Abstract
Drug-related mortality in the US grew dramatically in recent years, while mental health deteriorated among disadvantaged Americans and reported levels of pain increased over the same period. Here we investigate whether increased prevalence of drug misuse between the mid-1990s and early-2010s is associated with higher levels of mental distress and pain. Our results demonstrate higher drug misuse over this period, particularly for older and for socioeconomically disadvantaged Americans. After adjusting for sociodemographic characteristics, we estimate that the prevalence of drug misuse increased by 19 percentage points among those aged 50–76 in the bottom percentile of socioeconomic status (SES). Misuse increased much more at older than at younger ages for all drug types except sedatives, which increased to a similar degree in both age groups. Compared with measures of mental health, pain consistently accounted for a greater share of the period differential in drug misuse among both age groups and across all drug types. Misuse of prescription painkillers exhibited the largest difference in the contributions of pain versus mental health: among older individuals with the lowest SES, pain explained three times as much of the period trend as mental health (60% vs. 19%). Pain was more closely linked with the rise in misuse of prescription painkillers than other drugs. Mental health is a strong correlate of drug misuse (particularly sedative use), but growing drug misuse since the mid-1990s was more strongly linked with rising levels of reported pain than with deterioration in mental health. Pain could be a key factor underlying the association between trends in mental health and drug use: higher levels of pain may contribute to both mental distress and drug misuse. Given that pain, mental distress, and drug misuse are intertwined, successful intervention may require addressing all three factors.
Keywords: Drug misuse, non-medical drug use, mental health, psychological distress, pain, United States
INTRODUCTION
Mortality related to drug use, alcohol use, and suicide—known collectively as “deaths of despair” (Case, 2015; Khazan, 2015; Monnat, 2016)—has increased in the US since the late 1990s (Case & Deaton, 2017; Case & Deaton, 2015). Growth in drug-related mortality has been particularly dramatic: the age-adjusted rate increased by a factor of 3.6 between 1999 and 2017 (Hedegaard, Miniño, & Warner, 2018). Attention has focused on the opioid epidemic, but overdose rates involving psychostimulants with abuse potential (mostly methamphetamine) have risen faster (16-fold increase) since 1999 than rates for prescription opioids (nearly 4.3-fold increase) and heroin (7-fold increase) (National Center for Health Statistics, 2019). Overdose mortality from synthetic opioids (mostly fentanyl) grew the fastest (30-fold increase since 1999), accounting for 28,466 deaths in 2017, which represented 41% of all overdose deaths.
One explanation for the drug epidemic focuses on supply-side factors, including aggressive marketing of opioids by the pharmaceutical industry, changing norms toward prescribing opioids for chronic non-cancer pain, the expansion of insurance coverage of prescription opioids, and wider black market dissemination of heroin, fentanyl, and methamphetamine (Hadland, Cerda, Li, Krieger, & Marshall, 2018; Hadland, Rivera-Aguirre, Marshall, & Cerda, 2019; Mars et al., 2015; Masters, 2018; Ruhm, 2018; U.S. Department of Justice, Drug Enforcement Administration, 2018; Unick, Rosenblum, Mars, & Ciccarone, 2014; Van Zee, 2009). There is little doubt that increased availability and access to drugs have contributed to the problem. Nonetheless, some researchers have argued that the drug epidemic and wider mortality crisis may also stem from increased demand, resulting from a rising tide of despair especially among less-educated Americans (Case & Deaton, 2017; Case & Deaton, 2015; Cherlin, 2014; Cherlin, 2016; Dasgupta, Beletsky, & Ciccarone, 2018; Jalal et al., 2018; Stein, Gennuso, Ugboaja, & Remington, 2017).
Previous work suggests that mental health has deteriorated among Americans with low socioeconomic status since the mid-1990s (Goldman, Glei, & Weinstein, 2018). Co-morbidity between psychiatric disorders and substance abuse is common (National Institute on Drug Abuse, 2018a). According to the 2016 National Survey on Drug Use and Health, 43% of US adults aged 18 and older with a substance use disorder also had co-occurring mental illness (Substance Abuse and Mental Health Services Administration, 2017, see also Figure 68). Individuals with a serious mental illness were also much more likely to report a substance use disorder (25%) than those without serious mental illness (7%) (Substance Abuse and Mental Health Services Administration, 2017, see also Figure 69). While the cross-sectional association between mental health and substance abuse is well-established, here we test an underlying premise of the despair hypothesis: is growing drug misuse over recent decades related to deterioration in mental health among the US population?
The term “despair” has proliferated in media reports and academic literature since the Case and Deaton (2015) publication, but there is no agreed-upon definition. In our view, despair implies emotional distress, but a broader interpretation might include physical pain. For example, as evidence of “growing distress”, Case and Deaton (2015, p. 15078) cite not only rising levels of psychological distress but also concurrent increases in reported levels of pain along with declines in self-reported health and physical function. Whether you view pain as an indicator of “despair” or not, physical pain and psychological processes are intertwined. The biopsychosocial model of chronic pain posits that the experience of pain reflects a complex interaction of physiological, psychological, and social factors (Duenas, Ojeda, Salazar, Mico, & Failde, 2016; Gatchel, Peng, Peters, Fuchs, & Turk, 2007). Similarly, Garland et al. (2013) describes the feedback loop between chronic pain and emotions.
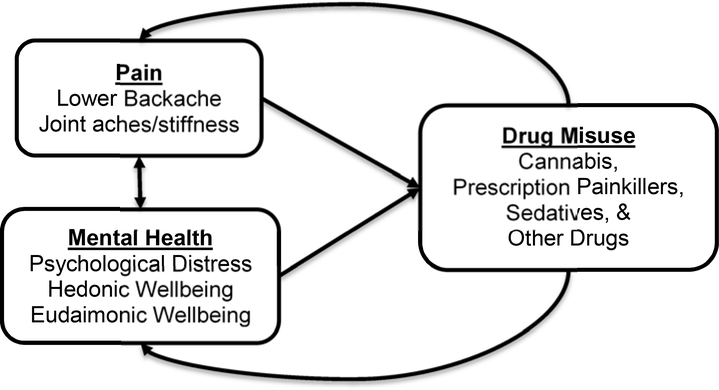
Drawing on the conceptual framework proposed by Garland et al. (2013), Figure 1 shows the bidirectional relationship between pain and mental health, the effects of those two variables on the risk of drug misuse, and the feedback effects of drug misuse on pain and mental health. Garland et al. (2013) suggest that recurrent pain initiates a downward spiral in which the individual’s attention becomes fixated on the pain, thus exacerbating the perception of pain and fueling negative affect. This maladaptive cognitive response to chronic pain is also referred to as “pain catastrophizing” (Institute of Medicine, 2011). Opioids may relieve not only physical pain but also associated mental distress, thereby reinforcing the drug habit even when it no longer provides pain relief because of increased tolerance (Garland et al., 2013). Some individuals with anxiety or depressive disorders may use drugs to relieve their psychiatric symptoms, which not only amplifies their risk of addiction, but can exacerbate the mental disorder over the long term (National Institute on Drug Abuse, 2018b); thus, Figure 1 shows a feedback loop from drug use and misuse to mental health. Prolonged opioid use also causes changes in the brain that increase sensitivity to pain and desensitize the dopamine system to naturally-rewarding experiences (Ballantyne & Mao, 2003; Volkow, Wang, Fowler, Tomasi, & Telang, 2011). All addictive drugs increase dopamine levels initially, which acts as positive reinforcement that encourages repeated use (Ross & Peselow, 2012). Drugs over-activate the basal ganglia, which is key to the brain’s “reward circuit”; with repeated use, this circuit becomes less sensitive making it difficult to gain pleasure from anything other than the drug (National Institute on Drug Abuse, 2018b).
Figure 1. Conceptual framework for the relationships among pain, mental health, and drug misuse.
We expect a bidirectional relationship between pain and mental health: pain negatively affects mental health, while psychological distress amplifies pain perception. In turn, pain and mental health are presumed to directly affect the risk of drug misuse. Yet, drug misuse can also have feedback effects on pain (by increasing pain sensitivity) and mental health (by desensitizing the dopamine system). Although not shown in this conceptual model, sociodemographic characteristics may act as potential confounders. Thus, our models control for sex, age, period, race/ethnicity, marital status, and SES, all of which may have direct effects of pain levels, mental health, and drug misuse.
In this paper, we use data from the Midlife Development in the US study (MIDUS) to investigate whether growing drug misuse is related to deterioration in mental health and increased levels of reported pain. We hypothesize that:
H1: The difference in drug misuse between the mid-1990s and early-2010s is associated with deterioration in mental health during the same period.
H2: The period difference in drug misuse (i.e., between the mid-1990s and early-2010s) is associated with higher levels of reported pain.
Whereas pain is likely to be a primary avenue through which a person initiates use of a prescription opioid, the pathway leading to use of other drugs is likely to be much more heterogeneous. Marijuana can be used to relieve pain, but is also used to treat a variety of other medical conditions (e.g., nausea/vomiting, multiple sclerosis, mental health disorders) as well as for recreational purposes. Similarly, people may use other drugs for reasons other than pain (e.g., sedatives or prescription anti-depressants for anxiety/depression; heroin, methamphetamine, and cocaine for recreational purposes). Nonetheless, pain may contribute indirectly to heroin use because individuals who become addicted to prescription opioids may later switch to heroin because it is cheaper and easier to obtain. Although prescription opioid use is a risk factor for heroin use, the progression from prescription opioids to heroin appears to be rare: less than four percent of those abusing prescription opioids started using heroin within five years (Muhuri, Gfroerer, & Davies, 2013). Thus, we pose the following additional hypothesis:
H3: Increases over time in pain are more strongly associated with the rise in misuse of prescription painkillers than other drugs.
One advantage of using MIDUS for this study is that the nationally-representative sample covers prime adult ages (25–74), including the middle-aged Americans who have been the focus of attention with respect to the US drug epidemic and related mortality crisis. Second, MIDUS includes repeated cross-sectional waves from 1994–95 (Period 1) and 2011–14 (Period 2) that allow us to identify period effects by comparing Americans of the same age range separated by nearly two decades. Finally, MIDUS offers a rich set of mental health measures, encompassing both psychological distress and well-being.
MATERIALS AND METHODS
Data
We use data from the two cross-sectional waves of MIDUS, each of which targeted a national probability sample of non-institutionalized, English-speaking adults aged 25–74 in the coterminous United States. In 1995–96, respondents were selected by random digit dialing with oversampling of older people and men ([dataset] Brim et al., 2016); 3487 respondents completed the phone interview (70% response rate, fielded January, 1995, through January, 1996) and 3034 also completed mail-in self-administered questionnaires (SAQ). In 2011–14, a new refresher cohort was sampled from the national population using a sampling frame that included both landlines and cell phones ([dataset] Ryff et al., 2016); 3577 individuals participated in the phone interview (59% response rate, fielded November, 2011, through May, 2014) and 2598 also completed the SAQ. Our analyses are restricted to respondents who completed the SAQ (pooled analysis sample: N=5632).
Measures
The SAQ asks the respondent if, during the past 12 months, s/he used various types of drugs and medications (i.e., sedatives, tranquilizers, amphetamines, prescription painkillers, inhalants, marijuana/hashish, cocaine/crack/free base, hallucinogens, heroin, prescription anti-depressants) “on your own”—that is, “without a doctor’s prescription, in larger amounts that prescribed, or for a long period than prescribed.” The most commonly used term for such drug usage is “misuse”, which we use throughout the remainder of the paper, but “nonmedical drug use” is sometimes used a synonym in the literature. The question about painkillers notes, “This does not include normal use of aspirin, Tylenol without codeine, etc., but does include use of Tylenol with codeine and other prescribed painkillers like Demerol, Darvon, and Percodan).” Because few respondents reported use of sedatives (<4%) or tranquilizers (<3%) and because the terms are often used as synonyms, we combine them into one group; hereafter, we refer to this category as “sedatives.” We refer to the category that includes marijuana and hashish as “cannabis,” the plant from which they are derived. Because very few respondents reported each of the remaining drug types (i.e., prescription anti-depressants, cocaine/crack/free base, amphetamines, heroin, hallucinogens, and inhalants), we combined them into a residual “other drugs” category.
Mental health
We include two measures of psychological distress (i.e., the Composite International Diagnostic Interview Short Form (CIDI-SF) major depression scale and the negative affect index) and two measures each of hedonic (i.e., the positive affect index, life satisfaction) and eudaimonic well-being (i.e., psychological well-being and social well-being indexes). See Supplementary Material for more details.
Pain
We include two measures related to pain. Respondents were asked how often they experienced lower backaches and aches/stiffness in joints during the past 30 days with responses coded on a six-point scale from “not at all” to “almost every day”.
Sociodemographic Control Variables
Our sociodemographic controls comprise age, sex, period, race/ethnicity (non-Latinx white vs. all else), whether the respondent is married/partnered, and a composite measure of relative socioeconomic status (SES). We first create an SES index based on education, occupation, income, and assets, which we then convert to a percentile rank representing the individual’s position within the distribution at that wave. For ease of interpretation, we rescale the SES variable to range from 0 (1st percentile) to 1 (99th percentile), such that a one-unit change denotes the difference between the bottom and top percentile of the SES continuum. See Supplementary Material for details.
Analytical Strategy
We addressed missing data using standard practices of multiple imputation (Rubin, 1996; Schafer, 1999). Among the pooled sample, variables with the most missing data were two components of the SES index: household income (18%) and assets (13%). The only other analysis variable for which more than 5% of the pooled sample was missing was social well-being (6.5%). We used multiple imputation to impute missing data on at least one analysis variable for 37% of the pooled sample. All of the variables used in this analysis as well as several auxiliary variables (e.g., measures of physical health, smoking status, alcohol abuse, employment status, measures of perceived economic distress) were included in the set of predictor variables for multiple imputation.
All analyses use post-stratification weights to ensure that the weighted samples show very similar distributions (in terms of age, sex, race, education and marital status) as the corresponding Current Population Survey. Trends in drug use (Substance Abuse and Mental Health Services Administration, 2017) and in overdose mortality (National Center for Health Statistics, 2019) differ by drug class, so we began by examining misuse by drug type (i.e., cannabis, prescription painkillers, sedatives, and all other drugs combined).
Prior work demonstrated that the increase in drug abuse between 1995–96 and 2011–14 was greater at older (50–76) than at younger (25–49) ages and was inversely associated with SES (Glei & Weinstein, 2019). Thus, we show plots of drug misuse at the two survey waves by relative SES and age group (< 50 vs. 50+) to demonstrate how age and SES interact with respect to the trends in drug misuse. For these plots, we performed local mean smoothing—also known as the Nadaraya-Watson estimator (Nadaraya, 1964; Watson, 1964)—using the lpolyci command in Stata 14.2 (StataCorp, 2015).
Next, we used logistic regression models to model the probability of reporting misuse of any drug and of each drug type. Model 1 included the sociodemographic controls. Based on the graphs demonstrating that changes in drug misuse varies by age and SES, we tested interactions between period and: 1) a dichotomous variable indicating that the respondent was aged 50 or older; and 2) the continuous measure of relative SES. The interaction between period and age group was significant for most outcomes—although it was only marginally significant for prescription painkillers and not significant for sedatives. The interaction between period and relative SES was significant for any drug use and cannabis, marginally significant for prescription painkillers and sedatives, and not significant for other drugs. We retained both of these interactions in all models for completeness. We also tested a two-way interaction between age group and relative SES as well as the three-way interaction between period, age group, and relative SES. Neither the three-way interaction nor the age by SES interaction was significant; thus, we excluded those interactions from the final models. Thus, Model 1 provides an estimate of the period effect (i.e., the difference in drug misuse between the mid-1990s and early-2010s) and how it varies by age group and relative SES.
In Models 2a, we adjust for mental health to determine the extent to which the period effect results from changes in those covariates at the population-level. If higher drug misuse resulted from deterioration in mental health (as predicted by H1), then we would expect the period effect to be substantially reduced after adjusting for mental health. Similarly, if increased drug misuse resulted from higher levels of pain (as predicted by H2), we would expect the period effect to be notably attenuated after adjusting for reported pain in Model 2b. Finally, in Model 3, we included measures of mental health and pain simultaneously.
In linear models, one can compare the period coefficient across models to determine the extent to which changes over time in specified covariates explain the period effect. In contrast, coefficients from nested nonlinear models are not comparable because of rescaling (i.e., the magnitude of the estimated coefficient depends on the error variance of the model, which depends on the selection of other covariates included in the model) (Karlson, Holm, & Breen, 2012; Kohler, Karlson, & Holm, 2011). The Karlson-Holm-Breen (KHB) method (Karlson et al., 2012) solves this problem by separating out the portion of the change in the coefficient that results from rescaling. To quantify the extent to which mental health and pain account for the period differential in drug misuse (i.e., indirect/mediating effect), we used the KHB method. We focus on those in the bottom percentile of SES to highlight this group, which prior literature has shown to be the most vulnerable (Case & Deaton, 2017; Case & Deaton, 2015).
To address H3, we compare the results from models by type of drug misuse to determine whether changes in pain level played a more important role in explaining the increase in misuse of prescription painkillers than other drugs. We expect pain to explain a much larger proportion of the period effect for prescription painkillers than it does for cannabis, sedatives, or other drugs.
The prevalence of drug misuse was generally low, and thus, the odds ratio was roughly similar to the relative risk ratio. However, misuse of any drug reached as high as 20% among the younger age group in the later wave. When we re-estimated the models using modified Poisson regression (Petersen & Deddens, 2008; Zou, 2004), the results remained similar.
RESULTS
The descriptive statistics presented in Table 1 demonstrate that the period differential in misuse of any drug was much larger among the older group (aged 50–76, 7.1% in 1995–96 vs. 17.2% in 2011–14) than the younger group (aged 20–49, 19.3% vs. 20.3%, respectively). Cannabis and sedatives were the only drug types for which the trend was in the same direction in both age groups, although the period differential for cannabis was much larger among older (0.1% in 1995–96 vs. 6.6% in 2011–14) than younger respondents (10.2% vs. 10.3%, respectively). For prescription painkillers and the residual category of all other drug types, misuse declined at younger ages, but increased at older ages.
Table 1.
Descriptive statistics for analysis variables by age group and survey wave (1995–96 versus 2011–14), weighted
| All Ages 20–76 | Aged 20–49 | Aged 50–76 | ||||
|---|---|---|---|---|---|---|
| 1995–96 | 2011–14 | 1995–96 | 2011–14 | 1995–96 | 2011–14 | |
| (N=3034) | (N=2598) | (N=1767) | (N=1104) | (N=1267) | (N=1494) | |
| Drug misuse | ||||||
| Any drug(s), % | 14.9 | 18.8 | 19.3 | 20.3 | 7.1 | 17.2 |
| Cannabis, % | 6.8 | 8.5 | 10.2 | 10.3 | 0.1 | 6.6 |
| Prescription painkillersa, % | 6.7 | 6.8 | 8.1 | 7.4 | 4.1 | 6.3 |
| Sedatives, % | 5.2 | 8.6 | 6.1 | 9.9 | 3.6 | 7.3 |
| Other drugsb, % | 3.6 | 4.2 | 5.0 | 4.7 | 0.1 | 3.7 |
| Control variables | ||||||
| Male, % | 47.7 | 48.0 | 48.6 | 49.2 | 46.0 | 46.7 |
| Age (20–76), mean (SD) | 45.5 (13.5) | 49.0 (13.6) | 36.9 (6.9) | 37.5 (6.9) | 60.8 (7.4) | 60.6 (7.3) |
| Non-Latinx white, % | 81.7 | 80.7 | 80.1 | 77.7 | 84.5 | 83.7 |
| Married or living with a partner, % | 72.2 | 69.1 | 72.7 | 68.0 | 71.2 | 70.1 |
| Relative SES (0–1), mean (SD) | 0.5 (0.3) | 0.5 (0.3) | 0.5 (0.3) | 0.5 (0.3) | 0.5 (0.3) | 0.5 (0.3) |
| Mental health | ||||||
| CIDI-SF major depression, mean (SD)c | 0.0 (1.0) | 0.0 (1.0) | 0.1 (1.1) | 0.1 (1.1) | −0.1 (0.8) | −0.1 (1.0) |
| Negative affect index, mean (SD)c | 0.0 (0.9) | 0.0 (1.1) | 0.0 (1.0) | 0.1 (1.1) | −0.1 (0.9) | −0.1 (1.0) |
| Positive affect index, mean (Sd)c | 0.0 (1.0) | 0.0 (1.0) | 0.0 (1.0) | −0.1 (1.1) | 0.2 (0.9) | 0.0 (1.0) |
| Life satisfaction, mean (SD)c | 0.0 (1.0) | 0.0 (1.0) | −0.1 (0.9) | −0.1 (1.0) | 0.2 (1.0) | 0.0 (1.0) |
| Psychological well-being index, mean (SD)c | 0.0 (1.0) | −0.1 (1.0) | 0.0 (1.0) | −0.1 (1.0) | 0.1 (1.0) | 0.0 (1.0) |
| Social well-being index, mean (SD)c | 0.0 (1.0) | 0.0 (1.0) | 0.0 (1.0) | 0.0 (1.0) | 0.0 (1.0) | 0.0 (1.0) |
| Pain | ||||||
| Lower backache, mean (SD)c | −0.1 (0.9) | 0.2 (1.0) | −0.1 (0.9) | 0.1 (1.0) | −0.2 (1.0) | 0.2 (1.1) |
| Not at all, % | 39.7 | 28.3 | 35.7 | 27.0 | 46.9 | 29.5 |
| Once a month, % | 22.8 | 20.2 | 27.0 | 22.4 | 15.3 | 18.0 |
| Several times a month, % | 14.9 | 16.3 | 14.8 | 15.8 | 15.0 | 16.8 |
| Once a week, % | 5.8 | 7.3 | 6.6 | 9.1 | 4.3 | 5.5 |
| Several times a week, % | 7.8 | 12.0 | 8.1 | 12.5 | 7.3 | 11.6 |
| Almost every day, % | 9.0 | 15.9 | 7.8 | 13.2 | 11.2 | 18.7 |
| Joint aches/stiffness, mean (SD)c | −0.2 (1.0) | 0.2 (1.0) | −0.4 (0.9) | 0.0 (1.0) | 0.1 (1.0) | 0.5 (0.9) |
| Not at all, % | 39.3 | 18.6 | 45.0 | 25.8 | 29.1 | 11.2 |
| Once a month, % | 13.8 | 15.0 | 15.2 | 18.5 | 11.3 | 11.5 |
| Several times a month, % | 17.2 | 17.7 | 16.3 | 17.0 | 18.9 | 18.4 |
| Once a week, % | 5.5 | 7.8 | 5.6 | 7.8 | 5.3 | 7.8 |
| Several times a week, % | 11.2 | 17.9 | 9.9 | 17.0 | 13.5 | 18.8 |
| Almost every day, % | 13.0 | 23.1 | 8.0 | 14.1 | 22.0 | 32.3 |
The question notes, “This does not include normal use of aspirin, Tylenol without codeine, etc., but does include use of Tylenol with codeine and other prescribed painkillers like Demerol, Darvon, and Percodan).”
For each of the following drug types, there are fewer than 10 users in some cells by survey wave and age group, therefore we have combined these drug types into one “other drugs” category: prescription anti-depressants, cocaine/crack/free base, amphetamines and other stimulants, heroin, hallucinogens, inhalants.
Standardized based on the weighted, pooled distribution.
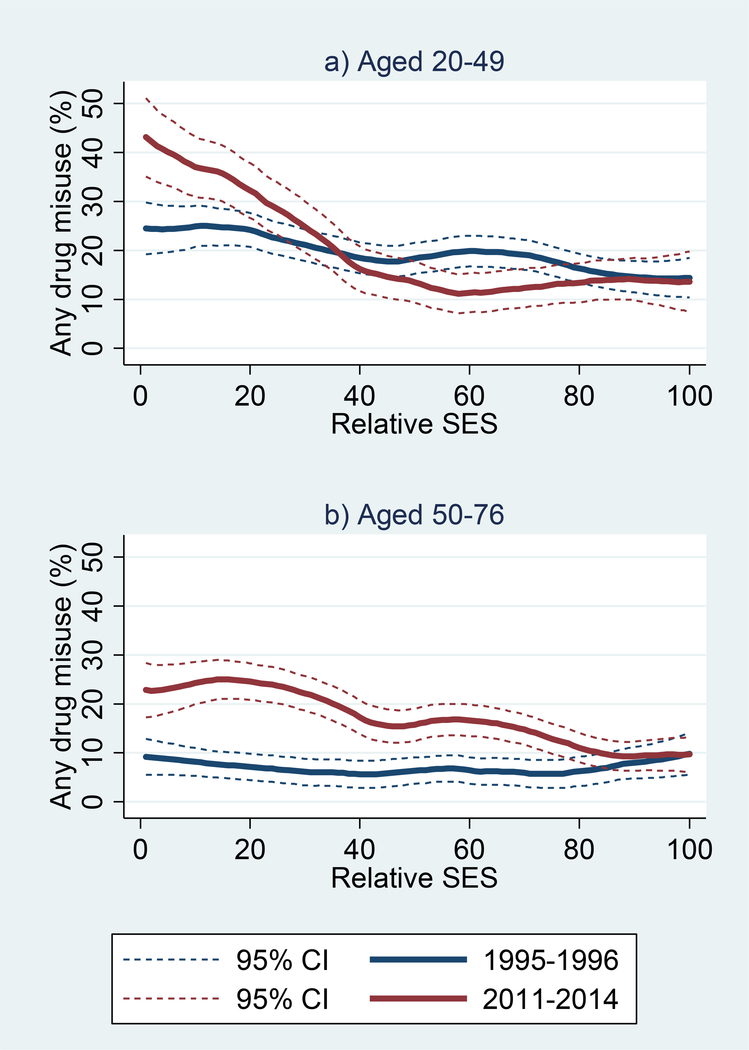
The period trend in drug misuse was much larger among those with low SES than their more advantaged counterparts (Figure 2). Among younger respondents, the difference was limited to those in the bottom quintile of SES, among whom the percentage reporting drug misuse was 24% in 1995–96 versus 38% by 2011–14. Among older respondents, the period difference was largest in the bottom quintile of SES (8% in 1995–96 vs. 22% in 2011–14), but prevalence of misuse was higher in 2011–14 than in 1995–96 up to the 80th percentile of SES.
Figure 2. Smoothed bivariate plots of drug misuse at 1995–96 and 2011–14 MIDUS waves by relative SES among respondents: a) aged 20–49; and b) aged 50–76.
Note: SES is based on education, occupation, income, and assets; values represent the respondents’ percentile rank within the distribution at the specified survey wave.
Similarly, levels of psychological distress (Figure S1) and hedonic well-being (Figure S2) deteriorated among those with low SES, whereas there was little difference or even improvement for those at the top of the SES spectrum. There was little change over time in levels of eudaimonic well-being (Figure S3).
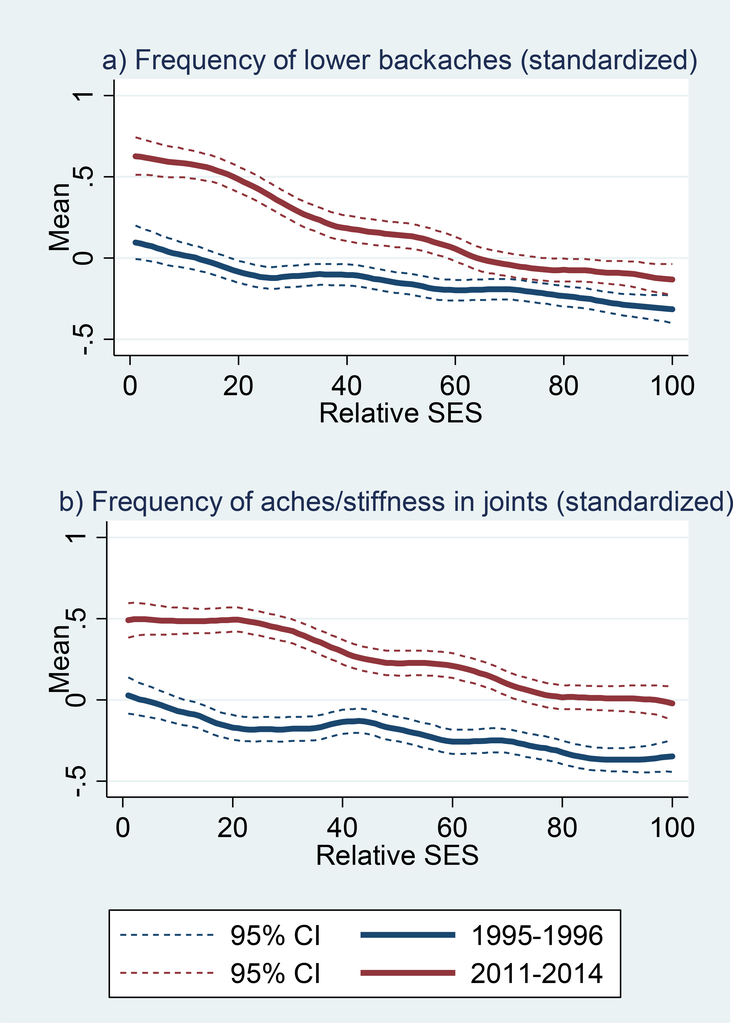
Overall reported levels of pain were notably higher in 2011–14 (Table 1). The percentage of respondents reporting any lower backache increased from 60% in 1995–96 to 72% in 2011–14, while the share reporting back pain “almost every day” nearly doubled (9% vs. 16%, respectively). The period difference in joint pain was even greater: the percentage reporting any aches/stiffness in their joints climbed from 61% in 1995–96 to 81% in 2011–14, while joint pain almost every day rose from 13% to 23%, respectively. Yet again, the period difference was larger at lower levels of SES (Figure 3).
Figure 3. Smoothed bivariate plots of pain measures by relative SES at 1995–96 and 2011–14 MIDUS waves: a) backache and b) joint ache.
Note: SES is based on education, occupation, income, and assets; values represent the respondents’ percentile rank within the distribution at the specified survey wave.
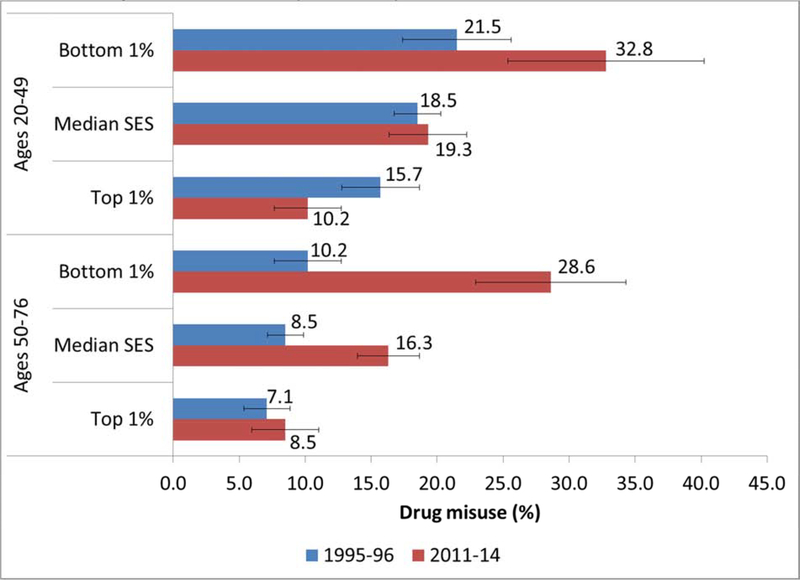
Table S1 presents results from the regression models for any drug misuse. Model 1 confirmed that the period difference varied by age and SES even after controlling for other sociodemographic characteristics. Among older respondents (aged 50–76), the period effect for drug misuse was much greater for those in the bottom percentile of SES (OR=3.6, p<0.001) than for those in the top percentile of SES (OR=3.61*0.34=1.2, p~0.36). In contrast, among younger respondents (aged 20–49), the period effect for those in the bottom percentile of SES was smaller (OR=1.8, p<0.01) than it was for the older group, while drug misuse was lower in the later period for younger respondents in the top percentile of SES (OR=1.81*0.34=0.6, p<0.01). Figure 4 shows these differences in terms of the predicted prevalence of drug misuse. Net of demographic controls, the biggest increase in drug misuse occurred among older adults with the lowest SES (from 10% in 1995–96 to 29% in 2011–14). The biggest decrease was in younger adults with the highest SES (from 16% in 1995–96 to 10% in 2011–14).
Figure 4. Estimated prevalence of drug misuse by survey wave, age group, and SES (bottom, median, and top percentile).
Note: SES is based on education, occupation, income, and assets and converted to a percentile rank denoting an individual’s position respondents’ percentile rank within the distribution at the specified survey wave. Predicted scores are computed using the coefficients from Model 1 (Table S1) for the two subpopulations (ages 20–49 and 50–76) and setting relative SES at the specified values (i.e., bottom and top percentile), while all other covariates (sex, race/ethnicity, and marital status) remain at the observed values among the pooled sample. Similar estimates could be shown for any level of SES. Error bars indicate the 95% confidence interval for each estimate.
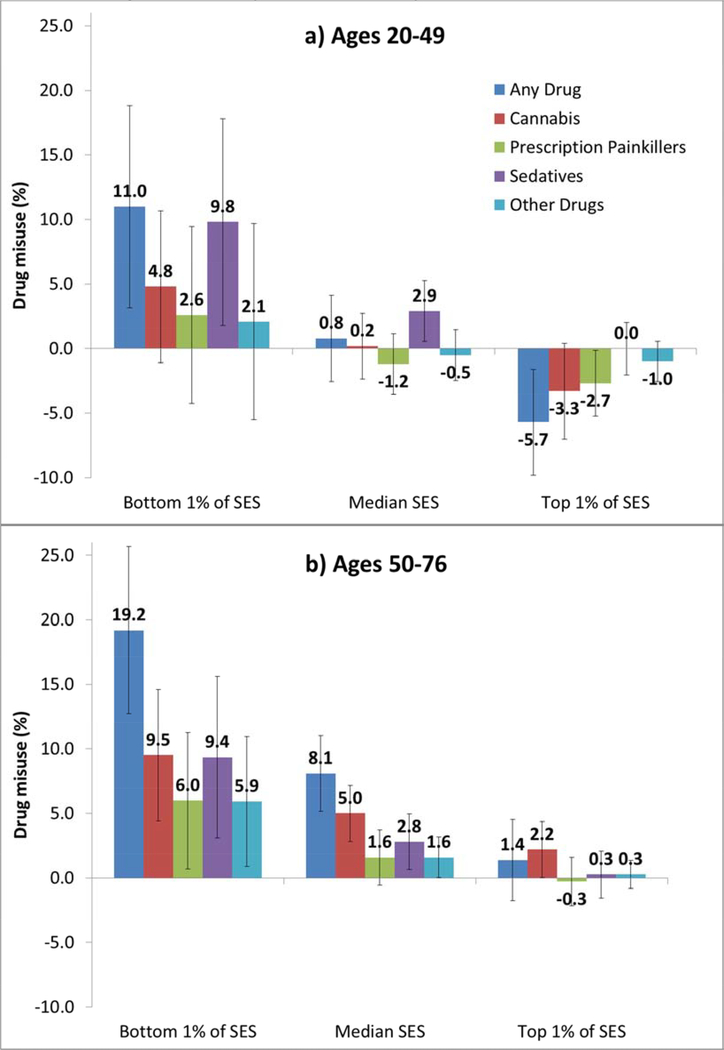
Models of misuse by drug type (Tables S2 and S3, Model 1) revealed that the age difference in the period trend was driven primarily by cannabis. Both age groups exhibited a similar period increase in misuse of sedatives (Table S3), as demonstrated graphically in Figure 5. In contrast, misuse of cannabis increased much more among older than younger individuals (Table S2). Misuse of prescription painkillers also appears to have increased faster at older ages, but the difference by age group was only marginally significant. The period increase in misuse of other drugs was also significantly greater among older than younger individuals (Table S3).
Figure 5. Period differential (2011–14 minus 1995–96) in estimated prevalence of drug misuse by type of drug, age group, and SES (bottom, median, and top percentile).
Note: SES is based on education, occupation, income, and assets and converted to a percentile rank denoting an individual’s position respondents’ percentile rank within the distribution at the specified survey wave. Values represent the average marginal effect of period based on Model 1 (Tables 2, S1, and S2) for the two subpopulations (ages 20–49 and 50–76) and setting relative SES at the specified values (i.e., bottom and top percentile), while all other covariates (sex, race/ethnicity, and marital status) remain at the observed values among the pooled sample. These period differentials represent the full range of SES, but one could show the corresponding period effect for any level of SES. Error bars indicate the 95% confidence interval for each estimate.
To test H1, we adjusted for mental health in Model 2a. Negative affect exhibited the strongest association with drug misuse (Table S1), but major depression and positive affect were also significant. The other measures of well-being were not significant. Overall, mental health was strongly associated with drug misuse—as indicated by a substantial improvement in variance explained (higher pseudo R2), model fit (lower BIC), and predictive ability (higher AUC) between Model 1 and Model 2a. The key question of interest, however, was whether the increase over time in drug misuse was linked with changes in mental health. We estimated that mental health accounted for 25% of the period differential in drug misuse among younger respondents in the bottom percentile of SES and 13% among their older counterparts, providing only partial support for H1 (Table 2). For those at the 50th percentile of SES (among whom the period effect was much smaller; see Figure 5), mental health explained an even smaller share of the period effect (8% for those aged 50–76; Table S4). Negative and positive affect accounted for most of the reduction in the period effects. Results were similar for misuse of prescription painkillers, sedatives, and other drugs (Tables S1 and S2, Model 2a). In the case of cannabis use, however, mental health accounted a much smaller share of the period effect (among older respondents) and the biggest contributor was life satisfaction followed by major depression (Tables 2 and S4).
Table 2.
Among those in the bottom percentile of SES, percent of the period effect for drug misuse explaineda by mental health and pain measures, by age group and drug type
| Age Group: | Aged 20–49b |
Aged 50–76 |
|||||
|---|---|---|---|---|---|---|---|
| Drug Type: | Any Drug | Sedatives | Any Drug | Cannabis | Prescription Painkillers | Sedatives | Other Drugs |
| Drug misuse | |||||||
| Among those in the bottom 1% of SES, percent of period effect explained by: | |||||||
| Mental health measures (Model 2a) | 25.0 | 21.9 | 13.2 | 7.2 | 18.7 | 20.9 | 18.9 |
| CIDI-SF major depression | 2.8 | 1.6 | 2.4 | 2.0 | 4.3 | 2.7 | 3.0 |
| Negative affect | 14.0 | 15.3 | 6.0 | 1.2 | 9.6 | 12.6 | 9.6 |
| Positive affect | 6.1 | 5.4 | 3.0 | 1.7 | 4.7 | 5.2 | −0.7 |
| Life satisfaction | 4.6 | 2.1 | 2.7 | 3.2 | 1.6 | 2.3 | 7.3 |
| Psychological well-being | −2.5 | −2.4 | −1.0 | −1.4 | 5.8 | −1.7 | 0.3 |
| Social well-being | 3.9 | −0.1 | 0.0 | 0.5 | 6.1 | −0.2 | −0.5 |
| Pain measures (Model 2b) | 45.5 | 35.2 | 24.9 | 14.1 | 59.8 | 37.5 | 35.8 |
| Lower backache | 22.2 | 13.5 | 11.8 | 8.6 | 26.5 | 13.8 | 10.4 |
| Joint aches/stiffness | 23.3 | 21.7 | 13.2 | 5.5 | 33.3 | 23.7 | 25.4 |
| Both mental health and pain (Model 3) | 50.7 | 38.8 | 26.8 | 15.6 | 62.5 | 37.9 | 40.4 |
| CIDI-SF major depression | 2.5 | 1.4 | 3.9 | 1.8 | 3.4 | 2.4 | 2.5 |
| Negative affect | 11.8 | 14.3 | 4.8 | 0.6 | 6.6 | 11.6 | 8.5 |
| Positive affect | 5.1 | 4.9 | 3.0 | 1.3 | 3.0 | 4.6 | −1.5 |
| Life satisfaction | 4.6 | 2.1 | 1.5 | 3.1 | −0.1 | 2.2 | 7.4 |
| Psychological well-being | −2.5 | −2.4 | 0.4 | −1.4 | 0.1 | −1.7 | 0.2 |
| Social well-being | 0.0 | −0.2 | 0.2 | 0.5 | −0.1 | −0.2 | −0.5 |
| Lower backache | 14.9 | 5.6 | 9.6 | 6.2 | 22.2 | 5.4 | 4.8 |
| Joint aches/stiffness | 14.3 | 13.1 | 3.4 | 3.5 | 27.4 | 13.6 | 19.0 |
The percent explained by mental health and by pain measures is calculated using the Karlson-Holm-Breen (KHB) method (Karlson et al., 2012) with the Stata user-written program “khb” (Kohler et al., 2011).
We do not compute the percent explained in cases where the period effect was not significant (i.e., misuse of cannabis, prescription painkillers and other drugs among those aged 20–49).
Model 2b tests H2 by substituting measures of pain in place of the mental health measures. Both pain measures were significantly associated with misuse of any drug (Table S1). More importantly, adjustment for pain levels explained a larger share of the period trend than adjustment for mental health (Tables 2 and S4). Thus, we find more support for H2 than for H1. Lower backaches and joint pain were both important contributors. Results were similar by drug type, although as predicted by H3, pain explained more of the period differential in misuse of prescription painkillers (60%) than it did for cannabis (14%), sedatives (38%), or other drugs (36%) among older respondents in the bottom percentile of SES (Table 2).[ Although sedative use was more strongly associated with mental health measures than with pain—as indicated by a bigger improvement between Model 1 and 2b in variance explained and predictive ability—changes over time in pain levels accounted for a larger share of the period increase in sedative use than deterioration in mental health (Tables 2 and S4).
Model 3 included both mental health and pain. Adjusted for the other covariates, negative affect, major depression, lower backache, and joint pain remained significantly associated with drug misuse (Table S1). Yet again, we are less interested in the cross-sectional associations between mental health, pain, and drug abuse than in the question of whether there is a link between period trends in mental health, pain, and drug use. Compared with pain alone, we found that the combination of pain and mental health accounted for only a slightly larger share of the period differences in misuse among those with the lowest SES across all drug types (Table 2). Among the individual measures of mental health and pain, the biggest contributors to the period differential in misuse of prescription painkillers for older respondents with low SES were joint pain (27%) and lower backache (22%), while negative affect (7%), major depression (3%), and positive affect (3%) played smaller roles. Negative affect, however, explained a larger share of growing misuse of sedatives in both age groups.
DISCUSSION
Contrary to H1, we found that deterioration in mental health accounted for only a small part of the growth in drug misuse between the mid-1990s and early 2010s. Measures of hedonic well-being (i.e., life satisfaction and positive affect) explained little of the period differential in drug misuse even though an earlier study demonstrated they were among the mental health measures that exhibited the greatest deterioration over this period among disadvantaged Americans (Goldman et al., 2018). Unsurprisingly, mental distress was strongly correlated with drug misuse, but growing psychological distress, particularly negative affect, accounted for only a small share of the rise in drug misuse. Furthermore, the direction of causation is unclear. Is drug misuse a response to psychological distress or is mental distress a consequence of drug use?
Our evidence is more consistent with the idea that rising drug misuse over the past couple decades relates to higher prevalence of pain (H2). The 2016 National Survey on Drug Use and Health showed that 62% of those who reported misusing prescription painkillers cited the main reason for misuse was to “relieve physical pain” (Substance Abuse and Mental Health Services Administration, 2017, Figure 33); some of the other reasons for misuse related to psychological health, but they were less frequently cited as the main reason: “relax or relieve tension” (11%), “help with feelings or emotions” (4%), and “help with sleep” (3%). We found that misuse of prescription painkillers exhibited the largest differential between the contributions of pain versus mental health: among older individuals with the lowest SES, pain explained three times as much of the period trend as mental health (60% vs. 19%). Furthermore, as predicted by H3, pain was more closely linked with the rise in misuse of prescription painkillers than other drugs.
We found dramatic increases since the mid-1990s in the reported prevalence of joint and back pain, which suggest that demand-side explanations may be at least partially responsible for the increase in drug misuse. Other recent nationally representative studies have identified secular increases in the prevalence of painful conditions, including both mild/moderate and severe (Zimmer & Zajacova, 2018), which may have increased demand for pain management. However, increased availability of drugs and more aggressive marketing of opioids to providers and to patients through direct-to-consumer advertising likely also contributed to addiction. Recent work showed that county-level opioid marketing by pharmaceutical companies was associated with county-level opioid prescribing rates and mortality from prescription opioid overdose (Hadland et al., 2018; Hadland et al., 2019). It is also possible that the demand and supply side factors acted synergistically to increase drug abuse. Consistent with this possibility, Van Zee (2009) documents the use of prescriber profiling data by Purdue Pharmaceutical to target OxyContin to physicians who were the highest prescribers of opioids and those with the highest volume of chronic-pain patients, which coincided with areas of rural Appalachia that became early epicenters of the opioid epidemic.
The finding that increased pain may contribute to growing drug misuse raises questions about the underlying causes of pain trends. A report by the Institute of Medicine (2011) attributes the rise of chronic pain to five factors: population aging, growing obesity, increased survivorship after catastrophic injury or serious illness, more outpatient surgeries which may result in inadequate management of acute postsurgical pain, and greater patient expectations for pain relief. However, increases in pain have been observed across all adult age groups (Institute of Medicine, 2011) suggesting that population aging cannot fully account for trends.
A link between growing obesity and upward trends in pain seems plausible. Data from the NHANES indicate that obesity rates more than doubled between 1976–1980 and 2015–2016; nearly 40% of U.S. adults are currently obese (Hales, Fryar, Carroll, Freedman. D.S., & Ogden, 2018; Ogden & Carroll, 2010). Obesity increases risk of developing a variety of painful conditions (e.g., osteoarthritis, lower back pain) by two mechanisms: 1) biomechanical effects on the joints and spine; and 2) inflammation (Koonce & Bravman, 2013; Shiri, Karppinen, Leino-Arjas, Solovieva, & Viikari-Juntura, 2010; Urban & Little, 2018). The extent to which obesity has contributed to the trends in pain and opioid use represent an important direction for future research.
Limitations
A key limitation of this paper is the inability to infer causal direction. Pain, emotional distress, and drug abuse are likely to be intertwined in a complicated, multi-directional relationship (Garland et al., 2013). It is difficult to establish a causal relationship between mental health and substance abuse because the relationship bi-directional and because mental illness and substance use disorders share many of the same risk factors, such as genetic vulnerability and exposure to stressors (National Institute on Drug Abuse, 2018a). Similarly, our data do not allow us to determine whether increases in pain precipitated drug misuse or whether drug misuse may have heightened reports of pain (e.g., respondents attempting to rationalize their use of painkillers or increased sensitivity to pain as a result of prolonged use of opioids). Disentangling the causal effects may be difficult, if not impossible.
Other limitations of this study include limited pain measures, possible under-reporting of drug misuse, and the availability of only two cross-sectional survey waves. MIDUS does not include a global measure of pain, nor do the questions identify the duration and severity of pain or the extent to which pain limits normal activities. While MIDUS does not capture all types of pain, prior literature suggests that lower back and joint pain are likely to be the most prevalent types of pain at the population level (Johannes, Le, Zhou, Johnston, & Dworkin, 2010). Nonetheless, given the crudeness of our pain measures, we suspect that our analysis may understate the extent to which drug misuse is linked with pain. Self-reported drug misuse almost certainly under-estimates true prevalence. Reporting accuracy may also vary across sub-groups, which could bias our results. Finally, with only two cross-sectional waves, we can only evaluate period differences between the mid-1990s and early-2010s in drug misuse, mental health, and pain. If we had more frequent cross-sectional survey waves throughout this period, we could better delineate the period trend in drug misuse and its association with corresponding trends in mental health and pain levels.
Implications
The problem of drug misuse cannot be solved by addressing mental health, pain, or drug availability in isolation. Although pain appears to play a bigger role than mental health in explaining the rise in drug misuse, the reciprocal relationship between pain and emotional distress suggests that successful intervention may require dealing with both factors. Attempts to cut off the drug supply may be futile if no attempt is made to treat those already addicted and address the pain and psychological distress that may contribute to and/or be amplified by drug misuse. As Dasgupta et al. note (2018, p. 182): “simplistic measures to cut access to opioids offer illusory solutions to this multidimensional societal challenge.” Evans et al. (2018) found that the introduction of abuse-deterrent formulation of OxyContin in 2010 simply increased mortality from heroin by a commensurate amount.
Drug users are already switching from prescription opioids to heroin—which is cheaper and easier to obtain—and synthetic opioids—which are much more potent and especially difficult to regulate. Overdose rates involving prescription opioids nearly quadrupled between 1999 and 2010, but since then, rates have risen faster for synthetic opioids (9-fold increase), heroin (5-fold), and psychostimulants (5-fold) while overdose rates from prescription opioids without other synthetic narcotics declined by 20% (National Center for Health Statistics, 2019). In 2017, 60% of all opioid overdose resulted from synthetic opioids (other than methadone) and another 16% from heroin (without synthetic narcotics) (National Center for Health Statistics, 2019).
CONCLUSION
Drug misuse was higher in the early 2010s than in the mid-1990s, particularly for older and for socioeconomically disadvantaged individuals. After adjusting for sociodemographic characteristics, we estimate that that prevalence of drug misuse increased by 19 percentage points among older persons aged 50–76 in the bottom 1% of SES. With the exception of sedatives—for which misuse increased to a similar degree in both ages groups—misuse of other drugs increased much more at older than at younger ages.
Pain measures consistently accounted for more of the period differential in drug misuse than mental health in both age groups and across all drug types. Some might interpret these results as casting doubt on the despair hypothesis, unless one considers physical pain a manifestation of despair. In any case, we find that deteriorating mental health explains only a small part of growing drug misuse. Instead, rising drug misuse—particularly among disadvantaged, older Americans—appears to be more strongly linked with increased levels of reported pain. Pain could be a key factor underlying the association between deteriorating mental health and growing drug use: higher levels of pain may contribute to both mental distress and drug misuse.
Supplementary Material
HIGHLIGHTS.
Drug misuse increased since 1995–96 for those with lower socioeconomic status.
Except for sedatives, misuse increased more at older than at younger ages.
Mental health accounted for only a small part of the increase in misuse.
Pain explained most of the period trend in misuse of prescription painkillers.
Pain contributed more than mental health to the rise in misuse of all drug types.
ACKNOWLEDGMENTS
This work was supported by the National Institute on Aging [grant numbers P01 AG020166, U19AG051426] and the Graduate School of Arts and Sciences, Georgetown University.
Footnotes
Declarations of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ballantyne JC, & Mao J (2003). Opioid therapy for chronic pain. The New England Journal of Medicine, 349(20), 1943–1953. doi: 10.1056/NEJMra025411 [doi] [DOI] [PubMed] [Google Scholar]
- Brim OG, et al. (2016). National Survey of Midlife Development in the United States (MIDUS), 1995–1996. ICPSR02760-v11. Retrieved December 20, 2016, from 10.3886/ICPSR02760.v11 [DOI]
- Case A (2015). ‘Deaths of despair’ are killing America’s white working class. Retrieved December 11, 2017, from https://qz.com/583595/deaths-of-despair-are-killing-americas-white-working-class/
- Case A, & Deaton A (2017). Mortality and morbidity in the 21st century. Brookings Papers on Economic Activity, (Spring), 397–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case A, & Deaton A (2015). Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proceedings of the National Academy of Sciences of the United States of America, 112(49), 15078–15083. doi: 10.1073/pnas.1518393112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherlin AJ (2014). Labor’s love lost: the rise and fall of the working class family in America. New York: Russell Sage. [Google Scholar]
- Cherlin AJ (2016, February 22, 2016). Why are white death rates rising? New York: Times, pp. A19. [Google Scholar]
- Dasgupta N, Beletsky L, & Ciccarone D (2018). Opioid Crisis: No Easy Fix to Its Social and Economic Determinants. American Journal of Public Health, 108(2), 182–186. 458 doi: 10.2105/AJPH.2017.304187 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duenas M, Ojeda B, Salazar A, Mico JA, & Failde I (2016). A review of chronic pain impact on patients, their social environment and the health care system. Journal of Pain Research, 9, 457–467. doi: 10.2147/JPR.S105892 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans WN, Lieber E, & Power P (2018). How the reformulation of oxycontin ignited the heroin epidemicWorking Paper 24475)National Bureau of Economic Research. doi: 10.3386/w24188 [DOI] [Google Scholar]
- Garland EL, Froeliger B, Zeidan F, Partin K, & Howard MO (2013). The downward spiral of chronic pain, prescription opioid misuse, and addiction: cognitive, affective, and neuropsychopharmacologic pathways. Neuroscience and Biobehavioral Reviews, 37(10 Pt 2), 2597–2607. doi: 10.1016/j.neubiorev.2013.08.006 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatchel RJ, Peng YB, Peters ML, Fuchs PN, & Turk DC (2007). The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychological Bulletin, 133(4), 581–624. doi:2007-09203-002 [pii] [DOI] [PubMed] [Google Scholar]
- Glei DA, & Weinstein M (2019). Drug and Alcohol Abuse: the Role of Economic Insecurity. American Journal of Health Behavior, 43(4), 838–853. doi: 10.5993/AJHB.43.4.16 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman N, Glei DA, & Weinstein M (2018). Declining mental health among disadvantaged Americans. Proceedings of the National Academy of Sciences of the United States of America, 115(28), 7290–7295. doi: 10.1073/pnas.1722023115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadland SE, Cerda M, Li Y, Krieger MS, & Marshall BDL (2018). Association of Pharmaceutical Industry Marketing of Opioid Products to Physicians With Subsequent Opioid Prescribing. JAMA Internal Medicine, 178(6), 861–863. doi: 10.1001/jamainternmed.2018.1999 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadland SE, Rivera-Aguirre A, Marshall BDL, & Cerda M (2019). Association of Pharmaceutical Industry Marketing of Opioid Products With Mortality From Opioid-Related Overdoses. JAMA Network Open, 2(1), e186007. doi: 10.1001/jamanetworkopen.2018.6007 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CM, Fryar CD, Carroll MD, Freedman DS, & Ogden CL (2018). Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007–2008 to 2015–2016. Jama, 319(16), 1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard H, Miniño AM, & Warner M (2018). Drug overdose deaths in the United States, 1999–20187No. 329). Hyattsville, MD: National Center for Health Statistics. [Google Scholar]
- Institute of Medicine. (2011). Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Jalal H, Buchanich JM, Roberts MS, Balmert LC, Zhang K, & Burke DS (2018). Changing dynamics of the drug overdose epidemic in the United States from 1979 through 2016. Science (New York, N.Y.), 361(6408), eaau1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes CB, Le TK, Zhou X, Johnston JA, & Dworkin RH (2010). The prevalence of chronic pain in United States adults: results of an Internet-based survey. The Journal of Pain, 11(11), 1230–1239. [DOI] [PubMed] [Google Scholar]
- Karlson KB, Holm A, & Breen R (2012). Comparing regression coefficients between same-sample nested models using logit and probit: A new method. Sociological Methodology, 42(1), 286–313. [Google Scholar]
- Khazan O (2015). Middle-aged white Americans are dying of despair. Retrieved December 11, 2017, from https://www.theatlantic.com/health/archive/2015/11/boomers-deaths-pnas/413971/
- Kohler U, Karlson KB, & Holm A (2011). Comparing coefficients of nested nonlinear probability models. The Stata Journal, 11(3), 420–438. [Google Scholar]
- Koonce RC, & Bravman JT (2013). Obesity and osteoarthritis: more than just wear and tear. The Journal of the American Academy of Orthopaedic Surgeons, 21(3), 161–169. doi: 10.5435/JAAOS-21-03-161 [doi] [DOI] [PubMed] [Google Scholar]
- Mars SG, Fessel JN, Bourgois P, Montero F, Karandinos G, & Ciccarone D (2015). Heroin-related overdose: The unexplored influences of markets, marketing and source-types in the United States. Social Science & Medicine (1982), 140, 44–53. doi: 10.1016/j.socscimed.2015.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters RK (2018). Explaining recent mortality trends among younger and middle-aged white Americans. International Journal of Epidemiology, 47(1), 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnat SM (2016). Deaths of despair and support for Trump in the 2016 Presidential Election. State College, PA: Pennsylvania State University. [Google Scholar]
- Muhuri PK, Gfroerer JC, & Davies MC (2013). Associations of nonmedical pain reliever use and initiation of heroin use in the United States CBHSQ Data Review. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. [Google Scholar]
- Nadaraya EA (1964). On estimate regression. Theory of Probability and its Application, 9, 141–142. [Google Scholar]
- National Center for Health Statistics. (2019). National drug overdose deaths, 1999–2017. Tabulations of data from CDC Wonder, Multiple Cause of Death (Detailed Mortality). Retrieved July 10, 2019, from https://www.drugabuse.gov/sites/default/files/overdose_data_1999-2017_0.xls
- National Institute on Drug Abuse. (2018a). Common comorbilities with substance use disorders. Retrieved 03/20, 2018, from https://www.drugabuse.gov/publications/research-reports/common-comorbidities-substance-use-disorders/introduction
- National Institute on Drug Abuse. (2018b). Drugs, Brains, and Behavior: The Science of AddictionNIH Publication No. 18-DA-5605). Bethesda, MD: U.S. Department of Health and Human Services. [Google Scholar]
- Ogden CL, & Carroll MD (2010). Prevalence of overweight, obesity, and extreme obesity among adults: United States, trends 1976–1980 through 2007–2008, Health E-Stats. Hyattsville, MD: National Center for Health Statistics. [Google Scholar]
- Petersen MR, & Deddens JA (2008). A comparison of two methods for estimating prevalence ratios. BMC Medical Research Methodology, 8, 9–2288-8–9. doi: 10.1186/1471-2288-8-9 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S, & Peselow E (2012). Co-occurring psychotic and addictive disorders: neurobiology and diagnosis. Clinical Neuropharmacology, 35(5), 235–243. doi: 10.1097/WNF.0b013e318261e193 [doi] [DOI] [PubMed] [Google Scholar]
- Rubin DB (1996). Multiple imputation after 18+ years (with discussion). Journal of the American Statistical Association, 91, 473–489. [Google Scholar]
- Ruhm CJ (2018). Deaths of despair or drug problems? National Bureau of Economic Research Working Paper Series, Working Paper 24188). Cambridge, MA: National Bureau of Economic Research. doi: 10.3386/w24188 [DOI] [Google Scholar]
- Ryff C, et al. (2016). Midlife in the United States (MIDUS Refresher), 2011–2014. ICPSR36532-v2. Retrieved September 12, 2016, from 10.3886/ICPSR36532.v2 [DOI] [Google Scholar]
- Schafer JL (1999). Multiple imputation: a primer. Statistical Methods in Medical Research, 8(1), 3–15. [DOI] [PubMed] [Google Scholar]
- Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, & Viikari-Juntura E (2010). The association between obesity and low back pain: a meta-analysis. American Journal of Epidemiology, 171(2), 135–154. doi: 10.1093/aje/kwp356 [doi] [DOI] [PubMed] [Google Scholar]
- StataCorp. (2015). Stata: Release 14 Statistical Software. College Station, TX: StataCorp LP. [Google Scholar]
- Stein EM, Gennuso KP, Ugboaja DC, & Remington PL (2017). The epidemic of despair among white Americans: trends in the leading causes of premature death, 1999–2015. American Journal of Public Health, 107(10), 1541–1547. doi: 10.2105/AJPH.2017.303941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2017). Key substance use and mental health indicators in the United States: Results from the 2016 National Survey on Drug Use and Health. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. [Google Scholar]
- U.S. Department of Justice, Drug Enforcement Administration. (2018). 2018 National Drug Threat Assessment. Washington, DC: U.S. Department of Justice. [Google Scholar]
- Unick G, Rosenblum D, Mars S, & Ciccarone D (2014). The relationship between US heroin market dynamics and heroin-related overdose, 1992–2008. Addiction (Abingdon, England), 109(11), 1889–1898. doi: 10.1111/add.12664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban H, & Little CB (2018). The role of fat and inflammation in the pathogenesis and management of osteoarthritis. Rheumatology (Oxford, England), 57(suppl_4), iv10–iv21. doi: 10.1093/rheumatology/kex399 [doi] [DOI] [PubMed] [Google Scholar]
- Van Zee A (2009). The promotion and marketing of oxycontin: commercial triumph, public health tragedy. American Journal of Public Health, 99(2), 221–227. doi: 10.2105/AJPH.2007.131714 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, & Telang F (2011). Addiction: beyond dopamine reward circuitry. Proceedings of the National Academy of Sciences of the United States of America, 108(37), 15037–15042. doi: 10.1073/pnas.1010654108 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson G (1964). Smooth regression analysis. Sankhya Series A, 26, 359–372. [Google Scholar]
- Zimmer Z, & Zajacova A (2018). Persistent, Consistent, and Extensive: The Trend of Increasing Pain Prevalence in Older Americans. The Journals of Gerontology.Series B, Psychological Sciences and Social Sciences, doi: 10.1093/geronb/gbx162 [doi] [DOI] [PubMed] [Google Scholar]
- Zou G (2004). A modified poisson regression approach to prospective studies with binary data. American Journal of Epidemiology, 159(7), 702–706. doi: 10.1093/aje/kwh090 [doi] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.