Abstract
Non-canonical sentence comprehension impairments are well-documented in aphasia. Studies of neurotypical controls indicate that prosody can aid comprehension by facilitating attention towards critical pitch inflections and phrase boundaries. However, no studies have examined how prosody may engage specific cognitive and neural resources during non-canonical sentence comprehension in persons with left hemisphere damage. Experiment 1 examines the relationship between comprehension of non-canonical sentences spoken with typical and atypical prosody and several cognitive measures in 25 persons with chronic left hemisphere stroke and 20 matched controls. Experiment 2 explores the neural resources critical for non-canonical sentence comprehension with each prosody type using region-of-interest-based multiple regressions. Lower orienting attention abilities and greater inferior frontal and parietal damage predicted lower comprehension, but only for sentences with typical prosody. Our results suggest that typical sentence prosody may engage attention resources to support non-canonical sentence comprehension, and this relationship may be disrupted following left hemisphere stroke.
Keywords: aphasia, prosody, attention, working memory, cognition, sentence comprehension, angular gyrus, Broca’s area
1. Introduction
Sentence comprehension impairments are prevalent in individuals with aphasia and are well-studied, yet the cognitive and neural mechanisms behind these impairments continue to be debated (Caplan, Michaud, & Hufford, 2013; Dronkers, Wilkins, Van Valin, Redfern, & Jaeger, 2004; Hartwigsen & Saur, 2017; Love, Swinney, Walenski, & Zurif, 2008; Magnusdottir et al., 2013; Minkina, Rosenberg, Kalinyak-Fliszar, & Martin, 2017; Oers et al., 2018; Pettigrew & Hillis, 2014; Potagas, Kasselimis, & Evdokimidis, 2011; Rogalsky et al., 2018; Villard & Kiran, 2017). Nonetheless, it is well-accepted that individuals with left hemisphere damage, both with and without aphasia, but particularly with Broca’s or conduction aphasia, have more difficulty comprehending sentences with non-canonical structure (e.g., subject-object-verb word order) compared to those with canonical structure (e.g., subject-verb-object word order; (Bradley, Garrett, & Zurif, 1980; Caramazza & Zurif, 1976). It has been proposed that the non-canonical structure of the sentence brings with it an additional processing load, as its non-linear structure requires active manipulation of clauses to assign thematic roles. This active manipulation has been shown to engage additional cognitive resources such as verbal short-term memory (maintenance of information; Pettigrew & Hillis, 2014; Potagas et al., 2011), verbal working memory (maintenance plus manipulation of information; Caspari, Parkinson, LaPointe, & Katz, 1998; Crosson et al., 1999; Gordon, Hendrick, & Levine, 2002; Just & Carpenter, 1992; King & Just, 1991; Newman, Malaia, Seo, & Cheng, 2013; Rogalsky, Matchin, & Hickok, 2008), attentional or cognitive control (adaptable responses necessary for goal directed behaviors; Alexander, 2006; January, Trueswell, & Thompson-Schill, 2009; LaCroix, Blumenstein, Houlihan, & Rogalsky, 2019; Murray, Holland, & Beeson, 1997; Novick, Kan, Trueswell, & Thompson-Schill, 2009; Novick, Trueswell, & Thompson-Schill, 2005; Villard & Kiran, 2017), and/or language-specific sentence processing resources (Caplan, Chen, & Waters, 2008; Caplan et al., 2013; Fedorenko, Duncan, & Kanwisher, 2012; Goucha & Friederici, 2015; Santi & Grodzinsky, 2007). Thus, it has been suggested that non-canonical sentence comprehension deficits in aphasia arise at least in part because of deficits (e.g., reduced capacities) in these cognitive resources, particularly verbal working memory: individuals with and without aphasia who have reduced verbal working memory capacities demonstrate poorer non-canonical sentence comprehension abilities, but canonical sentence comprehension is largely unaffected1 (e.g., Just & Carpenter, 1992; King & Just, 1991; Newman et al., 2013; Rogalsky et al., 2008). However, working memory is not an isolated process and attention towards specific pieces of information has been shown to be critical for encoding information into working memory (Baddeley, 2010; Cowan et al., 2005). This leads us to suggest that attention may be an important yet understudied dimension of non-canonical sentence comprehension in individuals with aphasia. This suggestion is supported by previous work finding that deficits in attention have been related to poorer language abilities in individuals with aphasia (Murray, 2012; Murray et al., 1997; Villard & Kiran, 2017), and evidence from computational modelling work that indicates that the cognitive contributions to non-canonical sentence comprehension in aphasia are multidimensional (Mätzig, Vasishth, Engelmann, Caplan, & Burchert, 2018; Patil, Hanne, Burchert, De Bleser, & Vasishth, 2016).
In parallel to this work, there is a body of literature in neurotypical participants and individuals with aphasia investigating the effects of typical sentence prosody (i.e., naturally occurring pitch and rhythm modulations in a spoken language that contribute to or convey sentence meaning) on syntactically ambiguous sentence comprehension. This work largely finds that typical sentence prosody yields faster and more accurate sentence comprehension performance than atypical prosodic patterns as it allows for the more efficient use of specific cognitive resources (Carlson, 2009; Kjelgaard & Speer, 1999; Perkins, Baran, & Gandour, 1996; Roncaglia-Denissen, Schmidt-Kassow, & Kotz, 2013; Sheppard, Love, Midgley, Holcomb, & Shapiro, 2017; Speer, Kjelgaard, & Dobroth, 1996). For example, pitch inflections have been shown to focus listener attention on important words and/or clauses and prosodic boundaries to divide sentences into smaller units of information (Frazier, Carlson, & Clifton Jr., 2006; Fromont, Soto-Faraco, & Biau, 2017; Schafer, 1996; Schafer, 1997). This division of sentences into smaller units of information may improve comprehension by decreasing cognitive demands such that more resources can be devoted towards manipulating clauses within non-canonical sentences during thematic role assignment. The idea that prosody can facilitate attention towards critical elements of a sentence may be particularly important for non-canonical sentences due to the additional verbal working memory resources they are known to recruit. Thus, we hypothesize that attention to prosodic cues (i.e., specific points in time within a sentence that provide pitch or rhythm information to convey meaning or facilitate comprehension), such as pitch inflections and prosodic boundaries, may increase the potential for the effective encoding of phrases and clauses into working memory, subsequently reducing the need for reanalysis.
In Experiment 1, we will examine how non-canonical sentence comprehension may be affected by prosody (by comparing typical sentence prosody and a list prosody control that lacks meaningful pitch inflections and prosodic boundaries), and how comprehension of sentences with each prosody type may be predicted by attentional control abilities in left hemisphere stroke survivors and matched-controls. While the focus of this study is on the relationship between prosody and attention, verbal working memory was additionally assessed as several studies identify a relationship between verbal working memory and sentence comprehension deficits (e.g., Caspari et al., 1998; Just & Carpenter, 1992; Newman et al., 2013); although we hypothesize that attention is at least, in part, accounting for this relationship (Baddeley, 2010; Cowan et al., 2005). The relationship between prosody and cognition is specifically explored within the context of non-canonical sentence comprehension because almost all individuals with aphasia have deficits in the comprehension of complex, non-canonical sentences, and individuals with Broca’s and conduction aphasia often exhibit relatively specific non-canonical sentence comprehension deficits (Bradley et al., 1980; Caramazza & Zurif, 1976). It was hypothesized that attentional control abilities will predict comprehension of non-canonical sentences spoken with sentence prosody while no relationship would be observed between attention and comprehension of non-canonical sentences spoken with list prosody as list prosody does not contain meaningful pitch inflections and prosodic boundaries previously shown to engage attention resources.
In addition to it being unclear what cognitive resources are engaged by prosody during sentence comprehension, it is also unclear what areas of damage affect prosody-related sentence comprehension performance. In other words, does a particular lesion location or pattern affect the use of prosody during sentence comprehension? Functional MRI (fMRI) studies and metaanalyses in neurotypical adults have identified reliable areas of activation sensitive to sentence prosody in bilateral left inferior and middle frontal gyri, bilateral posterior superior temporal gyrus, and bilateral inferior parietal cortex (including supramarginal gyrus and angular gyrus; Belyk & Brown, 2014; den Ouden, Dickey, Anderson, & Christianson, 2016; Fedorenko, Hsieh, & Balewski, 2015; Humphries, Love, Swinney, & Hickok, 2005; van der Burght, Goucha, Friederici, Kreitewolf, & Hartwigsen, 2019; Wildgruber et al., 2004). It is noteworthy that these regions of activation in response to prosodic manipulations in control subjects overlap with areas identified to be critical for auditory comprehension of non-canonical sentences spoken with typical sentence prosody (Dronkers et al., 2004; Magnusdottir et al., 2013; Pillay, Binder, Humphries, Gross, & Book, 2017; Rogalsky et al., 2018; Thothathiri, Kim, Trueswell, & Thompson-Schill, 2012). Furthermore, the inferior and middle frontal regions often implicated in non-canonical sentence comprehension also overlap with regions known to be engaged by attentional control (January et al. 2009), and verbal working memory is known to reliably engage inferior frontal and inferior parietal/posterior superior temporal regions (Buchsbaum et al., 2011). However, to our knowledge, no studies have examined the neural bases of receptive sentence prosody in sentence comprehension in individuals with left hemisphere strokes. Thus, in Experiment 2, we will identify the neural resources that support comprehension of sentences spoken with typical sentence prosody (and our list prosody control) in individuals with chronic left hemisphere strokes using region of interest-based multiple regression analyses (Caplan, Michaud, Hufford, & Makris, 2016; Caplan et al., 2007). We hypothesize that lower comprehension of non-canonical sentences spoken with either the sentence prosody or the list prosody control will be associated with left posterior superior temporal and inferior parietal damage, as these areas are reliably shown to be critical for sentence comprehension in several lesion studies (Dronkers et al., 2004; Pillay et al., 2017; Rogalsky et al., 2018; Thothathiri et al., 2012). We also hypothesize that lower comprehension of non-canonical sentences spoken only with sentence prosody (and not with the list prosody control) will be predicted by left inferior frontal damage, due to its known role in attentional control and in sentence comprehension in neurotypical controls (Caplan et al., 2016; January et al., 2009).
Experiment 1: Cognitive Predictors of Comprehension of Non-Canonical Sentences with Different Prosodies
2. Method
2.1. Participants
Participants were 25 individuals (14 females) who experienced a left hemisphere cerebral stroke2 at least 6 months prior to testing (Table 1). Stroke participants ranged in age from 28 to 80 years (M = 54.20, sd = 13.23). Stroke participants were pre-morbidly right-handed, native speakers of American English, 18+ years of age, with no history of neurological disease, head trauma, or psychiatric disturbances prior to their stroke. Aphasia classification was determined using the Boston Diagnostic Aphasia Evaluation-III (Goodglass, Kaplan, & Barresi, 2000); each stroke participant’s aphasia diagnosis is reported in Table 1.
Table 1.
Stroke group demographics.
| Participant | Gender | Age | Months Post Stroke |
Years of Education |
Aphasia Diagnosis |
|---|---|---|---|---|---|
| AZ1001 | Female | 57 | 77 | 18 | None |
| AZ1003 | Female | 48 | 110 | 19 | Broca’s |
| AZ1006 | Male | 60 | 138 | 14 | Broca’s |
| AZ1011 | Female | 73 | 53 | 16 | Anomic |
| AZ1012 | Male | 77 | 85 | 16 | Wernicke’s |
| AZ1013 | Female | 47 | 258 | 17 | Broca’s |
| AZ1016 | Male | 37 | 142 | 14 | Broca’s |
| AZ1018 | Female | 43 | 29 | 14 | Broca’s |
| AZ1022 | Female | 46 | 79 | 14 | Broca’s |
| AZ1026 | Male | 70 | 50 | 16 | None |
| AZ1028 | Female | 80 | 19 | 24 | Wernicke’s |
| AZ1029 | Female | 34 | 174 | 14 | None |
| AZ1030 | Male | 56 | 32 | 16 | Broca’s |
| AZ1031 | Female | 40 | 63 | 20 | Broca’s |
| AZ1032 | Male | 28 | 20 | 13 | Anomic |
| AZ1033 | Male | 57 | 180; 60 | 14 | Global |
| AZ1034 | Female | 59 | 110 | 15 | Anomic |
| AZ1035 | Female | 41 | 72 | 17 | Broca’s |
| AZ1036 | Male | 65 | 158 | 15 | Broca’s |
| AZ1037 | Male | 57 | 13 | 16 | Broca’s |
| AZ1038 | Male | 54 | 155 | 14 | Broca’s |
| AZ1039 | Female | 66 | 48 | 14 | Anomic |
| AZ1040 | Female | 54 | 45 | 14 | Broca’s |
| AZ1041 | Female | 59 | 24 | 12 | Anomic |
| AZ1042 | Male | 55 | 37 | 14 | Broca’s |
An additional 20 neurotypical control subjects (14 females) ranging in age from 31 to 79 years (M = 51.40, sd = 12.82) who were also right-handed, native speakers of American English, 18+ years of age, with no history of neurological disease, head trauma, or psychiatric disturbances were recruited. The stroke and control groups did not significantly differ from each other in terms of age, gender, education, and hearing status (Table 2). All participants were monetarily compensated for their participation. Arizona State University’s Institutional Review Board approved all procedures.
Table 2.
Demographic comparisons between stroke and control groups.
| Stroke (n=25) |
Controls (n=20) |
Statistic | |
|---|---|---|---|
| Age | 54.52 (13.23) | 51.40 (12.82) | t(43)=.80, p=.43 |
| Gender (male/female) | 11/14 | 6/14 | χ2(1)=.93, p=.34 |
| Education (years) | 15.60 (2.57) | 15.20 (2.17) | t(43)=.56, p=.58 |
| Hearing Statusa | 15.45 (12.42) | 13.43 (9.07) | t(43)=.61, p=.55 |
Pure tone average for better ear; 500-4000 Hz
2.3. Stimuli
2.3.1. Sentences.
Stimuli were canonical and non-canonical sentences from Wilson et al. (2010). All sentences contained two nouns (boy, girl), one of seven verbs (hit, push, kick, kiss, wash, pull, hug), and one of three color adjectives (blue, green, red); thematic role assignment, verb, and adjective use were balanced across all sentence structures. Each sentence contained 10 syllables. See Table 3 for descriptions and examples.
Table 3.
Sentence stimuli.
| Sentence Structure* |
Example | Description | Sentence Prosody Duration |
List Prosody Duration |
|---|---|---|---|---|
| Canonical | The boy who is red is kissing the girl. | Subject-verb-object word order. Active modifying clause can only be attached to the subject. | 3.81-4.45 seconds (M=4.07, sd= .13) | 4.52-4.79 seconds (M=4.68, sd= .07). |
| Non-Canonical | The girl who the boy is kissing is red. | Subject-object-verb word order. Active modifying clause can be attached to either the subject or object; correct parsing leads to attachment of modifying clause to the subject. | 3.88-4.73 seconds (M=4.29, sd= .15) | 4.53-4.80 seconds (M=4.72, sd= .07). |
The canonical sentences correspond to one of the two types of sentences within Wilson et al.’s (2010) “long easy” sentences. The non-canonical sentences correspond to Wilson et al.’s (2010) “long medium.”
2.3.2. Prosody manipulations.
All sentences were spoken with both sentence prosody (i.e., natural prosody with pitch inflections and prosodic boundaries that are slightly exaggerated, but still perceived to be within the range of normal) and list prosody (i.e., monotone prosody where each word contains equal emphasis and stress but typical pitch inflections and prosodic boundaries are absent). Stimuli were digitally recorded by a classically trained female vocalist in Audacity sound editing software using a 32-bit resolution and 44,100 Hz sampling rate. Sentences spoken with sentence prosody and list prosody had an average fundamental frequency of 243.61 Hz (sd=10.15) and 213.12 (sd=6.50), respectively. Loudness was perceptually matched across all stimuli, and then the mean intensities of each condition were inspected: sentences spoken with sentence prosody had an average intensity of 75.72 dB (sd=.96) while sentences spoken with list prosody had an average intensity of 75.30 dB (sd=.72). Representative examples and spectrograms are provided in the Supplementary Materials (Figure S1).
To generate the sentences with sentence prosody, the speaker spoke each sentence with natural intonation. The speaker was instructed to accentuate key words needed to parse the sentence with pitch inflections and prosodic boundaries. Sentences with list prosody were generated by recording each word in isolation, out of sentence context, and then concatenating the individual words in the order of the experimental sentence. The inter-word interval for the list prosody sentences was 20 milliseconds. Durations for sentences spoken with sentence prosody and sentences spoken with list prosody are reported in Table 3.
2.4. Experimental Design
As part of a larger study, participants completed a cognitive-linguistic battery measuring sentence comprehension, auditory attention, verbal working memory, processing speed, and auditory single-word comprehension. Pure tone audiometric thresholds (500-4000 Hz) were also measured on all participants.
2.4.1. Sentence-picture matching task.
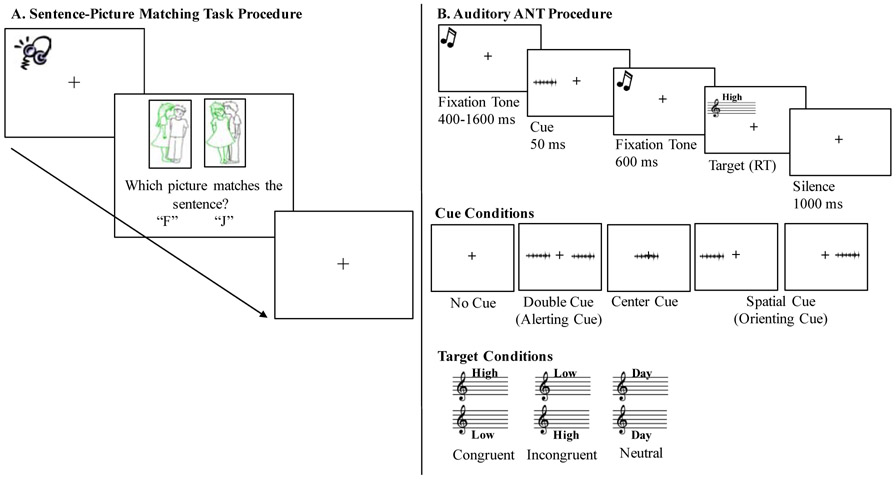
Sentence comprehension was measured using a sentence-picture matching task from Wilson et al. (2010), presented using E-prime 2.0 software (Psychology Software Tools, Pittsburg, PA). Each participant completed 40 experimental trials and 10 rest trials (i.e., four seconds of silence when no stimulus was presented which were included for better compatibility with possible task-based fMRI studies in the future). There were four experimental conditions (two sentence structures x two prosody manipulations), with each condition containing 10 sentence presentations. Each trial began with the simultaneous presentation of an auditory sentence and a target and foil picture (positioned left and right respectively and counterbalanced across trials). Foils were either thematic (i.e., role reversal of agent and patient) or color based (i.e., color assigned to wrong agent/patient) and were counterbalanced across trials. Participants were instructed to decide which picture matched the target sentence as quickly and accurately as possible, with accuracy being emphasized over speed. A response was made via a keyboard button press. Accuracy and reaction time (RT) were recorded for each trial by the E-prime 2.0 software. After the participant responded to a trial, a black fixation cross appeared for one second before initiation of the next trial (Figure 1A). Stimulus presentation was randomized for each participant. Verbal and written instructions, examples of all stimuli, and three practice trials preceded the start of the experiment.
Figure 1.
Illustration of the (A) sentence-picture matching task and (B) auditory ANT procedures.
2.4.2. Auditory Attention Network Task (ANT).
Three types of attention well-defined in the literature are alerting, orienting, and executive control (defined below; Posner & Petersen, 1990). Numerous studies in neurotypical and patient populations indicate that these three types (or networks) of attention are functionally and neuroanatomically distinct from one another (Chica et al., 2012; Fan, McCandliss, Sommer, Raz, & Posner, 2002; Fan & Posner, 2004; Petersen & Posner, 2012; Posner & Petersen, 1990; Roberts, Summerfield, & Hall, 2006). Alerting involves maintaining vigilance towards external stimuli and is supported by the thalamus, brainstem, and right fronto-parietal cortices. Orienting includes selection of specific information from a given stimulus and is supported by the right temporal-parietal junction, intraparietal sulcus, superior parietal lobe, and frontal eye fields. Executive control is supported by the bilateral prefrontal cortex and is a measure of how efficiently a correct response is achieved when relevant stimulus information conflicts with irrelevant stimulus information (Fan et al., 2002; Fan & Posner, 2004; Posner & Petersen, 1990; Rinne et al., 2013). To explore the potential role of each of these aspects of attention in sentence comprehension as a function of prosody, we used an auditory version (Figure 1B; Roberts, Summerfield, & Hall, 2006) of the well-studied Attention Network Test (ANT; Fan et al., 2002).
The auditory ANT administered was a replication of Roberts et al., (2006); please see their work for complete details. Briefly, the auditory ANT is a cued auditory Stroop task, where performance differences across cue types provide measures of alerting and orienting, and performance on the auditory Stroop task itself provides a measure of executive control. The auditory Stroop task consists of participants hearing the word “high,” “low,” or “day” spoken in either a high or a low-pitched voice. Participants were instructed to ignore the semantic content (i.e., the spoken word “high,” “low,” or “day”) and indicate via a button press whether the speaker's voice was high or low in pitch. A congruent trial occurred when the semantic content of the word corresponded with the vocal pitch (e.g., the word “high” spoken in a high-pitched voice). An incongruent trial occurred when the semantic content of the word conflicted with the vocal pitch (e.g., the word “high” spoken in a low-pitched voice). A neutral trial was when the semantically neutral (i.e., pitch neutral) word “day” was presented in either a high or low-pitched voice. A single female speaker recorded the auditory Stroop targets. The average fundamental frequency of the high-pitch words was 356.67 Hz (sd=5.96); the average for the low-pitch words was 211.17 Hz (sd=5.73). One of four auditory cues preceded each target. Auditory cues were 50 millisecond bursts of speech-shaped noise, cosine gated for 10 milliseconds at the onset and offset: (1) center cues (correlated noise bursts perceived in the center of the head), (2) double cues (uncorrelated noise bursts perceived as separate signals in each ear), (3) spatial cue (single noise burst) presented in the left or right ear (spatial cue always predicted the location of the auditory Stroop task), and (4) no cue. Participants completed 180 trials where all cue types and Stroop conditions were presented equally. Trial presentation was randomized for each participant. Verbal and written instructions, examples of all stimuli, and 10 practice trials preceded the start of the experiment.
As is standard in the attention literature, the combination of cues and targets embedded within the auditory ANT was used to calculate measures of alerting (no cue RT – double cue RT), orienting (center cue RT – spatial cue RT), and executive control attention (incongruent target RT – congruent target RT). Larger alerting and orienting RT difference scores indicate better alerting and orienting attention abilities (i.e., participants respond faster to the alerting or orienting cue compared to the comparison cue), while RT difference scores closer to zero reflect better executive control attention (i.e., similar levels of executive control are necessary for incongruent and congruent trials).
2.4.3. Repeatable Battery for the Assessment of Neuropsychological Status (RBANS).
Verbal working memory was assessed using a widely-used clinical measure, the RBANS Immediate Memory Index, which is comprised of the List Learning and Story Memory subtests (Randolph, 1998). In the List Learning subtest, participants are read a list of 10 words and asked to immediately recall the list; participants then hear the same list three more times and are asked to recall the words immediately following each list presentation. For the Story Memory subtest, participants hear a short story and are instructed to verbally recall the story; participants then hear the same story and are asked to recall it once more. Our verbal working memory measure was the combined raw scores of the first list recall and first story recall. Participants could achieve a maximum raw score of 22. Processing speed was also measured using the RBANS Coding subtest; in this task, participants matched specific numbers to certain symbols as quickly and accurately as possible. Participants could achieve a maximum raw score of 89. All raw scores were transformed into proportion correct for the analyses.
2.4.4. Boston Diagnostic Aphasia Examination-III (BDAE-III) Short Form.
Auditory single word comprehension was assessed using the Basic Word Discrimination subtest of the widely-used research and clinical measure, the BDAE-III short form (Goodglass et al., 2000). In this subtest, participants point to 16 familiar objects/pictures (e.g., body parts, animals, vehicles, etc.) following a verbal prompt from the examiner. The verbal prompt includes the carrier phrase “Point to” followed by the target word (e.g., Point to bear). The first two items in the subtest are body parts (i.e., shoulder, cheek), which the participant identifies on their own body. The next item is presented in a picture array of two (i.e., target: candle; foil: kite). The remaining 13 items are presented in a four-picture array, containing the target and three semantic foils. Participants could achieve a maximum raw score of 16. Raw scores were transformed into proportion correct for the analyses.
2.5. Data Analysis
Throughout the data analyses, both accuracy and RTs are dependent variables of interest because (1) speed-accuracy tradeoffs have been found in difficult sentence comprehension tasks (Brébion, 2001; McElree, Foraker, & Dyer, 2003), and (2) RTs may provide a more sensitive measure of the effects of prosody on sentence comprehension.
2.5.1. Sentence-picture matching task analysis.
To compare our results with previous work examining differences in non-canonical and canonical sentence comprehension, as well as the effects of prosody on sentence comprehension, we first used logistic regression to determine the effects and interactions of group (stroke or control), sentence structure (canonical or non-canonical) and prosody (sentence or list) on accuracy in the sentence-picture matching task. Similarly, a 2x2x2 mixed ANOVA was computed to determine the effects of these factors on RT in the sentence-picture matching task. Sentence structure and prosody were within-subjects factors; group was a between-subjects factor.
2.5.2. Cognitive measures to predict sentence comprehension.
To investigate the relationship between cognition and comprehension of sentences with different types of prosody in each group, hierarchical multiple regression models were calculated to identify independent variables that significantly predicted the dependent variables of interest. The regression models were computed in SPSS Version 24.0 (IBM Corp., Armonk, NY) and followed standard practices regarding treatment of covariates and thresholding (Feise, 2002; Perneger, 1998; Rothman, 1990). We focus on the non-canonical sentences as it is these sentences that have been reliably shown to yield non-ceiling performance in both chronic left hemisphere stroke survivors and neurotypical older adults. The dependent variables for accuracy were (1) non-canonical sentences spoken with sentence prosody and (2) non-canonical sentences spoken with list prosody. For RT, the dependent variables were: (1) mean RT of non-canonical – canonical sentences spoken with sentence prosody and (4) mean RT of non-canonical – canonical sentences spoken with list prosody. These mean RT difference scores were used because subtracting the canonical sentence mean RT from the non-canonical mean RT (our main interest) allows us to account for individual variability in general processing speed, response selection, and motor response speeds. RT difference scores closer to zero reflect less additional time required to respond to the non-canonical sentences compared to the canonical sentences. The independent variables were alerting attention (ANT no cue – double cue RT), orienting attention (ANT center cue – spatial cue RT), executive control attention (ANT incongruent – congruent RT), and verbal working memory (proportion correct from RBANS). Bivariate correlations were computed to identify covariates that significantly correlated with each dependent variable (p< .05); the significant covariates were included in the relevant model. Potential covariates were auditory single word comprehension from the BDAE-III, processing speed measured using the RBANS Coding subtest, pure tone audiometry (500-4000 Hz better ear), age, education, and months post-stroke.
For analyses regarding accuracy, all trials in a particular condition for each participant were included. For the RT analyses, RTs associated with incorrect responses and those greater than 2.5 standard deviations from each participant’s mean were excluded from the analyses to ensure that the process of interest is being captured, not other extraneous factors (e.g., brief distractions, button press mistakes, etc.). This data trimming procedure was determined a priori based on it being a standard, well-studied approach in psycholinguistic research (Baayen & Milin, 2010; Lachaud & Renaud, 2011; Ratcliff, 1993), and also because individuals with aphasia demonstrate abnormal online processing patterns for incorrect responses (Caplan, Waters, DeDe, Michaud, & Reddy, 2007; Dickey, Choy, & Thompson, 2007; Hanne, Sekerina, Vasishth, Burchert, & Bleser, 2011). Consistent with the above procedure, 14.62% (errors: 12.11%; outliers: 2.51%) of the data was removed for the auditory ANT and 26.1% (errors: 25.5%; outliers: .6%) from the sentence-picture matching task for the stroke group. For the control group, 4.39% (errors: 1.89%; outliers: 2.5%) of the data was removed for the auditory ANT and 3.0% (errors: 2.38%; outliers: .62%) from the sentence-picture matching task. The number of trials removed per participant per condition are reported in the Supplementary Materials (Tables S1 and S2).
3. Results
3.1. Sentence-Picture Matching Task
3.1.1. Accuracy.
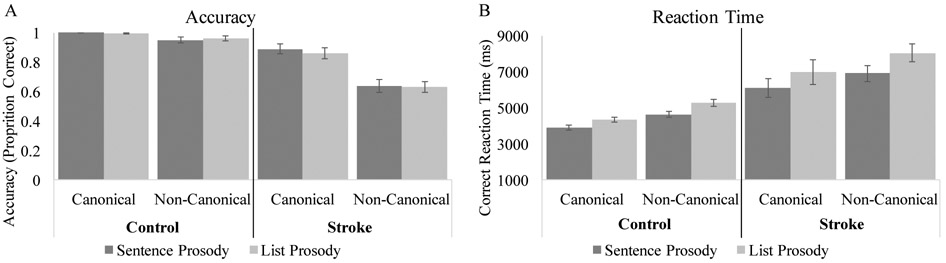
Scatterplots depicting each stroke participant’s sentence prosody and list prosody accuracy are displayed in Figure 2. Logistic regressions of the accuracy data revealed a significant between groups effect, χ2(1) = 15.86, p<0.001, with the control group being more accurate than the stroke group. The main effect of sentence structure was also significant, χ2(1) = 8.48, p=0.004, with non-canonical sentences yielding significantly lower accuracy compared to canonical structures (Figure 3; Table 4). The main effect of prosody was not significant, χ2(1) = 0,00, p=0.99, confirming that the two prosody conditions are of similar general difficulty on average across each group. None of the interactions were significant; sentence structure by group: χ2(1) = 1.62, p=0.20, prosody by group: χ2(1) = 0.15, p=0.69, sentence structure by prosody: χ2(1) = 0.15, p = 0.70, and sentence structure x prosody x group: χ2(1) = 0.00, p=0.96.
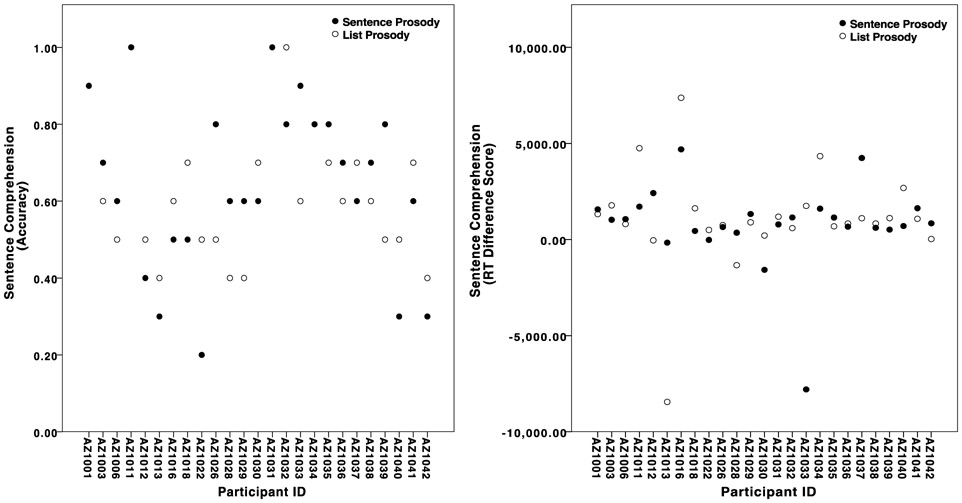
Figure 2.
Scatterplots showing individual variation in sentence prosody and list prosody (A) accuracy and (B) RT difference scores. Participants with a single solid marker achieved the same accuracy or RT difference score for sentence and list prosody.
Figure 3.
Sentence-picture matching task accuracy (A) and RT (B) for the control and stroke groups. Error bars show SEM.
Table 4.
Means and standard deviations for accuracy (proportion correct) and RT (milliseconds) for canonical and non-canonical sentences spoken in sentence prosody and list prosody.
| Condition | Sentence Prosody Accuracy Mean (sd) |
List Prosody Accuracy Mean (sd) |
Sentence Prosody RT Mean (sd) |
List Prosody RT Mean (sd) |
|
|---|---|---|---|---|---|
| Control Group (n=20) | Canonical | 1.0 (0.0) | 1.0 (.02) | 3895.75 (637.60) | 4355.56 (647.05) |
| Non-Canonical | .95 (.09) | .96 (.07) | 4618.34 (726.63) | 5254.79 (833.28) | |
| RT Difference Score | 786.60 (2189.26) | 1059.43 (2628.75) | |||
| Stroke Group (n=25) | Canonical | .88 (.17) | .86 (.17) | 6118.55 (2592.96) | 6966.18 (3392.18) |
| Non-Canonical | .64 (.22) | .63 (.19) | 6905.15 (2173.53) | 8042.40 (2460.26) | |
| RT Difference Score | 722.60 (758.55) | 899.24 (686.06) |
3.1.2. Reaction time.
Scatterplots depicting each stroke participant’s sentence prosody and list prosody RT difference scores are displayed in Figure 2. The between groups effect was significant, F(1, 43)=23,83, p<.001, with the control group having faster RTs than the stroke group. Main effects of sentence structure, F(1, 43)=15.27, p<.001, and prosody, F(1, 43)=12.43, p=.001, also were observed, with faster responses for canonical than non-canonical sentences, and for sentences spoken with sentence prosody than list prosody (Figure 3; Table 4). No interactions were significant; sentence structure by group: F(1, 43)=.07, p=.79, prosody by group: F(1, 43)=1.03, p=.32, sentence structure by prosody: F(1, 43)= .46, p= .50, and sentence structure x prosody x group: F(1, 43)= .03, p= .87.
3.2. Cognitive Measures Predicting Sentence Comprehension
Means and standard deviations of the cognitive predictors are reported in Table 5 for both groups. Of the potential covariates, auditory single-word comprehension significantly correlated with the RT difference scores for both sentence prosody and list prosody in the stroke group. For the control group, age and hearing status significantly correlated with accuracy in the list prosody condition (Table 6). No other correlations between the dependent variables and the possible covariates were significant.
Table 5.
Means and standard deviations for each cognitive predictor and potential covariate. Alerting, orienting, and executive control are measured in milliseconds; verbal working memory, processing speed, and single word comprehension in proportion correct; age and education in years; time post stroke in months; and hearing status is the pure tone average for the better ear for 500-4000 Hz.
| Cognitive Variable | Stroke Group Mean (sd) |
Control Group Mean (sd) |
|---|---|---|
| Alerting Attention | 21.27 (209.35) | 7.91 (44.28) |
| Orienting Attention | 20.38 (119.24) | −6.43 (44.75) |
| Executive Control Attention | 183.15 (232.22) | 123.09 (56.77) |
| Verbal Working Memory | .27 (.15) | .78 (.20) |
| Potential Covariates | Stroke Group Mean (sd) | Control Group Mean (sd) |
| Age | 54.52 (13.23) | 51.40 (12.82) |
| Single Word Comprehension | .92 (.13) | .99 (.02) |
| Hearing Status | 15.45 (12.42) | 13.43 (9.07) |
| Education | 15.60 (2.57) | 15.20 (2.17) |
| Processing Speed | .28 (.12) | .57 (.12) |
| Time Post Stroke | 86.84 (63.26) | n/a |
Table 6.
Bivariate correlations between potential covariates and the dependent variable for each group in accuracy and RT.
| Accuracy: Non-Canonical Sentences with Sentence Prosody | ||
|---|---|---|
| Covariate | Stroke Group | Control Group |
| Age | r(23)= .08, p=.70 | r(18)= .16, p=.49 |
| Single Word Comprehension | r(23)= .22, p=29 | r(18)= −.11, p=.66 |
| Hearing Status | r(23)= −.03, p=90 | r(18)= .22, p=.35 |
| Education | r(23)= .23, p=.28 | r(18)= −.05, p=.84 |
| Processing Speed | r(23)= .34, p=.09 | r(18)= .02, p=.95 |
| Time Post Stroke | r(23)= −.09, p=.69 | n/a |
| Accuracy: Non-Canonical Sentences with List Prosody | ||
| Covariate | Stroke Group | Control Group |
| Age | r(23)= −.22, p=.29 | r(18)= −.46, p=.04* |
| Single Word Comprehension | r(23)= .34, p=.10 | r(18)= −.25, p=.28 |
| Hearing Status | r(23)= −.03, p=88 | r(18)= −.74, p<.001* |
| Education | r(23)= .04, p=.86 | r(18)= −.09, p=.72 |
| Processing Speed | r(23)= .26, p=22 | r(18)= .25, p=.28 |
| Time Post Stroke | r(23)= −.33, p=.11 | n/a |
| RT: Non-Canonical - Canonical Sentences with Sentence Prosody | ||
| Covariate | Stroke Group | Control Group |
| Age | r(23)= −.07, p=.75 | r(18)= −.09, p=.70 |
| Single Word Comprehension | r(23)= .58, p=.003* | r(18)= −.07, p=.77 |
| Hearing Status | r(23)= −.08, p=.70 | r(18)= .14, p=.57 |
| Education | r(23)= .03, p=.89 | r(18)= .21, p=.37 |
| Processing Speed | r(23)= .25, p=.22 | r(18)= −.19, p=.41 |
| Time Post Stroke | r(23)= −.26, p=.21 | n/a |
| RT: Non-Canonical - Canonical Sentences with List Prosody | ||
| Age | r(23)= −.04, p=.84 | r(18)= −.19, p=.43 |
| Single Word Comprehension | r(23)= .41, p=.04* | r(18)= −.08, p=.74 |
| Hearing Status | r(23)= .01, p=.97 | r(18)= .09, p=.71 |
| Education | r(23)= −.26, p=.21 | r(18)= .07, p=.78 |
| Processing Speed | r(23)= .12, p=.57 | r(18)= −.08, p=.74 |
| Time Post Stroke | r(23)= −.26, p=.20 | n/a |
significant at p<.05
3.2.1. Accuracy.
3.2.1.1. Stroke group.
The overall regression model predicting non-canonical sentences spoken with sentence prosody was not significant, R2=.24, F(4,20)=1.60, p=.21. The overall regression model for non-canonical sentences with list prosody was significant, R2=.39, F(4,20)=3.24, p=.03, with verbal working memory being the only significant predictor: stroke participants with better verbal working memory scores demonstrated greater accuracy of non-canonical sentences spoken with list prosody (β= .63, p=.002) (Table 7).
Table 7.
Multiple regression models predicting accuracy and RT difference scores for the stroke and control groups. Whenever n/a is reported, the covariate did not significantly relate to the dependent variable in that group as reported in Table 6 and was therefore not included as a covariate in the model. Only regressions for which the model was significant are presented.
| Accuracy: Non-Canonical Sentences with List Prosody | ||||||
|---|---|---|---|---|---|---|
| Stroke Group | Control Group | |||||
| Predictors | β | t | p | β | t | p |
| Age | n/a | n/a | n/a | −.12 | −.57 | .58 |
| Hearing Status | n/a | n/a | n/a | −.56 | −2.57 | .02* |
| Alerting Attention | .11 | .51 | .61 | .05 | .26 | .80 |
| Orienting Attention | −.11 | −.47 | .64 | .28 | 1.67 | .12 |
| Executive Control Attention | −.16 | −.88 | .39 | −.07 | −.36 | .73 |
| Verbal Working Memory | .63 | 3.55 | .002* | −.30 | −1.61 | .13 |
| RT: Non-Canonical - Canonical Sentences with Sentence Prosody | ||||||
| Stroke Group | Control Group | |||||
| Predictors | β | t | p | β | t | p |
| Single Word Comprehension | .60 | 2.44 | .03* | n/a | n/a | n/a |
| Alerting Attention | .31 | 1.44 | .17 | −.40 | −2.20 | .04* |
| Orienting Attention | −.48 | −2.28 | .03* | −.38 | −1.99 | .07 |
| Executive Control Attention | −.03 | −.15 | .88 | .36 | 1.65 | .12 |
| Verbal Working Memory | −.11 | −.45 | .66 | .32 | 1.59 | .13 |
| RT: Non-Canonical - Canonical Sentences with List Prosody | ||||||
| Stroke Group | ||||||
| Predictors | β | t | p | |||
| Single Word Comprehension | .10 | .41 | .68 | |||
| Alerting Attention | .41 | 1.96 | .07 | |||
| Orienting Attention | .05 | .26 | .80 | |||
| Executive Control Attention | −.23 | −1.31 | .21 | |||
| Verbal Workine Memory | .52 | 2.19 | .04* | |||
significant at p<.05
3.2.1.2. Control group.
The overall regression model predicting non-canonical sentences spoken with sentence prosody was not significant, R2=.32, F(4,19)=1.77, p=.19. For non-canonical sentences spoken with list prosody, the overall model was significant, R2=.69, F(6,19)=4.81, p=.009, with the hearing covariate being the only significant predictor. Control participants with lower pure tone thresholds (i.e., better hearing abilities) demonstrated better comprehension of non-canonical sentences spoken with list prosody (β= −.56, p=.02) (Table 7).
3.2.2. Reaction Time.
3.2.2.1. Stroke group.
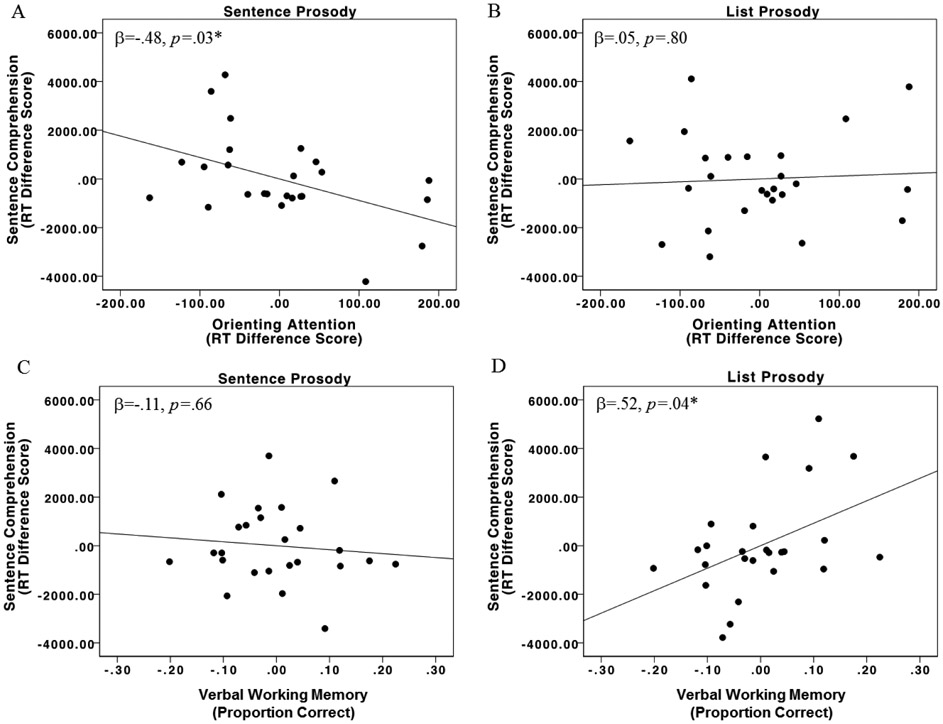
The overall regression model predicting RT for non-canonical sentences spoken with sentence prosody was significant, R2=.49, F(5,19)=3.65, p=.02, with single word comprehension (β=.60, p=.03) and orienting attention (β= −.48, p=.03) being the significant predictors. The single word comprehension predictor indicates that participants with lower single word comprehension scores demonstrated smaller RT difference scores in the sentence prosody condition; this is likely because participants with single word comprehension impairments do not exhibit longer RTs for non-canonical sentences than canonical sentences because their single word impairments prevent the words from being mapped onto the sentence structure (Caramazza & Zurif, 1976). For orienting attention, stroke participants with better orienting attention abilities demonstrated faster comprehension of non-canonical sentences spoken with sentence prosody. For non-canonical sentences spoken with list prosody, the overall regression model was significant, R2=.51, F(5,19)=3.96, p=.01, with the only significant predictor being verbal working memory: stroke survivors with better verbal working memory scores demonstrated slower RTs for non-canonical sentences spoken with list prosody (β=.52, p=.04) (Figure 4; Table 7).
Figure 4.
Stroke group partial regression plots for orienting attention predicting (A) sentence prosody RT and (B) list prosody RT, as well as for verbal working memory predicting (C) sentence prosody and (D) list prosody. Lower RT scores on the y-axes represent faster sentence comprehension abilities. Higher orienting attention scores (A, B) and verbal working memory scores (C, D) on the x-axes represent better performance on these tasks.
3.2.2.2. Control group.
The overall regression model predicting RT for non-canonical sentences spoken with sentence prosody was significant, R2=.52, F(4,15)=4.08, p=.02; participants with better alerting attention abilities demonstrated faster sentence comprehension (β= −.40, p=.04). For non-canonical sentences spoken with list prosody, the overall regression model was not significant, R2=.34, F(4,15)=1.89, p=16 (Table 7).
4. Discussion
Experiment 1 examined how cognitive performance predicts comprehension of sentences spoken with different types of prosody in individuals with a left hemisphere stroke and matched-control subjects. Replicating previous work (Rogalsky et al., 2018; Wilson et al., 2010), non-canonical sentences were more difficult to comprehend than canonical sentences as evidenced by lower accuracies and longer RTs for both groups. Sentence prosody elicited faster RTs than list prosody across groups, however, there were no differences in accuracy between the two types of prosody.
Regarding the relationship between attention and sentence prosody, the RT findings indicate that stroke participants with lower orienting attention abilities demonstrated slower comprehension of sentences spoken with sentence prosody; there were no significant predictors for accuracy. In the control group, orienting attention did not significantly predict sentence prosody comprehension RTs, but it did trend towards significance (p = .07). No measure of attention significantly predicted comprehension of sentences with list prosody in either group. Instead, verbal working memory abilities predicted comprehension of non-canonical sentences spoken with list prosody: stroke participants with better verbal working memory abilities demonstrated better accuracy but slower RTs when non-canonical sentences were spoken with list prosody.
4.1. Attention and Sentence Prosody
As hypothesized, attention abilities, specifically orienting attention, significantly predicted faster comprehension of non-canonical sentences spoken with sentence prosody but not list prosody in the stroke group, suggesting that the relationship between attention and non-canonical sentence comprehension is facilitated by sentence prosody. The relationship between orienting attention and non-canonical sentence RTs in the control group approached significance (p= .07), but alerting attention was a significant predictor (p = .04). It is noteworthy that the control and stroke group results identify different types of attention as related to sentence comprehension; this difference may reflect different attention demands during sentence comprehension in individuals with aphasia: In neurotypical individuals, it is likely that all types of attention contribute to sentence comprehension, to varying degrees likely based on context, sentence type, and individual differences. For the sentence task we used, it seems that when the language system is intact as is the case for our control group, better alerting is related to better sentence comprehension with typical sentence prosody, more so than other types of attention we investigated. In our individuals with aphasia, who by definition have impaired language systems, it seems that orienting may be particularly helpful in compensating for their impaired language system. Thus, it may be that inherent attention abilities, not just attention changes due to stroke, may affect the ability of an individual to use prosodic cues in sentence comprehension, which may be particularly critical in aphasia when other parts of the language system are compromised. Nonetheless, future work is needed to further parse apart the contributions of each aspect of attention to non-canonical sentence comprehension more generally.
Being able to orient attention to critical information (e.g., phrase boundaries as indicated by prosodic cues) facilitates comprehension, but only when attentional resources are adequate or preserved enough to utilize this benefit. Previous studies suggest that sentence prosody aids comprehension by helping individuals to chunk sentence-level information into smaller and more manageable units of information and that these smaller chunks reduce the load placed on working memory resources during sentence comprehension (Cohen, Douaire, & Elsabbagh, 2001; Kjelgaard & Speer, 1999; Roncaglia-Denissen et al., 2013; Speer et al., 1996). Our results extend these findings by indicating that sentence prosody may do so by reducing encoding demands by engaging orienting attention. Thus, we also suggest that sentence comprehension deficits previously attributed to deficits in short-term or working memory may possibly be explained by deficits in attention resources, which are also often impaired in aphasia (Alexander, 2006; Murray et al., 1997; Villard & Kiran, 2017). In line with computational modeling work (Mätzig et al., 2018; Patil et al., 2016), it may be that intermittent deficiencies in orienting attention (measured using RT) may extend the time needed for comprehension, but that eventual non-canonical sentence comprehension (measured using accuracy) is not affected due to the positive impact of typical prosodic cues. However, this slower processing may still have profound impacts on everyday communication (as nicely described by Love et al., 2008).
It is notable that orienting and alerting attention abilities were implicated in non-canonical sentence comprehension, but not executive control attention. To our knowledge orienting and alerting attention have not been previously examined in individuals with aphasia or in relation to sentence comprehension. Executive control has been investigated more in these areas, often using a Stroop task (as we did here with an auditory Stroop task), with mixed results (Brownsett et al., 2014; Green et al., 2010; January et al., 2009; Pompon, McNeil, Spencer, & Kendall, 2015). The present findings suggest that attentional measures such as our orienting and alerting measures, which measure the ability of participants to utilize attentional cues rather than the ability to inhibit conflicting information, may be a fruitful avenue to examine the role of attentional processes in speech comprehension.
4.2. Atypical (List) Prosody and Sentence Comprehension
As hypothesized, there was no relationship between attention and comprehension of sentences spoken with list prosody, further suggesting that attention resources are specifically engaged by sentence prosody. Our results do however identify a relationship between verbal working memory and comprehension of non-canonical sentences spoken with list prosody in the stroke group. Stroke participants with better verbal working memory abilities were more accurate for non-canonical sentences spoken with list prosody, yet were slower to respond. This finding of a speed-accuracy tradeoff for sentences presented with list prosody in relation to verbal working memory abilities suggests that without sentence prosody cues, strong verbal working memory abilities are needed, likely for reanalysis of the non-canonical sentence, which takes more time but is helpful for successful comprehension.
It could also be that list prosody facilitates the engagement of relatively intact verbal working memory resources due to its rhythmic properties. Previous work links regular (i.e., steady periodicity) speech rhythms with improved non-canonical sentence comprehension in neurotypical adults (Roncaglia-Denissen et al., 2013), as well as rhythm perception with auditory working memory abilities in individuals with aphasia (Zipse, Worek, Guarino, & Shattuck-Hufnagel, 2014). Thus, rhythm may also be able to play a role in encoding information into verbal working memory resources, assuming working memory is relatively intact. Additionally, the slower duration of list prosody may allow the sentence presentation rate to more closely align with an individual’s reduced processing speed (LaCroix et al., 2019). This may subsequently improve non-canonical sentence comprehension in aphasia as slower activation of syntactic information is also hypothesized to contribute to non-canonical sentence comprehension deficits in aphasia (Hanne et al., 2011; Love et al., 2008; Meyer, Mack, & Thompson, 2012; Patil et al., 2016). Importantly, both these possibilities may allow for sufficient reanalysis and thus successful comprehension, and explain the pattern of slower RTs but greater accuracy observed in stroke participants with relatively preserved verbal working memory. However, future work is needed to better understand the mechanisms underlying the relationship between list prosody and verbal working memory, and whether this relationship is driven by verbal task demands or working memory more broadly, particularly since non-verbal working memory abilities can also be impaired in aphasia and have been shown to relate to language abilities (Christensen & Wright, 2010; Potagas et al., 2011).
Experiment 2: Lesion Correlates of Non-Canonical Sentence Comprehension as a Function of Prosody Type
5. Method
5.1. Participants
Twenty-one participants in the stroke group (12 females) from Experiment 1 completed Experiment 2 (Figure 5). The remaining four participants from Experiment 1 (AZ1013, AZ1035, AZ1036, AZ1042) were excluded from Experiment 2 due to scanning contraindications. The remaining stroke participants ranged in age from 28 to 80 years (M = 55, sd = 13.86).
Figure 5.
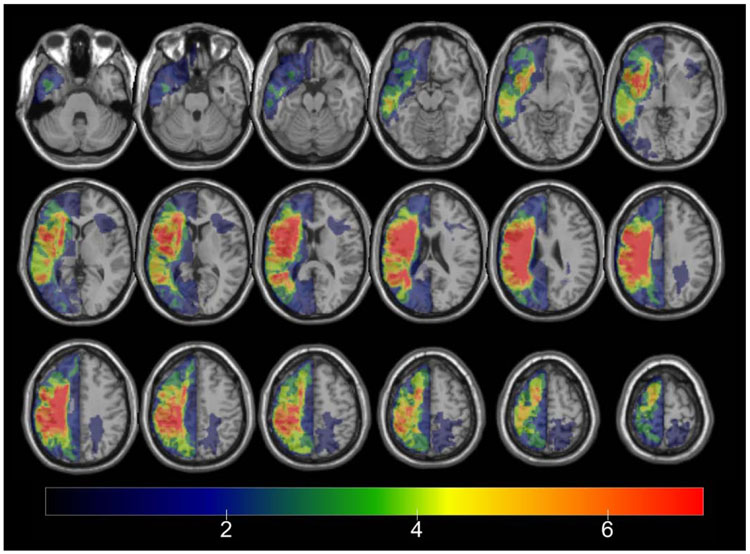
Lesion overlap map for all 21 stroke participants.
5.2. Data Collection
In addition to completing the behavioral measures reported in Experiment 1, participants underwent MRI scanning on a 3T Phillips Ingenia MRI scanner equipped with a 32 channel radiofrequency head coil located at the Keller Center for Imaging Innovation at the Barrow Neurological Institute in Phoenix, Arizona. In addition to other imaging collected for other studies, a T1 image (FOV = 270 X 252, TR = 6.7, flip angle = 9, voxel size = 1 x 1 x 1 mm) was collected for the present study’s lesion analyses.
5.2.1. Lesion identification and normalization.
Lesions were demarcated on the T1 image in MRIcron (Rorden & Brett, 2000). The resulting lesion maps were smoothed with a 3mm full-width half maximum Gaussian kernel to remove jagged edges associated with manual drawing. Enantiomorphic normalization (Nachev, Coulthard, Jäger, Kennard, & Husain, 2008) was conducted using SPM12 in accordance with procedures developed by Rorden et al., (2012 ) (i.e., NiiStat’s “nii_harvest”). First, a mirrored image of the T1 image (reflected across the midline) was co-registered to the native T1 image. Then, a chimeric image based on the native T1 image with the lesioned tissue replaced by tissue from the mirrored image (using the smoothed lesion map to modulate this blending, feathering the lesion edge) was created. SPM12's unified segmentation-normalization (Ashburner & Friston, 2005) was used to transform this chimeric image to standard space; the resulting spatial transformation was subsequently applied to the T1 image. The normalized lesion map was then binarized, using a 50% probability threshold.
5.2.2. Lesion symptom mapping.
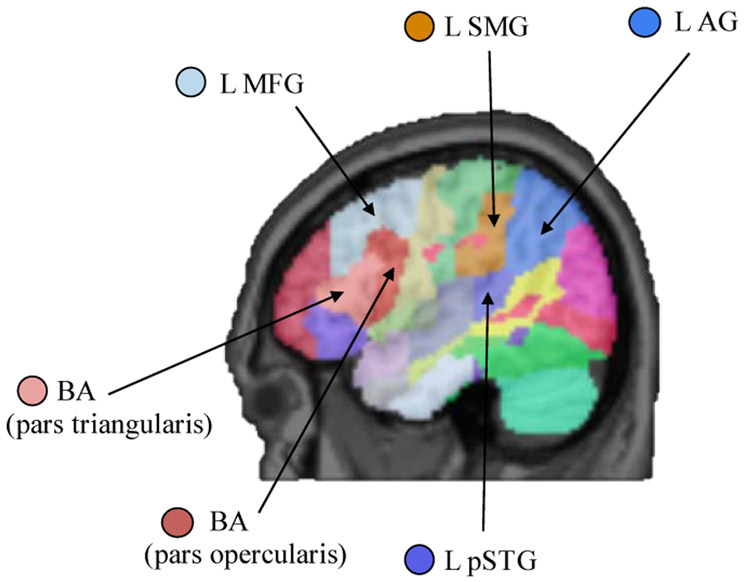
Lesion maps were parcellated into regions of interest using the Johns Hopkins University (JHU) brain atlas, which uses structural-anatomical boundaries to define regions (Faria et al., 2012). From the 189 potential regions of interest defined by the JHU brain atlas, six regions were chosen based on the previous literature and a priori hypotheses described in the introduction regarding left hemisphere regions shown to be sensitive to sentence prosody manipulations. These regions of interest are: the posterior half of the left middle frontal gyrus, Broca’s area (pars opercularis and the pars triangularis are each ROIs), left posterior superior temporal gyrus, left supramarginal gyrus, and left angular gyrus (Figure 6). The percentage of each region of interest that was damaged (i.e., marked as lesion in the participant’s lesion map) in each stroke participant was extracted using an in-house Matlab (MathWorks, Natick, MA) script.
Figure 6.
Sentence comprehension regions of interest derived from the JHU atlas (Faria et al., 2012). Center of mass coordinates for each region of interest in MNI space are as follows: BA pars triangularis (−43, 26, 10); BA pars opercularis (−45, 13, 15); L MFG (−36, 18, 38); L pSTG (−51, −34, 12); L SMG (−52, −29, 32); L AG (−42, −52, 38).
Key: L: left; AG: angular gyrus; BA: Broca’s area; MFG: middle frontal gyrus; pSTG: posterior superior temporal gyrus; SMG: supramarginal gyrus.
5.3. Data Analysis
Multiple regression analyses using percentage of lesioned voxels in ROIs as independent variables has been shown to be an effective means for investigating the neurobiology of sentence comprehension, particularly for sample sizes such as our own for which for voxel-based or support-vector regression lesion-symptom mapping are not suitable (Caplan, Michaud, Hufford, & Makris, 2016; Caplan et al., 2007; Sperber, Wiesen, & Karnath, 2018). We used the same hierarchical multiple regression approach as in Experiment 1 to again predict accuracy and RT difference scores for the non-canonical sentences spoken with each prosody type. Thus, the dependent variables were the same as in Experiment 1. The independent variables are now the percentage of lesioned voxels in each of the six ROIs described above and depicted in Figure 6. Lesion volume was the only potential covariate.
6. Results
Means and standard deviations for each sentence condition and cognitive measure for the 21 stroke participants are reported in Table 8. The potential covariate, lesion volume, did not significantly correlate with any of the dependent variables: non-canonical sentences spoken with sentence prosody accuracy, r(19)= −.25, p=.27; non-canonical sentences spoken with list prosody accuracy, r(19)= −.31, p=.18; non-canonical sentences spoken with sentence prosody RT, r(19)= −.22, p=.35, and non-canonical sentences spoken with list prosody RT, r(19)= .34, p=.13.
Table 8.
Means and standard deviations for each sentence condition (accuracy and RT difference score) and cognitive variable. Sentence comprehension accuracy and verbal working memory are proportion correct measurements. Sentence prosody and list prosody RT difference scores, alerting, orienting, and executive control are measured in milliseconds.
| Variable | Mean (sd) |
|---|---|
| Non-Canonical Sentence Prosody Accuracy | .66 (.21) |
| Non-Canonical List Prosody Accuracy | .65 (.19) |
| Sentence Prosody RT Difference Score | 817.32 (2383.78) |
| List Prosody RT Difference Score | 1589.44 (1882.58) |
| Auditory Alerting | 58.00 (173.37) |
| Auditory Orienting | 36.97 (112.23) |
| Auditory Executive Control | 187.29 (237.78) |
| Verbal Working Memory | .39 (.21) |
6.1. Brain Regions Predicting Comprehension of Non-Canonical Sentences Spoken with Each Prosody Type
6.1.1. Accuracy.
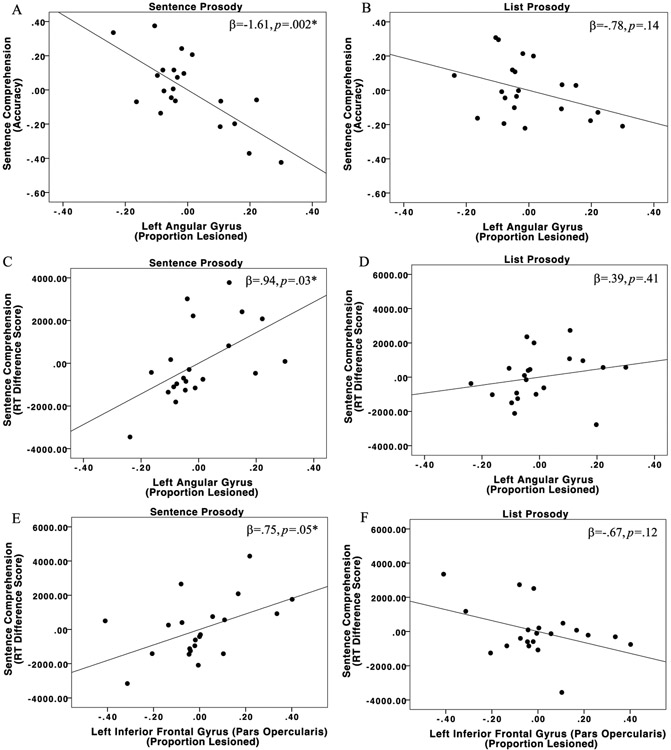
The overall model predicting accuracy for non-canonical sentences spoken with sentence prosody was significant, R2=.55, F(6,14)=2.80, p=.05; the left angular gyrus ROI was the only significant predictor (β= −1.61, p=.002). A larger proportion of the left angular gyrus demarcated as lesioned predicted lower accuracy when non-canonical sentences were spoken with sentence prosody. The overall model predicting accuracy for non-canonical sentences spoken with list prosody was not significant, R2=.38, F(6,14)=1.43, p=.27 (Figure 7; Table 9).
Figure 7.
Partial regression plots for the left angular gyrus predicting non-canonical sentence comprehension for (A) sentence prosody accuracy and (B) list prosody accuracy; left angular gyrus predicting (C) sentence prosody RT and (D) list prosody RT; and the left pars opercularis predicting (E) sentence prosody RT and (F) list prosody RT.
Table 9.
Multiple regression models for prosody (accuracy and RT difference scores) predicted from sentence comprehension regions of interest. Only significant regressions are presented.
| Non-Canonical Sentences with Sentence Prosody Accuracy | |||
|---|---|---|---|
| Predictors | β | t | p |
| Left middle frontal gyrus | 1.04 | 2.27 | .04a |
| Broca’s area (pars opercularis) | −.56 | −1.49 | .16 |
| Broca’s area (pars triangularis) | .07 | .17 | .87 |
| Left supramarginal gyrus | .47 | 1.83 | .09 |
| Left angular gyrus | −1.61 | −3.81 | .002* |
| Left posterior superior temporal gyrus | .40 | 1.55 | .14 |
| Non-Canonical - Canonical Sentences with Sentence Prosody RT | |||
| Predictors | β | t | p |
| Left middle frontal gyrus | −.52 | −1.21 | .25 |
| Broca’s area (pars opercularis) | .75 | 2.14 | .05* |
| Broca’s area (pars triangularis) | −.92 | −2.29 | .04a |
| Left supramarginal gyrus | −.75 | −3.13 | .007a |
| Left angular gyrus | .94 | 2.39 | .03* |
| Left posterior superior temporal gyrus | −.35 | −1.42 | .18 |
significant at p<.05
Region of interest elicits a statistically significant finding in the unexpected direction; likely driven by damage to other regions. This is a common finding in lesion-symptom mapping and aphasia research due to lesion locations not being independent of one another, but should not be interpreted as intact tissue equaling impairment.
6.1.2. Reaction Time.
The overall model predicting RT for non-canonical sentences spoken with sentence prosody was significant, R2=.60, F(6,14)=3.54, p=.02; the left pars opercularis and left angular gyrus were the two significant predictors. A larger proportion of lesion in Broca’s area (pars opercularis; β= .75, p=.05) and a larger proportion of lesion in the left angular gyrus (β= .94, p=.03) both predicted slower RTs for non-canonical sentences spoken with sentence prosody. The overall model predicting RT for non-canonical sentences spoken with list prosody was not significant, R2=.47, F(6,14)=2.04, p=.13 (Figure 7; Table 9).
7. Discussion
Experiment 2 investigated the neural resources which support comprehension of non-canonical sentences spoken with two types of prosody. As hypothesized, stroke participants with a larger proportion of Broca’s area (pars opercularis) damaged demonstrated slower RTs for non-canonical sentences spoken with sentence prosody, but not with list prosody. Additionally, left angular gyrus damage was associated with poorer and slower comprehension of non-canonical sentences spoken with sentence prosody. There were no brain regions which predicted accuracy or RT for sentences spoken with list prosody.
7.1. Frontal and Parietal Regions Support Sentence Prosody
Stroke participants with a larger proportion of the left pars opercularis and/or angular gyrus damaged exhibited poorer comprehension of sentences spoken with sentence prosody. Previous work indicates that persons with left hemisphere lesions have specific deficits in processing sentence prosody (Baum & Dwivedi, 2003; Baum & Pell, 1999; Pell, 1998), but that they also demonstrate gains in comprehension when sentences (Lasky, Weider, & Johnson, 1976) and paragraphs (Pashek & Brookshire, 1982) are spoken with an exaggerated linguistic stress. The results from the present study expand upon this previous work by indicating that the anterior portion of Broca’s area (pars opercularis) and the left angular gyrus, specifically, appear to be engaged in processing typical sentence prosody post-stroke. Our sentence prosody manipulation is somewhat exaggerated in that the pitch inflections and prosodic boundaries are over emphasized, but still perceived to be within the range of normal. Thus, it is possible that individuals with these inferior frontal and parietal regions preserved may benefit from exaggerated prosodic cues.
Typical sentence prosody has been hypothesized to facilitate sentence comprehension by reducing demands placed on cognitive resources (Kjelgaard & Speer, 1999; Roncaglia-Denissen et al., 2013; Speer et al., 1996), and our Experiment 1 indicates that attention, and perhaps orienting attention in particular, may be particularly important for sentence comprehension when typical sentence prosody cues are present. It is also noteworthy that fMRI studies of auditory orienting and alerting attention in control subjects implicate bilateral inferior frontal gyrus and inferior parietal regions (Huang, Belliveau, Tengshe, & Ahveninen, 2012; Mayer, Harrington, Adair, & Lee, 2006; Rossi, Huang, Furtak, Belliveau, & Ahveninen, 2014) that overlap with the ROIs identified here to be implicated in comprehension of sentences containing typical sentence prosody cues, but not for list prosody.
A post-hoc exploration using regression models to predict our alerting, orienting, and executive control attention measures from the same six ROIs used in the prosody regression models revealed none of the three overall models to be significant, which is not surprising given the known bilateral representation of attention resources. But, it is notable that although the orienting model did not reach overall significance (R2=.65, F(6,14)=1.74, p=.18), the angular gyrus was the top predictor of orienting attention (ß=1.11, p=.04). Given our finding linking angular gyrus damage to comprehension of sentences with typical sentence prosody, future work is needed to better understand the relationship between attention, and sentence prosody, and the underlying neural substrates supporting this relationship in individuals with aphasia.
7.2. Neural Resources Supporting List Prosody
No region of interest significantly predicted comprehension of sentences spoken with list prosody. This finding aligns with previous fMRI research indicating that list prosody recruits bilateral middle and posterior temporal cortices (Humphries et al., 2005), as well as other work demonstrating flattened, monotone speech to activate similar regions in neurotypical controls (Meyer, Steinhauer, Alter, Friederici, & von Cramon, 2004). Left hemisphere damage has also been generally associated with impairments in identifying and decoding sentence prosody, but not other types of pitch cues such as those in emotional prosody (Pell & Baum, 1997). Thus perhaps, list prosody, unlike typical sentence prosody, may be able to help recruit right hemisphere brain regions during sentence comprehension, possibly facilitating comprehension for some individuals with left hemisphere lesions affecting brain regions engaged in processing sentence prosody. However, future work is needed to better understand the spectral and temporal qualities of each prosody manipulation and how each affects the neural resources supporting non-canonical sentence comprehension.
7.3. Methodological Considerations and Future Directions
Two points related to our ROI-based lesion-symptom mapping approach that are important when interpreting our results are as follows. First, our independent variables of “percent damage” of each ROI is simply the structural proportion of binary “damage” versus “intactness” of a given brain region as seen on a T1 MRI scan; it is not a complete measure of that region’s structural or functional integrity, and does not account for diaschisis. There is one other caveat regarding our ROI approach: the frontal ROIs were anatomically adjacent to one another, as were the posterior temporal/parietal ROIs. Thus, damage between the anterior ROIs, and between the posterior ROIs, respectively, was significantly correlated with one another. As a result, the relative strengths or specificity of the contributions of two adjacent ROIs, such as posterior STG and the supramarginal gyrus, should be done with caution. Therefore, future work is particularly needed to investigate both structural and functional connectivity and specificity between and within these ROIs, in order to better understand the contributions of specific brain regions to comprehension of sentences spoken with sentence prosody.
The second point is regarding notable differences in our findings related to Broca’s area and previous studies mentioned in the introduction. Based on previous neuroimaging work in controls, we expected that Broca’s area would be implicated by sentence prosody, and in fact we did find that damage to the left pars opercularis was significantly related to slower non-canonical sentence comprehension when sentences were spoken with sentence prosody. However, it is noteworthy that several previous large-scale stroke studies of non-canonical sentence comprehension only implicate temporal-parietal regions, not Broca’s area (Dronkers et al., 2004; Magnusdottir et al., 2013; Pillay et al., 2017; Rogalsky et al., 2018; Thothathiri et al., 2012), and all of these studies presented auditory sentences with typical sentence prosody similar to ours. We suspect that the discrepancy between these studies and ours regarding Broca’s area contributions to non-canonical sentence comprehension are due to these previous studies all being voxel-based lesion symptom mapping (Bates et al., 2003), whereas we took an ROI-based approach (Caplan et al. 2016; Caplan et al., 2007).
It is well-established that Broca’s area is an anatomically diverse region (Amunts et al., 1999) and there is substantial inter-subject variability regarding the functional organization of Broca’s area in relation to speech comprehension and related cognitive abilities (Fedorenko et al., 2012; Fedorenko, Hsieh, Nieto-Castañón, Whitfield-Gabrieli, & Kanwisher, 2010; Rogalsky, Almeida, Sprouse, & Hickok, 2015). Thus, we suspect that an ROI-based approach, which does not require voxel-to-voxel alignment for an effect to be identified, may be more sensitive than VLSM approaches to Broca’s area involvement in sentence comprehension. In fact, ROI-based studies of individuals with aphasia by Caplan et al. (2007; 2016) do in fact implicate left pars opercularis in comprehension of some types of non-canonical sentences, including for sentence-picture matching tasks such as the one used in the present study. Clearly, a direct comparison of VLSM and ROI-approaches within the same sample is needed to verify this possibility, but it is worth considering when comparing ROI versus voxel-based findings in the lesion-symptom mapping literature.
8. Conclusions
In Experiment 1, it was found that stroke participants with poorer orienting attention demonstrated slower comprehension of non-canonical sentences spoken with sentence prosody, but not with list prosody. The results from Experiment 2 indicate that the left pars opercularis and angular gyrus support comprehension of sentences spoken with sentence prosody, but not with list prosody. No left hemisphere region of interest predicted comprehension of sentences spoken with list prosody. Overall, our findings indicate that while non-canonical sentence comprehension is supported by a large network of left hemisphere brain regions, prosody can affect the cognitive and neural resources that are recruited during non-canonical sentence comprehension, and that orienting attention may be a particularly important, yet mostly unexplored, cognitive resource for non-canonical sentence comprehension.
Supplementary Material
Highlights.
Effects of prosody on resources critical for sentence comprehension were examined.
Attention predicts comprehension with typical prosody, but not with list prosody.
Left frontal and parietal damage predict comprehension only with typical prosody.
Prosody may recruit intact cognitive resources during sentence comprehension.
Acknowledgments
Funding: This work was supported by the National Institutes of Health (DC009659; PI: G. Hickok) and the American Heart Association pre-doctoral fellowship (18PRE33990328; A. LaCroix).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There is considerable debate regarding the specificity and nature of working memory contributions to sentence comprehension that is out of the scope of the present study (Caplan & Waters, 1999; Fedorenko & Kanwisher, 2011; Just & Carpenter, 1992; Rogalsky & Hickok 2011; Van Dyke & Johns, 2012). What is relevant here is that verbal working memory is known to be impaired in most types of aphasia and the neural resources supporting verbal working memory overlap with those implicated in conduction aphasia and auditory-motor integration for speech more generally (Buchsbaum et al., 2011).
One participant had two strokes ten years apart (AZ1033) and two other participants report a single stroke, but a bilateral lesion was evident on an MRI scan (AZ1001 and AZ1040).
References
- Alexander MP (2006). Impairments of procedures for implementing complex language are due to disruption of frontal attention processes. Journal of the International Neuropsychological Society, 12(2), 236–247. 10.1017/S1355617706060309 [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings HBM, & Zilles K (1999). Broca’s region revisited: Cytoarchitecture and intersubject variability. Journal of Comparative Neurology, 412(2), 319–341. [DOI] [PubMed] [Google Scholar]
- Ashburner J, & Friston KJ (2005). Unified segmentation. NeuroImage, 26(3), 839–851. 10.1016/j.neuroimage.2005.02.018 [DOI] [PubMed] [Google Scholar]
- Baayen RH, & Milin P (2010). Analyzing reaction times. International Journal of Psychological Research, 3(2), 12–28. [Google Scholar]
- Baddeley A (2010). Working memory. Current Biology, 20(4), R136–R140. 10.1016/j.cub.2009.12.014 [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, & Dronkers NF (2003). Voxel-based lesion–symptom mapping. Nature Neuroscience, 6(5), 448–450. 10.1038/nn1050 [DOI] [PubMed] [Google Scholar]
- Baum SR, & Dwivedi VD (2003). Sensitivity to prosodic structure in left- and right- hemisphere-damaged individuals. Brain and Language, 87(2), 278–289. 10.1016/S0093-934X(03)00109-3 [DOI] [PubMed] [Google Scholar]
- Baum SR, & Pell MD (1999). The neural bases of prosody: Insights from lesion studies and neuroimaging. Aphasiology, 13(8), 581–608. [Google Scholar]
- Belyk M, & Brown S (2014). Perception of affective and linguistic prosody: An ALE meta-analysis of neuroimaging studies. Social Cognitive and Affective Neuroscience, 9(9), 1395–1403. 10.1093/scan/nst124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley DC, Garrett MF, & Zurif EB (1980). Syntactic deficits in Broca’s aphasia In Caplan David (Ed.), Biological Studies of Mental Processes. Cambridge, MA: MIT Press. [Google Scholar]
- Brébion G (2001). Language Processing, Slowing, and Speed/Accuracy Trade-Off in the Elderly. Experimental Aging Research, 27(2), 137–150. 10.1080/036107301750073999 [DOI] [PubMed] [Google Scholar]
- Brownsett SLE, Warren JE, Geranmayeh F, Woodhead Z, Leech R, & Wise RJS (2014). Cognitive control and its impact on recovery from aphasic stroke. Brain, 137(1), 242–254. 10.1093/brain/awt289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum BR, Baldo J, Okada K, Berman KF, Dronkers N, D’Esposito M, & Hickok G (2011). Conduction aphasia, sensory-motor integration, and phonological short-term memory – An aggregate analysis of lesion and fMRI data. Brain and Language, 119(3), 119–128. 10.1016/j.bandl.2010.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan D, Michaud J, Hufford R, & Makris N (2016). Deficit-lesion correlations in syntactic comprehension in aphasia., Deficit-Lesion Correlations in Syntactic Comprehension in Aphasia. Brain and Language, 152, 152, 14, 14–27. 10.1016/j.bandl.2015.10.005, 10.1016/j.bandl.2015.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan David, Chen E, & Waters G (2008). Task-dependent and task-independent neurovascular responses to syntactic processing. Cortex, 44(3), 257–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan David, Michaud J, & Hufford R (2013). Short Term Memory, Working Memory, and Syntactic Comprehension in Aphasia. Cognitive Neuropsychology, 30(2). 10.1080/02643294.2013.803958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan David, Waters G, DeDe G, Michaud J, & Reddy A (2007). A study of syntactic processing in aphasia I: Behavioral (psycholinguistic) aspects. Brain and Language, 101(2), 103–150. 10.1016/j.bandl.2006.06.225 [DOI] [PubMed] [Google Scholar]
- Caplan David, Waters G, Kennedy D, Alpert N, Makris N, DeDe G, … Reddy A (2007). A study of syntactic processing in aphasia II: Neurological aspects. Brain and Language, 101(2), 151–177. 10.1016/j.bandl.2006.06.226 [DOI] [PubMed] [Google Scholar]
- Caplan David, & Waters GS (1999). Verbal working memory and sentence comprehension. Behavioral and Brain Sciences, 22(1), 77–94. [DOI] [PubMed] [Google Scholar]
- Caramazza A, & Zurif EB (1976). Dissociation of algorithmic and heuristic processes in language comprehension: Evidence from aphasia. Brain and Language, 3(4), 572–582. [DOI] [PubMed] [Google Scholar]
- Carlson K (2009). How Prosody Influences Sentence Comprehension. Language and Linguistics Compass, 3(5), 1188–1200. 10.1111/j.1749-818X.2009.00150.x [DOI] [Google Scholar]
- Caspari I, Parkinson SR, LaPointe LL, & Katz RC (1998). Working Memory and Aphasia. Brain and Cognition, 37(2), 205–223. 10.1006/brcg.1997.0970 [DOI] [PubMed] [Google Scholar]
- Chica AB, Thiebaut de Schotten M, Toba M, Malhotra P, Lupiáñez J, & Bartolomeo P (2012). Attention networks and their interactions after right-hemisphere damage. Cortex, 48(6), 654–663. 10.1016/j.cortex.2011.01.009 [DOI] [PubMed] [Google Scholar]
- Christensen SC, & Wright HH (2010). Verbal and non-verbal working memory in aphasia: What three n-back tasks reveal. Aphasiology, 24(6–8), 752–762. 10.1080/02687030903437690 [DOI] [Google Scholar]
- Cohen H, Douaire J, & Elsabbagh M (2001). The role of prosody in discourse processing. Brain and Cognition, 46(1–2), 73–82. [DOI] [PubMed] [Google Scholar]
- Cowan N, Elliott EM, Saults JS, Morey CC, Mattox S, Hismjatullina A, & Conway ARA (2005). On the Capacity of Attention: Its Estimation and Its Role in Working Memory and Cognitive Aptitudes. Cognitive Psychology, 51(1), 42–100. 10.1016/j.cogpsych.2004.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson B, Rao SM, Woodley SJ, Rosen AC, Bobholz JA, Mayer A, … Stein EA (1999). Mapping of semantic, phonological, and orthographic verbal working memory in normal adults with functional magnetic resonance imaging. Neuropsychology, 13(2), 171–187. 10.1037/0894-4105.13.2.171 [DOI] [PubMed] [Google Scholar]
- den Ouden D-B, Dickey MW, Anderson C, & Christianson K (2016). Neural correlates of early-closure garden-path processing: Effects of prosody and plausibility. The Quarterly Journal of Experimental Psychology, 69(5), 926–949. 10.1080/17470218.2015.1028416 [DOI] [PubMed] [Google Scholar]
- Dickey MW, Choy JJ, & Thompson CK (2007). Real-time comprehension of whmovement in aphasia: Evidence from eyetracking while listening. Brain and Language, 100(1), 1–22. 10.1016/j.bandl.2006.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Redfern BB, & Jaeger JJ (2004). Lesion analysis of the brain areas involved in language comprehension. Cognition, 92(1–2), 145–177. 10.1016/j.cognition.2003.11.002 [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, & Posner MI (2002). Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience, 14(3), 340–347. [DOI] [PubMed] [Google Scholar]
- Fan J, & Posner M (2004). Human Attentional Networks. Psychiatrische Praxis, 31, 210–214 10.1055/s-2004-828484 [DOI] [PubMed] [Google Scholar]
- Faria AV, Joel SE, Zhang Y, Oishi K, van Zjil PCM, Miller MI, … Mori S (2012). Atlas-Based Analysis of Resting-State Functional Connectivity: Evaluation for Reproducibility and Multi-Modal Anatomy-Function Correlation Studies. Neuroimage, 61(3), 613–621. 10.1016/j.neuroimage.2012.03.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, & Kanwisher N (2012). Language-Selective and Domain-General Regions Lie Side by Side within Broca’s Area. Current Biology, 22(21), 2059–2062. 10.1016/j.cub.2012.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Hsieh P-J, & Balewski Z (2015). A possible functional localizer for identifying brain regions sensitive to sentence-level prosody. Language, Cognition and Neuroscience, 30(1–2), 120–148. 10.1080/01690965.2013.861917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Hsieh P-J, Nieto-Castañón A, Whitfield-Gabrieli S, & Kanwisher N (2010) New Method for fMRI Investigations of Language: Defining ROIs Functionally in Individual Subjects. Journal of Neurophysiology, 104(2), 1177–1194. 10.1152/jn.00032.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, & Kanwisher N (2011). Some Regions within Broca’s Area Do Respond More Strongly to Sentences than to Linguistically Degraded Stimuli: A Comment on Rogalsky and Hickok (2011). Journal of Cognitive Neuroscience, 23(10), 2632–2635. [DOI] [PubMed] [Google Scholar]
- Feise RJ (2002). Do multiple outcome measures require p-value adjustment? BMC Medical Research Methodology, 2(1), 8 10.1186/1471-2288-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier L, Carlson K, & Cliftonjr C (2006). Prosodic phrasing is central to language comprehension. Trends in Cognitive Sciences, 10(6), 244–249. 10.1016/j.tics.2006.04.002 [DOI] [PubMed] [Google Scholar]
- Fromont LA, Soto-Faraco S, & Biau E (2017). Searching High and Low: Prosodic Breaks Disambiguate Relative Clauses. Frontiers in Psychology, 8 10.3389/fpsyg.2017.00096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E, & Barresi B (2000). Boston Diagnostic Aphasia Examination Record Booklet. Lippincott Williams & Wilkins. [Google Scholar]
- Gordon PC, Hendrick R, & Levine WH (2002). Memory-load interference in syntactic processing. Psychological Science, 13(5), 425–430. [DOI] [PubMed] [Google Scholar]
- Goucha T, & Friederici AD (2015). The language skeleton after dissecting meaning: A functional segregation within Broca’s Area. NeuroImage, 114, 294–302. 10.1016/j.neuroimage.2015.04.011 [DOI] [PubMed] [Google Scholar]
- Green DW, Grogan A, Crinion J, Ali N, Sutton C, & Price CJ (2010). Language control and parallel recovery of language in individuals with aphasia. Aphasiology, 24(2), 188–209. 10.1080/02687030902958316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanne S, Sekerina IA, Vasishth S, Burchert F, & Bleser RD (2011). Chance in agrammatic sentence comprehension: What does it really mean? Evidence from eye movements of German agrammatic aphasic patients. Aphasiology, 25(2), 221–244. 10.1080/02687038.2010.489256 [DOI] [Google Scholar]
- Hartwigsen G, & Saur D (2017). Neuroimaging of stroke recovery from aphasia – Insights into plasticity of the human language network. 10.1016/j.neuroimage.2017.11.056 [DOI] [PubMed] [Google Scholar]
- Huang S, Belliveau JW, Tengshe C, & Ahveninen J (2012). Brain Networks of Novelty-Driven Involuntary and Cued Voluntary Auditory Attention Shifting. PLoS ONE, 7(8). 10.1371/journal.pone.0044062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries C, Love T, Swinney D, & Hickok G (2005). Response of anterior temporal cortex to syntactic and prosodic manipulations during sentence processing. Human Brain Mapping, 26(2), 128–138. 10.1002/hbm.20148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- January D, Trueswell JC, & Thompson-Schill SL (2009). Co-localization of Stroop and Syntactic Ambiguity Resolution in Broca’s Area. Journal of Cognitive Neuroscience, 21(12), 2434–2444. 10.1162/jocn.2008.21179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, & Carpenter PA (1992). A capacity theory of comprehension: Individual differences in working memory. Psychological Review, 99(1), 122. [DOI] [PubMed] [Google Scholar]
- King J, & Just MA (1991). Individual Differences in Syntactic Processing: The Role of Working Memory Journal of Memory and Language; New York, 30(5), 580–602. [Google Scholar]
- Kjelgaard MM, & Speer SR (1999). Prosodic facilitation and interference in the resolution of temporary syntactic closure ambiguity. Journal of Memory and Language, 40, 153–194. [Google Scholar]
- Lachaud CM, & Renaud O (2011). A tutorial for analyzing human reaction times: How to filter data, manage missing values, and choose a statistical model. Applied Psycholinguistics, 32(02), 389–416. 10.1017/S0142716410000457 [DOI] [Google Scholar]
- LaCroix AN, Blumenstein N, Houlihan C, & Rogalsky C (2019). The effects of prosody on sentence comprehension: Evidence from a neurotypical control group and seven cases of chronic stroke. Neurocase. Retrieved from https://www.tandfonline.com/doi/abs/10.1080/13554794.2019.1630447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky EZ, Weider WE, & Johnson JP (1976). Influence of Linguistic Complexity, Rate of Presentation, and Interphrase Pause Time on Auditory-Verbal Comprehension of Adult Aphasic Patients. Brain and Language, 3, 386–395. [DOI] [PubMed] [Google Scholar]
- Love T, Swinney D, Walenski M, & Zurif E (2008). How left inferior frontal cortex participates in syntactic processing: Evidence from aphasia. Brain and Language, 107(3), 203–219. 10.1016/j.bandl.2007.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusdottir S, Fillmore P, den Ouden DB, Hjaltason H, Rorden C, Kjartansson O, … Fridriksson J (2013). Damage to left anterior temporal cortex predicts impairment of complex syntactic processing: A lesion-symptom mapping study: ATL Damage Impairs Complex Syntax Processing. Human Brain Mapping, 34(10), 2715–2723. 10.1002/hbm.22096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzig P, Vasishth S, Engelmann F, Caplan D, & Burchert F (2018). A Computational Investigation of Sources of Variability in Sentence Comprehension Difficulty in Aphasia. Topics in Cognitive Science, 10(1), 161–174. 10.1111/tops.12323 [DOI] [PubMed] [Google Scholar]
- Mayer AR, Harrington D, Adair JC, & Lee R (2006). The neural networks underlying endogenous auditory covert orienting and reorienting. NeuroImage, 30(3), 938–949. 10.1016/j.neuroimage.2005.10.050 [DOI] [PubMed] [Google Scholar]
- McElree B, Foraker S, & Dyer L (2003). Memory structures that subserve sentence comprehension. Journal of Memory and Language, 48(1), 67–91. 10.1016/S0749-596X(02)00515-6 [DOI] [Google Scholar]
- Meyer AM, Mack JE, & Thompson CK (2012). Tracking Passive Sentence Comprehension in Agrammatic Aphasia. Journal of Neurolinguistics, 25(1), 31–43. 10.1016/j.jneuroling.2011.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Steinhauer K, Alter K, Friederici AD, & von Cramon DY (2004). Brain activity varies with modulation of dynamic pitch variance in sentence melody. Brain and Language, 89(2), 277–289. 10.1016/S0093-934X(03)00350-X [DOI] [PubMed] [Google Scholar]
- Minkina I, Rosenberg S, Kalinyak-Fliszar M, & Martin N (2017). Short-Term Memory and Aphasia: From Theory to Treatment. Seminars in Speech and Language, 38(1), 17–28. 10.1055/s-0036-1597261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray LL (2012). Attention and Other Cognitive Deficits in Aphasia: Presence and Relation to Language and Communication Measures. American Journal of Speech-Language Pathology, 21(2), S51–S64. 10.1044/1058-0360(2012/11-0067) [DOI] [PubMed] [Google Scholar]
- Murray LL, Holland AL, & Beeson PM (1997). Auditory processing in individuals with mild aphasia: A study of resource allocation. Journal of Speech, Language, and Hearing Research, 40(4), 792–808. [DOI] [PubMed] [Google Scholar]
- Nachev P, Coulthard E, Jager HR, Kennard C, & Husain M (2008). Enantiomorphic normalization of focally lesioned brains. NeuroImage, 39(3), 1215–1226. 10.1016/j.neuroimage.2007.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SD, Malaia E, Seo R, & Cheng H (2013). The effect of individual differences in working memory capacity on sentence comprehension: An fMRI study. Brain Topography, 26(3), 458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick JM, Kan IP, Trueswell JC, & Thompson-Schill SL (2009). A case for conflict across multiple domains: Memory and language impairments following damage to ventrolateral prefrontal cortex. Cognitive Neuropsychology, 26(6), 527–567. 10.1080/02643290903519367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick JM, Trueswell JC, & Thompson-Schill SL (2005). Cognitive control and parsing: Reexamining the role of Broca’s area in sentence comprehension. Cognitive, Affective, & Behavioral Neuroscience, 5(3), 263–281. 10.3758/CABN.5.3.263 [DOI] [PubMed] [Google Scholar]
- Oers CAMM, Worp HB, Kappelle LJ, Raemaekers MAH, Otte WM, & Dijkhuizen RM (2018). Etiology of language network changes during recovery of aphasia after stroke. Scientific Reports, 8(1), 856 10.1038/s41598-018-19302-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashek GV, & Brookshire RH (1982). Effects of Rate of Speech and Linguistic Stress on Auditory Paragraph Comprehension of Aphasic Individuals. Journal of Speech, Language, and Hearing Research, 25(3), 377–383. 10.1044/jshr.2503.377 [DOI] [PubMed] [Google Scholar]
- Patil U, Hanne S, Burchert F, De Bleser R, & Vasishth S (2016). A Computational Evaluation of Sentence Processing Deficits in Aphasia. Cognitive Science, 40(1), 5–50. 10.1111/cogs.12250 [DOI] [PubMed] [Google Scholar]
- Pell MD (1998). Recognition of prosody following unilateral brain lesion: Influence of functional and structural attributes of prosodic contours. Neuropsychologia, 36(8), 701–715. 10.1016/S0028-3932(98)00008-6 [DOI] [PubMed] [Google Scholar]
- Pell MD, & Baum SR (1997). Unilateral brain damage, prosodic comprehension deficits, and the acoustic cues to prosody. Brain and Language, 57(2), 195–214. [DOI] [PubMed] [Google Scholar]
- Perkins JM, Baran JA, & Gandour J (1996). Hemispheric specialization in processing intonation contours. Aphasiology, 10(4), 343–362. [Google Scholar]
- Perneger TV (1998). What’s wrong with Bonferroni adjustments. BMJ : British Medical Journal, 316(7139), 1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, & Posner MI (2012). The Attention System of the Human Brain: 20 Years After. Annual Review of Neuroscience, 35, 73–89. 10.1146/annurev-neuro-062111-150525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew C, & Hillis AE (2014). Role for Memory Capacity in Sentence Comprehension: Evidence from Acute Stroke. Aphasiology, 28(10), 1258–1280. 10.1080/02687038.2014.919436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay SB, Binder JR, Humphries C, Gross WL, & Book DS (2017). Lesion localization of speech comprehension deficits in chronic aphasia. Neurology, 88(10), 970–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompon RH, McNeil MR, Spencer KA, & Kendall DL (2015). Intentional and Reactive Inhibition During Spoken-Word Stroop Task Performance in People With Aphasia. Journal of Speech, Language, and Hearing Research : JSLHR, 58(3), 767–780. 10.1044/2015_JSLHR-L-14-0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, & Petersen SE (1990). The attention system of the human brain. Annual Review of Neuroscience, 13(1), 25–42. [DOI] [PubMed] [Google Scholar]
- Potagas C, Kasselimis D, & Evdokimidis I (2011). Short-term and working memory impairments in aphasia. Neuropsychologia, 49(10), 2874–2878. 10.1016/j.neuropsychologia.2011.06.013 [DOI] [PubMed] [Google Scholar]
- Randolph C (1998). Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). San Antonio, TX: Psychological Corporation. [Google Scholar]
- Ratcliff R (1993). Methods for dealing with reaction time outliers. Psychological Bulletin, 114(3), 510. [DOI] [PubMed] [Google Scholar]
- Rinne P, Hassan M, Goniotakis D, Chohan K, Sharma P, Langdon D, … Bentley P (2013). Triple dissociation of attention networks in stroke according to lesion location. Neurology, 81(9), 812–820. 10.1212/WNL.0b013e3182a2ca34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts KL, Summerfield AQ, & Hall DA (2006). Presentation modality influences behavioral measures of alerting, orienting, and executive control. Journal of the International Neuropsychological Society, 12(04). 10.1017/S1355617706060620 [DOI] [PubMed] [Google Scholar]
- Rogalsky C, Almeida D, Sprouse J, & Hickok G (2015). Sentence processing selectivity in Broca’s area: Evident for structure but not syntactic movement. Language, Cognition and Neuroscience, 30(10), 1326–1338. 10.1080/23273798.2015.1066831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalsky C, & Hickok G (2011). The role of Broca’s area in sentence comprehension. Journal of Cognitive Neuroscience, 23(7), 1664–1680. [DOI] [PubMed] [Google Scholar]
- Rogalsky C, LaCroix AN, Chen K-H, Anderson SW, Damasio H, Love T, & Hickok G (2018). The Neurobiology of Agrammatic Sentence Comprehension: A Lesion Study. Journal of Cognitive Neuroscience, 30(2), 234–255. 10.1162/jocn_a_01200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalsky C, Matchin W, & Hickok G (2008). Broca’s area, sentence comprehension, and working memory: An fMRI study. Frontiers in Human Neuroscience, 2(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncaglia-Denissen MP, Schmidt-Kassow M, & Kotz SA (2013). Speech Rhythm Facilitates Syntactic Ambiguity Resolution: ERP Evidence. PLoS ONE, 8(2), e56000 10.1371/journal.pone.0056000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden Chris, & Brett M (2000). Stereotaxic display of brain lesions. Behavioural Neurology, 12(4), 191–200. [DOI] [PubMed] [Google Scholar]
- Rorden Christopher, Bonilha L, Fridriksson J, Bender B, & Karnath H-O (2012). Age-specific CT and MRI templates for spatial normalization. Neuroimage, 61(4), 957–965. 10.1016/j.neuroimage.2012.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Huang S, Furtak SC, Belliveau JW, & Ahveninen J (2014). Functional connectivity of dorsal and ventral frontoparietal seed regions during auditory orienting. Brain Research, 1583, 159–168. 10.1016/j.brainres.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ (1990). No Adjustments Are Needed for Multiple Comparisons. Epidemiology, 1(1), 43–46. [PubMed] [Google Scholar]
- Santi A, & Grodzinsky Y (2007). Working memory and syntax interact in Broca’s area. NeuroImage, 37(1), 8–17. 10.1016/j.neuroimage.2007.04.047 [DOI] [PubMed] [Google Scholar]
- Schafer A (1996). Focus in relative clause construal. Language and Cognitive Processes, 11(1–2), 135–164. [Google Scholar]
- Schafer AJ (1997). Prosodic parsing: The role of prosody in sentence comprehension (PhD Thesis). University of Massachusetts at Amherst. [Google Scholar]
- Sheppard SM, Love T, Midgley KJ, Holcomb PJ, & Shapiro LP (2017). Electrophysiology of prosodic and lexical-semantic processing during sentence comprehension in aphasia. Neuropsychologia, 107, 9–24. 10.1016/j.neuropsychologia.2017.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer SR, Kjelgaard MM, & Dobroth KM (1996). The influence of prosodic structure on the resolution of temporary syntactic closure ambiguities. Journal of Psycholinguistic Research, 25(2), 249–271. 10.1007/BF01708573 [DOI] [PubMed] [Google Scholar]
- Sperber C, Wiesen D, & Karnath H-O (2018). An empirical evaluation of multivariate lesion behaviour mapping using support vector regression. BioRxiv, 446153 10.1101/446153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thothathiri M, Kim A, Trueswell JC, & Thompson-Schill SL (2012). Parametric effects of syntactic-semantic conflict in Broca’s area during sentence processing. Brain and Language, 120(3), 259–264. 10.1016/j.bandl.2011.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Burght CL, Goucha T, Friederici AD, Kreitewolf J, & Hartwigsen G (2019). Intonation guides sentence processing in the left inferior frontal gyrus. Cortex, 117, 122–134. 10.1016/j.cortex.2019.02.011 [DOI] [PubMed] [Google Scholar]
- Van Dyke JA, & Johns CL (2012). Memory Interference as a Determinant of Language Comprehension. Language and Linguistics Compass, 6(4), 193–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villard S, & Kiran S (2017). To what extent does attention underlie language in aphasia? Aphasiology, 31(10), 1226–1245. 10.1080/02687038.2016.1242711 [DOI] [Google Scholar]
- Wildgruber D, Hertrich I, Riecker A, Erb M, Anders S, Grodd W, & Ackermann H (2004). Distinct Frontal Regions Subserve Evaluation of Linguistic and Emotional Aspects of Speech Intonation. Cerebral Cortex, 14(12), 1384–1389. 10.1093/cercor/bhh099 [DOI] [PubMed] [Google Scholar]
- Wilson SM, Dronkers NF, Ogar JM, Jang J, Growdon ME, Agosta F, … Gorno-Tempini ML (2010). Neural Correlates of Syntactic Processing in the Nonfluent Variant of Primary Progressive Aphasia. Journal of Neuroscience, 30(50), 16845–16854. 10.1523/JNEUROSCI.2547-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipse L, Worek A, Guarino AJ, & Shattuck-Hufnagel S (2014). Tapped Out: Do People With Aphasia Have Rhythm Processing Deficits? Journal of Speech, Language, and Hearing Research, 57 10.1044/2014_jslhr-l-13-0309 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.