Abstract
Background:
Perfluorooctanoic acid (PFOA) is a poly- and perfluoroalkyl substance (PFAS) associated with adverse pregnancy outcomes in mice and humans, but little is known regarding one of its replacements, hexafluoropropylene oxide dimer acid (HFPO-DA, referred to here as GenX), both of which have been reported as contaminants in drinking water.
Objectives:
We compared the toxicity of PFOA and GenX in pregnant mice and their developing embryo–placenta units, with a specific focus on the placenta as a hypothesized target.
Methods:
Pregnant CD-1 mice were exposed daily to PFOA (0, 1, or ) or GenX (0, 2, or ) via oral gavage from embryonic day (E) 1.5 to 11.5 or 17.5 to evaluate exposure effects on the dam and embryo–placenta unit. Gestational weight gain (GWG), maternal clinical chemistry, maternal liver histopathology, placental histopathology, embryo weight, placental weight, internal chemical dosimetry, and placental thyroid hormone levels were determined.
Results:
Exposure to GenX or PFOA resulted in increased GWG, with increase in weight most prominent and of shortest latency with GenX exposure. Embryo weight was significantly lower after exposure to PFOA (9.4% decrease relative to controls). Effect sizes were similar for higher doses ( PFOA and GenX) and lower doses ( PFOA and GenX), including higher maternal liver weights, changes in liver histopathology, higher placental weights and embryo–placenta weight ratios, and greater incidence of placental abnormalities relative to controls. Histopathological features in placentas suggested that PFOA and GenX may exhibit divergent mechanisms of toxicity in the embryo–placenta unit, whereas PFOA- and GenX-exposed livers shared a similar constellation of adverse pathological features.
Conclusions:
Gestational exposure to GenX recapitulated many documented effects of PFOA in CD-1 mice, regardless of its much shorter reported half-life; however, adverse effects toward the placenta appear to have compound-specific signatures. https://doi.org/10.1289/EHP6233
Introduction
Perfluorooctanoic acid (PFOA) is a fully fluorinated, eight-carbon synthetic chemical belonging to the class of compounds known as poly- and perfluoroalkyl substances (PFAS). PFAS are used in a wide range of industrial processes and consumer products and are globally ubiquitous, persistent, and detectable in nearly all humans living in industrialized nations (ATSDR 2019; Kato et al. 2011). Although humans are exposed to PFAS through multiple routes, drinking water is one of the most well-understood sources of exposure (Hu et al. 2016).
Within the general U.S. population, serum levels of PFOA have declined from a geometric mean of in 1999–2000 (CDC 2009) to in 2015–2016 (CDC 2019). This shift is likely the result of efforts by the U.S. Environmental Protection Agency (U.S. EPA) to reduce environmental emissions and to phase out U.S. production and use of PFOA by 2015 (U.S. EPA 2006). Similarly, in 2017, the European Union placed restrictions on the production and use of PFOA (European Commission 2017). Despite such efforts, exposure to PFOA remains a concern due to its long human half-life () (Olsen et al. 2007), environmental persistence (Lindstrom et al. 2011), and the fact that longer-chain/precursor PFAS chemicals can degrade and form PFOA. In response to restrictions on PFOA, manufacturers have increased production on replacement compounds with alternative chemistries aimed at making the compounds less bioaccumulative and with shorter serum half-lives; however, toxicity data for these alternative PFAS are limited (Bao et al. 2018).
Hexafluoropropylene oxide dimer acid (HFPO-DA), referred to herein as GenX, is a PFOA replacement compound. GenX has received intense public scrutiny in North Carolina since its discovery in (Strynar et al. 2015), and contamination of, the Cape Fear River Basin following release from a manufacturing facility (Sun et al. 2016). GenX has also been measured in the environment in other regions of the United States, including the Ohio River (Hopkins et al. 2018), as well as in other countries, including the Xiaoqing River in China (Brandsma et al. 2018) and the Rhine River in Europe (Heydebreck et al. 2015).
PFAS are detectable in the serum of pregnant women and in cord blood, and the ratio of the concentration of PFOA in maternal serum to cord serum is typically (Kim et al. 2011; Monroy et al. 2008). Maternal exposure to PFOA has been associated with multiple adverse health outcomes, including increased gestational weight gain (GWG) (Ashley-Martin et al. 2016), pregnancy-induced hypertension (Darrow et al. 2013), preeclampsia (Savitz et al. 2012; Stein et al. 2009), and reduced birth weight (Apelberg et al. 2007; Fei et al. 2007; Johnson et al. 2014; Kobayashi et al. 2017; Lam et al. 2014; Rijs and Bogers 2017). Based on a systematic review of the literature and meta-analysis, the shift in birth weight associated with PFOA exposure has been estimated to be birth weight per increase in serum PFOA [95% confidence interval (CI): , ] (Johnson et al. 2014).
In mice, the reproductive and developmental effects of gestational exposure to PFOA are well documented. Previous studies have shown gestational exposure to PFOA in mice results in maternal liver damage (Lau et al. 2006), maternal hypolipidemia (Yahia et al. 2010), and reduced embryo weight (Koustas et al. 2014). It has been estimated from a meta-analysis of data from eight mouse studies that the shift in mice is pup birth weight per body weight (BW)/d increase in PFOA dose to pregnant dams (95% CI: , ) (Koustas et al. 2014). In contrast, there is a paucity of data regarding the reproductive and developmental effects of GenX. A previous reproductive and developmental toxicity study of GenX in CD-1 mice determined the no observed adverse effect level (NOAEL) for reproductive toxicity and maternal systemic toxicity (microscopic changes in maternal liver) was HFPO-DA (GenX; DuPont-18,405-1,037). A recent study in rats showed limited gestational exposure to HFPO-DA (GenX) resulted in a lowest observed adverse effect level (LOAEL) for disrupted maternal thyroid hormone (TH) (LOAEL: ) and lipids (LOAEL: ), up-regulated gene expression in peroxisome proliferator–activated receptor (PPAR) signaling pathways in both maternal and embryo liver (LOAEL: ), and lower BWs in gestationally exposed female offspring (LOAEL: ) (Conley et al. 2019). Additional studies examining the reproductive and developmental effects of GenX are needed.
The biological mechanism through which PFOA exerts adverse effects on embryo growth is not known, but the placenta is a suspected target tissue. The placenta is critical for embryo growth and development, and disruptions in placental development or function can lead to adverse outcomes for both maternal and embryo health. Previous animal studies have examined the effect of gestational exposure to PFOA on maternal mammary gland development and embryo growth (Macon et al. 2011; White et al. 2007), but effects on the placenta have yet to be evaluated. The aims of this study were to compare the effects of gestational exposure to PFOA and a replacement, GenX, on GWG, embryo growth, liver pathology, and placental development/morphology.
Methods
Animals
Naïve female CD-1 mice between 7.5 and 15.5 wk of age from the NIEHS colony were bred in-house on a single night, and copulatory plug–positive females were identified on embryonic day (E) 0.5. Pregnant dams were singly housed in ventilated polypropylene cages and received nesting materials, National Institutes of Health (NIH)-31 diet (Zeigler Bros., Inc.) and reverse osmosis deionized (RODI) water ad libitum. Animals were housed in humidity- and temperature-controlled rooms with 25°C and 45–60% average humidity and standard 12-h light cycles. All animal procedures were approved by the NIEHS Animal Care and Use Committee (ASP #2017-0022).
Dosing Solutions
PFOA ammonium salt (CAS #3825-26-1) was purchased from Millipore Sigma, and GenX [ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoate; CAS# 62,037-80-3] was purchased from SynQuest Laboratories. PFOA and GenX dosing solutions were prepared in RODI water and administered to mice once daily via oral gavage. Daily doses were administered between 0700 and 0800 hours and adjusted to the BW of the mouse based on the previous day’s weight at a volume of BW. PFOA doses of (high dose) and (low dose) were selected based on previous work that demonstrated a reduction in neonatal weight gain (Lau et al. 2006; White et al. 2007). The dose of PFOA, used in the mouse developmental toxicity study of Lau et al. 2006, provided a lowest effect dose that was used to set the reference dose within the U.S. EPA’s drinking water lifetime health advisory level (HAL) of PFOA (U.S. EPA 2016). Given that the state of North Carolina has a provisional health goal of GenX (double the PFOA HAL), we selected doses of GenX (, high dose; , low dose) to mirror doses of PFOA previously used in HAL decision-making.
Study Design
This experiment was conducted over two blocks (Block 1 and Block 2) to achieve a total of per treatment group and sacrifice time point (E11.5 and E17.5). The experimental design of the second block was identical to the first block of the study, and experimental methods were similar but expanded upon to include more rigorous and detailed measurements. Copulatory plug–positive mice (E0.5) were weighed to obtain a baseline BW and placed into one of five groups. Once all mice were assigned to groups, mean BWs were calculated, and a few animals were reassigned so that mean BWs in each group were similar. This was done to avoid confounding effects of baseline BW. Treatment groups were then randomly assigned a color by using a random sequence generator. Experimenters and dosing technicians were blinded to the treatment group to which the color groups corresponded throughout the duration of the study, including at necropsy. Randomly assigned treatment groups included in each block: vehicle control (deionized water only), PFOA, PFOA, GenX, and GenX. Pregnant dams were dosed via oral gavage from E1.5 to E11.5 or from E1.5 to E17.5. The sacrifice time points were selected a priori to examine effects of gestational PFOA or GenX exposure on embryo and placental growth prior to placental maturation (E11.5) as well as after full placental maturation (E17.5) (Watson and Cross 2005). The E11.5 early-gestation time point was selected because it overlaps a critical period of placental development in the mouse where the placenta undergoes vascularization with the uterine wall and chorioallantoic branching of vessels begins (Watson and Cross 2005). The E17.5 late-gestation time point was selected so that embryo weight changes that may be related to treatment would be evident.
Necropsy
On the day of necropsy, dams received daily oral gavage between 0700 and 0800 hours and were weighed and then euthanized humanely by swift decapitation, and serum was collected. In Block 1, necropsies were completed from 0800 to 1600 hours, and in Block 2, necropsies were completed from 0800 to 1200 hours. Serum from dams euthanized in Block 1 was snap frozen for internal dosimetry analyses. Serum and urine from Block 2 dams were reserved for clinical chemistry analyses. In both blocks, the uterus was removed, and total implantation sites were counted based on gross observation of an implantation nodule along the uterine horn. Viable embryos, nonviable embryos, and sites of resorption were counted based on gross observation. Embryos were considered viable if they were properly formed, were not pale in color, and were of similar size to neighboring embryos. Embryos that were poorly formed and pale in color (without heartbeat) were considered nonviable. Sites of resorption were defined as a dark red–appearing clot-like nodule apparent on gross observation.
From each uterus, first, viable embryos and their matched placentas were collected in succession within a horn and immediately snap frozen (), and subsequent embryos were collected for growth measurements (). Additional placentas were collected and placed in 4% paraformaldehyde (PFA) for histological analysis (Block 2 only). Amniotic fluid was collected by needle aspiration from litters euthanized at E11.5 and snap frozen in liquid nitrogen. Embryo livers were collected from litters euthanized at E17.5 and snap frozen in liquid nitrogen. Dam livers were weighed, a portion of the left lateral lobe was placed in 4% PFA for histology, and another portion of the same lobe was snap frozen in liquid nitrogen. A third liver section was obtained from Block 2 dams and fixed in McDowell and Trump’s fixative for electron microscopy (EM). Gross lesions were collected when observed and placed in 4% PFA for histology. Dam kidneys were removed, a cross section was prepared from the right kidney, a longitudinal section was prepared from the left kidney, and both sections were fixed in 4% PFA for histological analysis.
Tissue Preparation/Histology/Clinical Measures
Dam livers, kidneys, and placentas were trimmed and embedded by the NIEHS Mouse Embryo Phenotyping Core. Tissues collected at necropsy were fixed in 4% PFA for 72 h and paraffin embedded, and sections were prepared and stained with hematoxylin and eosin (H&E). Pathology was evaluated and a pathology review conducted by S.A.E. Diplomate American College of Veterinary Pathologists (DACVP). Pathology reviews were conducted as an informed approach analysis [e.g., nonblinded analysis; see Sills et al. (2019)]. Select tissue slides were scanned using the AT2 Scanner (Aperio). Images were then captured for publication using the ImageScope software; version 12.3.0.5056 (Aperio). Serum and urine obtained from dams in Block 2 were analyzed using the AU480 clinical chemistry analyzer (Beckman Coulter Inc.). Reagents and calibration standards used to measure alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), serum creatinine, urine creatinine, glucose (Glu), total protein (TP), triglyceride (Trig), high-density lipoprotein (HDL), cholesterol (Chol), and albumin (ALB) were purchased from Beckman Coulter Inc. Reagents for sorbitol dehydrogenase (SDH), total bile acid (TBA), and micro-TP were purchased from Sekisui Diagnostics. The reagent used to measure low-density lipoprotein (LDL) was purchased form Diazyme Laboratories.
Transmission Electron Microscopy
Block 2 dam liver portions stored in McDowell and Trump’s fixative (McDowell and Trump 1976) were processed using a Leica EM TP processor. Briefly, samples were rinsed with buffer, postfixed in 1% osmium tetroxide in phosphate buffer, rinsed in distilled water, dehydrated, and embedded in Ply/Bed® 812 (Polysciences, Inc.) epoxide resin. Blocks were trimmed, and semithin sections () were stained with 1% toluidine blue (Poly-scientific R&D Corp.) O in 1% sodium borate to ascertain areas of interest. Ultrathin sections () were cut from areas of interest and placed on 200-mesh copper grids and stained with uranyl acetate and lead citrate, and digital images were captured using an Orius® SC1000 side mount camera (Gatan) attached to a Techani T12 transmission electron microscope (TEM) (FEI Company). In general, peroxisomes were smaller than mitochondria and round with a dark, electron-dense, granular matrix and surrounded by a single membrane. Mitochondria were round to elongated, had a matrix that was less electron dense than peroxisomes and contained crista, and were surrounded by an inner and outer membrane. Samples were analyzed by R.D.K., Ph.D.
Placental Thyroid Hormone Quantification
Thyroid hormones (T3, triiodothyronine; T4, thyroxine; rT3, reverse triiodothyronine) in placenta were analyzed according to the methods described in Leonetti et al. (2016). Briefly, () of two to three pooled placental tissues of same-sex embryos was homogenized and digested for 16 h overnight in PRONASE® Protease (Streptomyces griseus) solution (EMD Millipore Corp.). Each pooled sample of two to three placentas was considered as one biological replicate and included placentas from the same litter when possible. Three biological replicates were used for each treatment group and each sex. Samples were spiked with an antioxidant solution (containing each of citric acid, ascorbic acid, and dithiothreitol) and isotopically labeled internal standards (T4, T3, and rT3), and cold acetone was added to stop the digestion reaction. Samples were vortex mixed and centrifuged three times for 2 min at 10,000 relative centrifugal force (rcf), and the supernatants were collected and combined. Sample pH was adjusted with hydrochloric acid to . A liquid–liquid extraction with cyclopentane was performed and the cyclopentane layer discarded; briefly, of cyclopentane was added to the supernatant and vortexed before the sample was centrifuged for 3 min at 3,000 rcf and the cyclopentane layer discarded, and this was repeated three times. A liquid–liquid extraction with ethyl acetate was performed; briefly, of ethyl acetate was added to the extract and vortexed before being shaken on a plate shaker for 30 min and centrifuged for 3 min at 3,000 rcf, and the ethyl acetate layer collected; this was repeated three times. Ethyl acetate extracts were dried down to under a gentle nitrogen stream and resuspended in of hydrochloric acid in 10% methanol. Samples were purified by solid-phase extraction using SampliQ Optimized Polymer Technology (OPT) cartridges (, ; Agilent Technologies). Final extracts in of 1:1 methanol:water were filtered using Whatman® Mini-UniPrep® Syringeless Filters [Polytetrafluoroethylene (PTFE), ; GE Healthcare]. Extracts were analyzed on an Agilent high-performance liquid chromatography (HPLC) 1260 with a Synergi™ Polar-RP column (; Phenomenex) coupled to an Agilent model 6460 tandem mass spectrometer with electrospray ionization (HPLC-MS/MS-ESI). Mobile phases consisted of formic acid in methanol and formic acid in water. Laboratory processing blanks were extracted alongside the placental tissues to monitor background levels. No TH were detected in the lab blanks. Method detection limits (MDLs) were calculated using a signal-to-noise value of 3 for each analyte (T3, T4, and rT3). Values were normalized to the wet weight of placenta extracted for a final value of nanogram hormone/gram placenta. Values below the MDL (T4, ; T3, ; rT3, ) were imputed using the calculation , and values lacking a quantifiable peak on mass spectrometry were excluded from the analysis.
Internal Dosimetry
Maternal serum, maternal liver, amniotic fluid, and whole embryos were analyzed for PFOA and GenX concentrations using methods similar to those previously reported (Conley et al. 2019; McCord et al. 2018; Reiner et al. 2009; Rushing et al. 2017). Solid tissues were homogenized in RODI water at a ratio of approximately 1:3 tissue mass (milligrams) to liquid volume (microliters). Maternal serum, amniotic fluid, and tissue homogenates () were spiked with internal standard suspended in formic acid in a denaturation step, followed by a subsequent protein crash using ice-cold acetonitrile. Samples were vortex mixed after addition of formic acid and acetonitrile and then centrifuged at for 5 min. Extract supernatants were separated using a Waters ACQUITY UPLC® (Waters Corporation) fitted with a Waters ACQUITY UPLC® BEH C18 Column (; ; ). Detection was performed using a Waters Quattro Premier™ XE tandem quadrupole mass spectrometer in negative ionization mode. Stable isotopes of PFOA (, MPFOA; Wellington Laboratories) or GenX (, M3HFPO-DA; Wellington Laboratories) were used as internal standards for quantification of vehicle control samples (run against a nine-point calibration curve of ) and experimental samples (run against a nine-point calibration curve of ). Vehicle control and dosed animal samples were quantified for both PFOA and GenX using respective isotope-labeled chemicals and calibration curves.
Embryo/Placental Growth Metrics
Gross observations were recorded at necropsy. Embryo sex was determined by polymerase chain reaction (PCR) amplification of the Sry gene (forward, 5′-GCTTCAGTAATCTCAGCACCTAGAA-3′, and reverse, 3′-CACATTGGCATGATAGCTCCAAATT-5′) using a snipped portion of tissue (TransnetYX®, Inc.). Embryos and their placentas were weighed separately as wet tissue. Images of embryos were obtained on a Leica Z16 APO imaging scope, and embryo length was measured as snout-to-rump distance using FIJI (Schindelin et al. 2012) and Zen 2 Blue (Zeiss).
Statistical Analysis
Data were analyzed in R (version 1.1.456; R Development Core Team). Sample sizes for each end point are reported in the accompanying figure legends or tables. A threshold of was used for determining statistical significance unless otherwise noted. Analyses combining data from both experimental blocks were performed after verifying the absence of experimental block effects. Single-observation dam outcomes (e.g., liver weight, relative liver weight, implantation sites, resorptions, viable embryos, and internal dose metrics) were analyzed by analysis of variance using the lme4 (Bates et al. 2014) and lmerTest packages (Kuznetsova et al. 2017). Simultaneous tests for general linear hypotheses were corrected for multiple comparisons of means using Tukey contrasts in the package multcomp (Hothorn et al. 2009).
For all statistical tests adjusting for litter size as a fixed effect in the model, litter size was defined as the number of viable embryos. GWG on the day of sacrifice was adjusted for litter size using a general linear model. To compare GWG growth curves, GWG was measured as the percent change in BW compared to E0.5 and analyzed using mixed-effects models controlling for litter size and accounting for repeated measures of dams over time.
Embryo and placental metrics were analyzed using mixed-effect models and included a priori fixed effects of treatment group and litter size and a random-effects term for the dam using the lme4 package. Embryo and placental metrics included embryo weight, embryo length, placental weight, and embryo:placenta weight ratios, a meaningful predictor of fetal birth outcomes in humans (Hayward et al. 2016). To account for potential introduction of random effects, the study block (Block 1 or Block 2) and experimenter handling of embryo/placental tissues (Experimenter A or Experimenter B) were included as additional random effects. Models were fit in a stepwise procedure for random effects, and final models included treatment group and litter size as fixed effects using the lmerTest package (Kuznetsova et al. 2017). All final models included dam as a random effect but were allowed to vary in the inclusion of experimenter and experimental block random effects based on likelihood ratio test results. Point estimates and 95% CIs were determined from the final model using the Wald method. The number of individual observations for each outcome (embryo weight, placenta weight, and embryo:placenta weight ratio) and the number of litters evaluated in the mixed-effect models are shown in Table S1.
To document the effects of PFOA and GenX on the placenta, placentas were assessed for histopathological lesions in five to six litters per treatment group for both time points, with an average of seven individual placentas evaluated per litter. Analyses of histopathological data included placentas collected from viable embryos and excluded fused placentas and placentas collected from sites of resorption, which did not occur more frequently than at expected background levels in this strain. Histopathological lesions of evaluated placenta were evaluated using two statistical approaches. The first approach assumed the absence of litter effects and considered each placenta evaluated within a treatment group to be a totally independent observation, regardless of its litter of origin. These data were analyzed as counts using a generalized linear model with a Poisson regression using the package lme4 (Bates et al. 2014). The second approach considered the litter as the biological unit and compared the relative incidence of placental lesions [e.g., percent within normal limits (WNL)] to adjust for differences in the total number of observations across litters within and between treatment groups. These data were analyzed using a linear model. Both approaches were subjected to simultaneous tests for general linear hypotheses to correct for multiple comparisons using Tukey contrasts in the package multcomp (Hothorn et al. 2009).
TH concentrations in the placenta were quantified, and the ratios of T3:T4 and rT3:T4 in E17.5 placentas were assessed to evaluate potential disruption of peripheral TH control (e.g., impacts on thyroid deiodinase activity). Each end point was analyzed for interaction or for an overall effect of sex. Placenta TH were analyzed by analysis of variance using lme4 (Bates et al. 2014). Simultaneous tests for general linear hypotheses were corrected for multiple comparisons of means using Tukey contrasts in the package multcomp (Hothorn et al. 2009). Placental TH and their ratios were initially analyzed with embryo sex as an interaction term in the model, with the dose group as the predictor variable. Inclusion of a sex interaction or sex covariate in the final model was examined in a stepwise fashion. Internal dosimetry data were analyzed by analysis of variance. Simultaneous tests for general linear hypotheses were corrected for multiple comparisons of means using Tukey contrasts in the package multcomp (Hothorn et al. 2009).
Results
Internal Dosimetry
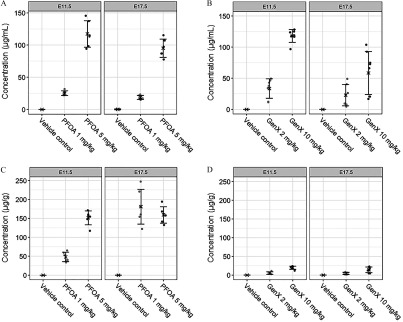
Maternal serum, maternal liver, amniotic fluid (E11.5 only), and whole-embryo dosimetry varied based on compound, dose, and time point. Urine collection was attempted at necropsy of pregnant dams exposed to GenX but was unable to be consistently collected in sufficient volume for dosimetry analysis. Concentrations of GenX in the serum of dams exposed daily to of GenX was equivalent to the concentration of PFOA in serum of dams exposed to of PFOA at E11.5 ( serum and serum, respectively; Figure 1A,B; Table S2). In contrast, GenX accumulation in the serum of dams exposed to GenX was 32% higher than the accumulation of PFOA in the serum of dams exposed to PFOA ( serum and serum, respectively; Figure 1A,B; Table S2). Serum levels of either dose of PFOA or GenX measured at E17.5 were lower from those measured at E11.5 (Figure 1A,B; Tables S2 and S3). This could be explained by a dilution effect caused by blood volume expansion over the course of gestation or may be due to increased transfer to embryos over time.
Figure 1.
Internal dosimetry of perfluorooctanoic acid (PFOA) and GenX [hexafluoropropylene oxide dimer acid (HFPO-DA)] in maternal serum and liver at embryonic day (E) 11.5 and E17.5. (A) Maternal serum concentration (microgram PFOA per milliliter serum) at E11.5 and E17.5, (B) maternal serum concentration (microgram GenX per milliliter serum) at E11.5 and E17.5, (C) maternal liver concentration (microgram PFOA per gram liver) at E11.5 and E17.5, and (D) maternal liver concentration (microgram GenX per gram liver) at E11.5 and E17.5 were determined by high-performance liquid chromatography–tandem mass spectrometry. Treatment group mean values are denoted with an “X” flanked above and below by error bars showing standard deviation, and individual data points are shown as gray circles (). Vehicle control (VC) samples were quantified for PFOA and GenX; all VC means were below the limit of detection (LOD) of for both PFOA and GenX except for maternal serum (). Statistical comparisons of internal dosimetry across all treatment groups are shown in Tables S2 and S3.
Accumulation of PFOA in the maternal liver was greater than the accumulation of GenX, regardless of dose level or collection time point (Figure 1C,D; Tables S2 and S3). While maternal serum levels of PFOA or GenX were surprisingly roughly equivalent at E11.5 in dams exposed to PFOA or GenX, respectively, the accumulation of PFOA in the maternal liver was markedly higher in mice exposed to PFOA than the accumulation of GenX in liver of mice exposed to GenX (Figure 1C,D; Tables S2 and S3). It appeared that bioaccumulation of PFOA in the liver had reached a maximum of approximately liver by E17.5 regardless of PFOA dose group (Figure 1C; Table S3). When comparing across low ( PFOA vs. ) and high ( PFOA vs. GenX) dose groups at each time point, the fold change comparing GenX accumulation in the liver to the PFOA accumulation in the liver was 7.6-fold lower ( GenX vs. PFOA; E11.5), 8.9-fold lower ( GenX vs. PFOA; E17.5), 11.2-fold lower ( GenX vs. PFOA; E17.5), and 39.7-fold lower ( GenX vs. PFOA; E17.5) (Figure 1C,D; Tables S2 and S3). Unlike PFOA, GenX did not significantly bioaccumulate further in dam livers between E11.5 and E17.5 (Figure 1D; Tables S2 and S3).
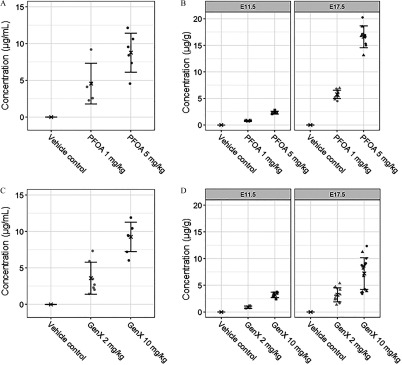
Amniotic fluid concentrations of PFOA and GenX were roughly equivalent when comparing the accumulation in dams exposed at the high ( PFOA vs. GenX) and low doses ( PFOA vs. GenX) (Figure 2A,C; Table S2). Comparing across PFOA and GenX dose groups, embryo accumulation at E11.5 was greatest in mice exposed to GenX (), followed by mice exposed to PFOA (), GenX (), and PFOA () (Figure 2B,D; Table S2). At E17.5, embryo accumulation was not different between sexes for either compound at the doses tested (Figure 2B,D; Table S3). Concentrations of PFOA or GenX in embryos were greater when measured at E17.5 than at E11.5, suggesting accumulation of both compounds over time in the embryo regardless of the shorter half-life of GenX (Figure 2B,D; Tables S2 and S3).
Figure 2.
Internal dosimetry of perfluorooctanoic acid (PFOA) and GenX [hexafluoropropylene oxide dimer acid (HFPO-DA)] in amniotic fluid and whole embryos. (A) Amniotic fluid concentration (microgram PFOA per milliliter amniotic fluid) at embryonic day (E) 11.5, (B) whole-embryo concentration (microgram PFOA per gram embryo) at E11.5 and E17.5, (C) amniotic fluid concentration (microgram GenX per milliliter amniotic fluid) at E11.5, and (D) whole-embryo concentration (microgram GenX per gram embryo) at E11.5 and E17.5 were determined by high-performance liquid chromatography–tandem mass spectrometry. Treatment group mean values are denoted with an “X” flanked above and below by error bars showing standard deviation, and individual data points are shown as gray squares, circles, or triangles (). Triangles, E17.5 male embryos; circles, E17.5 female embryos; squares, pooled E11.5 embryos (B and D). Vehicle control (VC) samples were quantified for PFOA and GenX; all VC means were below the limit of detection (LOD) of for both PFOA and GenX. Statistical comparisons of internal dosimetry across all treatment groups are shown in Tables S2 and S3.
Maternal Outcomes
Gross anomalies were visually evident in some dams upon necropsy; excess abdominal fluid, edematous tissues, clotted placentas, and two fetuses attached to a single placenta were noted. However, these findings were unexpected a priori and thus were not looked for in each animal, were not reported by dose group, and require further investigation in future studies.
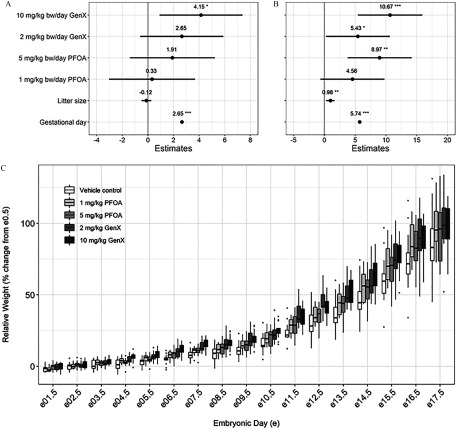
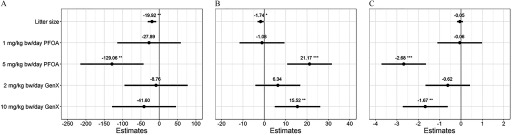
Mean dam BWs at E0.5 were similar across all treatment groups, including PFOA and GenX, for either sacrifice time point and did not differ from vehicle controls (Table 1). The relative change in dam BW from E0.5 to the time of collection (percent change in weight; GWG) was significantly greater after exposure to GenX at E11.5 (7.4% greater BW gain at E11.5 relative to vehicle controls; ; Table 1). The number of implantation sites, viable embryos, nonviable embryos, and resorptions did not significantly differ among treatment groups, including PFOA and GenX, at either time point relative to the vehicle controls, although GenX-treated dams had fewer implantation sites and viable embryos at E17.5 (Table S4). When controlling for litter size, relative GWG was significantly greater than controls in Gen-treated mice (E11.5: 7.1% greater compared to controls; E17.5: 19.1% greater compared to controls) and PFOA-treated mice (E17.5: 14.5% greater compared to controls; Table S5). Effect estimates from mixed-effect models adjusting for repeated measures of relative GWG (dataset shown in Figure 3C), litter size, and gestational/embryonic day showed significantly higher relative GWG in mice exposed to GenX (E11.5 and E17.5) (Figure 3A,B), GenX (E17.5) (Figure 3B), and PFOA (E17.5) (Figure 3B).
Table 1.
Maternal indices at embryonic day 11.5 and 17.5 [; ].
| Embryonic day | Maternal index | Vehicle control | (PFOA) | (PFOA) | GenX (HFPO-DA) | GenX (HFPO-DA) |
|---|---|---|---|---|---|---|
| 11.5 | E0.5 weight (g) | |||||
| 11.5 | Weight at necropsy (g) | |||||
| 11.5 | Weight at necropsy (% change from E0.5) | * | ||||
| 11.5 | Liver weight (g) | * | * | * | * | |
| 11.5 | Relative liver weight (% BW) | * | * | * | * | |
| 11.5 | Kidney weight (g) | |||||
| 11.5 | Relative kidney weight (% BW) | |||||
| 17.5 | E0.5 weight (g) | |||||
| 17.5 | Weight at necropsy (g) | |||||
| 17.5 | Weight at necropsy (% change from E0.5) | |||||
| 17.5 | Liver weight (g) | * | * | * | ||
| 17.5 | Relative liver weight (% BW) | * | * | * | * | |
| 17.5 | Kidney weight (g) | * | ||||
| 17.5 | Relative kidney weight (% BW) | * |
Note: BW, body weight. for kidney weight and relative kidney weight.
relative to vehicle control [analysis of variance (ANOVA) with post hoc multiple comparison correction using Tukey contrasts].
Figure 3.
Gestational weight gain (GWG) repeated-measure, mixed-effect model estimates for pregnant dams exposed to perfluorooctanoic acid (PFOA) and GenX [hexafluoropropylene oxide dimer acid (HFPO-DA)]. Effect estimates for pregnant dams exposed through embryonic day 11.5 (A) or 17.5 (B) are centered around the vehicle control group () and show the point estimate of the relative change in dam weight (percent change from E0.5) with 95% confidence intervals (CIs). (C) Boxplots of relative weight gain over time, with the upper and lower hinges corresponding to the first and third quartiles (25th and 75th percentiles), the middle hinge corresponding to the median, and the upper whisker extending to the highest value that is within 1.5 times the distance between the first and third quartiles [interquartile range (IQR)] of the hinge and the lower whisker extending to the lowest value within 1.5 times the IQR of the hinge. dams per treatment group. *. **. ***. Beta estimate 95% confidence intervals do not overlap zero. [Repeated-measures mixed-effect model adjusting a priori for litter size and gestational (embryonic) day as fixed effects and the dam as a random effect, vehicle control as reference group].
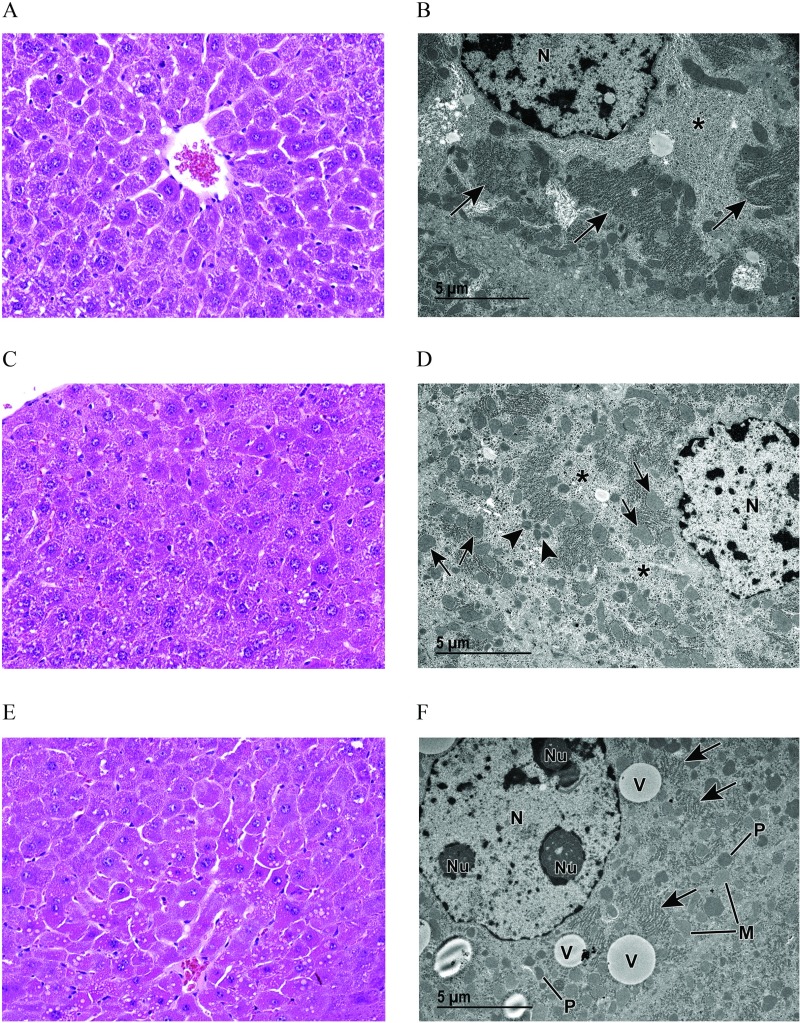
Dam liver weights were significantly higher in all treated groups compared to vehicle controls at E11.5 (Table 1). At E17.5, absolute liver weights of dams were significantly higher in the PFOA, GenX, and GenX-treatment groups than in vehicle controls (Table 1). Dam relative liver weight (as a percentage of BW) was significantly higher in both PFOA and GenX treatment groups relative to vehicle controls at E11.5 and E17.5 (Table 1). At E11.5, vehicle control livers exhibited either normal hepatocellular features with uniform hepatocellular size and cytoplasmic glycogen or minimal centrilobular hepatocellular hypertrophy with decreased glycogen, consistent with pregnancy at this stage of gestation. At E17.5, vehicle control livers exhibited hepatocellular changes consistent with pregnancy at this stage of gestation (minimal to mild centrilobular hepatocellular hypertrophy with karyomegaly, increased mitotic figures, decreased glycogen, and increased basophilic granular cytoplasm (Figures 4A and 5A). Compared with their respective controls, all livers (100% incidence) from both PFOA- and GenX-treated dams at E11.5 and E17.5 showed a variety of adverse outcomes (Figure S1), including some degree of cytoplasmic alteration, characterized by varying degrees of hepatocellular hypertrophy with decreased glycogen and intensely eosinophilic granular cytoplasm (Figures 4C,E and 5C,E; Tables S6 and S7). As the severity increased, there was extension of the cytoplasmic alteration into the midzonal and periportal regions. Also, as the cytoplasmic alteration increased in severity, there was an observed decrease in mitoses and increase in apoptotic cell death (Figures 4E and 5E). A few livers from exposed animals also had focal regions of classic necrosis. Incidence of liver lesions and vacuolation are reported in Tables S6 and S7.
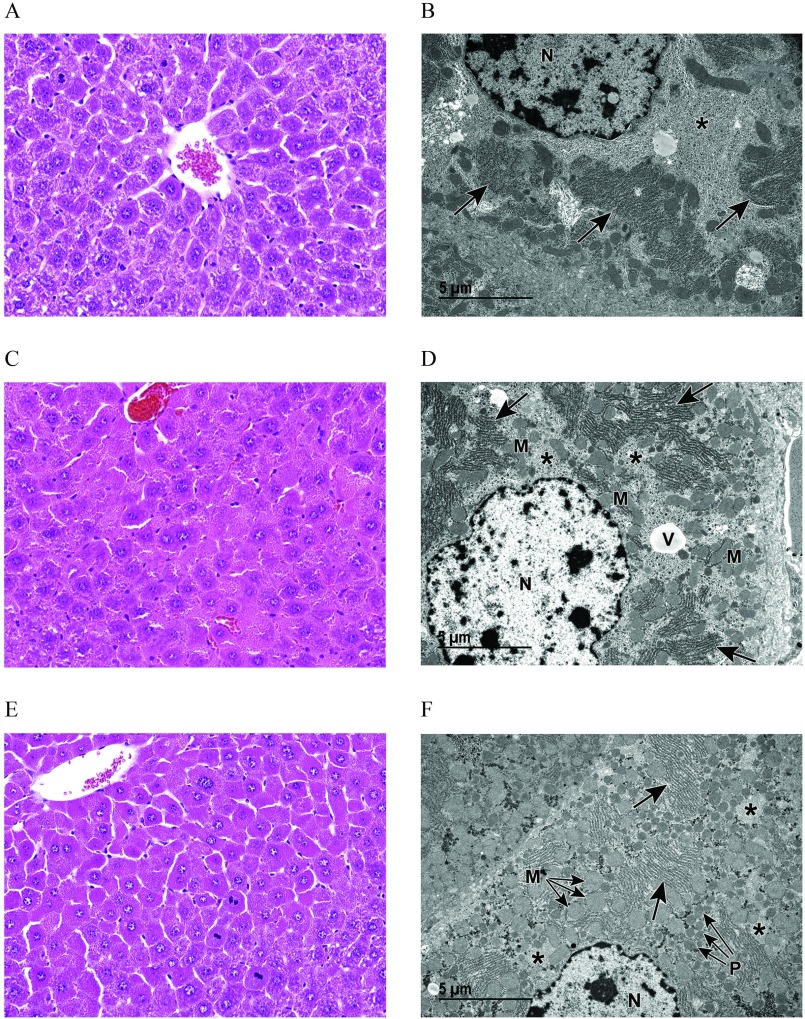
Figure 4.
Light and transmission electron microscopy (TEM) of liver from vehicle control (VC) and perfluorooctanoic acid (PFOA)–exposed pregnant dams at embryonic day (E) 17.5. (A) Light microscopic image at magnification of liver from a VC pregnant dam (control) showing centrilobular hepatocellular hypertrophy with karyomegaly, increased basophilic granular cytoplasm, and decreased glycogen. (B) Corresponding TEM magnification shows prominent rough endoplasmic reticulum (arrows) with abundant ribosomes and evenly dispersed, abundant glycogen (asterisk) (see Figure S2A). (C) Light microscopic image at magnification of liver from a pregnant dam at E17.5 and treated with PFOA. (D) Although this liver appears to be within normal limits when viewed with light microscopy, TEM reveals an increase in scattered vacuoles (see Figure S2B); decreased, evenly dispersed glycogen (asterisks); as well as abundant mitochondria (arrows) and peroxisomes (arrowheads). (E) Light microscopic image at magnification of liver from a pregnant dam at E17.5 and treated with PFOA. Increased cytoplasmic vacuoles are evident at this light microscopic level. (F) TEM reveals abundant cytoplasmic organelles consistent with mitochondria (M) and peroxisomes (P), extensive vacuoles (V), less prominent rough endoplasmic reticulum (arrows) with fewer ribosomes and less abundant glycogen (see Figure S2C,S2D). Note: N, nucleus; NU, nucleolus; TEM, transmission electron microscopy.
Figure 5.
Light and transmission electron microscopy (TEM) of liver from vehicle control (VC) and GenX-exposed pregnant dams at embryonic day (E) 17.5. (A) Light microscopic image at magnification of liver from a VC pregnant dam showing centrilobular hepatocellular hypertrophy with karyomegaly, increased basophilic granular cytoplasm, and decreased glycogen. (B) Corresponding medium TEM magnification shows prominent rough endoplasmic reticulum (arrows) with abundant ribosomes and evenly dispersed, abundant glycogen (asterisk) (see Figure S2A). (C) Light microscopy at magnification, and (D) transmission electron microscopy of liver from a pregnant dam at E17.5 treated with GenX [hexafluoropropylene oxide dimer acid (HFPO-DA)] or GenX (E and F). Marked cytoplasmic alteration is evident in (C) and (E). TEM (D and F; see Figure S2E and S2F, respectively) reveals an abundance of cytoplasmic organelles, consistent with mitochondria (M) and peroxisomes (P) that increase with increasing dose (D compared to F). Note also the decreased glycogen (asterisks) as well as the vacuole (V) and rough endoplasmic reticulum (arrows). N, nucleus.
Histopathological liver findings from a subset of E17.5 dams, including all dose groups for PFOA, GenX, and vehicle controls for comparison, were further evaluated using TEM. All vehicle control livers exhibited normal ultrastructure for this stage of gestation. In the centrilobular regions with hepatocellular hypertrophy, there was abundant glycogen, prominent rough endoplasmic reticulum (RER) with abundant ribosomes, numerous lysosomes, and minimal vacuolation with vacuoles often containing remnant membrane material as myelin figures (Figures 4B and 5B). Livers from mice exposed to PFOA exhibited enlarged hepatocytes with increased cytoplasmic organelles consistent with mitochondria and peroxisomes, evenly dispersed glycogen, and small vacuoles in the centrilobular regions (Figure 4D) compared to vehicle controls. Livers from mice exposed to PFOA exhibited abnormal ultrastructure with abundant organelles consistent with mitochondria and peroxisomes, highly prevalent cytoplasmic vacuolation, reduced RER with fewer ribosomes, and less abundant glycogen (Figure 4F). Livers from mice exposed to GenX exhibited abnormal ultrastructure with enlarged hepatocytes containing more abundant cytoplasmic organelles consistent with mitochondria and peroxisomes, and vacuolation (Figure 5D). Livers from mice exposed to GenX exhibited abnormal ultrastructure with enlarged hepatocytes containing abundant organelles consistent with mitochondria and peroxisomes, and prevalent vacuolation often with remnant membrane material as myelin figures, abundant RER with few ribosomes present, and unevenly dispersed glycogen appearing as clustered clumps (Figure 5F). At the level of TEM, PFOA and GenX generally caused a variety of cellular alterations: increased vacuolation, increased numbers of cytoplasmic organelles consistent with mitochondria and peroxisomes, reduced glycogen stores and reduction of RER ribosomes (Figure S2). Marked clumping of glycogen was a unique observation in livers of mice exposed to GenX, likely a secondary effect due to abundant mitochondria, peroxisomes, and RER.
Kidney weights and relative kidney weights of dams exposed to either dose of PFOA or GenX did not differ from vehicle controls at E11.5 (Table 1). At E17.5, GenX-exposed mice exhibited higher kidney weight relative to vehicle controls (both absolute kidney weight and relative kidney weight) (Table 1). Kidney cross sections and longitudinal sections were histopathologically evaluated at E11.5 and E17.5 time points, and diagnoses were made with no threshold: cortical glomeruli; cortical and medullary tubules; papillary collecting ducts; parenchyma; and vascular tree including renal artery, interlobar artery, interlobular artery, arcuate artery, and renal veins. Kidneys from vehicle control and treated animals were histologically WNL.
Clinical Chemistry
Dam serum Trig levels were significantly lowered at E11.5 across all treatment groups compared to controls in a dose–response manner ( PFOA and GenX lowered Trigs by 58% and 61%, respectively; PFOA and GenX lowered Trigs by 37% and 43%, respectively; Table 2). At E17.5, dam serum Trigs were significantly lower in PFOA and GenX-treated mice (66% lower and 74% lower, respectively) (Table 3).
Table 2.
Clinical chemistry panel of dam serum at embryonic day 11.5.
| Measurement | Vehicle control [ ()] | PFOA [ ()] | PFOA [ ()] | GenX [ ()] | GenX [ ()] |
|---|---|---|---|---|---|
| ALB (g/dL) | (5) | (5) | (5) | (4) | (3) |
| ALP (U/L) | (5) | (5) | (5) | (5) | (5) |
| ALT (U/L) | (5) | (5) | (5) | (5) | (5) |
| AST (U/L) | (5) | (5) | (5) | (5) | (4) |
| BUN (mg/dL) | (5) | (5) | (5) | (4) | (3) |
| Chol (mg/dL) | (5) | (5) | (5) | * (5) | (4) |
| Cre (mg/dL) | (5) | (5) | (5) | (4) | (3) |
| Glu (mg/dL) | (5) | (5) | (5) | (4) | (3) |
| HDL (mg/dL) | (5) | (5) | (5) | * (5) | (4) |
| LDL (mg/dL) | (5) | (5) | (5) | (4) | (4) |
| SDH (U/L) | (5) | (5) | (5) | (4) | (4) |
| TBA () | (5) | (4) | (5) | (5) | (4) |
| TP (g/dL) | (5) | (5) | (5) | (4) | (3) |
| Trig (mg/dL) | (5) | * (5) | * (5) | * (5) | * (4) |
| Ucrea (mg/dL) | (1) | (4) | (4) | (3) | (5) |
Note: ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Chol, cholesterol; Cre, creatinine; Glu, glucose; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SD, standard deviation; SDH, sorbitol dehydrogenase; TBA, total bile acids; TP, total protein; Trig, triglycerides; Ucrea, urinary creatinine; U/L, units per liter.
relative to vehicle control [analysis of variance (ANOVA) with post hoc multiple comparison correction using Tukey contrasts].
Table 3.
Clinical chemistry panel of dam serum at embryonic day 17.5.
| Measurement | Vehicle control [ ()] | PFOA [ ()] | PFOA [ ()] | GenX [ ()] | GenX [ ()] |
|---|---|---|---|---|---|
| ALB (g/dL) | (4) | (5) | * (6) | (5) | (5) |
| ALP (U/L) | (4) | (5) | (6) | (5) | * (5) |
| ALT (U/L) | (4) | (5) | (6) | (5) | (5) |
| AST (U/L) | (4) | (5) | * (6) | (5) | (5) |
| BUN (mg/dL) | (4) | (5) | (6) | (5) | (5) |
| Chol (mg/dL) | (4) | (5) | (6) | (5) | (5) |
| Cre (mg/dL) | (4) | (5) | (6) | (5) | (5) |
| Glu (mg/dL) | (4) | (5) | (6) | (5) | (5) |
| HDL (mg/dL) | (4) | (5) | (6) | (5) | (5) |
| LDL (mg/dL) | (4) | (5) | (5) | (5) | (5) |
| SDH (U/L) | (4) | (5) | * (6) | (5) | (5) |
| TBA () | (4) | (5) | (6) | (5) | (5) |
| TP (g/dL) | (4) | (5) | * (6) | (5) | (5) |
| Trig (mg/dL) | (4) | (5) | * (6) | (5) | * (5) |
| Ucrea (mg/dL) | (2) | (2) | (3) | (4) | (4) |
Note: ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Chol, cholesterol; Cre, creatinine; Glu, glucose; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SD, standard deviation; SDH, sorbitol dehydrogenase; TBA, total bile acids; TP, total protein; Trig, triglycerides; Ucrea, urinary creatinine.
relative to vehicle control (ANOVA with post hoc multiple comparison correction using Tukey contrasts).
At E11.5, serum Glu levels in dams exposed to PFOA and GenX were lower relative to controls (20% and 18% lower, respectively), but this shift did not reach statistical significance (Table 2; and , respectively). By E17.5, serum Glu remained lower in PFOA-exposed mice and GenX-exposed mice, but this shift was also not statistically significant (Table 3; and , respectively).
At E11.5, dams exposed to GenX exhibited higher Chol and HDL compared with controls (66% and 56% higher, respectively) (Table 2). E11.5 dams exposed to PFOA and GenX similarly exhibited higher Chol and HDL levels relative to controls, but this shift did not reach statistical significance ( and , respectively) (Table 3). By E17.5, treatment-related effects on Chol and HDL appeared to be generally attenuated (Table 3). At E17.5, mice exposed to PFOA and GenX exhibited lower LDL (50% lower and 31% lower, respectively), but only the shift in PFOA-exposed mice was significant (Table 3).
Dams exposed to PFOA and GenX exhibited higher ALT relative to controls (a 172% increase and a 200% increase, respectively), but these shifts were not statistically significant with post hoc corrections (Table 2). By E17.5, treatment group–related effects on ALT were attenuated. At E17.5, dams exposed to PFOA exhibited lower serum ALB, increased AST, increased SDH, and lower total serum protein relative to controls (Table 3). Similar shifts occurred in mice exposed to GenX with respect to AST, SDH, and TP, but were not statistically significant (Table 3). Overall, GenX and PFOA liver pathology was consistent across dose groups and time points (100% incidence of cytoplasmic alteration) (Table S6 and S7), while changes in ALT, AST, and SDH measurements were not statistically significant across all GenX or PFOA dose groups or time points.
Embryo and Placenta Outcomes
Although the number of implantation sites, viable embryos, nonviable embryos, or resorptions did not significantly differ across treatment groups at E11.5 or E17.5 (Table S4), we evaluated embryos and their placentas for differences in weight. At E11.5, there were no significant differences in viable embryo weight, placental weight, or embryo:placenta weight ratios across treatment groups relative to vehicle controls (Table S8). At E17.5, significantly lower viable embryo weight was observed in PFOA-treated mice ( PFOA embryos were lower in BW than vehicle control embryos based on mixed-effect model estimates; Figure 6A and Table S8). At E17.5, placental weight was significantly higher in PFOA- and GenX-treated mice relative to vehicle controls (an estimated and increase in placental weight relative to controls, respectively; Figure 6B and Table S8). Embryo:placenta weight ratios (mg:mg) were significantly reduced relative to controls in PFOA- and GenX-treated mice at E17.5 (Figure 6C and Table S8).
Figure 6.
Mixed-effect model estimates for (A) embryo weight (mg), (B) placental weight (mg), and (C) embryo:placenta weight ratios (mg:mg) after exposure in utero to perfluorooctanoic acid (PFOA) or GenX [hexafluoropropylene oxide dimer acid (HFPO-DA)] at embryonic day (E) 17.5. Effect estimates are centered around the vehicle control group () and show the point estimate of the relative change in weight (in milligrams; A and B) or weight ratio (mg:mg; C) with 95% confidence intervals (CIs). *. **. ***. Beta estimate 95% confidence intervals do not overlap zero (mixed-effect model adjusting a priori for litter size as a fixed effect and the dam as a random effect, vehicle control as reference group). Adjusted estimates and 95% CIs are shown in Table S8.
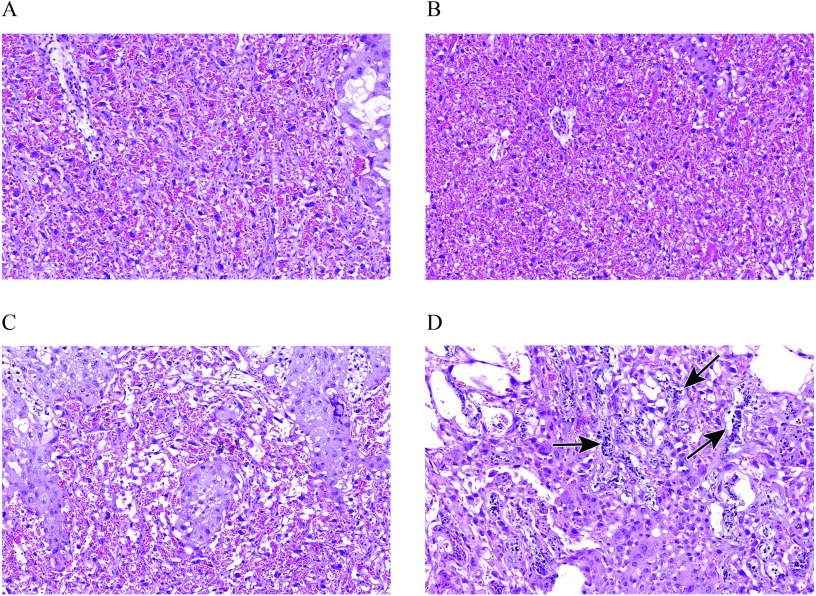
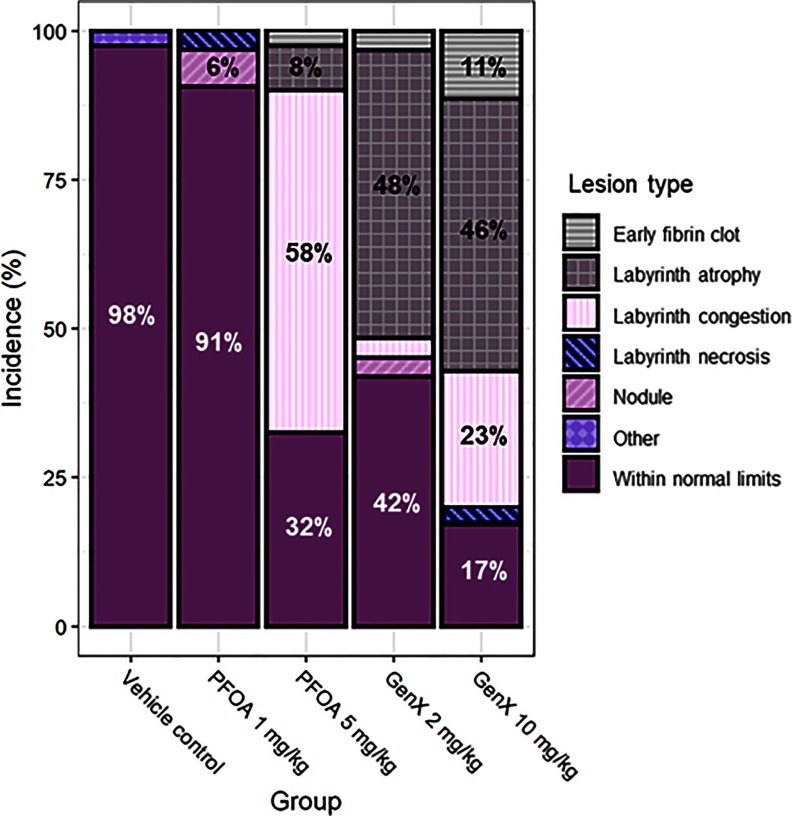
At E11.5, placental lesions were relatively sparse and mostly included labyrinth atrophy, labyrinth necrosis, or early fibrin clot formation. At E11.5, there were no differences in the incidence of placentas WNL across treatment groups (Table S9). At E17.5, placental abnormalities were observed in all treatment groups and tended to occur as litter-specific effects (e.g., most or all placenta within one litter were affected), and the most common lesions included labyrinth congestion (Figure 7B), labyrinth atrophy (Figure 7C), early fibrin clots (Figure S3A), labyrinth necrosis (Figure 7D), and placental nodules (Figure S3B). Placental nodules were most likely resorption of an adjacent twin. Placentas of mice exposed to PFOA exhibited labyrinth congestion as the most common lesion, whereas placentas of mice exposed to either or GenX primarily exhibited atrophy of the labyrinth (Figure 8 and Table S10). Early fibrin clots were most common in placentas of mice exposed to GenX (Figure 8 and Table S10). At E17.5, placentas WNL were significantly lower in mice exposed to PFOA or GenX when all evaluated placentas were considered as independent observations (regardless of litter of origin) (Table S10). Placental lesions were also evaluated to account for litter effects by using the proportion of placenta within a litter that was WNL (percent WNL). Comparing placenta using this method showed a reduction in placenta WNL in mice exposed to PFOA, GenX, and GenX (Table S10).
Figure 7.
Representative examples of histopathological placenta findings observed in dams at embryonic day (E) 11.5 and E17.5, treated with perfluorooctanoic acid (PFOA) or GenX [hexafluoropropylene oxide dimer acid (HFPO-DA)]. (A) Normal labyrinth from a vehicle control dam at E17.5. (B) Labyrinth congestion in a dam at E17.5 that was treated with GenX (C) Moderate labyrinth atrophy of the trilaminar trophoblast layer at E17.5 in a dam treated with GenX. (D) Labyrinth necrosis (arrows) in an E17.5 dam that was treated with GenX. All images at magnification.
Figure 8.
Incidence of placenta lesions across treatment groups at embryonic day 17.5. with 31–41 placentas evaluated per treatment group (an average of 6–8 placentas per litter). Incidence values are not numerically indicated, but all values and statistical comparisons of placenta lesion incidences across treatment groups at E17.5 are shown in Table S10.
Placental Thyroid Hormones
For all placental TH endpoints, interaction and sex as a covariate did not significantly influence model fit and were not incorporated in the final linear model (Table S11). Placentas exposed to GenX had significantly higher T4 relative to controls (60% increase) (Table 4). This effect occurred in both male and female placentas, but statistical significance was attenuated post hoc in sex-stratified models likely due to low sample sizes. There was a trend towards a significant effect of higher T4 in placentas exposed to GenX (38% increase; Table 4), but this effect was attenuated after applying post hoc corrections for multiple tests. Similarly, a trend toward a lower T3:T4 ratio was observed in placentas exposed to GenX, but this effect was attenuated after applying post hoc corrections. There were no other significant effects of sex or treatment on placental rT3, T3, T3:T4 ratio, or rT3:T4 ratio.
Table 4.
Placental thyroid hormone measurements at embryonic day 17.5.
| Hormone | Vehicle control { [ (a, b)]} | PFOA { [ (a, b)]} | PFOA { [ (a, b)]} | GenX { [ (a, b)]} | GenX { [ (a, b)]} |
|---|---|---|---|---|---|
| rT3 (ng/g) | [5 (4, 1)] | [6 (3, 3)] | [5 (5, 0)] | [6 (6, 0)] | [6 (6, 0)] |
| T3 (ng/g) | [6 (1, 5)] | [6 (0, 6)] | [4 (0, 4)] | [5 (0, 5)] | [6 (0, 6)] |
| T4 (ng/g) | [6 (6, 0)] | [6 (6, 0)] | [6 (6, 0)] | [6 (6, 0)] | * [6 (6, 0)] |
| T3:T4 ratio | [6] | [6] | [4] | [5] | [6] |
| rT3:T4 ratio | [5] | [6] | [5] | [6] | [6] |
Note: Sample sizes are expressed as the total number of samples (n) as well as the number of samples above the MDL (a) and below the MDL (b). Nonquantifiable samples below the MDL were imputed using the calculation . MDL values were: T4, ; T3, ; rT3, . MDL, method detection limit; rT3, reverse triiodothyronine; SD, standard deviation; T3, triiodothyronine; T4, thyroxine.
relative to vehicle control [analysis of variance (ANOVA) with post hoc multiple comparison correction using Tukey contrasts].
Discussion
Our prior work in mice has consistently shown reduced birth weight resulting from gestational exposure to PFOA (Macon et al. 2011; White et al. 2007), but we did not examine effects on the placenta, a critical organ that facilitates embryo growth, nor did we examine the effects of replacement PFAS congeners. Here we present evidence consistent with previous reports of PFOA-reduced embryo growth and provide novel evidence indicating that the pregnant dam liver and placenta are sensitive targets of both PFOA and a replacement PFAS, GenX. Adverse placental and maternal effects were most prominent in late gestation (E17.5) in mice gestationally exposed to PFOA and GenX, but GenX also exhibited significant effects on maternal liver and placenta. Future studies should investigate adverse effects at doses lower than GenX to determine more precise percent responses at different lower dose levels using a benchmark dose approach.
It is well documented in humans and animal models that PFAS readily pass from maternal serum to the developing embryo via the placenta (Chen et al. 2017; Yang et al. 2016a, 2016b) and that PFOA transplacentally transfers to the mouse offspring (Fenton et al. 2009). Here, we report transplacental transfer of both PFOA and GenX, higher placenta weight, higher incidence of placental lesions, and lower embryo–placenta weight ratios in mice exposed to PFOA or GenX.
In humans, placenta weight and placental-to-fetal (also reported as feto-placental) weight ratios are clinically relevant end points that have been associated with adverse pregnancy outcomes (Hutcheon et al. 2012; Risnes et al. 2009; Thornburg et al. 2010). The placenta is a critical organ that mediates the transport of nutrients, oxygen, waste, and xenobiotics between mother and embryo, and it is rarely evaluated in reproductive toxicity studies. We chose the placenta as a focal end point due to its importance in studies of human pregnancy outcomes (Hutcheon et al. 2012; Risnes et al. 2009), its role as a programming agent of latent health outcomes in both the mother and child (Thornburg et al. 2010), and our own hypothesis that it is a key target tissue of PFAS.
Placental insufficiency (PI) occurs when functional capacity of the placenta is limited or deteriorates, resulting in reduced transplacental transfer of oxygen and nutrients to the fetus (Gagnon 2003). Reduction or impairment of placental blood flow (Chaddha et al. 2004), aberrant fibrin depositions or other thrombo-occlusive damage in the placenta (Chaddha et al. 2004), and disruption of maternal–placental THs (Belet et al. 2003) are all believed to contribute to PI pathogenesis in women. We provide evidence illustrating pathological and physiological features that are concordant with PI in our experimental mouse model. Here we show maternal exposure to PFOA- or GenX-induced atrophy, necrosis, and congestion of the murine placental labyrinth (suggestive of impaired transplacental transfer of nutrients and/or oxygen), aberrant formation of early fibrin clots, and disruption of placental TH (GenX only). These data are suggestive of a PI phenotype induced by maternal exposure to PFAS in mice that deserves further investigation.
In epidemiological studies, disproportionately large placentas increase the risk for adverse health outcomes in neonates (Hutcheon et al. 2012) and adult offspring (Risnes et al. 2009). The placenta influences cardiovascular disease (CVD) risk in the offspring (Risnes et al. 2009), and the functional capacity of the placenta is likely the driver of fetal heart fitness (Thornburg et al. 2010). Placentas that are disproportionately large relative to fetal size tend to exhibit reduced functional capacity with respect to optimal blood flow and vascular resistance (Risnes et al. 2009; Salafia et al. 2006), which could lead to both adverse perinatal (Hutcheon et al. 2012) and adult CVD outcomes (Thornburg et al. 2010). Here we show higher placenta weights that were disproportionate to embryo weights in mice exposed to PFOA and GenX. Whether the increased placental weight is due to pathological changes or is a compensatory mechanism to protect the developing fetus is not known. The extent to which gestational exposure to these environmental contaminants could adversely impact perinatal and adult offspring health outcomes, especially cardiovascular outcomes, should be the focus of future studies.
A previous report has shown dose-dependent necrotic changes in the placenta of mice exposed to and PFOA, and pup mortality and gestational weight loss were evident (Suh et al. 2011). Here, placental lesions in mice exposed to GenX, GenX, and PFOA at E17.5 occurred at a significantly higher incidence compared to controls, and the labyrinth was the specific target. This is significant because the maternal–embryo exchange of oxygen, nutrients, and waste occurs in the placental labyrinth. Adverse placental effects of PFOA and GenX occurred at both the litter level as well as across all placenta evaluated, regardless of litter, and adverse placental effects of GenX were significant when considered at the level of the litter as a unit. The lowest doses tested in this study resulting in adverse placental pathology were GenX and PFOA. Given that maternal serum accumulation and embryo deposition of PFOA and GenX were similar at the high ( PFOA vs. GenX) and low doses ( PFOA vs. GenX) and that the placenta is at the interface between these two compartments, the disparate patterns in adverse placenta histopathology suggest that the placenta may be more sensitive to the effects of GenX vs. PFOA. The mechanisms of toxicity towards the placenta may also differ between the two PFAS and will be pursued in ongoing studies.
TH play a critical role in neurodevelopment (de Escobar et al. 2004; Porterfield 1994). PFAS are well-documented thyroid disrupters in humans (Coperchini et al. 2017; Webster et al. 2016), including in pregnant women (Ballesteros et al. 2017; Berg et al. 2015; Wang et al. 2014; Webster et al. 2014). Generally, maternal PFAS levels during pregnancy are associated with shifts in TH levels consistent with hypothyroidism (e.g., elevated thyroid-stimulating hormone), which is associated with increased risk for low birth weight (Alexander et al. 2017). It is possible that PFAS chemicals exert some adverse effects on embryo growth via TH disruption across the maternal–placental–embryo unit. Indeed, Conley et al. (2019) reported maternal serum total triiodothyronine (T3) and thyroxine (T4) were reduced in rats exposed to HFPO-DA (GenX) during gestational days 14–18. Maternal serum TH could not be measured due to volume constraints in our study. As the placenta regulates the degree to which maternal THs pass to the developing fetus, and it maintains the optimal balance of the TH throughout embryo development (Chan et al. 2009), the relationship between PFAS-induced maternal TH changes and placental function requires additional study, especially given the role of TH in fetal neurodevelopment.
In a systematic review and meta-analysis of nonhuman evidence for effects of PFOA on BW, it was estimated that a 1-unit ( BW/d) increase in PFOA is associated with a (95% CI: , ) shift in pup birth weight (Koustas et al. 2014). Here we report a (95% CI: , 0.586) shift in embryo weight on E17.5 in mice exposed to PFOA and a (95% CI: , ) shift in mice exposed to PFOA. Effects on embryo weight at E17.5 in this study can be summarized as most severe to least severe: PFOA (), GenX (), PFOA (), and GenX (). An industry study of CD-1 mice exposed to HFPO-DA (GenX) from preconception through weaning showed reduced pup weight at postnatal day (PND) 1 that persisted through PND 21 with effects more severe in male offspring (DuPont-18,405-1,037). In rats, mean embryo weights were decreased in rats exposed to HFPO-DA (GenX) for 15 d of gestation (Edwards 2010a), and in a different study, female birth weights were reduced after 5 d of gestational exposure at (Conley et al. 2019). To our knowledge, there are no human data showing associations between maternal GenX exposure and birth weight outcomes.
Several human cohort studies have shown that higher levels of prenatal or early-life PFOA exposure is associated with increased adiposity in childhood (Braun et al. 2016; Fleisch et al. 2017) and metabolic disruption in young adulthood (Domazet et al. 2016). Additionally, it is known that low birth weight is associated with adult diseases, including metabolic syndrome in both humans and animals (Barker 2004). Due to the environmental ubiquity of a mixture of PFAS chemicals, it is difficult to unravel the relative contributions of prenatal and postnatal (e.g., chronic, lifelong) exposure and adverse health outcomes. Animal studies allow for discrete measurement of health outcomes associated with specific critical periods of exposure, and future work should investigate metabolic disruption in offspring exposed in utero to provide key insights on the metabolic programming capacity of PFAS.
In the present study, PFOA () and GenX ( or ) exposures resulted in significantly higher GWG in mice, with significant effects emerging at an earlier point in gestation in mice exposed to GenX and occurring at a lower dose than PFOA ( GenX vs. PFOA). In contrast, a decrease in mean maternal weight gain was reported in a recent study of gestational exposure to GenX in rats exposed to 250 or (Conley et al. 2019). Although these findings are not consistent with the higher GWG reported here, it is possible that statistical methods (absolute change in maternal weight vs. relative change in weight analyzed using repeated measures models), differing windows of exposure (5 d during mid- to late gestation vs. exposure throughout gestation), and interspecies differences in preliminary PFAS elimination rates [GenX elimination half-life in rats: vs. in mice, (Gannon et al. 2016)] could explain these disparate results. It is possible that different elimination rates of the compound make the comparison of equivalent or similar external doses a challenge. In fact, dam serum concentrations of rats exposed to from gestation day (GD) 14-18 reported in Conley et al. (2019) were of similar magnitude to those observed in mice exposed to throughout gestation in the present study (). Similarly, serum concentrations from pregnant mice in the current study exposed to GenX were roughly equivalent () to serum concentrations obtained from rat dams exposed to GenX in the study by Conley et al. (2019).
Higher GWG observed in our PFOA-exposed mice is consistent with findings reported in humans; interquartile range increases in GWG were associated with elevated cord blood levels of PFOA (; 95% CI: 1.13,1.56) (Ashley-Martin et al. 2016). Similarly, other legacy PFAS compounds such as perfluorooctanesulfonic acid are positively associated with GWG (Jaacks et al. 2016). However, our data describing the relationship between maternal exposure to GenX and increased GWG in a mouse model are novel. Importantly, higher GWG is associated with adverse outcomes for both mother and infant in humans, including increased risk for pregnancy-associated hypertension (with or without smaller birth weights), gestational diabetes, postpartum weight retention, increased risk for unsuccessful breastfeeding, and increased risk for stillbirth, infant mortality, and preterm birth (Rasmussen and Yaktine 2009). These disorders share many risk factors, but it is not fully understood to what extent their etiologies are interrelated and/or interdependent (Villar et al. 2006) or what mechanisms may be driving them. Our data suggest a need for additional study of the adverse maternal and offspring health outcomes associated with GenX exposure.
Liver toxicity is a consistent finding in animal studies of PFOA (Li et al. 2017) and other PFAS, but studies examining GenX are limited. Here, we report similar histopathological findings in livers of exposed pregnant dams to those previously described by our group (and others) in offspring prenatally exposed to PFOA, including increased extent of hepatocellular hypertrophy, cytoplasmic alteration, and increased mitochondria (Filgo et al. 2015; Lau et al. 2006). We hypothesize that the consistent and persistent hepatic cytoplasmic alterations seen following PFAS exposures lead to increased incidence and/or distribution of cell death, which is consistent with the decrease in mitotic figures compared to control liver sections. This constellation of lesions is considered adverse and is incompatible with long-term normal liver function. The maternal liver responds to estrogen produced by the placenta and produces thyroid-binding globulin, which, in turn, regulates the level of maternal circulating TH (Nader et al. 2009). It is possible that altered maternal liver function due to PFOA or GenX exposure plays an important role in mediating placental and embryo outcomes.
In addition to consistently observed histopathological changes in the liver induced by either PFOA or GenX, maternal clinical chemistry indicated shifts in liver enzymes, including higher ALT ( GenX; E11.5), higher ALP ( GenX; E17.5), higher AST ( PFOA; E17.5), and higher SDH ( PFOA; E17.5). Our TEM findings build upon a growing body of evidence demonstrating potential mechanisms of PFAS-induced hepatic toxicity other than PPAR and demonstrate this for the first time with GenX.
In a previous reproductive and developmental toxicity study of HFPO-DA (GenX) in CD-1 mice, was determined to be the NOAEL for reproductive toxicity and maternal systemic toxicity (based on microscopic changes in maternal liver; DuPont-18,405-1,037) (Edwards 2010b). Here, we are not able to report a NOAEL, as significant adverse effects occurred in the lowest GenX dose group evaluated in this study (). We demonstrate adverse systemic toxicity of dams exposed to GenX, which include microscopic alterations in the liver, higher GWG, and higher incidence of placental lesions. Dam serum GenX concentrations obtained at E17.5 in the present study were comparable to dam plasma concentrations reported by DuPont-18,405-1,037: (present study, on E17.5), (DuPont-18,405-1,037, on lactation day 21), and (present study, on E17.5; compared in Figure S4). However, it should be noted that in the present study at all tested doses, both PFOA and GenX, maternal serum concentrations were higher at E11.5 than E17.5. This could be explained by maternal off-loading of body burden to developing embryos and other maternal tissues (i.e., liver) and rapid expansion of maternal blood volume throughout the course of pregnancy.
There are several limitations to this study regarding experimental design, sample sizes, and interspecies differences. Due to performing the experiment over two experimental blocks, some end points were only evaluated from one of the two blocks, limiting statistical power. It is possible that some effects would achieve statistical significance with a larger number of observations. The two-block design did not impair the strength of the effect when significant effects were present in end points evaluated at both time points, which was verified by statistical analysis. It is possible that variance in half-life, amount of exposure to these chemicals, and other interspecies differences may limit the human relevance of the findings reported here. Although the mouse and human both have discoid hemochorial placenta, the maternal–placental–embryo unit in mice differs from that in humans in other ways, including the labyrinthine vs. villous structure, the number of offspring carried during each pregnancy ( vs. ), and gestation length ( vs. ). Although there are distinct interspecies differences between humans and mice, the outbred CD-1 mouse was selected in the current study due to its genetic diversity. While the CD-1 mouse is sensitive to PFOA, compared to other inbred mouse strains (Tucker et al. 2015), significant treatment-related effects were still detectable despite its greater biologic variability in response. It is not known whether there are strain differences in sensitivity to GenX, which should be investigated in future studies.
Conclusion
In a comparative reproductive and developmental study in mice of PFOA and a replacement, GenX, we report adverse effects of both compounds against the maternal–embryo–placenta unit. Both PFOA and GenX induced elevated GWG, higher maternal liver weights, adverse microscopic pathological changes in the maternal liver, and abnormal histopathological lesions in mature placenta. Importantly, we provide evidence that illustrates GenX (as low as ) significantly affects the maternal–embryo–placenta unit differently than its predecessor PFOA and that this alternative compound may have a unique mechanism(s) of reproductive toxicity in this model system. Lastly, we build a case for the importance of evaluating the placenta as a critical tissue in studies of developmental and reproductive toxicity through utilizing clinically relevant, translational end points to illustrate the unique susceptibility of this organ to the adverse effects of GenX.
Supplementary Material
Acknowledgments
The authors would like to thank Katrina Loper (NIEHS) for animal breeding support, Keith Shockley (NIEHS) for guidance in statistical analyses, Lois Wyrick (NIEHS) for assistance with generation of manuscript figures, Tina Jones (NIEHS) for sectioning histology tissues, Deloris Sutton (NIEHS) for TEM sample preparation, and Matthew Ruis (Duke University) for TH quantification support. We also want to thank Georgia Roberts (NTP) for coordinating an external data audit of this study. This document has been reviewed by the National Toxicology Program, NIEHS, and the U.S. EPA, Office of Research and Development, and approved for publication. The research presented was not performed or funded by the U.S. EPA and was not subject to the U.S. EPA’s quality system requirements. Consequently, the views, interpretations, and conclusions expressed do not necessarily reflect or represent the U.S. EPA’s views or policies.
This research was supported in part by the NIH, National Institute of Environmental Health Sciences. This work was funded under NIEHS Z0ES102785 (to S.E.F.) and University of North Carolina at Chapel Hill T32 ES007126 (to B.E.B.).
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP6233).
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 27(3):315–389, PMID: 28056690, 10.1089/thy.2016.0457. [DOI] [PubMed] [Google Scholar]
- Apelberg BJ, Witter FR, Herbstman JB, Calafat AM, Halden RU, Needham LL, et al. 2007. Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ Health Perspect 115(11):1670–1676, PMID: 18008002, 10.1289/ehp.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley-Martin J, Dodds L, Arbuckle TE, Morisset AS, Fisher M, Bouchard MF, et al. 2016. Maternal and neonatal levels of perfluoroalkyl substances in relation to gestational weight gain. Int J Environ Res Public Health 13(1):E146, PMID: 26805861, 10.3390/ijerph13010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry). 2019. PFAS exposure assessments. https://www.atsdr.cdc.gov/pfas/PFAS-Exposure-Assessments.html [accessed July 7, 2019].
- Ballesteros V, Costa O, Iñiguez C, Fletcher T, Ballester F, Lopez-Espinosa MJ. 2017. Exposure to perfluoroalkyl substances and thyroid function in pregnant women and children: a systematic review of epidemiologic studies. Environ Int 99:15–28, PMID: 27884404, 10.1016/j.envint.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Bao Y, Deng S, Jiang X, Qu Y, He Y, Liu L, et al. 2018. Degradation of PFOA substitute: GenX (HFPO–DA ammonium salt): oxidation with UV/persulfate or reduction with UV/sulfite? Environ Sci Technol 52(20):11728–11734, PMID: 30207460, 10.1021/acs.est.8b02172. [DOI] [PubMed] [Google Scholar]
- Barker DJ. 2004. The developmental origins of chronic adult disease. Acta Paediatr Suppl 93(446):26–33, PMID: 15702667, 10.1111/j.1651-2227.2004.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S. 2014. Fitting Linear Mixed-Effects Models Using lme4. https://arxiv.org/abs/1406.5823 [accessed 15 January 2019].
- Belet N, Imdat H, Yanik F, Küçüködük S. 2003. Thyroid function tests in preterm infants born to preeclamptic mothers with placental insufficiency. J Pediatr Endocrinol Metab 16(8):1131–1135, PMID: 14594173, 10.1515/jpem.2003.16.8.1131. [DOI] [PubMed] [Google Scholar]
- Berg V, Nøst TH, Hansen S, Elverland A, Veyhe AS, Jorde R, et al. 2015. Assessing the relationship between perfluoroalkyl substances, thyroid hormones and binding proteins in pregnant women; a longitudinal mixed effects approach. Environ Int 77:63–69, PMID: 25647630, 10.1016/j.envint.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Brandsma SH, Koekkoek JC, van Velzen MJM, de Boer J. 2018. The PFOA substitute GenX detected in the environment near a fluoropolymer manufacturing plant in the Netherlands. Chemosphere 220:493–500, PMID: 30594801, 10.1016/j.chemosphere.2018.12.135. [DOI] [PubMed] [Google Scholar]
- Braun JM, Chen A, Romano ME, Calafat AM, Webster GM, Yolton K, et al. 2016. Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: the HOME study. Obesity (Silver Spring) 24(1):231–237, PMID: 26554535, 10.1002/oby.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) 2009. Fourth National Report on Human Exposure to Environmental Chemicals. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Environmental Health. [Google Scholar]
- CDC 2019. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Environmental Health. [Google Scholar]
- Chaddha V, Viero S, Huppertz B, Kingdom J. 2004. Developmental biology of the placenta and the origins of placental insufficiency. Semin Fetal Neonatal Med 9(5):357–369, PMID: 15691771, 10.1016/j.siny.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Chan SY, Vasilopoulou E, Kilby MD. 2009. The role of the placenta in thyroid hormone delivery to the fetus. Nat Clin Pract Endocrinol Metab 5(1):45–54, PMID: 19079273, 10.1038/ncpendmet1026. [DOI] [PubMed] [Google Scholar]
- Chen F, Yin S, Kelly BC, Liu W. 2017. Isomer-specific transplacental transfer of perfluoroalkyl acids: results from a survey of paired maternal, cord sera, and placentas. Environ Sci Technol 51(10):5756–5763, PMID: 28434222, 10.1021/acs.est.7b00268. [DOI] [PubMed] [Google Scholar]
- Conley JM, Lambright CS, Evans N, Strynar MJ, McCord J, McIntyre BS, et al. 2019. Adverse maternal, fetal, and postnatal effects of hexafluoropropylene oxide dimer acid (GenX) from oral gestational exposure in Sprague-Dawley rats. Environ Health Perspect 127(3):37008, PMID: 30920876, 10.1289/EHP4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coperchini F, Awwad O, Rotondi M, Santini F, Imbriani M, Chiovato L. 2017. Thyroid disruption by perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA). J Endocrinol Invest 40(2):105–121, PMID: 27837466, 10.1007/s40618-016-0572-z. [DOI] [PubMed] [Google Scholar]
- Darrow LA, Stein CR, Steenland K. 2013. Serum perfluorooctanoic acid and perfluorooctane sulfonate concentrations in relation to birth outcomes in the mid-Ohio Valley, 2005–2010. Environ Health Perspect 121(10):1207–1213, PMID: 23838280, 10.1289/ehp.1206372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Escobar GM, Obregón MJ, del Rey FE. 2004. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab 18(2):225–248, PMID: 15157838, 10.1016/j.beem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Domazet SL, Grøntved A, Timmermann AG, Nielsen F, Jensen TK. 2016. Longitudinal associations of exposure to perfluoroalkylated substances in childhood and adolescence and indicators of adiposity and glucose metabolism 6 and 12 years later: the European Youth Heart Study. Diabetes Care 39(10):1745–1751, PMID: 27489335, 10.2337/dc16-0269. [DOI] [PubMed] [Google Scholar]
- Edwards TL. 2010a. An Oral (Gavage) Prenatal Developmental Study of H-28548 in Rats (DuPont). Ashland, OH: WIL Research Laboratories, LLC. [Google Scholar]
- Edwards TL. 2010b. An Oral (Gavage) Reproduction/Developmental Toxicity Screening Study of H-28548 in Mice (DuPont). Ashland, OH: WIL Research Laboratories, LLC. [Google Scholar]
- European Commission. 2017. Commission Regulation (EU) 2017/1000 of 13 June 2017 Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Perfluorooctanoic Acid (PFOA), Its Salts and PFOA-Related Substances. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R1000&from=EN [accessed 1 July 2019].
- Fei C, McLaughlin JK, Tarone RE, Olsen J. 2007. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ Health Perspect 115(11):1677–1682, PMID: 18008003, 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton SE, Reiner JL, Nakayama SF, Delinsky AD, Stanko JP, Hines EP, et al. 2009. Analysis of PFOA in dosed CD-1 mice. Part 2. Disposition of PFOA in tissues and fluids from pregnant and lactating mice and their pups. Reprod Toxicol 27(3–4):365–372, PMID: 19429407, 10.1016/j.reprotox.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filgo AJ, Quist EM, Hoenerhoff MJ, Brix AE, Kissling GE, Fenton SE. 2015. Perfluorooctanoic acid (PFOA)-induced liver lesions in two strains of mice following developmental exposures: PPARalpha is not required. Toxicol Pathol 43(4):558–568, PMID: 25398757, 10.1177/0192623314558463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch AF, Rifas-Shiman SL, Mora AM, Calafat AM, Ye X, Luttmann-Gibson H, et al. 2017. Early-life exposure to perfluoroalkyl substances and childhood metabolic function. Environ Health Perspect 125(3):481–487, PMID: 27586368, 10.1289/EHP303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon R. 2003. Placental insufficiency and its consequences. Eur J Obstet Gynecol Reprod Biol 110:S99–S107, PMID: 12965097, 10.1016/S0301-2115(03)00179-9. [DOI] [PubMed] [Google Scholar]
- Gannon SA, Fasano WJ, Mawn MP, Nabb DL, Buck RC, Buxton LW, et al. 2016. Absorption, distribution, metabolism, excretion, and kinetics of 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoic acid ammonium salt following a single dose in rat, mouse, and cynomolgus monkey. Toxicology 340:1–9, PMID: 26743852, 10.1016/j.tox.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Hayward CE, Lean S, Sibley CP, Jones RL, Wareing M, Greenwood SL, et al. 2016. Placental adaptation: what can we learn from birthweight: placental weight ratio? Front Physiol 7:28, PMID: 26903878, 10.3389/fphys.2016.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydebreck F, Tang J, Xie Z, Ebinghaus R. 2015. Correction to alternative and legacy perfluoroalkyl substances: differences between European and Chinese river/estuary systems. Environ Sci Technol 49(24):14742–14743, PMID: 26636476, 10.1021/acs.est.5b05591. [DOI] [PubMed] [Google Scholar]
- Hopkins ZR, Sun M, DeWitt JC, Knappe DRU. 2018. Recently detected drinking water contaminants: GenX and other per‐and polyfluoroalkyl ether acids. J Am Water Works Assoc 110(7):13–28. [Google Scholar]
- Hothorn T, Bretz F, Hothorn MT. 2009. The multcomp Package. Technical Report 1.0-6. The R Project for Statistical Computing. [Google Scholar]
- Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, et al. 2016. Detection of poly-and perfluoroalkyl substances (PFASs) in U.S. drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ Sci Technol Lett 3(10):344–350, PMID: 27752509, 10.1021/acs.estlett.6b00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheon JA, McNamara H, Platt RW, Benjamin A, Kramer MS. 2012. Placental weight for gestational age and adverse perinatal outcomes. Obstet Gynecol 119(6):1251–1258, PMID: 22617591, 10.1097/AOG.0b013e318253d3df. [DOI] [PubMed] [Google Scholar]
- Jaacks LM, Boyd Barr D, Sundaram R, Grewal J, Zhang C, Buck Louis GM. 2016. Pre-pregnancy maternal exposure to persistent organic pollutants and gestational weight gain: a prospective cohort study. Int J Environ Res Public Health 13(9):905, PMID: 27626435, 10.3390/ijerph13090905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PI, Sutton P, Atchley DS, Koustas E, Lam J, Sen S, et al. 2014. The navigation guide - evidence-based medicine meets environmental health: systematic review of human evidence for PFOA effects on fetal growth. Environ Health Perspect 122(10):1028–1039, PMID: 24968388, 10.1289/ehp.1307893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Wong L-Y, Jia LT, Kuklenyik Z, Calafat AM. 2011. Trends in exposure to polyfluoroalkyl chemicals in the U.S. population: 1999–2008. Environ Sci Technol 45(19):8037–8045, PMID: 21469664, 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- Kim S, Choi K, Ji K, Seo J, Kho Y, Park J, et al. 2011. Trans-placental transfer of thirteen perfluorinated compounds and relations with fetal thyroid hormones. Environ Sci Technol 45(17):7465–7472, PMID: 21805959, 10.1021/es202408a. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Azumi K, Goudarzi H, Araki A, Miyashita C, Kobayashi S, et al. 2017. Effects of prenatal perfluoroalkyl acid exposure on cord blood IGF2/H19 methylation and ponderal index: The Hokkaido study. J Expo Sci Environ Epidemiol 27(3):251–259, PMID: 27553991, 10.1038/jes.2016.50. [DOI] [PubMed] [Google Scholar]
- Koustas E, Lam J, Sutton P, Johnson PI, Atchley DS, Sen S, et al. 2014. The navigation guide - evidence-based medicine meets environmental health: systematic review of nonhuman evidence for PFOA effects on fetal growth. Environ Health Perspect 122(10):1015–1027, PMID: 24968374, 10.1289/ehp.1307177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB. 2017. LmerTest package: tests in linear mixed effects models. J Stat Soft 82(13), 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- Lam J, Koustas E, Sutton P, Johnson PI, Atchley DS, Sen S, et al. 2014. The navigation guide - evidence-based medicine meets environmental health: integration of animal and human evidence for PFOA effects on fetal growth. Environ Health Perspect 122(10):1040–1051, PMID: 24968389, 10.1289/ehp.1307923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C, Thibodeaux JR, Hanson RG, Narotsky MG, Rogers JM, Lindstrom AB, et al. 2006. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol Sci 90(2):510–518, PMID: 16415327, 10.1093/toxsci/kfj105. [DOI] [PubMed] [Google Scholar]
- Leonetti C, Butt CM, Hoffman K, Hammel SC, Miranda ML, Stapleton HM. 2016. Brominated flame retardants in placental tissues: associations with infant sex and thyroid hormone endpoints. Environ Health 15(1):113, PMID: 27884139, 10.1186/s12940-016-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Gao P, Xiang P, Zhang X, Cui X, Ma LQ. 2017. Molecular mechanisms of PFOA-induced toxicity in animals and humans: implications for health risks. Environ Int 99:43–54, PMID: 27871799, 10.1016/j.envint.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Lindstrom AB, Strynar MJ, Libelo EL. 2011. Polyfluorinated compounds: past, present, and future. Environ Sci Technol 45(19):7954–7961, PMID: 21866930, 10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- Macon MB, Villanueva LR, Tatum-Gibbs K, Zehr RD, Strynar MJ, Stanko JP, et al. 2011. Prenatal perfluorooctanoic acid exposure in CD-1 mice: low-dose developmental effects and internal dosimetry. Toxicol Sci 122(1):134–145, PMID: 21482639, 10.1093/toxsci/kfr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J, Newton S, Strynar M. 2018. Validation of quantitative measurements and semi-quantitative estimates of emerging perfluoroethercarboxylic acids (PFECAs) and hexfluoroprolyene oxide acids (HFPOAs). J Chromatogr A 1551:52–58, PMID: 29628221, 10.1016/j.chroma.2018.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell EM, Trump BF. 1976. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med 100(8):405–414, PMID: 60092. [PubMed] [Google Scholar]
- Monroy R, Morrison K, Teo K, Atkinson S, Kubwabo C, Stewart B, et al. 2008. Serum levels of perfluoroalkyl compounds in human maternal and umbilical cord blood samples. Environ Res 108(1):56–62, PMID: 18649879, 10.1016/j.envres.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Nader S. 2009. Thyroid disease and pregnancy. In: Creasy and Resnik‘s Maternal-Fetal Medicine: Principles and Practice. Creasy R, Resnik R, Iams J, Lockwood C, Moore T, eds. 6th ed Philadelphia, PA: Saunders Elsevier, 995–1014. [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. 2007. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115(9):1298–1305, PMID: 17805419, 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porterfield SP. 1994. Vulnerability of the developing brain to thyroid abnormalities: environmental insults to the thyroid system. Environ Health Prespect 102(suppl 2):125–130, 10.1289/ehp.94102125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen KM, Yaktine AL. 2009. Weight Gain during Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Reiner JL, Nakayama SF, Delinsky AD, Stanko JP, Fenton SE, Lindstrom AB, et al. 2009. Analysis of PFOA in dosed CD1 mice. Part 1. Methods development for the analysis of tissues and fluids from pregnant and lactating mice and their pups. Reprod Toxicol 27(3–4):360–364, PMID: 19028561, 10.1016/j.reprotox.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijs KJ, Bogers RP. 2017. PFOA and Possible Health Effects: A Review of Scientific Literature. RIVM report 2017-0086. Bilthoven, Netherlands: The Netherlands National Institute for Public Health and the Environment. [Google Scholar]
- Risnes KR, Romundstad PR, Nilsen TI, Eskild A, Vatten LJ. 2009. Placental weight relative to birth weight and long-term cardiovascular mortality: findings from a cohort of 31,307 men and women. Am J Epidemiol 170(5):622–631, PMID: 19638481, 10.1093/aje/kwp182. [DOI] [PubMed] [Google Scholar]
- Rushing BR, Hu Q, Franklin JN, McMahen RL, Dagnino S, Higgins CP, et al. 2017. Evaluation of the immunomodulatory effects of 2, 3, 3, 3-tetrafluoro-2-(heptafluoropropoxy)-propanoate in C57BL/6 mice. Toxicol Sci 156:179–189, PMID: 28115649, 10.1093/toxsci/kfw251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salafia CM, Charles AK, Maas EM. 2006. Placenta and fetal growth restriction. Clin Obstet Gynecol 49(2):236–256, PMID: 16721104, 10.1097/00003081-200606000-00007. [DOI] [PubMed] [Google Scholar]
- Savitz DA, Stein CR, Bartell SM, Elston B, Gong J, Shin HM, et al. 2012. Perfluorooctanoic acid exposure and pregnancy outcome in a highly exposed community. Epidemiology 23(3):386–392, PMID: 22370857, 10.1097/EDE.0b013e31824cb93b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9(7):676–682, PMID: 22743772, 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sills RC, Cesta MF, Willson CJ, Brix AE, Berridge BR. 2019. National Toxicology Program position statement on informed (“non-blinded”) analysis in toxicologic pathology evaluation. Toxicol Pathol 47(7):887–890, 10.1177/0192623319873974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CR, Savitz DA, Dougan M. 2009. Serum levels of perfluorooctanoic acid and perfluorooctane sulfonate and pregnancy outcome. Am J Epidemiol 170(7):837–846, PMID: 19692329, 10.1093/aje/kwp212. [DOI] [PubMed] [Google Scholar]
- Strynar M, Dagnino S, McMahen R, Liang S, Lindstrom A, Andersen E, et al. 2015. Identification of novel perfluoroalkyl ether carboxylic acids (PFECAs) and sulfonic acids (PFESAs) in natural waters using accurate mass time-of-flight mass spectrometry (TOFMs). Environ Sci Technol 49(19):11622–11630, PMID: 26392038, 10.1021/acs.est.5b01215. [DOI] [PubMed] [Google Scholar]
- Suh CH, Cho NK, Lee CK, Lee CH, Kim DH, Kim JH, et al. 2011. Perfluorooctanoic acid-induced inhibition of placental prolactin-family hormone and fetal growth retardation in mice. Mol Cell Endocrinol 337(1–2):7–15, PMID: 21241770, 10.1016/j.mce.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Sun M, Arevalo E, Strynar M, Lindstrom A, Richardson M, Kearns B, et al. 2016. Legacy and emerging perfluoroalkyl substances are important drinking water contaminants in the Cape Fear River Watershed of North Carolina. Environ Sci Technol Lett 3:415–419, 10.1021/acs.estlett.6b00398. [DOI] [Google Scholar]
- Thornburg KL, O’Tierney PF, Louey S. 2010. Review: the placenta is a programming agent for cardiovascular disease. Placenta 31 suppl:S54–S59, PMID: 20149453, 10.1016/j.placenta.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker DK, Macon MB, Strynar MJ, Dagnino S, Andersen E, Fenton S. 2015. The mammary gland is a sensitive pubertal target in CD-1 and C57BL/6 mice following perinatal perfluorooctanoic acid (PFOA) exposure. Reprod Toxicol 54:26–36, PMID: 25499722, 10.1016/j.reprotox.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA (Environmental Protection Agency). 2006. 2010/2015 PFOA Stewardship Program Washington, DC: U.S. Environmental Protection Agency; http://www.epa.gov/oppt/pfoa/pubs/stewardship. [Google Scholar]
- U.S. EPA. 2016. Drinking Water Health Advisory for Perfluorooctanoic Acid (PFOA). 822-R-16-005 UEJEDN. Washington, DC: U.S. Environmental Protection Agency. [Google Scholar]
- Villar J, Carroli G, Wojdyla D, Abalos E, Giordano D, Ba’aqeel H, et al. 2006. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? Am J Obstet Gynecol 194(4):921–931, PMID: 16580277, 10.1016/j.ajog.2005.10.813. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rogan WJ, Chen PC, Lien GW, Chen HY, Tseng YC, et al. 2014. Association between maternal serum perfluoroalkyl substances during pregnancy and maternal and cord thyroid hormones: Taiwan maternal and infant cohort study. Environ Health Perspect 122(5):529–534, PMID: 24577800, 10.1289/ehp.1306925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson ED, Cross JC. 2005. Development of structures and transport functions in the mouse placenta. Physiology (Bethesda) 20:180–193, PMID: 15888575, 10.1152/physiol.00001.2005. [DOI] [PubMed] [Google Scholar]
- Webster GM, Rauch SA, Marie NS, Mattman A, Lanphear BP, Venners SA. 2016. Cross-sectional associations of serum perfluoroalkyl acids and thyroid hormones in U.S. adults: variation according to TPOAb and iodine status (NHANES 2007–2008). Environ Health Perspect 124(7):935–942, PMID: 26517287, 10.1289/ehp.1409589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster GM, Venners SA, Mattman A, Martin JW. 2014. Associations between perfluoroalkyl acids (PFASs) and maternal thyroid hormones in early pregnancy: a population-based cohort study. Environ Res 133:338–347, PMID: 25019470, 10.1016/j.envres.2014.06.012. [DOI] [PubMed] [Google Scholar]
- White SS, Calafat AM, Kuklenyik Z, Villanueva L, Zehr RD, Helfant L, et al. 2007. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol Sci 96(1):133–144, PMID: 17132714, 10.1093/toxsci/kfl177. [DOI] [PubMed] [Google Scholar]
- Yahia D, El-Nasser MA, Abedel-Latif M, Tsukuba C, Yoshida M, Sato I, et al. 2010. Effects of perfluorooctanoic acid (PFOA) exposure to pregnant mice on reproduction. J Toxicol Sci 35(4):527–533, PMID: 20686339, 10.2131/jts.35.527. [DOI] [PubMed] [Google Scholar]
- Yang L, Li J, Lai J, Luan H, Cai Z, Wang Y, et al. 2016a. Placental transfer of perfluoroalkyl substances and associations with thyroid hormones: Beijing prenatal exposure study. Sci Rep 6:21699, PMID: 26898235, 10.1038/srep21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wang Z, Shi Y, Li J, Wang Y, Zhao Y, et al. 2016b. Human placental transfer of perfluoroalkyl acid precursors: levels and profiles in paired maternal and cord serum. Chemosphere 144:1631–1638, PMID: 26517392, 10.1016/j.chemosphere.2015.10.063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.