Abstract
Gestational diabetes mellitus is a condition similar to type 2 diabetes mellitus (T2DM) in that patients are unable to compensate for the degree of insulin resistance and both conditions are often treated with metformin. The comparative pharmacodynamic (PD) response to metformin in these two populations has not been studied. This study characterized insulin sensitivity, β-cell responsivity, and disposition index following a mixed meal tolerance test utilizing a minimal model of glucose, insulin and C-peptide kinetics before and during treatment with metformin. The study included women with gestational diabetes mellitus (n=34), T2DM (n=14) and healthy pregnant women (n=30). Prior to treatment, the gestational diabetes mellitus group had significantly higher baseline (45%), dynamic (68%), static (71%) and total β-cell responsivity (71%) than the T2DM group. Metformin significantly increased insulin sensitivity (51%) as well as disposition index (97%) and decreased mixed meal tolerance test peak glucose concentrations (8%) in women with gestational diabetes mellitus after adjusting for gestational age dependent effects; however, in women with T2DM metformin only significantly affected peak glucose concentrations (22%) and had no significant effect on all other parameters. Metformin had a greater effect on the change in disposition index (Δ disposition index) in women with gestational diabetes mellitus than in those with T2DM (p=0.01). In conclusion, response to metformin in women with gestational diabetes mellitus is significantly different than in women with T2DM, which is likely related to the differences in disease severity.
Keywords: gestational diabetes mellitus, type 2 diabetes mellitus, metformin, pregnancy, pharmacodynamics, insulin sensitivity, β-cell responsivity, disposition index, insulin, glucose
Introduction
Gestational diabetes mellitus is a common complication during pregnancy and is associated with significant adverse effects for the mother, fetus and neonate.1–4 The underlying abnormalities and risk factors are similar in women with gestational diabetes mellitus and those with type 2 diabetes mellitus (T2DM); although, in general, disease severity is greater with T2DM.5,6 In both gestational diabetes and T2DM, patients have marked insulin resistance and an inability to compensate for the degree of insulin resistance. Metformin is a drug that lowers glucose concentrations primarily by decreasing insulin resistance through increasing peripheral glucose uptake and utilization. Metformin also decreases intestinal glucose absorption and hepatic glucose production. Metformin is commonly used in the treatment of women with T2DM and in women with gestational diabetes mellitus.7 During pregnancy, the majority of medications, including oral glucose lowering drugs like metformin, are utilized in a similar manner to their FDA approved indication in the non-pregnant population, without comparative clinical pharmacological studies being conducted during pregnancy to determine if this approach is appropriate.5–7 Physiological, biochemical and hormonal changes during pregnancy are known to alter the pharmacokinetics (PK) of drugs throughout gestation.8 The renal clearance of metformin is 49% higher in mid-pregnancy and 29% higher in late-pregnancy compared to the non-pregnant state.9 In addition, we know that pregnancy is associated with insulin resistance, hyperinsulinemia and changes in glucose handling.7 With differences in metformin exposure, glucose handling and disease severity between gestational diabetes mellitus and T2DM, 7 it is likely that the PD response to metformin will also differ between pregnant women with gestational diabetes mellitus and non-pregnant women with T2DM. No data exist on how the PD of metformin in the treatment of women with gestational diabetes mellitus compares to that in women with T2DM. The objective of this study was to describe and provide preliminary data comparing the PD response to metformin in women with gestational diabetes mellitus and non-pregnant women with T2DM. Mathematical modeling of glucose, insulin and C-peptide dynamics following a mixed meal tolerance test was used to estimate insulin sensitivity, β-cell responsivity and disposition index in pregnant women with gestational diabetes mellitus and non-pregnant women with T2DM.5,10 Insulin sensitivity and β-cell responsivity are hyperbolically related and informative in understanding the underlying pathology of gestational diabetes mellitus and T2DM as well as understanding metformin’s pharmacology.10,11
Methods
Subjects.
The study was approved by the institutional review boards at the University of Washington, Madigan Army Medical Center, University of Texas Medical Branch in Galveston, University of Pittsburgh, Indiana University, University of Utah Health Care, University of Alabama at Birmingham and RTI International and conducted in accordance with their guidelines. All subjects gave written, informed consent. This was a multicenter, prospective, Phase I/II longitudinal PD study (clinicaltrials.gov identifier NCT01329016). There were three groups of women recruited for this study: pregnant women with a diagnosis of gestational diabetes mellitus (n=34), gestational age-matched healthy pregnant women (n=30) and non-pregnant women with a new diagnosis of T2DM (n=14). Only women who completed the study and adhered to the protocol were included in the results. Subjects were determined to be non-adherent based on unacceptable study pill counts (any doses missed or time of dosage > 1 hour deviation from expected time of dosing in the 3 days prior to study day 2), physician clinical impression, or subject’s admittance to not following study protocol.
Entry Criteria.
Gestational diabetes mellitus entry criteria included: pregnant women prior to 32 weeks gestation, singleton pregnancy, 18–45 years of age, failed diet therapy and required drug treatment. Gestational diabetes mellitus diagnosis was made in one of 3 ways based on serum or plasma glucose concentrations: 1) 3-hour oral glucose tolerance test (100 Gm glucose orally with 2 or more values meeting or exceeding targets: fasting glucose ≥ 95 mg/dL, 1-hour ≥ 180 mg/dL, 2-hour ≥ 155 mg/dL and 3-hour ≥ 140 mg/dL), 2) 2-hour oral glucose tolerance test (75 Gm glucose orally with 1 or more values meeting or exceeding targets, fasting glucose ≥ 92 mg/dL, 1-hour glucose ≥ 180 mg/dL, 2-hour glucose > 153 mg/dL) or 3) 1-hour oral glucose tolerance test (50 Gm glucose orally with 1-hour glucose ≥ 185). The women with gestational diabetes mellitus who received metformin for treatment in this study came from another study in which women with gestational diabetes mellitus were randomized to one of 3 treatment arms (metformin, glyburide or combination therapy with metformin and glyburide).10 Only the women assigned to metformin monotherapy are included in this study. T2DM entry criteria included non-pregnant women, 18–45 years of age, new diagnosis of T2DM, hemoglobin A1C > 7% and expected to receive metformin treatment. Exclusion criteria for women with gestational diabetes mellitus or T2DM included: medications expected to interact with metformin; medications expected to alter blood glucose concentrations; serum creatinine > 1.2 mg/dL; hematocrit < 28%; allergy to metformin; significant liver disease (diagnosis of liver disease other than Gilbert’s syndrome); congestive heart failure or history of myocardial infarction; moderate to severe pulmonary disease (any pulmonary disease requiring drug treatment other than mild exercise induced asthma requiring only intermittent pharmacologic treatment for which the patient was not currently receiving drug therapy); and adrenal or pituitary insufficiency. Healthy pregnant women entry criteria included: singleton pregnancy, 18–45 years of age, between 20–32 weeks gestation and a normal 1-hour or 2-hour oral glucose tolerance test. Exclusion criteria for healthy pregnant women included: hematocrit < 28% or known kidney, liver, heart, pulmonary, adrenal or pituitary disease as well as those receiving glucose-lowering agents or corticosteroids.
Treatment.
All subjects with gestational diabetes mellitus and T2DM in this study were treated with metformin. Titration schematic for subjects with gestational diabetes mellitus can be found in the previously published parent study.10 In brief, metformin was started at 500 mg orally twice daily and titrated to clinical control or treatment failure. For women with gestational diabetes mellitus, blood glucose concentrations were considered controlled when ≥75% of fasting glucose concentrations were ≤95 mg/dL and ≥75% of either 1-hour postprandial glucose concentrations were <140 mg/dL or 2-hour postprandial glucose concentrations were <120 my/dL. For women with type 2 diabetes mellitus, clinical control was based on hemoglobin A1C (target A1C < 7%). Provider discretion was allowed for dosage titration. Dosage initiation and titration for women with T2DM was entirely based on clinical need without regard to the study. Metformin was provided to all subjects with gestational diabetes mellitus and T2DM for the 3 days prior to study day 2. Metformin was at steady-state (consistent dosage for a minimum of 1 week) prior to evaluation of insulin sensitivity, β-cell responsivity, and disposition index during therapy (study day 2). Healthy pregnant subjects did not receive metformin.
Mixed Meal Tolerance Test.
Insulin sensitivity, β-cell responsivity, and disposition index were estimated prior to (study day 1) and during-treatment (study day 2) utilizing a mixed meal tolerance test, which involved consuming one can of Boost Plus® energy drink and two slices of whole wheat toast with two teaspoons of margarine and consumed within 10 minutes. Serial blood samples were collected pre- mixed meal tolerance test (time = 0), and 10, 20, 30, 60, 90, 120, 150, 180, 210, and 240 minutes following the initiation of the mixed meal tolerance test to measure serum glucose, insulin, and C-peptide concentrations. Blood glucose concentrations were also measured at each timepoint in real time on each study day for safety assessment. Study day 2 took place once subjects achieved clinical control or prior to switching therapy if they failed to achieve glycemic control. On study day 2, the metformin morning dose was given at time 0 (pre- mixed meal tolerance test) and blood sampling was conducted as during study day 1. Glucose concentrations were measured using a glucose oxidase/peroxidase assay 12. Insulin and C-peptide concentrations were measured using a previously described radioimmunoassays.13,14
Mixed Meal Tolerance Test Parameter Estimation.
Insulin sensitivity; total, baseline, static and dynamic β-cell responsivity; and disposition index were estimated pre- and during metformin treatment as previously described utilizing SAAM II software (version 2.3, The Epsilon Group, Charlottesville, VA).5,10,15–20 In brief, insulin sensitivity, defined as the ability of insulin to normalize glucose concentrations by stimulating uptake of glucose and suppressing its production, was estimated from glucose and insulin concentrations using the minimal model of glucose kinetics after the mixed meal tolerance test. Model structure and equations have been previously published.15 β-cell responsivity during the mixed meal tolerance test was estimated from serum glucose and C-peptide concentrations using the minimal model of C-peptide kinetics. C-peptide was used in the model to provide an accurate reconstruction of pre-hepatic secretion. The equations for the model have been published previously.19,20 Disposition index was calculated for each individual subject as the product of insulin sensitivity and total β-cell responsivity. It allows for evaluation of an individual’s glucose tolerance as a function of both insulin sensitivity and β-cell function.10,15–20
The area under the concentration-time curves (AUCs) for glucose, C-peptide, insulin and metformin over the 240-min study period were calculated using the trapezoidal rule. PD response to metformin was based on an expected increase in insulin sensitivity. Gestational age-matched healthy pregnant subjects were studied to estimate gestational age-dependent changes in insulin sensitivity and β-cell responsivity between study day 1 and study day 2. Since the PD parameters are gestational age dependent and to evaluate specifically the drug effect, we subtracted out the gestational age effect from study day 2 in the women with gestational diabetes mellitus. The correction for gestational age-dependent effect was accomplished by subtracting the average difference between study day 2 and study day 1 in the healthy pregnant subjects from individual study day 2 parameters for the subjects with gestational diabetes mellitus.10 Changes in PD parameters (Δ) were determined by subtracting PD parameters estimated pre-treatment on study day 1 from those estimated post-treatment on study day 2 corrected for gestational age-dependent effects in women with gestational diabetes mellitus.
Plasma Metformin Concentration Analysis.
Serial blood samples were collected pre-dose, then 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10 and 12 hours post-dose, truncated to the dosing interval, for measurement of metformin plasma concentrations utilizing a validated LC-MS/MS assay as previously described.21 Metformin area under the concentration-time curve over one dosing interval was determined utilizing trapezoidal rule.
Statistical analyses.
Statistical comparisons of PD parameters between study day 1 and study day 2 utilized paired Student’s t test. The mean percent difference from study day 1 to study day 2 was calculated as the mean change divided by the mean value on study day 1. Statistical comparisons between gestational diabetes mellitus and T2DM utilized unpaired Student’s t test. P-values were not adjusted for multiple comparisons. Statistical results are reported where appropriate as mean ± standard deviation (95% confidence interval). All statistical analyses were done using the R-based programs,22 and graphs were generated in R using the package ggplot2.23
Power analysis.
This study is one component of a larger study evaluating the effects of oral glucose lowering agents in the treatment of women with gestational diabetes mellitus and type 2 diabetes mellitus. Thirty subjects were anticipated to be needed to detect a 50% change in insulin sensitivity.10
Results
Demographics.
Demographics for adherent subjects with gestational diabetes mellitus, T2DM and healthy pregnant subjects who completed the study are reported in Table 1. Demographics for all gestational diabetes mellitus and T2DM subjects can be found in Supplementary Table S1. No significant differences were found in race or ethnicity between study arms (p ≈ 1). Age, weight and BMI were not significantly different between gestational diabetes mellitus and T2DM groups. Similar race and ethnicity distributions were reported for healthy pregnant subjects that completed the study. There were an average of 5 weeks between study days for the subjects with gestational diabetes mellitus and 15 weeks for the subjects with T2DM.
Table 1.
Demographics, metformin dosing and area under the concentration-time curve for subjects with gestational diabetes, non-pregnant subjects with type 2 diabetes mellitus and healthy pregnant subjects
| GDM | T2DM | HP | |
|---|---|---|---|
| n | 25 | 12 | 28 |
| Age SD1 (years) | 31 ± 5 (22 to 39) |
33 ± 7 (23 to 45) |
25 ± 5 (18 to 38) |
| Height SD1 (cm) | 163 ± 6 (155 to 179) |
160 ± 8 (147 to 175) |
162 ± 8 (147 to 178) |
| Body weight SD1 (kg) | 90 ± 20 (70 to 100) |
100 ± 30 (60 to 100) |
80 ± 10 (50 to 109) |
| BMI Pre-pregnancy (kg/m2) | 31 ± 6 (21 to 43) |
36 ± 7 (24 to 46) |
27 ± 5 (20 to 40) |
| GA, SD1 (weeks) | 31 ± 2 (20 to 33) |
NA | 30 ± 1 (28 to 33) |
| GA, SD2 (weeks) | 35 ± 1 (32 to 38) |
NA | 36 ± 1 (34 to 38) |
| Metformin dose SD2 (mg/day) | 1,400 ± 500 (1,000 to 2,000) |
800 ± 200 (500 to 1,000) |
NA |
| Metformin AUC (μg•hr/mL) | 11 ± 4 | 12 ± 3 | NA |
| White | 80% | 75% | 82% |
| Black | 16% | 25% | 18% |
| Asian | 4% | 0% | 0% |
| Hispanic/Latina | 36% | 67% | 32% |
| Baseline C-peptide AUC (pmol•hr/L) | 12,000 ± 4000 | 8000 ± 4000 | 9000 ± 3000 |
| Baseline glucose AUC (mg•hr/L) | 500 ± 70 | 1000 ± 400 | 380 ± 40 |
| Baseline insulin AUC (uU•hr /mL) | 300 ± 100 | 200 ± 200 | 190 ± 80 |
BMI = Body Mass Index, GA = gestational age, GDM = gestational diabetes mellitus, T2DM = type 2 diabetes mellitus, HP = healthy pregnant subjects, SD1 = study day 1, SD2 = study day 2. Results reported as mean ± standard deviation.
Gestational Age Correction.
In the subjects with gestational diabetes, the correction for gestational age dependent changes between study day 1 and 2 can be found in Supplementary Table S2.
Pharmacodynamic Parameters within Group Comparisons.
In the women with gestational diabetes mellitus, metformin increased insulin sensitivity 51% (p=0.005), total β-cell responsivity 26% (p = 0.04), static β-cell responsivity 29% (p = 0.04) and disposition index 97% (p = 0.003) compared to baseline (i.e., study day 2 vs. study day 1), as well as decreased baseline β-cell responsivity 24% (p = 0.004) and mixed meal tolerance test peak glucose concentration 8% (p = 0.006) but had no significant effect on dynamic β-cell responsivity. In the subjects with T2DM, there was a 22% decrease in peak glucose concentration (p = 0.04, Table 2) with metformin, but none of the estimated PD parameters were significantly altered by metformin in this group.
Table 2.
Pharmacodynamic parameters following a mixed meal tolerance test on study day 1 (SD1, pre-treatment) and study day 2 (SD2, with metformin treatment) in non-pregnant control subjects with type 2 diabetes mellitus and women with gestational diabetes mellitus, corrected for gestational-age-dependent changes.
| Parameter | GDM (n = 25) |
T2DM (n = 12) |
||||
|---|---|---|---|---|---|---|
| SD1 | SD2 | Δ | SD1 | SD2 | Δ | |
| SI (10−4 min−1 μU−1 mL) | 3 ± 2 | 4 ± 2 | 2 ± 2 (0.5 to 3) (p=0.005)* |
5 ± 8 | 6 ± 5 | 1 ± 5 (−2 to 4) (p=0.4) |
| Φtotal (10−9 min−1) | 100 ± 50 | 130 ± 70 | 30 ± 60 (1 to 50) (p=0.04)* |
30 ± 30 | 40 ± 30 | 10 ± 20 (−0.4 to 30) (p=0.06) |
| Φstatic (10−9 min−1) | 90 ± 40 | 120 ± 70 | 30 ± 60 (1 to 50) (p=0.04)* |
30 ± 30 | 40 ± 30 | 10 ± 20 (−0.6 to 30) (p=0.06) |
| Φdynamic (10−9) | 2,000 ± 1,000 | 2,000 ± 1,000 | 100 ± 1,000 (−300 to 600) (p=0.5) |
600 ± 900 | 600 ± 700 | 50 ± 700 (−400 to 500) (p=0.8) |
| Φbaseline (10−9 min−1) | 11 ± 5 | 9 ± 6 | −3 ± 4 (−4 to −0.9) (p=0.004)* |
6 ± 4 | 7 ± 4 | 1 ± 2 (−0.3 to 2) (p=0.1) |
| DI (10−13 min−2 μU−1 mL) | 300 ± 300 | 600 ± 400 | 300 ± 500 (100 to 500) (p=0.003)* |
200 ± 300 | 200 ± 200 | 40 ± 100 (−50 to 100) (p=0.4) |
| MMTT Peak glucose (mg/dL) | 150 ± 20 | 140 ± 20 | −10 ± 20 (−20 to −4) (p=0.006)* |
280 ± 80 | 220 ± 80 | −60 ± 90 (−100 to −4) (p=0.04)* |
GDM = gestational diabetes mellitus group, T2DM = type 2 diabetes mellitus group, SD = study day, Δ = average change between SD1 and SD2, SI = insulin sensitivity, Φ = β-cell responsivity, DI = disposition index, MMTT = mixed-meal tolerance test. Results reported as mean ± standard deviation (95% confidence interval).
Statistically different comparing SD2 to SD1.
Gestational diabetes mellitus vs. T2DM Disease Severity.
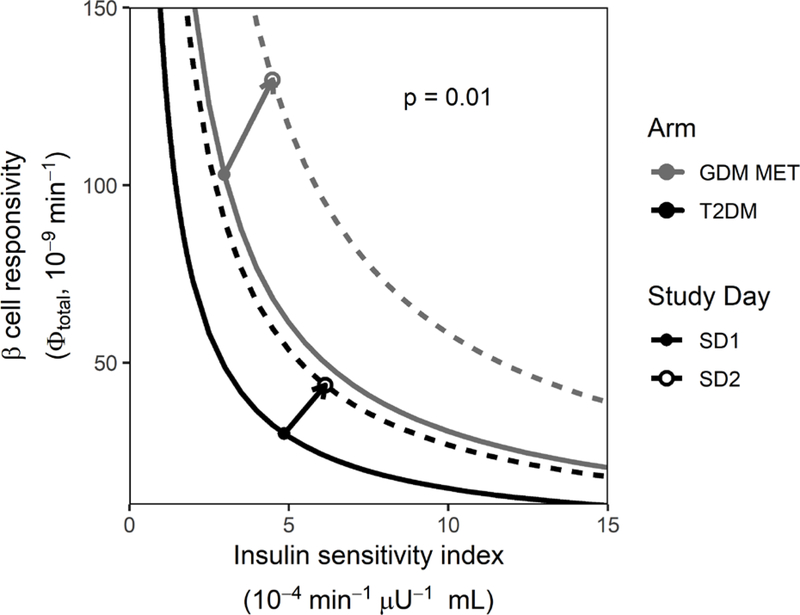
Table 3 provides mean difference data for mixed meal tolerance test baseline parameters between pregnant women with gestational diabetes mellitus and non-pregnant women with T2DM. Mean β-cell responsivity parameters were higher in the women with gestational diabetes mellitus at baseline than in the non-pregnant women with T2DM. Specifically, baseline β-cell responsivity was an average of 45% higher (p = 0.002), dynamic β-cell responsivity 68% higher (p = 0.0009), static β-cell responsivity 71% higher (p = 0.000007) and total β-cell responsivity 71% higher (p = 0.000003) in the women with gestational diabetes mellitus as compared to the non-pregnant women with T2DM. In addition, mean mixed meal tolerance test peak glucose concentrations were on average 48% lower (p = 0.0002) in women with gestational diabetes mellitus than in women with T2DM. The pre-treatment mean disposition index curves for subjects with T2DM and gestational diabetes mellitus are depicted in Figure 1. Although the mean disposition index curve for subjects with T2DM relative to those with gestational diabetes mellitus on study day 1 appears shifted down and to the left, because of inter-subject variability, there were no significant differences in disposition index or insulin sensitivity between groups at baseline.
Table 3.
Disease Severity – Mean difference in pre-treatment, baseline mixed meal tolerance test parameters for women with gestational diabetes mellitus and non-pregnant women with type 2 diabetes mellitus
| Parameter | Mean difference | p value |
|---|---|---|
| SI (10−4 min−1 μU−1 mL) | −2 (−7 to 3) |
0.4 |
| Φtotal (10−9 min−1) | 73 (47 to 99) |
0.000003 |
| Φstatic (10−9 min−1) | 63 (39 to 87) |
0.000007 |
| Φdynamic (10−9) | 1302 (597 to 2007) |
0.0009 |
| Φbaseline (10−9 min−1) | 5 (2 to 8) |
0.002 |
| DI (10−13 min−2 μU−1 mL) | 157 (−52 to 366) |
0.1 |
| MMTT peak glucose (mg/dL) | −136 (−190 to −82) |
0.0002 |
GDM = gestational diabetes mellitus, T2DM = type 2 diabetes mellitus, SI = insulin sensitivity, Φtotal = total β-cell responsivity, Φstatic = static β-cell responsivity, Φdynamic = dynamic β-cell responsivity, Φbaseline = baseline β-cell responsivity, DI = disposition index, MMTT = mixed meal tolerance test. Results reported as mean (95% confidence interval). Refer to Table 2 baseline actual parameter values.
Figure 1. Pharmacodynamic effects of metformin in women with gestational diabetes mellitus (GDM) and type 2 diabetes mellitus (T2DM).
Mean disposition index for all adherent subjects who completed the study in the GDM metformin arm at baseline (solid gray line) and on study day 2 (dashed gray line) as well as for subjects with T2DM at baseline (solid black line) and on study day 2 (dashed black line). The vectors depict the mean pharmacodynamic effect of metformin in subjects with GDM (gray arrow) and subjects with T2DM (black arrow). Solid dots represent mean baseline disposition index (study day 1) and open circles represent study day 2 mean disposition index (GDM corrected for mean gestational age-dependent change).
Metformin Dose/Day.
Average metformin dose/day on study day 2 for subjects with gestational diabetes mellitus and T2DM can be seen in Table 1. The mean metformin dose/day in the women with gestational diabetes mellitus was higher than that for the women with T2DM (difference: 607 (95% CI: 369 to 844) mg, p < 0.001). However, due to differences in the pharmacokinetics during pregnancy as compared with the non-pregnant state, mean metformin area under the concentration-time curve (AUC) was 14% smaller in the women with gestational diabetes mellitus as compared with the women with T2DM. This difference is not statistically significant (gestational diabetes mellitus : 11 ± 4 μg•hr/mL vs. T2DM: 12 ± 3 μg•hr/mL, p=0.2).
Metformin’s Pharmacodynamic Effect in Subjects with gestational diabetes mellitus vs. Non-Pregnant Subjects with T2DM.
Figure 1 depicts the average disposition index curves for each group on study day 1 and study day 2. Figure 1 also depicts the mean metformin pharmacologic PD response vectors for subjects with gestational diabetes mellitus and non-pregnant subjects with T2DM starting at their pre-treatment average disposition index on study day 1 and extending to the concomitant treatment average disposition index on study day 2. With metformin treatment, the women with T2DM disposition index curve shifted up and to the right but only reached the pre-treatment disposition index curve for the women with gestational diabetes mellitus. Comparing the change from study day 1 to study day 2 with metformin (Table 4), the mean Δ disposition index was greater for the women with gestational diabetes mellitus (300 ± 500 10−13 min−2 μU−1 mL) than for the women with T2DM (40 ± 100 10−13 min−2 μU−1 mL, p = 0.01). There was no significant difference in Δ insulin sensitivity (p = 0.9) or Δβ-cell responsivity (p = 0.4) with metformin in women with gestational diabetes mellitus vs. non-pregnant women with T2DM. Mean peak glucose concentrations decreased with metformin treatment in both T2DM (mean difference: 60 ± 90 mg/dL, p = 0.04) and in gestational diabetes mellitus (mean difference: 10 ± 20 mg/dL, p = 0.006) groups. Eighty-four percent of the subjects with gestational diabetes mellitus and 83% of the subjects with T2DM had some pharmacologic response to metformin (i.e. increase in insulin sensitivity).
Table 4.
Mean difference in mixed meal tolerance test parameters during treatment with metformin (study day 2 and change from study day 1 to study day 2) for women with gestational diabetes mellitus and non-pregnant women with type 2 diabetes mellitus
| Parameter | Mean difference on SD2 between study arms |
p value comparing mean value on SD2 between study arms |
p value comparing mean change from SD1 to SD2 between study arms |
|---|---|---|---|
| SI (10−4 min−1 μU−1 mL) | −1.6 (−5.2 to 1.86) |
0.3 | 0.9 |
| Φtotal (10−9 min−1) | 86 (51 to 121) |
0.00001 | 0.4 |
| Φstatic (10−9 min−1) | 76 (43 to 109) |
0.00005 | 0.4 |
| Φdynamic (10−9 min−1) | 1400 (779 to 2020) |
0.00006 | 0.7 |
| Φbaseline (10−9 min−1) | 1.4 (−2.3 to 5.1) |
0.4 | 0.001 |
| DI (10−13 min−2 μU−1 mL) | 422 (227 to 617) |
0.0001 | 0.01 |
| Peak glucose (mg/dL) | −85 (−133 to −37) |
0.002 | 0.08 |
GDM = gestational diabetes mellitus, T2DM = type 2 diabetes mellitus, SI = insulin sensitivity, Φtotal = total β-cell responsivity, Φstatic = static β-cell responsivity, Φdynamic = dynamic β-cell responsivity, Φbaseline = baseline β-cell responsivity, DI = disposition index, MMTT = mixed meal tolerance test. Results reported as mean ± standard deviation. The values for women with GDM were adjusted to account for normal gestational age-dependent. Refer to Table 2 for actual parameter values.
Mean differences in PD parameters following the mixed meal tolerance test on study day 2 while on treatment with metformin in subjects with gestational diabetes mellitus and subjects with newly diagnosed T2DM are shown in Table 4. While receiving metformin treatment, average disposition index for women with gestational diabetes mellitus was ~3-fold higher than average disposition index for women with T2DM (mean difference: 421 × 10−13 min−2 μU−1 mL, p = 0.0001). In addition, while on metformin treatment, the average peak mixed meal tolerance test glucose concentration was 85 mg/dL lower in the women with gestational diabetes mellitus than those with T2DM (p = 0.002).
Safety.
The adverse effects associated with the mixed meal tolerance test were limited to occasional bruising from phlebotomy. No hypoglycemia was noted during the mixed meal tolerance test with either group.
Discussion
For the most part, clinical trials for new drugs have been conducted in non-pregnant individuals. Our understanding of the clinical pharmacology of medications during pregnancy is growing,5,9,10,24 but most medication prescribing during pregnancy is based on data collected from the non-pregnant population. Normal pregnancy is associated with lower fasting and pre-prandial glucose concentrations and much higher peak insulin concentrations than in the non-pregnant population.7 This is likely due in part to the insulin resistance that occurs during normal pregnancy.6 Metformin is approved by the Food and Drug Administration for treatment of patients with T2DM. There has been discussion in the literature as to whether or not gestational diabetes mellitus and T2DM are the same condition.25 Both conditions are associated with insulin resistance and decreased pancreatic β-cell compensation ability.6,26
Insulin sensitivity, β-cell responsivity, and disposition index with glyburide has been reported for patients with gestational diabetes mellitus and T2DM.5 With glyburide treatment, women with gestational diabetes mellitus have greater total (i.e. overall insulin response) and static (i.e. second phase insulin response) β-cell responsivity than women with T2DM.5 In this study, prior to metformin treatment, women with gestational diabetes mellitus demonstrated not only better total and static β-cell responsivity, but also dynamic (i.e. first phase insulin response) and baseline (i.e. basal, non-stimulated index of insulin secretion) β-cell responsivity compared to those with T2DM. This is consistent with more severe pancreatic β-cell dysfunction in women with T2DM than in those with gestational diabetes mellitus. However, overall disposition index at baseline for women with gestational diabetes mellitus and non-pregnant women with T2DM was not significantly different in either this study or our previous work.6 With these underlying differences in disease severity prior to treatment, it is reasonable to question whether the clinical pharmacological response to treatment will also differ during pregnancy. Data is extremely limited on the PD effects of medications during pregnancy, including MET. This study provides preliminary data comparing the pharmacodynamics of metformin treatment in women with gestational diabetes mellitus and non-pregnant women with T2DM.
Women with gestational diabetes mellitus have a 7-fold increased risk for developing T2DM later in life than women without gestational diabetes mellitus during pregnancy.27 This is not surprising given the similar risk factors such as obesity as well as similar pathology, including increased insulin resistance and decreased ability to compensate for the degree of insulin resistance in women with gestational diabetes mellitus and those with T2DM. Metformin is the most commonly utilized treatment for patients with T2DM. The difference in metformin response in women with gestational diabetes mellitus as compared to non-pregnant women with T2DM is complicated by baseline differences in pregnancy status, which increases insulin resistance, as well as disease state. That being said, we report for the first time that a similar percent of women receiving metformin experienced some positive effect on insulin sensitivity if they had gestational diabetes mellitus (84%) or T2DM (83%). Despite this similarity, the magnitude of response to metformin differed between women with gestational diabetes mellitus and those with T2DM for some PD parameters. On average metformin treatment in women with gestational diabetes mellitus had a greater effect on the change in disposition index than women with T2DM (p=0.01), but there was no significant difference in the change in peak glucose concentrations between women with gestational diabetes mellitus and T2DM (p = 0.08). Of note, although average metformin dosage was higher in the women with gestational diabetes mellitus, due to the pharmacokinetic differences in pregnancy as compared to the non-pregnant state,9 there was no significant difference in metformin exposure as measured by area under the concentration-time curve compared to the non-pregnant women with T2DM in this study.
Metformin decreases insulin resistance through decreased glycogenolysis, lipolysis and activity of hepatic glucose-6-phosphatase as well as increased glycogen synthesis, insulin receptor tyrosine kinase activity and glucose transporter type 4 (GLUT4) activity.28,29 Our data with metformin in the treatment of women with gestational diabetes mellitus demonstrates improvement in insulin sensitivity, consistent with its primary mechanism of action. Metformin can also increase insulin secretion through increasing the release of glucagon-like-peptide-1 (GLP-1).29 GLP-1 is known to improve β-cell activity, which should translate into increased β-cell responsivity.30,31 Interestingly, in contrast to expectations for a drug that has been reported to increase release of GLP-1, metformin significantly decreased the baseline β-cell responsivity in women with gestational diabetes mellitus, which likely reflect the changes seen in insulin sensitivity. Consistent with expectations, metformin increased total β-cell responsivity by 26% and static β-cell responsivity by 29%, although there was no significant change in dynamic β-cell responsivity. Most importantly, metformin improved the disposition index, a measure of the overall metabolic state, by 97%, shifting the disposition index curve up and to the right in women with gestational diabetes mellitus. In contrast to the results in women with gestational diabetes mellitus, the women with T2DM had no significant change in any of the PD parameters with MET. This is to some extent a result of the smaller number of subjects studied with T2DM, a limitation of this preliminary work.
However, in comparing our data in patients with T2DM to previously reported effects of metformin in non-pregnant patients with T2DM, our subjects with T2DM were similar, but had less disease severity based on mean insulin sensitivity (this study: 5 X 10−4 min−1 μU−1 mL vs previous work: 2.8–2.1 X 10−4 min−1 μU−1 mL), total β-cell responsivity (this study: 30 X 10−9 min−1 vs previous work: 6.4–7.4 X 10−9 min−1), static β-cell responsivity (this study: 30 X 10−9 min−1 vs previous work: 12.2–15.5 X 10−9 min−1), dynamic β-cell responsivity (this study: 600 X 10−9 min−1 vs previous work: 418–480 X 10−9 min−1), baseline β-cell responsivity (this study: 6 X 10−9 min−1 vs previous work: 4.6–5.5 X 10−9 min−1) and disposition index (this study: 200 X 10−13 min−2 μU−1 mL vs previous work: 17.4–20 X 10−13 min−2 μU−1 mL). 32 Response to metformin was also similar in our subjects with T2DM to that reported in the literature based on insulin sensitivity (this study: 1 X 10−4 min−1 μU−1 mL vs previous work: 0.1–0.7 X 10−4 min−1 μU−1 mL), total β-cell responsivity (this study: 10 X 10−9 min−1 vs previous work: 1.2–2.4 X 10−9 min−1), static β-cell responsivity (this study: 10 X 10−9 min−1 vs previous work: 5.9–13 X 10−9 min−1), dynamic β-cell responsivity (this study: 50 X 10−9 min−1 vs previous work: 31–54 X 10−9 min−1), baseline β-cell responsivity (this study: 1 X 10−9 min−1 vs previous work: 0.5–1.3 X 10−9 min−1) and disposition index (this study: 40 X 10−13 min−2 μU−1 mL vs previous work: 5.2–11.6 X 10−13 min−2 μU−1 mL).32
On study day 2 (during metformin treatment) in a similar pattern to study day 1 (prior to metformin treatment), women with gestational diabetes mellitus had closer to normal (i.e. significantly higher) β-cell responsivity (total, dynamic and static) than women with T2DM. However, whereas there was a significant difference prior to treatment in baseline β-cell responsivity, with metformin treatment this difference was no longer observable. This appears to be driven by the decrease in baseline β-cell responsivity with metformin treatment in the women with gestational diabetes mellitus and no change from baseline in β-cell responsivity in the women with T2DM. In addition, the overall metabolic states (i.e. disposition index) were not different between the two groups at baseline, but became markedly different with metformin treatment. Women in the gestational diabetes mellitus group were much better able to compensate for the degree of insulin resistance than the women with T2DM. Since the ability to compensate for the degree of insulin resistance is key to maintaining glycemic control, this difference is critically important and is an apt illustration of the need for not only rigorous PK studies in pregnancy, but that PD studies comparing response to the non-pregnant population are also needed.
Limitations of Study
This study provides preliminary data on the pharmacodynamics of metformin during pregnancy as compared to non-pregnant patients with type 2 diabetes mellitus and should be interpreted with care. A major limitation of this study is the small sample size in the T2DM study group. This group was intended to be larger and equivalent in number to the gestational diabetes mellitus study group, but due to slow enrollment in this arm and the end of the funding cycle, the funding agency closed the study for further enrollment.
Another limitation of this study is the multiple testing performed without adjusting for multiple comparisons. This report focused on two arms of a larger study, and it is unclear what should be considered a “family” of tests for this report when analyzing and interpreting a subset of those data. Further, adjusting p values for comparisons and analyses not presented herein would be confusing for the reader and challenging to explain. Therefore, we did not make any adjustments for multiple testing, but did make it clear to the reader that this was the case.
Lastly, the use of the mixed meal tolerance test method to derive the PD parameters provides the advantages that it is well tolerated by patients, easy to administer, includes the effects of gastrointestinal incretins, and correlates well with the gold standard hyperglycemic clamp studies. However, it is limited because it does require making assumption regarding the rate of nutrient absorption and requires significant mathematical modeling.
Conclusion
This study describes and compares the clinical pharmacologic pharmacodynamic response to metformin in women with gestational diabetes mellitus and non-pregnant women with T2DM. The clinical pharmacological response to metformin treatment is significantly different in pregnant women with gestational diabetes mellitus than in non-pregnant women with T2DM, which likely reflects differences in disease severity. With similar metformin exposure, women with gestational diabetes mellitus have a greater improvement in overall disposition index with metformin treatment than non-pregnant women with T2DM.
Supplementary Material
Acknowledgements
In memoriam we acknowledge the tremendous contribution of David A. Flockhart, Ph.D., M.D. to the Obstetric-fetal Pharmacology Research Unit Network. We also thank the research coordinators and nurses including Alisha Bouge, Claudine Hernandez, Karen Hays, Ira Kantrowitz-Gordon, Anna Lemchen, Heather Johnson, Holly West, Julie Croxford, Dawn Fisher, Wenchen Zhao, Becky Cypher and Janie Klank for their hard work in completing this study. This research was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development grant #U10HD063094, U10HD047892, U10HD097905, U10HD047891, U10HD057753 and the NIH National Center for Advancing Translational Science through the Clinical and Translational Science Awards Program grant # ULITR000423, TLITR000422, ULITR001108, National Institute of General Medical Sciences R01GM124264 and unrestricted research funds from the University of Washington. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health. In addition, the views expressed are those of the author(s) and do not reflect the official policy or position of the US Army Medical Department, Department of the Army, Department of Defense or the U.S. Government. The investigators have adhered to the policies for protection of human subjects as prescribed in 45 CFR 46.
Footnotes
Disclosure
The D.L.S. declares that at the time of the conduct and analysis of the study, she had no conflict of interest. However, since the completion of the study, D.L.S.’s affiliation has changed to PRAHealthSciences. X.M. is an employee of Eli Lilly and Company. All other authors declare that at the time of the conduct and analysis of the study, they had no conflict of interest.
A professional medical writing company was not used for manuscript preparation.
Data Sharing
Readers should contact Dr. Mary Hebert (mhebert@uw.edu) for questions regarding data sharing.
References
- (1).Jovanovic L, Pettitt DJ. Gestational diabetes mellitus. JAMA. 2001;286:2516–2518. [DOI] [PubMed] [Google Scholar]
- (2).Paglia MJ, Coustan DR. Gestational diabetes: evolving diagnostic criteria. Curr Opin Obstet Gynecol. 2011;23:72–75. [DOI] [PubMed] [Google Scholar]
- (3).HAPO Study Cooperative Research Group. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Int J Gynaecol Obstet. 2002;78:69–77. [DOI] [PubMed] [Google Scholar]
- (4).American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces Practice Bulletin Number 180, July 2017). Gestational diabetes mellitus. Obstet Gynecol. 2018. February;131(2):e49–e64. [PubMed] [Google Scholar]
- (5).Hebert MF, Ma X, Naraharisetti SB, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85, 607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Buchanan TA. Pancreatic B-cell defects in gestational diabetes: implications for the pathogenesis and prevention of type 2 diabetes. J Clin Endocrinol Metab 2001;86:989–993. [DOI] [PubMed] [Google Scholar]
- (7).Phelps RL, Metzger BE, Freinkel N. Carbohydrate metabolism in pregnancy. XVII. Diurnal profiles of plasma glucose, insulin, free fatty acids, triglycerides, cholesterol and individual amino acids in late normal pregnancy. Am J Obstet Gynecol. 1981;140(7):730–6. [PubMed] [Google Scholar]
- (8).Klieger C, Pollex E, Kazmin A, Koren G. Hypoglycemics: pharmacokinetic considerations during pregnancy. Ther Drug Monit. 2009;31:533–541. [DOI] [PubMed] [Google Scholar]
- (9).Eyal S, Easterlin TR, Carr D. et al. Pharmacokinetics of metformin during pregnancy. Drug Metab Dispos. 2010;38:833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Shuster DL, Shireman LM, Ma X, et al. Pharmacodynamics of glyburide, metformin and glyburide/metformin combination therapy in the treatment of gestational diabetes mellitus. Clin Pharmacol Ther. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Bergman RN Minimal model: perspective from 2005. Hormone Res. 2005;64:8–15. [DOI] [PubMed] [Google Scholar]
- (12).Bandi ZL, Fuller JB, Bee DE, James GP. Extended clinical trial and evaluation of glucose determination with the Eastman Kodak Ektachem GLU/BUN Analyzer. Clin Chem. 1981;27:27–34. [PubMed] [Google Scholar]
- (13).Haffner SM, Mykkanen L, Stern MP, Valdez RA, Heisserman JA Bowsher RR. Relationship of proinsulin and insulin to cardiovascular risk factors in nondiabetic subjects. Diabetes. 1993;42:1297–1302. [DOI] [PubMed] [Google Scholar]
- (14).Wiedmeyer HM, Polonsky KS, Myers GL et al. International comparison of C-peptide measurements. Clinical chemistry 2007;53:784–787. [DOI] [PubMed] [Google Scholar]
- (15).Dalla Man C, Caumo A, Cobelli C. The oral glucose minimal model: estimation of insulin sensitivity from a meal test. IEEE Trans Biomed Eng. 2002;49:419–429. [DOI] [PubMed] [Google Scholar]
- (16).Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes 2001;50:150–8. [DOI] [PubMed] [Google Scholar]
- (17).Cobelli C, Toffolo GM, Dalla Man C, et al. Assessment of beta-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab. 2007;293:E1–E15. [DOI] [PubMed] [Google Scholar]
- (18).Caumo A, Bergman RN, Cobelli C. Insulin sensitivity from meal tolerance tests in normal subjects: a minimal model index. J Clin Endocrinol Metab. 2000;85:4396–4402. [DOI] [PubMed] [Google Scholar]
- (19).Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C. Minimal model estimation of glucose absorption and insulin sensitivity from oral test: validation with a tracer method. American journal of physiology Endocrinol Metab. 2004;287:E637–E643. [DOI] [PubMed] [Google Scholar]
- (20).Dalla Man C, Campioni M, Polonsky KS, et al. Two-hour seven-sample oral glucose tolerance test and meal protocol: minimal model assessment of beta-cell responsivity and insulin sensitivity in nondiabetic individuals. Diabetes. 2005;54:3265–3273. [DOI] [PubMed] [Google Scholar]
- (21).Zhang X, Wang X, Vernikovskaya DI, et al. Quantitative determination of metformin, glyburide and its metabolites in plasma and urine of pregnant patients by LC-MS/MS. Biomed Chromatogr. 2015;29:560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).R Core Team (2018). R: A language and environment for statistical computing. R Foundation for StatisticalComputing, Vienna, Austria: URL https://www.R-project.org/. [Google Scholar]
- (23).Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag; New York, 2016. [Google Scholar]
- (24).Yerby MS, Friel PN, McCormick K et al. Pharmacokinetics of anticonvulsants in pregnancy: alterations in plasma protein binding. Epilepsy Res. 1990;5(3):223–228. [DOI] [PubMed] [Google Scholar]
- (25).Zajdenverg L, Negrato CA. Gestational diabetes mellitus and type 2 diabetes: same disease in a different moment of life? Maybe not. Arch Endocrinol Metab. 2017;61:208–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Xiang AH, Peters RK, Kjos SL, et al. Pharmacological treatment of insulin resistance at two different stages in the evolution of type 2 diabetes: impact on glucose tolerance and β-cell function. J Clin Endocrin Metab. 2004;89:2846–2851. [DOI] [PubMed] [Google Scholar]
- (27).Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773–1779. [DOI] [PubMed] [Google Scholar]
- (28).Wiernsperger NF, Bailey CJ. Antihyperglycaemic effect of metformin: therapeutic and cellular mechanisms. Drugs. 1999;58:31–82. [DOI] [PubMed] [Google Scholar]
- (29).Scarpello JH, Howlett HC. Metformin therapy and clinical uses. Diabetes Vasc Dis Res. 2008;5:157–167. [DOI] [PubMed] [Google Scholar]
- (30).Vilsboll T, Krarup T, Madsbad S, Holst JJ. Defective amplification of the late phase insulin response to glucose by GIP in obese type II diabetic patients. Diabetologia. 2002;45:1111–1119. [DOI] [PubMed] [Google Scholar]
- (31).Tran KL, Park YI, Pandya S, et al. Overview of glucagon-like peptide-1 receptor agonists or the treatment of patients with type 2 diabetes. Am Health Drug Benefits. 2017;10:178–188. [PMC free article] [PubMed] [Google Scholar]
- (32).Williams-Herman D, Xu L, Teng R, et al. Effect of initial combination therapy with sitagliptin and metformin on β-cell function in patients with type 2 diabetes. Diabetes Obesity Metab 2012;14:67–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.