Abstract
Breathing practices are often incorporated into treatments for tobacco dependence, but there is little direct research testing the efficacy of breathing practices. This study examined the effects of a mindfulness-based yogic breathing intervention (MB) versus active treatment (Cognitive Strategy; CS) and no-treatment (NT) control groups on craving, affect, withdrawal, and smoking behavior. Smokers (N = 60; 50% female; 83% African American) were randomized to receive 20 minutes of MB, CS, or NT. Participants completed self-report measures before and after the manipulation and then took part in a 50-minute smoking choice procedure. Afterwards, participants were advised to use the techniques they learned and self-monitor smoking for 24 hours. They received three reminder text messages and returned to the lab the following day. MB and CS were more effective than NT in decreasing craving to smoke and perceived nicotine withdrawal. MB, but not CS, was more effective than NT in reducing negative affect. MB reduced the risk of smoking by more than two-fold relative to both CS and NT during the smoking choice procedure. Participants in the MB condition smoked fewer cigarettes than those in the CS and NT conditions in the 24 hours following the manipulation. There were no differential effects of the manipulations on state mindfulness or positive affect. Mindful yogic breathing appears to be particularly effective in alleviating the acute negative effects of smoking abstinence and decreasing smoking behavior. Mindful breathing techniques are safe, simple, and cost-effective strategies that deserve additional research attention, especially among underserved populations of smokers.
Keywords: Smoking Cessation, Yogic Breathing, Mindfulness, Craving, Negative Affect
Cigarette smoking remains the leading cause of preventable death, disease, and disability in the United States (U.S. Department of Health and Human Services, 2014). Although a majority of adult smokers want to quit smoking and many try to do so, cessation rates remain low (Babb, Malarcher, Schauer, Asman, & Jamal, 2017). Aversive nicotine withdrawal symptoms, including craving, irritability, negative affect, and difficulty concentrating are prominent in the early stages of smoking abstinence (Hughes, 2007a,b; Welsch et al., 1999). A better understanding of effective strategies for actively coping with such aversive experiences is warranted, as individuals who use coping strategies during smoking cessation attempts have greater success (e.g., Fiore et al., 2008; Hall, Rugg, Tunstall, & Jones, 1984; O’Connell, Hosein, Schwartz, & Leibowitz, 2007).
Breathing practices may be particularly effective strategies for smoking cessation. Widely used and publicly available smoking cessation programs recommend “taking deep breaths” as a coping strategy during cessation (e.g., American Lung Association, 2009; Fiore et al., 2008; Mayo Clinic, 2016; U.S. National Cancer Institute, 2013). Moreover, breathing is one of the most commonly reported useful coping strategies among smokers attempting abstinence, especially during the early stages of abstinence (O’Connell, Fears, Cook, Gerkovich, Zechmann, 1991). Paying attention to one’s breath or mindful breathing is also a core component of mindfulness-based approaches (e.g., Mindfulness Meditation, Mindfulness-Based Stress Reduction [MBSR], Kabat-Zinn, 1994), and yoga practices, which also may involve various controlled breathing exercises (Brown & Gerbarg, 2005, 2009; Khanna and Greeson, 2013). The focus of mindful breathing is paying attention to the experience of breathing and gently bring attention back to the breath, irrespective of the breathing technique. This focus on breathing is a way of anchoring attention to present-moment experiences (Kabat-Zinn, 1990; 1994).
While there has been little controlled research directly investigating breathing practices and smoking, there are various possible explanations for why breathing practices may decrease one’s desire to smoke and improve abstinence outcomes. Various types of mindfulness meditation, deep breathing, and yogic breathing practices (e.g., Ujjayi breathing) have been shown to reduce stress, anxiety, depression, and other negative emotional states (reviewed in Jerath, Crawford, Barnes & Harden, 2015), and negative emotional states appear to play a central role in tobacco withdrawal, smoking behavior, and cessation outcomes (Leventhal, Piper, Japuntich, Baker, & Cook, 2014; Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; Kassel, Stroud, & Paronis, 2003). Such breathing practices have also been shown to reduce physiological arousal, increase parasympathetic activity, and increase GABA levels (i.e., a major inhibitory neurotransmitter), which may inhibit stress and negative emotions (Brown & Gerbarg, 2005, Busch et al., 2012; Jerath, Edry, Barnes, & Jerath, 2006; Jerath, Crawford, Barnes & Harden, 2015; Streeter et al., 2007). Furthermore, deep breathing may reduce smoking urge by mimicking actions associated with inhaling cigarette smoke, serving as a distracting activity (Shahab, Sarkar & West, 2013), or producing a relaxation response similar to smoking (Dai & Sharma, 2014). Mindful attention to breath is hypothesized to enhance self-regulation (a defining feature of addiction; Sayette and Griffin, 2011) via various mechanisms including greater attentional control and body awareness (Hölzel et al., 2011; Malinowski, 2013). Mindfulness skills in general have been hypothesized to help smokers quit by learning to tolerate unpleasant withdrawal symptoms and learning to observe but not react to smoking-related cues and feelings of stress or discomfort (Witkiewitz, Lustyk, & Bowen, 2013).
There has been a significant amount of research investigating mindfulness-based treatments for smoking and other addictions, which typically involve core practices of MBSR, including mindful breathing, as well as specific strategies for coping with cravings and high-risk situations (e.g., urge surfing; Bowen, Chawla, & Marlatt, 2010). Mindfulness-based interventions have been shown to produce higher smoking abstinence rates than standard or sham treatments (e.g., Brewer et al., 2011; de Souza et al., 2015; Oikonomou, Arvanitis, & Sokolove, 2017; Ruscio, Muench, Brede, & Waters, 2016; but also see Maglione et al., 2017) and quitline-delivered treatment (Davis et al., 2014), as well as higher rates of lapse recovery (Vidrine et al., 2016). Decreased cravings, stress, and negative affect (e.g., Davis, Manley, Goldberg, Smith, & Jorenby, 2014; de Souza et al., 2015; Ruscio et al., 2016; Spears et al., 2017), and reduced neural reactivity to stress and smoking-related cues after mindfulness training have also been documented (Janes et al., 2019; Kober, Brewer, Height, & Sinha, 2017). Prior studies have also tested the efficacy of specific mindfulness components (Serfaty, Gale, Beadman, Froeliger, & Kamboj, 2018). For example, 10 minutes of practicing the body scan (mindful attention to breath and other areas of the body) has been shown to reduce craving and withdrawal symptoms (Cropley, Ussher, & Charitou, 2007; Ussher, Cropley, Playle, Mohidin, & West, 2009) and increase state mindfulness (Luberto and Mcleish, 2018) compared to no treatment control conditions. While these clinical and laboratory studies demonstrate the potential effectiveness of mindfulness-based treatments for smoking, the independent effects of mindful breathing (when not combined with other mindfulness practices like the body scan) on smoking behavior is unknown.
It has also been suggested that yoga may aid smoking cessation and some mindfulness-based treatments for smoking cessation have incorporated yoga practices. While there are various types of practices (e.g., Hatha, Bikrim), yoga generally encompasses breathing techniques, postures, strengthening exercises, mindfulness meditation, and sometimes spiritual teachings. Yoga and mindfulness share common elements including mindful awareness of experiences and emotions without reacting, as well as breath and body awareness (Khanna & Greeson, 2013). Controlled studies have demonstrated that, compared to no treatment, Hatha yoga decreases smoking cravings (Elibero, Janse Van Rensburg, & Drobes, 2011), and that Vinyasa yoga improves abstinence rates when added to cognitive behavior therapy for smoking cessation (Bock et al., 2012). While there have been other trials of yoga for smoking cessation reported in the literature (reviewed in Khanna & Greeson, 2013; Kuppili, Parmar, Gupta, & Balhara, 2018; Sarkar & Varshney, 2017), well controlled studies are lacking, and individual components including yogic breathing practices have not been examined with just one exception (Shahab et al., 2013).
Despite the common use of breathing strategies in a wide array of treatments for smoking cessation, just two prior studies have directly tested the effects of breathing practices among abstaining smokers. McClernon, West and Rose (2004) reported that controlled breathing exercises (5 deep breaths every 30 minutes for 4 hours) produced lower levels of craving and negative affect compared to no-treatment among overnight abstinent smokers. Shahab and colleagues (2013) found that a controlled yogic breathing exercise (10 minutes of diaphragmatic breathing and alternate nostril breathing with the assistance of a trainer) produced greater reductions in smoking craving, but not other withdrawal symptoms, compared to a 10-minute video about yogic breathing. There were no differences in craving or withdrawal between the groups at the 24-hour follow-up; albeit, the authors noted low out-of-session adherence to the breathing instructions.
The present study investigated the effects of a yogic breathing technique (i.e., Ujjayi breathing or “ocean breath”) along with explicit mindful attention to breath instructions on indices of smoking motivation and actual smoking behavior in a controlled laboratory setting. This mindful breathing strategy was compared to a cognitive coping strategy (thinking about the long-term consequences of smoking; CS) and no treatment (NT) control. A cognitive strategy was chosen as an active control condition as cognitive coping strategies are associated with reduced craving and relapse among smokers (Bliss, Garvey, Heinold, & Hitchcock, 1989; Bliss Garvey, & Ward, 1999; Kober, Kross, Mischel, Hart, & Ochsner, 2010; O’Connell et al. 2007; Shiffman, Paty, Gnys, Kassel, & Hickcox, 1996) and smokers report using cognitive strategies to overcome cravings (Glasgow, Klesges, Mizes, & Pechacek, 1985; O’Connell et al., 1991). Including an active control group in addition to a no-treatment control group allows for a more rigorous test of the effects of mindful yogic breathing. Smoking craving, withdrawal, and negative affect were assessed as these subjective outcomes have been reliably shown to predict smoking behavior and relapse (e.g., Kenford et al., 2002; Piasecki, Jorenby, Smith, Fiore, & Baker, 2003). State mindfulness was assessed as it has been shown to increase after mindfulness interventions (Luberto & Mcleish, 2018; Ruscio et al., 2016; Tanay & Bernstein, 2013). Smoking behavior was assessed using a 50-minute smoking versus money choice task followed by 24 hours of smoking self-monitoring outside of the laboratory.
It was hypothesized that compared to both CS and NT, MB would produce: a) greater reductions in smoking craving, withdrawal, and negative affect; b) greater increases in state mindfulness; c) lower risk of smoking during the choice task; and d) lower smoking rate 24 hours post-manipulation.
Method
Participants
Smokers were recruited from the local Washington DC metro area using print and web-based advertisements. Individuals were eligible to enroll based on the following: a) ages 18–65; b) able to read and write in English and use a computer, c) smoke at least five cigarettes per day for at least the past year, and d) not currently seeking smoking cessation treatment. Individuals were excluded if they reported pregnancy, smoking-related health problems, and/or the use of smoking cessation pharmacotherapies. One hundred and thirteen individuals were screened by phone. Eighteen individuals were ineligible due to insufficient smoking rate or duration (n = 16), health problems (n = 1), or seeking treatment for smoking (n = 1), and 30 were not interested or not scheduled because they couldn’t be reached or had scheduling conflicts. Sixty-five individuals were scheduled and 62 attended the first session. One participant was ineligible upon arrival based on an elevated CO reading and one dropped out prior to randomization resulting in a final sample of 60 smokers (30 male and 30 female). Participants had a mean age of 40.38 (SD = 12.79) years and 12.7 years of education (SD = 2.48). Participants had a mean FTND score of 4.63 (SD = 1.97) and smoked 12.36 (SD = 6.96) cigarettes per day for the past 19.51 (SD = 12.00) years. Race was reported as follows: 83% African-American, 8% Caucasian, 3% Asian, and 6% other. Three percent identified as Hispanic. One-third of participants were employed, with 45% reporting an average annual income of $10,000 or less. Eighty-eight percent smoked menthol cigarettes. This study was approved by the Institutional Review Board.
Procedures
During phone screening participants were told that the purpose of the study was to examine “factors associated with smoking behavior” and would require two visits to the laboratory on two consecutive days. Interested and eligible participants were scheduled and instructed to abstain from smoking for 12 hours prior to the first session and bring their preferred brand of cigarettes.
Session 1.
The experimental session took place in a room that contained a desk, chairs, and desktop computer. Participants provided informed consent, reported the time of their last cigarette, and provided a breath sample to assess carbon monoxide (CO) to validate 12 hours of abstinence (cut-off <8ppm). Participants then completed baseline measures as shown in Table 1. Cigarette packs were collected and placed in view of participants for the remainder of the session. Based on random assignment, participants then received one of three experimental manipulations lasting approximately 20 minutes: 1) practicing the mindful breathing exercise (MB, n = 20), 2) thinking about the long-term consequences associated with regular smoking (CS, n = 20), or 3) waiting in the room without distractions (NT, n = 20). Randomization was determined using a web-based randomization tool (i.e., Research Randomizer) and was stratified by gender.
Table 1.
Baseline Sample Characteristics
| MB (n = 20) Mean (SD) |
CS (n = 20) Mean (SD) |
NT (n = 20) Mean (SD) |
|
|---|---|---|---|
| Age | 39.55 (12.78) | 40.65 (13.58) | 40.95 (12.61) |
| Sex (% female) | 50% | 50% | 50% |
| Race (% Black/African American) | 80% | 85% | 85% |
| Years of Education | 12.00 (2.20) | 13.45 (2.52) | 12.65 (2.60) |
| Income (% < $10,000) | 60% | 40% | 35% |
| Cigarettes per day | 12.25 (8.44) | 12.70 (7.29) | 11.83 (5.07) |
| Years of smoking | 18.58 (10.21) | 20.05 (13.84) | 19.90 (12.25) |
| FTND | 4.25 (2.00) | 5.10 (2.20) | 4.55 (1.70) |
| Prior serious quit attempts | 5.30 (6.78) | 2.82 (2.58) | 3.41 (2.65) |
| Trait Mindfulness (FFMQ) | 3.56 (.51) | 3.33 (.34) | 3.60 (.46) |
| State Mindfulness Total (SMS) | 3.73 (.92) | 3.83 (.60) | 3.88 (.70) |
| Contemplation Ladder | 6.40 (2.39) | 5.70 (3.39) | 6.15 (2.84) |
| Craving (URS) | 6.73 (2.04) | 7.01 (2.19) | 6.06 (2.02) |
| Withdrawal (single-item) | 5.50 (3.07) | 5.55 (2.65) | 4.10 (2.45) |
| Withdrawal (MTWQ) | 1.34 (.84) | 1.30 (1.02) | 1.30 (.94) |
| Negative Affect (PANAS) | 1.64 (.57) | 1.60 (.56) | 1.52 (.68) |
| Positive Affect (PANAS) | 3.03 (.96) | 2.57 (.80) | 2.53 (1.08) |
Note. There were no baseline differences among the groups on any of the variables. MB = Mindful Breathing, CS = Cognitive Strategy, NT = No Treatment, FTND = Fagerström Test for Nicotine Dependence, FFMQ = Five Facet Mindfulness Questionnaire, SMS = State Mindfulness Questionnaire, URS = Urge Rating Scale, MTWQ = Minnesota Tobacco Withdrawal Questionnaire, PANAS = Positive and Negative Affect Schedule
In the MB condition, participants were instructed by the researcher to sit in a comfortable position and close their eyes. Participants were told, “In this technique we breathe consciously, paying attention to the flow of our breath throughout our body. We also sit consciously with our back straight, feet flat on the ground and hands resting flat on our knees or clasped in our lap. Our breaths are steady and controlled.” Participants were shown to take breaths through the nose and to contract the back of one’s throat to make an “ocean” sound. This is consistent with ujjayi breathing, which is a slow diaphragmatic breath that involves gentle contraction of the throat muscles to create resistance and has been associated with increased physical and mental calmness (Brown & Gerbarg, 2009; Cappo & Holmes, 1984). Participants were instructed to first concentrate on the natural inhalations and exhalations of their breath. Then they were instructed through a series of progressive steps and then repetitions to inhale deeply through the nose, expand the belly and ribcage with air, and exhale through the nose. They were told to gently and nonjudgmentally bring their attention back to the breath whenever their mind wandered.
In the CS condition, participants were told, “We are aware that there are many long-term health consequences associated with smoking cigarettes. For example, we know that long-term smoking increases the risk of cardiovascular disease, cancer, shortness of breath, emphysema and so on. In this portion of the study, we would like to invite you to actively think about the longterm health consequences of smoking.” Participants were instructed to sit and breathe as they normally would. Then they were instructed to close their eyes and imagine the long-term health consequences of smoking. They were told to gently bring their attention back to the purpose of this activity whenever their mind wandered.
In both the MB and CS conditions, researchers spent 5 minutes instructing and practicing the exercises with the participants and then provided 15 minutes for participants to do the exercises. A Smartphone was programmed to make a soft gong-like sound every 30 seconds as a gentle reminder for participants to bring their attention back to the exercises.
In NT, participants were instructed to wait in the room for the researcher to come back and to refrain from using any distractors (e.g., cell phones).
Afterwards all participants completed post-manipulation measures of craving, affect, withdrawal, and state mindfulness. Participants then took part in a smoking-versus-money choice procedure based on a laboratory model of smoking lapse behavior (McKee, Krishnan-Sarin, Shi, Mase, & O’Malley, 2006; McKee, Weinberger, Shi, Tetrault, & Coppola, 2012). Participants were told that they could choose to smoke or earn $.50 for each five-minute period they chose to delay smoking. The participant’s pack of cigarettes, a lighter, and an ashtray were present. A timer sounded every 5 minutes, which alerted participants to make a choice between smoking a cigarette or earning $0.50 for 50 minutes. Participants in the MB and CS conditions were encouraged to use the practices that they learned during this period. The amount of time before smoking was initiated was recorded by the research assistant who observed through a one-way mirror.
Before leaving the laboratory, participants were encouraged to use the practices they learned for the next 24 hours but were not given any instructions to change their smoking. They were provided self-monitoring forms to record each time they smoked. To increase out-of-session compliance with the exercises, participants were informed that they would receive three text messages (two during the evening of the first day and one the following morning) as reminders to engage in the practices. The MB text messages stated: “This is a reminder to engage in the breathing exercises you practiced in the lab.” The CS text messages stated “This is a reminder to think about the long-term health consequences of smoking like you did in the lab.”
Session 2.
Fifty-seven participants returned to the laboratory 24 hours later (2 participants in MB and 1 participant in CS dropped out prior to session 2). They were given a CO breath test, reported the time of their last cigarette, submitted self-monitoring forms, and completed measures of smoking craving, affect, and withdrawal. Afterwards they completed an experiment evaluation form and were paid ($50 plus up to an additional $5 based on the smoking choice task) and debriefed about the purpose of the study.
Measures
Smoking Abstinence.
Smoking abstinence was biochemically confirmed based on a breath CO reading of < 8ppm using the Bedfont Micro III Smokerlyzer (Kent, UK) to capture expired carbon monoxide (CO) levels in parts per million (ppm).
Demographics and Smoking History Questionnaire.
This measure developed in our laboratory assesses demographic variables including age, gender, race, household income, and educational level. Smoking-related variables included the number of prior quit attempts, smoking rate, years smoked, and cigarette preferences. Participants were also asked to rate their intention to quit smoking from 0- “No thought of quitting” to 10- “Taking action to quit” (Contemplation Ladder; Biener & Abrams, 1991).
Fagerström Test of Nicotine Dependence (FTND, Heatherton, Kozlowski, Frecker, & Fagerström, 1991).
This widely used measure of nicotine dependence consists of six items (e.g., number of cigarettes per day; how soon after waking do you smoke) with the total score ranging from 0–10 (Cronbach’s alpha = .53).
Five-Facet Mindfulness Questionnaire (FFMQ).
This is a 39-item self-report measure of dispositional mindfulness (Baer, Smith, Hopkins, Krietemeyer, & Toney, 2006). Participants rate how often each item describes them from 1 (never or rarely true) to 5 (very often or always true). The FFMQ yields five subscales: 1) Observing (e.g., “I pay attention to sensations, such as the wind in my hair or the sun on my face”), 2) Describing (e.g., “I’m good at findings words to describe my feelings”), 3) Acting with Awareness (e.g., “I rush through activities without really being attentive to them [reverse-scored]), 4) Nonjudging (e.g., “I think some of my emotions are bad or inappropriate and I shouldn’t feel them” [reverse-scored]), and 5) Nonreactivity (e.g., “I perceive my feelings and emotions without having to react to them”). In this study Cronbach’s alpha for the subscales ranged from .76 – .89.
State Mindfulness Scale (SMS; Tanay & Bernstein, 2013).
This is a 21-item self-report measure of state mindfulness ranked on a 5-point Likert scale that load onto two factors: Mind (e.g., “I noticed pleasant and unpleasant thoughts;” “I was aware of what was going on in my mind”) and Body (e.g., “I noticed some pleasant and unpleasant physical sensations;” “I clearly physically felt what was going on in my body”), in addition to a higher-order state mindfulness total score (average Cronbach’s alpha = .95).
Urge Rating Scale (URS).
This three-item self-report measure assessed participants’ cravings, wants, and desires to smoke on a scale from 1 (none at all) to 10 (greatest ever experienced). These items have demonstrated adequate reliability and validity in assessing smoking craving (Kozlowski, Pillitteri, Sweeney, Whitfield & Graham, 1996). Internal consistency in the present study was high (Cronbach’s alpha = .95).
Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988).
This measure consists of 10 positive affect (e.g., enthusiastic, proud; average Cronbach’s alpha = .95) and 10 negative affect (e.g., upset, distressed; average Cronbach’s alpha = .93) items rated on a scale from 1 (very slightly or not at all) to 5 (extremely).
Minnesota Tobacco Withdrawal Scale (MTWS; Hughes & Hatsukami, 1986).
This measure consists of seven withdrawal symptoms consistent with the DSM-5: angry/irritable/frustrated; anxious/nervous; depressed mood/sad; difficulty concentrating; restless; increased appetite/hungry and insomnia/sleep problems/awakening at night rated on a scale ranging from 0 (none) to 4 (severe). The first 6 items were combined for analyses. The scale had high internal consistency (average Cronbach’s alpha = .89).
Perceived Nicotine Withdrawal.
In this one-item subjective measure of nicotine withdrawal, participants were asked to respond to the question “How much are you experiencing nicotine withdrawal symptoms at this time?” rated on a scale from 0 (Not at all) to 10 (Greatest ever experienced).
Statistical Analyses
All analyses were conducted using SPSS version 24. A series of mixed repeated measures ANOVAs were conducted to test for treatment effects on craving, affect, withdrawal, and state mindfulness. Pre and post scores on the dependent measure were entered as the within-subjects variable (i.e., time), and treatment condition (MB, CS, and NT) was entered as the between-subjects variable. A significant interaction of time and condition indicated a differential effect of the treatment on the dependent measure. Significant omnibus F tests were followed up with post-hoc comparisons. Cox proportional hazards models were run to examine differential effects of the treatment on smoking risk during the choice procedure. Self-reported smoking in the 24 hours between sessions 1 and 2 was analyzed using ANCOVA controlling for baseline smoking rate. An equal number of men and women were assigned to each of the conditions and preliminary analyses were conducted including gender as a between-subjects factor. There were no significant effects based on gender and thus this variable was collapsed for all analyses.
Results
Participant Characteristics
A series of one-way ANOVAs were run to test for possible baseline differences among participants in the three conditions. As shown in Table 1, there were no baseline differences on any of the demographic variables, smoking history variables, trait or state mindfulness, or dependent measures (all p’s > .10).
Treatment Effects on Self-Reported Measures
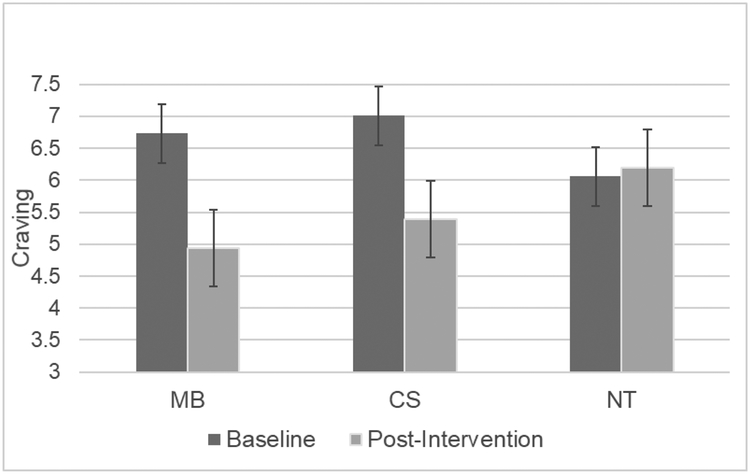
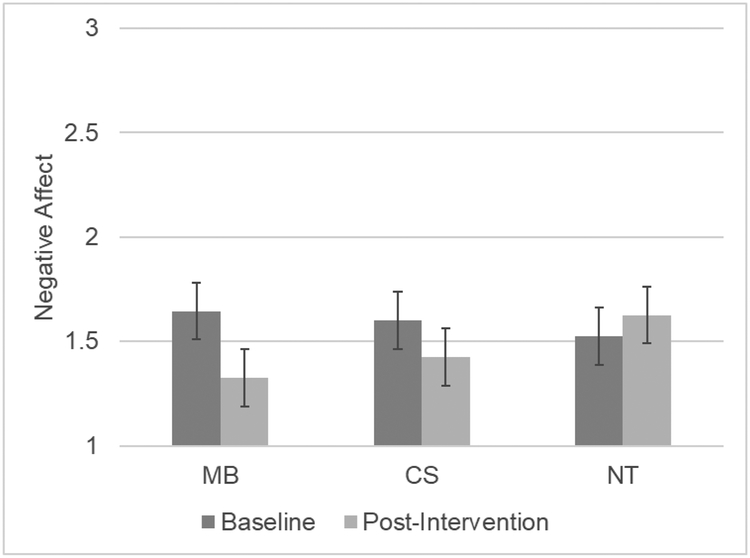
There was a significant time by condition interaction for smoking craving [F(2, 57) = 5.96, p = .004, ηp2 = .173]. Post-hoc comparisons revealed that both MB [F(1, 38) = 12.51, p = .001, ηp2 = .248] and CS [F(1, 38) = 6.85, p = .013, ηp2 = .153] produced greater decreases in craving than NT (see Figure 1). There was also a significant time by condition interaction for negative affect [F(2, 57) = 3.75, p = .030, ηp2 = .116]. As shown in Figure 2, post-hoc comparisons indicated that MB produced greater reductions in negative affect than NT [F(1, 38) = 7.78, p = .008, ηp2 = .170] but CS did not [F(1, 38) = 2.89, p =.098, ηp2 = .071]. There were also effects on the single-item perceived nicotine withdrawal question [F(2, 57) = 5.44, p = .007, ηp2 = .160] with those in the MB group [F(1, 38) = 10.20, p = .003, ηp2 = .212] and CS group [F(1, 38) = 7.91, p = .008, ηp2 = .172] reporting greater decreases in withdrawal than the NT group. There were no time by condition interactions for positive affect or the MTWS. There were no differences between the MB and CS groups on any of the subjective measures. There were no differences among the treatments in changes in overall state mindfulness [F(2, 57) = 1.41, p =.251, ηp2 =.047] or the mind [F(2, 57) = .742, p = .481, ηp2 =.025] or body subscales ([F(2, 57) = 2.57, p = .085, ηp2 = .083].
Figure 1.
Baseline to post-intervention craving change
Figure 2.
Baseline to post-intervention negative affect change
Treatment Effects on Smoking Behavior
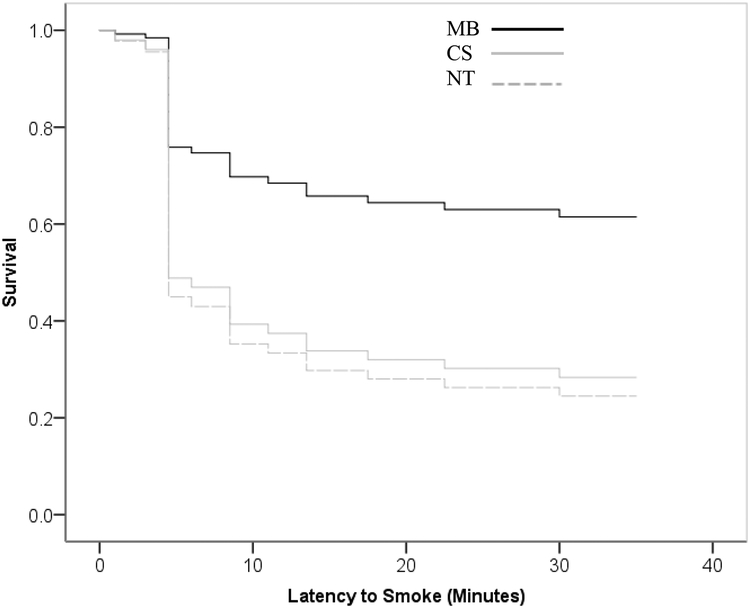
Participants in the MB condition delayed smoking by an average of 34.10 minutes, compared to 18.50 minutes and 18.65 minutes for the CS and NT conditions respectively (or a median of 50 mins, 10 mins, and 5 mins). Sixty percent of participants in the MB condition abstained for the full 50 minutes compared to just 25% of participants in both CS and NT. Cox proportional hazards analysis revealed that MB resulted in a lower risk of smoking relative to both CS and NT. As shown in Figure 3 the estimated risk of smoking for NT was 2.89 times that for MB (1.17 to 7.13 with 95% confidence, Wald = 5.324, HR = 2.89, p = 0.021). The risk of smoking for CS was estimated to be 2.59 times that for MB (1.050 to 6.390 with 95% confidence, Wald = 4.272, p = 0.039). There was no difference between NT and CS conditions.
Figure 3:
Latency to smoke during the smoking choice procedure
Self-monitoring data revealed that the smoking rate in the 24 hours following the manipulation was lowest among individuals in MB. Those in the MB condition smoked significantly fewer cigarettes than those in the CS condition controlling for baseline smoking rate [M = 6.32 (SD = 5.0) vs 10.89 (SD = 6.29), F(1,34) = 7.52, p = .010, ηp2 = .181]. There was no difference between MB and NT and CS and NT.
Discussion
This controlled laboratory study provides evidence for the value of a mindfulness-based yogic breathing strategy in reducing smoking craving, negative affect, and smoking behavior. Relative to NT, both MB and CS were associated with greater reductions in craving and perceived nicotine withdrawal among 12-hr abstinent smokers in the presence of smoking-related cues. However, only MB was superior in reducing negative affect relative to the no treatment control group. Additionally, those in the MB condition had more than a twofold reduction in risk of smoking during the laboratory choice procedure. Only 35% of participants in the MB condition chose to smoke during the choice procedure compared to 75% in the CS and NT groups. Controlling for baseline smoking rate, participants in the MB condition also smoked fewer cigarettes in the 24 hours following the manipulation compared to those in the CS condition. There were no effects of the treatments on state mindfulness, positive affect, or specific withdrawal symptoms measured by the MTWQ.
Our findings are consistent with two previous laboratory studies that have shown beneficial effects of deep and yogic breathing in which craving (McClernon et al., 2004; Shahab et al., 2013) and negative affect (McClernon et al., 2004) were reduced among smokers. The present study adds to these prior findings by demonstrating the effectiveness of 20 minutes of yogic breathing with explicit mindfulness instructions in reducing self-reported craving and negative affect. Consistent with these prior studies there were no effects on other specific withdrawal symptoms (e.g., appetite, concentration, irritability). However, in the present study participants were also asked to rate how much they were experiencing “nicotine withdrawal” and these ratings decreased more in the MB and CS conditions compared to NT. A particular strength of the current study was the inclusion of both active and no treatment control conditions. Moreover, the present study is the first to show that MB was superior to the other conditions in decreasing actual smoking behavior as demonstrated by both the laboratory choice task and self-reported out-of-session smoking.
The breathing exercise tested in the present study was different than the two prior laboratory studies. In McClernon et al.’s (2004) study, participants were instructed to take 5 deep breaths (inhaling for 5 seconds, holding for 2 seconds, and exhaling for 5 seconds) in 30 second intervals every 30 minutes for 4 hours. In Shahab et al.’s (2013) study, participants engaged in 10 minutes of yogic breathing involving a three-part breath and alternate nostril inhalation–exhalation. Neither of these studies explicitly used mindfulness instructions (i.e., focusing one’s attention on breathing and nonjudgmentally bringing attention back to the breath whenever the mind wanders). In the present study participants engaged in a 20-minute mindfulness-based yogic ujjayi breathing exercise. As such this study tested a breathing technique that has elements of deep slow controlled diaphragmatic breathing and mindfulness of breath. While it may be that breathing practices of any sort are quite robust and may have distinct and overlapping mechanisms of effectiveness, future research could evaluate different types of breathing practices during smoking abstinence (e.g., parsing mindful breathing and controlled deep breathing) to isolate the active elements. Future research could also test the ideal dose and timing of breathing as well as the relative effectiveness of ad-lib versus scheduled breathing exercises. Additionally, future research could examine the underlying mechanisms by which various types of breathing are effective for reducing smoking cravings, negative affect, and smoking behavior.
Contrary to predictions, the mindful breathing practice in this study did not produce greater increases in state mindfulness relative to the other conditions. This is inconsistent with a laboratory study by Luberto and Mcleish (2018) who found that a 10-minute mindfulness exercise involving focusing on breath, body, sounds, and thoughts produced increased scores on the State Mindfulness Scale (SMS) relative to a no treatment control group. It is possible that the different interventions may account for different findings. It is also possible that the present study had less power due to the smaller sample size and the three-group design including an active control condition.
Additional limitations of the study should be noted. We recruited a sample of nontreatment seeking smokers and thus findings may not generalize to individuals seeking smoking cessation treatment. However, it should be noted that participants in the sample were long-term smokers (mean = ~ 20 years) who appeared to have interest in quitting and reported a mean of about four prior serious quit attempts. Participants were encouraged to practice the strategies in their own environment for 24 hours and received text message reminders, but they were not given instructions to decrease smoking. Nevertheless, individuals in the MB condition reported smoking fewer cigarettes. However, this finding is based on self-report, which could be prone to biases and inaccuracies. This study is also limited by a short follow-up period and future studies could examine whether breathing strategies are effective over a longer timeframe. Also, we did not ask participants to self-report on how often they used the techniques in the 24 hours post-manipulation. Future studies could include a measure of how often individuals used the skills to determine adherence and if the rate of use predicts outcomes. Furthermore, future research could determine the treatment acceptability of breathing practices relative to other interventions.
Most of our participants were African American, and 45% of the sample reported that their average annual income was $10,000 or less. African American and low-income smokers often have greater difficulty quitting smoking and exhibit disproportionately higher rates of tobacco-related morbidity and mortality compared to Caucasians and those with higher income (U.S. National Cancer Institute, 2017). Mindfulness-based smoking cessation treatment shows promise in low-income (Davis, Goldberg et al., 2014; Davis, Manley et al., 2014) and racially/ethnically diverse samples (Brewer et al., 2011; Vidrine et al., 2016), but these sociodemographic groups have traditionally been underrepresented in mindfulness research (Waldron, Hong, Moskowitz, & Burnett-Zeigler, 2018). The current results suggest that mindful yogic breathing may be useful for promoting smoking cessation in low-income and African American adults, and additional research is warranted with these underserved populations.
In conclusion, the current study suggests that a mindfulness-based yogic deep breathing technique may have promise for decreasing craving, negative affect and smoking behavior. More research is needed to examine the effectiveness of different breathing strategies and underlying mechanisms, the ideal dose and timing, and ways to promote adherence to the breathing practices outside of the laboratory or clinic. Breathing strategies are safe, simple, highly disseminable, and cost-effective treatment approaches that warrant more research attention.
Acknowledgments
This study was funded in part by a Mellon Award from the College of Arts and Sciences at American University. Claire A. Spears is supported by grant number K23AT008442 from the National Center for Complementary and Integrative Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Portions of these data were presented at the 50th Annual Meeting of the Association for Behavioral and Cognitive Therapies, New York, NY.
Contributor Information
Sadaf Lotfalian, Department of Psychology, American University, Washington, D.C.;.
Claire A. Spears, Department of Health Policy & Behavioral Sciences, School of Public Health, Georgia State University, Atlanta, Georgia;
Laura M. Juliano, Department of Psychology, American University, Washington, D.C.
References
- American Lung Association (2009). Freedom From Smoking: The guide to help you quit smoking. Retrieved from https://www.messa.org/Portals/0/PDF/ALA-FFS-A-Guide-to-Help-You-Quit%20Smoking.pdf
- Babb S, Malarcher A, Schauer G, Asman K, & Jamal A (2017). Quitting smoking among adults--United States, 2000–2015. Morbidity and Mortality Weekly Report, 65(52), 1457–1464. 10.15585/mmwr.mm6552a1 [DOI] [PubMed] [Google Scholar]
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L (2006). Using self-report assessment methods to explore facets of mindfulness. Assessment, 13, 27–45. 10.1177/1073191105283504 [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, & Fiore MC (2004). Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review, 111(1), 33. [DOI] [PubMed] [Google Scholar]
- Biener L, & Abrams DB (1991). The Contemplation Ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology, 10, 360 10.1037/0278-6133.10.5.360 [DOI] [PubMed] [Google Scholar]
- Bliss RE, Garvey AJ, Heinold JW, & Hitchcock JL (1989). The influence of situation and coping on relapse crisis outcomes after smoking cessation. Journal of Consulting and Clinical Psychology, 57, 443 10.1037/0022-006X.57.3.443 [DOI] [PubMed] [Google Scholar]
- Bliss RE, Garvey AJ, & Ward KD (1999). Resisting temptations to smoke: Results from within-subjects analyses. Psychology of Addictive Behaviors, 13, 143–151. 10.1037/0893-164X.13.2.143 [DOI] [Google Scholar]
- Bock BC, Fava JL, Gaskins R, Morrow KM, Williams DM, Jennings E, … & Marcus BH (2012). Yoga as a complementary treatment for smoking cessation in women. Journal of Women’s Health, 21(2), 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen S, Chawla N, Marlatt GA (2010). Mindfulness-based relapse prevention for the treatment of substance use disorders: A clinician’s guide. New York, NY: Guilford Press. [Google Scholar]
- Brewer JA, Mallik S, Babuscio TA, Nich C, Johnson HE, Deleone CM, … Rounsaville BJ (2011). Mindfulness training for smoking cessation: Results from a randomized controlled trial. Drug and Alcohol Dependence, 119, 72–80. 10.1016/j.drugalcdep.2011.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RP, & Gerbarg PL (2005). Sudarshan kriya yogic breathing in the treatment of stress, anxiety, and depression: Part I-neurophysiologic model. Journal of Alternative and Complementary Medicine (New York, N.Y.), 11, 189–201. http://doi.org10.1089/acm.2005.11.189 [DOI] [PubMed] [Google Scholar]
- Brown RP, & Gerbarg PL (2009). Yoga breathing, meditation, and longevity. Annals of the New York Academy of Sciences, 1172, 54–62. 10.1111/j.17496632.2009.04394.x [DOI] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR, & Zvolensky MJ (2005). Distress tolerance and early smoking lapse. Clinical Psychology Review, 25(6), 713–733. 10.1016/j.cpr.2005.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch V, Magerl W, Kern U, Haas J, Hajak G, & Eichhammer P (2012). The effect of deep and slow breathing on pain perception, autonomic activity, and mood processing--an experimental study. Pain Medicine, 13, 215–228. 10.1111/j.15264637.2011.01243.x [DOI] [PubMed] [Google Scholar]
- Cropley M, Ussher M, & Charitou E (2007). Acute effects of a guided relaxation routine (body scan) on tobacco withdrawal symptoms and cravings in abstinent smokers. Addiction, 102, 989–993. 10.1111/j.1360-0443.2007.01832.x [DOI] [PubMed] [Google Scholar]
- Dai CL, & Sharma M (2014). Between inhale and exhale: Yoga as an intervention in smoking cessation. Journal of Evidence-based Complementary & Alternative Medicine, 19(2), 144–149. [DOI] [PubMed] [Google Scholar]
- Davis JM, Goldberg SB, Anderson MC, Manley AR, Smith SS, & Baker TB (2014). Randomized trial on mindfulness training for smokers targeted to a disadvantaged population. Substance Use & Misuse, 49, 571–585. 10.3109/10826084.2013.770025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Manley AR, Goldberg SB, Smith SS, & Jorenby DE (2014). Randomized trial comparing mindfulness training for smokers to a matched control. Journal of Substance Abuse Treatment, 47(3), 213–221. 10.1016/j.jsat.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza ICW, de Barros VV, Gomide HP, Miranda TCM, de Paula Menezes V, Kozasa EH, & Noto AR (2015). Mindfulness-based interventions for the treatment of smoking: a systematic literature review. The Journal of Alternative and Complementary Medicine, 21(3), 129–140. [DOI] [PubMed] [Google Scholar]
- Elibero A, Janse Van Rensburg K, & Drobes DJ (2011). Acute effects of aerobic exercise and Hatha yoga on craving to smoke. Nicotine & Tobacco Research, 13(11), 1140–1148. [DOI] [PubMed] [Google Scholar]
- Fiore M, Jaén C, Baker T, Bailey W, Benowitz N, Curry S, … Leitzke C (2008). Treating tobacco use and dependence: 2008 update U.S. Public Health Service Clinical Practice Guideline executive summary. Respiratory Care, 53(9), 1217–1222. Retrieved from http://search.proquest.com/docview/69588144/ [PubMed] [Google Scholar]
- Glasgow RE, Klesges RC, Mizes JS, & Pechacek TF (1985). Quitting smoking: Strategies used and variables associated with success in a stopsmoking contest. Journal of Consulting and Clinical Psychology, 53, 905–912. 10.1037/0022-006X.53.6.905 [DOI] [PubMed] [Google Scholar]
- Hall SM, Rugg D, Tunstall C, & Jones RT (1984). Preventing relapse to cigarette smoking by behavioral skill training. Journal of Consulting and Clinical Psychology, 52, 372–382. 10.1037/0022-006X.52.3.372 [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerström KO (1991). The Fagerströmtest for nicotine dependence: A revision of the Fagerström tolerance questionnaire. British Journal of Addiction, 86, 11–19. 10.1111/j.13600443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- Hughes JR (2007a). Measurement of the effects of abstinence from tobacco: A qualitative review. Psychology of Addictive Behaviors, 21, 127–137. 10.1037/0893-164X.21.2.127 [DOI] [PubMed] [Google Scholar]
- Hughes JR (2007b). Effects of abstinence from tobacco: Valid symptoms and time course. Nicotine & Tobacco Research, 9, 315–327. 10.1080/14622200701188919 [DOI] [PubMed] [Google Scholar]
- Hughes JR, & Hatsukami D (1986). Signs and symptoms of tobacco withdrawal. Archives of general psychiatry, 43(3), 289–294. 10.1001/archpsyc.1986.01800030107013 [DOI] [PubMed] [Google Scholar]
- Janes AC, Datko M, Roy A, Barton B, Druker S, Neal., … Brewer JA (2019). Quitting starts in the brain: A randomized controlled trial of app-based mindfulness shows decreases in neural responses to smoking cues that predict reductions in smoking. Neuropsychoopharmacology, 44, 1631–1638. 10.1038/s41386-019-0403-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerath R, Edry JW, Barnes VA, & Jerath V (2006). Physiology of long pranayamic breathing: neural respiratory elements may provide a mechanism that explains how slow deep breathing shifts the autonomic nervous system. Medical hypotheses, 67(3), 566–571. [DOI] [PubMed] [Google Scholar]
- Jerath R, Crawford MW, Barnes VA, & Harden K (2015). Self-regulation of breathing as a primary treatment for anxiety. Applied psychophysiology and biofeedback, 40(2), 107–115. [DOI] [PubMed] [Google Scholar]
- Hölzel BK, Lazar SW, Gard T, Schuman-Olivier Z, Vago DR, & Ott U (2011). How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspectives on Psychological Science, 6(6), 537–559. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J (1990). Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness. New York: Delacorte Press. [Google Scholar]
- Kabat-Zinn J (1994). Wherever you go. There You Are: Mindfulness Meditation in Everyday Life. New York: Hyperion. [Google Scholar]
- Kassel JD, Stroud LR, & Paronis CA (2003). Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychological Bulletin, 129(2), 270. [DOI] [PubMed] [Google Scholar]
- Kober H, Brewer JA, Height KL, & Sinha R (2017). Neural stress reactivity relates to smoking outcomes and differentiates between mindfulness and cognitive-behavioral treatments. Neuroimage, 151, 4–13. 10.1016/j.neuroimage.2016.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Kross EF, Mischel W, Hart CL, & Ochsner KN (2010). Regulation of craving by cognitive strategies in cigarette smokers. Drug and Alcohol Dependence, 106, 52–55. 10.1016/j.drugalcdep.2009.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski LT, Pillitteri JL, Sweeney CT, Whitfield KE, & Graham JW (1996). Asking questions about urges or cravings for cigarettes. Psychology of Addictive Behaviors, 10, 248 10.1037/0893-164X.10.4.248 [DOI] [Google Scholar]
- Kuppili PP, Parmar A, Gupta A, & Balhara YPS (2018). Role of yoga in management of substance-use disorders: A narrative review. Journal of neurosciences in rural practice, 9(01), 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Piper ME, Japuntich SJ, Baker TB, & Cook JW (2014). Anhedonia, depressed mood, and smoking cessation outcome. Journal of Consulting and Clinical Psychology, 82(1), 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luberto CM, & McLeish AC (2018). The effects of a brief mindfulness exercise on state mindfulness and affective outcomes among adult daily smokers. Addictive behaviors, 77, 73–80. [DOI] [PubMed] [Google Scholar]
- Maglione MA, Maher AR, Ewing B, Colaiaco B, Newberry S, Kandrack R, … & Hempel S (2017). Efficacy of mindfulness meditation for smoking cessation: A systematic review and meta-analysis. Addictive Behaviors, 69, 27–34. [DOI] [PubMed] [Google Scholar]
- Mayo Clinic (2016). Quitting smoking: 10 ways to resist tobacco cravings. Retrieved from https://www.mayoclinic.org/healthy-lifestyle/quit-smoking/in-depth/nicotine-craving/art-20045454?pg=2
- Malinowski P (2013). Neural mechanisms of attentional control in mindfulness meditation. Frontiers in Neuroscience, 7, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Westman EC, & Rose JE (2004). The effects of controlled deep breathing on smoking withdrawal symptoms in dependent smokers. Addictive Behaviors, 29, 765–772. 10.1016/j.addbeh.2004.02.005 [DOI] [PubMed] [Google Scholar]
- McKee SA, Krishnan-Sarin S, Shi J, Mase T, & O’Malley SS (2006). Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology, 189, 201–210. 10.1177/0269881110376694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Weinberger AH, Shi J, Tetrault J, & Coppola S (2012). Developing and validating a human laboratory model to screen medications for smoking cessation. Nicotine & Tobacco Research, 14, 1362–1371. 10.1093/ntr/nts090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell KA, Fears BA, Cook MR, Gerkovich MM, & Zechmann A (1991). Overcoming the urge to smoke: The strategies of long-term abstainers and late relapsers. Psychology of Addictive Behaviors, 5, 1 10.1037/h0080579 [DOI] [Google Scholar]
- O’Connell KA, Hosein VL, Schwartz JE, & Leibowitz RQ (2007). How does coping help people resist lapses during smoking cessation? Health Psychology, 26, 77 10.1037/0278-6133.26.1.77 [DOI] [PubMed] [Google Scholar]
- Oikonomou MT, Arvanitis M, & Sokolove RL (2017). Mindfulness training for smoking cessation: A meta-analysis of randomized-controlled trials. Journal of Health Psychology, 22, 1841–1850. 10.1177/1359105316637667 [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Jorenby DE, Smith SS, Fiore MC, & Baker TB (2003). Smoking withdrawal dynamics: I. Abstinence distress in lapsers and abstainers. Journal of Abnormal Psychology, 112(1), 3–13. [PubMed] [Google Scholar]
- Ruscio AC, Muench C, Brede E, MacIntyre J, & Waters AJ (2016). Administration and assessment of brief mindfulness practice in the field: A feasibility study using ecological momentary assessment. Mindfulness, 7(4), 988–999. 10.1007/s12671-016-0538-4 [DOI] [Google Scholar]
- Ruscio AC, Muench C, Brede E, & Waters AJ (2016). Effect of brief mindfulness practice on self-reported affect, craving, and smoking: A pilot randomized controlled trial using ecological momentary assessment. Nicotine & Tobacco Research, 18(1), 64–73. [DOI] [PubMed] [Google Scholar]
- Sarkar S, & Varshney M (2017). Yoga and substance use disorders: A narrative review. Asian Journal of Psychiatry, 25, 191–196. doi: 10.1093/ntr/ntv074 [DOI] [PubMed] [Google Scholar]
- Sayette MA, & Griffin KM (2011). Self-regulatory failure and addiction In Vohs KD & Baumeister RF (Eds.), Handbook of self-regulation: Research, theory, and applications (pp. 505–521). New York, NY, US: Guilford Press. [Google Scholar]
- Serfaty S, Gale G, Beadman M, Froeliger B, & Kamboj SK (2018). Mindfulness, acceptance and defusion strategies in smokers: a systematic review of laboratory studies. Mindfulness, 9(1), 44–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahab L, Sarkar BK, & West R (2013). The acute effects of yogic breathing exercises on craving and withdrawal symptoms in abstaining smokers. Psychopharmacology, 225, 875–882. 10.1007/s00213-012-2876-9 [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, & Hickcox M (1996). First lapses to smoking: Within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology, 64, 366–379. 10.1037/0022-006X.64.2.366 [DOI] [PubMed] [Google Scholar]
- Spears CA, Hedeker D, Li L, Wu C, Anderson NK, Houchins SC, … & Waters AJ (2017). Mechanisms underlying mindfulness-based addiction treatment versus cognitive behavioral therapy and usual care for smoking cessation. Journal of Consulting and Clinical Psychology, 85, 1029 10.1037/ccp0000229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter CC, Jensen JE, Perlmutter RM, Cabral HJ, Tian H, Terhune DB, … & Renshaw PF (2007). Yoga Asana sessions increase brain GABA levels: a pilot study. The Journal of Alternative and Complementary Medicine, 13(4), 419–426. [DOI] [PubMed] [Google Scholar]
- Tanay G & Bernstein A (2013). State Mindfulness Scale (SMS): Development and Initial Validation. Psychological Assessment, 25(4), 1286–1299. 10.1037/a0034044 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (2014). The health consequences of smoking—50 years of progress: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 17. [Google Scholar]
- U.S. National Cancer Institute (2013). A quit-smoking guide for people 50 and older. Retrieved April 5, 2019, from https://smokefree.gov/sites/default/files/pdf/clear-horizonsaccessible.pdf
- U.S. National Cancer Institute. (2017). A sociological approach to addressing tobacco-related health disparities. National Cancer Institute Tobacco Control Monograph 22. NIH Publication No. 17-CA-8035A. U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. [Google Scholar]
- Ussher M, Cropley M, Playle S, Mohidin R, & West R (2009). Effect of isometric exercise and body scanning on cigarette cravings and withdrawal symptoms. Addiction, 104, 1251–1257. 10.1111/j.1360-0443.2009.02605.x [DOI] [PubMed] [Google Scholar]
- Vidrine JI, Spears CA, Heppner WL, Reitzel LR, Marcus MT, Cinciripini PM, … Wetter DW (2016). Efficacy of mindfulness-based addiction treatment (MBAT) for smoking cessation and lapse recovery: A randomized clinical trial. Journal of Consulting Clinical Psychology, 84, 824–838. 10.1037/ccp0000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron EM, Hong S, Moskowitz JT, & Burnett-Zeigler I (2018). A Systematic Review of the Demographic Characteristics of Participants in US-Based Randomized Controlled Trials of Mindfulness-Based Interventions. Mindfulness, 9, 1671–1692. 10.1007/s12671-018-0920-5 [DOI] [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of personality and social psychology, 54, 1063 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, & Baker TB (1999) Development and validation of the Wisconsin Smoking Withdrawal Scale. Experimental and Clinical Psychopharmacology, 7, 354–61. 10.1037/1064-1297.7.4.354 [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Lustyk MKB, & Bowen S (2013). Retraining the addicted brain: A review of hypothesized neurobiological mechanisms of mindfulness-based relapse prevention. Psychology of Addictive Behaviors, 27(2), 351. [DOI] [PMC free article] [PubMed] [Google Scholar]