Abstract
Background:
Greater affordability and accessibility of noninvasive brain imaging techniques have led to an increased interest to identify biomarkers of various cognitive processes, particularly in the field of neurodevelopmental disabilities. Autism spectrum disorder (ASD) is one area of research where strong claims in support of brain-based biomarkers, such as the face-sensitive N170 event-related potential response, are currently emerging. This study systematically examined the possibility of the N170 amplitude and latency measures serving as a biomarker of social information processing in ASD.
Methods:
The N170 response to faces and houses was recorded during passive picture viewing in 77 children with ASD, age 7–16 years, at two time points (before and after a social skills intervention) three months apart. Social functioning was assessed using standardized behavioral tests, caregiver reports, and observational measures of naturalistic social interactions.
Results:
The results replicated prior findings of larger N170 amplitudes in response to faces than houses, but the associations with the behavioral measures of social functioning were modest and not consistently present across the two assessment time points. Neither the amplitude nor latency of the N170 response to faces was sensitive to the effects of a social skills intervention that produced behavioral improvements.
Conclusions:
The N170 is a reliable ERP response reflecting the sensory-perceptual stage of face processing, but it does not fit the definition of a biomarker of social deficits in ASD because it is not sufficiently informative about heterogeneity of social functioning and is not sensitive to treatment effects.
Keywords: autism, biomarker, N170, face, social, treatment
Greater affordability and accessibility of noninvasive brain imaging techniques have led to a dramatic increase in the interest to identify biomarkers of various cognitive processes. The appeal of an objective brain-based measure that could predict risk, assist with a diagnosis, or evaluate treatment effects is particularly strong in the field of neurodevelopmental disorders, where standardized behavioral assessment options are often limited (e.g., due to intellectual, motor, or language difficulties) and access to clinical expertise for diagnosis and management is not always readily available.
Autism spectrum disorder (ASD) is one area where strong claims in support of brain-based biomarkers are currently emerging. ASD is a neurodevelopmental disorder characterized by impairment in social competence, restricted, repetitive behavior and sensory processing problems(1), involving cognitive, neural, behavioral, and functional components(2). Behavioral studies in ASD often noted atypical social information processing, mainly using face stimuli in tasks of detection, recognition, or emotion identification(3–6). However, the results were highly variable, leading some to question whether face processing is uniformly impaired in ASD(7; 8). The need for a reliable measure that could be used across ages and functioning levels makes biological data highly attractive. Indeed, biological differences that may be clinically relevant are not always detected in overt behaviors(9) but could be captured in measures of brain activity and/or peripheral physiology(10).
Event-related potentials (ERP) offer an affordable and widely accessible means to noninvasively monitor information processing with millisecond-level precision. Among the ERP responses, a negative peak occurring over occipito-temporal scalp at 170 ms (N170) has been established in typical populations as sensitive to faces, which elicit larger amplitudes than nonsocial stimuli(11; 12). Following the initial reports of atypical N170 characteristics in ASD(13), the past 10 years have seen a 10-fold increase in the number of empirical studies and opinion papers considering the possibility of the N170 response serving as a biomarker of social information processing in ASD, from 15 papers in 2000–2009 to 149 papers in 2010–2019 (Google Scholar search with “ASD, N170, biomarker” keywords).
Recently, Kang et al.(14) conducted the first meta-analysis of the N170 studies comparing persons with ASD to typical individuals. They identified no consistent group differences in the N170 amplitude, but noted a small but significant effect size for delays in the N170 latency in ASD. Age, sex, cognitive ability, or diagnostic process differences did not explain variability in the timing of the N170 response to faces. These findings led the authors to conclude that the N170 latency could serve as a possible biomarker of social information processing in ASD(14).
However, this conclusion was challenged by Vettori et al.(15), who pointed out that a slower than typical N170 latency to faces in ASD may reflect general delays in visual processing speed, because the meta-analysis noted a similar pattern of prolonged latencies (non-significant but with medium effect size) for nonsocial stimuli. They further argued that the N170 latency does not fulfill the criteria for a clinically valuable biomarker because of difficulties effectively categorizing individuals and questions whether it is measuring a specific impairment (face processing) related to a specific clinical profile (e.g., ASD). Considering the latter point, Kang et al.(14) highlighted the need for further research connecting the N170 response and the mechanisms of social difficulties in ASD.
This discussion brought to the forefront the conversation about the possible diversity in the types of biomarkers. In the case of ASD, a complex clinical condition with multiple symptoms affecting numerous physiological systems, the current opinion is that a diagnostic biomarker is not yet available(16). In the meantime, the N170 response is often suggested as a promising sample stratification or a target engagement biomarker of social deficits(9; 17). Nevertheless, even in this more limited context, a measure aiming for classification as a biomarker needs to be sensitive to heterogeneity within the target population, developmental differences, and treatment effects(17). To date, this has not been clearly demonstrated for the N170 response.
Most studies examining the N170 response in persons with ASD did not test the associations between its amplitude or latency and behavioral measures of social functioning. The few studies that performed explicit correlational analyses yielded inconsistent results. Some reported that more accurate performance on face recognition tasks in persons with ASD was associated with slower left hemisphere N170 responses (Wechsler Face Recognition test)(13) or faster right hemisphere N170 (Benton Facial Recognition Test)(18). The strength of such correlations (e.g., with DANVA-2) diminished after controlling for age and IQ(19). Others reported no significant associations between the N170 amplitude or latency and performance on the Wechsler Face Recognition test(20; 21) or DANVA-2(22). Furthermore, small-to-medium correlations between the N170 latency and a subset of DANVA-2 items (e.g., child angry faces) were attenuated when a behavioral measure of social motivation was included in the statistical model(22). The N170 amplitude or latency also did not correlate with autism diagnostic scores, IQ, language, adaptive behavior(23) or with measures of social motivation(22), social cognition, and social behavior(24). In adults with ASD, the N170 amplitude or latency did not change following intensive face recognition training that produced significant improvements in behavioral performance(25).
This variability in the results across studies could be attributable to differences in the sample size, age, ASD diagnostic procedures and severity, equipment, choice of tasks, analyses, and data quality. Nevertheless, it highlights the need for further research that would validate the N170 response as a biomarker of social deficits in ASD.
In the current study using a large sample of children with ASD, we aimed to systematically examine whether the N170 face response fits the definition of a stratification or a treatment effect biomarker by evaluating its sensitivity to (1) individual differences in social functioning, and (2) the effects of a behavioral intervention targeting social skills. Specifically, based on the suggestions by Kang et al.(14) that the N170 response reflects neural processes relevant to social functioning in ASD, we hypothesized that larger amplitudes and shorter latencies in response to faces would be associated with more optimal performance on the standardized behavioral measures and during real-life social interactions. Improvements in social functioning following treatment would be associated with acceleration of N170 latency and/or increase in amplitude in response to faces. Additionally, we examined psychometric properties of the N170 response, such as test-retest stability of its amplitude and latency as well as of its associations with behavioral measures of social functioning. Given our focus on heterogeneity within the ASD population and not on group differences from typical peers, this study did not include a typical comparison group.
Method
Participants
Seventy-seven youth with ASD, age 7–16 years, representing three consecutive cohorts of participants in a randomized clinical trial of a social skills treatment (SENSE Theatre®; www.clinicaltrials.gov ID# NCT02276534) contributed ERP data for this study. The sample included 44 individuals randomized into the treatment (EXP) group and 33 participants placed into the waitlist control (WLC) group. Participants were recruited from the university clinic, support groups and schools. The diagnosis of ASD was made in accordance with the Diagnostic and Statistical Manual-5(1) based on: (1) a previous diagnosis by a psychologist, psychiatrist, or pediatrician with autism expertise; (2) current clinical judgment (B.A.C.); and (3) the Autism Diagnostic Observation Schedule (ADOS)(26), administered by research-reliable personnel. Social Communication Questionnaire (SCQ)(27) further corroborated the diagnosis (scores of ≥15). Co-occurring conditions included ADHD (19.5%), Learning Disability (1.3%), Language Disorder (1.3%), Sensory (3.9%), Anxiety (6.5%), and Medical diagnosis (13.0%), and were evenly distributed across the two groups, χ2 (6) = 2.69, p = 0.85. All participants had an intelligence quotient of 70 or greater, as measured by the Wechsler Abbreviated Scale of Intelligence (WASI)(28). The demographic information is presented in Table 1.
Table 1.
Demographic and diagnostic characteristics of the treatment (EXP) and waitlist control (WLC) Groups.
| EXP | WLC | p value | |
|---|---|---|---|
| Psychotropic | 18 | 14 | |
| Medications: > 1 | 11 | 7 | |
| Age | 11.12 (2.54) | 10.58 (2.32) | 0.34 |
| Gender F/M | 11/32 | 8/25 | 0.82 |
| ADOS Algorithm | 10.57 (4.73) | 11.83 (5.43) | 0.32 |
| WASI | 104.18 (19.27) | 96.49 (17.50) | 0.07 |
| SCQ | 20.95 ( 6.70) | 20.69 (7.18) | .872 |
Note: EXP = Experimental Group, WLC = Waitlist Control Group. Means, SD by group. F/M = Female/Male, ADOS = Autism Diagnostic Observation Schedule, WASI = Wechsler Abbreviated Scale of Intelligence, SCQ = Social Communication Questionnaire.
All participants had normal or corrected-to-normal vision, no medical history of seizures, traumatic head injury, or other serious medical conditions affecting the central nervous system (confirmed during screening). Parents/guardians of the participants provided written informed consent, and participants provided assent. The study was approved by the Institutional Review Board of Vanderbilt University Medical Center.
Procedures
Participants completed ERP, neuropsychological, and social behavior measures at baseline and at the end of a treatment period, approximately three months later. All assessments for the EXP and the WLC groups were conducted concurrently.
N170 ERP Acquisition
Following the procedures of Key & Corbett(29), participants viewed a sequence of 51 color photographs of unfamiliar young adult faces (Radboud Faces Database(30)) mixed with 51 color photographs of unfamiliar house façades (obtained from realtor websites). Unbeknownst to the participants, one of the stimuli in each category was randomly selected and repeated 50 times throughout the experiment, yielding a unique set of 50 repeated faces and houses for each person. The remaining stimuli were presented once. The participants were instructed to watch the screen “like TV” and had no stimulus-specific task. To verify attention, a button press was required in response to a drawing of a yellow smiley face (10 probes). All stimuli were presented in random order for 1500 ms with a varied inter-stimulus interval of 1300–1600 ms to prevent habituation. The onscreen size of faces and houses was 30 cm × 25 cm (visual angle of 19°×16° from the viewing distance of 90 cm). The attention probe was 14.5 cm (9.21°) in diameter. E-prime (v.2.0, PST, Inc., Pittsburgh, PA) controlled stimulus presentation. The entire task included 210 trials and lasted approximately 12 minutes. If participants became inattentive or restless, stimulus presentation was suspended until the participant was ready to continue with the task.
A 128-channel Geodesic Sensor net (EGI, Inc., Eugene, OR) was used to record the ERPs. Data were sampled at 250Hz with impedance levels at or below 50 kOhm. All electrodes were referred to vertex and then re-referenced during data analysis to an average reference(31).
Neuropsychological assessments
NEPSY: Memory for Faces(32) assessed face perception. Participants viewed a series of 16 pictures of children’s faces presented for 5 seconds each and then were asked to identify them amidst an array of 3 non-presented choices, immediately and following a 20-minute delay. The scaled scores for immediate and delayed memory for faces (average: 7–13) were used in the analyses.
Social Responsiveness Scale(33) is a 65-item questionnaire completed by caregivers to measure social functioning (e.g., communication, cognition,). The Total T-score was used in the analyses. T-scores between 60–75 are clinically significant, above 76 indicate more severe ASD symptoms.
Adaptive Behavior Assessment System (ABAS)(34) is a caregiver questionnaire that assesses 10 areas of adaptive functioning. For this study, the ABAS ascertained adaptive functioning related to social skills. Scaled scores between 7–13 fall within the average range, scores between 3–6 are clinically relevant.
Social Behavior Assessment
The Peer Interaction Paradigm (PIP)(35) is a 20-minute playground interaction in which the participant with ASD engages in play with two unfamiliar trained, gender- and age-matched typically developing confederates who provide behavioral structure to the play by initiating interactive sequences (i.e., cooperative and group play) in an otherwise natural setting. The Observer XT(36) was used for the analysis of observational data.
Continuous timed-event coding of two primary behaviors (Cooperative Play, Verbal Bout) was conducted by coders blind to group membership and study time periods. Cooperative play was defined as the percentage of time the participant with ASD was engaged in a reciprocal activity for enjoyment that involved participation of other children. Verbal bout was defined as an interaction between the participant with ASD and one or more children that began with a verbal overture and continued in reciprocal to-and-fro communication. Inter-rater reliability was comparable to previous studies(35) with k=.82 and .88 for Cooperative Play and Verbal Bout, respectively.
Social Skills Treatment
The SENSE Theatre® social skills intervention(37–39) was implemented over 10 4-hour group sessions. It used theatre games, role-play, improvisation, and character development activities in the context of putting on a play. Trained peer actors served as expert models of reciprocal social communication, flexible thinking and behavior(40). Prior studies examining the efficacy of SENSE Theatre® showed significant and sustained gains in behavioral (e.g., social communication), cognitive (e.g., theory-of-mind) and neurophysiological measures of social functioning (e.g., face memory)(37–39).
ERP Data Analysis
Continuous EEG recordings were filtered using a 0.1–30Hz bandpass filter and segmented on stimulus onset to include a 100-ms prestimulus baseline and a 900-ms poststimulus interval. Trials contaminated by ocular and movement artifacts were excluded from analysis using an automated screening algorithm in NetStation 5.3 followed by a manual review. Data for electrodes with poor signal quality were reconstructed using spherical spline interpolation(41). If more than 20% of the electrodes within a trial required interpolation, the entire trial was discarded. The retention rates were comparable across conditions, groups and test sessions (EXP: Baseline: M=20.22, SD=6.20; Posttest: M=21.26, SD=7.21; WLC: Baseline: M=20.71, SD=7.19; Posttest M=19.09, SD=7.04; all p-values >.05) and similar to those reported in prior studies using the same paradigm(29; 42; 43).
Following artifact removal, individual ERPs were averaged for repeated and single presentations of faces and houses, re-referenced to an average reference, and baseline-corrected by subtracting the average microvolt value across the 100-ms prestimulus interval from the poststimulus segment. Next, mean N170 amplitudes and peak latencies were derived within the 150–240 ms interval for occipito-temporal electrodes within each hemisphere (left: 57,58,63,64,65,69,70; right: 90,91,95,96,97,100,101; Figure 1). These scalp locations and time intervals were selected a priori based on published N170 studies in children with autism(29; 44) and confirmed by visual inspection of the grand-averaged waveforms. The resulting values were averaged across the electrodes within each cluster and entered into separate repeated-measures ANOVAs with Group (2: EXP, WLC) as the between-subject factor and Time (2: baseline, posttest) × Stimulus (2: faces, houses) × Memory condition (2: single, repeated) × Hemisphere (2: left/right) within-subject factors with Huynh-Feldt correction. Significant interactions were further explored using one-way ANOVAs and pairwise comparisons with Bonferroni correction.
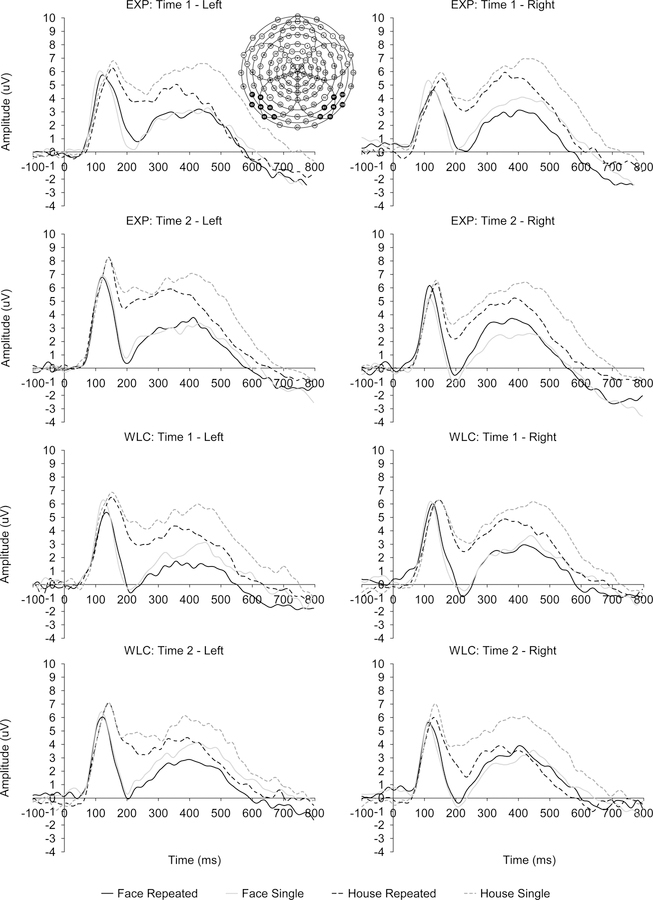
Figure 1.
Event-related potential (ERP) waveforms in response to repeated and single stimuli at left and right occipito-temporal clusters for youth with autism spectrum disorder in the treatment (EXP) and waitlist control (WLC) groups at baseline (Time 1) and posttest (Time 2).
Exploratory analyses examined correlations between the N170 characteristics, age, ASD symptoms and social functioning, as well as test-retest reliability. To provide the least conservative evaluation of possible brain-behavior associations, no correction for multiple significance testing was applied for this analysis.
Results
Summary data for the neuropsychological and social behavior assessments are presented in Table 2. The N170 amplitude and latency data are presented in Table 3.
Table 2.
Baseline (pre) and posttest (post) performance on behavioral measures of social functioning in the treatment (EXP) and waitlist control (WLC) groups.
| Measure | Time | WLC | EXP | ||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| NEPSY MF-I | pre | 7.46 | 3.69 | 8.50 | 3.66 |
| post | 9.52* | 2.98 | 10.35* | 3.30 | |
| NEPSY MFD | pre | 7.88 | 3.57 | 8.96 | 3.59 |
| post | 9.42* | 4.30 | 11.05* | 3.55 | |
| ABAS-Social | pre | 3.16 | 3.00 | 2.80 | 2.26 |
| post | 3.28 | 2.62 | 3.77* | 2.93 | |
| SRS Total | pre | 78.28 | 9.41 | 78.82 | 6.60 |
| post | 76.97 | 9.66 | 75.36* | 9.12 | |
| SCQ Total | pre | 20.69 | 7.18 | 20.95 | 6.90 |
| post | 19.64 | 7.39 | 19.12* | 7.21 | |
| PIP T2 | pre | 49.52 | 38.53 | 65.63 | 30.99 |
| Verbal Bout | post | 53.62 | 37.25 | 61.80 | 31.63 |
| PIP T4 | pre | 57.38 | 39.57 | 56.39 | 37.28 |
| Verbal Bout | post | 49.05 | 36.24 | 64.37 | 31.50 |
| PIP T2 | pre | 33.74 | 33.22 | 59.45 | 28.18 |
| Cooperative Play | post | 31.51 | 33.83 | 34.54* | 34.85 |
| PIP T4 | pre | 33.62 | 33.24 | 41.47 | 27.06 |
| Cooperative Play | post | 34.29 | 32.05 | 56.87* | 29.35 |
indicate posttest values that are significantly different from baseline in each group.
MF-I – memory for faces immediate, MFD – memory for faces delayed, PIP – Playground Interaction Paradigm, T2/T4 – PIP periods with elicited social interactions.
See Corbett et al. (under review) for further details regarding the treatment and its outcomes.
Table 3.
Mean amplitude and peak latency for the N170 in the left and right hemisphere for all stimulus conditions and at baseline (Time 1) and posttest (Time 2).
| N170 amplitude | N170 latency | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WLC (n=33) | EXP (n=44) | Total (N=77) | WLC (n=33) | EXP (n=44) | Total (N=77) | |||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||
| Baseline | Face | single | left | 0.03 | 4.23 | 0.01 | 3.82 | 0.02 | 3.98 | 207.65 | 18.61 | 206.64 | 20.32 | 207.07 | 19.49 | |
| right | −0.39 | 3.16 | −0.38 | 3.57 | −0.39 | 3.37 | 208.45 | 23.54 | 201.01 | 23.61 | 204.20 | 23.72 | ||||
| repeated | left | 0.24 | 3.92 | 0.86 | 4.36 | 0.60 | 4.16 | 211.22 | 17.19 | 206.74 | 25.89 | 208.66 | 22.55 | |||
| right | 0.05 | 3.39 | −0.26 | 3.75 | −0.13 | 3.58 | 211.58 | 24.79 | 202.78 | 27.64 | 206.55 | 26.65 | ||||
| House | single | left | 4.62 | 5.33 | 4.03 | 4.46 | 4.29 | 4.83 | 202.70 | 26.93 | 203.51 | 25.79 | 203.16 | 26.11 | ||
| right | 3.46 | 3.86 | 3.02 | 4.54 | 3.21 | 4.24 | 207.08 | 26.20 | 201.71 | 28.66 | 204.01 | 27.59 | ||||
| repeated | left | 3.69 | 5.06 | 3.16 | 4.15 | 3.39 | 4.54 | 206.75 | 25.97 | 202.73 | 23.26 | 204.45 | 24.37 | |||
| right | 3.02 | 4.48 | 2.50 | 3.96 | 2.72 | 4.17 | 212.45 | 18.89 | 203.56 | 28.27 | 207.37 | 24.94 | ||||
| Posttest | Face | single | left | 0.49 | 3.50 | 0.64 | 5.01 | 0.58 | 4.39 | 200.59 | 23.40 | 202.79 | 17.44 | 201.83 | 20.12 | |
| right | −0.70 | 2.64 | −0.62 | 3.26 | −0.65 | 2.99 | 201.30 | 21.56 | 196.81 | 22.73 | 198.76 | 22.20 | ||||
| repeated | left | −0.03 | 3.38 | 0.26 | 4.23 | 0.13 | 3.86 | 203.58 | 20.22 | 199.67 | 21.48 | 201.37 | 20.90 | |||
| right | −0.36 | 3.39 | −0.51 | 3.46 | −0.44 | 3.41 | 202.58 | 25.48 | 195.92 | 22.90 | 198.81 | 24.12 | ||||
| House | single | left | 4.00 | 4.68 | 4.12 | 5.75 | 4.07 | 5.28 | 201.06 | 22.47 | 197.70 | 22.16 | 199.16 | 22.21 | ||
| right | 3.24 | 4.04 | 2.43 | 3.43 | 2.78 | 3.71 | 200.10 | 25.86 | 194.91 | 25.37 | 197.17 | 25.54 | ||||
| repeated | left | 3.55 | 4.61 | 3.38 | 6.14 | 3.45 | 5.49 | 212.43 | 21.84 | 199.40 | 24.86 | 205.06 | 24.33 | |||
| right | 1.75 | 4.70 | 1.44 | 3.41 | 1.57 | 4.00 | 209.44 | 24.78 | 198.67 | 21.98 | 203.35 | 23.69 | ||||
WLC – waitlist control group; EXP – treatment group
N170 amplitude:
There were main effects of Stimulus, F(1,74)=117.197, p<.001, ηp2=.613, Memory, F(1,74)=6.856, p=.011, ηp2=.085, and Hemisphere, F(1,74)=10.202, p=.002, ηp2=.121, as well as Stimulus × Memory, F(1,74)=12.977, p=.001, ηp2=.149, Stimulus × Hemisphere, F(1,74)=4.115, p=.046, ηp2=.053, and Time × Stimulus × Memory × Hemisphere interactions, F(1,74)=5.516, p=.022, ηp2=.069. There was no significant Group effect. Follow-up analysis of the 4-way interaction first contrasted the N170 responses at baseline vs. posttest and revealed no significant differences in the left or right hemisphere for any of the stimulus conditions (p=.031-.999). Therefore, the remaining analyses were performed on data pooled across the two test sessions.
Paired t-tests indicated that in the repeated and single presentation conditions, faces elicited larger (more negative) N170 responses than houses in both hemispheres, t(75)=6.475–11.196, p<.001, d=.74–1.28. Larger right than left hemisphere N170 responses were observed for repeated and single presentations of faces and house, but only the latter remained statistically significant after correction for multiple comparisons (single houses: t(75)=3.224, p=.002, d=.37; repeated houses: t(75)=3.445, p=.001, d=.40). Differences between the single and repeated presentations were present only for the house images, with the repeated stimuli eliciting more negative amplitudes both in the left and right hemisphere, t(75)=3.695, p<.001, d=.42 and t(75)=3.470, p=.001, d=.40, respectively.
N170 latency:
The analyses identified main effects of Time, F(1,74)=7.186, p=.009, ηp2=.089, and Memory, F(1,74)=11.619, p=.001, ηp2=.136, as well as a Memory × Group interaction, F(1,74)=7.194, p=.009, ηp2=.089. Follow-up paired t-tests noted slightly faster N170 latencies at posttest (200ms) compared to baseline (205ms), t(75)=2.735, p =.008, d=.31. Across the two time points, the latencies were slightly longer for the repeated than single presentations for all stimulus types, 204 vs. 201 ms, t(75)=2.963, p =.004, d=.34. This result was driven primarily by the WLC group, 208 vs. 203 ms, t(32)=4.539, p <.001, d=.79, while the EXP group did not show a significant difference, 201 vs. 200 ms (p=.611). The between-group one-way ANOVA indicated that the groups were not significantly different in the N170 latency for the single presentations (p=.357), while the N170 response to all repeated stimuli was delayed in the WLC compared to the EXP group (p=.029).
Brain-Behavior associations:
Exploratory analyses of the brain-behavior associations at baseline and posttest revealed low-to-moderate concurrent and predictive correlations between the N170 amplitude and latency and behavioral metrics of age, autism severity, intellectual and social functioning (Table 4). Similar strength of associations was also noted between the N170 characteristics and real-life social behaviors during naturalistic social interactions (Table 5). Of note, a large portion of the observed correlations involved non-social stimuli.
Table 4.
Concurrent and predictive brain-behavior associations between the standardized measures of social functioning and the N170 amplitude and latency at baseline and posttest.
| Baseline | Post-Treatment | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Face | House | Face | House | |||||||||||||
| single | repeated | single | repeated | single | repeated | single | repeated | |||||||||
| Amplitude | left | right | left | right | left | right | left | right | left | right | left | right | left | right | left | right |
| Age (yrs) | 0.06 | 0.10 | 0.12 | 0.16 | −0.22 | −0.15 | −0.12 | −0.02 | −0.05 | 0.21 | 0.19 | 0.17 | −0.21 | −0.14 | −0.19 | −0.01 |
| ADOS communication | −.25* | −0.23 | −0.23 | −0.21 | −0.06 | −0.11 | −0.15 | −0.12 | −0.13 | 0 | −0.14 | −0.11 | −0.11 | −0.17 | −0.02 | 0.06 |
| ADOS social interaction | 0.14 | −0.06 | −0.01 | −0.08 | 0.18 | 0.21 | 0.16 | 0.15 | −0.01 | −0.06 | −0.06 | −0.09 | 0.01 | −0.02 | 0.04 | 0.06 |
| ADOS stereotyped behaviors | 0.11 | 0.06 | 0.06 | −0.05 | 0.15 | −0.01 | 0.13 | 0.06 | 0.01 | −0.02 | −0.04 | −0.07 | −0.08 | −0.06 | −0.07 | −0.17 |
| ADOS Total | −0.03 | −0.14 | −0.11 | −0.19 | −0.01 | −0.01 | −0.03 | 0.02 | −0.04 | −0.03 | −0.15 | −0.19 | −0.07 | −0.10 | −0.01 | −0.07 |
| ADOS algorithm score | 0.13 | −0.02 | −0.01 | −0.12 | 0.23 | 0.19 | 0.13 | 0.18 | 0.09 | 0.02 | 0 | 0.03 | 0.08 | 0.15 | 0.11 | 0.17 |
| WASI composite IQ | 0.11 | 0.15 | 0.05 | 0.07 | 0.19 | 0.19 | .23* | 0.03 | 0.07 | −0.07 | −0.03 | −0.02 | .30** | 0.15 | .27* | 0.11 |
| WASI verbal IQ | 0.04 | 0.11 | 0.03 | 0.08 | 0.11 | 0.15 | 0.12 | −0.06 | 0.13 | 0.01 | 0.03 | 0.07 | .29* | 0.17 | .26* | 0.10 |
| WASI performance IQ | 0.18 | 0.22 | 0.07 | 0.1 | .24* | .24* | .31** | 0.17 | 0.02 | −0.09 | −0.03 | −0.09 | .25* | 0.12 | .25* | 0.14 |
| NEPSY MF-I (baseline) | −0.08 | 0.10 | −0.17 | 0.06 | −0.07 | 0.11 | −0.04 | 0.01 | −0.18 | −0.08 | −0.06 | 0.13 | −0.06 | 0.12 | −0.04 | 0.22 |
| NEPSY MF-I (posttest) | −0.08 | 0.10 | −0.01 | 0.16 | −0.15 | 0.15 | −0.11 | −0.03 | −0.06 | 0 | −0.09 | 0.11 | 0.04 | .25* | 0.09 | .26* |
| NEPSY MFD (baseline) | −0.10 | 0.03 | −0.14 | −0.06 | −0.02 | 0.01 | −0.1 | −0.08 | −0.12 | −0.14 | −0.10 | −0.02 | −0.07 | 0.10 | −0.1 | 0.15 |
| NEPSY MFD (posttest) | −0.11 | −0.03 | −0.04 | 0.04 | −0.13 | 0.02 | −0.12 | −0.12 | −0.06 | −0.04 | −0.11 | 0.04 | −0.01 | 0.09 | 0.03 | 0.20 |
| SCQ total (baseline) | 0.07 | 0.02 | 0.02 | 0.05 | 0.10 | −0.01 | .29* | 0.17 | 0.07 | 0.09 | −0.01 | 0.01 | −0.03 | 0.02 | −0.03 | −0.07 |
| SCQ total (posttest) | 0.16 | −0.01 | 0.06 | 0.01 | 0.21 | 0.07 | .29* | .32** | 0.15 | 0.07 | 0.07 | 0.01 | 0.10 | 0.11 | 0.10 | 0.07 |
| ABAS social (baseline) | −0.2 | −0.03 | −0.19 | −0.01 | −0.04 | −0.05 | −0.12 | −0.03 | −0.11 | 0.03 | −0.04 | −0.01 | 0.07 | 0.13 | −0.02 | 0.09 |
| ABAS social (posttest) | 0.01 | 0.13 | 0.03 | 0.07 | 0.01 | 0.02 | −0.10 | −0.07 | −0.07 | −0.05 | 0.02 | 0.06 | 0.05 | 0.03 | −0.04 | 0.07 |
| SRS total (baseline) | 0.10 | −0.11 | 0.12 | −0.01 | 0.17 | 0.14 | .23* | 0.15 | 0.10 | 0.07 | 0.05 | 0.05 | −0.05 | 0.07 | 0.03 | 0 |
| SRS total (posttest) | 0.15 | −0.09 | 0.15 | 0.07 | 0.19 | 0.16 | .29* | .34** | .26* | 0.15 | .23* | −0.05 | 0.12 | 0.09 | 0.21 | 0.03 |
| Latency | ||||||||||||||||
| Age (yrs) | −0.13 | −0.13 | −0.15 | −.32** | −0.06 | −0.13 | −0.09 | −0.18 | −0.20 | −.34** | −.29* | −.39** | −0.13 | −0.14 | −0.18 | −.25* |
| ADOS communication | 0.11 | 0.10 | −0.04 | −0.02 | .25* | .28* | .28* | .254* | 0.08 | .26* | 0.20 | 0.17 | 0.22 | 0.23 | 0.17 | 0.19 |
| ADOS social interaction | 0.21 | 0.04 | 0.19 | 0.09 | 0.19 | 0.05 | 0.21 | 0.13 | .28* | 0.19 | .32** | .28* | .30* | .29* | 0.13 | 0.21 |
| ADOS stereotyped behaviors | 0.10 | −0.02 | 0.12 | 0.13 | 0.21 | 0.18 | 0.21 | 0.14 | 0.20 | 0.02 | .36** | 0.20 | 0.22 | 0.22 | 0.21 | 0.13 |
| ADOS Total | .24* | 0.14 | 0.14 | 0.16 | .24* | .25* | .30* | .286* | .24* | .24* | .34** | .33** | .29* | .33** | 0.19 | .25* |
| ADOS algorithm score | 0.23 | 0.15 | 0.13 | 0.15 | 0.19 | 0.14 | 0.14 | 0.07 | 0.11 | 0.21 | .26* | 0.14 | 0.24 | .25* | 0.12 | 0.24 |
| WASI composite IQ | −0.07 | −0.03 | −0.06 | −0.06 | −.34** | −.27* | −.46** | −0.17 | −0.03 | −0.01 | −0.05 | −0.06 | −0.20 | −0.22 | −.24* | −0.21 |
| WASI verbal IQ | −0.01 | 0.05 | 0.04 | 0.05 | −0.27 | −0.2 | −.36** | −0.15 | −0.04 | 0.03 | −0.06 | 0.01 | −0.19 | −0.19 | −0.22 | −0.11 |
| WASI performance IQ | −0.07 | −0.08 | −0.16 | −0.12 | −.39** | −.33** | −.50** | −0.17 | −0.05 | −0.03 | −0.04 | −0.1 | −0.16 | −0.22 | −.25* | −.27* |
| NEPSY MF-I (baseline) | −.26* | −0.16 | −0.1 | −0.08 | −.24* | −.24* | −0.1 | −0.08 | −0.05 | 0.03 | −0.09 | −0.03 | −0.09 | −0.13 | −0.14 | −0.17 |
| NEPSY MF-I (posttest) | −0.21 | −0.15 | −0.01 | −0.16 | −.30** | −.32** | −0.2 | −0.15 | −0.03 | 0.02 | −0.08 | −0.05 | −0.08 | −0.08 | −0.05 | −0.14 |
| NEPSY MFD (baseline) | −0.11 | −0.06 | 0.04 | −0.07 | −0.11 | −0.10 | −.24* | −0.02 | −0.12 | 0.01 | −0.01 | −0.02 | 0.07 | −0.06 | 0.08 | −0.09 |
| NEPSY MFD (posttest) | −0.13 | −0.06 | 0.08 | −0.14 | −0.22 | −0.13 | −0.18 | −0.04 | −0.05 | 0.01 | −0.02 | −0.06 | −0.02 | 0.01 | 0.10 | −0.14 |
| SCQ total (baseline) | −0.09 | −0.17 | −0.15 | −0.12 | −0.07 | −0.04 | −0.11 | −0.11 | −0.01 | −0.22 | 0.05 | −0.17 | −0.05 | −0.05 | −0.11 | −0.07 |
| SCQ total (posttest) | −0.02 | −0.04 | −0.11 | 0.03 | −0.08 | 0.03 | −0.05 | −0.18 | 0.05 | −0.11 | 0 | −0.14 | −0.07 | −0.06 | −0.12 | −0.02 |
| ABAS social (baseline) | −0.07 | 0.10 | −0.16 | 0 | −0.04 | −0.02 | −0.18 | −0.03 | 0.07 | .23* | −0.04 | 0.15 | −0.16 | −0.11 | 0.17 | 0.10 |
| ABAS social (posttest) | −0.07 | 0.06 | −0.06 | −0.09 | −0.22 | −.26* | −0.20 | −0.14 | −0.02 | 0.15 | −0.12 | 0.10 | −0.14 | 0.04 | 0.01 | −0.09 |
| SRS total (baseline) | −0.14 | −0.02 | −0.11 | −0.04 | 0.09 | −0.06 | −0.11 | −0.03 | −0.22 | −0.14 | −0.10 | −0.09 | −0.16 | 0.03 | −0.13 | 0.04 |
| SRS total (posttest) | −0.02 | 0.06 | −0.10 | 0.02 | 0.09 | −0.03 | 0.02 | −0.09 | −0.07 | −0.07 | −0.12 | −0.09 | −0.09 | 0.15 | −.24* | 0.12 |
MF-I – memory for faces immediate; MFD – memory for faces delayed
p<.05
p<.01
Table 5.
Concurrent and predictive associations between the N170 amplitude/latency and real-life social behaviors during two elicited social interaction periods (T2/T4) of the Peer Interaction Protocol at baseline (PRE) and posttest (POST).
| Baseline | Posttest | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Face single | Face repeated | House single | House repeated | Face single | Face repeated | House single | House repeated | |||||||||
| left | right | left | right | left | right | left | right | left | right | left | right | left | right | left | right | |
| Amplitude | ||||||||||||||||
| T2 PRE Verbal Bout | 0.019 | 0.124 | 0.033 | 0.107 | 0.131 | 0.133 | −0.004 | −0.01 | 0.068 | 0.003 | 0.028 | 0.05 | 0.148 | 0.167 | 0.12 | 0.074 |
| T4 PRE Verbal Bout | 0.024 | 0.112 | 0.11 | 0.056 | 0.084 | 0.146 | 0.068 | 0.117 | 0.022 | 0.076 | −0.022 | 0.139 | 0.143 | .256* | 0.128 | 0.031 |
| T2 PRE Cooperative Play | −.235* | −0.09 | −0.153 | 0.044 | −0.215 | −0.068 | −0.209 | −0.185 | −0.18 | 0.004 | −0.059 | −0.05 | −0.157 | −0.192 | −0.131 | −0.119 |
| T4 PRE Cooperative Play | −0.159 | −0.102 | −0.037 | −0.067 | −.243* | −0.13 | −.254* | −0.12 | −.242* | 0 | −0.164 | 0.061 | −0.215 | −0.047 | −.231* | −0.07 |
| T2 POST Verbal Bout | −0.024 | 0.074 | 0.075 | 0.071 | −0.047 | 0.051 | −0.035 | 0.054 | 0.073 | 0.122 | 0.091 | 0.036 | 0.098 | 0.125 | 0.135 | 0.03 |
| T4 POST Verbal Bout | −0.03 | 0.081 | 0.034 | −0.02 | −0.005 | 0.035 | 0.052 | 0.036 | 0.036 | 0.002 | 0.024 | 0.007 | 0.118 | 0.028 | 0.152 | −0.007 |
| T2 POST Cooperative Play | −.227* | −0.142 | −.239* | −0.145 | −0.123 | −0.148 | −0.039 | −0.218 | −0.068 | −.232* | −0.061 | −0.212 | −0.021 | −.246* | −0.048 | −0.071 |
| T4 POST Cooperative Play | −0.038 | 0.146 | 0.121 | 0.133 | −0.116 | −0.093 | −0.128 | −0.144 | −0.027 | 0.171 | 0.019 | 0.108 | 0.029 | 0.045 | −0.001 | 0.042 |
| Latency | ||||||||||||||||
| T2 PRE Verbal Bout | −0.058 | 0.044 | −0.041 | −0.036 | 0.15 | −0.003 | 0.003 | −0.113 | −0.123 | 0.024 | −0.034 | 0.003 | −0.153 | −0.048 | −0.123 | 0.065 |
| T4 PRE Verbal Bout | −0.106 | 0.107 | 0.026 | −0.016 | −0.052 | −0.05 | −.303** | −0.029 | −0.093 | 0.044 | −0.08 | 0.002 | −0.175 | 0.005 | −0.056 | 0.003 |
| T2 PRE Cooperative Play | −.338** | −0.167 | −0.198 | −0.208 | 0.04 | 0.002 | 0.018 | −0.123 | 0.087 | −0.187 | 0.064 | −0.067 | −0.13 | −0.17 | −0.144 | −0.166 |
| T4 PRE Cooperative Play | 0.037 | 0 | 0.063 | −0.138 | .239* | 0.154 | 0.088 | 0.057 | −0.082 | −0.12 | −0.053 | −0.095 | 0.018 | −0.032 | −0.001 | 0.032 |
| T2 POST Verbal Bout | −0.211 | −0.161 | −0.194 | −.343** | −.227* | −0.213 | −0.179 | −.229* | −.311** | −.281* | −.413** | −.271* | −0.201 | −0.059 | −.320** | −0.221 |
| T4 POST Verbal Bout | −.246* | −0.187 | −0.208 | −0.221 | −0.183 | −0.091 | −0.18 | −0.036 | −0.191 | −0.076 | −.363** | −.232* | −0.188 | −0.078 | −.254* | −0.107 |
| T2 POST Cooperative Play | −0.081 | −0.028 | 0.02 | 0.067 | −0.058 | 0.184 | 0.014 | 0.084 | 0.048 | 0.015 | 0.007 | −0.062 | 0.103 | −0.22 | −0.046 | −0.114 |
| T4 POST Cooperative Play | −0.106 | −0.143 | −0.182 | −.297** | 0.019 | −0.014 | −0.015 | −0.126 | −.278* | −.276* | −0.158 | −.274* | −0.112 | −.273* | −0.066 | −.268* |
p<.05
p<.01
Test-retest reliability:
Exploratory intraclass correlations examined test-retest reliability of the N170 metrics in the EXP and WLC groups, as well as in the combined sample. The results suggested moderate-to-high reliability for the N170 amplitude in response to faces and houses, while the latency was moderately reliable for faces only (Table 6).
Table 6.
Intraclass correlations indexing test-retest reliability between baseline and posttest values for the N170 amplitude and latency in the treatment (EXP), waitlist control (WLC), and the combined samples. 95% confidence intervals are provided in the parentheses. Shaded cells identify non-significant values.
| EXP | WLC | Combined | ||||
|---|---|---|---|---|---|---|
| left | right | left | right | left | right | |
| N170 amplitude | ||||||
| Face single | 0.72 (.49–.85) | 0.73 (.50–.85) | 0.73 (.45–.87) | 0.53 (.05–.77) | 0.72 (.56–.83) | 0.66 (.47–.79) |
| Face repeated | 0.58 (.23–.77) | 0.81 (.64–.89) | 0.70 (.40–.85) | 0.57 (.13–.79) | 0.63 (.41–.76) | 0.72 (.56–.82) |
| House single | 0.76 (.55–.87) | 0.78 (.59–.88) | 0.71 (.40–.85) | 0.53 (.05–.77) | 0.74 (.58–.83) | 0.68 (.50–.80) |
| House repeated | 0.64 (.33–.80) | 0.58 (.22–.77) | 0.79 (.57–.89) | 0.36 (−.29–.69) | 0.70 (.53–.80) | 0.47 (.16–.66) |
| N170 latency | ||||||
| Face single | 0.24 (−.40–.59) | 0.52 (.11–.74) | 0.54 (.08–.77) | 0.58 (.14–.79) | 0.40 (.05–.62) | 0.55 (.30–.72) |
| Face repeated | 0.17 (−.53–.55) | 0.49 (.07–.73) | 0.64 (.27–.82) | 0.59 (.16–.80) | 0.36 (−.01–.60) | 0.55 (.29–.72) |
| House single | 0.50 (.08–.73) | 0.59 (.24–.78) | 0.37 (−.27–.69) | 0.13 (−.77–.57) | 0.44 (.12–.65) | 0.44 (.11–.64) |
| House repeated | 0.48 (.03–.72) | 0.48 (.03–.72) | 0.28 (−.45–.65) | 0.40 (−.21–.71) | 0.42 (.08–.63) | 0.49 (.19–.67) |
Discussion
This study evaluated the N170 response as a potential biomarker of social deficits in ASD. We examined its sensitivity to individual differences in social functioning (measured using standardized and naturalistic tools), developmental stage (age), and treatment effects of an established social skills intervention in a large (n=77) sample of youth with ASD. Participants were diagnosed using the gold-standard tools and represented a wide range of ages (7–16 years), intellectual ability (70–141), and ADOS scores (total: 6–25). We also assessed test-retest stability of the N170 amplitude and latency across two visits conducted approximately 3 months apart.
N170 to Faces vs. Nonsocial Stimuli
Our results replicated prior findings of larger N170 amplitudes to faces than houses in both repeated and single stimulus presentation conditions, consistent with the interpretation of the N170 amplitude as a face-sensitive response in typical populations(11) and in persons with ASD(45; 46). We also observed the expected hemisphere differences with larger N170 amplitudes in response to faces over the right than left occipito-temporal regions(11; 46; 47). Test-retest stability analysis of the N170 amplitude replicated prior evidence(48) of its good reliability for both faces and houses, suggesting that it is a robust perceptual response that can be obtained in typical and atypical populations across ages, ability levels, testing settings, and equipment types.
Within-session repetition-related amplitude enhancement was detected for the houses only, replicating our previous findings(29) and possibly reflecting increased perceptual experience due to repeated exposures to the same image(49). The lack of a comparable enhancement for the repeated faces suggests that face perception in ASD may be less modifiable by short-term exposure, with the N170 amplitude reflecting a stable trait characteristic. It is also possible that face perception mechanisms in participants with ASD were consistently engaged regardless of face familiarity (see also(46)). This interpretation is further supported by the lack of significant differences in the N170 amplitude between the baseline and posttest assessments for any of the stimulus conditions or hemisphere sites. The meta-analysis findings(14) of absent group differences in the N170 amplitude to faces between participants with ASD and typical peers further support the interpretation that it may not be the optimal measure of social perception deficits in ASD.
The N170 latency did not appear to differentiate between social and nonsocial stimuli in children with ASD at either of the two time points (see(18) for similar findings). It did show slight acceleration (5 ms) from baseline to posttest for all stimuli, but the effect size was small, raising concerns about its clinical significance. Within-session stimulus repetition was associated with slight delays in the N170 latency compared to the stimuli presented once, but this finding was not specific to faces. Test-retest reliability of the N170 latency was moderate. Our results are consistent with the comments by Vettori et al.(15) that the N170 latency may be less face-specific than its amplitude.
Sensitivity to heterogeneity in social functioning
After replicating the established N170 response characteristics in our passive viewing paradigm, we examined sensitivity of the N170 to individual differences in social functioning. The extensive battery of standardized behavioral measures included gold-standard assessments of autism symptomatology, direct testing of social information processing (face memory), and caregiver reports of social skills and adaptive functioning. Real-life social behavior was systematically characterized using the naturalistic Playground Interaction Paradigm(35).
The exploratory correlational analysis involving the N170 amplitude for repeated and single presentations of faces and houses revealed sporadic and mostly weak (r <.4) brain-behavior associations at baseline and posttest. Applying statistical correction for multiple significance testing to these results would have further reduced the number of detected associations. The N170 amplitude was not significantly associated with age at baseline, while at posttest, a small partial correlation was observed after controlling for the EXP group membership, with increasing age being associated with smaller N170 responses to faces and larger responses to houses. The most consistent pattern of significant effects across the two testing times was between the smaller N170 amplitude to houses and higher IQ scores, particularly the performance IQ. Of particular note, few correlations were observed between the N170 amplitude and standardized measures of social cognition (NEPSY Memory for Faces) or daily social functioning (SRS, SCQ) at baseline: smaller N170 responses to houses were associated with higher scores on all of these measures. The same correlations were not present at posttest, where only reduced N170 amplitude to faces was related to higher SRS scores.
Compared to the amplitude measures, the N170 latency appeared to be more sensitive to individual differences in social functioning at baseline. Yet, similar to patterns observed for the amplitude, most of the significant correlations were with the N170 latency to the nonsocial stimuli. Delayed N170 response to houses was related to higher ADOS scores, while faster latencies were associated with higher IQ scores and better NEPSY Memory for Faces. Similar to the amplitudes, most of these associations were not observed at posttest.
Correlations between the N170 amplitude and real-life social interactions revealed largely the same pattern of a few weak associations that were not consistently present across baseline and posttest. Correlations with the N170 latency reached significance mainly at posttest, when faster responses to faces and houses were associated with longer periods of verbal interaction and cooperative play.
In combination, these results suggest that the N170 amplitude and latency in ASD may be weakly associated with distinct aspects of social and adaptive functioning: the amplitudes reflected more general nonverbal intelligence, while the latencies showed associations with autism symptomatology, memory for faces, and real-life social behavior. Importantly, for both measures, the observed correlations were not specific to faces – the greatest number of brain-behavior associations was with the nonsocial stimuli. Furthermore, the correlations were small, and generally not repeatable across two time points. Thus, while the N170 characteristics appear to be sensitive to some aspects of individual differences in social functioning, the reliability of such connections may be low.
Sensitivity to treatment effects
In the context of a social skills training program with known efficacy(37–39), analyses revealed no clear evidence of sensitivity to treatment effects (no Time × Group interactions) for the N170 amplitude or latency. Yet, there were significant increases in behavioral performance on NEPSY Memory for Faces (immediate and delayed) in both groups, and the EXP group also showed improvements on the SRS, SCQ, and ABAS. Previously, Faja et al.(25) reported a similar lack of the N170 sensitivity to treatment effects following a perceptual expertise training that resulted in behavioral improvements in adults with ASD. Thus, the N170 metrics may not be sensitive to changes in social functioning, and instead reflect a stable perceptual trait in ASD.
This observation extends support for the idea that purely perceptual deficits may not fully explain social difficulties in ASD(7; 8). We previously identified a parietal “old/new” response elicited within 250–500ms after stimulus onset that indexed spontaneous recognition of stimulus repetition(29). That response was specific to faces (no effect for houses), greater in typical children than those with ASD, and correlated with the aforementioned behavioral measures of social functioning. It was also sensitive to treatment effects (i.e., increased in the EXP group, unchanged in the WLC group(39)), including in the current sample (Corbett et al., under review). Consideration of the EEG/ERP metrics indexing face recognition (see also(50)) as potential biomarkers of social information processing in ASD would fit with the social motivation theory of ASD(20; 51). Social salience can be indexed by incidental memory for faces, a cognitive ability dependent on sufficient engagement with the stimuli and allocation of adequate cognitive processing resources (i.e., beyond initial sensory-perceptual processes associated with stimulus detection).
Conclusions
This study aimed to examine whether the N170 response could serve as a stratification or treatment effects biomarker of social functioning in ASD. Many ERP responses have known neural sources and well-established functional interpretations: in case of the N170, it reflects activity of the fusiform gyrus associated with expert visual processing and is typically larger for faces than other stimuli. In the current study, the N170 response was successfully recorded using a passive viewing task in youth with ASD and varied intellectual and adaptive functioning. We replicated the larger N170 amplitude to faces than nonsocial stimuli and observed moderate test-retest stability across two time points.
However, the N170 amplitude and latency showed limited sensitivity to individual differences in social functioning in youth with ASD. The observed correlations were small, not consistently repeatable across the two time points, and often involved the N170 response to houses rather than faces. Therefore, the N170 response does not fit the definition of a social deficit biomarker in ASD that could be used for sample characterization or stratification. Of note, our exploratory correlational analyses included a variety of behavioral assessments commonly used in ASD research and deliberately minimized Type 2 error. Our sample (n=77) was at least twice the size of that in the previous studies that reported significant correlations between N170 latency and face processing in ASD (e.g., n=15 in(13); 36 in(18); 34 in(19)), and therefore provided sufficient power to detect even small correlations. Thus, the lack of strong and consistent brain-behavior associations for the N170 response is not likely to be explained by low statistical power. Replication of the canonical N170 characteristics (larger amplitude for faces than houses, particularly in the right hemisphere) also rules out the possibility that our passive viewing paradigm or the selected electrode clusters were not optimal for eliciting the N170 response.
Our data also did not support the use of the N170 as a biomarker of treatment effects. Neither amplitude nor latency measures were sensitive to change following a social skills intervention that resulted in improved behavioral performance on standardized measures and in real-life social interactions. It is possible that the N170 response reflects a basic social perceptual process that may not be malleable by a treatment targeting social behaviors rather than basic face detection.
In sum, our results do not support the notion that the N170 latency is a biomarker of social deficits in ASD. It may be a frequently used and psychometrically stable measure of one domain of functioning - basic perceptual face processing, but it is not sufficiently informative about heterogeneity of social functioning and other characteristics of autism. Therefore, the search for a “brain signature” of ASD or social difficulties in general must continue and expand to include other measures to move the field forward.
Acknowledgements
This work was supported, in part, by NIMH R34 MH097793 (Corbett), NICHD U54HD083211 to Vanderbilt Kennedy Center, and a VKC Hobbs Discovery Award (Corbett & Key).
Financial Disclosures
Alexandra Key reported no biomedical financial interests or potential conflicts of interest. Blythe Corbett is the founder of SENSE Theatre® but derives no financial compensation from the nonprofit 501(c)(3) entity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association (2013): Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) American Psychiatric Pub. [Google Scholar]
- 2.Kennedy DP, Adolphs R (2012): The social brain in psychiatric and neurological disorders. Trends in Cognitive Sciences 16: 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop-Fitzpatrick L, Mazefsky CA, Eack SM, Minshew NJ (2017): Correlates of Social Functioning in Autism Spectrum Disorder: The Role of Social Cognition. Research in Autism Spectrum Disorders 35: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klin A, Sparrow SS, de Bildt A, Cicchetti DV, Cohen DJ, Volkmar FR (1999): A normed study of face recognition in autism and related disorders. J Autism Dev Disord 29: 499–508. [DOI] [PubMed] [Google Scholar]
- 5.Kuusikko S, Haapsamo H, Jansson-Verkasalo E, Hurtig T, Mattila M-L, Ebeling H, et al. (2009): Emotion recognition in children and adolescents with autism spectrum disorders. Journal of Autism and Developmental Disorders 39: 938–945. [DOI] [PubMed] [Google Scholar]
- 6.Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J (2002): Visual scanning of faces in autism. J Autism Dev Disord 32: 249–261. [DOI] [PubMed] [Google Scholar]
- 7.Jemel B, Mottron L, Dawson M (2006): Impaired Face Processing in Autism: Fact or Artifact? J Autism Dev Disord 36: 91–106. [DOI] [PubMed] [Google Scholar]
- 8.Weigelt S, Koldewyn K, Kanwisher N (2012): Face identity recognition in autism spectrum disorders: A review of behavioral studies. Neuroscience & Biobehavioral Reviews 36: 1060–1084. [DOI] [PubMed] [Google Scholar]
- 9.Kang E, McPartland JC, Keifer CM, Foss-Feig JH, Levy EJ, Lerner MD (2019): Reply to: Can the N170 Be Used as an Electrophysiological Biomarker Indexing Face Processing Difficulties in Autism Spectrum Disorder? Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 4: 324–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woo C-W, Chang LJ, Lindquist MA, Wager TD (2017): Building better biomarkers: brain models in translational neuroimaging. Nat Neurosci 20: 365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bentin S, Allison T, Puce A, Perez E (1996): Electrophysiological studies of face perception in humans. J Cogn Neurosci 8: 551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossion B (2014): Understanding face perception by means of human electrophysiology. Trends in Cognitive Sciences 18: 310–318. [DOI] [PubMed] [Google Scholar]
- 13.McPartland J, Dawson G, Webb SJ, Panagiotides H, Carver LJ (2004): Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. J Child Psychol & Psychiat 45: 1235–1245. [DOI] [PubMed] [Google Scholar]
- 14.Kang E, Keifer CM, Levy EJ, Foss-Feig JH, McPartland JC, Lerner MD (2018): Atypicality of the N170 Event-Related Potential in Autism Spectrum Disorder: A Meta-analysis. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 3: 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vettori S, Jacques C, Boets B, Rossion B (2019): Can the N170 Be Used as an Electrophysiological Biomarker Indexing Face Processing Difficulties in Autism Spectrum Disorder? Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 4: 321–323. [DOI] [PubMed] [Google Scholar]
- 16.Uddin LQ, Dajani DR, Voorhies W, Bednarz H, Kana RK (2017): Progress and roadblocks in the search for brain-based biomarkers of autism and attention-deficit/hyperactivity disorder. Translational Psychiatry 7: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McPartland JC (2017): Developing Clinically Practicable Biomarkers for Autism Spectrum Disorder. J Autism Dev Disord 47: 2935–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McPartland JC, Wu J, Bailey CA, Mayes LC, Schultz RT, Klin A (2011): Atypical neural specialization for social percepts in autism spectrum disorder. Social Neuroscience 6: 436–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lerner MD, McPartland JC, Morris JP (2013): Multimodal emotion processing in autism spectrum disorders: An event-related potential study. Developmental Cognitive Neuroscience 3: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dawson G, Webb SJ, McPartland J (2005): Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Developmental Neuropsychology 27: 403–424. [DOI] [PubMed] [Google Scholar]
- 21.Webb SJ, Merkle K, Murias M, Richards T, Aylward E, Dawson G (2012): ERP responses differentiate inverted but not upright face processing in adults with ASD. Social cognitive and affective neuroscience 7: 578–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garman HD, Spaulding CJ, Webb SJ, Mikami AY, Morris JP, Lerner MD (2016): Wanting it Too Much: An Inverse Relation Between Social Motivation and Facial Emotion Recognition in Autism Spectrum Disorder. Child Psychiatry Hum Dev 1–13. [DOI] [PMC free article] [PubMed]
- 23.Dawson G, Jones EJH, Merkle K, Venema K, Lowy R, Faja S, et al. (2012): Early Behavioral Intervention Is Associated With Normalized Brain Activity in Young Children With Autism. J Am Acad Child Adolesc Psychiatry 51: 1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hileman CM, Henderson H, Mundy P, Newell L, Jaime M (2011): Developmental and Individual Differences on the P1 and N170 ERP Components in Children With and Without Autism. Developmental Neuropsychology 36: 214–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faja S, Webb SJ, Jones E, Merkle K, Kamara D, Bavaro J, et al. (2012): The Effects of Face Expertise Training on the Behavioral Performance and Brain Activity of Adults with High Functioning Autism Spectrum Disorders. J Autism Dev Disord 42: 278–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. (2000): The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 30: 205–223. [PubMed] [Google Scholar]
- 27.Rutter M, Bailey A, Lord C (2003): The Social Communication Questionnaire Los Angeles: Western Psychological Services. [Google Scholar]
- 28.Wechsler D (1999): Manual for the Wechsler Abbreviated Scale of Intelligence New York. [Google Scholar]
- 29.Key AP, Corbett BA (2014): ERP Responses to Face Repetition During Passive Viewing: A Nonverbal Measure of Social Motivation in Children With Autism and Typical Development. Developmental Neuropsychology 39: 474–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langner O, Dotsch R, Bijlstra G, Wigboldus D, Hawk S, van Knippenberg A (2010): Presentation and validation of the Radboud Faces Database. Cognition & Emotion 24: 1377–1388. [Google Scholar]
- 31.Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, et al. (2000): Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology 37: 127–152. [PubMed] [Google Scholar]
- 32.Korkman M, Kirk U, Kemp S (2007): NEPSY 2nd Edition. San Antonio, TX: Harcourt Assessment. [Google Scholar]
- 33.Constantino JN, Gruber CP (2005): Social Responsiveness Scale Los Angeles, CA: Western Psychological Services. [Google Scholar]
- 34.Harrison P, Oakland T (2000): Adaptive Behavior Assessment System San Antonio, TX: Psychological Corporation. [Google Scholar]
- 35.Corbett BA, Swain DM, Newsom C, Wang L, Song Y, Edgerton D (2014): Biobehavioral profiles of arousal and social motivation in autism spectrum disorders. J Child Psychol & Psychiat 55: 924–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noldus (2008): The Observer XT (Vol. 10.5) Wageningen, The Netherlands: Noldus Information Technology. [Google Scholar]
- 37.Corbett BA, Gunther JR, Comins D, Price J, Ryan N, Simon D, et al. (2011): Brief report: theatre as therapy for children with autism spectrum disorder. J Autism Dev Disord, 2nd ed. 41: 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corbett BA, Swain DM, Coke C, Simon D, Newsom C, Houchins-Juarez N, et al. (2014): Improvement in social deficits in autism spectrum disorders using a theatre-based, peer-mediated intervention. Autism Res 7: 4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corbett BA, Key AP, Qualls L, Fecteau S, Newsom C, Coke C, Yoder P (2016): Improvement in Social Competence Using a Randomized Trial of a Theatre Intervention for Children with Autism Spectrum Disorder. J Autism Dev Disord 46: 658–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corbett BA, Qualls LR, Valencia B, Fecteau S-M, Swain DM (2014): Peer-mediated theatrical engagement for improving reciprocal social interaction in autism spectrum disorder. Front Pediatr 2: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perrin F, Pernier J, Bertrand O, Echallier JF (1989): Spherical splines for scalp potential and current density mapping. Electroencephalography and clinical Neurophysiology 72: 184–187. [DOI] [PubMed] [Google Scholar]
- 42.Key AP, Dykens EM (2016): Face repetition detection and social interest: An ERP study in adults with and without Williams syndrome. Social Neuroscience 11: 652–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Key AP, Dykens EM (2017): Incidental memory for faces in children with different genetic subtypes of Prader-Willi syndrome. Social cognitive and affective neuroscience 12: 918–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grice SJ, Spratling MW, Karmiloff-Smith A, Halit H, Csibra G, de Haan M, Johnson MH (2001): Disordered visual processing and oscillatory brain activity in autism and Williams syndrome. NeuroReport 12: 2697–2700. [DOI] [PubMed] [Google Scholar]
- 45.Bentin S, Deouell LY (2000): Structural encoding and identification in face processing: ERP evidence for separate mechanisms. Cognitive Neuropsychology 17: 35–55. [DOI] [PubMed] [Google Scholar]
- 46.Webb SJ, Jones EJH, Merkle K, Murias M, Greenson J, Richards T, et al. (2010): Response to familiar faces, newly familiar faces, and novel faces as assessed by ERPs is intact in adults with autism spectrum disorders. International Journal of Psychophysiology 77: 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rossion B (2003): A network of occipito-temporal face-sensitive areas besides the right middle fusiform gyrus is necessary for normal face processing. Brain 126: 2381–2395. [DOI] [PubMed] [Google Scholar]
- 48.Cassidy SM, Robertson IH, O’Connell RG (2012): Retest reliability of event-related potentials: Evidence from a variety of paradigms. Psychophysiology 49: 659–664. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka JW, Curran T (2001): A Neural Basis for Expert Object Recognition. Psychological Science 12: 43–47. [DOI] [PubMed] [Google Scholar]
- 50.Vettori S, Dzhelyova M, Van der Donck S, Jacques C, Steyaert J, Rossion B, Boets B (2019): Reduced neural sensitivity to rapid individual face discrimination in autism spectrum disorder. Neuroimage: Clinical 21: 101613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT (2012): The social motivation theory of autism. Trends in Cognitive Sciences 16: 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]