To the Editor:
The composition of the intestinal microbiota has been linked to the risk for developing allergic disease. Although the mechanism by which the microbiota prevents allergic sensitization is unclear, evidence suggests that it is through modulation of the immune system.1 In this study we investigated the ability of the spore-forming gram-positive bacterium Bacillus subtilis to prevent the development of allergic disease. Previous work from our lab established B. subtilis as an immune suppressive bacterium that can prevent diarrheal disease from Citrobacter rodentium infection.2, 3
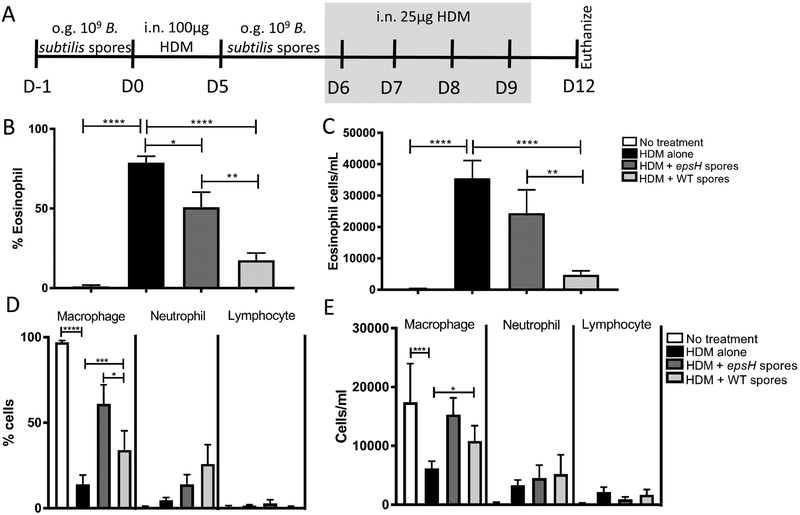
In this study we tested the ability of B. subtilis spores to prevent house dust mite (HDM)-induced eosinophilic inflammation using the treatment protocol shown in Figure 1A, that was approved by the Midwestern University Institutional Animal Care and Use Committee. C57Bl/6 mice were orally gavaged with 109 WT B. subtilis spores or 109 epsH spores that are unable to produce exopolysaccharide (EPS), due to a mutation in the eps operon4. Our previous work found that EPS was the component of B. subtilis required for protection from C. rodentium induced inflammation4, 5. Following the final HDM treatment, bronchial alveolar lavage (BAL) was collected from euthanized mice, by flushing the lungs with 0.8mL PBS containing 10% FCS, 1mM EDTA) and immune cell infiltration was assessed by DiffQuick (Dade Behring) staining of cytospun cells (Figure 1 B–E). HDM from Dermatophagoides pteronyssinus (XPB82D3A2.5, Stallergenes Greer) alone induces a significant eosinophilia, shown as both percent and total cells/mL (Figure 1B–C). WT B. subtilis spore treatment significantly reduced the eosinophilia in HDM-treated mice. The B. subtilis-mediated protection was partially dependent on the production of EPS, evidenced by epsH B. subtilis spores being unable to significantly reduce the HDM-induced eosinophilia.
Figure 1.
Prevention of HDM-induced eosinophilia by Bacillus subtilis.(A) Timeline for B. subtilis and HDM treatment of 6 week-old C57Bl/6 mice. (B-E) Quantification of cells from bronchial alveolar lavage (BAL) cytospun and stained with DiffQuick on Day 12. Data represent mean ± SEM (n = 7–14) from 3 combined experiments. **** p < 0.0001, *** p < 0.0005 ** p < 0.005, * p < 0.05 by Student t test. o.g. = oral gavage; i.n. = intranasal.
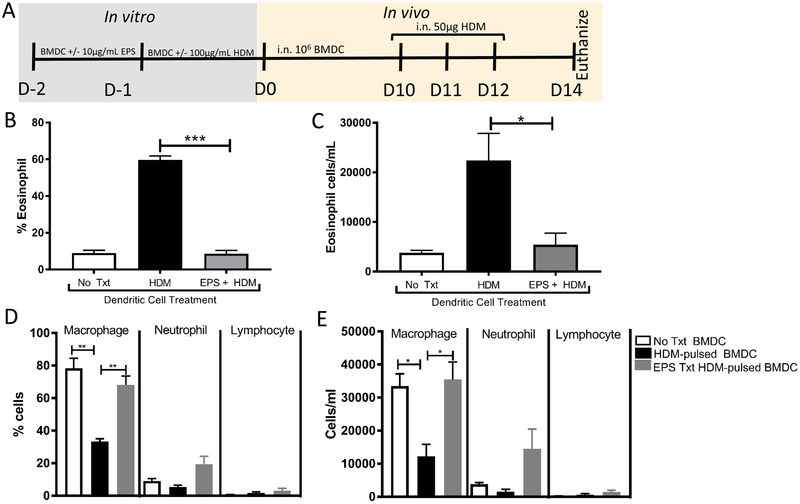
To understand the mechanism of B. subtilis suppression of eosinophilia, we tested the effect of EPS treatment on dendritic cells. Dendritic cells are critical in the development of eosinophilia and are responsible for activating T cells. Bone marrow derived dendritic cells (BMDC) were pre-treated with or without EPS for 24 hours, followed by the presence or absence of HDM for 24 hours, and transferred intranasally into naïve C57Bl/6 mice (according to protocol timeline, Figure 2A). EPS was isolated according to a previously published protocol by Jones et al. The purified EPS had undetectable nucleic acid (O.D.260) and protein (O.D.280). Quantification of carbohydrate content was determined by phenol-sulfuric acid method. Mice were then challenged intranasally with HDM, daily for 3 days. Dendritic cells treated with EPS in the absence of HDM failed to elicit eosinophilia (data not shown). We found that while HDM-pulsed dendritic cells elicit eosinophilia, HDM-pulsed dendritic cells treated with EPS failed to induce eosinophilia, shown as both percent and total cells/mL (Figure 2B–C). Other infiltrating immune cells including neutrophils and lymphocytes were not significantly different between the transferred dendritic cell treatment groups, while the resident macrophage population remained significantly higher in both no treatment and EPS treatment groups (Figure 2D–E). These results suggest that B. subtilis EPS-treated BMDC can prevent the induction of allergic eosinophilia.
Figure 2.
Prevention of eosinophilia by EPS-treated dendritic cells.(A) Timeline for BMDC incubation with exopolysaccharide and HDM prior to i.n. transfer into 7–10 week-old C57Bl/6 mice. BMDC were generated from C57Bl/6 mouse bone marrow in the presence of 20ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) for 6 days to >85% CD11c+ purity. Dendritic cell transfer groups included 1) No Treatment (No Txt) with BMDC receiving neither EPS nor HDM 2) HDM group with BMDC receiving HDM alone on D-2 and 3) EPS + HDM group of BMDC receiving EPS on D-2, followed by HDM on D-1. (B-E) Quantification of cells from BAL cytospun and stained with DiffQuick on Day 14. Data represent mean ± SEM (n = 3). *** p < 0.0005, ** p < 0.005, * p < 0.05 by Student t test. i.n.= intranasal. These data are representative of 4 independent BMDC transfer experiments.
The results of these experiments support the hypothesis that bacteria can help educate the immune system to prevent allergic disease. Specifically, we show that B. subtilis-derived EPS can suppress dendritic cell function to elicit allergic eosinophilia. Future work is required to understand how EPS-treated dendritic cells inhibit eosinophilia. We suggest that EPS-treated dendritic cells impair Th2 cell polarization and subsequent IL-5 production, which is needed for activation of eosinophils. Probiotics have been previously identified as methods for reducing allergic eosinophilia,6 however our work is unique in the fact that we required only two treatments with B. subtilis to prevent allergic disease and this was in the presence of a normal microbiota. Additional work will be focused on elucidating other, currently unknown, molecules or metabolites, that contribute to B. subtilis-mediated protection that is EPS-independent. In conclusion, we have established B. subtilis as a novel bacterium for the treatment of allergic disease and we show B. subtilis-derived EPS is involved in this protection.
Acknowledgments
This research was funded by the Midwestern University One Health Research Award (A.J.), Midwestern University startup support (J.A.S) and by NIH AI110586 (K.L.K.)
Footnotes
Disclosure of potential conflict of interest: All authors declare that they have no relevant conflicts of interest.
Contributor Information
Julie A Swartzendruber, Email: jswart@midwestern.edu.
Ryan W Incrocci, Email: rincro@midwestern.edu.
Samantha A Wolf, Email: swolf87@midwestern.edu.
Ariee Jung, Email: ajung54@midwestern.edu.
Katherine L Knight, Email: kknight@luc.edu.
REFERENCES
- 1.Hooper LV, Littman DR & Macpherson AJ Interactions between the microbiota and the immune system. Science (New York, N.Y.) 336, 1268–1273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones SE & Knight KL Bacillus subtilis-Mediated Protection from Citrobacter rodentium-Associated Enteric Disease Requires espH and Functional Flagella. Infection and Immunity 80, 710–719 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Arienzo R et al. Bacillus subtilis spores reduce susceptibility to Citrobacter rodentium-mediated enteropathy in a mouse model. Research in Microbiology 157, 891–897 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Jones SE, Paynich ML, Kearns DB & Knight KL Protection from Intestinal Inflammation by Bacterial Exopolysaccharides. The Journal of Immunology 192, 4813–4820 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paynich ML, Jones-Burrage SE & Knight KL Exopolysaccharide from Bacillus subtilis Induces Anti-Inflammatory M2 Macrophages That Prevent T Cell-Mediated Disease. Journal of immunology (Baltimore, Md. : 1950) 198, 2689–2698 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang YJ et al. The microbiome in allergic disease: Current understanding and future opportunities-2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. The Journal of allergy and clinical immunology 139, 1099–1110 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]