Abstract
Significant evidence demonstrates that aging is associated with variability in cognitive performance, even among individuals who are cognitively normal. In this study, we examined measures from magnetic resonance imaging (MRI) and cerebrospinal fluid (CSF) to investigate which measures, alone or in combination, were associated with individual differences in episodic memory performance. Using hierarchical linear regressions, we compared the ability of diffusion tensor imaging (DTI) metrics, CSF measures of amyloid and tau, and gray matter volumes to explain variability in memory performance in a cohort of cognitively normal older adults. Measures of DTI microstructure were significantly associated with variance in memory performance, even after accounting for the contribution of the CSF and MRI gray matter volume measures. Significant associations were found between DTI measures of the hippocampal cingulum and fornix with individual differences in memory. No such relationships were found between memory performance and CSF markers or gray matter volumes. These findings suggest that DTI metrics may be useful in identifying changes associated with aging or age-related diseases.
Keywords: cognitive aging, biomarker, diffusion tensor imaging, cerebrospinal fluid, episodic memory
1. Introduction
Alzheimer’s disease (AD) is the most common form of dementia and the sixth-leading cause of death in the United States (Alzheimer’s Association, 2017). Mounting evidence suggests that neuropathological changes associated with AD may be present up to decades before any clinical symptoms arise (Sperling et al., 2011). Recent research has therefore focused on examining cognitively normal individuals across a range of ages in an effort to identify subtle alterations in cognition, and related biomarkers, that may precede clinical symptom onset of AD. In one such study, the Biomarkers of Cognitive Decline Among Normal Individuals (BIOCARD) study (Albert et al., 2014), cognitively normal individuals were enrolled primarily during middle-age and followed longitudinally for over 20 years. Assessments included a comprehensive clinical and cognitive evaluation, as well magnetic resonance imaging (MRI) and cerebrospinal fluid (CSF) collection. The present study utilized cross-sectional data from this cohort to examine the relationship of MRI and CSF measures to episodic memory performance in cognitively normal middle aged and older individuals.
A large number of studies have examined volumetric MRI changes associated with aging and the early phases of AD (e.g. Miller et al., 2015a, 2015b, 2013; Soldan et al., 2015). Recently, more attention has been drawn to alterations in white matter fiber bundles, the structural pathways that connect disparate neural regions throughout the brain, and changes associated with aging and early AD (for reviews, see Bennett and Madden, 2014; Caso et al., 2016; Contreras et al., 2015; Oishi et al., 2011; Radanovic et al., 2013). Diffusion tensor imaging (DTI) is a well-suited technique for such investigations, since DTI methodologies allow for the quantification of white matter microstructure, which may enable better detection of subtle variations that may be present even among cognitively normal individuals, compared to changes on the macrostructural level. In fact, microstructural aberrations have been demonstrated in cognitively normal older adults, as well as patients with mild cognitive impairment (MCI; Amlien and Fjell, 2014; Clerx et al., 2012; Gold et al., 2012; Lam et al., 2017; Nobili et al., 2014; Teipel et al., 2016; Zhang et al., 2014), and suggest a spatiotemporal pattern to the microstructural changes, with the earliest alterations beginning in limbic tracts, followed by more lateral temporoparietal association tracts, and ultimately progressing to long-range frontal pathways (Teipel et al., 2016). Teipel and colleagues (2016) also noted that the early limbic tract alterations tend to be the most severe and can be detected years before gray matter atrophy or cognitive impairment is observed.
Microstructural variation in limbic white matter pathways has also been consistently associated with episodic memory function, which is typically the earliest cognitive manifestation of AD. The fornix, for example, which connects the hippocampus to the mammillary bodies, is known to play a critical role in facilitating episodic memory processing, and its microstructure has been related to variability in memory performance in both cognitively normal older adults and patients with MCI. Specifically, fornix microstructure was associated with performance on tests of verbal free recall in cognitively normal participants (Metzler-Baddeley et al., 2011a), as well as MCI patients (Mielke et al., 2012; Oishi et al., 2012), and further associated with visual recall performance in cognitively normal participants (Metzler-Baddeley et al., 2011a). Similarly, the hippocampal cingulum, which connects the hippocampus and parahippocampus to the inferior parietal lobe, has been associated with verbal recall performance in non-demented older adults (Ezzati et al., 2016; Metzler-Baddeley et al., 2011a), and both verbal and visual recall in MCI patients (Lin et al., 2014). Finally, associations have been found with the uncinate fasciculus (UF), which connects the anterior and medial temporal lobes (MTLs; including portions of the entorhinal cortex, parahippocampal cortex, and perirhinal cortex) to the orbitofrontal cortex (OFC). Although fewer studies have examined these relationships in cognitively normal older adults, reports have demonstrated that UF microstructure is related to performance on a paired associates task among cognitively normal participants (Metzler-Baddeley et al., 2011a), while in MCI patients, the relationship has been demonstrated with verbal recall (Hiyoshi-Taniguchi et al., 2015). Taken together, these findings suggest that the microstructural properties of these three pathways may be particularly relevant in the context of age- and disease-related changes in cognition.
CSF measures are well-established as biomarkers of AD. They have been used as proxies for quantification of the two hallmark neuropathological features of AD: amyloid plaques and neurofibrillary tangles (Braak and Braak, 1991; Thal et al., 2002). CSF Aß42 is used to index amyloid-beta (Aß) protein deposition, while CSF total tau (t-tau) and phosphorylated tau (p-tau) are measures associated with neurodegeneration and the pathological build-up of tau proteins within neurofibrillary tangles, respectively (Fagan et al., 2007; Gustafson et al., 2007). Recent evidence indicates that CSF AD biomarkers predict longitudinal cognitive decline among cognitively normal individuals (Soldan et al., 2016; Vos et al., 2013), as well as progression to MCI (Moghekar et al., 2013). Evidence remains mixed, however, as to whether cross-sectional measures of CSF and cognitive performance are related in cognitively normal participants. A few studies have detected cross-sectional correlations between CSF markers and episodic memory performance (Aß42: Stomrud et al., 2010; t-tau and p-tau, but not Aß42: Pettigrew et al., 2015). However, it has been more common for studies to report no evidence of a relationship with Aß42 or tau markers (Li et al., 2014; Rami et al., 2011; Rolstad et al., 2011; Schott et al., 2010; Vemuri et al., 2011), at least for cognitively normal participants.
Although CSF markers may demonstrate weak associations with cross-sectional cognitive performance when examined in isolation, they may add meaningful information when examined in combination with other biomarkers, such as DTI metrics. However, research comparing the relative contributions of DTI and CSF measures to variability in cognition has been very limited (for a review, see Alm and Bakker, 2019). The present investigation, therefore, sought to explore the combined use of DTI and CSF metrics in explaining variance in episodic memory performance cross-sectionally. We aimed to test whether measures of DTI microstructure, CSF markers, gray matter volumes, white matter volumes, or a combination of these metrics were significantly related to delayed memory performance in cognitively normal participants in the BIOCARD cohort. Hierarchical regression analyses were used, so that each class of metrics could be added as independent variables within the overall model in a step-wise fashion. This allowed us to assess the relative contributions of each category of variables to the proportion of explained variance in episodic memory performance. Gray matter volumes were included in our models to ensure any potential DTI effects were present above and beyond the potential effects of gray matter volume. In a separate analysis, we tested whether white matter volume of the structures of interest related to variability in delayed episodic memory.
2. Materials and Methods
2.1. Study design
Data included in the present investigation were obtained from a subsample of participants enrolled in the BIOCARD study, a longitudinal study designed to identify biomarkers of the earliest phases of AD. The BIOCARD study began in 1995 under the auspices of the intramural program of the Geriatric Psychiatry Branch of the National Institutes of Mental Health (NIMH). During this time, participants underwent annual assessments comprised of a comprehensive neuropsychological battery and clinical evaluation. Approximately every two years, MRI data, CSF samples, and blood specimens were collected. The study was stopped in 2005 for administrative reasons. In 2009, a research team at Johns Hopkins University (JHU) was funded to re-initiate the study. Participants again began to undergo annual cognitive and clinical assessments, and in 2015, biennial collection of MRI (including diffusion-weighted imaging) and CSF data was initiated, and amyloid PET data were acquired. The data presented here were collected beginning in 2015. Data collection is ongoing.
2.2. Participants
The overall BIOCARD study sample consists of 349 individuals who were recruited between 1995 and 2005. At the time of enrollment, participants were primarily middle-aged (M = 57.3, SD = 10.4, range = 20 – 85). Participants underwent a comprehensive evaluation comprised of a battery of neuropsychological tests, physical and neurological examinations, an electrocardiogram, and standard laboratory tests. At the time of enrollment, all participants were cognitively normal, as ascertained by cognitive testing, and free from any significant medical problems (e.g. severe cerebrovascular disease, epilepsy, or substance abuse). By design, approximately 75% of enrolled participants had a first degree relative with AD dementia. Additional information on the overall BIOCARD cohort is presented elsewhere (see Albert et al., 2014).
The current study sample consisted of 109 cognitively normal participants (60.6% female) who underwent MRI scanning, CSF collection, and cognitive testing between January 2015 and January 2017 as part of the ongoing longitudinal assessments of the larger BIOCARD cohort. The participants had a mean age of 69.22 (SD = 8.60, range = 34 – 89) and a mean of 17.52 years of education (SD = 2.09, range = 12–20). All of these participants were judged to be cognitively normal, based on the consensus diagnoses procedures completed by the staff of the JHU BIOCARD Clinical Core, which included neurologists, neuropsychologists, research nurses, and research assistants. All cases were handled in a manner comparable to those employed at the National Institute on Aging Alzheimer’s Disease Centers program. First, a syndromic diagnosis was established (i.e., normal, MCI, Impaired Not MCI, Dementia). The syndromic diagnoses used three sources of information: (1) clinical data pertaining to the medical, neurological, and psychiatric status of the individual; (2) reports of changes in cognition by the individual and by collateral sources; and (3) decline in cognitive performance, based on review of longitudinal testing from multiple domains (and comparison to published norms). Within the context of this study, the diagnosis of Impaired Not MCI typically reflected contrasting information from the CDR interview and the cognitive test scores (i.e., the subject or collateral source expressed concerns about cognitive changes in daily life, but the cognitive testing did not show changes, or vice versa, the test scores provided evidence for declines in cognition, but neither the subject nor the collateral source reported changes in daily life; see Albert et al., 2014 for details). Subjects with a diagnosis of Impaired Not MCI (n = 20) were therefore included among the group of cognitively normal participants. If a subject was not considered to be normal, then an etiologic diagnosis was made (e.g., AD, Frontotemporal Dementia, Lewy Body Dementia, etc.). These diagnostic procedures follow the guidelines of the National Institute on Aging – Alzheimer’s Association working group (Albert et al., 2011; McKhann et al., 2011). All diagnoses were made without knowledge of the MRI or CSF biomarker measures.
2.3. Image acquisition and processing
MRI imaging was conducted on a 3T Philips Achieva scanner (Eindhoven, The Netherlands). Diffusion-weighted images were acquired from a spin echo sequence (TR = 7.5 s, TE = 75 ms, FOV = 260 × 260, slice thickness = 2.2 mm, flip angle = 90, b-value = 700, number of gradients = 33, axial plane). The image analysis was performed on an atlas-based matrix, which was generated by MRICloud (https://braingps.mricloud.org; Mori et al., 2016). DTI reconstruction and quality control were also performed using MRICloud, which follows the pipeline of DTIStudio (Jiang, Van Zijl, Kim, Pearlson, & Mori, 2006). MRICloud offers a fully automated multi-atlas image parcellation algorithm, which combines the image transformation algorithm, Large Deformation Diffeomorphic Metric Mapping (LDDMM; Christensen et al., 1997; Grenander and Miller, 1998; Miller et al., 1997) based on complementary contrasts (mean diffusivity (MD), fractional anisotropy (FA), and fiber orientation; Ceritoglu et al., 2009), and a likelihood fusion algorithm for DTI multi-atlas mapping and parcellation (Tang et al., 2014). The DTI multi-atlas template set contains 12 healthy adult brains, and results in the parcellation of 168 brain structures, from which vectors of DTI scalar metrics (three eigenvalues) and volumes were extracted.
We focused our analyses on MD, which is the average of the three eigenvalues, and radial diffusivity (RD), which is the average of the two minor eigenvalues, perpendicular to the major axis of diffusion. These diffusivity indices have been shown to be more sensitive to specific microstructural changes than FA, which is quite sensitive to general changes in microstructure, but is less likely to detect the particular type of change (Alexander et al., 2011). Although FA remains the most commonly reported measure, certain studies have shown that it is a suboptimal measure of underlying microstructure (e.g. Acosta-Cabronero et al., 2010; Leow et al., 2009). In addition, we sought to a priori restrict our number of comparisons in order to minimize inflated false positive rates due to multiple comparisons. Therefore, MD and RD were chosen as our specific microstructural measures of interest.
Five structures were defined for these analyses as a priori regions of interest (ROIs) based on their known involvement in episodic memory processes. DTI metrics of MD and RD, as well as white matter volumes were used in our statistical analyses for the following ROIs: (1) the fornix (restricted to the body and column due to resolution constraints; see Supplementary Figure S1), (2) the hippocampal cingulum, and (3) the uncinate fasciculus (UF). The gray matter volumes of two ROIs were also included in the analyses: (1) the entorhinal area and (2) the hippocampus. ROI-specific MD and RD measures were obtained by averaging the MD or RD values across all of the voxels within an ROI. Volumes were measured by counting the number of voxels within an ROI. ROIs for each participant were visually inspected to ensure that the automated segmentation process yielded accurate delineations of the structures of interest.
2.4. CSF assessments
CSF specimens in the present study were collected beginning in 2015, when biospecimen collection was re-initiated at Johns Hopkins. CSF and MRI data were collected during the same visit. 20 ml CSF was collected in the morning between 8 and 10 am after an overnight fast into a 50 ml polypropylene tube. After mixing and centrifugation at 2000 rpm for 15 minutes, 500 ul aliquots of CSF were frozen at −80 °C within 60 minutes of collection. CSF Aβ42 (picograms/ml) and CSF t-tau (picograms/ml) were measured using the Lumipulse G1200 assay (Fujirebio, Malvern, PA). Assays were run in duplicate, and all samples were run in a single batch. Intra-assay coefficient of variation for this assay was 3.1% for Aβ42 and 4.3% for t-tau.
2.5. Delayed episodic memory composite score
A delayed verbal episodic memory composite score was derived from performance on tasks within the neuropsychological battery administered during the same visit as MRI data acquisition and lumbar punctures. Specifically, z-scored performance was calculated for the California Verbal Learning Test (CVLT) long delay free recall and the Wechsler Memory Scale Logical Memory (LM) delayed recall measures. For each participant, these z-scored measures were then averaged to yield a single delayed memory composite score.
2.6. Statistical analyses
Hierarchical linear regression analyses were used to examine the relationship between biomarker measures – including DTI metrics, CSF markers, and gray matter volume measures – and the composite measure of delayed episodic memory. The variabilities for each of the independent variables of interest are plotted in Supplementary Figure S2.
Separate models were constructed for each microstructural measure (i.e. MD and RD) in order to avoid multicollinearity. For all hierarchical regressions, the dependent measure was the composite delayed episodic memory score, and independent variables were added in a step-wise manner. Step 1 included independent variables corresponding to demographics, including age, sex, and years of education. In Step 2, DTI microstructural measures were entered as additional separate independent variables, including MD or RD of the hippocampal cingulum, fornix, and UF. To reduce the number of comparisons, microstructural measures were collapsed across hemispheres; however, it should be noted that comparable results were obtained when considering left and right hemispheres separately (data not shown). In Step 3, CSF measures of Aß42 and t-tau were entered as additional separate independent variables. Finally, in Step 4, entorhinal and hippocampus volume measures (collapsed across hemispheres) were entered as additional separate independent variables. We included gray matter volume of the hippocampus and entorhinal area to determine whether associations with the white matter tracts were driven by volume differences in the gray matter regions where the pathways originate (Caso et al., 2016).
The same general procedure was followed to construct a hierarchical model to examine white matter volumes. The dependent measure remained composite delayed episodic memory score. The three demographic variables were entered in Step 1, followed by white matter volumes of the hippocampal cingulum, fornix, and UF (collapsed across hemispheres) in Step 2. Next, CSF Aß42 and t-tau were entered in Step 3, and gray matter volumes of the entorhinal area and hippocampus were entered in Step 4. Statistical analyses were performed using SPSS (version 24).
3. Results
3.1. Mean diffusivity explains variability in delayed episodic memory performance
First, a hierarchical regression model was constructed using mean MD of the MTL ROIs as the white matter measures of interest. Results are presented in Table 1. Step 1, including age, sex, and education was significant (R2 = 0.11, F(3, 105) = 4.27, p = .01), with sex showing a significant association with delayed memory (ß = 0.26, t(105) = 2.79, p = .01) such that female participants tended to perform better than males. Age and education did not reach significance (p’s > .07).
Table 1.
Mean MTL MD explaining variability in delayed episodic memory composite (N = 109)
| Delayed memory composite score | Independent variables | ß | t-value | F | ΔF | R2 | ΔR2 |
|---|---|---|---|---|---|---|---|
| Step 1 | 4.27** | 0.11 | |||||
| Age | −0.17 | −1.85† | |||||
| Sex | 0.26 | 2.79** | |||||
| Education | −0.06 | −0.62 | |||||
| Step 2 | 3.53** | 2.60† | 0.17 | 0.06 | |||
| Age | −0.02 | −0.17 | |||||
| Sex | 0.28 | 3.05** | |||||
| Education | −0.05 | −0.58 | |||||
| Hippocampal Cingulum MD | 0.26 | 2.38* | |||||
| Fornix MD | −0.29 | −2.18* | |||||
| Uncinate MD | −0.05 | −0.45 | |||||
| Step 3 | 2.74** | 0.46 | 0.18 | 0.01 | |||
| Age | 0.01 | 0.10 | |||||
| Sex | 0.29 | 3.13** | |||||
| Education | −0.06 | −0.59 | |||||
| Hippocampal Cingulum MD | 0.26 | 2.32* | |||||
| Fornix MD | −0.31 | −2.29* | |||||
| Uncinate MD | −0.06 | −0.51 | |||||
| Aβ42 | −0.01 | −0.08 | |||||
| t-tau | −0.09 | −0.89 | |||||
| Step 4 | 2.33* | 0.77 | 0.19 | 0.01 | |||
| Age | 0.01 | 0.05 | |||||
| Sex | 0.27 | 2.47* | |||||
| Education | −0.05 | −0.55 | |||||
| Hippocampal Cingulum MD | 0.25 | 2.22* | |||||
| Fornix MD | −0.34 | −2.43* | |||||
| Uncinate MD | −0.06 | −0.50 | |||||
| Aβ42 | −0.01 | −0.12 | |||||
| t-tau | −0.09 | −0.91 | |||||
| Entorhinal volume | −0.10 | −0.94 | |||||
| Hippocampal volume | −0.07 | −0.62 |
p < .07;
p < .05;
p < .01;
MD: mean diffusivity; MTL: medial temporal lobe
In Step 2, the addition of mean MD measures resulted in a significant overall model (R2 = 0.17, F(6, 102) = 3.53, p = .003), with a trend towards an enhanced proportion of variance explained by the model from the inclusion of these microstructural measures (ΔR2 = 0.06, ΔF(3, 102) = 2.60, p = .06). Sex remained significant (ß = 0.28, t(102) = 3.05, p = .003). Additionally, hippocampal cingulum MD and fornix MD both emerged as significant variables, each uniquely contributing to the proportion of explained variance (ß = 0.26, t(102) = 2.38, p = .02 and ß = −0.29, t(102) = −2.18, p = .03, respectively). Specifically, higher hippocampal cingulum MD and lower fornix MD were associated with better delayed memory performance. No other variables reached significance (p’s > .56).
In Step 3, the addition of CSF Aβ42 and t-tau yielded a significant overall model (R2 = 0.18, F(8, 100) = 2.74, p = .01). However, adding these measures did not significantly increase the variance explained by the overall model (ΔR2 = 0.01, ΔF(2, 100) = 0.46, p = .63). Sex (ß = 0.29, t(100) = 3.13, p = .002), hippocampal cingulum MD (ß = 0.26, t(100) = 2.32, p = .02), and fornix MD (ß = −0.31, t(100) = −2.29, p = .02) remained significant in this step, but neither Aβ42 or t-tau were significantly related to delayed memory performance (ß = −0.01, t(100) = −0.08, p = .93 and ß = −0.09, t(100) = −0.89, p = .38, respectively).
In Step 4, inclusion of the gray matter volumes yielded a significant overall model (R2 = 0.19, F(10, 98) = 2.33, p = .02), but adding these variables did not change the amount of variance explained by the model (ΔR2 = 0.01, ΔF(2, 98) = 0.77, p = .47). Therefore, the addition of gray matter volumes did not improve the model. Sex (ß = 0.27, t(98) = 2.47, p = .02), hippocampal cingulum MD (ß = 0.25, t(98) = 2.22 p = .03), and fornix MD (ß = −0.34, t(98) = −2.43, p = .02) all remained significant. No other variables reached significance (p’s > .35). Partial regression plots for the significant associations derived from Step 4, which represent the unique relationship between the independent variable of interest and delayed memory performance after controlling for all other variables in the model, are depicted in Figure 1a. Partial regression plots for non-significant associations are presented in Supplementary Figure S3.
Figure 1. Delayed memory performance associations with white matter microstructure.
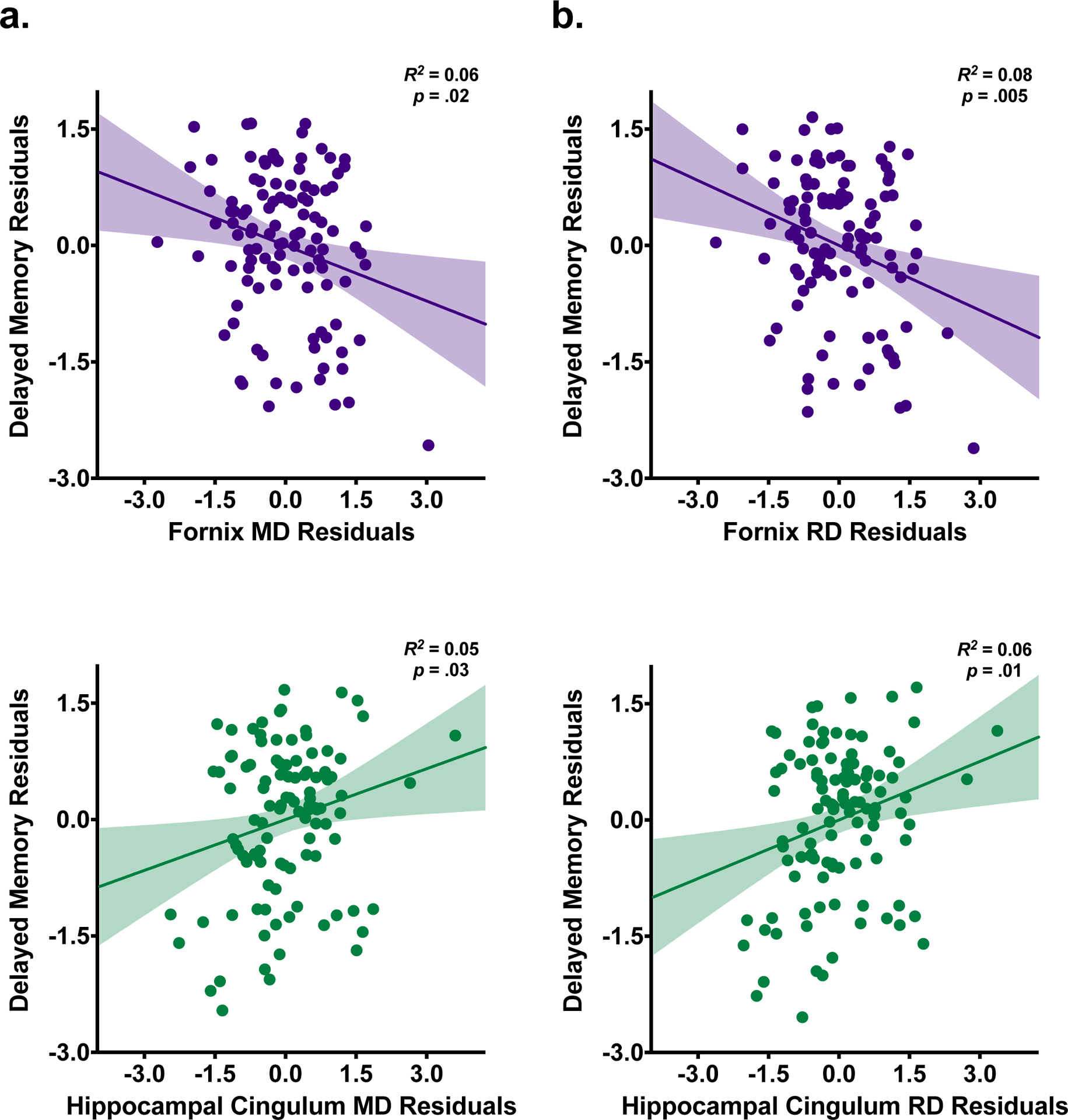
Partial regression plots with standardized residuals depicting the relationship of delayed episodic memory performance with fornix and hippocampal cingulum microstructure from the final step of the hierarchical regression model using mean diffusivity (MD) as the primary DTI metric of interest (a) and the final step of the hierarchical regression model using radial diffusivity (RD) as the primary DTI metric of interest (b).
In order to ensure that this pattern of results was not dependent on the order in which the diffusion metrics were entered into the model, we also examined a hierarchical model where the MTL MD metrics were entered during Step 4, rather than Step 2. The results were consistent with the findings reported above. Only the addition of the MD metrics significantly increased the amount of variance explained by the model above and beyond the demographic variables (ΔR2 = 0.07, ΔF(3, 98) = 2.84, p = .04). The full results of this model are presented in Supplementary Table S1.
3.2. Radial diffusivity explains variability in delayed episodic memory performance
For the next hierarchical model, mean RD values of the MTL structures served as the primary variables of interest. Results are presented in Table 2. For Step 1 (which included age, sex, and education as independent variables), the results were the same as described above. In Step 2, the inclusion of mean RD of the MTL structures yielded a significant overall model (R2 = 0.19, F(6, 102) = 4.03, p < .001), as well as a significant increase in the amount of explained variance accounted for by the model (ΔR2 = 0.08, ΔF(3, 102) = 3.48, p = .02). The addition of microstructural RD indices significantly improved the model’s ability to explain variability in memory performance. Female sex (ß = 0.27, t(102) = 2.99, p = .003), greater hippocampal cingulum RD (ß = 0.31, t(102) = 2.71, p = .01), and lower fornix RD (ß = −0.37, t(102) = −2.65, p = .01) were all significantly associated with better delayed memory performance. No other variables reached significance (p’s > .54).
Table 2.
Mean MTL RD explaining variability in delayed episodic memory composite (N = 109)
| Delayed memory composite score | Independent variables | ß | t-value | F | ΔF | R2 | ΔR2 |
|---|---|---|---|---|---|---|---|
| Step 1 | 4.27** | 0.11 | |||||
| Age | −0.17 | −1.85† | |||||
| Sex | 0.26 | 2.79** | |||||
| Education | −0.06 | −0.62 | |||||
| Step 2 | 4.03*** | 3.48* | 0.19 | 0.08 | |||
| Age | −0.03 | −0.21 | |||||
| Sex | 0.27 | 2.99** | |||||
| Education | −0.06 | −0.62 | |||||
| Hippocampal Cingulum RD | 0.31 | 2.71** | |||||
| Fornix RD | −0.37 | −2.65** | |||||
| Uncinate RD | 0.02 | 0.16 | |||||
| Step 3 | 3.16** | 0.62 | 0.20 | 0.01 | |||
| Age | 0.02 | 0.13 | |||||
| Sex | 0.29 | 3.10** | |||||
| Education | −0.06 | −0.63 | |||||
| Hippocampal Cingulum RD | 0.31 | 2.72** | |||||
| Fornix RD | −0.40 | −2.80** | |||||
| Uncinate RD | 0.01 | 0.07 | |||||
| Aβ42 | −0.01 | −0.11 | |||||
| t-tau | −0.10 | −1.03 | |||||
| Step 4 | 2.63** | 0.61 | 0.21 | 0.01 | |||
| Age | 0.01 | 0.10 | |||||
| Sex | 0.26 | 2.43* | |||||
| Education | −0.06 | −0.60 | |||||
| Hippocampal Cingulum RD | 0.30 | 2.58** | |||||
| Fornix RD | −0.43 | −2.88** | |||||
| Uncinate RD | 0.002 | 0.02 | |||||
| Aβ42 | −0.02 | −0.16 | |||||
| t-tau | −0.11 | −1.06 | |||||
| Entorhinal volume | −0.08 | −0.80 | |||||
| Hippocampal volume | −0.06 | −0.60 |
p < .07;
p < .05;
p < .01;
p < .001;
RD: radial diffusivity; MTL: medial temporal lobe
In Step 3, the overall model was significant (R2 = 0.20, F(8, 100) = 3.16, p = .003), but the amount of variance explained did not change significantly from Step 2 to Step 3 (ΔR2 = 0.01, ΔF(2, 100) = 0.62, p = .54), indicating that adding measures of CSF Aβ42 and t-tau did not improve the proportion of explained variance. In Step 3, sex, hippocampal cingulum RD, and fornix RD each remained significant (sex: ß = 0.29, t(100) = 3.10, p = .003; hippocampal cingulum RD: ß = 0.31, t(100) = 2.72, p = .01; fornix RD: ß = −0.40, t(100) = −2.80, p = .01). By contrast, neither Aβ42 nor t-tau were found to be significantly related to delayed memory (ß = −0.01, t(100) = −0.11, p = .91 and ß = −0.10, t(100) = −1.03, p = .31, respectively). No other variables reached significance (p’s > .53).
In Step 4, a similar pattern of results emerged. The overall model was significant (R2 = 0.21, F(10, 98) = 2.63, p = .01), but the inclusion of entorhinal and hippocampal volumes did not change the proportion of variance explained by the model (ΔR2 = 0.01, ΔF(2, 98) = 0.61, p = .55). Once again, sex, hippocampal cingulum RD, and fornix RD remained significant, even after accounting for gray matter volumes (sex: ß = 0.26, t(98) = 2.43, p = .02; hippocampal cingulum RD: ß = 0.30, t(98) = 2.58, p = .01; fornix RD: ß = −0.43, t(98) = −2.88, p = .01). No other variables reached significance (p’s > .29). The partial regression plots from Step 4 for the fornix and hippocampal cingulum are depicted in Figure 1b. Partial regression plots for non-significant associations are presented in Supplementary Figure S3.
To confirm that these findings were not simply due to the order of variable entry into the hierarchical model, we also examined a model in which the DTI measures were entered last. Even when the MTL RD measures were entered in Step 4, this remained the only step where the proportion of explained variance increased significantly beyond the initial model with demographics only (ΔR2 = 0.09, ΔF(3, 98) = 3.70, p = .01). The full hierarchical model is presented in Supplementary Table S2.
3.3. Examining potential partial volume effects
In order to account for potential confounds due to partial volume effects, we examined models in which both DTI microstructural measures and white matter volumes were included. This is especially a concern for smaller pathways, like the fornix, as structures with lower volumes tend to show higher partial volume effects (Vos et al., 2011). At Step 1, the regression model included the demographic variables. At Steps 2 and 3, DTI microstructural measures and white matter volumes corresponding to the structures of interest were included, respectively. With these variables in the model, the DTI microstructural relationships with delayed memory remained significant (hippocampal cingulum MD: ß = 0.23, t(99) = 2.04, p = .05; fornix MD: ß = −0.34, t(99) = −2.52, p = .01; hippocampal cingulum RD: ß = 0.26, t(99) = 2.23, p = .03; fornix RD: ß = −0.40, t(99) = −2.83, p = .01), suggesting that these associations were not merely the result of size differences in the white matter ROIs.
3.4. Examining the relationship of CSF ratios to variability in delayed episodic memory performance
Prior evidence suggests that the ratio of CSF tau to Aß42 is a better predictor of clinical decline than each measure independently (Moghekar et al., 2013). Therefore, we also examined models in which this ratio was included. In this analysis, we used the ratio of t-tau/Aß42 as an independent variable in the regression models, instead of separate variables for each measure. T-tau/Aß42 was not significantly related to delayed memory in either model (MD model: ß = −0.09, t(101) = −0.99, p = .32; RD model: ß = −0.10, t(101) = −1.05, p = .30), nor did it change the overall pattern of results.
3.5. Examining the relationship of white matter volume to variability in delayed episodic memory performance
Finally, we tested whether the volume of the white matter ROIs explained variation in delayed memory composite scores. The general procedure for constructing the hierarchical model was the same, except that Step 2 was now comprised of white matter volume measures corresponding to the hippocampal cingulum, fornix, and UF. Results are presented in Table 3.
Table 3.
Mean MTL white matter volumes explaining variability in delayed episodic memory composite (N = 109)
| Delayed memory composite score | Independent variables | ß | t-value | F | ΔF | R2 | ΔR2 |
|---|---|---|---|---|---|---|---|
| Step 1 | 4.27** | 0.11 | |||||
| Age | −0.17 | −1.85† | |||||
| Sex | 0.26 | 2.79** | |||||
| Education | −0.06 | −0.62 | |||||
| Step 2 | |||||||
| Age | −0.19 | −2.04* | 3.24** | 2.08 | 0.16 | 0.05 | |
| Sex | 0.30 | 3.01** | |||||
| Education | −0.05 | −0.55 | |||||
| Hippocampal Cingulum volume | −0.20 | −1.87† | |||||
| Fornix volume | 0.12 | 1.30 | |||||
| Uncinate volume | 0.19 | 1.69 | |||||
| Step 3 | 2.53* | 0.48 | 0.17 | 0.01 | |||
| Age | −0.17 | −1.84† | |||||
| Sex | 0.32 | 3.12** | |||||
| Education | −0.06 | −0.63 | |||||
| Hippocampal Cingulum volume | −0.20 | −1.90† | |||||
| Fornix volume | 0.13 | 1.37 | |||||
| Uncinate volume | 0.19 | 1.72 | |||||
| Aβ42 | −0.03 | −0.33 | |||||
| t-tau | −0.08 | −0.79 | |||||
| Step 4 | 2.29* | 1.26 | 0.19 | 0.02 | |||
| Age | −0.20 | −2.08* | |||||
| Sex | 0.29 | 2.57** | |||||
| Education | −0.06 | −0.60 | |||||
| Hippocampal Cingulum volume | −0.19 | −1.76 | |||||
| Fornix volume | 0.14 | 1.50 | |||||
| Uncinate volume | 0.26 | 2.18* | |||||
| Aβ42 | −0.04 | −0.36 | |||||
| t-tau | −0.08 | −0.85 | |||||
| Entorhinal volume | −0.09 | −0.90 | |||||
| Hippocampal volume | −0.15 | −1.28 |
p < .07;
p < .05;
p < .01;
MTL: medial temporal lobe
Given that Step 1 was the same as in the previous hierarchical models (i.e., demographic variables entered as separate independent variables, with delayed memory composite score as the dependent measure), the results are identical to those observed in Step 1 above.
In Step 2, the inclusion of white matter volumes yielded a significant overall model (R2 = 0.16, F(6, 102) = 3.24, p = .01); however, adding these variables did not lead to a significant change in the proportion of explained variance (ΔR2 = 0.05, ΔF(3, 102) = 2.08, p = .11), signifying that demographic variables alone explained a greater proportion of the variance in delayed memory than volume of the MTL white matter ROIs. Only age and sex were significantly related to delayed memory (ß = −0.19, t(102) = −2.04, p = .04 and ß = 0.30, t(102) = 3.01, p = .003, respectively). None of the white matter volumes reached significance; however, hippocampal cingulum volume exhibited a trending effect (ß = −0.20, t(102) = −1.87, p = .06).
In Step 3, adding CSF markers yielded a significant overall model (R2 = 0.17, F(8, 100) = 2.53, p = .02), but once again, did not lead to a significant change in the proportion of explained variance (ΔR2 = 0.01, ΔF(2, 100) = 0.48, p = .62). After accounting for the influence of CSF Aβ42 and t-tau, sex remained significant (ß = 0.32, t(100) = 3.12, p = .002), but age did not (ß = −0.17, t(100) = −1.84, p = .07). No other variables reached significance, but hippocampal cingulum volume remained trending (ß = −0.20, t(100) = −1.90, p = .06).
Similar to the previous steps, in Step 4, the overall model was significant (R2 = 0.19, F(10, 98) = 2.29, p = .02), but the addition of gray matter volumes did not result in a change in the overall proportion of explained variance (ΔR2 = 0.02, ΔF(2, 98) = 1.26, p = .29). In this final step, age and sex were significant (ß = −0.20, t(98) = −2.08, p = .04 and ß = 0.29, t(98) = 2.57, p = .01, respectively), and volume of the UF was also found to uniquely contribute to the relationship with delayed memory performance (ß = 0.26, t(98) = 2.18, p = .03). Specifically, higher uncinate volume was related to better performance. No other variables reached significance (p’s > .08).
4. Discussion
Despite the growing number of investigations of DTI and CSF markers, very few have examined the relative contributions of each type of measure to individual differences in cognition (for a review, see Alm and Bakker, 2019). In this study, we sought to investigate whether MRI and CSF measures, alone or in combination, provide a neurobiological basis for individual differences in episodic memory performance in a group of cognitively normal older adults. We utilized hierarchical linear regression analyses in order to tease apart the proportion of variance in episodic memory accounted for by each class of variables. Our findings demonstrate that DTI measures of MTL white matter tracts (specifically the fornix and hippocampal cingulum) account for a significant amount of the variance explained in a composite score of delayed episodic memory above and beyond demographic variables. Moreover, the DTI metrics for these ROIs were the only set of variables in the hierarchical regression model that significantly improved the proportion of explained variance, even with measures of AD CSF markers, gray matter volumes, and white matter volumes included in the models. This remained true even in models where the DTI variables were entered in the last step of the hierarchy, indicating that the findings were not merely an effect influenced by the order of variable entry into the models. This indicates that DTI microstructural metrics for MTL regions exhibit a robust relationship with individual differences in episodic memory.
It should be noted that while RD measures yielded a significant increase in the variability explained by the overall regression model, the increase associated with MD metrics was only marginal (p = .06). However, the RD and MD models accounted for very similar proportions of explained variance (19% and 17% respectively), and the change in R2 for MD measures surpassed the threshold for statistical significance in the model when MD values were entered last. Therefore, it seems that RD may be a slightly more robust measure in terms of relating brain structure to cognitive function. In line with this, RD is typically considered to be more sensitive to specific alterations in microstructure, as well as a more direct index of microstructure (Alexander et al., 2011).
Across the MD and RD indices, both the hippocampal cingulum and the fornix were uniquely associated with variability in delayed memory. Importantly, these associations were present across each of the steps within the hierarchical regression models, signifying robust relationships with episodic memory, above and beyond any effects of age, sex, education, CSF markers, and gray matter volumes. This suggests a strong association between individual differences in delayed memory and variability in the microstructure of hippocampal white matter tracts. Given that both pathways terminate in the hippocampus, this adds to the body of literature implicating the hippocampus in the facilitation of long term memory function (e.g. Nadel & Moscovitch, 1997; Squire & Alvarez, 1995; Squire, Genzel, Wixted, & Morris, 2015) by providing evidence that hippocampal white matter also seems to be critically involved in supporting episodic memory processes. Microstructural changes in the fornix and the hippocampal cingulum have also been previously reported across the stages of the AD continuum (Oishi et al., 2012; Zhang et al., 2007). Moreover, the associations with memory performance are consistent with prior studies relating microstructural variations to individual differences in episodic memory. Microstructure of the fornix and hippocampal cingulum have both demonstrated previous associations with memory performance in non-demented older adults (which may include individuals with MCI; Ezzati et al., 2016; Metzler-Baddeley et al., 2011a), as well as across the AD continuum (Lin et al., 2014; Mielke et al., 2012; Oishi et al., 2012).
Our findings extend the prior literature by demonstrating that these relationships for the fornix and hippocampal cingulum are present in a cohort of cognitively normal participants, even after accounting for potential contributions from CSF AD biomarkers and MTL gray matter volumes (adjusted for demographics). Furthermore, these brain-behavior associations were specific to fornix and hippocampal cingulum microstructure, since the same relationships did not emerge for the corresponding white matter volumes or the UF. Given that microstructural measures index the underlying properties of white matter tissue on a finer scale, it is possible that these metrics are better able to detect subtle alterations not present at the macrostructural level in cognitively normal individuals. Age-related microstructural changes in the UF may be more subtle and therefore not associated with episodic memory in cognitively normal individuals.
It is important to note the discrepancy in the directions of the hippocampal cingulum and fornix relationships with delayed memory. For the fornix, decreases in MD and RD were associated with better memory performance. In contrast, elevated MD and RD were both indicative of better performance for the hippocampal cingulum. However, this inconsistency was maintained in sensitivity analyses removing both 5% and 50% of cases, based on the Mahalanobis distance test for multivariate outliers (data not shown); therefore, it does not seem to merely be due to the influence of a few outliers. Additionally, the hippocampal cingulum relationships were not necessarily in the expected direction. MD is a measure of the average diffusion in a given voxel; thus, heightened MD values can indicate increased free diffusion, which is often interpreted as the presence of tissue damage (Soares et al., 2013). It was therefore surprising to find a positive relationship between hippocampal cingulum MD and delayed memory. Similarly, RD is a measurement that represents the degree of diffusion occurring perpendicular to the primary axis of diffusion, such that increases in RD tend to be associated with myelin loss (Alexander et al., 2011; Beaulieu, 2011; Jones et al., 2013; Tournier et al., 2011). However, these interpretations are based largely on animal studies and may not hold true for the much more complex connectome of the human brain. For instance, the assumption that fiber bundles run parallel to the primary axis of diffusion, and therefore increased diffusion in the perpendicular direction (i.e. RD) indicates some sort of damage does not always hold true for the human brain (Jones et al., 2013). An axon bundle consisting of fanning or crossing fibers will have varying diffusion orientations within the bundle. This could lead to a microstructure that appears to be less coherent and less anisotropic relative to a fiber bundle with the same number and quality of fibers, but containing axons all aligned in the same direction (Jones et al., 2013). Yet, this does not signify that one of the fiber bundles has higher “integrity” than the other, only that they differ in appearance. It is therefore critical to be particularly cautious when interpreting microstructural DTI effects.
With respect to the CSF biomarkers, the addition of Aß42 and t-tau to the hierarchical regression (and the ratio of t-tau/Aß42) did not improve the overall model. Furthermore, neither CSF marker was independently associated with delayed memory performance. This was not completely unexpected, however, since past examinations of cross-sectional relationships between CSF markers and episodic memory have yielded mixed results in cognitively normal cohorts. Some studies have reported significant cross-sectional associations (e.g. Pettigrew et al., 2015; Stomrud et al., 2010), while others have found no evidence of such relationships (e.g. Li et al., 2014; Rami et al., 2011; Rolstad et al., 2011; Schott et al., 2010; Vemuri et al., 2011); therefore, further research is needed on this topic. Of note, Pettigrew et al. (2015) reported an association between CSF t-tau and p-tau with visuospatial episodic memory, but not with verbal episodic memory as examined in the present study. It is possible, therefore, that measures of tau are more directly related to visuospatial episodic memory but not to measures of verbal episodic memory. It is also possible that alterations in CSF measurements (which reflect global rather than localized changes) are less likely to demonstrate associations with episodic memory performance among cognitively normal individuals.
Similar to the CSF markers, the inclusion of gray matter volumes corresponding to the entorhinal area and hippocampus did not change the amount of variance accounted for by the overall model. Additionally, neither entorhinal volume nor hippocampal volume demonstrated a significant relationship with memory performance. This was surprising, given both regions’ importance in episodic memory function, and the significance of the entorhinal cortex as one of the earliest regions to undergo pathological changes in AD (Braak and Braak, 1991). It is possible that macrostructural measures of these brain regions are significant predictors of progression to MCI, but are not strongly associated with cross-sectional differences in cognition among cognitively normal individuals. It is also possible that more subtle shape-based gray matter measures of the hippocampus or entorhinal cortex may be more sensitive to individual differences in episodic memory among cognitively normal individuals (e.g. Miller et al., 2013).
Finally, in a hierarchical model with MTL white matter volumes as the independent measures of interest, the addition of the other variables described above did not improve the overall model. In general, this again suggests that detecting effects in a cognitively normal cohort may necessitate a more sensitive measure than volume; however, there was one particular white matter volume ROI that did yield a significant effect. Greater UF volume was associated with better delayed memory performance, but only in the final step. This finding requires further replication.
4.1. Limitations and Future Directions
The primary limitation of the present study is the cross-sectional nature of the investigation. Future studies will be able to take advantage of the longitudinal data currently being collected, which can examine not only the relationship between white matter microstructure and episodic memory, but also how the relationship may change over time. Longitudinal assessments will also aid in disentangling whether DTI measures are predictive of clinical decline or whether they may reflect age-related changes that are independent of AD.
Several technical issues are also potential limitations. One concerns conclusions drawn from correlations between DTI indices and behavior. As discussed above, much is still unknown with respect to interpreting directionality of brain-behavior relationships in the context of DTI microstructure. Thicker myelination in general may not always lead to enhanced performance on a cognitive task (Scholz et al., 2009). Although myelination is typically thought to enhance signal transduction, improved neural efficiency may also be achieved through mechanisms like synaptic pruning, which could yield local decreases in myelination. Microstructural findings are also vulnerable to overinterpretations about the overall integrity of structural connections throughout the brain. DTI metrics are derived on a voxel by voxel basis; thus, conclusions drawn from such local changes may not be generalizable to the human connectome on a broader scale. We must therefore be wary not to over-attribute changes in local microstructure to alterations in more global integrity of white matter.
Additionally, it should be noted that ROI-based analyses of small fiber pathways, such as the fornix, are particularly prone to partial volume effects that occur from signal contamination due to the close proximity of CSF (Metzler-Baddeley et al., 2011b; Vos et al., 2011). Although this is more of a concern in samples with known atrophy, since volume reductions can exacerbate partial volume effects (Oishi and Lyketsos, 2014), we still sought to mitigate this issue. We conducted a follow-up analysis controlling for the volumes of the white matter ROIs, and our pattern of results was unchanged. Therefore, it does not seem that our findings were solely due to the influence of partial volume effects; however, we still caution against overinterpretation of the anatomical changes that may be represented by changes in diffusivity. Future studies would benefit from utilization of recent advances in DTI sequence development that can facilitate the reduction of partial volume effects and improve the ability to distinguish particular pathways, such as multi-shell acquisitions with higher b-values.
It is also possible that variation in gray matter volumes may contribute to the relationships found between episodic memory and white matter microstructure. Volume differences in the gray matter regions from which the white matter pathways emanate may be driving the DTI effects. However, the effects in the hippocampal cingulum and fornix remained significant even after controlling for the potential influence of gray matter volume differences across participants, suggesting that our findings cannot solely be explained by volume differences.
4.2. Conclusions
Very few studies have directly compared the relative contributions of DTI and CSF markers to individual differences in cognition. The hierarchical regression analyses used in the present study allowed us to assess the unique contribution of each class of variables to variability in episodic memory. Our findings suggest that DTI metrics can provide indices of the underlying microstructure of white matter pathways in the medial temporal lobe that are sensitive enough to capture individual differences in episodic memory performance among cognitively normal individuals. These subtle microstructural alterations exhibited robust associations with delayed episodic memory, suggesting that they may be useful in identifying changes associated with the initial phases of AD.
It is possible that the relationships exhibited between DTI metrics and individual differences in memory function could be used to improve the selection of clinical trial candidates, as well as provide critical co-variates in the analysis of outcome measures. These relationships could also be used to track subject-specific trajectories of disease progression.
Supplementary Material
Acknowledgements
This work was supported in part by grants from the National Institutes of Health (U19-AG033655, P50-AG005146, and T32-AG027668). We thank the entire BIOCARD study team at Johns Hopkins University for their support, the BIOCARD participants for continuing to participate in the study, and the Geriatric Psychiatry Branch of the intramural program of the NIMH who initiated this study (PI: Dr. Trey Sunderland).
Footnotes
Disclosure statement
AM receives funding from Fujirebio Diagnostics Ltd. (no direct payments, salary support, or consultation fees). SM is part owner and CEO of “AnatomyWorks”. This arrangement is being managed by the Johns Hopkins University in accordance with its conflict of interest policies. The remaining authors declare no competing interests.
References
- Acosta-Cabronero J, Williams GB, Pengas G, Nestor PJ, 2010. Absolute diffusivities define the landscape of white matter degeneration in Alzheimer’s disease. Brain 133, 529–539. 10.1093/brain/awp257 [DOI] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH, 2011. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 7, 270–279. 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, Soldan A, Gottesman R, McKhann G, Sacktor N, Farrington L, Grega M, Turner R, Lu Y, Li S, Wang M-C, Selnes O, 2014. Cognitive changes preceding clinical symptom onset of mild cognitive impairment and relationship to ApoE genotype. Curr. Alzheimer Res 11, 773–84. 10.2174/156720501108140910121920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Hurley SA, Samsonov AA, Adluru N, Hosseinbor AP, Mossahebi P, Tromp DPM, Zakszewski E, Field AS, 2011. Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connect. 1, 423–446. 10.1089/brain.2011.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm KH, Bakker A, 2019. Relationships between diffusion tensor imaging and cerebrospinal fluid metrics in early stages of the Alzheimer’s disease continuum. J. Alzheimer’s Dis 70, 965–981. 10.3233/jad-181210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association, 2017. 2017 Alzheimer’s disease facts and figures. Alzheimers Dement. 13, 325–373. 10.1016/j.jalz.2017.02.001 [DOI] [Google Scholar]
- Amlien IK, Fjell AM, 2014. Diffusion tensor imaging of white matter degeneration in Alzheimer’s disease and mild cognitive impairment. Neuroscience 276, 206–215. 10.1016/j.neuroscience.2014.02.017 [DOI] [PubMed] [Google Scholar]
- Beaulieu C, 2011. What makes diffusion anisotropic in the nervous system, in: Jones DK (Ed.), Diffusion MRI: Theory, Methods, and Applications. Oxford University Press, New York, pp. 92–109. [Google Scholar]
- Bennett IJ, Madden DJ, 2014. Disconnected aging: Cerebral white matter integrity and age-related differences in cognition. Neuroscience 276, 187–205. 10.1016/j.neuroscience.2013.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E, 1991. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259. 10.1007/BF00308809 [DOI] [PubMed] [Google Scholar]
- Caso F, Agosta F, Filippi M, 2016. Insights into white matter damage in Alzheimer’s disease: From postmortem to in vivo diffusion tensor MRI studies. Neurodegener. Dis 16, 26–33. 10.1159/000441422 [DOI] [PubMed] [Google Scholar]
- Ceritoglu C, Oishi K, Li X, Chou MC, Younes L, Albert M, Lyketsos C, van Zijl PCM, Miller MI, Mori S, 2009. Multi-contrast large deformation diffeomorphic metric mapping for diffusion tensor imaging. Neuroimage 47, 618–627. 10.1016/j.neuroimage.2009.04.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen GE, Joshi SC, Miller MI, 1997. Volumetric transformation of brain anatomy. IEEE Trans. Med. Imaging 16, 864–877. 10.1109/42.650882 [DOI] [PubMed] [Google Scholar]
- Clerx L, Visser PJ, Verhey F, Aalten P, 2012. New MRI markers for Alzheimer’s disease: A meta-analysis of diffusion tensor imaging and a comparison with medial temporal lobe measurements. J. Alzheimer’s Dis 29, 405–429. 10.3233/JAD-2011-110797 [DOI] [PubMed] [Google Scholar]
- Contreras JA, Goñi J, Risacher SL, Sporns O, Saykin AJ, 2015. The Structural and Functional Connectome and Prediction of Risk for Cognitive Impairment in Older Adults. Curr. Behav. Neurosci. Reports 2, 234–245. 10.1007/s40473-015-0056-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati A, Katz MJ, Lipton ML, Zimmerman ME, Lipton RB, 2016. Hippocampal volume and cingulum bundle fractional anisotropy are independently associated with verbal memory in older adults. Brain Imaging Behav. 10, 652–659. 10.1007/s11682-015-9452-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM, 2007. Cerebrospinal fluid tau/beta-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Arch. Neurol 64, 343–349. [DOI] [PubMed] [Google Scholar]
- Gold BT, Johnson NF, Powell DK, Smith CD, 2012. White matter integrity and vulnerability to Alzheimer’s disease: Preliminary findings and future directions. Biochim. Biophys. Acta 1822, 416–422. 10.1016/j.bbadis.2011.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenander U, Miller MI, 1998. Computational anatomy: An emerging discipline. Q. Appl. Math 56, 617–694. [Google Scholar]
- Gustafson DR, Skoog I, Rosengren L, Zetterberg H, Blennow K, 2007. Cerebrospinal fluid β-amyloid 1–42 concentration may predict cognitive decline in older women. J. Neurol. Neurosurg. Psychiatry 78, 461–464. 10.1136/jnnp.2006.100529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyoshi-Taniguchi K, Oishi N, Namiki C, Miyata J, Murai T, Cichocki A, Fukuyama H, 2015. The uncinate fasciculus as a predictor of conversion from aMCI to Alzheimer disease. J. Neuroimaging 25, 748–753. 10.1111/jon.12196 [DOI] [PubMed] [Google Scholar]
- Jiang H, Van Zijl PCM, Kim J, Pearlson GD, Mori S, 2006. DtiStudio: Resource program for diffusion tensor computation and fiber bundle tracking. Comput. Methods Programs Biomed 81, 106–116. 10.1016/j.cmpb.2005.08.004 [DOI] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, Turner R, 2013. White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. Neuroimage 73, 239–254. 10.1016/j.neuroimage.2012.06.081 [DOI] [PubMed] [Google Scholar]
- Lam CLM, Yiend J, Lee TMC, 2017. Imaging and neuropsychological correlates of white matter lesions in different subtypes of Mild Cognitive Impairment: A systematic review. NeuroRehabilitation 41, 189–204. 10.3233/NRE-171471 [DOI] [PubMed] [Google Scholar]
- Leow AD, Zhan L, Zhu S, Hageman N, Barysheva M, Toga AW, McMahon KL, de Zubicaray GI, Wright MJ, Thompson PM, 2009. White matter integrity measured by fractional anisotropy correlates poorly with actual individual fiber anisotropy. 2009 IEEE Int. Symp. Biomed. Imaging From Nano to Macro 622–625. 10.1109/ISBI.2009.5193124 [DOI] [Google Scholar]
- Li G, Millard SP, Peskind ER, Zhang J, Yu CE, Leverenz JB, Mayer C, Shofer JS, Raskind MA, Quinn JF, Galasko DR, Montine TJ, 2014. Cross-sectional and longitudinal relationships between cerebrospinal fluid biomarkers and cognitive function in people without cognitive impairment from across the adult life span. JAMA Neurol. 71, 742–751. 10.1001/jamaneurol.2014.445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-C, Shih Y-C, Tseng W-YI, Chu Y-H, Wu M-T, Chen T-F, Tang P-F, Chiu M-J, 2014. Cingulum correlates of cognitive functions in patients with mild cognitive impairment and early Alzheimer’s disease: A diffusion spectrum imaging study. Brain Topogr. 27, 393–402. 10.1007/s10548-013-0346-2 [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH, 2011. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 7, 263–269. 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler-Baddeley C, Jones DK, Belaroussi B, Aggleton JP, O’Sullivan MJ, 2011a. Frontotemporal connections in episodic memory and aging: A diffusion MRI tractography study. J. Neurosci 31, 13236–13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler-Baddeley C, Jones DK, Pasternak O, Bells S, O’Sullivan MJ, 2011b. How and how not to correct for CSF-contamination in diffusion MRI. Neuroimage 59, 1394–1403. 10.1016/j.neuroimage.2011.08.043 [DOI] [PubMed] [Google Scholar]
- Mielke MM, Okonkwo OC, Oishi K, Mori S, Tighe S, Miller MI, Ceritoglu C, Brown T, Albert M, Lyketsos CG, 2012. Fornix integrity and hippocampal volume predict memory decline and progression to Alzheimer’s disease. Alzheimer’s Dement. 8, 105–113. 10.1016/j.jalz.2011.05.2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Banerjee A, Christensen G, Joshi S, Khaneja N, Grenander U, Matejic L, 1997. Statistical methods in computational anatomy. Stat. Methods Med. Res 6, 267–299. 10.1191/096228097673360480 [DOI] [PubMed] [Google Scholar]
- Miller MI, Ratnanather JT, Tward DJ, Brown T, Lee DS, Ketcha M, Mori K, Wang M-C, Mori S, Albert MS, Younes L, 2015a. Network Neurodegeneration in Alzheimer’s Disease via MRI Based Shape Diffeomorphometry and High-Field Atlasing. Front. Bioeng. Biotechnol. 3, 1–16. 10.3389/fbioe.2015.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MI, Younes L, Ratnanather JT, Brown T, Trinh H, Lee DS, Tward D, Mahon PB, Mori S, Albert M, 2015b. Amygdalar atrophy in symptomatic Alzheimer’s disease based on diffeomorphometry: The BIOCARD cohort. Neurobiol. Aging 36, S3–S10. 10.1016/j.neurobiolaging.2014.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MI, Younes L, Ratnanather JT, Brown T, Trinh H, Postell E, Lee DS, Wang MC, Mori S, O’Brien R, Albert M, 2013. The diffeomorphometry of temporal lobe structures in preclinical Alzheimer’s disease. NeuroImage Clin. 3, 352–360. 10.1016/j.nicl.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghekar A, Li S, Lu Y, Li M, Wang MC, Albert M, O’Brien R, 2013. CSF biomarker changes precede symptom onset of mild cognitive impairment. Neurology 81, 1753–1758. 10.1212/01.wnl.0000435558.98447.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wu D, Li Y, Kolasny A, Vaillant MA, Faria AV, Oishi K, Miller MI, 2016. MRICloud: Delivering high-throughput MRI neuroinformatics as cloud-based software as a service. Comput. Sci. Eng 18, 21–35. [Google Scholar]
- Nadel L, Moscovitch M, 1997. Memory consolidation and the hippocampal complex. Curr. Opin. Neurobiol 7, 217–227. 10.1016/S0959-4388(97)80010-4 [DOI] [PubMed] [Google Scholar]
- Nobili F, Arnaldi D, Roccatagliata L, Chincarini A, Accardo J, Picco A, Ferrara M, Buschiazzo A, Morbelli S, 2014. Neuroimaging findings in mild cognitive impairment, in: Dierckx RAJO, Leenders KL, De Vries EFJ, Van Waarde A (Eds.), Pet and Spect in Neurology. pp. 271–306. 10.1007/978-3-642-54307-4 [DOI] [Google Scholar]
- Oishi K, Lyketsos CG, 2014. Alzheimer’s disease and the fornix. Front. Aging Neurosci 6, 1–9. 10.3389/fnagi.2014.00241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Mielke MM, Albert M, Lyketsos CG, 2011. DTI analyses and clinical applications in Alzheimer’s disease. J. Alzheimer’s Dis 26, 287–296. 10.3233/JAD-2011-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Mielke MM, Albert M, Lyketsos CG, Mori S, 2012. The fornix sign: A potential sign for alzheimer’s disease based on diffusion tensor imaging. J. Neuroimaging 22, 365–374. 10.1111/j.1552-6569.2011.00633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew C, Soldan A, Moghekar A, Wang MC, Gross AL, O’Brien R, Albert M, 2015. Relationship between cerebrospinal fluid biomarkers of Alzheimer’s disease and cognition in cognitively normal older adults. Neuropsychologia 78, 63–72. 10.1016/j.neuropsychologia.2015.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radanovic M, Pereira FRSR, Stella F, Aprahamian I, Ferreira LKK, Forlenza OVV, Busatto GF, 2013. White matter abnormalities associated with Alzheimer’s disease and mild cognitive impairment: A critical review of MRI studies. Expert Rev. Neurother 13, 483–493. 10.1586/ern.13.45 [DOI] [PubMed] [Google Scholar]
- Rami L, Fortea J, Bosch B, Solé-Padullés C, Lladó A, Iranzo A, Sánchez-Valle R, Molinuevo JL, 2011. Cerebrospinal fluid biomarkers and memory present distinct associations along the continuum from healthy subjects to AD patients. J. Alzheimer’s Dis 23, 319–326. 10.3233/JAD-2010-101422 [DOI] [PubMed] [Google Scholar]
- Rolstad S, Berg AI, Bjerke M, Blennow K, Johansson B, Zetterberg H, Wallin A, 2011. Amyloid-β42 is associated with cognitive impairment in healthy elderly and subjective cognitive impairment. J. Alzheimer’s Dis 26, 135–142. 10.3233/JAD-2011-110038 [DOI] [PubMed] [Google Scholar]
- Scholz J, Tomassini V, Johansen-Berg H, 2009. Individual differences in white matter microstructure in the healthy brain, in: Johansen-Berg H, Behrens TEJ (Eds.), Diffusion MRI: From Quantitative Measurement to In Vivo Neuroanatomy. Elsevier Inc, London, UK, pp. 237–249. 10.1016/B978-0-12-396460-1.00014-7 [DOI] [Google Scholar]
- Schott JM, Bartlett JW, Fox NC, Barnes J, 2010. Increased brain atrophy rates in cognitively normal older adults with low cerebrospinal fluid Aβ1–42. Ann. Neurol 68, 825–834. 10.1002/ana.22315 [DOI] [PubMed] [Google Scholar]
- Soares JM, Marques P, Alves V, Sousa N, 2013. A hitchhiker’s guide to diffusion tensor imaging. Front. Neurosci 7, 1–14. 10.3389/fnins.2013.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldan A, Pettigrew C, Cai Q, Wang MC, Moghekar AR, O’Brien RJ, Selnes OA, Albert MS, Rodzon B, Gottesman R, Sacktor N, McKhann G, Turner S, Farrington L, Grega M, Rudow G, D’Agostino D, Rudow S, Miller M, Mori S, Ratnanather T, Brown T, Chi H, Kolasny A, Oishi K, Reigel T, Younes L, Spangler A, Scherer R, Shade D, Ervin A, Jones J, Toepfner M, Parlett L, Patterson A, Mohammed A, Lu D, Troncoso J, Crain B, Pletnikova O, Fisher K, 2016. Hypothetical preclinical Alzheimer disease groups and longitudinal cognitive change. JAMA Neurol. 73, 698–705. 10.1001/jamaneurol.2016.0194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldan A, Pettigrew C, Lu Y, Wang M-C, Selnes O, Albert M, Brown T, Ratnanather JT, Younes L, Miller MI, 2015. Relationship of medial temporal lobe atrophy, APOE genotype, and cognitive reserve in preclinical Alzheimer’s disease. Hum. Brain Mapp 36, 2826–2841. 10.1002/hbm.22810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH, 2011. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 7, 280–292. 10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Alvarez P, 1995. Retrograde amnesia and memory consolidation: A neurobiological perspective. Curr. Opin. Neurobiol 5, 169–177. [DOI] [PubMed] [Google Scholar]
- Squire LR, Genzel L, Wixted JT, Morris RG, 2015. Memory Consolidation. Cold Spring Harb. Perspect. Biol 7, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stomrud E, Hansson O, Zetterberg H, Blennow K, Minthon L, Londos E, 2010. Correlation of longitudinal cerebrospinal fluid biomarkers with cognitive decline in healthy older adults. Arch. Neurol 67, 217–223. 10.1001/archneurol.2009.316 [DOI] [PubMed] [Google Scholar]
- Tang X, Yoshida S, Hsu J, Huisman TAGM, Faria AV, Oishi K, Kutten K, Poretti A, Li Y, Miller MI, Mori S, 2014. Multi-contrast multi-atlas parcellation of diffusion tensor imaging of the human brain. PLoS One 9, e96985 10.1371/journal.pone.0096985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teipel SJ, Grothe MJ, Zhou J, Sepulcre J, Dyrba M, Sorg C, Babiloni C, 2016. Measuring cortical connectivity in Alzheimer’s disease as a brain neural network pathology: Toward clinical applications. J. Int. Neuropsychol. Soc 22, 138–163. 10.1017/S1355617715000995 [DOI] [PubMed] [Google Scholar]
- Thal DR, Rüb U, Orantes M, Braak H, 2002. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 58, 1791–800. 10.1212/WNL.58.12.1791 [DOI] [PubMed] [Google Scholar]
- Tournier J-D, Mori S, Leemans A, 2011. Diffusion tensor imaging and beyond. Magn. Reson. Med 65, 1532–1556. 10.1002/mrm.22924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P, Weigand SD, Przybelski SA, Knopman DS, Smith GE, Trojanowski JQ, Shaw LM, Decarli CS, Carmichael O, Bernstein MA, Aisen PS, Weiner M, Petersen RC, Jack CR, 2011. Cognitive reserve and Alzheimer’s disease biomarkers are independent determinants of cognition. Brain 134, 1479–1492. 10.1093/brain/awr049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos SB, Jones DK, Viergever MA, Leemans A, 2011. Partial volume effect as a hidden covariate in DTI analyses. Neuroimage 55, 1566–1576. 10.1016/j.neuroimage.2011.01.048 [DOI] [PubMed] [Google Scholar]
- Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, Cairns NJ, Morris JC, Holtzman DM, Fagan AM, 2013. Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol. 12, 957–965. 10.1016/S1474-4422(13)70194-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Xu Y, Zhu B, Kantarci K, 2014. The role of diffusion tensor imaging in detecting microstructural changes in prodromal Alzheimer’s disease. CNS Neurosci. Ther 20, 3–9. 10.1111/cns.12166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Schuff N, Jahng G-H, Bayne W, Mori S, Schad L, Mueller S, Du A-T, Kramer JH, Yaffe K, Chui H, Jagust WJ, Miller BL, Weiner MW, 2007. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology 68, 13–19. 10.1212/01.wnl.0000250326.77323.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


