Abstract
Chemotherapy-induced peripheral neuropathy (henceforth, neuropathy) is often dose limiting and is generally managed by empirical dose modifications. We aimed to (1) identify an early time point that is predictive of future neuropathy using a patient-reported outcome and (2) propose a dose-adjustment algorithm based on simulated data to manage neuropathy. In previous work, a dose-neuropathy model was developed using dosing and patient-reported outcome data from Cancer and Leukemia Group B 40502 (Alliance), a randomized phase III trial of paclitaxel, nanoparticle albumin–bound paclitaxel or ixabepilone as first-line chemotherapy for locally recurrent or metastatic breast cancer. In the current work, an early time point that is predictive of the future severity of neuropathy was identified based on predictive accuracy of the model. Using the early data and model parameters, simulations were conducted to propose a dose-adjustment algorithm for the prospective management of neuropathy in individual patients. The end of the first 3 cycles (12 weeks) was identified as the early time point based on a predictive accuracy of 75% for the neuropathy score after 6 cycles. For paclitaxel, nanoparticle albumin–bound paclitaxel, and ixabepilone, simulations with the proposed dose-adjustment algorithm resulted in 61%, 48%, and 35% fewer patients, respectively, with neuropathy score ≥8 after 6 cycles compared to no dose adjustment. We conclude that early patient-reported outcome data on neuropathy can be used to guide dose adjustments in individual patients that reduce the severity of future neuropathy. Prospective validation of this approach should be undertaken in future studies.
Keywords: chemotherapy, breast cancer, modeling and simulation, neuropathy, personalized
Chemotherapy-induced peripheral neuropathy (henceforth, neuropathy) is a dose-limiting toxicity caused by several chemotherapeutic agents, such as platinum drugs, taxanes, and vinca alkaloids.1 Neuropathy decreases quality of life, and may impact survival by necessitating the discontinuation of effective therapies.2 A recent study showed that 47% of women had symptoms of neuropathy many years after treatment, along with functional deficits, disability, and a higher incidence of falls.3 There are no effective therapies for the prevention of neuropathy and only a single therapy (duloxetine) with modest benefits for painful neuropathy.4,5 Because there are no reliable factors available to predict at-risk patients,6 neuropathy is usually managed by dose reduction and/or interruption once it develops, at the discretion of the treating physician.
There is substantial interpatient variability in the timing of onset and severity of neuropathy.6 Therefore, there is a need for dose individualization to manage neuropathy. Some researchers have postulated that early recognition and management through dose interruption and/or reduction might prevent discontinuation of therapy.7 We propose to use a patient’s early neuropathy data to predict the future severity of neuropathy in that patient, so that oncologists can make dose adjustments prospectively to mitigate future neuropathy.
In previous work,8 we developed a model quantifying the relationship between dosing history and longitudinal neuropathy score using data from Cancer and Leukemia Group B (CALGB) 40502, a randomized phase III trial of paclitaxel, nanoparticle albumin–bound (nab)-paclitaxel or ixabepilone as first-line chemotherapy for locally recurrent or metastatic breast cancer.9 Now, we have utilized the same model to (1) identify an early time point that can predict the future severity of neuropathy in a patient and (2) propose a dose-adjustment algorithm to manage neuropathy.
Methods
Data and Dose-Neuropathy Model
The data were obtained from CALGB 40502 (Alliance), a randomized phase III trial of paclitaxel, nab-paclitaxel, or ixabepilone as first-line chemotherapy (along with bevacizumab) for locally recurrent or metastatic breast cancer.9 Paclitaxel, nab-paclitaxel, or ixabepilone was administered intravenously at doses of 90 mg/m2, 150 mg/m2, and 16 mg/m2, respectively, on days 1, 8, and 15 of each 28-day cycle. A validated, patient-reported scale (the Functional Assessment of Cancer Therapy/Gynecologic Oncology
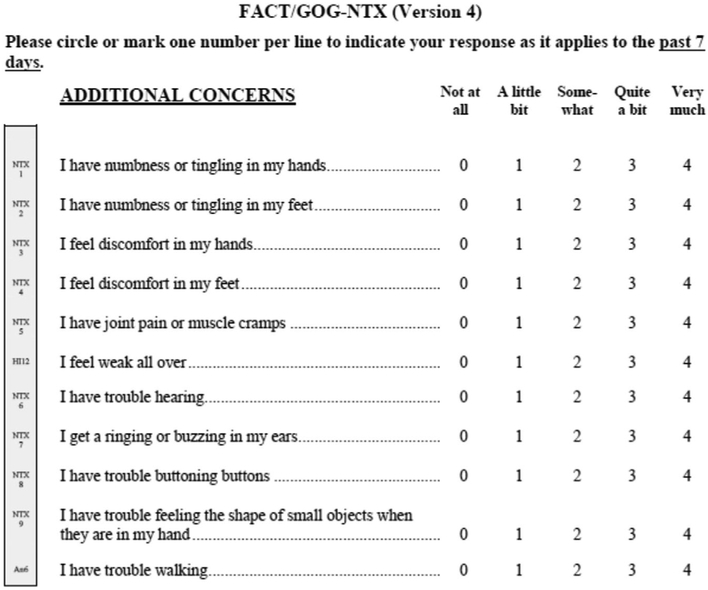
Group [FACT/GOG] Neurotoxicity Subscale) was used for assessing neuropathy, as shown in Figure 1.10-12 Neuropathy was evaluated at baseline and at the start of each chemotherapy cycle (every 28 days). It has been reported that the first 4 questions pertaining to sensory neuropathy (numbness/tingling in hands, numbness/tingling in feet, discomfort in hands, discomfort in feet) account for the majority of changes in neuropathy over time,11 and we verified that this was the case in the current data set.8 Therefore, a phenotype (neuropathy score) was derived by adding the scores from the 4 items related to sensory neuropathy. Neuropathy scores ranged from 0 to 16, with higher scores indicating higher severity. Because pharmacokinetic data were not collected, a kinetic-pharmacodynamic model was used to quantitate the dose-neuropathy relationship.8
Figure 1.
FACT/GOG neurotoxicity scale for neuropathy.
Early Time Point Identification
A model-based analysis for identification of the optimal early time point was conducted. The population parameter estimates (mean and variability) obtained from the dose-neuropathy model along with the baseline (cycle [C] 1; day [D] 1) and early neuropathy scores from a patient were used to calculate that patient’s parameter estimates. The patient’s parameter estimates were then used to predict their neuropathy scores for later cycles at the same doses administered in the trial. In the first iteration, the observed C1D1 and C2D1 scores were used to predict neuropathy scores on C3D1, C4D1, C5D1, C6D1, and C7D1. In each subsequent iteration, observed data from 1 additional cycle were used to predict neuropathy scores in future cycles. The predictive accuracy of the model and false-positive rates with the early neuropathy data were calculated as in the equations below. A neuropathy score ≥8 was selected as the cutoff because this is 50% of the maximum possible score, and getting this score means that two or more of the four sensory neuropathy symptoms are being felt “somewhat” (2 points), “quite a bit” (3 points), or “very much” (4 points).
Simulations to Explore the Effects of Dose Reduction Scenarios
After identification of the optimal early time point after 3 cycles (see Results), simulations were conducted in all patients with observed data on C4D1 using various dose reduction scenarios for managing neuropathy. A reference simulation was conducted in which no dose reduction occurred, such that C3 doses were administered for the next 3 cycles (C4, C5, and C6). Patients who were predicted to have a neuropathy score ≥8 on C7D1 were identified. Following this, 3 dose-reduction scenarios as described in Table 1 were evaluated.
Table 1.
Dose-Reduction Scenarios Evaluated
| Dose Modification Scenario |
% Reduction From C3 Dose (Total % Dose Reduction) |
C4 |
C5 |
C6 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C4D1 | C4D8 | C4D15 | C5D1 | C5D8 | C5D15 | C6D1 | C6D8 | C6D15 | ||
| No dose reduction Scenario 1: Reduction | 0 | x | x | x | x | x | x | x | x | x |
| 10 | 0.9x | 0.9x | 0.9x | 0.9x | 0.9x | 0.9x | 0.9x | 0.9x | 0.9x | |
| 20 | 0.8x | 0.8x | 0.8x | 0.8x | 0.8x | 0.8x | 0.8x | 0.8x | 0.8x | |
| 30 | 0.7x | 0.7x | 0.7x | 0.7x | 0.7x | 0.7x | 0.7x | 0.7x | 0.7x | |
| 40 | 0.6x | 0.6x | 0.6x | 0.6x | 0.6x | 0.6x | 0.6x | 0.6x | 0.6x | |
| 50 | 0.5x | 0.5x | 0.5x | 0.5x | 0.5x | 0.5x | 0.5x | 0.5x | 0.5x | |
| Scenario 2: Skip D8 +/− reduction | Skip D8 (33) | x | 0 | x | x | 0 | x | x | 0 | x |
| Skip D8 + 10% reduction (40) | 0.9x | 0 | 0.9x | 0.9x | 0 | 0.9x | 0.9x | 0 | 0.9x | |
| Skip D8 + 20% reduction (47) | 0.8x | 0 | 0.8x | 0.8x | 0 | 0.8x | 0.8x | 0 | 0.8x | |
| Skip D8 + 30% reduction (53) | 0.7x | 0 | 0.7x | 0.7x | 0 | 0.7x | 0.7x | 0 | 0.7x | |
| Skip D8 + 40% reduction (60) | 0.6x | 0 | 0.6x | 0.6x | 0 | 0.6x | 0.6x | 0 | 0.6x | |
| Skip D8 + 50% reduction (67) | 0.5x | 0 | 0.5x | 0.5x | 0 | 0.5x | 0.5x | 0 | 0.5x | |
| Scenario 3: Skip C4 +/− reduction | Skip C4 (33) | 0 | 0 | 0 | x | X | x | x | x | x |
| Skip C4 + 10% reduction (40) | 0 | 0 | 0 | 0.9x | 0.9x | 0.9x | 0.9x | 0.9x | 0.9x | |
| Skip C4 + 20% reduction (47) | 0 | 0 | 0 | 0.8x | 0.8x | 0.8x | 0.8x | 0.8x | 0.8x | |
| Skip C4 + 30% reduction (53) | 0 | 0 | 0 | 0.7x | 0.7x | 0.7x | 0.7x | 0.7x | 0.7x | |
| Skip C4 + 40% reduction (60) | 0 | 0 | 0 | 0.6x | 0.6x | 0.6x | 0.6x | 0.6x | 0.6x | |
| Skip C4 + 50% reduction (67) | 0 | 0 | 0 | 0.5x | 0.5x | 0.5x | 0.5x | 0.5x | 0.5x | |
C, cycle; D, day.
“x” is C3 dose.
Scenario 1 (reduction): Doses for C4, C5, and C6 were reduced by 10%, 20%, 30%, 40%, or 50% compared to C3 doses.
Scenario 2 (skip D8 ± reduction): Skipping the D8 doses in C4, C5, and C6, with and without also reducing the D1 and D15 doses by 10%, 20%, 30%, 40%, or 50%.
Scenario 3 (skip C4 ± reduction): Skipping all C4 doses, with and without reducing the C5 and C6 doses by 10%, 20%, 30%, 40%, or 50%.
The results of different scenarios were compared by calculating the percentage of patients with a neuropathy score ≥8 on C7D1.
Neuropathy Dose-Adjustment Algorithm
Dose-adjustment algorithms for paclitaxel, nab-paclitaxel, and ixabepilone were proposed based on the simulation results. A patient with a higher neuropathy score at C4D1 requires a larger dose reduction to manage neuropathy compared to a patient with a lower neuropathy score on C4D1. Therefore, patients who were predicted to develop neuropathy score ≥8 on C7D1 without dose reduction were further stratified based on their C4D1 score. The patients were grouped into 4 categories based on their C4D1 neuropathy scores: <4 (category 1), 4-7 (category 2), 8-9 (category 3) and ≥10 (category 4). In the trial, the maximum allowed dose reductions for paclitaxel, nab-paclitaxel, and ixabepilone were 33%, 40%, and 37.5%, respectively.8 Therefore, the following criteria were adopted for dose reductions:
A 33% dose reduction was considered adequate if the predicted proportion of patients with neuropathy score ≥8 on C7D1 was at least 30% fewer compared to no dose reduction.
If a 33% dose reduction did not result in at least 30% fewer patients with neuropathy score ≥8 on C7D1, further dose reduction scenarios were evaluated that resulted in at least 30% fewer patients compared to no dose reduction.
Results
Early Time Point Identification
The optimal early time point was selected based on the ability of the model to correctly identify patients with and without neuropathy score ≥8 at later cycles (predictive accuracy). Table 2 shows the predictive accuracy of the model for the future severity of neuropathy using early data. As expected, the more data included from earlier cycles, the greater the predictive accuracy of the model. Using baseline and observed neuropathy scores through C4D1, the predictive accuracy of the model was 80%, 74%, and 75% for C5D1, C6D1, and C7D1, respectively. The false-positive rate (incorrectly predicting a patient to have neuropathy score ≥8) at C7D1 was 12%. Only 17%, 23%, and 16% of patients had observed neuropathy score ≥8 on C4D1 for paclitaxel, nab-paclitaxel, and ixabepilone, respectively, indicating that this time point for prospectively managing neuropathy would be feasible in most patients.
Table 2.
Identification of the Optimal Early Time Point to Predict the Future Severity of Neuropathy
| Predictive Accuracy of Early Data to Predict Neuropathy On | |||||
|---|---|---|---|---|---|
| Early Data | C3D1 (%) | C4D1 (%) | C5D1 (%) | C6D1 (%) | C7D1 (%) |
| Baseline + C1 | 90 | 83 | 72 | 69 | 66 |
| Baseline + C1-C2 | N/A | 85 | 76 | 69 | 73 |
| Baseline + C1-C3 | N/A | N/A | 80 | 74 | 75 |
| Baseline + C1-C4 | N/A | N/A | N/A | 80 | 80 |
| Baseline + C1-C5 | N/A | N/A | N/A | N/A | 85 |
C, cycle; D, day; N/A, not applicable.
The end of C3 (baseline + C1-C3) was selected as the optimal early time point.
Simulations to Explore the Effects of Dose-Reduction Scenarios
Observed neuropathy scores on C4D1 were available in 173, 146, and 132 patients in the paclitaxel, nab-paclitaxel, and ixabepilone arms, respectively. Simulations show that, without dose reduction for cycles 4 through 6, 29%, 40%, and 33% of the patients on paclitaxel, nab-paclitaxel, and ixabepilone, respectively, would develop a neuropathy score ≥8 on C7D1. These predictions are consistent with the observed data, in which 25%, 40%, and 44% of the patients on paclitaxel, nab-paclitaxel, and ixabepilone, respectively, had a neuropathy score ≥8 on C7D1.
Table 3 shows the percentage of simulated patients who had a neuropathy score ≥8 on C7D1 under different dose-reduction scenarios. For example, a 30% dose reduction in C4, C5, and C6 resulted in 14%, 23%, and 27% of the simulated patients on paclitaxel, nab-paclitaxel, and ixabepilone, respectively, developing a neuropathy score ≥8 on C7D1. Skipping D8 doses in C4, C5, and C6 or skipping all 3 doses of C4 resulted in a similar proportion of simulated patients with a neuropathy score ≥8 on C7D1.
Table 3.
Simulations of the Effect of the Dose-Reduction Scenarios on Neuropathy
| Dose Modification Scenario |
% Reduction From C3 Dose (Total % Dose Reduction) |
% of Patients With Neuropathy Score ≥8 at C7D1 | ||
|---|---|---|---|---|
| Paclitaxel (n = 173) | Nab-Paclitaxel (n = 146) | Ixabepilone (n = 132) | ||
| No dose reduction Scenario 1: Reduction | 0 | 29 | 40 | 33 |
| 10 | 28 | 34 | 33 | |
| 20 | 22 | 30 | 30 | |
| 30 | 14 | 23 | 27 | |
| 40 | 13 | 21 | 23 | |
| 50 | 10 | 18 | 20 | |
| Scenario 2: Skip D8 ± reduction | Skip D8 (33) | 14 | 23 | 25 |
| Skip D8 + 10% reduction (40) | 13 | 21 | 23 | |
| Skip D8 + 20% reduction (47) | 10 | 20 | 21 | |
| Skip D8 + 30% reduction (53) | 8 | 17 | 20 | |
| Skip D8 + 40% reduction (60) | 5 | 15 | 18 | |
| Skip D8 + 50% reduction (67) | 4 | 12 | 17 | |
| Scenario 3: Skip C4 ± reduction | Skip C4 (33) | 16 | 26 | 24 |
| Skip C4 + 10% reduction (40) | 14 | 24 | 22 | |
| Skip C4 + 20% reduction (47) | 13 | 21 | 22 | |
| Skip C4 + 30% reduction (53) | 10 | 17 | 19 | |
| Skip C4 + 40% reduction (60) | 7 | 16 | 18 | |
| Skip C4 + 50% reduction (67) | 3 | 13 | 16 | |
C, cycle; D, day.
For paclitaxel (Table S1A), of 51 patients predicted to develop a neuropathy score ≥8 on C7D1, 23, 14, and 14 patients were in categories 2, 3, and 4, respectively. In patients predicted to develop a neuropathy score ≥8 on C7D1 without dose reduction, a 30% reduction in C4, C5, and C6 doses resulted in 13%, 57%, and 100% of those simulated patients with a neuropathy score ≥8 on C7D1 for categories 2, 3, and 4, respectively. Results for nab-paclitaxel and ixabepilone are presented in Table S1B and S1C, respectively. In any dose-reduction scenario, ixabepilone produced the highest percentage of patients with a neuropathy score ≥8 on C7D1.
Neuropathy Dose-Adjustment Algorithm
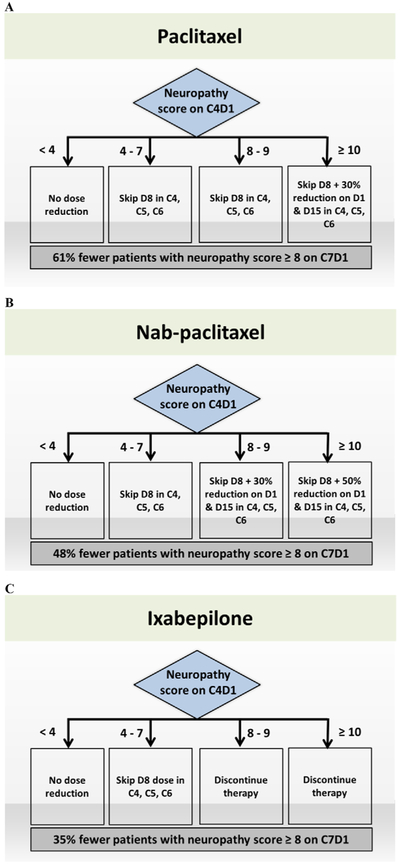
Based on the criteria defined in methods section, dose-adjustment algorithms are proposed for paclitaxel, nab-paclitaxel, and ixabepilone (Figure 2). In all cases, no dose adjustment is necessary for patients with C4D1 scores <4 (category 1).
Figure 2.
Proposed neuropathy dose-adjustment algorithm for (A) paclitaxel, (B) nab-paclitaxel, and (C) ixabepilone. Dose reduction or discontinuation scenarios were chosen for the algorithm such that the predicted proportion of patients with a neuropathy score ≥8 on C7D1 is at least 30% fewer compared to no dose reduction. C, cycle; D, day.
For paclitaxel, skipping D8 doses in C4, C5, and C6 in patients with C4D1 scores of 4 to 7 (category 2) and C4D1 scores of 8 to 9 (category 3) resulted in only 13% and 50% of such patients with a neuropathy score ≥8 at C7D1, respectively, compared to 100% for those with no dose adjustment (Table S1). For patients with C4D1 scores of ≥10 (category 4), skipping D8 doses along with 30% reduction of remaining doses is necessary to obtain the target 30% reduction in neuropathy score ≥8 at C7D1. Overall (combining all categories), our dosing strategy for paclitaxel resulted in 61% fewer simulated patients with a neuropathy score ≥8 at C7D1 compared to no dose reduction (Figure 2).
For nab-paclitaxel, skipping D8 doses in C4, C5, and C6 resulted in only 35% of category 2 patients with a neuropathy score ≥8 at C7D1 (Table S2). For category 3, skipping D8 doses along with 30% reduction of remaining doses resulted in the target of at least 30% fewer patients with a neuropathy score ≥8 at C7D1. For category 4, skipping D8 doses with 50% reduction of remaining doses was required. Overall (combining all categories), our dosing strategy for nab-paclitaxel resulted in 48% fewer patients with a neuropathy score ≥8 at C7D1 compared to no dose adjustment (Figure 2).
For ixabepilone, skipping D8 doses in C4, C5, and C6 resulted in 71% of category 2 simulated patients with a neuropathy score ≥8 at C7D1 (Table S3). For categories 3 and 4, even skipping D8 doses along with 50% reduction of remaining doses did not achieve the target 30% reduction. When no doses were administered to the simulated patients in C4, C5, and C6, there were only 17% and 70% with neuropathy score ≥8 at C7D1 in categories 3 and 4, respectively. Overall (combining all categories), our dosing strategy for ixabepilone resulted in 35% fewer patients with a neuropathy score ≥8 at C7D1 compared to no dose adjustment (Figure 2).
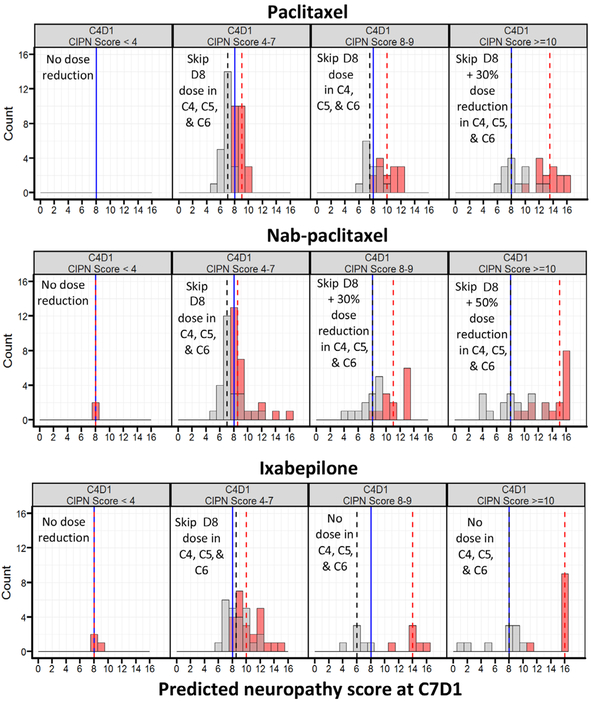
Figure 3 shows the distribution of simulated neuropathy scores with and without the recommended dose adjustments stratified by C4D1 neuropathy score. For patients with higher scores at C4D1, reducing the dose (in the cases of paclitaxel and nab-paclitaxel) or discontinuing the drug (in the case of ixabepilone) resulted in lower neuropathy scores at C7D1.
Figure 3.
Comparison of neuropathy scores (labeled CIPN scores) after no dose reduction in C4, C5, and C6 vs the proposed dose-adjustment algorithm in patients with a predicted neuropathy score ≥8 at C7D1, stratified by C4D1 neuropathy scores.
Discussion
We used a dose-neuropathy model to demonstrate how a personalized dosing strategy can be used to decrease the severity of neuropathy in patients receiving neurotoxic chemotherapy for metastatic breast cancer. If validated when compared to usual care, the proposed algorithm would provide oncologists with a quantitative method for making dose adjustments. The approach has the potential to preserve the quality of life and functionality of patients, while allowing them to continue to receive drugs that are effective. It is noteworthy that modeling and simulation studies have growing importance in drug approval and labeling, highlighted by the example of the nivolumab label change to include every 4 weeks’ dosing.13,14
The end of 3 cycles (12 weeks) was identified as the optimal early time point that could predict the future severity of neuropathy, with a predictive accuracy of 75% to predict neuropathy after 6 cycles. Furthermore, the false-positive rate is only 12%, indicating that oncologists would unnecessarily reduce the dose in only 1 of every 8 patients. There are no baseline risk factors that are known to have this level of predictive accuracy.6 Oncologists often order a computed tomography scan to assess the response to therapy after 3 cycles, so this is an ideal time to make dose adjustments if the drug is going to be continued.
The first 3 cycles of data on neuropathy can be used to guide dose adjustments that are aimed at decreasing the severity of neuropathy at later time points. Our recommended dose-adjustment algorithm shows that, for some patients, omitting the D8 dose (with or without a reduction in doses on D1 and D15) can substantially reduce the severity of neuropathy at a later time point. This is a patient-friendly approach, as it results in 33% fewer trips to the infusion center. There are some patients, such as those on ixabepilone with high neuropathy scores after 3 cycles, for which permanent discontinuation of the drug is the recommended strategy, and it is noteworthy that the neuropathy is partially reversible when the drug is discontinued. In those cases, the patient and oncologist would need to discuss alternate therapies. Although the work here has highlighted dose adjustments after C3, the same model and additional observed data could guide adjustments after C6 or at other time points.
Patients and oncologists are likely to have concerns about reducing doses of effective therapies without any guarantee of similar efficacy at lower doses. While this is a valid concern, in the status quo, empirical dose adjustments are implemented for severe toxicity, often when it is already too late for dose adjustments to make a substantial difference. It is possible that patients with prospective dose adjustments to mitigate neuropathy could continue effective therapy for a longer period of time.
The approach used in this study is unique compared to dose adjustment algorithms that have previously been proposed. For example, a pharmacokinetic-based dosing algorithm to manage neuropathy based on time of paclitaxel concentration >0.05 μmol was proposed.15 This dosing algorithm was developed in 200 patients with various solid malignancies, primarily esophageal cancer (72.5%). Neuropathy was measured using the Common Terminology Criteria for Adverse Events version 4.0, and there was no information on time at which neuropathy occurred. The proposed dosing algorithm reduced the incidence of grade ≥2 neuropathy from 9.6% of patients to 4.4%. However, in CALGB 40502, the proportion of patients with grade 2 and grade 3 neuropathy (by Common Terminology Criteria for Adverse Events version 4.0) in the paclitaxel arm were 28% and 17%, respectively.9 The higher proportions of patients with grade 2 and 3 sensory neuropathy may be attributed to the different patient population and differences in the dosing of paclitaxel in CALGB 40502 compared to the previous study.12 Furthermore, from an implementation standpoint, it may not be practical to draw pharmacokinetic samples and adjust doses based on results that come back days later.
There are several limitations to our approach. The first limitation is that we compared the proposed dose-adjustment algorithm to no dose reduction, whereas usual care is for oncologists to make dose reductions based on their clinical judgment (and for reasons other than neuropathy). Second, the feasibility of using the algorithm in clinical practice and the impact of the proposed dose adjustments on observed neuropathy and efficacy are unknown. Therefore, the strategy needs to be prospectively evaluated in a randomized trial of usual care vs the dose-adjustment algorithm with a primary end point of neuropathy score and secondary efficacy end points. Third, the algorithm is based on 4 questions from the FACT/GOG neurotoxicity scale, so its utility would be limited to settings in which these patient-reported outcome data can be collected. Fourth, the choice of a 30% reduction in risk of a neuropathy score ≥8 compared to no dose reduction is arbitrary, and the algorithm might be different if another threshold (such as 20% or 50%) had been chosen. Finally, the proposed algorithm may not apply to other populations or to patients who are being treated on a different dose/schedule of these drugs, as the baseline risk and dose-neuropathy relationship may be different in those scenarios.
Conclusions
We have proposed a dose-adjustment algorithm for neurotoxic chemotherapy drugs in patients with metastastic breast cancer that has the potential to reduce the severity of future neuropathy in individual patients. This approach warrants prospective validation in comparison to usual care.
Supplementary Material
Acknowledgments
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA031946, U10CA033601, U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), U10CA025224, U10CA032291, U10CA041287, U10CA077651, U10CA108068, U10CA138561, U10CA180790, U10CA180791, 5UG1CA189830, U10CA180836, U10CA180867, and 5UG1CA190000. Manish R. Sharma was supported by K12CA139160 from the National Cancer Institute, by a Young Investigator Award from the Cancer Research Foundation, and by K23GM112128 from the National Institute of General Medical Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest
The authors have declared no conflicts of interest.
Data Sharing
The data supporting the results in this manuscript cannot be made publicly available.
Supplemental Information
Additional supplemental information can be found by clicking the Supplements link in the PDF toolbar or the Supplemental Information section at the end of web-based version of this article.
References
- 1.Schloss JM, Colosimo M, Airey C, et al. Nutraceuticals and chemotherapy induced peripheral neuropathy (CIPN): a systematic review. Clin Nutr. 2013;32:888–893. [DOI] [PubMed] [Google Scholar]
- 2.Mantyh PW. Cancer pain and its impact on diagnosis, survival and quality of life. Nat Rev Neurosci. 2006;7:797–809. [DOI] [PubMed] [Google Scholar]
- 3.Winters-Stone KM, Horak F, Jacobs PG, et al. Falls, functioning, and disability among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. J Clin Oncol. 2017;35:2604–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32:1941–1967. [DOI] [PubMed] [Google Scholar]
- 5.Smith EML, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy. JAMA. 2013;309:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Argyriou AA, Kyritsis AP, Makatsoris T, et al. Chemotherapy-induced peripheral neuropathy in adults: a comprehensive up-date of the literature. Cancer Manag Res. 2014;6:135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velasco R, Bruna J. Chemotherapy-induced peripheral neuropathy: an unresolved issue. Neurologia. 2010;25:116–131. [PubMed] [Google Scholar]
- 8.Mehrotra S, Sharma MR, Gray E, et al. Kinetic-pharmacodynamic model of chemotherapy induced peripheral neuropathy in patients with metastatic breast cancer treated with paclitaxel, nab-paclitaxel or ixabepilone: CALGB 40502 (Alliance). AAPS J. 2017;19(5):1411–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rugo HS, Barry WT, Aspitia AM, et al. Randomized phase III trial of paclitaxel once per week compared with nanoparticle albumin-bound nab-paclitaxel once per week or ixabepilone with bevacizumab as first-line chemotherapy for locally recurrent or metastatic breast cancer: CALGB40502/NCCTGN063H. J Clin Oncol. 2015;33:2361–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasane M, Tencer T, French A, et al. Patient-reported out-comes in chemotherapy-induced peripheral neuropathy: a review. J Support Oncol. 2010;8:e15–e21. [Google Scholar]
- 11.Huang HQ, Brady MF, Cella D, et al. Validation and reduction of FACT/GOG-Ntx subscale for platinum/paclitaxel-induced neurologic symptoms: a gynecologic oncology group study. Int J Gynecol Cancer. 2007;17:387–393. [DOI] [PubMed] [Google Scholar]
- 12.Cella D, Peterman A, Hudgens S, et al. Measuring the side effects of taxane therapy in oncology: the functional assessment of cancer therapy-taxane (FACT-taxane). Cancer. 2003;98:822–831. [DOI] [PubMed] [Google Scholar]
- 13.Prescribing information of Opdivo (nivolumab). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125554s058lbl.pdf. Accessed February 24, 2019.
- 14.Ratain MJ, Goldstein DA. Time is money: optimizing the scheduling of nivolumab. J Clin Oncol. 2018;36:3074–3076. [DOI] [PubMed] [Google Scholar]
- 15.Kraff S, Nieuweboer AJM, Mathijssen RHJ, et al. Pharmacokinetically based dosing of weekly paclitaxel to reduce drug-related neurotoxicity based on a single sample strategy. Cancer Chemother Pharmacol. 2015;75:975–983. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.