Abstract
Context
Skeletal fragility is a significant complication of type 1 diabetes (T1D), with an increased risk of fracture observed starting in childhood. Altered bone accrual and microarchitectural development during the critical peripubertal years may contribute to this fragility.
Objective
To evaluate differences in skeletal microarchitecture between girls with T1D and controls and to assess factors associated with these differences.
Design
Cross-sectional comparison.
Participants
Girls ages 10–16 years, 62 with T1D and 61 controls.
Results
Areal bone mineral density (BMD) measured by dual-energy x-ray absorptiometry did not differ between girls with and without T1D. At the distal tibia, trabecular BMD was 7.3 ± 2.9% lower in T1D (P = 0.013), with fewer plate-like and axially-aligned trabeculae. Cortical porosity was 21.5 ± 10.5% higher, while the estimated failure load was 4.7 ± 2.2% lower in T1D (P = 0.043 and P = 0.037, respectively). At the distal radius, BMD and microarchitecture showed similar differences between the groups but did not reach statistical significance. After stratifying by HbA1c, only those girls with T1D and HbA1c > 8.5% differed significantly from controls. P1NP, a marker of bone formation, was lower in T1D while CTX and TRAcP5b, markers of bone resorption and osteoclast number, respectively, did not differ. The insulin-like growth factor 1 (IGF-1) Z-score was lower in T1D, and after adjustment for the IGF-1 Z-score, associations between T1D status and trabecular microarchitecture were largely attenuated.
Conclusions
Skeletal microarchitecture is altered in T1D early in the course of disease and among those with higher average glycemia. Suppressed bone formation and lower circulating IGF-1 likely contribute to this phenotype.
Keywords: type 1 diabetes, bone density, microarchitecture, pediatrics
Individuals with T1D have a substantially higher fracture risk than that of the general population (1–3). Interestingly, this increase in fracture risk is not limited to adults with long-standing disease but begins in childhood (3). Several studies of biochemical markers of bone turnover in both children and adults indicate that T1D is a low bone-turnover state (4, 5); in particular, bone formation markers are lower among T1D patients compared with nondiabetic controls. Given that childhood is a period of rapid bone growth, with approximately 40% of peak bone mass accrued in the 4 years surrounding peak height velocity (6), and because marked changes in bone geometry and microarchitecture occur during puberty (7, 8), impaired bone accrual during this critical period may lower peak bone mass and alter bone microarchitecture, thereby conferring a life-long increased risk of fracture.
While areal bone mineral density (aBMD) measured by dual-energy x-ray absorptiometry (DXA) is lower among those with T1D compared with controls (9), this relatively small decrement is not sufficient to explain the magnitude of increase in fracture risk (1). Studies of volumetric BMD (vBMD) using peripheral quantitative computed tomography (pQCT) have suggested that T1D is associated with lower cortical area, but data are conflicting regarding associations with trabecular BMD (10–12). More recently, a study using high-resolution pQCT (HRpQCT), which enables detailed evaluation of skeletal microarchitecture, demonstrated that adults with T1D have lower trabecular bone volume (Tb.BV/TV). However, this association was observed only in adults with clinically diagnosed diabetic microvascular disease (13). Recent data indicate that bone microstructure, including cortical BMD and trabecular number and thickness, predict incident fracture independent of aBMD (14), suggesting that altered microstructure may contribute to increased fracture risk in T1D.
To better understand the skeletal fragility of patients with T1D and its etiology, we are studying bone accrual and microarchitectural development in girls with T1D and nondiabetic controls during the critical peripubertal years. We report results from our baseline comparison of girls with T1D and healthy controls. In addition, we examine the effects of glycemic control and diabetes-associated alterations in IGF-1 and its binding proteins on skeletal parameters.
Materials and Methods
Study participants
We enrolled girls ages 10–16 years for this study. Subjects with T1D (n = 62) had a duration of disease of ≥ 1 year and either had positive autoantibodies or were diagnosed by their primary endocrinologist based on clinical history. Type 1 diabetes subjects were recruited from local pediatric endocrine clinics and diabetes summer camps. Control subjects (n = 61) were recruited from the community via flyers and a direct mail campaign. Exclusion criteria included medical conditions other than T1D anticipated to affect skeletal metabolism, including hypogonadism, hyperthyroidism, celiac disease, renal disease, vitamin D deficiency (25OHD < 20 ng/mL with a parathyroid hormone [PTH] above the upper limit of normal), and underweight or obesity (BMI ≤ 5th or ≥ 95th percentile for age respectively); subjects with controlled hypothyroidism (TSH < 7.0 uIU/mL) were included. Subjects were additionally excluded for use of bone-active medications (oral glucocorticoids for greater than 3 months, lithium, and hormonal birth control). We limited enrollment to girls given well-described differences in the timing and trajectory of bone microarchitecture development between boys and girls (7, 15). Informed consent and assent were obtained from a parent/guardian and the subjects, respectively. This study was approved by the Partners Human Research Committee and was registered with ClinicalTrials.gov (ID NCT02140424).
Clinical and biochemical investigation
A standardized physical examination was performed, including breast Tanner staging by a single pediatric endocrinologist (DMM), height, and metabolic weight. Subjects self-identified race. Fasting blood samples were obtained in the morning (prior to 10:00 am), and fasting second-voided urine samples were obtained. Fasting blood concentrations of glucose, hemoglobin A1C (HbA1c), calcium, phosphate, creatinine, bicarbonate, PTH, and urine calcium, phosphate, and creatinine were measured by a reference laboratory with standard methods (LabCorp, Burlington, North Carolina). 25OHD was measured by liquid chromatography-mass spectrometry (Mayo Medical Laboratories, Rochester, Minnesota) with a lower limit of detection of 6 ng/mL and an interassay CV of 6–9%. Procollagen type I N terminal propeptide (P1NP) and CTX were measured by chemiluminescent immunoassay (IDS, Tyne & Wear, United Kingdom), with intraassay CVs of 2.9% and 3.2%, respectively, and interassay CVs of 4.6% and 6.2% respectively. TRAcP5b was measured by ELISA (IDS), with intraassay and interassay CVs of 9.0% and 7.6%, respectively. IGF-1 was measured by liquid chromatography-mass spectrometry, with CVs of 6.0–8.8%. and an IGF-1 Z-score (normalized for age given substantial physiologic variation in childhood and adolescence) was calculated (Quest Diagnostics, San Juan Capistrano, USA). IGFBP-1 was measured by ELISA (R&D, CVs not determined) and IGFBP-3 was measured by chemiluminescence (IDS, intraassay CV 1.9%, interassay CV 6.4%) (Maine Medical Center Research Institute, Scarborough, Maine). The molar ratio of IGF-1 to IGFBP-3 was calculated as described (16). Medical records for subjects with T1D were reviewed for age and antibody status at diagnosis. An x-ray of the left hand was obtained for bone age (17). Dietary and supplemental calcium intake was evaluated with a validated food frequency questionnaire (18). Physical activity was assessed with the Children’s Physical Activity Questionnaire (cPAQ) (19).
DXA imaging
Scans of the whole body, spine, hip, and distal radius were obtained (Hologic Horizon-A, Marlborough, Maine) for bone mineral density and body composition (least significant change 0.042 g/cm2, 0.024 g/cm2, 0.048 g/cm2, 0.024 g/cm2 for spine, total hip, femoral neck, and distal radius, respectively). Z-scores were generated with Hologic Apex 3.3 software. Trabecular bone score was assessed with TBS Insight software (version 3.0.2.0, Medimaps Group, Geneva, Switzerland).
HRpQCT imaging
We measured compartment-specific volumetric densities and microarchitecture of the distal radius (7% site) and distal tibia (8% site) with HRpQCT (XtremeCT, Scanco Medical AG, Brüttisellen, Switzerland) (20, 21). A region of interest spanning 9.02 mm was scanned with an isotropic voxel size of 82 μm. We scanned the non-dominant limb unless there was a history of fracture in which case the dominant side was scanned. Same-day reproducibility for repeated measurements is 0.2% to 1.4% for volumetric bone mineral density (vBMD) values, 0.3% to 8.6% for trabecular microarchitecture parameters, and 0.6% to 2.4% for cortical microarchitecture parameters. We excluded 14 radius scans (4 from the T1D group and 10 from the control group) from our analysis due to motion artifact. The trabecular compartment was further evaluated using individual trabecula segmentation to assess rod-like and plate-like trabeculae and their microarchitectural features (22) given the strong correlations of plate-like trabeculae specifically with empirically determined bone strength (23). Micro-finite element analysis (FEA) was used to estimate failure load in the setting of simulated axial compression (24).
Statistical analyses
Study data were collected and managed using the REDCap electronic data capture software (https://projectredcap.org/resources/citations) hosted at Partners Healthcare (25). Analyses were performed with Stata 12.1 (StataCorp LP, College Station, Texas). Statistical significance was defined as 2-sided P < 0.05. This paper reports the baseline data for an ongoing longitudinal study of bone accrual; our power calculations were based on longitudinal changes in vBMD and are thus not directly applicable to this analysis. Demographic and clinical characteristics are reported as mean ± standard deviation for continuous data and as percentages for categorical data. Continuous data were compared by the student’s t-test and proportions by Fisher’s exact test. DXA data were compared by the student’s t-test for unadjusted comparisons and repeated using multivariable regression when adjusting for height and weight. HRpQCT data were compared using multivariable regression, adjusting for height, weight, and bone age, and where specified, for 25OHD or the IGF-1 Z-score. Bone turnover markers, IGF-1 and IGFBPs, were compared using multivariable regression after adjusting for bone age, with the exception of the IGF1 Z-score, which incorporates age in its calculation. For comparisons among 3 groups, an ANCOVA was performed and, if significant at P < 0.05, pairwise comparisons were then performed. Testing for a linear trend across groups (control, T1D with low HbA1c, T1D with high HbA1c) was performed after linear regression with orthogonal polynomial contrasts.
Results
Subject characteristics
Table 1 shows the clinical characteristics of the 123 study subjects; the groups were well-matched for age, bone age, race, and pubertal status, and there was no difference in prevalent fracture history. Of the 62 subjects with T1D, the mean age at diagnosis was 8.8 ± 3.0 years and the mean duration of disease was 4.8 ± 3.2 years. The mean HbA1c of 8.6% among the T1D subjects is comparable to that seen in youth in the T1D Exchange, a large US-based registry, in which the mean HbA1c was 8.5% and 9.2% in patients ages 6–12 and 13–17 years, respectively (26). No subjects with T1D had persistent microalbuminuria, nephropathy, neuropathy, or retinopathy. As expected, HbA1c was higher among subjects with T1D than the control subjects. T1D subjects had a significantly lower mean 25OHD and correspondingly higher PTH than control subjects, though there were no differences in serum calcium or phosphate, nor were there differences in urine calcium or phosphate excretion. Z-scores for both weight and BMI were higher among the T1D subjects; on examination of body composition as measured by DXA, this appeared to be driven by nonsignificant increases in both fat and lean mass, as the percent body fat did not differ between the groups.
Table 1.
Clinical Characteristics of Controls and Subjects With Type 1 Diabetes (T1D)
| Variable | Control (n = 61) | T1D (n = 62) | P |
|---|---|---|---|
| Age (y) | 13.6 ± 1.9 | 13.6 ± 1.7 | 0.908 |
| Age at menarche (y) | 12.2 ± 1.0 | 12.5 ± 1.1 | 0.139 |
| Bone age (y) | 13.9 ± 2.2 | 13.9 ± 1.0 | 0.934 |
| History of fracture (n, %) | 29 (48%) | 22 (35%) | 0.175 |
| Calcium intake (mg/day) | 864.0 ± 457.4 | 950.2 ± 488.2 | 0.314 |
| Vitamin D intake (IU/day) | 336.2 ± 769.9 | 191.7 ± 267.1 | 0.165 |
| Physical activity score | 2.3 ± 0.5 | 2.2 ± 0.5 | 0.067 |
| Fasting glucose (mg/dL) | 88.7 ± 6.0 | 192.7 ± 65.5 | <0.001 |
| Creatinine (mg/dL) | 0.60 ± 0.10 | 0.60 ± 0.09 | 0.792 |
| Calcium (mg/dL) | 9.6 ± 0.3 | 9.6 ± 0.4 | 0.898 |
| Phosphate (mg/dL) | 4.3 ± 0.6 | 4.2 ± 0.5 | 0.390 |
| Bicarbonate (mmol/L) | 22.1 ± 2.0 | 22.6 ± 2.1 | 0.146 |
| HbA1c (%) | 5.4 ± 0.3 | 8.6 ± 1.3 | <0.001 |
| PTH (pg/mL) | 33.4 ± 9.6 | 38.9 ± 11.0 | 0.004 |
| 25OHD (ng/mL) | 33.2 ± 9.6 | 26.3 ± 7.6 | <0.001 |
| Urine calcium/creatinine (mg/mg) | 0.08 ± 0.06 | 0.06 ± 0.06 | 0.049 |
| TP/GFR (mg/dL) | 4.0 ± 0.6 | 3.8 ± 0.6 | 0.185 |
| Height Z-score | 0.4 ± 0.9 | 0.3 ± 1.1 | 0.420 |
| Weight Z-score | 0.4 ± 0.9 | 0.7 ± 0.8 | 0.043 |
| BMI Z-score | 0.3 ± 0.8 | 0.7 ± 0.7 | 0.002 |
| Fat mass (kg) | 13.1 ± 5.7 | 18.4 ± 6.3 | 0.199 |
| Lean mass (kg) | 34.2 ± 6.8 | 36.5 ± 6.1 | 0.054 |
| Percent body fat | 31.7 ± 5.7 | 31.9 ± 5.7 | 0.836 |
| Race (n) | |||
| White | 56 (92%) | 53 (85%) | 0.399 |
| Black | 2 (3%) | 1 (2%) | |
| Multiple/other | 3 (5%) | 8 (13%) | |
| Tanner stage (n) | |||
| 1 | 4 (7%) | 1 (2%) | 0.319 |
| 2 | 13 (21%) | 10 (16%) | |
| 3 | 3 (5%) | 9 (15%) | |
| 4 | 9 (15%) | 13 (21%) | |
| 5 | 31 (51%) | 29 (47%) |
Data presented as mean ± standard deviation or n (%). Comparisons evaluated by t-test (continuous variables) or Fisher’s exact test (categorical variables). Of subjects who identified race as multiple/other, controls self-identified as “White and Brazilian” (n = 1), “White and Black” (n = 1), and “White and Asian” (n = 1). Subjects with T1D self-identified as “Hispanic” (n = 5), “White, Black, and Native American” (n = 1), “White and Pacific Islander” (n = 1), and “Black and Native American” (n = 1). Abbreviations: 25OHD, 25-hydroxyvitamin D; HbA1c, hemoglobin A1C; TP/GFR, tubular reabsorption of phosphate normalized to glomerular filtration rate; PTH, parathyroid hormone.
Areal BMD
We observed no differences in aBMD Z-scores as measured by DXA at the whole body less head, spine, total hip, femoral neck, or radius between the control and T1D subjects, both in univariate comparisons and after adjustment for height and weight (Table 2). In addition, the trabecular bone score did not differ between groups.
Table 2.
Comparison of Areal Bone Mineral Densities Between Control and T1D Subjects
| DXA parameter | Control (n = 61) | T1D (n = 62) | P | P a |
|---|---|---|---|---|
| WBLH BMD Z-score | -0.36 ± 0.84 | -0.52 ± 1.01 | 0.337 | 0.177 |
| Spine BMD Z-score | 0.00 ± 0.84 | -0.05 ± 1.00 | 0.764 | 0.264 |
| Total hip BMD Z-score | 0.20 ± 0.75 | 0.13 ± 1.03 | 0.679 | 0.239 |
| Femoral neck BMD Z-score | -0.09 ± 0.97 | -0.25 ± 1.08 | 0.380 | 0.096 |
| Radius BMD Z-score | 0.08 ± 1.07 | -0.02 ± 1.06 | 0.600 | 0.077 |
| Trabecular bone scoreb | 1.33 ± 0.11 | 1.32 ± 0.10 | 0.771 | 0.434 |
Data presented as mean ± standard deviation. Comparisons evaluated by t-test for univariate comparisons and by linear regression adjusting for height and weight. Abbreviations: BMD, bone mineral density; T1D, type 1 diabetes; WBLH, whole body less head. aAdjusted for height and weight. bTrabecular bone score was measured in a subset of n = 55 control and n = 36 T1D subjects.
Volumetric BMD and microarchitecture
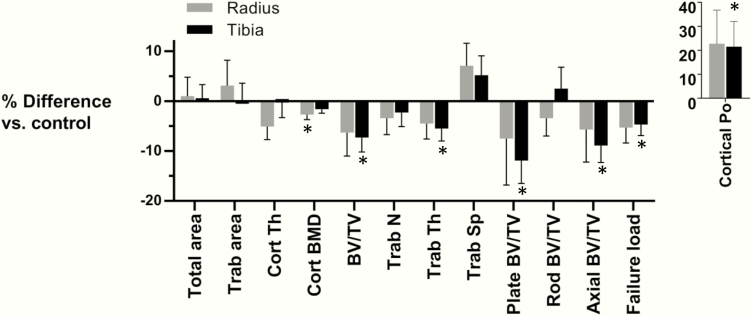
HRpQCT imaging of the distal radius and tibia demonstrated numerous differences in the vBMD and microarchitecture between the groups, with comparisons adjusted for height, weight, and bone age (Fig. 1). We did not observe differences in bone size, with similar total area, trabecular area, and cortical thickness among T1D subjects compared to the control subjects. However, cortical vBMD was 2.7% lower at the radius and was borderline significantly lower at the tibia among subjects with T1D, in conjunction with 21–22% increases in cortical porosity at both sites, statistically significant only at the tibia.
Figure 1.
Percent differences in volumetric bone mineral density, microarchitecture, and estimated failure load at the distal radius (gray bars) and tibia (black bars) between T1D and control subjects. Percent differences are adjusted for bone age, height, and weight, and error bars represent standard error of marginal effect. Abbreviations: Cort, cortical; BMD, bone mineral density; BV/TV, bone volume over total volume; Junc D, junction density; L, length; N, number; Po, porosity; PP, plate-plate; RP, rod-plate; RR, rod-rod; SA, surface area; Sp, spacing; Trab, trabecular; Th, thickness. *P < 0.05 compared to control subjects.
At the tibia, trabecular BV/TV was 7.3% lower (P = 0.013) and trabecular thickness was 5.5% lower (P = 0.027) among T1D subjects. Using individual trabecula segmentation to evaluate trabecular morphology, we observed decrements of 11.9% (P = 0.011) and 8.9% (P = 0.011) in plate-like and axial BV/TV at the tibia among T1D subjects, with no difference in rod-like BV/TV (P = 0.566). FEA-generated estimates of bone strength showed a 4.7% lower failure load at the tibia among T1D subjects (P = 0.037). Notably, the relative differences in trabecular microarchitecture and failure load between T1D and control subjects were numerically similar at the distal radius, though these differences were not statistically significant. We did not observe an association of age at diagnosis nor duration of disease with HRpQCT parameters among T1D subjects.
Skeletal outcomes and glycemic control
To investigate the effect of glycemic control on skeletal outcomes, we divided our T1D cohort at the median HbA1c, generating two groups: T1D-low (n = 31, HbA1c 6.1–8.5%) and T1D-high (n = 31, HbA1c 8.6–13.6%). The two T1D groups did not differ by age, bone age, height Z-score, weight Z-score, race, or concentrations of 25OHD or PTH.
At the distal tibia, trabecular vBMD and microarchitecture were more severely affected among subjects with higher HbA1c (Fig. 2). In particular, we observed a significant 9.8% decrement in Tb.BV/TV among T1D-high vs. the control subjects (P = 0.008). This difference appeared to be driven primarily by differences in trabecular thickness, which was 7.1% lower (P = 0.023) in T1D-high vs. the control subjects. Individual trabecula segmentation revealed differences of greater magnitude, with a 15.7% (P = 0.007) and 12.5% (P = 0.004) decrement in plate-like and axial trabeculae among T1D-high subjects, respectively, compared to the control subjects. Conversely, cortical porosity was 33.9% higher (P = 0.011) among T1D-high compared to controls. Estimated bone strength also tracked with glycemic control, with a 7.0% lower failure load among T1D-high vs. the control subjects (P = 0.013). Notably, T1D-low subjects were numerically intermediate between control and T1D-high subjects for all trabecular parameters, cortical porosity, and failure load (P-value for linear trend 0.008 for BV/TV, 0.011 for cortical porosity, and 0.013 for failure load), but were not significantly different from either group in pairwise comparisons.
Figure 2.
Least square mean estimates of volumetric bone mineral density, microarchitecture, and estimated failure load at the distal radius and tibia in control (open bar), T1D-low (gray bars), and T1D-high (black bars) subjects. Means adjusted for bone age, height, and weight. Error bars represent standard error of the marginal effect.
At the distal radius, we did not observe differences in any trabecular parameter among T1D-high, T1D-low, and the control subjects. However, cortical vBMD and cortical thickness were lower, and rod trabecular length was higher among T1D-low vs. the control subjects, with no significant differences between T1D-high and control subjects (27). Given baseline differences in mean 25OHD among groups (27.0 ± 8.2, 25.7 ± 7.0, and 33.2 ± 9.6 ng/mL in T1D-high, T1D-low, and control subjects, respectively), we repeated these analyses additionally adjusting for 25OHD and found similar results (27).
Bone turnover markers and glycemic control
P1NP, a marker of bone formation, was lower in T1D vs. the control subjects (367.9 ± 229.5 vs. 444.5 ± 329.8 ng/mL, P = 0.002 adjusted for bone age). We did not observe differences in CTX, a measure of bone resorption (1.38 ± 0.68 vs. 1.50 ± 0.74 ng/mL, P = 0.075), or TRAcP5b, a measure of osteoclast number (8.60 ± 4.13 vs. 8.21 ± 4.25 U/L, P = 0.576). Neither PTH nor 25OHD were significantly associated with these markers. To investigate the relationship of bone turnover markers with glycemic control, we compared T1D-high, T1D-low, and the control subjects (Fig. 3) and observed a significantly lower P1NP among T1D-high subjects vs. the control subjects, with an intermediate value in T1D-low subjects that was not significantly different from that of the control subjects (P for linear trend <0.001). Neither CTX nor TRAcP5b differed significantly among groups.
Figure 3.
Least square mean estimates of bone turnover markers in control (open bar), T1D-low (gray bars), and T1D-high (black bars) subjects. Means adjusted for bone age. Error bars represent standard error of the marginal effect.
Correlations of IGF-1 and its binding proteins with microarchitecture
IGF-1 and the IGF-1 Z-score were both lower in T1D subjects compared with the control subjects (301.6 ± 101.8 and 395.1 ± 121.2 ng/mL, P < 0.001, and -0.74 ± 1.10 vs. 0.11 ± 1.00, P < 0.001, respectively). IGFBP-3 did not differ between groups (4966.5 ± 834.5 vs. 5005.1 ± 650.8 ng/mL, P = 0.793), but the IGF-1/IGFBP-3 molar ratio, an index of free IGF-1 (16), was lower among T1D subjects (0.24 ± 0.06 vs. 0.32 ± 0.07, P < 0.001). IGFBP-1, a binding protein believed to inhibit IGF-1 action (28), was conversely higher among T1D subjects (72.9 ± 78.7 vs. 18.2 ± 29.0 ng/mL, P < 0.001). Tb.BV/TV correlated positively with the IGF-1 Z-score and the IGF-1/IGFBP-3 molar ratio at both the radius and the tibia, and with IGF-1 at the tibia. Cortical porosity was positively associated with IGF-1, the IGF-1 Z-score, and the IGF-1/IGFBP-3 molar ratio at the tibia only. We observed no correlation of IGF-1 or any of its binding proteins with estimated failure load (27). After re-running these models replacing weight with lean mass, the associations weakened such that Tb.BV/TV remained positively correlated only with the IGF1 Z-score and only at the tibia (standardized β = 0.25, P = 0.011). Tibia cortical porosity remained positively associated with IGF-1, the IGF-1 Z-score, and the IGF-1/IGFBP-3 molar ratio (standardized β = 0.17, P = 0.027; standardized β = 0.19, P = 0.009; and standardized β = 0.17, P = 0.038, respectively).
Given that among the indices of IGF-1 action—the IGF-1 Z-score was most tightly correlated with microarchitectural parameters—we assessed the association of T1D with key parameters after adjusting for the IGF-1 Z-score. After adjusting for the IGF-1 Z-score, the associations of T1D with trabecular vBMD, morphology, and failure load at the tibia were substantially attenuated, though most retained a trend towards statistical significance, while the association of T1D with cortical porosity became tighter (Table 3). Differences in the IGF-1 Z-score explained an additional 2% to 7% of the variation in trabecular densities, and 2% to 3% of the variation in cortical porosity among controls and subjects with diabetes, with height, weight, and bone age included in all models.
Table 3.
Association of T1D Status with Key Microarchitectural Parameters with and without Controlling for IGF-1 Z-Score
| Base model | Model with IGF-1 Z-scorea | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | Parameter | T1D-low vs. control | T1D-high vs. control | T1D-low vs. control | T1D-high vs. control | ||||||
| Adjusted R2 | β | P | β | P | Adjusted R2 | β | P | β | P | ||
| Radius | Cort Po (%) | 0.34 | 0.15 | 0.091 | 0.08 | 0.374 | 0.36 | 0.23 | 0.018 | 0.13 | 0.192 |
| BV/TV (%) | 0.00 | -0.13 | 0.237 | -0.12 | 0.305 | 0.02 | -0.07 | 0.530 | -0.04 | 0.753 | |
| Plate BV/TV (%) | 0.01 | -0.05 | 0.653 | -0.11 | 0.345 | 0.05 | 0.01 | 0.918 | -0.01 | 0.926 | |
| Axial BV/TV (%) | 0.00 | -0.04 | 0.691 | -0.13 | 0.261 | 0.04 | 0.03 | 0.824 | -0.03 | 0.794 | |
| Failure Load (N) | 0.63 | -0.09 | 0.142 | -0.09 | 0.174 | 0.62 | -0.10 | 0.183 | -0.11 | 0.152 | |
| Tibia | Cort Po (%) | 0.50 | 0.06 | 0.365 | 0.19 | 0.011 | 0.53 | 0.15 | 0.053 | 0.25 | 0.002 |
| BV/TV (%) | 0.11 | -0.14 | 0.128 | -0.26 | 0.008 | 0.18 | -0.09 | 0.362 | -0.19 | 0.063 | |
| Plate BV/TV (%) | 0.24 | -0.14 | 0.113 | -0.25 | 0.007 | 0.27 | -0.12 | 0.198 | -0.19 | 0.058 | |
| Axial BV/TV (%) | 0.23 | -0.13 | 0.150 | -0.27 | 0.004 | 0.25 | -0.12 | 0.222 | -0.22 | 0.030 | |
| Failure Load (N) | 0.68 | -0.06 | 0.296 | -0.15 | 0.013 | 0.66 | -0.01 | 0.912 | -0.12 | 0.084 | |
Data presented as adjusted R2 for the model, standardized beta coefficient, P value. All comparisons adjusted for height, weight, and bone age. Abbreviations: Cort po, cortical porosity; BV/TV, bone volume over total volume. aAdjusted additionally for IGF-1 Z-score.
Discussion
We present here the first study describing bone microarchitecture using HRpQCT in girls with T1D and demonstrate several differences, including lower cortical vBMD at the radius, higher cortical porosity at the tibia, and lower total, plate-like, and axial trabecular BV/TV at the tibia among girls with T1D compared with healthy controls. These differences are consistent with increased skeletal fragility, an observation borne out by a significantly lower estimated failure load at the distal tibia and numerically similar (though not statistically significant) decrements at the distal radius.
The lower trabecular BV/TV is consistent with findings in adults with T1D (13, 29) though, notably, in adults, low trabecular BV/TV is observed only in those with diabetic microvascular disease, whereas none of our pediatric subjects had clinically evident microvascular disease. More recently, estimates of BV/TV using quantitative MRI at the proximal tibia in boys and girls ages 10–18 demonstrated lower BV/TV and trabecular number as well as increased trabecular separation in this younger cohort (30). An increase in cortical porosity has not been observed in adults with T1D (13), though this has been observed in adults with T2D (31–34).
In addition, we demonstrate that glycemic control is associated with the magnitude of the effect of T1D on bone, with greater decrements in trabecular density, microarchitecture, and morphology, and increases in cortical porosity seen at the tibia in those T1D subjects with a higher HbA1c. These data are consistent with previous smaller studies using pQCT that demonstrated an inverse relationship between trabecular bone density and HbA1c (35, 36), as well as a recent study of 36 children with new-onset T1D, in which those with higher HbA1c over the following 12 months had slower total body bone mineral accrual (37). In parallel with these findings, we observed a HbA1c-dependent decrease in bone formation as measured by serum P1NP among T1D subjects, consistent with previous reports of an inverse association of bone formation markers and glycemic control in youth (5). These observations may be explained by the fact that hyperglycemia and associated hyperosmolality are directly toxic to osteoblasts, interfering with their differentiation and gene expression (38, 39). In addition, hyperglycemia interferes with the osteoblast and osteocyte response to mechanical loading in cell culture models (40, 41). If the effect of hyperglycemia is to impair the anabolic response to mechanical loading, this may explain the lack of an observed gradient of effect of HbA1c on trabecular architecture and cortical porosity at the radius—a site with less mechanical loading than the tibia. This lack of an effect may also reflect poorer statistical power given the smaller sample size and greater variability at the radius due to greater motion artifact at this site.
As expected, we observed lower circulating IGF-1 and higher IGFBP-1 among T1D subjects (28), thought to be due to relative insulin deficiency in the portal circulation (42). Our regression models suggest that alterations in circulating IGF-1 mediate a substantial portion of the effect of T1D on trabecular microarchitecture. These data are consistent with those of Kirmani et al., who found IGF-1 to be associated with trabecular BMD in children, though, after stratification by sex, this association was seen only in boys (7). In that study, similar to our data, IGF-1 was also positively associated with cortical porosity in both boys and girls, possibly due to delayed cortical consolidation in the face of rapid longitudinal growth (7, 43). These data are also consistent with those of Moyer-Mileur et al., who found circulating IGF-1 to be associated with bone strength estimates by pQCT in adolescent girls with T1D (44). Notably, studies in mouse models have demonstrated a temporal effect of IGF-1 deficiency: an inducible knock-out in young mice leads to pronounced deficits in both cortical and trabecular bone, while a knock-out in mature mice has minimal effect (45). This finding is consistent with our hypothesis that childhood is a unique period of susceptibility to the deleterious effects of T1D on skeletal health.
Our study has several strengths. This is one of the larger cohorts of youth with T1D who have undergone detailed skeletal phenotyping, and our subjects with T1D have a wide spectrum of glycemic control, reflecting patients seen in real-world practice (26). Limitations include the lack of boys in our cohort. In addition, we did not obtain volumetric imaging of the hip or spine, sites with potentially more clinical relevance, but which would require substantially more radiation exposure. Given that bones are elongating in growing children, there is ongoing debate regarding optimal positioning of the region of interest for HRpQCT scans (46); the sites used in this study are consistent with those used in previous pediatric studies (47, 48) and, importantly, are proximal to the physes in order to prevent excess radiation exposure to this potentially more sensitive organ. Finally, these data are cross-sectional; we are continuing to follow this cohort in order to evaluate the effects of hyperglycemia, low IGF-1, and other metabolic derangements on bone accrual and microarchitecture development.
In summary, we have demonstrated biomechanically significant alterations in the skeletal microarchitecture of girls with T1D relatively early in the course of their disease and prior to clinical evidence of other diabetic complications. The association of elevated HbA1c with decreased bone formation and impaired microarchitecture suggests that poor glycemic control in childhood may affect fracture risk decades later given the importance of this period for bone mass acquisition (6). Longitudinal studies will help clarify the contribution of glycemic control to ongoing bone accrual.
Acknowledgments
Financial support: The work described in this article was supported by NIH grants K23DK105350, K24HD071843, S10RR023405, and UL1RR025758, Harvard Clinical and Translational Science Center, and the National Center for Research Resources. This work was also supported by a Massachusetts General Hospital Department of Pediatrics Pilot and Feasibility Award, a Massachusetts General Hospital Executive Committee on Research Fund for Medical Discovery Award, and the American Society for Bone and Mineral Research Rising Star award.
ClinicalTrials.gov ID: NCT02140424
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int. Apr 2007;18(4):427–444. [DOI] [PubMed] [Google Scholar]
- 2. Hothersall EJ, Livingstone SJ, Looker HC, et al. Contemporary risk of hip fracture in type 1 and type 2 diabetes: a national registry study from Scotland. J Bone Miner Res. 2014;29(5):1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weber DR, Haynes K, Leonard MB, Willi SM, Denburg MR.. Type 1 diabetes is associated with an increased risk of fracture across the life span: a population-based cohort study using The Health Improvement Network (THIN). Diabetes Care. Oct 2015;38(10):1913–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hygum K, Starup-Linde J, Harslof T, Vestergaard P, Langdahl BL.. MECHANISMS IN ENDOCRINOLOGY: Diabetes mellitus, a state of low bone turnover - a systematic review and meta-analysis. Eur J Endocrinol. Mar 2017;176(3):R137–R157. [DOI] [PubMed] [Google Scholar]
- 5. Madsen JOB, Jorgensen NR, Pociot F, Johannesen J.. Bone turnover markers in children and adolescents with type 1 diabetes-A systematic review. Pediatr Diabetes. Aug 2019;20(5):510–522. [DOI] [PubMed] [Google Scholar]
- 6. Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA.. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res. Aug 2011;26(8):1729–1739. [DOI] [PubMed] [Google Scholar]
- 7. Kirmani S, Christen D, van Lenthe GH, et al. Bone structure at the distal radius during adolescent growth. J Bone Miner Res. Jun 2009;24(6):1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nishiyama KK, Macdonald HM, Moore SA, Fung T, Boyd SK, McKay HA.. Cortical porosity is higher in boys compared with girls at the distal radius and distal tibia during pubertal growth: an HR-pQCT study. J Bone Miner Res. Feb 2012;27(2):273–282. [DOI] [PubMed] [Google Scholar]
- 9. Shah VN, Harrall KK, Shah CS, Gallo TL, Joshee P, Snell-Bergeon JK, Kohrt WM.. Bone mineral density at femoral neck and lumbar spine in adults with type 1 diabetes: a meta-analysis and review of the literature. Osteoporos Int. Sep 2017;28(9):2601–2610. [DOI] [PubMed] [Google Scholar]
- 10. Moyer-Mileur LJ, Dixon SB, Quick JL, Askew EW, Murray MA.. Bone mineral acquisition in adolescents with type 1 diabetes. J Pediatr. Nov 2004;145(5):662–669. [DOI] [PubMed] [Google Scholar]
- 11. Bechtold S, Dirlenbach I, Raile K, Noelle V, Bonfig W, Schwarz HP.. Early manifestation of type 1 diabetes in children is a risk factor for changed bone geometry: data using peripheral quantitative computed tomography. Pediatrics. Sep 2006;118(3):e627–634. [DOI] [PubMed] [Google Scholar]
- 12. Roggen I, Gies I, Vanbesien J, Louis O, De Schepper J.. Trabecular bone mineral density and bone geometry of the distal radius at completion of pubertal growth in childhood type 1 diabetes. Horm Res Paediatr. 2013;79(2):68–74. [DOI] [PubMed] [Google Scholar]
- 13. Shanbhogue VV, Hansen S, Frost M, et al. Bone geometry, volumetric density, microarchitecture, and estimated bone strength assessed by HR-pQCT in adult patients with type 1 diabetes mellitus. J Bone Miner Res. Dec 2015;30(12):2188–2199. [DOI] [PubMed] [Google Scholar]
- 14. Samelson EJ, Broe KE, Xu H, et al. Cortical and trabecular bone microarchitecture as an independent predictor of incident fracture risk in older women and men in the Bone Microarchitecture International Consortium (BoMIC): a prospective study. The Lancet. Diabetes & Endocrinol. Jan 2019;7(1):34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burrows M, Liu D, McKay H.. High-resolution peripheral QCT imaging of bone micro-structure in adolescents. Osteoporos Int. Mar 2010;21(3):515–520. [DOI] [PubMed] [Google Scholar]
- 16. Juul A, Scheike T, Nielsen CT, Krabbe S, Muller J, Skakkebaek NE.. Serum insulin-like growth factor I (IGF-I) and IGF-binding protein 3 levels are increased in central precocious puberty: effects of two different treatment regimens with gonadotropin-releasing hormone agonists, without or in combination with an antiandrogen (cyproterone acetate). J Clin Endocrinol Metab. Oct 1995;80(10):3059–3067. [DOI] [PubMed] [Google Scholar]
- 17. Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. 2nd ed Stanford, CA: Stanford University Press; 1959. [Google Scholar]
- 18. Taylor C, Lamparello B, Kruczek K, Anderson EJ, Hubbard J, Misra M.. Validation of a food frequency questionnaire for determining calcium and vitamin D intake by adolescent girls with anorexia nervosa. J Am Diet Assoc. Mar 2009;109(3):479–485, 485 e471-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nor Aini J, Poh BK, Chee WS.. Validity of a children’s physical activity questionnaire (cPAQ) for the study of bone health. Pediatrics international: official journal of the Japan Pediatric Society. Apr 2013;55(2):223–228. [DOI] [PubMed] [Google Scholar]
- 20. Boutroy S, Bouxsein ML, Munoz F, Delmas PD.. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. Dec 2005;90(12):6508–6515. [DOI] [PubMed] [Google Scholar]
- 21. Burghardt AJ, Kazakia GJ, Ramachandran S, Link TM, Majumdar S.. Age- and gender-related differences in the geometric properties and biomechanical significance of intracortical porosity in the distal radius and tibia. J Bone Miner Res. May 2010;25(5):983–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu XS, Sajda P, Saha PK, et al. Complete volumetric decomposition of individual trabecular plates and rods and its morphological correlations with anisotropic elastic moduli in human trabecular bone. J Bone Miner Res. Feb 2008;23(2):223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou B, Liu XS, Wang J, Lu XL, Fields AJ, Guo XE.. Dependence of mechanical properties of trabecular bone on plate-rod microstructure determined by individual trabecula segmentation (ITS). J Biomech. Feb 7 2014;47(3):702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD.. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res. Mar 2008;23(3):392–399. [DOI] [PubMed] [Google Scholar]
- 25. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG.. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. Apr 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Foster NC, Beck RW, Miller KM, et al. State of Type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther. Feb 2019;21(2):66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mitchell DM, Caksa S, Joseph T, Bouxsein ML, Misra M. Data from: Supplemental Tables. Figshare. Deposited 8 June 2019. https://figshare.com/articles/Supplemental_Tables/9274187.
- 28. Clemmons DR. Metabolic actions of insulin-like growth factor-I in normal physiology and diabetes. Endocrinol Metab Clin North Am. Jun 2012;41(2):425–443, vii-viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abdalrahaman N, McComb C, Foster JE, et al. Deficits in trabecular bone microarchitecture in young women with type 1 diabetes mellitus. J Bone Miner Res. Aug 2015;30(8):1386–1393. [DOI] [PubMed] [Google Scholar]
- 30. Chen SC, Shepherd S, McMillan M, et al. Skeletal fragility and its clinical determinants in children with type 1 diabetes. J Clin Endocrinol Metab. Aug 1 2019;104(8):3585–3594. [DOI] [PubMed] [Google Scholar]
- 31. Patsch JM, Burghardt AJ, Yap SP, et al. Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. J Bone Miner Res. Feb 2013;28(2):313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu EW, Putman MS, Derrico N, Abrishamanian-Garcia G, Finkelstein JS, Bouxsein ML.. Defects in cortical microarchitecture among African-American women with type 2 diabetes. Osteoporos Int. Feb 2015;26(2):673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shanbhogue VV, Hansen S, Frost M, et al. Compromised cortical bone compartment in type 2 diabetes mellitus patients with microvascular disease. Eur J Endocrinol. Feb 2016;174(2):115–124. [DOI] [PubMed] [Google Scholar]
- 34. Samelson EJ, Demissie S, Cupples LA, et al. Diabetes and deficits in cortical bone density, microarchitecture, and bone size: framingham HR-pQCT study. J Bone Miner Res. Jan 2018;33(1):54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lettgen B, Hauffa B, Mohlmann C, Jeken C, Reiners C.. Bone mineral density in children and adolescents with juvenile diabetes: selective measurement of bone mineral density of trabecular and cortical bone using peripheral quantitative computed tomography. Horm Res. 1995;43(5):173–175. [DOI] [PubMed] [Google Scholar]
- 36. Heap J, Murray MA, Miller SC, Jalili T, Moyer-Mileur LJ.. Alterations in bone characteristics associated with glycemic control in adolescents with type 1 diabetes mellitus. J Pediatr. Jan 2004;144(1):56–62. [DOI] [PubMed] [Google Scholar]
- 37. Weber DR, Gordon RJ, Kelley JC, et al. Poor glycemic control is associated with impaired bone accrual in the year following a diagnosis of type 1 diabetes. J Clin Endocrinol Metab. Apr 29 2019;104(1):4511–4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zayzafoon M, Stell C, Irwin R, McCabe LR.. Extracellular glucose influences osteoblast differentiation and c-Jun expression. J Cell Biochem. Aug 2 2000;79(2):301–310. [DOI] [PubMed] [Google Scholar]
- 39. Botolin S, McCabe LR.. Chronic hyperglycemia modulates osteoblast gene expression through osmotic and non-osmotic pathways. J Cell Biochem. Oct 1 2006;99(2):411–424. [DOI] [PubMed] [Google Scholar]
- 40. Parajuli A, Liu C, Li W, et al. Bone’s responses to mechanical loading are impaired in type 1 diabetes. Bone. Dec 2015;81:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seref-Ferlengez Z, Maung S, Schaffler MB, Spray DC, Suadicani SO, Thi MM.. P2X7R-Panx1 complex impairs bone mechanosignaling under high glucose levels associated with type-1 diabetes. PLoS One. 2016;11(5):e0155107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Phillips LS, Pao CI, Villafuerte BC.. Molecular regulation of insulin-like growth factor-I and its principal binding protein, IGFBP-3. Prog Nucleic Acid Res Mol Biol. 1998;60:195–265. [DOI] [PubMed] [Google Scholar]
- 43. Ramchand SK, Seeman E.. The influence of cortical porosity on the strength of bone during growth and advancing age. Curr Osteoporos Rep. Oct 2018;16(5):561–572. [DOI] [PubMed] [Google Scholar]
- 44. Moyer-Mileur LJ, Slater H, Jordan KC, Murray MA.. IGF-1 and IGF-binding proteins and bone mass, geometry, and strength: relation to metabolic control in adolescent girls with type 1 diabetes. J Bone Miner Res. Dec 2008;23(12):1884–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Courtland HW, Elis S, Wu Y, et al. Serum IGF-1 affects skeletal acquisition in a temporal and compartment-specific manner. PLoS One. 2011;6(3):e14762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Seeman E, Ghasem-Zadeh A.. Challenges in the acquisition and analysis of bone microstructure during growth. J Bone Miner Res. Dec 2016;31(12):2239–2241. [DOI] [PubMed] [Google Scholar]
- 47. Gabel L, Macdonald HM, McKay HA.. Sex differences and growth-related adaptations in bone microarchitecture, geometry, density, and strength from childhood to early adulthood: a mixed longitudinal HR-pQCT study. J Bone Miner Res. Feb 2017;32(2):250–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mitchell DM, Caksa S, Yuan A, Bouxsein ML, Misra M, Burnett-Bowie SM.. Trabecular bone morphology correlates with skeletal maturity and body composition in healthy adolescent girls. J Clin Endocrinol Metab. Jan 1 2018;103(1):336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]