Abstract
Cardiac CT offers several approaches to establish the hemodynamic severity of coronary artery obstructions. Dynamic myocardial perfusion CT (MPICT) is based on serial CT imaging to measure the inflow of contrast medium into the myocardium and calculate absolute measures of myocardial perfusion. This review describes the MPICT acquisition protocol, post-image acquisition processing and calculation of quantitative parameters, the diagnostic performance of MPICT and the potential incremental value of this technique in comparison to alternative approaches. Further technical innovation using different scanner platforms and establishment of reproducible diagnostic thresholds to differentiate significant coronary artery disease will be crucial in the path to broader clinical implementation.
Keywords: computed tomography, myocardial perfusion imaging, coronary artery disease, myocardial blood flow
1.1. Principles of dynamic CT myocardial perfusion imaging
Dynamic stress myocardial perfusion CT (MPICT) is performed by acquiring a series of CT images after injection of a bolus of contrast medium during pharmacological hyperemia, similar to perfusion imaging technique by MRI or PET. Dynamic MPICT was first demonstrated in the late 1970s using electron-beam CT, but this technique never reached broad clinical application, partly due to the inability of scanners at the time to image the entire myocardium in a single acquisition1. To measure blood flow throughout the left ventricle, today dynamic MPICT is performed on scanners with a detector-row width that covers the entire ventricle in 1 or 2 acquisitions. This requirement restricts dynamic MPICT to the latest generation wide-detector CT systems and 2nd/3rd generation dual-source CT systems. In order to limit the cumulative dose of a serial acquisition protocol, individual datasets are acquired using a low tube potential. Nevertheless, exposure is generally higher than what can be achieved with static MPICT protocols. By measuring regional myocardial attenuation over time quantitative measures of myocardial perfusion can be calculated throughout the myocardium of the left ventricle and displayed as volumetric perfusion maps. Due to the high iodine concentration in the ventricular cavity, beam hardening artifacts will inappropriately reduce attenuation values within the myocardium, particularly in the basal inferior wall positioned between the contra-enhanced ventricle and descending aorta. Correction of these beam hardening artifacts is essential for accurate myocardial perfusion imaging. Dynamic iterative correction algorithms that take into account different tissue types and changes in iodine content over time perform better than conventional beam-hardening correction algorithms2, 3.
1.2. Scan protocol
Patients are required to abstain from caffeine 12-24h prior to the examination. If CT angiography is performed prior to the perfusion scan, a 10-15 minutes delay is recommended for washout of contrast medium. Adenosine or another vasodilators are administered through an intravenous cannula for 3-5 minutes. Regadenoson is administrated as a single bolus. The cardiac rhythm is continuously monitored and the blood pressure is measured at regular intervals. Just before the scan, a short, high-rate iodine contrast bolus is injected through a separate intravenous cannula followed by a saline bolus. Imaging starts just before the contrast reaches the right ventricle. Dual-source CT scanners have a longitudinal coverage of 3.8-5.8 cm, which is sufficient to cover the heart in two scans. The table moves back and forth between acquisitions resulting in a sampling rate of one complete dataset every 4 seconds, or every 6 seconds in cases of very fast heart rates. During a 30s breath hold, between 10 to 15 complete, low-dose datasets are acquired. Some MPICT protocols on wide-detector systems allow for superficial breathing by providing a post-acquisition displacement correction4. If a CT system with full cardiac coverage is used, a full dataset could be acquired every heart cycle. However, to limit roentgen exposure, the sampling rate can be altered during the exam with the objective of acquiring a maximum sampling rate during the crucial phase of myocardial contrast inflow and a lower sampling rate before and after4. In most studies, end-systolic datasets have been acquired to minimize beam-hardening artifacts from the contrast-filled LV cavity.
When MPICT is performed as part of a combined examination with coronary CTA, the so-called stress-rest protocol, this has logistic advantages because no delay is needed between the MPICT and CTA for washout of contrast medium from the myocardium. However, in clinical practice CTA effectively rules out coronary disease in the majority of patients with new suspected ischemic heart disease, in which case a perfusion scan is redundant and unnecessarily exposes patients to contrast medium and radiation. Particularly for patients without known coronary disease, an approach that starts with CTA, selectively followed by stress MPICT if angiographic lesions are detected on CTA, would be a more efficient approach in terms of effort and radiation exposure. The need for immediate CTA interpretation represents a logistic drawback to this approach, as well as the required contrast wash-out delay before MPICT can be performed5.
1.3. Calculation of quantitative parameters of myocardial perfusion
Despite best efforts by patients to hold still, often there will be some degree of displacement of the myocardium during the long scan period. Because it is important to compare attenuation values over time for the same tissue, correction of this gradual displacement is a crucial step in the data processing. Rigid and non-rigid transformation techniques can be applied to realign the 2D cross-sections, ideally compensating for in-plane as well as through-plane displacement of the ventricle. Rhythm irregularities that result in data acquisition during a different phase of contraction are more difficult to correct and may require exclusion from the quantitative analyses. The next step is to isolate the ventricle and discretize the myocardium into small volumetric elements. Within each myocardial element the measured attenuation values are plotted against time. The arterial input function is derived from a sample volume in the descending aorta. The myocardial time-attenuation curves are coupled with the arterial-input function using a hybrid deconvolution model, which uses a simplified impulse-residue function for modeling the interaction between the intravascular and extravascular compartments, after which the myocardial blood flow can be calculated by dividing the convoluted maximal slope of the myocardial time-attenuation curve by the maximum arterial input function6. Myocardial blood flow, as well as other parameters of myocardial perfusion like perfused capillary blood volume, and first-pass distribution volume, are reconstructed as color-coded volumetric maps. Regions of interest can be sampled manually to obtain the average myocardial blood flow. Alternatively, polar maps or bull-eye plots, can be created, which summarize myocardial perfusion parameters in a single image and report myocardial MBF per standardized myocardial segment using the 16/17-segment AHA classification. MBF measured by CT is generally lower than by other perfusion techniques, which is partly related to lower sampling rates of dual-source systems. MBF also varies between individuals, for various clinical and technical reasons7, 8. Reported MBF cut-off values that signify hemodynamic significance vary substantially from 75 and 164 ml/min/100ml between studies [Table 1]. Due to these inconsistencies, normalization of regional MBF values relative to a measure of global MBF appears to improve the diagnostic accuracy of MPICT6, 9-11.
Table 1: Diagnostic performance of dynamic MPICT.
Summary of studies comparing dynamic stress CT myocardial perfusion imaging (MPI) against invasive coronary angiography (ICA) with fractional flow reserve (FFRcath) or other noninvasive functional tests. Sensitivity (sens), specificity (spec) and area under the curve (AUC) are listed on a per-vessel basis, and compared to CT angiography, if data available. Myocardial blood flow (MBF, ml/min/100cc myocardium), single/dual source computed tomography (SSCT/DSCT), coronary flow rate (CFR).
| Author (year) |
N | CT system (vendor) |
Dose (mSv) |
MBF Cut-off |
Reference | CTA | MPICT | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sens (%) |
Spec (%) |
AUC | Sens (%) |
Spec (%) |
AUC | ||||||
| Bamberg 2011 | 33 | 128-slice DSCT (Siemens) | 75 | ICA/FFRcath | 100 (88-100) | 51 (39-63) | 93 (77-99) | 87 (76-94) | |||
| Wang 2012 | 30 | 128-slice DSCT (Siemens) | 9.5±1.3 | NR | MPISPECT | 90 | 51 | 100 | 75.7 | ||
| Weiniger 2012 | 10 | 128-slice DSCT (Siemens) | 12.8±2.4 | Visual | MPIMRI | 86 | 98 | ||||
| MPISPECT | 84 | 92 | |||||||||
| Huber 2013 | 32 | 256 SDCT (Philips) | 9.5 | 164 | ICA >75% FFRcath ≤ 0.75 | 76 (57-90) | 100 (95-100) | 0.86 | |||
| Rossi 2014 | 99 | 128-slice DSCT (Siemens) | 78 | FFRcath ≤ 0.75 | 61 (77-94) | 77 (84-97) | 85 (69-94 | 89 (78-95) | 0.95 | ||
| Kono 2014 | 42 | 128-slice DSCT (Siemens) | 9.4 | 0.85 (index) | FFRcath ≤ 0.80 | 98 | 70 | 0.85 | |||
| Kikuchi 2014 | 32 | 320-slice SSCT (Canon) | 12.8±2.9 | 2.97 (CFR) | MPIPET | 86 | 92 | 0.90 | |||
| Tanabe 2016 | 53 | 256 MDCT (Philips) | 10.5-10.6 | 92 | MPISPECT (N=25) | 95 (52-100) | 72 (53-91) | 0.87 | |||
| 98 | MPIMRI (N=28) | 78 (67-97) | 80 (58-86) | 0.89 | |||||||
| Coenen 2017 | 74 | 128-slice DSCT (Siemens) | 9.3-1.8 | 0.71 (index) | ICA/FFRcath ≤0.80 | 78 (65-90) | 49 (38-61) | 0.70 | 73 (61-86) | 68 (56-80 | 0.75 |
| Rossi 2017 | 115 | 128/192-DSCT (Siemens) | 6.0-10.3 | 75 (index) | ICA/FFRcath ≤0.80 | 35 (21–53) | 95 (81–99) | 0.65 | 89 (76-96) | 73 (59-83 | 0.85 |
| Pontone 2019 | 85 | 256-slice SSCT (GE) | 5.3±0.7 | 101 | ICA/FFRcath | 83 75-91) | 66 (9-73) | 0.83 | 73 (63-83) | 86 (81-91) | 0.88 |
| Nishiyama 2019 | 38 | 256-slice SSCT (Philips) | 10.2±1.2 | 126 | ICA/FFRcath <0.75 | 96 (88–100) | 57 (46–67) | 0.84 | 83 (68–98) | 93 (88–98) | 0.96 |
| Alessio 2019 | 34 | 256-slice SSCT (GE) | 8.4±1.1 | 126 | 82-Rubidium PET | 75 | 83 | NR | |||
2.1. Diagnostic performance
The diagnostic performance of MPICT has been validated in several animal studies6, 12, 13. Myocardial blood flow measured by CT demonstrated good correlation with directly measured coronary flow, fractional flow reserve and MBF determined by microspheres. In human patients the performance of MPICT has been compared against catheter-based FFR as well as other myocardial perfusion imaging techniques4, 6, 9-11, 14-22, summarized in table 1. The studied cohorts were typically referred for clinically indicated invasive angiography and therefore had a relatively high coronary disease burden. MBF or indexed MBF demonstrated superior and incremental discriminatory value over CTA in most studies. According to a meta-analysis by Lu et al, dynamic MPICT identifies hemodynamically significant coronary artery disease with a sensitivity and specificity of 85% and 81% on a per-vessel basis, compared to 82% and 61% by CTA23. MPICT relies on consistent myocardial sampling and the diagnostic performance is negatively affected by gross cardiac motion and arrhythmia. Incidental rhythm irregularities can be corrected by excluding a specific phase from the analysis. Several studies reported improved diagnostic performance using indexed MBF values, which are relative MBF values normalized against remote myocardium or the 75th percentile of the left ventricle MBF, to neutralize global differences in measured MBF values between individuals and examinations10, 11.
To differentiate reversible ischemia from prior infarction some centers perform both a rest and a stress perfusion scan. To avoid an additional scan, the coronary CT angiogram can often serve as a static resting perfusion scan24. In case of prior infarction, the dynamic MPICT will show very low MBF values25. CT imaging of late iodine enhancement represents an alternative option to identify myocardial scar26-28.
2.2. Prognostic value
There have been several registry reports from modest-size cohorts on the prognostic value of MPICT. Nakamura et al followed 332 patients with suspected coronary disease for 2.5 years and showed that the summed stress score based on normalized MBF by MPICT predicted a composite endpoint of cardiac death, nonfatal myocardial infarction, unstable angina, or hospitalization for congestive heart failure, with an incremental prognostic value on CTA based coronary stenosis: hazard ratio 5.7; 95% confidence interval: 1.9 to 16.9; p = 0.00229. In a cohort of 81 patients, Assen et al reported that indexed MBF by MPICT predicted adverse events, including cardiac death, nonfatal myocardial infarction, unstable angina requiring hospitalization, or revascularization, over 18 months: hazard ratio 11.4 (95% confidence interval: 3.4 to 38.2; p<0.001), with superior and independent predictive value compared to CTA and CT-FFR8.
2.3. Clinical effectiveness
In one prospective, randomized controlled trial between a tiered cardiac CT protocol that included dynamic MPICT and conventional stress testing for patients with stable chest pain symptoms5, the cardiac CT approach was associated with a higher rate of coronary disease with a class I indication for revascularization by ESC standards (88% vs 50%), without increasing catheterization rates.
3.1. Comparison of dynamic MPICT to other noninvasive functional tests
Dynamic MPICT is technically somewhat more demanding than static MPICT, but has the conceptual advantage of absolute blood flow measurements. Quantitative measures are potentially valuable for differentiating degrees of ischemia and changes over time, and may allow for quantification of microvascular disease. Anecdotally, dynamic perfusion MPICT is less susceptible to beam-hardening artifacts. No large-scale direct comparative studies have been performed between dynamic and static MPICT in humans. However, a meta-analysis by Danad et al, suggests that dynamic MPICT may be more accurate, albeit at the expense of a higher radiation dose30. At the time of their analysis, the radiation dose of MPICT was around 10mSv, based largely on studies with 2nd generation dual-source CT systems and higher kVp settings. More recent studies using contemporary CT technology show that dynamic MPICT is possible at doses around 5mSv or less4, 14, 31. Meta-analyses indicate that the diagnostic accuracy of static/dynamic MPICT is comparable to other stress imaging modalities using invasive FFR as reference32 . On a per-vessel level, Takx et al reported that the diagnostic accuracy of MPICT (AUC 0.93) was in the same range as perfusion imaging by MRI (AUC 0.94) and PET (AUC 0.93), and these perfusion imaging modalities outperformed stress echocardiography (AUC 0.82) and SPECT perfusion imaging (AUC 0.83)32. Although meta-analyses cannot be considered conclusive, there is currently no indication that MPICT performs inferior to other stress imaging techniques. MPICT also has the practical advantage that it can be performed in conjunction with coronary CTA, and images may be merged for a comprehensive interpretation of coronary artery disease. Additional radiation and contrast medium exposure represent a drawback of MPICT compared to MRI, echocardiography and stress testing without imaging.
CT-derived FFR represents an alternative approach to assess the hemodynamic significance of coronary disease on CTA. Dynamic MPICT has been compared to CT-FFR in several studies, and showed comparable performance and complementary value4, 9. Coenen, et al. concluded that for patients with an on-site performed CT-FFR result within the diagnostic grey zone, MPICT provided an efficient approach to improve diagnostic accuracy in the detection of hemodynamically significant coronary lesions9. Pontone, et al. confirmed these observations using a commercially available CT-FFR solution and showed that a similar stepwise approach increased diagnostic performance (AUC increased from 0.88 to 0.92, P<0.05)4.
4.1. Future developments
The current evidence for dynamic MPICT is based on relatively small, often single-center cohorts. More than half of these studies were performed on dual-source CT systems from one specific manufacturer. For dynamic MPICT to mature into a clinically used diagnostic tool it will be important to validate the technique in larger multicenter cohorts using different CT systems33. Inter-individual variation in measured global myocardial blood flow challenges interpretation of absolute perfusion values. This necessitates the use of normalized MBF indexes to identify inducible myocardial ischemia. Further research is needed to identify perfusion parameters that best identify significant myocardial ischemia. Comprehensive interpretation and revascularization decision making would benefit from a robust infarct imaging technique for CT. Finally, it will be important to develop more efficient scan protocols to achieve the highest diagnostic value with the lowest effective dose. Prospective studies in larger cohorts are needed to determine the most efficient deployment of MPICT and assess its performance against other diagnostic tests for the diagnostic evaluation of patients with suspected or known coronary disease.
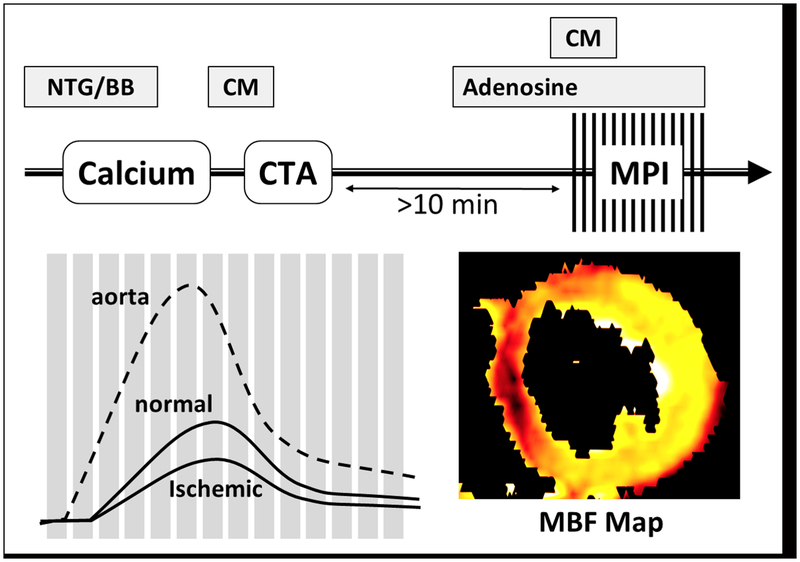
Figure 1: Acquisition protocol.
For a “rest-stress approach”, CTA is performed first, followed by dynamic stress CT myocardial perfusion imaging in case of abnormalities. A delay between both scans is required for contrast medium washout. A low-dose native scan is performed during systole to plan the perfusion scan, which is also performed during systole. Attenuation values throughout the myocardium and the aorta are plotted against time, from which myocardial perfusion maps can be reconstructed. Nitroglycerin (NTG), beta-blocker (BB), contrast medium (CM), myocardial perfusion imaging (MPI), myocardial blood flow (MBF).
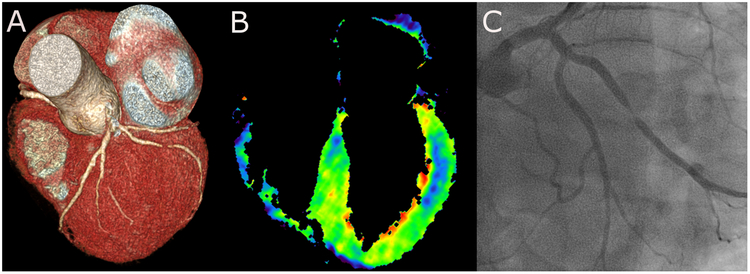
Figure 2: MPICT case example.
CT angiography demonstrating a severe lesion in a large marginal branch off the left circumflex coronary artery (A). The myocardial blood flow map obtained by dynamic MPICT shows a distinct defect in the mid antero-lateral wall indicating hemodynamic significance of the coronary lesion (B). Confirmation of a high-grade stenotic lesion in the marginal branch by invasive angiography.
Abbreviations
- AUC
Area under the curve
- CT
Computed tomography
- CTA
Computed tomography angiography
- ESC
European Society of Cardiology
- FFR
Fractional Flow Reserve
- MBF
Myocardial blood flow
- MPI
Myocardial perfusion imaging
- MRI
Magnetic resonance imaging
- PET
Positron emission tomography
- SPECT
Single-photon emission computed tomography
Footnotes
Disclosures: Koen Nieman received institutional research support from Siemens Healthineers, Bayer Healthcare, GE Healthcare and Heartflow Inc.
References
- 1.Robb RA and Ritman EL. High speed synchronous volume computed tomography of the heart. Radiology. 1979;133:655–61. [DOI] [PubMed] [Google Scholar]
- 2.Stenner P, Schmidt B, Allmendinger T, Flohr T and Kachelrie M. Dynamic iterative beam hardening correction (DIBHC) in myocardial perfusion imaging using contrast-enhanced computed tomography. Investigative radiology. 2010;45:314–23. [DOI] [PubMed] [Google Scholar]
- 3.So A, Hsieh J, Li JY and Lee TY. Beam hardening correction in CT myocardial perfusion measurement. Physics in medicine and biology. 2009;54:3031–50. [DOI] [PubMed] [Google Scholar]
- 4.Pontone G, Baggiano A, Andreini D, Guaricci AI, Guglielmo M, Muscogiuri G, Fusini L, Soldi M, Del Torto A, Mushtaq S, Conte E, Calligaris G, De Martini S, Ferrari C, Galli S, Grancini L, Olivares P, Ravagnani P, Teruzzi G, Trabattoni D, Fabbiocchi F, Montorsi P, Rabbat MG, Bartorelli AL and Pepi M. Dynamic Stress Computed Tomography Perfusion With a Whole-Heart Coverage Scanner in Addition to Coronary Computed Tomography Angiography and Fractional Flow Reserve Computed Tomography Derived. JACC Cardiovascular imaging. 2019. [DOI] [PubMed] [Google Scholar]
- 5.Lubbers M, Coenen A, Kofflard M, Bruning T, Kietselaer B, Galema T, Kock M, Niezen A, Das M, van Gent M, van den Bos EJ, van Woerkens L, Musters P, Kooij S, Nous F, Budde R, Hunink M and Nieman K. Comprehensive Cardiac CT With Myocardial Perfusion Imaging Versus Functional Testing in Suspected Coronary Artery Disease: The Multicenter, Randomized CRESCENT-II Trial. JACC Cardiovascular imaging. 2018;11:1625–1636. [DOI] [PubMed] [Google Scholar]
- 6.Rossi A, Dharampal A, Wragg A, Davies LC, van Geuns RJ, Anagnostopoulos C, Klotz E, Kitslaar P, Broersen A, Mathur A, Nieman K, Hunink MG, de Feyter PJ, Petersen SE and Pugliese F. Diagnostic performance of hyperaemic myocardial blood flow index obtained by dynamic computed tomography: does it predict functionally significant coronary lesions? European heart journal cardiovascular Imaging. 2014;15:85–94. [DOI] [PubMed] [Google Scholar]
- 7.Yokoi T, Tanabe Y, Kido T, Kurata A, Kido T, Uetani T, Ikeda S, Izutani H, Miyagawa M and Mochizuki T. Impact of the sampling rate of dynamic myocardial computed tomography perfusion on the quantitative assessment of myocardial blood flow. Clinical imaging. 2019;56:93–101. [DOI] [PubMed] [Google Scholar]
- 8.van Assen M, De Cecco CN, Eid M, von Knebel Doeberitz P, Scarabello M, Lavra F, Bauer MJ, Mastrodicasa D, Duguay TM, Zaki B, Lo GG, Choe YH, Wang Y, Sahbaee P, Tesche C, Oudkerk M, Vliegenthart R and Schoepf UJ. Prognostic value of CT myocardial perfusion imaging and CT-derived fractional flow reserve for major adverse cardiac events in patients with coronary artery disease. Journal of cardiovascular computed tomography. 2019. [DOI] [PubMed] [Google Scholar]
- 9.Coenen A, Rossi A, Lubbers MM, Kurata A, Kono AK, Chelu RG, Segreto S, Dijkshoorn ML, Wragg A, van Geuns RM, Pugliese F and Nieman K. Integrating CT Myocardial Perfusion and CT-FFR in the Work-Up of Coronary Artery Disease. JACC Cardiovascular imaging. 2017;10:760–770. [DOI] [PubMed] [Google Scholar]
- 10.Kono AK, Coenen A, Lubbers M, Kurata A, Rossi A, Dharampal A, Dijkshoorn M, van Geuns RJ, Krestin GP and Nieman K. Relative myocardial blood flow by dynamic computed tomographic perfusion imaging predicts hemodynamic significance of coronary stenosis better than absolute blood flow. Investigative radiology. 2014;49:801–7. [DOI] [PubMed] [Google Scholar]
- 11.Rossi A, Wragg A, Klotz E, Pirro F, Moon JC, Nieman K and Pugliese F. Dynamic Computed Tomography Myocardial Perfusion Imaging: Comparison of Clinical Analysis Methods for the Detection of Vessel-Specific Ischemia. Circulation Cardiovascular imaging. 2017;10. [DOI] [PubMed] [Google Scholar]
- 12.Bamberg F, Hinkel R, Schwarz F, Sandner TA, Baloch E, Marcus R, Becker A, Kupatt C, Wintersperger BJ, Johnson TR, Theisen D, Klotz E, Reiser MF and Nikolaou K. Accuracy of dynamic computed tomography adenosine stress myocardial perfusion imaging in estimating myocardial blood flow at various degrees of coronary artery stenosis using a porcine animal model. Investigative radiology. 2012;47:71–7. [DOI] [PubMed] [Google Scholar]
- 13.George RT, Jerosch-Herold M, Silva C, Kitagawa K, Bluemke DA, Lima JA and Lardo AC. Quantification of myocardial perfusion using dynamic 64-detector computed tomography. Investigative radiology. 2007;42:815–22. [DOI] [PubMed] [Google Scholar]
- 14.Nishiyama H, Tanabe Y, Kido T, Kurata A, Uetani T, Kido T, Ikeda S, Miyagawa M and Mochizuki T. Incremental diagnostic value of whole-heart dynamic computed tomography perfusion imaging for detecting obstructive coronary artery disease. Journal of cardiology. 2019;73:425–431. [DOI] [PubMed] [Google Scholar]
- 15.Tomizawa N, Chou S, Fujino Y, Kamitani M, Yamamoto K, Inoh S, Nojo T, Kumamaru KK, Aoki S and Nakamura S. Feasibility of dynamic myocardial CT perfusion using single-source 64-row CT. Journal of cardiovascular computed tomography. 2019;13:55–61. [DOI] [PubMed] [Google Scholar]
- 16.Tanabe Y, Kido T, Uetani T, Kurata A, Kono T, Ogimoto A, Miyagawa M, Soma T, Murase K, Iwaki H and Mochizuki T. Differentiation of myocardial ischemia and infarction assessed by dynamic computed tomography perfusion imaging and comparison with cardiac magnetic resonance and single-photon emission computed tomography. European radiology. 2016;26:3790–3801. [DOI] [PubMed] [Google Scholar]
- 17.Kikuchi Y, Oyama-Manabe N, Naya M, Manabe O, Tomiyama Y, Sasaki T, Katoh C, Kudo K, Tamaki N and Shirato H. Quantification of myocardial blood flow using dynamic 320-row multi-detector CT as compared with (1)(5)O-H(2)O PET. European radiology. 2014;24:1547–56. [DOI] [PubMed] [Google Scholar]
- 18.Huber AM, Leber V, Gramer BM, Muenzel D, Leber A, Rieber J, Schmidt M, Vembar M, Hoffmann E and Rummeny E. Myocardium: Dynamic versus Single-Shot CT Perfusion Imaging. Radiology. 2013;269:378–386. [DOI] [PubMed] [Google Scholar]
- 19.Weininger M, Schoepf UJ, Ramachandra A, Fink C, Rowe GW, Costello P and Henzler T. Adenosine-stress dynamic real-time myocardial perfusion CT and adenosine-stress first-pass dual-energy myocardial perfusion CT for the assessment of acute chest pain: initial results. European journal of radiology. 2012;81:3703–10. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Qin L, Shi X, Zeng Y, Jing H, Schoepf UJ and Jin Z. Adenosine-stress dynamic myocardial perfusion imaging with second-generation dual-source CT: comparison with conventional catheter coronary angiography and SPECT nuclear myocardial perfusion imaging. AJR American journal of roentgenology. 2012;198:521–9. [DOI] [PubMed] [Google Scholar]
- 21.Bamberg F, Becker A, Schwarz F, Marcus RP, Greif M, von Ziegler F, Blankstein R, Hoffmann U, Sommer WH, Hoffmann VS, Johnson TR, Becker HC, Wintersperger BJ, Reiser MF and Nikolaou K. Detection of hemodynamically significant coronary artery stenosis: incremental diagnostic value of dynamic CT-based myocardial perfusion imaging. Radiology. 2011;260:689–98. [DOI] [PubMed] [Google Scholar]
- 22.Alessio AM, Bindschadler M, Busey JM, Shuman WP, Caldwell JH and Branch KR. Accuracy of Myocardial Blood Flow Estimation From Dynamic Contrast-Enhanced Cardiac CT Compared With PET. Circulation Cardiovascular imaging. 2019;12:e008323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu M, Wang S, Sirajuddin A, Arai AE and Zhao S. Dynamic stress computed tomography myocardial perfusion for detecting myocardial ischemia: A systematic review and meta-analysis. International journal of cardiology. 2018;258:325–331. [DOI] [PubMed] [Google Scholar]
- 24.Nieman K, Cury RC, Ferencik M, Nomura CH, Abbara S, Hoffmann U, Gold HK, Jang IK and Brady TJ. Differentiation of recent and chronic myocardial infarction by cardiac computed tomography. The American journal of cardiology. 2006;98:303–8. [DOI] [PubMed] [Google Scholar]
- 25.Bamberg F, Hinkel R, Marcus RP, Baloch E, Hildebrandt K, Schwarz F, Hetterich H, Sandner TA, Schlett CL, Ebersberger U, Kupatt C, Hoffmann U, Reiser MF, Theisen D and Nikolaou K. Feasibility of dynamic CT-based adenosine stress myocardial perfusion imaging to detect and differentiate ischemic and infarcted myocardium in an large experimental porcine animal model. The international journal of cardiovascular imaging. 2014;30:803–12. [DOI] [PubMed] [Google Scholar]
- 26.Mahnken AH, Koos R, Katoh M, Wildberger JE, Spuentrup E, Buecker A, Gunther RW and Kuhl HP. Assessment of myocardial viability in reperfused acute myocardial infarction using 16-slice computed tomography in comparison to magnetic resonance imaging. Journal of the American College of Cardiology. 2005;45:2042–7. [DOI] [PubMed] [Google Scholar]
- 27.Lardo AC, Cordeiro MA, Silva C, Amado LC, George RT, Saliaris AP, Schuleri KH, Fernandes VR, Zviman M, Nazarian S, Halperin HR, Wu KC, Hare JM and Lima JA. Contrast-enhanced multidetector computed tomography viability imaging after myocardial infarction: characterization of myocyte death, microvascular obstruction, and chronic scar. Circulation. 2006;113:394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurobe Y, Kitagawa K, Ito T, Kurita Y, Shiraishi Y, Nakamori S, Nakajima H, Nagata M, Ishida M, Dohi K, Ito M and Sakuma H. Myocardial delayed enhancement with dual-source CT: advantages of targeted spatial frequency filtration and image averaging over half-scan reconstruction. Journal of cardiovascular computed tomography. 2014;8:289–98. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura S, Kitagawa K, Goto Y, Omori T, Kurita T, Yamada A, Takafuji M, Uno M, Dohi K and Sakuma H. Incremental Prognostic Value of Myocardial Blood Flow Quantified With Stress Dynamic Computed Tomography Perfusion Imaging. JACC Cardiovascular imaging. 2018. [DOI] [PubMed] [Google Scholar]
- 30.Danad I, Szymonifka J, Schulman-Marcus J and Min JK. Static and dynamic assessment of myocardial perfusion by computed tomography. European heart journal cardiovascular Imaging. 2016;17:836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan J, Yuan M, Yu M, Gao Y, Shen C, Wang Y, Lu B and Zhang J. Myocardial Blood Flow Quantified by Low-Dose Dynamic CT Myocardial Perfusion Imaging Is Associated with Peak Troponin Level and Impaired Left Ventricle Function in Patients with ST-Elevated Myocardial Infarction. Korean journal of radiology. 2019;20:709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takx RA, Blomberg BA, El Aidi H, Habets J, de Jong PA, Nagel E, Hoffmann U and Leiner T. Diagnostic accuracy of stress myocardial perfusion imaging compared to invasive coronary angiography with fractional flow reserve meta-analysis. Circulation Cardiovascular imaging. 2015;8. [DOI] [PubMed] [Google Scholar]
- 33.Dynamic Stress Perfusion CT for Detection of Inducible Myocardial Ischemia (SPECIFIC) https://ClinicalTrials.gov/show/NCT02810795.