2,3 In perhaps one of the largest studies comprised of 1600 patients, for example, Elefteriades et al. estimated an annual risk of death of 10.8% in patients with nondissected, atherosclerotic thoracic and thoracoabdominal aneurysms measuring 6 cm or greater.1 Figure 3 compares the observed Kaplan-Meier survival among the nondissected, atherosclerotic aneurysms in this study to that estimated by Elefteriades et al. The observed mortality rate in our study doubles that estimated by Elefteriades et al. at two years. In a series of 89 patients deemed unfit for surgery by the Scottish National Thoraco-abdominal aneurysm service, Hansen et al. reported a mortality rate of 55% over a median follow-up period of 12 months with more than half of the mortality due to causes other than aneurysm.2 Taken together, these findings suggest that about half of the mortality in the patient population deemed unfit for surgery is due to causes other than aneurysm rupture. Regardless of technique, TAAA repair is a high-risk operation that is resource intensive and prophylactic in most cases. Among patients with advanced comorbidities, such an operation, even if successful, would not improve medium-term survival due to these other competing medical issues. Second, it is likely that those deemed to be unfit for operation would have rates of survival and good outcome that would be lower than what is already observed among the “healthier” operative candidates in this study. Stated another way, attempted repair in a poor surgical candidate carries a higher risk of shortening lifespan and/or severely decreasing quality of life compared to that of a standard operative candidate. Patients who do not undergo surgery do not incur the risks of surgery and therefore retain their current quality of life. Nonoperation for some of these patients may therefore strike a better balance between preserving both quantity and quality of life.
Figure 3.
Comparison between observed survival and 10.8% annual risk of death reported by Elefteriades et al. in patients with asymptomatic, non-dissecting, atherosclerotic degenerative TAAA with maximal diameter > 6 cm (Elefteriades J. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann. Thorac. Surg.; 2002;74:S1877-S1880)
Among operative patients, a wide difference between survival and a good outcome was observed. In all operative groups, the percentage of patients alive at one year exceeded the percentage of patients with a good outcome by a difference of 13% (81% vs 68%, p<0.0016). Survival alone therefore underestimated the expected benefit gained from operative intervention, even with minimally invasive techniques. Permanent loss of independence at the expense of survival or freedom from major complications may be viewed by many patients as treatment failure and this should be taken into account during preoperative counseling.9
Baseline patient characteristics also differed widely between operative groups, which demonstrates that patients were selected for a particular operative approach. Thus, the population of patients selected for a particular operative approach represents a small fraction of the overall cohort. It is likely that the same selection bias is present in national centers of excellence that specialize in a particular surgical approach. In addition, the remarkable results achieved at these high-volume centers not only represent the experience of those institutions and aforementioned selection bias, but also some degree of referral bias as patients are screened to some degree prior to such referral centers.5,6,8,7
Two further comments regarding the operative groups deserve mention. First, a survival advantage was observed in all operative groups other than the hybrid approach, suggesting that repair does improve survival among appropriately selected patients. The hybrid group, however, had similar rates of survival to the nonoperative group despite the former being an overall younger cohort with fewer comorbidities. Assuming that survival equals a good outcome in patients managed nonoperatively, this would indicate that patients in the hybrid group had a lower rate of good outcome compared to patients that had no operation at all in the short to medium term. The French AURC multicenter retrospective study of 76 patients undergoing hybrid repair reported similarly poor outcomes including perioperative mortality of 34% and incidence of bowel ischemia at 17% in a relatively young cohort of patients (mean age 68).15 Although others have reported some degree of success with this approach16,17, hybrid repair at our institution has fallen out of favor due to poor overall outcomes.
The second comment concerns those patients undergoing partial repair, in particular the large difference between rates of survival and good outcome observed in this group. A relatively large proportion of these patients presented with symptoms or rupture for extensive aneurysms (Extent I or II) and dissection as the underlying etiology, and thus many of these patients were treated with thoracic endografts that that did not treat the paravisceral aorta. The high rate of survival in this group came as a surprise because TAAA treated in this manner are expected to eventually experience further aneurysmal degeneration distal to the graft and subsequent treatment failure. However, patients in this group had an 80% survival at one year, which was significantly greater than that observed in the nonoperative group. On the other hand, the percentage of patients with a good outcome differed widely from the percentage of patients who merely survived at one year (59% versus 80%). These findings warrant further investigation.
This retrospective observational study describes outcomes in all patients with TAAA presenting to a multi-hospital academic institution regardless of management strategy and includes those managed nonoperatively. In this geographic region, there are few other providers who routinely manage TAAA, and the patient screening strategy was not dependent on consultation with a surgeon but by both ICD and radiographic diagnosis, so this cohort represents a less selected group of patients with TAAA than is seen in many published series from very high-volume referral centers. In contrast, many operative series report outcomes in a highly select group of patients chosen for a particular method of repair and therefore offer little insight into the patients not chosen in such studies.18,5,19,7,16,17,20 In addition, the present study included only patients with pre-specified anatomic criteria confirmed by review of CT imaging; namely, aneurysmal degeneration of the thoracoabdominal aorta that traverses the paravisceral segment. In contrast, studies using administrative datasets rely solely on diagnosis codes to define TAAA, while others combine thoracoabdominal aortic aneurysms with aortic aneurysms in other anatomic distributions including within the ascending, transverse arch, isolated descending thoracic aorta.3,21,6,8 This study demonstrated that CT review identified TAAA in only 432 of 718 (60%) patients whose ICD codes indicated TAAA, demonstrating the poor reliability of ICD coding in administrative database studies evaluating TAAA. Finally, few studies—with the notable exception of Rectenwald, et al.—include functional outcomes after recovery from TAAA repair.4 As stated above, permanent loss of independence at the expense of survival may be viewed by many patients as treatment failure.9 Patient counseling during the decision-making process for or against surgery should therefore take into account the preservation of preoperative functional status after recovery from TAAA repair.
This study has several limitations. First, an accurate cause of death was not able to be obtained. Based on the burden of major comorbid disease and data from other studies, it is reasonable to infer that about half of nonsurgical candidates ultimately succumb to comorbid illness before aneurysm rupture.2,3 Second, the baseline characteristics in the various management groups differed from one another with respect to age, etiology, presence of symptoms or rupture, extent of aneurysm, and burden of comorbid disease—all of which have been shown to affect surgical outcomes.5,6,22 A number of variables that are also known to affect surgical outcomes were similarly omitted in this study, including individual surgeon volume and details regarding operative techniques.23 As such, caution must be used in attempting to compare outcomes by the different surgical approaches. Finally, the cohort of patients in this study represents some degree of referral bias despite the efforts made to minimize that effect.
CONCLUSIONS
Close to half of the patients in this inclusive cohort study did not undergo repair despite access to a variety of operative approaches, and many likely die due to a high burden of major comorbid disease rather than aneurysm rupture. Among patients undergoing operation, a wide difference was observed between survival and a patient centered outcome. Although appropriately selected patients appeared to have survival advantage following elective repair with open, endovascular and partial approaches, higher risk patients with TAAA appear unlikely to benefit from operative repair due to limited baseline survival and low probability of good outcome.
Acknowledgments
Funding sources: No outside sources of support, including pharmaceutical or industry support were received for this study
Footnotes
Conflicts of Interest: None
REFERENCES
- 1.Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg. 2002;74(5):S1877–80; discussion S1892–8. http://www.ncbi.nlm.nih.gov/pubmed/12440685. Accessed April 8, 2017. [DOI] [PubMed] [Google Scholar]
- 2.Hansen PA, Richards JMJ, Tambyraja AL, Khan LR, Chalmers RTA. Natural history of thoraco-abdominal aneurysm in high-risk patients. Eur J Vasc Endovasc Surg. 2010;39(3):266–270. doi: 10.1016/j.ejvs.2009.12.023 [DOI] [PubMed] [Google Scholar]
- 3.Kim JB, Kim K, Lindsay ME, et al. Risk of rupture or dissection in descending thoracic aortic aneurysm. Circulation. 2015;132(17):1620–1629. doi: 10.1161/CIRCULATIONAHA.114.015177 [DOI] [PubMed] [Google Scholar]
- 4.Rectenwald JE, Huber TS, Martin TD, et al. Functional outcome after thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2002;35(4):640–647. doi: 10.1067/mva.2002.119238 [DOI] [PubMed] [Google Scholar]
- 5.Coselli JS, LeMaire SA, Preventza O, et al. Outcomes of 3309 thoracoabdominal aortic aneurysm repairs. J Thorac Cardiovasc Surg. 2016;151(5):1323–1338. doi: 10.1016/j.jtcvs.2015.12.050 [DOI] [PubMed] [Google Scholar]
- 6.Acher C, Wynn M. Outcomes in open repair of the thoracic and thoracoabdominal aorta. J Vasc Surg. 2010;52(4):3S–9S. doi: 10.1016/J.JVS.2010.06.137 [DOI] [PubMed] [Google Scholar]
- 7.Oderich GS, Ribeiro M, Hofer J, et al. Prospective, nonrandomized study to evaluate endovascular repair of pararenal and thoracoabdominal aortic aneurysms using fenestrated-branched endografts based on supraceliac sealing zones. J Vasc Surg. 2017;65(5):1249–1259.e10. doi: 10.1016/j.jvs.2016.09.038 [DOI] [PubMed] [Google Scholar]
- 8.Greenberg RK, Lu Q, Roselli EE, et al. Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair: a comparison of endovascular and open techniques. Circulation. 2008;118(8):808–817. doi: 10.1161/CIRCULATIONAHA.108.769695 [DOI] [PubMed] [Google Scholar]
- 9.Schwarze ML, Taylor LJ. Managing Uncertainty — Harnessing the Power of Scenario Planning. N Engl J Med. 2017;377(3):206–208. doi: 10.1056/NEJMp1704149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sweet MP, Fillinger MF, Morrison TM, Abel D. The influence of gender and aortic aneurysm size on eligibility for endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2011;54(4):931–937. doi: 10.1016/j.jvs.2011.02.054 [DOI] [PubMed] [Google Scholar]
- 11.Howard DPJ, Marron CD, Sideso E, Puckridge PJ, Verhoeven ELG, Spark JI. Editor’s Choice – Influence of Proximal Aortic Neck Diameter on Durability of Aneurysm Sealing and Overall Survival in Patients Undergoing Endovascular Aneurysm Repair. Real World Data from the Gore Global Registry for Endovascular Aortic Treatment (GREAT. Eur J Vasc Endovasc Surg. 2018;56(2):189–199. doi: 10.1016/J.EJVS.2018.03.027 [DOI] [PubMed] [Google Scholar]
- 12.Schanzer A, Greenberg RK, Hevelone N, et al. Vascular Medicine Predictors of Abdominal Aortic Aneurysm Sac Enlargement After Endovascular Repair. 2011. doi: 10.1161/CIRCULATIONAHA.110.014902 [DOI] [PubMed] [Google Scholar]
- 13.Sweet MP, Starnes BW, Tatum B. Endovascular treatment of thoracoabdominal aortic aneurysm using physician-modified endografts. J Vasc Surg. 2015;62(5):1160–1167. doi: 10.1016/j.jvs.2015.05.036 [DOI] [PubMed] [Google Scholar]
- 14.Crawford ES, DeNatale RW. Thoracoabdominal aortic aneurysm: observations regarding the natural course of the disease. J Vasc Surg. 1986;3(4):578–582. http://www.ncbi.nlm.nih.gov/pubmed/3959256. Accessed March 5, 2018. [DOI] [PubMed] [Google Scholar]
- 15.Rosset E, Ben Ahmed S, Galvaing G, et al. Editor’s choice--hybrid treatment of thoracic, thoracoabdominal, and abdominal aortic aneurysms: a multicenter retrospective study. Eur J Vasc Endovasc Surg. 2014;47(5):470–478. doi: 10.1016/j.ejvs.2014.02.013 [DOI] [PubMed] [Google Scholar]
- 16.Quinones-Baldrich W, Jimenez JC, DeRubertis B, Moore WS. Combined endovascular and surgical approach (CESA) to thoracoabdominal aortic pathology: A 10-year experience. J Vasc Surg. 2009;49(5):1125–1134. doi: 10.1016/j.jvs.2008.12.003 [DOI] [PubMed] [Google Scholar]
- 17.Benrashid E, Wang H, Andersen ND, Keenan JE, McCann RL, Hughes GC. Complementary roles of open and hybrid approaches to thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2016;64(5):1228–1238. doi: 10.1016/j.jvs.2016.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crawford ES, Crawford JL, Safi HJ, et al. Thoracoabdominal aortic aneurysms: preoperative and intraoperative factors determining immediate and long-term results of operations in 605 patients. J Vasc Surg. 1986;3(3):389–404. http://www.ncbi.nlm.nih.gov/pubmed/3951025. Accessed March 5, 2018. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg R, Eagleton M, Mastracci T. Branched endografts for thoracoabdominal aneurysms. J Thorac Cardiovasc Surg. 2010;140(6):S171–S178. doi: 10.1016/j.jtcvs.2010.07.061 [DOI] [PubMed] [Google Scholar]
- 20.Schanzer A, Simons JP, Flahive J, et al. Outcomes of fenestrated and branched endovascular repair of complex abdominal and thoracoabdominal aortic aneurysms. J Vasc Surg. 2017;66(3):687–694. doi: 10.1016/j.jvs.2016.12.111 [DOI] [PubMed] [Google Scholar]
- 21.Rigberg DA, McGory ML, Zingmond DS, et al. Thirty-day mortality statistics underestimate the risk of repair of thoracoabdominal aortic aneurysms: a statewide experience. J Vasc Surg. 2006;43(2):217–22; discussion 223. doi: 10.1016/j.jvs.2005.10.070 [DOI] [PubMed] [Google Scholar]
- 22.O’Callaghan A, Mastracci TM, Eagleton MJ. Staged endovascular repair of thoracoabdominal aortic aneurysms limits incidence and severity of spinal cord ischemia. J Vasc Surg. 2015;61(2). doi: 10.1016/j.jvs.2014.09.011 [DOI] [PubMed] [Google Scholar]
- 23.Cowan JA, Dimick JB, Henke PK, Huber TS, Stanley JC, Upchurch GR. Surgical treatment of intact thoracoabdominal aortic aneurysms in the United States: hospital and surgeon volume-related outcomes. J Vasc Surg. 2003;37(6):1169–1174. http://www.ncbi.nlm.nih.gov/pubmed/12764260. Accessed November 29, 2016. [DOI] [PubMed] [Google Scholar]