ABSTRACT
High-mobility group box 1 (HMGB1) can act as a structural protein of the chromatin and at the same time as a mediator of the immune system. Its high correlation with the graft acceptance in pancreatic islet recipients makes it a biomarker in islet transplantation. With the suspicion that preexisting HMGB1 in the fetal bovine serum (FBS) would be detrimental to the viability and function of murine beta cells, HMGB1 was removed from FBS and its impact was investigated. Interestingly, the elimination of HMGB1 from FBS seemed unfavorable to the viability and function of cultured murine beta cells, suggesting that the preexisting HMGB1 in the FBS may be an indispensable component of islet cell culture.
KEYWORDS: Pancreatic islet, transplantation, HMGB1, FBS
High-mobility group box 1 (HMGB1) is one of the chromatin proteins in the nucleus that is involved in arrangement of DNA structure and hence in regulation of gene transcription, but can also act as damage-associated molecular patterns (DAMPs) when secreted by activated immune cells or dead cells, thereby regarded as a useful biomarker in various inflammatory disorders.1 These proteins as DAMPs bind to specific receptors such as Toll-like receptors (TLR) or receptor for advanced glycation end products (RAGE) and activate downstream signaling pathways leading to pro-inflammatory response or cell migration.2 Notably, these proteins are highly expressed in pancreatic islets and can be secreted from damaged islets to induce damages that contribute to early loss of transplanted islets.3 Therefore, it would be crucial to monitor and effectively control HMGB1 in pre- and post-islet transplantation period.
In islet transplantation, both clinical and pre-clinical, the healthiness of the donor islets would be one of the most crucial factors for successful engraftment.4 In addition to the means for appropriate procurement and isolation of the tissue,5 optimal culture condition for the isolated islets prior to implantation is vital, and FBS is often included in the culture medium of murine and human islet culture media to reach the goal.6,7 Even though some literatures point out that FBS might be harmful to cell cultures due to unknown, xenogeneic substances,8 its specific effect has not been studied well in pre-transplantation islet cultures.
Undoubtedly, HMGB1 has been known as the marker of islet injury9,10 and the released HMGB1, in turn, can damage other islets as described above. Hence, we hypothesized that FBS harbored HMGB1 due to its presence in almost all eukaryotic cells11 and this preexisting HMGB1 would negatively affect cultured islet beta cells. First, the existence of HMGB1 in commercial FBS (Young In Frontier, Seoul, Korea) was checked by ELISA and western blot (Supplementary Figure 1). Sandwich ELISA protocol for HMGB1 detection was devised based on a previous study.12 FBS signal, independently or within the cell culture media (10% in RPMI 1640), was assessed. To our expectation, the HMGB1 signal in FBS rose dose-dependently following a semi-log curve (Supplementary Figure 1A). Moreover, the recombinant human HMGB1 (rHMGB1; R&D Biosystems, Minneapolis, MN) signals in FBS-free RPMI 1640 showed almost identical trend as that in standard dilution buffer compared to that in 10% FBS-supplemented RPMI 1640 (Supplementary Figure 1B), indicating that the FBS was the sole factor that had affected the rHMGB1 signal detected by ELISA. Nonetheless, at this point, it could not be guaranteed that other confounding factors in the FBS may have interfered with the ELISA signals.
To explore further into the effects of preexisting HMGB1 in FBS, the HMGB1 was depleted from the FBS via immunoprecipitation, and its absence was confirmed by SDS-PAGE and western blot analysis. Briefly, for the elimination of preexisting HMGB1, Pierce™ Classic Magnetic IP/Co-IP Kit (Thermo Fisher Scientific, Waltham, MA) was used with mouse anti-human rHMGB1/HMG-1 IgG2B according to the manufacturer’s protocol. Half of the FBS samples were processed without the antibodies to serve as the negative control, and after centrifugation, only the supernatant was used for the experiments. The amount of remaining HMGB1 in the FBS was checked by 12% SDS-PAGE and western blot (Supplementary Figure 1C). The immunoprecipitation and subsequent ELISA has proven that there was a certain amount of preexisting HMGB1 in the FBS: extrapolation from the OD of rHMGB1 in 10% FBS-supplemented RPMI 1640 produced the background HMGB1 concentration difference which was almost equal to 8 ng/mL with or without HMGB1 removal from the FBS (Supplementary Figure 1D; 15.28 ± 1.683 vs. 23.16 ± 1.496). It was noteworthy that in contrast to the results in Supplementary Figure 1B, the depletion of HMGB1 did not rescue the ELISA signal specificity and sensitivity (Supplementary Figure 2), confirming that factors other than preexisting HMGB1 had interfered with ELISA signals.
Since the detrimental effect of extracellular HMGB1 on pancreatic islet cells has been well known,3,13 we sought to investigate whether this ‘background’ HMGB1 in FBS had physiologically influenced cultured beta cells. For primary islet experiment, female C57BL/6N (B6) mice were purchased from Jackson Laboratories (Bar Harbor, ME). The mice were housed in Institute for Experimental Animals at Seoul National University College of Medicine. All the animal experiments were approved by the Seoul National University Institutional Animal Care and Use Committee (IACUC; IACUC no. SNU-170518-3-2). Murine islet isolation was performed as previously described,14 and the isolated islets were cultured in RPMI 1640 media supplemented with Anti-Anti and 10% of either normal FBS or HMGB1-removed FBS. In addition, mouse insulinoma cells (MIN6) were cultured in DMEM supplemented with Antibiotic-Antimycotic (Anti-Anti; Thermo Fisher Scientific) and 15% of either normal FBS or HMGB1-removed FBS for 48 h prior to the experiments.
To account for the change in viability, a modified MTT assay was carried out using Cell Counting Kit 8 (CCK8; Dojindo Molecular Technologies, Inc., Rockville, MD) according to the manufacturer’s protocols. The absorbance of the culture supernatant was read at 450 nm with 650 nm as reference using a Sunrise absorbance microplate reader (Tecan Life Sciences). After the 48-h culture, it was revealed that the removal of preexisting HMGB1 resulted in a significant reduction of the viability of cultured primary islet cells (P < .0001; Figure 1A). The same phenomenon could be observed in MIN6 cells (P < .0001; Figure 1B). To complement the viability evaluation by CCK8, flow cytometry analysis was performed on MIN6 cells using Annexin V-APC Apoptosis Detection Kit with Propidium Iodide (PI; Biolegend, San Diego, CA) according to the manufacturer’s protocols. MIN6 cells (5 × 105 cells) or single cell-dissociated murine islets (3 × 104 cells) were stained with Annexin V-APC and PI and analyzed with FACSII Canto (BD Biosciences, San Jose, CA). The apoptotic state of MIN6 cells was also determined by the tetramethylrhodamine ethyl ester (TMRE) staining method,15 as reported previously.16 Consistent with CCK8 data, flow cytometry analysis also indicated that more cells became non-viable with preexisting HMGB1 removal (P < .05; Figure 1C). Invariably, culturing with or without preexisting HMGB1 resulted in a significant difference in TMRE-positive MIN6 cells (P < .001; Figure 1D). TMRE-positivity indicates general mitochondria healthiness. Though the flow cytometry analysis of islet single cells did not demonstrate a consistent, significant difference in viability (Supplementary Figure 3), much of these data indicated that the absence of preexisting HMGB1 from FBS affected the cultured pancreatic beta cells negatively.
Figure 1.
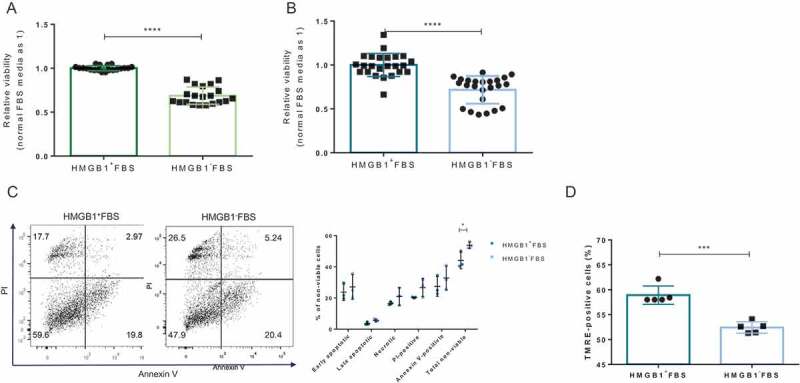
The effect of preexisting HMGB1 removal on the viability of cultured beta cells. 1 × 105 MIN6 cells or 300 IEQs of murine islets were incubated with the CCK8 reagent for 2 h in a 37°C, 5% CO2 incubator. (A) CCK8 assay results of cultured primary islets. Data are presented as mean ± SD. ****, P < .0001 as calculated by unpaired t-test. (B) CCK8 assay results of cultured MIN6 cells. Data are presented as mean ± SD. ****, P < .0001 as calculated by unpaired t-test. (C) Viability of MIN6 cells determined by Annexin V and PI staining. A representative scatter plot is shown. *, P < .05 as calculated by unpaired t-test. (D) Cell viability of MIN6 cells was also determined by TMRE staining. 5 × 105 MIN6 cells were trypsinized and stained with 150 nM of TMRE for 5 min at RT in the dark. Data are presented as mean ± SD. ***, P < .0001 as calculated by unpaired t-test.
To characterize the changed viability of culture beta cells, qRT-PCR was performed on MIN6 cells as described by others16,17 to quantify pro- and antiapoptotic gene expressions. After normalization of the results, it was revealed that HMGB1 removal from FBS did not result in the upregulation of proapoptotic factors or downregulation of antiapoptotic factors in culture islet beta cells (Supplementary Figure 4). Meanwhile, the protein levels of inflammatory markers in the culture supernatants of primary islets were assessed via Mouse Th1/Th2/Th17 Cytokine Kit (BD Biosciences) as recommended by the manufacturer. After the cytometric bead assay (CBA), it was discovered that the IL-6 levels in the cell culture supernatants differed significantly depending on the existence of preexisting HMGB1 in the FBS (P < .01; Figure 2). The significant difference in the level of other cytokines was not detected.
Figure 2.
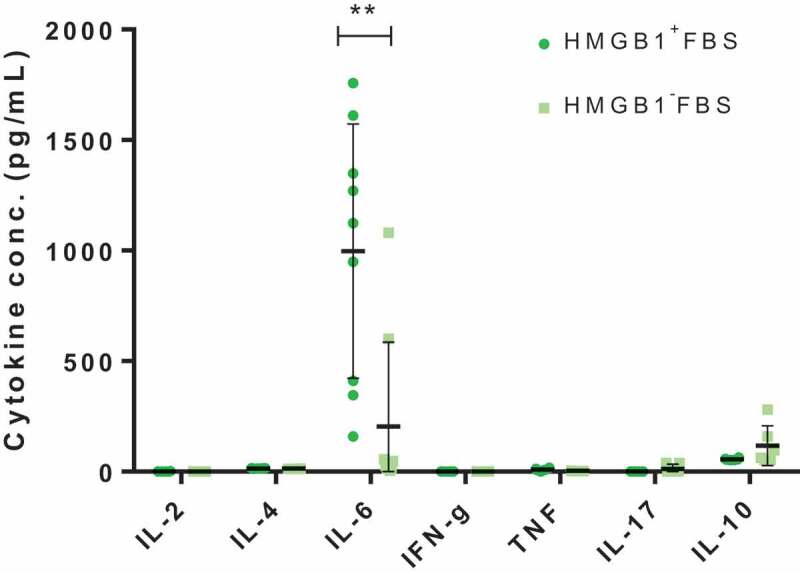
The effect of HMGB1-depletion from the FBS on pancreatic islet cell culture. The amount of cytokines within culture supernatants of primary islets (150 IEQs) incubated 48 h with or without preexisting HMGB1 removal, measured via CBA. Data are presented as mean ± SD. **, P < .01 as calculated by unpaired t-test.
Subsequently, the effect of preexisting removal of HMGB1 from FBS on islets was functionally assessed via ex vivo static glucose-stimulated insulin secretion (GSIS) assay. Briefly, the assay was conducted using a 96-well plate islet insulin secretion assay protocol essentially as in Truchan et al.’s study.18 Mouse insulin ELISA (ALPCO, Salem, NH) was run according to the manufacturer’s protocol (the dilution factors were pre-determined by candidate dilution factor test). To account for differences in islet size, the total insulin content was used to normalize the amounts of insulin secreted to 2 mM- and 20 mM-glucose solutions. Notably, the results of GSIS indicated that HMGB1 removal from FBS was unfavorable to the islet function: islets cultured with HMGB1-removed FBS secreted significantly less insulin upon stimulation with glucose at 20 mM (P < .0001; Figure 3). In the meantime, there was no significant difference in the steady-state insulin secretion (2 mM) although the mean insulin secretion was reduced (P = .4237).
Figure 3.
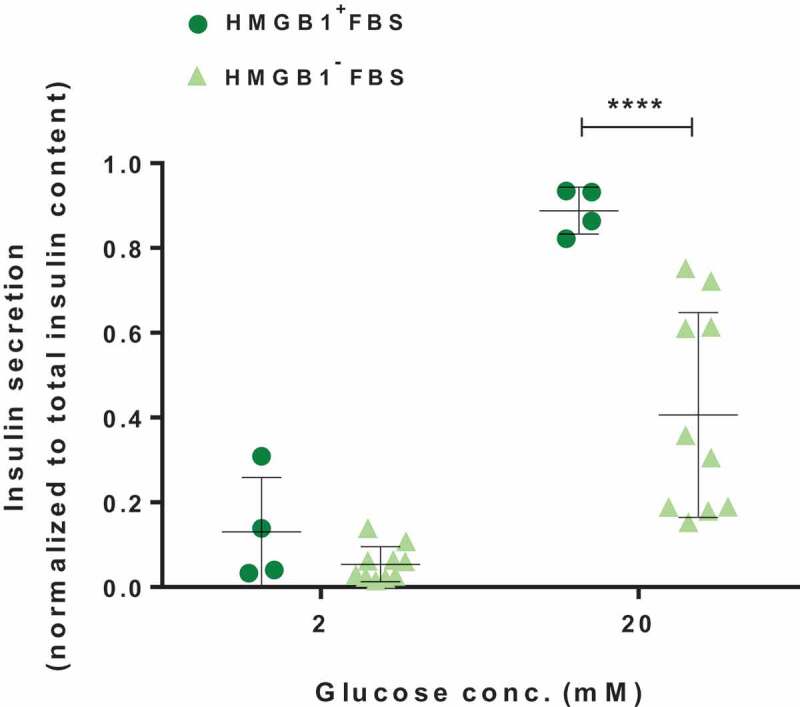
GSIS of pancreatic islet cells cultured in media with or without preexisting HMGB1 removal. The islets were equilibrated for 1 h in 2 mM glucose solution, and then incubated sequentially for 1 h each in 2 mM and 20 mM glucose solutions. Data are presented as mean ± SD. ****, P < .0001 as calculated by unpaired t-test.
In sum, it was discovered that the depletion of HMGB1 from FBS resulted in reduced viability and function of cultured islet beta cells. Consequently, it was inevitable to test whether the re-addition of complementary amount of HMGB1 could restore the viability and function of cultured islet beta cells. For every islet beta cell culture supplemented with HMGB1-depleted FBS, 10 ng/mL of rHMGB1 was introduced to investigate the effect. To eliminate any confounding factor, the culture media were 0.2 μm-filtered before culture experiments and the endotoxin level was tested with ToxinSensorTM Chromogenic LAL Endotoxin Assay Kit (Genscript, Piscataway, NJ) according to the manufacturer’s protocols to ensure the purity of FBS and rHMGB1, which were far under 0.1 EU/μg (Supplementary Figure 5).
Interestingly, the addition of 10 ng/mL of rHMGB1 enhanced the viability of primary islets significantly (P < .05; Figure 4A), nearly to the level before the depletion of preexisting HMGB1 within the FBS. However, the addition of an excessive amount of rHMGB1 (100 ng/mL) could not enhance the viability to a higher degree. The change in the viability of MIN6 cells also showed a consistent pattern (P < .05; Figure 4B). Nonetheless, just as in primary islets, an excessive amount of rHMGB1 (100 ng/mL) could not raise the viability of MIN6 cells (Supplementary Figure 6A). We then went on to check if rHMGB1 addition alone (10 ng/mL) to the FBS-free media could sustain MIN6 cells. As shown in Supplementary Figure 6B, serum starvation decreased MIN6 cell viability (P < .0001) and rHMGB1 addition exacerbated the effect (P < .01), indicating that preexsiting HMGB1 was probably not the dominant factor in FBS for optimal beta cell culture. In addition, the 7-AAD assay was performed to evaluate the cell viability via flow cytometry.19 The analysis after 7-AAD staining similarly indicated improved viability after rHMGB1 addition in MIN6 cells (Supplementary Figure 7). The function of primary islets also demonstrated a difference after the addition of rHMGB1 (Figure 4C). Contrarily to the results of culture experiments with HMGB1-depleted FBS (Figure 3), the steady-state insulin secretion increased significantly (P < .05) but the stimulated-state insulin secretion did not (P = .3170). Altogether, our results showed that re-addition of rHMGB1 to the media rescued the viability and function of pancreatic islet cells, pointing to the possibility and HMGB1 in the FBS could indeed be an essential component for islet cell culture.
Figure 4.
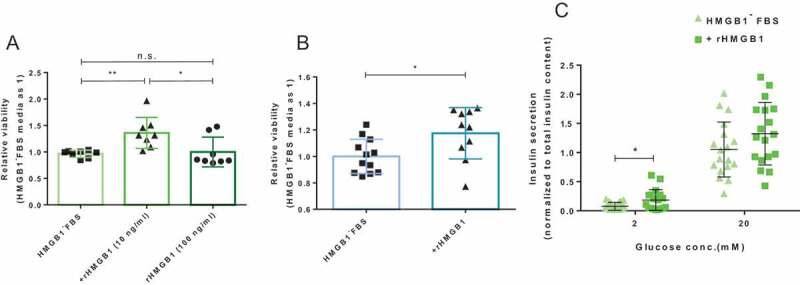
The effects of rHMGB1 re-addition on beta cell viability and function. After the cells were cultured for 48 h with various amounts of rHMGB1 (10 ng/mL or 100 ng/mL) cells were incubated with the CCK8 reagent for 2 h in a 37°C, 5% CO2 incubator. (A) CCK8 assay results of cultured primary islets (300 IEQs). Data are presented as mean ± SD. **, P < .01; *, P < .05 as calculated by unpaired t-test. (B) CCK8 assay results of cultured MIN6 cells (1 x 105). Data are presented as mean ± SD. *, P < .05 as calculated by unpaired t-test. (C) GSIS of pancreatic islet cells cultured in HMGB1-depleted FBS-supplemented media with or without the addition of rHMGB1 (10 ng/mL). The islets were equilibrated for 1 h in 2 mM glucose solution and then incubated sequentially for 1 h each in 2 mM and 20 mM glucose solutions. Data are presented as mean ± SD. *, P < .05 as calculated by unpaired t-test.
In our study, the presence of HMGB1 in commercial FBS was checked primarily by sandwich ELISA experiments. The preexisting HMGB1 in the FBS was the alleged factor that hindered the specific and sensitive detection of HMGB1 in the cell culture supernatant (Supplementary Figure 1). However, immunoprecipitation and subsequent ELISA revealed that preexisting HMGB1 contributed only partly to the deterioration of signal specificity and sensitivity (Supplementary Figure 2), which should simply make other components of FBS the culprit of ELISA signal disturbance. It has been known that quantification of HMGB1 is difficult due to its inherent characteristics, such as its nonspecific binding to other proteins.20 This phenomenon seems very natural considering the promiscuity of HMGB1 which makes it a proficient DAMP.21 Still, we believe that a more accurate method should be formulated and standardized to circumvent the confounding factors for HMGB1 measurement in FBS-supplemented media. Mass spectrometry might be an alternative in this instance.22 Nevertheless, to our knowledge, HMGB1 ELISA signal interference by FBS has never been addressed before, presumably because the subtle difference of HMGB1 in the cell culture medium has rarely been the center of scientific research.
Even though the actual amount could be arbitrary depending on the chosen measurement method, we assumed that the presence of HMGB1 in FBS could raise concerns about its potential effects on cultured cells, given its high conservedness among mammals where murine HMGB1 (UniProt: P63158) and bovine HMGB1 (UniProt: P10103) were found to share 98.6% homology on BLASTP and because omitting FBS from the cell culture medium could not be a practical option in murine islet cultures. The implication of this notion is that the ‘background HMGB1ʹ that had been detected by ELISA might have had ‘background effects’ on the cultured cells. Intriguingly, the removal of preexisting HMGB1 from FBS actually had a negative effect on pancreatic islet viability (Figure 1), a result opposite of what we had hypothesized. In other words, HMGB1, a seemingly harmful protein to pancreatic islets, might have been an indispensable cell culture component. Extracellular HMGB1 has been long known to be harmful to islet beta cells, but some studies have indicated that the A-box fragment of HMGB1 could increase the survival of pancreatic islets.23,24 Also, HMGB1 was reported to be pro-autophagic in cells other than pancreatic islets by which enhanced cell survival.25,26 Whether the relationship between the preexisting HMGB1 in FBS and murine islets is associated with the A-box fragments’ effect or the change in viability is linked to the autophagy-apoptosis axis would be interesting subjects of research.
To delineate the phenomenon of viability shift, qRT-PCR and CBA were implemented. Although the differential expression of genes that might affect the viability of beta cells was not observed, we consequently went to check the protein-level difference of mediators, particularly cytokines. It has been well known that islets can produce and respond to various cytokines.27 CBA results indicated that the elimination of HMGB1 from FBS actually resulted in increased IL-6 levels in the primary islet cell culture supernatants (Figure 2). This observation seemed reasonable because the production of IL-6 by pancreatic beta cells has been documented before28 and our group previously reported that IL-6 showed islet-protective effects in vitro and in vivo.14 However, it should be noted that despite rare discussions on the relationship between HMGB1 and IL-6 concerning pancreatic islet biology, Itoh et al. recently reported that anti-IL-6R antibody treatment in mice prevented the HMGB1-mediated loss of transplanted islets.29 There were also reports that indicated the potential harmful effects of IL-6 on pancreatic islets.30 Therefore, we believe that a more in-depth study should be performed on the underlying mechanisms of the phenomenon we discovered, which might help explain the contradictory effects of IL-6 on pancreatic islet cells.
Undoubtedly, the viability and function of cells are closely linked, and our data demonstrated that pancreatic beta cells cultured in HMGB1-depleted FBS also showed decreased insulin-secretion function (Figure 3). Nevertheless, it should be noted that the reduced level of HMGB1 in the media could have impaired the secretion of insulin from beta cells. Guzman-Ruiz et al. reported that HMGB1 levels coincided with insulin release and intracellular Ca2+ concentrations in rat beta cell-line,31 although it should be investigated whether the same phenomenon could be observed in non-pathological conditions.
We believe this study led us to question our general assumptions on common in vitro experiments, where the knowledge and insights we had gathered via in vitro experiments could have had HMGB1’s hidden effect on it. Even at what seemed a negligible amount of difference (~10 ng/mL measured by our ELISA), the absence of preexisting HMGB1 affected the islet viability and function substantially and the re-addition restored them (Figure 4). It could be argued that omitting a factor from a well-designed culture medium would negatively affect the viability of cultured cells. Nonetheless, HMGB1 has been deemed very unfavorable to pancreatic islet cells and its depletion must have been beneficial to the cultured islet cells, yet we discovered a rather paradoxical phenomenon. Hence, this discovery alerts us to always question our previous knowledge and assumptions in the field of science.
Some literatures have pointed out that FBS might be harmful to cell cultures due to unknown, xenogeneic substances,8 and preventing the use of FBS would also be desirable in the socio-ethical context. The advent of chemically defined media could have been the ideal alternative in this case, but the insufficient capability to sustain cells besides its unaffordable price has hampered its wide application.32 According to our research, the addition of an appropriate amount of rHMGB1 could be the solution to this dilemma. Further analyses of this phenomenon could help find the more proper culture condition for maintaining pancreatic islets prior to transplantation.
In conclusion, we investigated the effect of this FBS-derived HMGB1 on the viability and function of pancreatic islets and uncovered that its removal was indeed detrimental to the islets. This discovery indicates the contradictory roles of HMGB1 in pancreatic islet physiology, which is under active investigation in other fields of biology. Also, this notion could shed light on the optimal culture condition for pancreatic islets, especially in the clinics where maintaining the wellness of donor islets is very important.
Funding Statement
This work was supported by a grant from the Korea Healthcare Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) and funding from the Ministry for Health and Welfare, Republic of Korea (Grant No. HI13C0954). Authors Sung Ji Hong and So Won Choi received a scholarship from the BK21-plus education program provided by the National Research Foundation of Korea.
Disclosure of potential conflicts of interest
The authors report no conflict of interest.
Acknowledgments
The authors would like to thank Boo Yeon Won and Jihyun Lee for their contributions in experimental setup and execution.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Venereau E, De Leo F, Mezzapelle R, Careccia G, Musco G, Bianchi ME.. HMGB1 as biomarker and drug target. Pharmacol Res. 2016;111:534–544. doi: 10.1016/j.phrs.2016.06.031. [DOI] [PubMed] [Google Scholar]
- 2.Braza F, Brouard S, Chadban S, Goldstein DR. Role of TLRs and DAMPs in allograft inflammation and transplant outcomes. Nat Rev Nephrol. 2016;12:281–290. doi: 10.1038/nrneph.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuoka N, Itoh T, Watarai H, Sekine-Kondo E, Nagata N, Okamoto K, Mera T, Yamamoto H, Yamada S, Maruyama I, et al. High-mobility group box 1 is involved in the initial events of early loss of transplanted islets in mice. J Clin Invest. 2010;120:735–743. doi: 10.1172/JCI41360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shapiro AJ, Pokrywczynska M, Ricordi C. Clinical pancreatic islet transplantation. Nat Rev Endocrinol. 2017;13:268. doi: 10.1038/nrendo.2016.178. [DOI] [PubMed] [Google Scholar]
- 5.Itoh T, Sugimoto K, Takita M, Shimoda M, Chujo D, SoRelle JA, Naziruddin B, Levy MF, Matsumoto S. Low temperature condition prevents hypoxia-induced islet cell damage and HMGB1 release in a mouse model. Cell Transplant. 2012;21:1361–1370. doi: 10.3727/096368912X637514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avgoustiniatos ES, Scott WE, Suszynski TM, Schuurman H-J, Nelson RA, Rozak PR, Mueller KR, Balamurugan AN, Ansite JD, Fraga DW, et al. Supplements in human islet culture: human serum albumin is inferior to fetal bovine serum. Cell Transplant. 2012;21:2805–2814. doi: 10.3727/096368912X653138. [DOI] [PubMed] [Google Scholar]
- 7.Noguchi H, Miyagi-Shiohira C, Kurima K, Kobayashi N, Saitoh I, Watanabe M, Noguchi Y, Matsushita M. Islet culture/preservation before islet transplantation. Cell Med. 2015;8:25–29. doi: 10.3727/215517915X689047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Valk J, Bieback K, Buta C, Cochrane B, Dirks WG, Fu J, Hickman JJ, Hohensee C, Kolar R, Liebsch M, et al. Fetal bovine serum (FBS): past–present–future. ALTEX-Altern Anim Exp. 2018;35:99–118. doi: 10.14573/altex.1705101. [DOI] [PubMed] [Google Scholar]
- 9.Itoh T, Iwahashi S, Kanak MA, Shimoda M, Takita M, Chujo D, Tamura Y, Rahman AM, Chung WY, Onaca N, et al. Elevation of high-mobility group box 1 after clinical autologous islet transplantation and its inverse correlation with outcomes. Cell Transplant. 2014;23:153–165. doi: 10.3727/096368912X658980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itoh T, Takita M, SoRelle JA, Shimoda M, Sugimoto K, Chujo D, Qin H, Naziruddin B, Levy MF, Matsumoto S, et al. Correlation of released HMGB1 levels with the degree of islet damage in mice and humans and with the outcomes of islet transplantation in mice. Cell Transplant. 2012;21:1371–1381. doi: 10.3727/096368912X640592. [DOI] [PubMed] [Google Scholar]
- 11.Li G, Liang X, Lotze MT. HMGB1: the central cytokine for all lymphoid cells. Front Immunol. 2013;4:68. doi: 10.3389/fimmu.2013.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davé SH, Tilstra JS, Matsuoka K, Li F, DeMarco RA, Beer‐Stolz D, Sepulveda AR, Fink MP, Lotze MT, Plevy SE, et al. Ethyl pyruvate decreases HMGB1 release and ameliorates murine colitis. J Leukocyte Biol. 2009;86:633–643. doi: 10.1189/jlb.1008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itoh T, Iwahashi S, Shimoda M, Chujo D, Takita M, SoRelle J, Naziruddin B, Levy MF, Matsumoto S. High-mobility group box 1 expressions in hypoxia-induced damaged mouse islets. Transplant Proc. Elsevier, 2011;43:3156–3160. doi: 10.1016/j.transproceed.2011.09.100. [DOI] [PubMed] [Google Scholar]
- 14.Choi S-E, Choi K-M, Yoon I-H, Shin J-Y, Kim J-S, Park W-Y, Han D-J, Kim S-C, Ahn C, Kim J-Y, et al. IL-6 protects pancreatic islet beta cells from pro-inflammatory cytokines-induced cell death and functional impairment in vitro and in vivo. Transplant Immunol. 2004;13:43–53. doi: 10.1016/j.trim.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Crowley LC, Christensen ME, Waterhouse NJ. Measuring mitochondrial transmembrane potential by TMRE staining. Cold Spring Harb Protoc. 2016;2016:pdb. prot087361. [DOI] [PubMed] [Google Scholar]
- 16.Jin S-M, Kim KS, Lee S-Y, Gong C-H, Park SK, Shin JS, Park C-G, Kim S-J. The sequential combination of a JNK inhibitor and simvastatin protects porcine islets from peritransplant apoptosis and inflammation. Cell Transplant. 2011;20:1139–1151. doi: 10.3727/096368910X550170. [DOI] [PubMed] [Google Scholar]
- 17.Saliba Y, Bakhos JJ, Itani T, Farès N. An optimized protocol for purification of functional islets of Langerhans. Lab Invest. 2017;97(1):70. [DOI] [PubMed] [Google Scholar]
- 18.Truchan NA, Brar HK, Gallagher SJ, Neuman JC, Kimple ME. A single-islet microplate assay to measure mouse and human islet insulin secretion. Islets. 2015;7:e1076607. doi: 10.1080/19382014.2015.1076607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson S, Nguyen V, Coder D. Assessment of cell viability. Curr Protoc Cytom. 2013;64:(9.2. 1–9.2):26. doi: 10.1002/0471142956.cy0902s64. [DOI] [PubMed] [Google Scholar]
- 20.Dintilhac A, Bernués J. HMGB1 interacts with many apparently unrelated proteins by recognizing short amino acid sequences. J Biol Chem. 2002;277:7021–7028. doi: 10.1074/jbc.M108417200. [DOI] [PubMed] [Google Scholar]
- 21.Yanai H, Ban T, Wang Z, Choi MK, Kawamura T, Negishi H, Nakasato M, Lu Y, Hangai S, Koshiba R, et al. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009;462:99. doi: 10.1038/nature08512. [DOI] [PubMed] [Google Scholar]
- 22.Lu B, Antoine DJ, Kwan K, Lundbäck P, Wähämaa H, Schierbeck H, Robinson M, Van Zoelen MAD, Yang H, Li J, et al. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc Natl Acad Sci. 2014;111:3068–3073. doi: 10.1073/pnas.1316925111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang YH, Kim MJ, Lee Y-K, Lee M, Lee DY. HMGB1 modulation in pancreatic islets using a cell-permeable A-box fragment. J Control Release. 2017;246:155–163. doi: 10.1016/j.jconrel.2016.12.028. [DOI] [PubMed] [Google Scholar]
- 24.Jo EH, Hwang YH, Lee DY. Encapsulation of pancreatic islet with HMGB1 fragment for attenuating inflammation. Biomate Res. 2015;19:1. doi: 10.1186/s40824-015-0042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang D, Kang R, Livesey KM, Cheh C-W, Farkas A, Loughran P, Hoppe G, Bianchi ME, Tracey KJ, Zeh HJ, et al. Endogenous HMGB1 regulates autophagy. J Cell Biol. 2010;190:881–892. doi: 10.1083/jcb.200911078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrović A, Bogojević D, Korać A, Golić I, Jovanović-Stojanov S, Martinović V, Ivanović-Matić S, Stevanović J, Poznanović G, Grigorov I.. Oxidative stress-dependent contribution of HMGB1 to the interplay between apoptosis and autophagy in diabetic rat liver. J Physiol Biochem. 2017;73(4):511––521.. [DOI] [PubMed] [Google Scholar]
- 27.Donath MY, Böni-Schnetzler M, Ellingsgaard H, Halban PA, Ehses JA. Cytokine production by islets in health and diabetes: cellular origin, regulation and function. Trends Endocrinol Metab. 2010;21:261–267. doi: 10.1016/j.tem.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Campbell I, Cutri A, Wilson A, Harrison L. Evidence for IL-6 production by and effects on the pancreatic beta-cell. J Immunol. 1989;143:1188–1191. [PubMed] [Google Scholar]
- 29.Itoh T, Nitta T, Nishinakamura H, Kojima D, Mera T, Ono J, Kodama S, Yasunami Y. HMGB1-mediated early loss of transplanted islets is prevented by anti–IL-6R antibody in mice. Pancreas. 2015;44:166–171. doi: 10.1097/MPA.0000000000000188. [DOI] [PubMed] [Google Scholar]
- 30.Campbell IL, Hobbs MV, Dockter J, Oldstone M, Allison J. Islet inflammation and hyperplasia induced by the pancreatic islet-specific overexpression of interleukin-6 in transgenic mice. Am J Pathol. 1994;145:157. [PMC free article] [PubMed] [Google Scholar]
- 31.Guzmán-Ruiz R, Ortega F, Rodríguez A, Vázquez-Martínez R, Díaz-Ruiz A, Garcia-Navarro S, Giralt M, Garcia-Rios A, Cobo-Padilla D, Tinahones FJ, et al. Alarmin high-mobility group B1 (HMGB1) is regulated in human adipocytes in insulin resistance and influences insulin secretion in β-cells. Int J Obesity. 2014;38:1545. doi: 10.1038/ijo.2014.36. [DOI] [PubMed] [Google Scholar]
- 32.Karnieli O, Friedner OM, Allickson JG, Zhang N, Jung S, Fiorentini D, Abraham E, Eaker SS, Yong TK, Chan A, et al. A consensus introduction to serum replacements and serum-free media for cellular therapies. Cytotherapy. 2017;19:155–169. doi: 10.1016/j.jcyt.2016.11.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


