Abstract
Literature suggests vascular endothelial growth factor A (VEGFA) is protective among those at highest risk for Alzheimer’s disease (AD). Apolipoprotein E (APOE) ε4 allele carriers represent a highly susceptible population for cognitive decline, and VEGF may confer distinct protection among APOE-ε4 carriers. We evaluated interactions between cortical expression of 10 VEGF gene family members and APOE-ε4 genotype to clarify which VEGF genes modify the association between APOE-ε4 and cognitive decline. Data were obtained from the Religious Orders Study and Rush Memory and Aging Project (N=531). Linear regression assessed interactions on global cognition. VEGF genes NRP1 and VEGFA interacted with APOE-ε4 on cognitive performance (p.fdr<0.05). Higher NRP1 expression correlated with worse outcomes among s4 carriers but better outcomes among s4 non-carriers, suggesting NRP1 modifies the risk for poor cognitive scores based upon APOE-ε4 status. NRP1 regulates angiogenesis, and literature suggests vessels in APOE-ε4 brains are more prone to leaking, perhaps placing young vessels at risk for ischemia. Results suggest that future therapeutics targeting brain angiogenesis should also consider ε4 allele status.
Keywords: Cognition, Aging, APOE-ε4, Vascular Endothelial Growth Factor (VEGF), Gene expression
1. Introduction
Alzheimer’s disease (AD) is one of the most devastating and fastest growing neurological disorders in the world. With no available treatments to halt the progression of this disease, it is of monumental importance that novel insights into the underlying biology surrounding AD-associated cognitive decline are elucidated to generate effective therapeutic targets. Vascular endothelial growth factor A (VEGFA) has been studied as an emerging therapeutic candidate for AD (Hohman et al., 2015; Religa et al., 2013; Shim and Madsen, 2018; Storkebaum and Carmeliet, 2004), however the role of VEGFA in the development and progression of AD is debated. The vascular endothelial growth factor (VEGF) family plays a critical role in neuronal as well as vascular processes and is heavily involved in angiogenic regulation, neurogenesis and neuronal survival (Beazley-Long et al., 2013; Lange et al., 2016; Zacchigna et al., 2008). Some studies have found decreased protein levels of VEGFA in serum and cerebrospinal fluid (CSF) are associated with increased risk of AD and cognitive decline (Almodovar et al., 2009; Hohman et al., 2015; Huang et al., 2013), while others have found the opposite (Chiappelli et al., 2006; Tarkowski et al., 2002). In support of VEGFA’s neuroprotective role, studies have shown that AD model mice treated VEGFA recover from cognitive deficits (Garcia et al., 2014; Spuch et al., 2010). Additionally, our group has demonstrated that higher CSF VEGFA concentration is associated with slower rates of hippocampal atrophy and cognitive decline, particularly among AD biomarker-positive participants (Hohman et al., 2015). These studies suggest VEGFA is especially protective among participants at highest risk for AD and cognitive decline.
The polymorphic apolipoprotein E (APOE) gene is a strong genetic risk factor for late-onset AD, with the ε4 allele conferring the greatest risk and the ε2 allele conferring protection relative to the most common isoform, ε3 (Corder et al., 1993; Hohman et al., 2018; Liu et al., 2013). The molecular mechanism by which ApoE contributes to AD pathophysiology is still debated (Wu et al., 2018); however, well-characterized effects of APOE-ε4 include compromised blood-brain barrier integrity (Halliday et al., 2016), increased amyloid-p accumulation (Lim et al., 2017), and alterations in amyloid-β metabolism (Kim et al., 2009). ApoE4 has been strongly associated with cerebrovascular deficits, including a greater decline in cerebral blood flow with aging (Tai et al., 2016) and a significantly enhanced risk for ischemic stroke (Belloy et al., 2019).
Interestingly, VEGFA has also shown a comparable neuroprotective effect in humanized APOE-ε4 mice, whereby treatment with VEGF results in a recovery of behavioral deficits (Salomon-Zimri S, 2016). Given that APOE-ε4 carriers are at heightened risk for clinical AD, it may be that VEGF-mediated neuroprotection is particularly beneficial among this high-risk population. An increase in angiogenesis through VEGF signaling can initiate the growth of new vessels, which may serve as a mechanism to protect against APOE-related cognitive decline by preventing ischemia and downstream neurodegeneration. We hypothesize that APOE-ε4 carriers will show protection against AD and cognitive decline as a result of high angiogenesis-related VEGF gene expression in the brain, which may act to compensate against the multitude of biological vulnerabilities that make this population susceptible to cognitive decline.
The VEGF signaling family is large, with five genes encoding ligands (VEGFA, VEGFB, VEGFC, VEGFD, and PGF), 3 receptor genes (FLT1, KDR and FLT4), and 2 co-receptor genes (NRP1, NRP2), each with multiple isoforms (Almodovar et al., 2009; Carmeliet and de Almodovar, 2013). VEGFA, the most studied protein of the VEGF family, is a known key regulator of blood vessel growth (Shibuya, 2011, 2014) that is alternatively spliced into pro-or anti-angiogenic isoforms that also show opposing actions on vascular permeability and vasodilation (Beazley-Long et al., 2013). Additionally, the VEGFR-1 (FLT1), VEGFR-2 (KDR) and NRP1 receptors can be spliced into either transmembrane forms that signal through intracellular receptor tyrosine kinase (RTK) cascades or into soluble forms that act as scavengers of free ligand (Almodovar et al., 2009; Cackowski et al., 2004; Ebos et al., 2004). These points highlight the complexity of the VEGF signaling family and the need for comprehensive investigations of the entire VEGF gene family in the context of AD.
The present study investigates the interaction of prefrontal cortex VEGF gene and isoform expression with APOE-ε4 allele status on clinical AD, cognition and cognitive decline, as well as AD-related neuropathology. We hypothesize that higher expression of angiogenesis-specific VEGF genes and isoforms will modify the association between APOE-ε4 status and AD-related outcomes, such that ε4 carriers will show enhanced protection compared to non-carriers.
2. Methods
2.1. Participants
Data collected as part of the Rush University Religious Orders Study (ROS) and Memory and Aging Project (MAP) were utilized for this study. ROS data collection began in 1994 with Catholic clergy from across the USA, and MAP data collection began in 1997 across the Chicago area (Bennett et al., 2018). In both studies, older participants were non-demented at the time of enrollment, agreed to yearly clinical evaluation, and signed an informed consent, a repository consent for resource sharing, and an Anatomical Gift Act. The goal of these studies was to identify factors important for cognitive health during aging while monitoring the development of cognitive impairment, AD, and pathology of related disorders. Both studies were approved by an Institutional Review Board of Rush University Medical Center. Data sharing was carried out within the guidelines of Institutional Review Board (IRB)-protocols, and analyses were approved by the Vanderbilt University Medical Center IRB.
2.2. Neuropsychological Composites
Neuropsychological testing details have been previously published (Bennett et al., 2012a; Bennett et al., 2012b). Multiple aspects of cognition and memory were assessed using established protocols (Bennett et al., 2018). Z-score composites were then calculated in the domains of episodic memory, perceptual orientation, perceptual speed, semantic memory, and working memory. An average score across all 17 neuropsychological tests was calculated to represent global cognition.
2.3. Genotyping
DNA was extracted from peripheral blood monocytes (PBMCs) or brain tissue and underwent quality control measures as previously described (Keenan et al., 2012). APOE genotyping was performed by Polymorphic DNA Technologies using high-throughput sequencing of codons 112 and 158 of APOE exon 4, located on chromosome 19 (Oveisgharan et al., 2018).
2.4. Autopsy Measures of VEGF and APOE Gene Expression
Autopsies were performed to dissect and preserve tissue blocks from discrete brain regions. RNA was extracted from prefrontal cortices and sequenced using the Illumina HiSeq platform with 101 base paired-end reads. Details of sample processing and quality control measures have been previously published (Lim et al., 2014; Mostafavi et al., 2018). We analyzed RNA isoform expression of VEGF genes that interacted with APOE-ε4 on cognitive outcomes with the following number of isoforms available for each gene: VEGFA (14), NRP1 (12), VEGFB (3), VEGFC (2), VEGFD (2), NRP2 (13), FLT1 (3), FLT4 (8), KDR (1), PGF (5). RNA-Seq by Expectation Maximization (RSEM) was used in secondary analyses to assess isoform abundance, and isoforms of very low abundance (<10% expression in the cohort) were removed. Outliers, classified as values four standard deviations in either direction from the combined sample mean, were removed.
2.5. Neuropathological Measures
All neuropathological marker quantifications have been previously described (Bennett et al., 2012a; Bennett et al., 2012b). Briefly, amyloid load and paired helical filament tau density were quantified in eight brain regions (Bennett et al., 2006). Quantification of neuritic plaques and neurofibrillary tangles was based on silver staining of five brain regions (midfrontal cortex, midtemporal cortex, inferior parietal cortex, entorhinal cortex and hippocampus) to calculate the overall burden (Bennett et al., 2012b). TDP-43 immunoreactivity was assessed in the amygdala, nucleus accumbens, middle frontal gyrus, cingulate gyrus, dentate fascia, and inferior temporal cortex and scored on a graded scale (0=no pathology, 4=pathology in all regions) (Amador-Ortiz et al., 2007). Cerebral amyloid angiopathy (CAA) was measured by p-amyloid immunostaining in the midfrontal, midtemporal, angular, and calcarine cortices, and was scored on a scale from 0 – 4 (0=no pathology, 4=severe pathology) (Boyle et al., 2015; Love et al., 2014). Assessment of atherosclerosis was performed by visual inspection of the vertebral, basilar, posterior cerebral, middle cerebral, and anterior cerebral arteries of the Circle of Willis, as well as proximal branches and graded based on severity (0=no pathology, 4=severe pathology) (Arvanitakis et al., 2017). Arteriolosclerosis severity was classified by a semi-quantitative grading scale (0=no pathology, 3=severe pathology) after characterization of histologic changes in the vascular lumen (Buchman et al., 2011). Gross and micro infarcts were categorized as present (1) or absent (0) based upon visual inspection in nine brain regions (midfrontal, middle temporal, entorhinal, hippocampal, inferior parietal and anterior cingulate cortices, anterior basal ganglia, midbrain, and thalamus; (Arvanitakis et al., 2011; Schneider et al., 2007; Schneider et al., 2003).
2.6. Statistical Analyses
Data were analyzed using R (version 3.5.1, https://www.r-project.org) with APOE-ε4 allele status categorized using a dominant model (absence or presence). Linear regression models covaried for age at death, sex, postmortem interval, and interval between final visit and death assessed VEGF family gene expression associations with APOE-ε4 allele status and with APOE expression. A linear mixed effects regression model covaried for sex, age at death, postmortem interval, and interval between final visit and death, assessed VEGF family gene expression interactions with APOE expression on global cognitive change.
A binary logistic regression model assessed APOE-ε4 by VEGF family gene expression interactions on diagnosis (normal cognition [NC] compared to AD, mild cognitive impairment subjects were excluded from this analysis). Covariates included sex, age at time of death, postmortem interval, and interval between the last documented clinical visit and time of death.
A linear regression model covaried for sex, age at death, postmortem interval, and interval between final visit and death, was used to test for APOE-ε4 × VEGF family gene expression interactions on global cognition. Secondary analyses stratified by APOE-ε4 status investigated VEGF family gene expression associations with global cognition in ε4 carriers and non-carriers. This model was also used to investigate VEGF expression associations with APOE genotype and VEGF × APOE expression on global cognition. Additionally, a linear regression model covaried for sex, age at death, postmortem interval, and interval between final visit and death, was used to assess APOE-ε4 × VEGF family gene expression interactions on the following cognitive domains: episodic memory, perceptual orientation, perceptual speed, semantic memory, and working memory. Further, this linear regression model was also used to assess genome-wide interactions with the APOE-ε4 allele on cross-sectional global cognition.
A mixed effects regression model was used to analyze APOE-ε4 × VEGF family gene expression interactions on annual cognitive change. Fixed effects included age at death, APOE-ε4 status, sex, VEGF family expression, postmortem interval, years before death, and interval (years between last visit and the current visit). A three-way APOE-ε4 × VEGF × interval interaction was the term of interest. Random effects included the interval and intercept. Secondary analyses were stratified by APOE-ε4 status. All models were subjected to the Benjamini-Hochberg false discovery rate procedure (Benjamini and Hochberg, 1995) to correct for multiple comparisons (ie, correction for all 10 VEGF family genes).
AD-related neuropathological outcomes included amyloid load, paired helical filament tau density, neuritic plaques, and neurofibrillary tangles, all of which were square-root transformed. Linear models, covaried for age at death, postmortem interval, and sex, assessed APOE-ε4 × VEGF expression interactions on AD-related neuropathological outcomes. Non-AD neuropathological outcomes were assessed for APOE-ε4 × VEGF expression interactions using a binary logistic model for hippocampal sclerosis, gross infarcts and microinfarcts. A proportional odds logistic regression model evaluated APOE-ε4 × VEGF expression interactions on cerebral amyloid angiopathy (CAA), atherosclerosis, arteriolosclerosis, and TDP-43 reactivity. Macroinfarct count was analyzed using a Poisson regression model, and macroinfarct volume was square-root transformed and assessed using linear regression.
Sensitivity analyses were carried out for the cognitive and neuropathology models described above excluding individuals diagnosed with clinical AD to test if diagnostic status accounted for significant results. In addition, models were run using unique isoforms of VEGF genes that showed significant interactions with APOE-ε4 to determine if isoform-specific expression was responsible for significant interaction results. Isoform-specific models were corrected for multiple comparisons using the FDR procedure with the a priori threshold for statistical significance set to P.fdr<0.05.
Additional sensitivity analyses were performed to determine if cell-type marker expression, included as a covariate, would significantly alter model predictions. We first analyzed correlations between VEGF family expression and cell-type marker expression. Models were then re-run covarying for expression of either neuronal marker ENO2 or expression of all other available cell-type markers (OLIG2, oligodendrocytes; GFAP, astrocytes; CD34, endothelial cells; CD38, microglia,). These cell-type markers have been previously validated after comparisons of expression profiles and cell population frequency in cortical tissue in this cohort (Mostafavi et al., 2018; Patrick et al., 2019) and have been utilized to examine cell-type effects in previous analyses (Mahoney et al., 2019). Additionally, we calculated adjusted VEGF expression by residualizing the association between each gene and cell-type marker. This adjusted expression was then used to re-run the models described above.
3. Results
3.1. Participant Demographics
Summary demographic data are presented in Table 1. This cohort was long-lived, highly educated, with the majority self-identifying as non-Hispanic White. As expected, the proportion of APOE-ε4 carriers was higher among AD cases (35%) compared to NC (14%), and baseline global cognition scores declined across diagnostic groups (NC highest, AD lowest). It is noteworthy that the prevalence of APOE-ε4 carriers among participants diagnosed with clinical AD is less than other AD cohorts (Frisoni et al., 1998; Heffernan et al., 2016; Ward et al., 2012), however this is likely due to enrollment criteria that required participants to be non-demented at time of enrollment and the community-based nature of studies. The average age of AD diagnosis in this cohort was 82.1 ± 6.3 years of age. Age at time of death was also significantly different across diagnostic groups, with AD cases being the oldest at time of death.
Table 1.
Cohort demographics and summary statistics
| Clinical Diagnosis | Total (N=531) |
P | |||
|---|---|---|---|---|---|
| Normal Cognition (N=180) |
Mild Cognitive Impairment (N=148) |
Alzheimer’s Disease (N=203) |
|||
| Age of death, years | 86±7 | 89±6 | 91±6 | 89±7 | <0.001 |
| Male, no. (%) | 70 (39) | 54 (36) | 70 (34) | 194 (37) | 0.67 |
| Non-Hispanic white, no. (%) | 177 (98) | 146 (99) | 195 (96) | 518 (98) | 0.21 |
| Education, years | 17±4 | 16±3 | 17±4 | 17±4 | 0.59 |
| Global cognition composite z score (at last visit) | 0.14±0.42 | −0.49±0.45 | −1.85±0.91 | −0.80±1.09 | <0.001 |
| Average number of visits | 7.12±4.04 | 6.93±3.65 | 7.55±3.69 | 7.23±3.8 | 0.26 |
| APOE-ε4 carriers, no. (%) | 25 (14) | 30 (20) | 72 (35) | 127 (24) | <0.001 |
| APOE-ε2 carriers, no. (%) | 32 (18) | 19 (13) | 36 (18) | 87 (16) | 0.39 |
Values are presented as mean±standard deviation, unless otherwise indicated. Boldface indicates P<0.05.
3.2. VEGF Gene Expression Associations with APOE-ε4 Allele Status and APOE Expression
No VEGF ligand or receptor genes were differentially expressed between APOE-ε4 carriers and non-carriers (p-values>0.09, Supplemental Table 1). Additionally, no VEGF genes interacted with APOE expression on global cognition prior to death or cognitive change (p-values>0.06, Supplemental Table 2).
3.3. VEGF Gene Expression Interactions with APOE-ε4 Allele Status on Diagnosis
Using a binary logistic regression model, we found that NRP1 (β=0.77, p.fdr=0.037) expression interacted with APOE-ε4 status on clinical diagnosis (NC compared to AD). NRP2 expression fell just beyond the threshold for APOE-ε4 interaction significance after correction for multiple comparisons (p.fdr=0.060). After stratifying participants by APOE-ε4 status, lower NRP1 expression was significantly associated with AD diagnosis in ε4 non-carriers. Interaction and stratified results are presented in Supplemental Table 3.
3.4. VEGF Gene Expression Interactions with APOE-ε4 Allele Status on Cognitive Performance
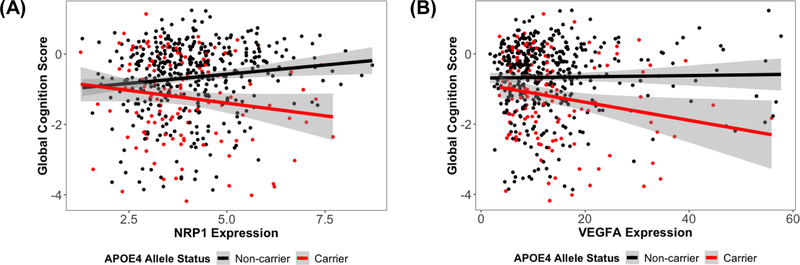
Cross-sectional analyses revealed NRP1 and VEGFA interacted with APOE-ε4 on global cognitive performance at the final neuropsychological assessment (NRP1: β=−0.287, p.fdr=0.004; VEGFA: β=−0.03, p.fdr=0.026; Table 2, Figure 1). We interpreted this interaction as evidence that NRP1 and VEGFA expression associations with late life cognition differ by APOE-ε4 status. To clarify the nature of these interaction results on cross-sectional cognition, stratified analyses showed that in APOE-ε4 carriers, higher expression of NRP1 (β=−0.176, p=0.034) and VEGFA (β=−0.027, p=0.019) were associated with worse global cognition scores; whereas in APOE-ε4 non-carriers, higher NRP1 (β=0.112, p=0.003) expression predicted better global cognition scores. Both interaction and stratified results on cognitive performance are summarized in Table 2. These APOE-ε4 interactions on global cognition did not survive a genome-wide correction in this cohort (Supplemental Figure 1), however the power for genome-wide analyses was quite low given the sample size.
Table 2.
Cross-sectional VEGF × APOE-ε4 interactions and stratified results on global cognition
| Interaction | APOE-ε4 Carriers (N=127) | APOE-ε4 Non-Carriers (N=404) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | β | SE | P | P.fdr | β | SE | P | P.fdr | β | SE | P | P.fdr |
| NRP1 | −0.287 | 0.080 | 3.58E-04* | 0.004 | −0.176 | 0.082 | 0.034 | 0.114 | 0.112 | 0.037 | 0.003 | 0.015 |
| VEGFA | −0.030 | 0.011 | 0.005 | 0.026 | −0.027 | 0.011 | 0.019 | 0.097 | 0.004 | 0.005 | 0.416 | 0.594 |
| FLT1 | −0.055 | 0.026 | 0.035 | 0.118 | −0.065 | 0.026 | 0.015 | 0.097 | −0.005 | 0.013 | 0.711 | 0.790 |
| FLT4 | −0.237 | 0.164 | 0.148 | 0.304 | −0.333 | 0.168 | 0.049 | 0.124 | −0.110 | 0.085 | 0.196 | 0.538 |
| VEGFB | 0.005 | 0.004 | 0.152 | 0.304 | −0.003 | 0.004 | 0.522 | 0.663 | −0.007 | 0.002 | 3.48E-05* | 3.48E-04* |
| KDR | 0.292 | 0.258 | 0.259 | 0.431 | 0.229 | 0.280 | 0.416 | 0.663 | 0.017 | 0.120 | 0.884 | 0.884 |
| VEGFD | 0.240 | 0.305 | 0.433 | 0.618 | 0.140 | 0.316 | 0.657 | 0.720 | −0.153 | 0.147 | 0.299 | 0.594 |
| PGF | −0.023 | 0.062 | 0.707 | 0.809 | −0.069 | 0.065 | 0.294 | 0.589 | −0.038 | 0.030 | 0.215 | 0.538 |
| VEGFC | −0.136 | 0.391 | 0.728 | 0.809 | −0.267 | 0.424 | 0.531 | 0.663 | −0.072 | 0.169 | 0.669 | 0.790 |
| NRP2 | 0.023 | 0.153 | 0.883 | 0.883 | 0.058 | 0.160 | 0.720 | 0.720 | 0.056 | 0.069 | 0.416 | 0.594 |
P.fdr represents corrected P-values. Boldface signifies P≤0.05.
denotes results that were significant after adjusting for all models tested for main outcomes (cognition, diagnosis)
Figure 1.
(A) NRP1 expression associations with global cognitive performance at the final neuropsychological assessment, stratified by APOE-ε4 allele status. Overall interaction: NRP1 × APOE-ε4, β=−0.28, p.fdr=0.007; APOE-ε4 carriers, β=−0.17, p=0.038; APOE-ε4 non-carriers, β=0.11, p=0.004. (B) VEGFA expression associations with global cognitive performance at the final neuropsychological assessment, stratified by APOE-ε4 allele status. Overall interaction: VEGFA × APOE-ε4, β=−0.03, p.fdr=0.026; APOE-ε4 carriers, β=−0.03, p=0.019; APOE-ε4 non-carriers, β=0.004, p=0.4.
Additional models to assess VEGF × APOE-ε4 interactions on specific cognitive domains did not reveal novel interactions compared to the global cognition results. These separated cognitive domain analyses did reveal that working memory, semantic memory and perceptual orientation appear to drive the NRP1 × APOE-ε4 interaction on global cognition. Further, stratified analyses of this model showed the same interaction trend among APOE-ε4 non-carriers as the global cognition results. Specifically, higher NRP1 expression was associated with better working and semantic memory, as well as better perceptual orientation among APOE-ε4 non-carriers (Supplemental Figure 2, Supplemental Tables 4 – 7).
Longitudinally, no VEGF genes interacted with APOE-ε4 on global cognitive change (Table 3). These results indicate that VEGF family expression associations with cognitive decline did not differ by APOE-ε4 status.
Table 3.
Longitudinal VEGF × APOE-ε4 interactions and stratified results on global cognitive change
| Interaction | APOE-ε4 Carriers (N=127) | APOE-ε4 Non-Carriers (N=404) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | β | SE | P | P.fdr | β | SE | P | P.fdr | β | SE | P | P.fdr |
| NRP1 | −0.015 | 0.009 | 0.084 | 0.439 | −0.008 | 0.009 | 0.388 | 0.583 | 0.007 | 0.004 | 0.104 | 0.207 |
| KDR | 0.044 | 0.028 | 0.120 | 0.439 | 0.034 | 0.030 | 0.260 | 0.544 | −0.015 | 0.012 | 0.211 | 0.351 |
| VEGFB | 0.001 | 4.16E-04 | 0.155 | 0.439 | −3.28E-04 | 4.48E-04 | 0.465 | 0.583 | −0.001 | 1.92E-04 | 1.46E-06* | 1.46E-05* |
| VEGFA | −0.002 | 0.001 | 0.176 | 0.439 | −0.002 | 0.001 | 0.135 | 0.544 | −1.95E-04 | 0.001 | 0.709 | 0.788 |
| VEGFD | 0.036 | 0.034 | 0.285 | 0.571 | 0.021 | 0.035 | 0.546 | 0.607 | −0.012 | 0.016 | 0.441 | 0.552 |
| NRP2 | 0.012 | 0.017 | 0.492 | 0.819 | 0.013 | 0.018 | 0.466 | 0.583 | −7.41E-06 | 0.008 | 0.999 | 0.999 |
| VEGFC | 0.023 | 0.044 | 0.608 | 0.869 | 0.006 | 0.048 | 0.909 | 0.909 | −0.020 | 0.018 | 0.282 | 0.403 |
| FLT4 | −0.007 | 0.018 | 0.723 | 0.904 | −0.032 | 0.019 | 0.090 | 0.544 | −0.025 | 0.009 | 0.006 | 0.021 |
| FLT1 | −4.65E-04 | 0.003 | 0.874 | 0.918 | −0.004 | 0.003 | 0.229 | 0.544 | −0.004 | 0.001 | 0.011 | 0.028 |
| PGF | 0.001 | 0.007 | 0.918 | 0.918 | −0.008 | 0.007 | 0.272 | 0.544 | −0.009 | 0.003 | 0.004 | 0.021 |
P.fdr represents corrected P-values. Boldface signifies P≤0.05.
denotes results that were significant after adjusting for all models tested for main outcomes (cognition, diagnosis)
3.5. VEGF Gene Expression Interactions with APOE-ε4 Allele Status on Neuropathology
No significant interactions were observed between APOE-ε4 and VEGF expression on AD neuropathology (Supplemental Table 8). Models to assess VEGF × APOE interactions on other neuropathological measures showed no significant interaction on CAA, cerebral atherosclerosis, arteriolosclerosis, TDP-43, hippocampal sclerosis, gross infarcts, or microinfarcts (Supplemental Table 9).
3.6. Sensitivity Analyses
NRP1 and VEGFA interacted with APOE-ε4 on cross-sectional global cognition, so we analyzed VEGFA and NRP1 isoform-specific interactions with APOE-ε4 on this outcome, which showed that pro-angiogenic coding transcripts of VEGFA (VEGFA-207, VEGFA-205) and several protein coding transcripts of NRP1 interacted with APOE-ε4 on global cognition (Supplemental Table 10).
Additionally, models were run using an adjusted VEGF gene expression value that was calculated by residualizing the association between expression and a given cell marker on global cognition. A correlation matrix for VEGF family and cell-type marker expression can be found in Supplemental Figure 3. Due to the fact that expression data were derived from tissue homogenate, we re-analyzed cross-sectional and longitudinal cognition interaction models to determine if significant VEGF expression × APOE-ε4 allele status interaction results persisted after adjusting for cell-specific effects. Cross-sectional results were generally consistent across cell-type marker adjustments and additional covariate models. Longitudinal results were consistent between the adjusted and unadjusted expression models. Cross-sectional results can be found in Supplemental Table 11 and longitudinal results can be found in Supplemental Table 12.
4. Discussion
We set out to determine how differences in VEGF gene family expression might interact with one of the strongest genetic risk factors for sporadic AD, APOE-ε4 status, to predict age-related cognitive decline and clinical AD diagnosis. NRP1 and VEGFA interacted with APOE-ε4 to modify the association between ε4 and the final global cognition score. Interestingly, effects of NRP1 expression on cognition in ε4 carriers compared to non-carriers was the opposite of expectation, such that higher expression of NRP1 was associated with worse outcomes in carriers and better outcomes in non-carriers. VEGF × APOE-ε4 interactions were not observed on AD pathology, suggesting these gene expression interactions were not driven by neuropathological changes. Further, no significant VEGF × APOE-ε4 interactions on pathological outcomes such as CAA suggest that amyloid build-up in vasculature does not drive the associations we observe on cognition.
The VEGF genes that modified the association between APOE-ε4 and cross-sectional cognition (NRP1 and VEGFA) are positive modulators of angiogenic signaling (Almodovar et al., 2009; Lee et al., 2011; Soker et al., 1998; Zhang et al., 2010). VEGFA binds NRP1, which forms a complex with KDR on endothelial cells to initiate intracellular signaling associated with the proliferation, migration, and survival of endothelial cells (Lee et al., 2011; Lee et al., 1996; Soker et al., 1998). Cerebrovascular deficits are an early feature of AD and cognitive decline with aging, and cerebrovascular ischemic disease has been found to contribute to the severity of cognitive decline (Love and Miners, 2016; Serrano-Pozo, 2019). The protective effects associated with high expression of angiogenesis relevant genes in ε4 non-carriers could reflect a mechanism to prevent ischemia and downstream neurodegeneration. However, angiogenic mechanisms may become damaging in the presence of the ε4 allele due to an over production of new vessels that are especially prone to leaking, as APOE-ε4 has been associated with increased blood-brain barrier leakiness resulting in cognitive decline (Serrano-Pozo, 2019; Tai et al., 2016). It is also possible that an increase in NRP1 expression in ε4 carriers causes an over-permeabilization of existing vessels as VEGF signaling is closely tied to vascular permeability (Bates, 2010). In opposition to our original hypothesis for this study, results suggest that VEGF signaling may be beneficial in APOE-ε4 non-carriers but detrimental in carriers, and it seems most plausible that this effect is mediated through angiogenic or endothelial cell remodeling processes.
It is interesting to note that we did not observe significant VEGF × APOE-ε4 interactions on neuropathology. Beta-amyloid peptides have been shown to antagonize VEGFR-2 (Patel et al., 2010), suggesting amyloid accumulation could represent a potential modulator of receptor mRNA expression. Additionally, the build-up of amyloid plaques has been hypothesized to trap free VEGFA and contribute to an up-regulation in expression (Almodovar et al., 2009), but our data do not suggest that the interactions between any VEGF family genes and APOE are driven primarily by alterations in amyloid, tau, or any of the other measured neuropathologies. It is notable that, as reported in earlier work from our group (Mahoney et al., 2019), there are main effects of VEGF family genes on AD neuropathology, but these associations do not differ by ε4 status. Thus, it is likely that the APOE-specific vulnerability is due either to a process downstream of neuropathology, such as a unique vulnerability to repair processes highlighted above, or a process that is entirely independent of measured neuropathology. It is also possible that differences in VEGF expression could influence subclinical brain alterations that may not be overtly detectable upon post-mortem observation but could manifest differentially between ε4-carriers and non-carriers. Future studies which incorporate markers of angiogenesis or vascular health may help elucidate underlying brain or vascular changes which may be influenced by VEGF expression.
While significant cross-sectional cognition interaction results did not survive genome-wide correction, results of this study contribute insight for the main effects of the VEGF family on global cognition in this cohort (Mahoney et al., 2019). Main effects results compared with APOE-ε4 interaction results showed that VEGFA and NRP1 expression are not associated with global cognition unless the APOE-ε4 allele is taken into account. This is particularly interesting given the literature that connects VEGFA to cognition without consideration of APOE-ε4. It is notable that our observed results appear to be counter to the protective effects of VEGFA that have been reported in humanized APOE-ε4 mouse models (Salomon-Zimri S, 2016). It is possible that the association between high VEGF expression and worse cognitive trajectories in ε4 carriers is reflective of upregulation by inflammatory cytokines (Angelo and Kurzrock, 2007; Maloney and Gao, 2015; Nagineni et al., 2012), which are also associated with AD progression (Heppner et al., 2015; Kinney et al., 2018), and that the observed VEGF expression effects could be a consequence of AD-related inflammation. The reparative role of angiogenesis in other conditions, such as cerebral ischemia and stroke, has also been well characterized (Li WW, 2005; Uccelli Andrea, 2019), and it is possible that the upregulation in VEGF expression is a compensatory mechanism that fails to rescue cognitive decline. It could also be the case that transcript levels are not reflective of protein VEGF levels. Future proteomic analyses could help to shed light on the underlying expression differences we observed.
Due to the heterogeneity of cell types in brain homogenates, we considered models that covaried or residualized for expression of a neuronal-specific marker (ENO2) as well as cell-type markers for astrocytes (GFAP), microglia (CD68), endothelial cells (CD34), and oligodendrocytes (OLIG2). Results were not significantly altered by adjusting for these cell-type markers.
Another interesting result was the lack of interaction between APOE expression and VEGF expression. Previous literature has debated the influence of genotype on APOE expression (Beffert et al., 1999; Harr et al., 1996; Kim et al., 2009) and the significance of APOE expression in AD (Bertrand et al., 1995; Pirttilä et al., 1996). Our study suggests that the interactions between VEGF genes and APOE-ε4 on cognition are driven by genotype-specific effects of APOE rather than brain APOE expression levels.
Several factors of this study limit generalizability, including the high level of participant education, lack of racial diversity and use of brain homogenate data which limits cell-type specific conclusions. An additional consideration is the inability to discern causal relationships given that the expression levels likely reflect a combination of cause and consequence of disease. It is also important to note the preliminary nature of the findings reported in this study, as the global cognition findings have not been replicated in another dataset. As no comparable data sets exist in the public domain, replication of this study remains a future goal.
However, this analysis also possesses several strengths, including the rich longitudinal cognitive data, measurable expression of all genes in the VEGF family in brain tissue, ample neuropathological data, and the comprehensive clinical characterization of the cohort. Future work should replicate these findings in other cohorts and investigate the underlying biological mechanisms driving these interactions on cognition through detailed proteomic and angiogenesis pathway analyses.
5. Conclusion
In summary, we found that NRP1 and VEGFA interacted with APOE-ε4 on cognition. Interestingly, higher expression of NRP1 was associated with beneficial outcomes in ε4 non-carriers and cognitive decline in ε4 carriers. These results suggest that angiogenic signaling may have different effects based upon an individual’s APOE-ε4 status. Further investigation of the biological interaction between the VEGF family, especially components relevant to angiogenesis, and APOE genotype, as well as replication of cognitive associations in an independent data set, is warranted to better understand how these genes and proteins impact cognitive outcomes in older adults.
Supplementary Material
Acknowledgements
This research was supported in part by K01-AG049164, R01-AG059716, R01-AG061518, R21-AG05994, K12-HD043483, K24-AG046373, HHSN311201600276P, S10-0D023680, R01-AG034962, R01-NS100980, R01-AG056534, P30-AG010161, R01-AG15819, R01-AG17917, U01-AG46152, UL1-TR000445, T32-AG058524, and the Vanderbilt Memory & Alzheimer's Center.
Footnotes
Declarations of interest: none.
References
- Allen M, Carrasquillo MM, Funk C, Heavner BD, Zou F, Younkin CS, Burgess JD, Chai H-S, Crook J, Eddy JA, Li H, Logsdon B, Peters MA, Dang KK, Wang X, Serie D, Wang C, Nguyen T, Lincoln S, Malphrus K, Bisceglio G, Li M, Golde TE, Mangravite LM, Asmann Y, Price ND, Petersen RC, Graff-Radford NR, Dickson DW, Younkin SG, Ertekin-Taner N, 2016. Human whole genome genotype and transcriptome data for Alzheimer’s and other neurodegenerative diseases. Sci Data 3, 160089–160089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M, Wang X, Burgess JD, Watzlawik J, Serie DJ, Younkin CS, Nguyen T, Malphrus KG, Lincoln S, Carrasquillo MM, Ho C, Chakrabarty P, Strickland S, Murray ME, Swarup V, Geschwind DH, Seyfried NT, Dammer EB, Lah JJ, Levey AI, Golde TE, Funk C, Li H, Price ND, Petersen RC, Graff-Radford NR, Younkin SG, Dickson DW, Crook JR, Asmann YW, Ertekin-Taner N, 2018. Conserved brain myelination networks are altered in Alzheimer’s and other neurodegenerative diseases. Alzheimers Dement 14(3), 352–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almodovar C.R.d., Lambrechts D, Mazzone M, Carmeliet P, 2009. Role and Therapeutic Potential of VEGF in the Nervous System. 89(2), 607–648. [DOI] [PubMed] [Google Scholar]
- Amador-Ortiz C, Lin W-L, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW, 2007. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Annals of neurology 61(5), 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelo LS, Kurzrock R, 2007. Vascular Endothelial Growth Factor and Its Relationship to Inflammatory Mediators. Clinical Cancer Research 13(10), 2825. [DOI] [PubMed] [Google Scholar]
- Arvanitakis Z, Capuano AW, Leurgans SE, Buchman AS, Bennett DA, Schneider JA, 2017. The Relationship of Cerebral Vessel Pathology to Brain Microinfarcts. Brain pathology (Zurich, Switzerland) 27(1), 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA, 2011. Microinfarct pathology, dementia, and cognitive systems. Stroke 42(3), 722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates DO, 2010. Vascular endothelial growth factors and vascular permeability. Cardiovascular research 87(2), 262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beazley-Long N, Hua J, Jehle T, Hulse RP, Dersch R, Lehrling C, Bevan H, Qiu Y, Lagreze WA, Wynick D, Churchill AJ, Kehoe P, Harper SJ, Bates DO, Donaldson LF, 2013. VEGF-A165b is an endogenous neuroprotective splice isoform of vascular endothelial growth factor A in vivo and in vitro. The American journal of pathology 183(3), 918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffert U, Cohn JS, Petit-Turcotte C, Tremblay M, Aumont N, Ramassamy C, Davignon J, Poirier J, 1999. Apolipoprotein E and β-amyloid levels in the hippocampus and frontal cortex of Alzheimer’s disease subjects are disease-related and apolipoprotein E genotype dependent. Brain Research 843(1), 87–94. [DOI] [PubMed] [Google Scholar]
- Belloy ME, Napolioni V, Greicius MD, 2019. A Quarter Century of APOE and Alzheimer’s Disease: Progress to Date and the Path Forward. Neuron 101(5), 820–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological) 57(1), 289–300. [Google Scholar]
- Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA, 2018. Religious Orders Study and Rush Memory and Aging Project. Journal of Alzheimer’s disease: JAD 64(s1), S161–S189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS, 2012a. Overview and findings from the religious orders study. Current Alzheimer research 9(6), 628–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS, 2012b. Overview and findings from the rush Memory and Aging Project. Current Alzheimer research 9(6), 646–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS, 2006. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. The Lancet Neurology 5(5), 406–412. [DOI] [PubMed] [Google Scholar]
- Bertrand P, Poirier J, Oda T, Finch CE, Pasinetti GM, 1995. Association of apolipoprotein E genotype with brain levels of apolipoprotein E and apolipoprotein J (clusterin) in Alzheimer disease. Molecular Brain Research 33(1), 174–178. [DOI] [PubMed] [Google Scholar]
- Boyle PA, Yu L, Nag S, Leurgans S, Wilson RS, Bennett DA, Schneider JA, 2015. Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology 85(22), 1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman AS, Leurgans SE, Nag S, Bennett DA, Schneider JA, 2011. Cerebrovascular disease pathology and parkinsonian signs in old age. Stroke 42(11), 3183–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cackowski FC, Xu L, Hu B, Cheng S-Y, 2004. Identification of two novel alternatively spliced Neuropilin-1 isoforms. Genomics 84(1), 82–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, de Almodovar CR, 2013. VEGF ligands and receptors: implications in neurodevelopment and neurodegeneration. Cellular and Molecular Life Sciences 70(10), 1763–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappelli M, Borroni B, Archetti S, Calabrese E, Corsi MM, Franceschi M, Padovani A, Licastro F, 2006. VEGF Gene and Phenotype Relation with Alzheimer’s Disease and Mild Cognitive Impairment. Rejuvenation Research 9(4), 485–493. [DOI] [PubMed] [Google Scholar]
- Corder E, Saunders A, Strittmatter W, Schmechel D, Gaskell P, Small G, Roses A, Haines J, Pericak-Vance M, 1993. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. 261(5123), 921–923. [DOI] [PubMed] [Google Scholar]
- Ebos JML, Bocci G, Man S, Thorpe PE, Hicklin DJ, Zhou D, Jia X, Kerbel RS, 2004. A Naturally Occurring Soluble Form of Vascular Endothelial Growth Factor Receptor 2 Detected in Mouse and Human Plasma<a id="xref-fn-1–1" class="xref-"href="#fn-1"><sup>1</sup></a>1Ontario Graduate Scholarship in Science and Technology (J.M.L. Ebos); Sunnybrook Trust for Medical Research (G. Bocci); and NIH grant CA41223, Canadian Institutes for Health Research, and National Cancer Institute of Canada (R.S. Kerbel). Molecular Cancer Research 2(6), 315. [PubMed] [Google Scholar]
- Frisoni GB, Manfredi M, Geroldi C, Binetti G, Zanetti O, Bianchetti A, Trabucchi M, 1998. The prevalence of apoE-ε4 in Alzheimer’s disease is age dependent. Journal of Neurology, Neurosurgery & Psychiatry 65(1), 103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia KO, Ornellas FLM, Martin PKM, Patti CL, Mello LE, Frussa-Filho R, Han SW, Longo BM, 2014. Therapeutic effects of the transplantation of VEGF overexpressing bone marrow mesenchymal stem cells in the hippocampus of murine model of Alzheimer’s disease. Frontiers in aging neuroscience 6, 30–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday MR, Rege SV, Ma Q, Zhao Z, Miller CA, Winkler EA, Zlokovic BV, Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism 36(1), 216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harr SD, Uint L, Hollister R, Hyman BT, Mendez AJ, 1996. Brain Expression of Apolipoproteins E, J, and A-I in Alzheimer’s Disease. Journal of Neurochemistry 66(6), 2429–2435. [DOI] [PubMed] [Google Scholar]
- Heffernan AL, Chidgey C, Peng P, Masters CL, Roberts BR, 2016. The Neurobiology and Age-Related Prevalence of the ε4 Allele of Apolipoprotein E in Alzheimer’s Disease Cohorts. J Mol Neurosci 60(3), 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner FL, Ransohoff RM, Becher B, 2015. Immune attack: the role of inflammation in Alzheimer disease. Nature Reviews Neuroscience 16, 358. [DOI] [PubMed] [Google Scholar]
- Hohman TJ, Bell SP, Jefferson AL, Alzheimer’s Disease Neuroimaging I., 2015. The role of vascular endothelial growth factor in neurodegeneration and cognitive decline: exploring interactions with biomarkers of Alzheimer disease. JAMA neurology 72(5), 520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohman TJ, Dumitrescu L, Barnes LL, et al. , 2018. Sex-specific association of apolipoprotein e with cerebrospinal fluid levels of tau. JAMA Neurology 75(8), 989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Jia J, Liu R, 2013. Decreased serum levels of the angiogenic factors VEGF and TGF-β1 in Alzheimer’s disease and amnestic mild cognitive impairment. Neuroscience Letters 550, 60–63. [DOI] [PubMed] [Google Scholar]
- Keenan BT, Shulman JM, Chibnik LB, Raj T, Tran D, Sabuncu MR, Alzheimer’s Disease Neuroimaging I, Allen AN, Corneveaux JJ, Hardy JA, Huentelman MJ, Lemere CA, Myers AJ, Nicholson-Weller A, Reiman EM, Evans DA, Bennett DA, De Jager PL, 2012. A coding variant in CR1 interacts with APOE-ε4 to influence cognitive decline. Human molecular genetics 21(10), 2377–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM, 2009. The role of apolipoprotein E in Alzheimer’s disease. Neuron 63(3), 287–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT, 2018. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s & Dementia: Translational Research & Clinical Interventions 4, 575–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C, Storkebaum E, de Almodovar CR, Dewerchin M, Carmeliet P, 2016. Vascular endothelial growth factor: a neurovascular target in neurological diseases. Nature Reviews Neurology 12, 439. [DOI] [PubMed] [Google Scholar]
- Lee HK, Chauhan SK, Kay E, Dana R, 2011. Flt-1 regulates vascular endothelial cell migration via a protein tyrosine kinase-7-dependent pathway. Blood 117(21), 5762–5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Gray A, Yuan J, Luoh SM, Avraham H, Wood WI, 1996. Vascular endothelial growth factor-related protein: a ligand and specific activator of the tyrosine kinase receptor Flt4. Proc Natl Acad Sci U S A 93(5), 1988–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WW TK, Zhai AW, Kruger EA, Li VW, 2005. The role of therapeutic angiogenesis in tissue repair and regeneration. Adv Skin Wound Care 18(9), 491–500. [DOI] [PubMed] [Google Scholar]
- Lim ASP, Srivastava GP, Yu L, Chibnik LB, Xu J, Buchman AS, Schneider JA, Myers AJ, Bennett DA, De Jager PL, 2014. 24-hour rhythms of DNA methylation and their relation with rhythms of RNA expression in the human dorsolateral prefrontal cortex. PLoS genetics 10(11), e1004792-e1004792. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lim YY, Mormino EC, Alzheimer’s Disease Neuroimaging I, 2017. APOE genotype and early p-amyloid accumulation in older adults without dementia. Neurology 89(10), 1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C-C, Liu C-C, Kanekiyo T, Xu H, Bu G, 2013. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nature reviews. Neurology 9(2), 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love S, Chalmers K, Ince P, Esiri M, Attems J, Jellinger K, Yamada M, McCarron M, Minett T, Matthews F, Greenberg S, Mann D, Kehoe PG, 2014. Development, appraisal, validation and implementation of a consensus protocol for the assessment of cerebral amyloid angiopathy in post-mortem brain tissue. American journal of neurodegenerative disease 3(1), 19–32. [PMC free article] [PubMed] [Google Scholar]
- Love S, Miners JS, 2016. Cerebrovascular disease in ageing and Alzheimer’s disease. Acta neuropathologica 131(5), 645–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney ER, Dumitrescu L, Moore AM, Cambronero FE, De Jager PL, Koran MEI, Petyuk VA, Robinson RAS, Goyal S, Schneider JA, Bennett DA, Jefferson AL, Hohman TJ, 2019. Brain expression of the vascular endothelial growth factor gene family in cognitive aging and alzheimer’s disease. Molecular Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney JP, Gao L, 2015. Proinflammatory Cytokines Increase Vascular Endothelial Growth Factor Expression in Alveolar Epithelial Cells %J Mediators of Inflammation. 2015, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafavi S, Gaiteri C, Sullivan SE, White CC, Tasaki S, Xu J, Taga M, Klein H-U, Patrick E, Komashko V, McCabe C, Smith R, Bradshaw EM, Root DE, Regev A, Yu L, Chibnik LB, Schneider JA, Young-Pearse TL, Bennett DA, De Jager PL, 2018. A molecular network of the aging human brain provides insights into the pathology and cognitive decline of Alzheimer’s disease. Nature Neuroscience 21(6), 811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagineni CN, Kommineni VK, William A, Detrick B, Hooks JJ, 2012. Regulation of VEGF expression in human retinal cells by cytokines: Implications for the role of inflammation in age-related macular degeneration. Journal of Cellular Physiology 227(1), 116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oveisgharan S, Buchman AS, Yu L, Farfel J, Hachinski V, Gaiteri C, De Jager PL, Schneider JA, Bennett DA, 2018. APOE ε2ε4 genotype, incident AD and MCI, cognitive decline, and AD pathology in older adults. 90(24), e2127–e2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NS, Mathura VS, Bachmeier C, Beaulieu-Abdelahad D, Laporte V, Weeks O, Mullan M, Paris D, 2010. Alzheimer’s (3-amyloid peptide blocks vascular endothelial growth factor mediated signaling via direct interaction with VEGFR-2. Journal of Neurochemistry 112(1), 66–76. [DOI] [PubMed] [Google Scholar]
- Patrick E, Taga M, Ergun A, Ng B, Casazza W, Cimpean M, Yung C, Schneider JA, Bennett DA, Gaiteri C, Jager PLD, Bradshaw EM, Mostafavi S, 2019. Deconvolving the contributions of cell-type heterogeneity on cortical gene expression. bioRxiv, 566307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirttilä T, Soininen H, Heinonen O, Lehtimaki T, Bogdanovic N, Paljärvi L, Kosunen O, Winblad B, Riekkinen P, Wisniewski HM, Mehta PD, 1996. Apolipoprotein E (apoE) levels in brains from Alzheimer disease patients and controls. Brain Research 722(1), 71–77. [DOI] [PubMed] [Google Scholar]
- Religa P, Cao R, Religa D, Xue Y, Bogdanovic N, Westaway D, Marti HH, Winblad B, Cao Y, 2013. VEGF significantly restores impaired memory behavior in Alzheimer’s mice by improvement of vascular survival. Scientific reports 3, 2053–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon-Zimri S GM, Barhum Y, Luz I, Boehm-Cagan A, Liraz O, Ben-Zur T, Offen D, Michaelson DM, 2016. Reversal of ApoE4-Driven Brain Pathology by Vascular Endothelial Growth Factor Treatment. J Alzheimers Dis. 53(4), 1443–1458. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL, Bennett DA, 2007. Subcortical infarcts, Alzheimer’s disease pathology, and memory function in older persons. 62(1), 59–66. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Wilson RS, Cochran EJ, Bienias JL, Arnold SE, Evans DA, Bennett DA, 2003. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology 60(7), 1082. [DOI] [PubMed] [Google Scholar]
- Serrano-Pozo AG, John H, 2019. Is Alzheimer’s Disease Risk Modifable? Journal of Alzheimer’s Disease 67(3), 795–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M, 2011. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti-and Pro-Angiogenic Therapies. Genes Cancer 2(12), 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M, 2014. VEGF-VEGFR Signals in Health and Disease. Biomol Ther (Seoul) 22(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JW, Madsen JR, 2018. VEGF Signaling in Neurological Disorders. 19(1), 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M, 1998. Neuropilin-1 Is Expressed by Endothelial and Tumor Cells as an Isoform-Specific Receptor for Vascular Endothelial Growth Factor. Cell 92(6), 735–745. [DOI] [PubMed] [Google Scholar]
- Spuch C, Antequera D, Portero A, Orive G, Hernández RM, Molina JA, Bermejo-Pareja F, Pedraz JL, Carro E, 2010. The effect of encapsulated VEGF-secreting cells on brain amyloid load and behavioral impairment in a mouse model of Alzheimer’s disease. Biomaterials 31(21), 5608–5618. [DOI] [PubMed] [Google Scholar]
- Storkebaum E, Carmeliet P, 2004. VEGF: a critical player in neurodegeneration. The Journal of clinical investigation 113(1), 14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai LM, Thomas R, Marottoli FM, Koster KP, Kanekiyo T, Morris AWJ, Bu G, 2016. The role of APOE in cerebrovascular dysfunction. Acta neuropathologica 131(5), 709–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkowski E, Issa R, Sjögren M, Wallin A, Blennow K, Tarkowski A, Kumar P, 2002. Increased intrathecal levels of the angiogenic factors VEGF and TGF-β in Alzheimer’s disease and vascular dementia. Neurobiology of Aging 23(2), 237–243. [DOI] [PubMed] [Google Scholar]
- Uccelli Andrea WT, Valente Paoloa, Di Maggio Nunzia, Pellegrino Matteo, Gürke Lorenz, Banfi Andrea, Gianni-Barrera Roberto, 2019. Vascular endothelial growth factor biology for regenerative angiogenesis. Swiss Med Wkly 149. [DOI] [PubMed] [Google Scholar]
- Wang M, Beckmann ND, Roussos P, Wang E, Zhou X, Wang Q, Ming C, Neff R, Ma W, Fullard JF, Hauberg ME, Bendl J, Peters MA, Logsdon B, Wang P, Mahajan M, Mangravite LM, Dammer EB, Duong DM, Lah JJ, Seyfried NT, Levey AI, Buxbaum JD, Ehrlich M, Gandy S, Katsel P, Haroutunian V, Schadt E, Zhang B, 2018. The Mount Sinai cohort of large-scale genomic, transcriptomic and proteomic data in Alzheimer’s disease. Sci Data 5, 180185–180185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A, Crean S, Mercaldi CJ, Collins JM, Boyd D, Cook MN, Arrighi HM, 2012. Prevalence of Apolipoprotein E4 Genotype and Homozygotes (APOE e4/4) among Patients Diagnosed with Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology 38(1), 1–17. [DOI] [PubMed] [Google Scholar]
- Wu L, Zhang X, Zhao L, 2018. Human ApoE Isoforms Differentially Modulate Brain Glucose and Ketone Body Metabolism: Implications for Alzheimer’s Disease Risk Reduction and Early Intervention. 38(30), 6665–6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacchigna S, Lambrechts D, Carmeliet P, 2008. Neurovascular signalling defects in neurodegeneration. Nature Reviews Neuroscience 9, 169. [DOI] [PubMed] [Google Scholar]
- Zhang S, Yu H, Zhang L, 2010. Role of vascular endothelial growth factor receptor-3/Flt-4 in early-stage cervical cancer. Oncology letters 1(3), 453–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.