Abstract
As antiretroviral therapy (ART) becomes increasingly affordable and accessible to women of childbearing age across the globe, the number of children who are exposed to Human Immunodeficiency Viruses (HIV) but uninfected is on the rise, almost all of whom were also exposed to ART perinatally. Although ART has successfully aided in the decline of mother-to-child-transmission of HIV, the long-term effects of in utero exposure to ART on fetal and postnatal neurodevelopment remain unclear. Evaluating the safety and efficacy of therapeutic drugs for pregnant women is a challenge due to the historic limitations on their inclusion in clinical trials and the dynamic physiological states during pregnancy that can alter the pharmacokinetics of drug metabolism and fetal drug exposure. Thus, much of our data on the potential consequences of ART drugs on the developing nervous system comes from preclinical animal models and clinical observational studies. In this review, we will discuss the current state of knowledge and existing approaches to investigate whether ART affects fetal brain development and describe novel human stem cell-based strategies that may provide additional information to better predict the impact of specific drugs on the human central nervous system.
Keywords: Neurodevelopment, antiretroviral drugs, organoids, iPSCs
Graphical Abstract
Approaches to evaluate impact of drugs on the developing brain
Dysregulation of the developing nervous system can lead to long-lasting changes. The integration of data from animal models, clinical observations, and cell culture studies is needed to predict the safety of therapeutic antiretroviral drugs during pregnancy. New approaches include human induced pluripotent stem cell (iPSC)-based 2D and 3D models of neuronal networks and brain regions, as well as single cell profiling in response to drug exposure.
Introduction
In the early 1980s, populations from five different continents were suffering from an unexplained infection, and the identity of the pathogen and mode of transmission was unknown. Human Immunodeficiency Viruses (HIV) were first isolated in 1983 (Barre-Sinoussi et al., 1983) and it is now understood that without treatment, HIV eventually progresses into acquired immunodeficiency syndrome (AIDS), a chronic disease characterized by fevers, unintentional weight loss, severe fatigue, opportunistic infections, as well as neurological symptoms such as HIV-associated dementia. To combat the rapid spread of HIV, the scientific community responded with the development of antiretroviral therapy (ART), which has now evolved to include more than 20 drugs. Although ART has proven to be highly effective in reducing viral load, approximately 30-50% of patients experience persistent neurological symptoms and cognitive deficits, collectively known as HIV-Associated Neurocognitive Disorders (Griffin, Kang, Ma, & Zhang, 2015; Heaton et al., 2010; Saylor et al., 2016). Because the frequency and severity of these symptoms can occur even when the viral load is undetectable in patients, recent studies have begun to question whether ART could have a direct impact on the nervous system (Decloedt, Rosenkranz, Maartens, & Joska, 2015). In support of this hypothesis, several studies have reported changes in brain morphology and cellular function in animal and cell culture models following exposure to ART alone (Akay et al., 2014; Brown et al., 2014; Etherton, Lyons, & Ard, 2015; Ma et al., 2016; K. Robertson, Liner, & Meeker, 2012; Tovar-y-Romo et al., 2012). Given the emerging evidence that some ART drugs may impact the mature brain, a critical question is whether and how ART may also affect the developing human nervous system. Women with HIV are encouraged to maintain or initiate an ART regimen throughout pregnancy, resulting in a rising number of children who were exposed to ART in utero. It is therefore important to determine whether exposure to ART affects neurodevelopmental processes in this growing population.
To date, much of the data on the acute and long-term effects of ART exposure on the central nervous system (CNS) and fetal development is based on observational studies of patients, animal models, and in vitro studies. Currently the U.S. Department of Health and Human Services (HHS), as well as international health agencies, integrate data from these studies to determine the potential teratogenicity of these drugs and to issue guidelines and recommendations for the usage of ART drugs for pregnant women and women of reproductive age with HIV. In this review, we will describe what we have learned from these studies about the impact of perinatal exposure to ART on the developing brain, as well as the limitations of these approaches and current gaps in our knowledge. Finally, we will discuss recently developed human stem cell-based technologies that provide new models to investigate the effects of ART on fetal and postnatal brain development, as well as on specific cell types in the mature CNS.
History and development of ART to combat HIV infection
Transmitted through direct contact with bodily fluids of an infected individual, HIV is a retrovirus that primarily targets immune cells such as macrophages and CD4+ helper T-lymphocytes, yielding a weakened immune system and a decreased ability to fight off other infections. HIV accomplishes this through a seven stage life cycle that includes the following steps: 1) binding, 2) fusion, 3) reverse transcription, 4) integration, 5) replication, 6) assembly, and 7) budding (Figure 1) (Goodsell, 2015). Once this life cycle is complete, the viral proteins are active and able to infect additional cells. Since the start of the HIV/AIDS epidemic, 77.3 million people have become infected with HIV, and 35.4 million people have died from AIDS-related illnesses (UNAIDS, 2018). Today, HIV and AIDS continue to represent a major health concern worldwide.
Fig. 1. Impact of antiretroviral therapy (ART) drugs on the HIV life cycle.
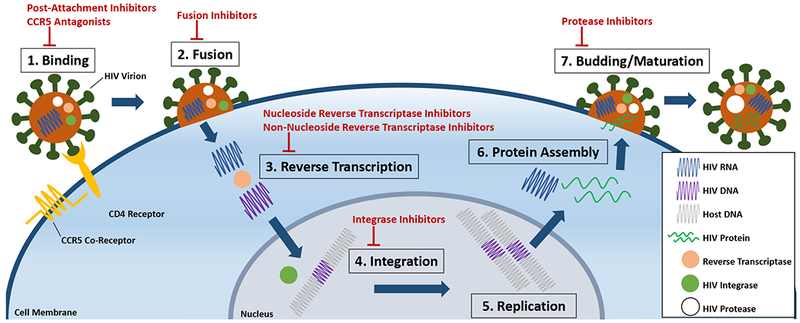
HIV infects and replicates in a seven-stage life cycle. (1) Once HIV is in the host bloodstream, the HIV virion binds to the host’s T-cell membrane via the CD4 receptor and the CCR5 co-receptor. Entry inhibitors, comprised of post-attachment inhibitors and CCR5 antagonists, block this stage of the life cycle by preventing HIV virion binding to the CD4 receptor and CCR5 co-receptor, respectively. (2) After binding, the HIV virion fuses with the T-cell membrane to release its RNA into the host cell. Fusion inhibitors block this stage. (3) In the cell cytoplasm, the HIV RNA is reverse transcribed into DNA. Nucleoside reverse transcriptase inhibitors (NRTI) or non-nucleoside reverse transcriptase inhibitors (NNRTI) block this stage by competitively and non-competitively binding reverse transcriptase, respectively. (4) In the nucleus, HIV DNA can integrate into the host’s DNA using a viral enzyme, HIV integrase. HIV integrase and host DNA integration can be blocked with integrase inhibitors. (5) Following integration, viral DNA undergoes replication (6) and the generation of additional HIV RNA that encodes for HIV proteins, which are assembled by translation in the cytoplasm. (7) After protein assembly, the new HIV virions form and bud from the host cell. The HIV protease enzyme cleaves immature viral proteins, allowing for maturation and infection of other cells, which can be blocked by protease inhibitors (PI).
In 1985, zidovudine (AZT or ZDV), a potential cancer therapeutic, was found to suppress HIV replication in HIV-infected human derived T-lymphocytes in vitro without adversely affecting non-infected T-lymphocytes (Mitsuya et al., 1985). Following this discovery, it was found that AZT also decreased AIDS-related symptoms such as opportunistic infections, increased the number of CD4+ T-lymphocytes, and reduced mortality in patients with AIDS. These data led the United States Food and Drug Administration (FDA) to approve AZT as the first treatment for HIV and AIDS in 1987 (Fischl et al., 1987).
Over the past three decades, the FDA has approved approximately two dozen antiretroviral drugs in eight different classes, and several drug combinations, for the treatment of HIV and AIDS (Table 1). Each class functions to block the HIV life cycle at different stages (Figure 1) (Arts & Hazuda, 2012). Drugs that prevent HIV from binding to the CD4+ T-lymphocytes are entry inhibitors, a class comprised of post-attachment inhibitors and CCR5 antagonists. Post-attachment inhibitors such as ibalizumab-uiyk (I BA) block the CD4 receptors, while CCR5 antagonists such as maraviroc (MVC) block the CCR5 co-receptors on the T-lymphocytes preventing HIV from binding to the cells. Drugs that prevent the fusion of HIV with the T-lymphocyte membrane and the subsequent entry of HIV into the cell, such as enfuvirtide (T-20), are known as fusion inhibitors. Nucleoside reverse transcriptase inhibitors (NRTIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs) both block reverse transcriptase in different ways, rendering HIV unable to transcribe its viral RNA into DNA. NRTIs, like AZT, abcavir (ABC), emtricitabine (FTC), lamivudine (3TC), tenofovir alafenamide (TAF), and tenofovir disoproxil fumarate (TDF) are analogues of nucleotides, binding to the active site of reverse transcriptase, and therefore competitively inhibit the enzyme’s function. NNRTIs, like doravine (DOR), efavirenz (EFV), etravirine (ETR), nevirapine (NVP), and rilpivirine (RPV), induce a conformational change in reverse transcriptase by binding at a hydrophobic pocket distant from the active site, and therefore non-competitively inhibit the enzyme’s function. Integrase inhibitors such as dolutegravir (DTG), raltegravir (RAL), and bictegravir (BIC) prevent further HIV replication by blocking HIV integrase, an enzyme that aids in the insertion of the reverse transcribed viral RNA into the host cell DNA. Protease inhibitors, which include atazanavir (ATV), darunavir (DRV), fosamprenavir (FPV), lopinavir (LPV), ritonavir (RTV), saquinavir (SQV), and tipranavir (TPV), prevent HIV from becoming active and able to infect additional cells by inhibiting HIV protease, a viral enzyme used to cleave newly synthesized viral proteins after assembly and budding from the cell. Lastly, pharmacokinetic enhancers such as cobicistat (COBI) are used to increase the effectiveness of a particular HIV medicine included in an ART regimen.
Table 1.
Approved ART drugs and select drug recommendations for ART-naïve pregnant women
| Drug class and generic name | Abbreviation | Recommendations for ART-naïve Pregnant Women | Rationale for recommendation |
|---|---|---|---|
| Post-Attachment Inhibitors | |||
| Ibalizumab-uiyk | IBA | Not recommended | Insufficient data |
| CCR5 Antagonists | |||
| Maraviroc | MVC | Not recommended | Insufficient data |
| Fusion Inhibitors | |||
| Enfuvirtide | T-20 | Not recommended | Insufficient data |
| Nucleoside Reverse Transcriptase Inhibitors (NRTIs) | |||
| Abcavir | ABC | Preferred | ~ |
| Emtricitabine | FTC | Preferred | ~ |
| Lamivudine | 3TC | Preferred | ~ |
| Tenofovir Alafenamide | TAF | Not recommended | Insufficient data |
| Tenofovir Disoproxil Fumarate | TDF | Preferred | ~ |
| Zidovudine | AZT/ZDV | Alternative | Possible association with cardiovascular, mitochondrial, and metabolic defects |
| Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs) | |||
| Doravine | DOR | Not recommended | Insufficient data |
| Efavirenz | EFV | Alternative | Possible association with neural tube defects |
| Etravirine | ETR | Not recommended | Insufficient data |
| Nevirapine | NVP | Not recommended | Possible association with liver toxicity in women |
| Rilprivirine | RPV | Alternative | Not recommended in some combinations |
| Integrase Inhibitors | |||
| Bictegravir | BIC | Not recommended | Insufficient data |
| Dolutegravir | DTG | Not in 1st trimester/Alternative in 2nd and 3rd trimesters | Possible association with neural tube defects |
| Raltegravir | RAL | Preferred | ~ |
| Protease Inhibitors (PIs) | |||
| Atazanavir | ATV | Preferred | ~ |
| Darunavir | DRV | Preferred | ~ |
| Lopinavir/Ritonavir | LPV/r | Alternative | Requires twice daily dose and increased dose in 3rd trimester |
| Fosamprenavir | FPV | Not recommended | Insufficient data |
| Saquinavir | SQV | Not recommended | Insufficient data |
| Tipranavir | TPV | Not recommended | Insufficient data |
| Pharmacokinetic Enhancers | |||
| Cobicistat | COBI | Not recommended | Pharmacokinetic changes in pregnancy decrease efficacy |
Currently, first-line ART regimens typically consist of two NRTIs, along with at least one drug from another class such as NNRTIs, protease inhibitors, or integrase inhibitors, often combined with a pharmacokinetic enhancer. Combination ART provides better protection against HIV infection by blocking the HIV life cycle at multiple stages simultaneously. The advent of ART, and combination ART, has dramatically decreased mortality rates and often results in undetectable viral loads in those infected (Teeraananchai, Kerr, Amin, Ruxrungtham, & Law, 2017), turning what was once a fatal disease into a manageable condition.
Special considerations during pregnancy
In determining ART recommendations for pregnant women or women planning to conceive who have HIV, the two main concerns are the efficacy of the drug regimen in preventing mother-to-child transmission (MTCT) and the potential harm to the fetus via off-target effects. Currently, 44% of all adults with HIV worldwide are women of reproductive age and there are 1.8 million children living with HIV, a majority of whom contracted the virus from their HIV-infected mothers in utero, or during childbirth or breastfeeding (UNAIDS, 2018). Without ART or any intervention to the mother during pregnancy, the rate of MTCT of HIV is between 15 to 45%. ART intervention decreases this rate to below 5% worldwide, and to 1% or less in the United States and Europe, due to the increased availability and affordability of ART in these areas (Caniglia et al., 2018; W.H.O., 2018).
Worldwide in 2016, over 160,000 infants acquired HIV through MTCT, but over 1 million infants exposed to HIV were uninfected (Fowler, Flynn, & Aizire, 2018; Slogrove et al., 2018; UNAIDS, 2018). And cumulatively, as of 2016, there were approximately 9 million children under the age of 16 who were born HIV-exposed, but uninfected, and were exposed to ART in utero or postnatally (Slogrove et al., 2018). Together, these data suggest that adherence to an ART regimen has drastically reduced vertical transmission, but increased the population of children exposed to ART.
Treatment guidelines and supporting data
Acquiring the data to support decisions regarding the safety and efficacy of therapeutic drugs for pregnant women has been challenging due to the ethical considerations surrounding their enrollment in clinical trials. Although the first double-blind and controlled clinical trial conducted in 1994 showed that AZT reduced MTCT of HIV by 67.5% with minimal short-term toxic effects (Connor et al., 1994; Volberding et al., 1990), there have been a limited number of controlled trials since that time. Placebo-controlled trials cannot be performed due to placing the mother’s own health at risk, but clinical trials comparing approved drugs have been conducted to evaluate the relative efficacy of different regimens in preventing MTCT. Among these, the PROMISE 1077 trial was a large study conducted between 2011 and 2016 comparing different antepartum and postpartum treatments with either AZT alone or followed by NVP in early postpartum stages or a triple combination of three ART drugs throughout the antepartum and postpartum stages. Both strategies were effective in reducing MTCT to below 2%, with the combination therapy showing lower transmission rates but an increased risk for preterm delivery and low birth weight (Fowler et al., 2018). Other major clinical trials that have focused on pregnant women exclusively and the prevention of MTCT include the PRIMEVA trial (Sibiude et al., 2015), and studies in countries such as Mozambique (De Schacht et al., 2014), Tanzania (Kilewo et al., 2009), South Africa (Becquet et al., 2009), and Kenya (Nyandiko et al., 2010) where HIV is more prevalent.
Currently, the main source of clinical data on the effects of ART for MTCT, as well as off-target effects on fetal development, comes from observational studies of infants born to HIV-infected women taking ART. Several such observational studies are underway around the world, with one of the largest being the prospective Antiretroviral Pregnancy Registry, which began in 1989 and continues to provide much of the data used to determine recommendations by the HHS for pregnant women. Other ongoing studies worldwide include the Pediatric HIV/AIDS Cohort Study’s Surveillance Monitoring of ART Toxicities (SMARTT) (Williams et al., 2015) and the Women’s Interagency HIV Study (WIHS) in the United States and Puerto Rico, the French Perinatal Cohort (EPF), the European Collaborative Study (ECS), the Swiss Mother and Child HIV Cohort Study (MoCHiV), and the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPACCT). Many of these observational studies utilize the same cohorts of mothers and children, but analyze patient data with different outcome measures. While this can provide critical information about the clinical outcomes following ART exposure on both the mother and the fetus, this approach also yields discrepancies in the data due to varying inclusion criteria among studies and confounding variables among participants such as adherence to ART regimens and comorbid conditions.
Today, of the more than 20 drugs that are FDA approved for treatment in adults, only seven are also recommended by HHS as a preferred initial ART regimen in ART-naïve pregnant women with HIV (Table 1), although others may be recommended in certain combinations or as an alternative ART regimen. Many of the restrictions have been issued due to a lack of information about the teratogenicity or tropism of the drug. Because individual responses to ART drugs vary widely in terms of efficacy and tolerability, there is a need to develop new formulations of drugs within each of the major classes. Several recently developed drugs in different classes of ART that have been approved for use either alone or in combinations, such as ibalizumab-uiyk and doravine, bictegravir, tenofovir alafenamide, appear to have high efficacy and are well-tolerated by most individuals. However, there are insufficient data at this time to justify recommending these drugs at any stage of pregnancy. There is typically a long latency between the initial approval of drugs for adults and evidence-based recommendations for women of reproductive age, illustrating the need for new avenues to generate preclinical data on the safety and efficacy of ART drugs during pregnancy.
Off-target effects of ART on the developing and adult CNS
ART prevents vertical transmission of HIV by both reducing the viral load in the mother, as well as providing prophylaxis through transplacental exposure. Maternal plasma concentrations show that the pharmacokinetics of a given drug can change dramatically over the different trimesters of pregnancy, which can alter its efficacy in suppressing the maternal viral load. Often, the efficacy decreases in later stages of pregnancy, prompting recommendations for dosage increases or drug switching in the second or third trimester for some ART drugs (Gilbert, Darin, Scarsi, & McLaughlin, 2015; Pariente et al., 2016). For example, cobicistat, which generally functions as a pharmacokinetic enhancer in adult HIV patients, actually decreases maternal drug exposure during later stages of pregnancy (Boyd et al., 2019). Cobicistat is therefore is not recommended by HHS for use at any stage of pregnancy, which excludes many combinations that are used effectively in the non-pregnant population.
For prophylaxis, drugs with high placental transfer efficacy are desired to protect the fetus from infection directly. Placental transfer of various drugs can be measured by the ratio of ART concentration in cord blood versus maternal plasma or directly from ex vivo placental tissue after birth (McCormack & Best, 2014). However, these measurements are limited to a single time point and we have little data on the dynamics of fetal exposure in utero. Although high placental transfer of effective ART drugs is desirable to prevent vertical transmission, the increased exposure to these drugs may have unintended effects on the developing fetal nervous system. Fetal development is extremely sensitive to factors such as maternal exposure to drugs, toxins, infections, and changes in physiological states such as acute or chronic stress. Perturbations of coordinated neurodevelopmental processes can lead to profound congenital abnormalities in early development, or more subtle changes in neural circuit formation or function that can impact cognitive function later in life (Bale, 2015; Shallie & Naicker, 2019). In terms of evaluating the safety of drugs, there are several critical periods of development for the CNS and distinct consequences of disruption at each stage (Figure 2).
Fig. 2. Overview of human brain developmental processes.
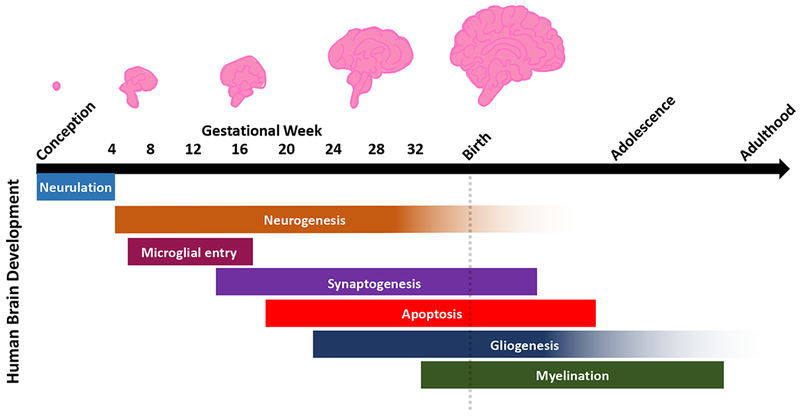
Human brain development undergoes many critical stages of maturation from conception through late adolescence and early adulthood. Neurodevelopment begins approximately two to three weeks after conception with the formation of the neural tube, or neurulation, in which the lateral ends of the neural plate fold and fuse. Following neurulation is the process of neurogenesis in which morphogen gradients determine the identity of neural progenitors that give rise to neurons. This stage continues through gestation until birth, with some evidence of neurogenesis occurring after birth. Between four to 16 gestational weeks, microglia migrate into the developing brain to establish the pool of resident immune cells. Following neurogenesis, synaptogenesis begins at approximately 12 weeks in utero, and pruning of excess synapses and dendritic processes continue after birth. At 18 weeks, apoptosis eliminates overproduction of cells to refine cell populations and to ensure proper development and synaptic connectivity in the mature brain. At 22 weeks, gliogenesis results in the production of region- and subtype-specific glia, which continues through adulthood. At approximately 32 gestational weeks, glial cells aid in myelination of the neurons, and this process continues after birth into adulthood.
Acute effects of ART on early brain development
In humans, neurodevelopment begins two to three weeks after conception with the formation of the neural tube (Tau & Peterson, 2010) through the folding and fusing of the lateral ends of the neural plate (Nikolopoulou, Galea, Rolo, Greene, & Copp, 2017). This process, known as neurulation, is one of the most crucial periods of neural development. If fusion of the neural tube is incomplete, the neuroepithelium on the ends of the neural plate remain exposed, and may be subject to degeneration. By the fourth week, the rostral portion of the neural tube forms vesicles that eventually give rise to the forebrain, midbrain, and hindbrain (Tau & Peterson, 2010). Following neurulation, morphogen gradients along the dorsal-ventral and anterior-posterior axes determine the identity of neural progenitors that give rise to region- and subtype-specific neurons and glia (Tao & Zhang, 2016; Tau & Peterson, 2010). Postmitotic cells then migrate to their final destinations, extending axons and dendrites to their synaptic partners. Disturbances at these early stages can range from severe neural tube defects (NTD) such as anencephaly and spina bifida/myelomeningocele (Greene & Copp, 2014) to more modest changes in brain structure, abnormal migration of neural cell types, and ocular abnormalities.
Most data on ART-related congenital abnormalities are generated through observational studies. Currently, there are enough data from the Antiretroviral Pregnancy Registry to rule out a 1.5-fold increase in birth defects from first trimester exposure to AZT. Similar results have been obtained from observational studies in Spain (Prieto et al., 2014). However, in a secondary analysis of Antiretroviral Pregnancy Registry data, ART regimens without AZT had a higher proportion of nondefect adverse outcomes, but AZT-containing combinations were associated with lower birth weight (Vannappagari, Koram, Albano, Tilson, & Gee, 2016). And in a recent clinical trial that compared AZT alone to combination ART that included AZT or TDF, ART with AZT had the lowest rate of vertical transmission but both combination strategies had higher rates of preterm delivery and adverse events than AZT alone (Fowler et al., 2016). These data illustrate the tradeoff that often occurs between viral suppression and the potential risk to the fetus from direct ART exposure. It also suggests that there may be synergistic interactions among specific ART drugs that can lead to qualitatively or quantitatively different effects. Overall, there appears to be minimal risk of acute or long-term neurological outcomes from in utero AZT exposure (Culnane et al., 1999; Sperling et al., 1998), but potential effects on fetal cardiac development, mitochondria, and metabolic profiles (Garcia-Otero et al., 2016; Sibiude et al., 2015; Van Dyke, Chadwick, Hazra, Williams, & Seage, 2016) have reclassified AZT from a preferred to an alternative component of a NRTI backbone.
Another drug that has been reclassified with regard to its recommended use during pregnancy is EFV, an NNRTI initially approved in 1998. In 2005, the FDA advised against its use in the first trimester of pregnancy due to its suspected association with NTDs and other congenital abnormalities. After nonhuman primate studies were reported to have shown an increased risk for NTDs in the form of anencephaly and other CNS anomalies such as unilateral anopthalmia, micro-opthalmia, and cleft palate following EFV exposure in utero, early observational studies reported incidences of myelomeningocele with Arnold-Chiari Malformation Type II (Saitoh, Hull, Franklin, & Spector, 2005; Williams et al., 2010) and congenital abnormalities including ventricular dilation, parietal agenesis of the corpus callosum, subependymal cysts, or pachygyria (Knapp et al., 2012; Sibiude et al., 2014). However, a metaanalysis that gathered data from 23 different observational studies reported a lack of evidence to support an increased risk of congenital abnormalities or NTDs with first trimester use of EFV (Ford et al., 2014). In 2013, the World Health Organization revised its guidelines to allow EFV throughout pregnancy, and the current HHS guidelines list EFV as an alternative option for use during pregnancy or preferred in some contexts depending on drug interaction considerations.
Currently, DTG is the subject of international concern based on an interim report from an observational study in Botswana designed to evaluate the incidence of NTDs for ART taken at the time of conception. DTG is generally a well-tolerated and highly effective drug that can be very effective in lowering viral loads and preventing MTCT. In 2016, DTG had become a first-line ART in Botswana and by 2018, a higher than expected number of children were born with NTDs, in the form of encephalocele, myelomeningocele, iniencephaly, or anencephaly, to pregnant women who initiated DTG-based ART prior to conception (Zash, Makhema, & Shapiro, 2018). Although it is too early to conclude that DTG is associated with a significant increase in the risk of NTDs, HHS no longer recommends DTG during the first trimester or for women planning to conceive. Another interim analysis scheduled for 2019 should provide data on an additional 1,200 women who were taking DTG at the time of conception. It is also important to note that the other currently approved integrase inhibitor, RAL, has not been observed to yield an increased risk of NTDs (Shamsuddin et al., 2019).
Developmental delay associated with in utero ART exposure
Less obvious but potentially still debilitating consequences of ART could arise from more subtle changes in brain development that lead to a long-lasting impact on cognition. At the end of the first trimester, at approximately 12 weeks in utero, synaptogenesis begins and initially results in an overabundance of synapses, a subset of which are later eliminated to refine neural networks and ensure proper connectivity (Figure 2). Just prior to birth, glial cells begin to aid in myelination, and synaptic pruning and myelination continue after birth, peaking at later time points in childhood through adolescence (Tau & Peterson, 2010). These prolonged phases of neural maturation define an extended critical window of brain development in humans that is vulnerable to disruption. In light of this, recent studies have begun to investigate whether children with perinatal exposure to ART are subject to developmental delays or neurological impairments (Caniglia et al., 2018; Whitehead, Potterton, & Coovadia, 2014). Longitudinal studies to track effects of in utero exposure to ART in children that are HIV-exposed but uninfected (CHEU) into adolescence or adulthood can take advantage of standardized tests to measure cognitive and developmental milestones to reveal emergent effects of fetal and early childhood exposure to ART.
Early stage development in children can be measured using many different assays focused on different cognitive and sensorimotor domains. Common examinations include the Bayley Scales of Infant and Toddler Development (Bayley), which aims to identify developmental delays in children between the ages of one and 42 months, the MacArthur-Bates Communicative Development Inventory (MB-CDI) to assess language development in children between 8 and 30 months, and the Ages and Stages Questionnaire (ASQ) to assess language development in children between 2 and 60 months. The Bayley has multiple subtests, including cognitive, language, and motor scales, as well as adaptive behavior and social-emotional scales. Some studies have reported lower cognitive, language, adaptive behavior, and social-emotional performances, as well as lower mental development and psychomotor indices on the Bayley subtests in children between the ages of 9 and 15 months that were perinatally exposed to various ART drugs, compared to unexposed children (Rice et al., 2013; Van Dyke et al., 2016; Williams et al., 2010), despite median scores being within the same range as population norms (Van Dyke et al., 2016). Specifically, children perinatally exposed to ATV were associated with lower language performance, those exposed to the combination lopinavir/RTV were associated with lower adaptive behavior performance, and those exposed to TDF were associated with lower social-emotional performance (Sirois et al., 2013; Van Dyke et al., 2016), suggesting there may be domain-specific effects of specific drugs. Children between 18 and 36 months of age exposed to several combinations of ART in utero were also reported to have lower development and adaptive behavior scores compared to non-exposed children, although these differences were not significant after correcting for confounding variables, such as maternal drug use, maternal IQ, and sociodemographic status (Alimenti et al., 2006). Additional studies report late language emergence in children perinatally exposed to ATV at one year, but not at two years using the MB-CDI and ASQ, respectively (Van Dyke et al., 2016).
Later stages of development can be monitored using the Wechsler Preschool and Primary Scale of Intelligence, which assesses verbal IQ, performance IQ, and full-scale IQ. Using this tool, studies have tested children at ages 3.5 and 5.5 years that were exposed in utero to ART, and reported significantly lower adaptive behavior and socialization scores in children at 3.5 years that were perinatally exposed to ART, but at 5.5 years, their scores did not diverge from population norms (Smith, Puka, Sehra, Read, & Bitnun, 2017). However, at 5.5 years, children exposed to ART were reported to have significantly lower verbal IQ scores compared to non-exposed children (Smith et al., 2017). Yet, other studies observe no adverse effects on cognition from in utero exposure to ART in children at 5.6 years old (Culnane et al., 1999). In a cross-sectional study of 687 children between the ages of 5 and 13 years old, mean scores on age-appropriate Weschler scales in children perinatally exposed to ART were slightly below population norms, but after controlling for confounding variables, cognitive and academic outcomes in these children did not differ significantly from the outcomes in unexposed children (Nozyce et al., 2014). Together, these results suggest that cognitive deficits may emerge in infancy and early school-age years, but normalize over time, indicating that ART exposure may be associated with a transient developmental delay, rather than a permanent impairment (Smith et al., 2017). However, it is important to note that there are limitations to these standardized neurocognitive assessments that may not be tailored for local populations or control for region-specific variables, which may impact the accuracy of the measurement of cognitive development.
Long-term behavioral effects of prenatal exposure to ART in animal models
Measuring specific neurodevelopmental processes in young children with developing nervous systems can also be challenging as neurodevelopment is extremely dynamic, and it is difficult to control for diet or co-morbidities, which makes it hard to compare developmental processes across different populations. However, dependent upon the degree of homology that exists between developing nervous systems in many species, animal models can provide an opportunity to study the mechanisms behind human neurodevelopment and behavior observed and tested in infants and young children (Tierney & Nelson, 2009). In several species, in utero exposure to ART has been found to alter innate and learned behaviors at later stages in life. These behavioral changes cover a wide range of domains including social interactions, cognitive functions, sensorimotor processing, and exploratory behaviors. For example, mice exposed to AZT in utero exhibit decreased aggression, increased pain sensitivity, decreased social investigation and exploratory behaviors, and an enhanced fear response to novelty (Calamandrei, Valanzano, Puopolo, & Aloe, 2002; Rondinini, Venerosi, Branchi, Calamandrei, & Alleva, 1999; Venerosi, Cirulli, Capone, & Alleva, 2003; Venerosi et al., 2000), as well as impaired acquisition of avoidance responses as adults (Rondinini et al., 1999). Additional studies report decreased affiliative interactions amongst female mice that were exposed to AZT and 3TC in combination in utero (Venerosi, Valanzano, Alleva, & Calamandrei, 2001). Mice exposed to EFV show delayed reflex and motor development in infancy and adolescence (van de Wijer et al., 2019). And Macaca nemestrina exposed to AZT in utero exhibited altered rooting and snouting reflexes, and lacked the ability to fixate on and follow near visual stimuli (Ha et al., 1998). However, these deficits disappeared over time (Ha et al., 1998), similar to the transient impairments observed in humans in terms of developmental delays in language emergence and academic performance.
ART-induced cognitive and psychiatric effects in adults
Due to the persistence of cognitive deficits in adults with HIV taking ART that can be independent of viral suppression (Rubin et al., 2017), it is possible that ART itself has negative effects on the adult brain. There are conflicting results about whether CNS penetration of ART correlates with improved (Carvalhal et al., 2016; Smurzynski et al., 2011) or impaired (Caniglia et al., 2014; Marra et al., 2009) cognition. Although cognitive deficits, or HIV-Associated Neurocognitive Disorders, have long been recognized in HIV patients on successful ART regimens, the development of psychiatric symptoms in a subset of patients is an emergent concern. Some observational studies of HIV-infected adults taking EFV-based ART regimens have reported increased cognitive impairments (Ciccarelli et al., 2011), increased hazard of suicidality, and increased occurrence of bad dreams and anxiety (Marra et al., 2009). Some adults experience adverse neuropsychiatric events after switching from a regimen of EFV without TDF to a regimen of EFV with TDF (Allavena, Le Moal, Michau, Chiffoleau, & Raffi, 2006). Further, EFV has been used as a recreational drug and reported to elicit similar effects to LSD (Gatch et al., 2013). RAL administration has been found to be associated with exacerbation of depressive symptoms (Harris, Larsen, & Montaner, 2008) and insomnia (Eiden, Peyriere, Peytavin, & Reynes, 2011; Gray & Young, 2009). Additional studies have reported that exposure to NVP in HIV-infected adults with no history of mental illness may be associated with low mood, cognitive impairments, clouding of consciousness, paranoia, visual hallucinations, delusions of persecution and infestation, command hallucinations leading to suicide attempts, and depressive thoughts, which decrease upon discontinuation of the drug (Wise, Mistry, & Reid, 2002). Another observational study reported that scores on the Wechsler Adult Intelligence Scales and the Trail-Making Test, which is used to test for dementia, improved for up to 96 weeks after discontinuation of ART in HIV-infected adults (K. R. Robertson et al., 2010). Of concern for pregnant women specifically, EFV has been associated with intra- and post-partum depression and suicide ideation (Jones, Rodriguez, Alcaide, Weiss, & Peltzer, 2019). We know very little about the risk factors for cognitive and psychiatric sequelae of ART. In the future, clinical data obtained from prospective studies of maternal and infant health could potentially be mined to identify any clinical signatures that are predictive of patient outcome or individual susceptibility.
Mechanisms of ART-induced pathology
Although reviewed extensively elsewhere (Underwood, Robertson, & Winston, 2015), there are multiple cellular processes that may be affected by different ART drugs that could be relevant to understanding the potential for developmental effects on cognition and other symptomatic domains related to neural function. Several model systems have contributed to our understanding of the potential for ART drugs to act on neural cell types. In vitro cultures of primary neurons, astrocytes, oligodendrocytes, microglia, as well as progenitor cells that are important in early development, can be obtained from rodent and human sources by dissociation of freshly obtained tissue and subsequent enrichment (Chen et al., 2007; Gordon, Amini, & White, 2013; Lange, Bak, Waagepetersen, Schousboe, & Norenberg, 2012; Lian, Roy, & Zheng, 2016; Millet & Gillette, 2012). Non-invasive imaging of HIV patients and studies of post-mortem tissue have also provided valuable data, but the variability associated with comorbid conditions, different intervals between HIV infection and ART initiation, and highly individual treatment history make it difficult to identify potential causal pathology. Finally, animal studies are a critical means of establishing a causal link between cellular and systems-level pathology and behavioral outcomes, but it is more difficult to test for psychiatric and higher-order cognitive impairments. However, by integrating data from these complementary approaches, several mechanisms have been proposed that could impact neuronal integrity and neural function.
Cell proliferation and cell death
In the developing nervous system, neural stem cells give rise to both neurons and glia and thus serve as a primary determinant of the neural cell populations. A recent study in rats found that exposure to EFV decreased proliferation of neural stem cells, activated p38 signaling pathways, and disrupted mitochondrial function (Jin et al., 2016). In primary fetal mouse forebrain neural progenitor cells, combination treatment with TDF, emtricitabine, and RAL led to decreased proliferation in neural progenitors and increased apoptosis, with TDF as the main causative agent in this cocktail (Xu et al., 2017). Finally, a study of AZT exposure in primary neonatal mice forebrain NPCs found reduced proliferation, increased senescence, and decreased production of neuroblasts as well as impaired neurogenesis in the adult brain (Demir & Laywell, 2015).
ART-mediated induction of cell death has been observed in higher concentrations of some drugs in culture. In primary cultures of rat neurons, it was found that abcavir, EFV, etravirine, NVP, and ATV have a high risk for inducing toxicity, and the lowest risk was associated with darunavir, emtricitabine, TDF, and maraviroc (K. Robertson et al., 2012). Subsequent studies have found EFV to be more neurotoxic than other frontline drugs (Ciavatta et al., 2017). Another study revealed that the inclusion of RTV into various combinations induced statistically significant neuronal damage and death (Akay et al., 2014). Additionally, treatment with either RTV or SQV, alone or in combination with AZT, led to the activation of the calcium-activated death protease, calpain, indicated by the increase of calpain-cleaved spectrin in these cultures. However, none of these treatment combinations caused increases in cleaved-caspase 3, which may indicate that neuronal death observed in these models are due to necrotic cell death, rather than apoptotic cell death (Akay et al., 2014).
These results suggest that ART drugs may impact the building blocks of the nervous system, potentially leading to subtle differences in the number and functional properties of constitutive populations. As many of these intrinsic cellular processes are highly dynamic and sensitive to fluctuations in physiological states and exposure to exogenous facts, it is critical to study the effects of individual and combination ART drugs on early proliferation and fate specification. And as the differing results on the impact of TDF suggests, there may be species-specific differences in response to drug exposure, as well as context-dependent effects of drug combinations that highlight the need to study ART in human neural progenitor cells. In addition, there are specialized neurogenic niches in the adult brain, such as the hippocampus, that could be impacted by ART drugs, leading to changes in the homeostatic balance of neural stem cell proliferation and quiescence in the mature nervous system that may contribute to cognitive impairments.
Mitochrondrial deficits and oxidative stress
Perhaps the most consistent finding with respect to ART-mediated effects on cellular processes is cellular stress associated with mitochondrial dysfunction (White, 2001). Mitochondria are involved in many metabolic processes including energy production and biomolecule synthesis, and are implicated in neurodevelopment (Son & Han, 2018). Cells undergoing development change metabolic states and require energy in order to support newly developed structural and functional properties, as well as to maintain a homeostatic state (Son & Han, 2018). Because of this, in developing and differentiating cells, energy metabolism switches from glycolysis, which occurs in the cell cytoplasm, to oxidative phosphorylation, which occurs in the mitochondria (Son & Han, 2018). Previous research has found that mitochondria undergo morphological changes and mature during neurogenesis, which reflects the metabolic shift to oxidative phosphorylation and the increase in cellular energy for developing cells (Son & Han, 2018). Therefore, it is possible that human neurodevelopment may be negatively impacted through mitochondrial dysfunction arising from cellular stress.
Some studies have reported that EFV exposure increased levels of reactive oxygen species (ROS) and induced mitochondrial dysfunction in primary neonatal rat cortical neurons (Funes et al., 2014). Additional studies in primary rat cortical astrocytes demonstrate that EFV exposure increases the expression of induced nitric oxide synthase (iNOS) and the production of nitric oxide, which may further contribute to mitochondrial dysfunction (Apostolova et al., 2015). Studies in primary embryonic rat neuron, glia, and mixed neuroglial cells showed that exposure to RTV, SQV, and ATV led to the accumulation of ROS and activation of cell-death pathways (Akay et al., 2014). It was also observed that in cultures with both neurons and astrocytes, ROS were present when treated with RTV, SQV, and AZT, solely in the neurons (Akay et al., 2014).
Although exposure to many ART drugs results in cellular stress, in vitro studies have suggested that individual drugs may act via different pathways in cell type-specific manner. For example, studies in mixed primary embryonic rat neuron and glia culture showed that lopinavir induced the endogenous antioxidant response whereas elvitegravir (EVG) activated the integrated stress response, indicated by increased eIF2a phosphorylation (Stern et al., 2018). To counteract oxidative stress induced by exposure to ART, a targeted delivery strategy to introduce Coenzyme Q10, an endogenous antioxidant, directly to the mitochondria of affected NPCs attenuated mitochondrial ROS generation and decreased cell proliferation induced by treatments with various ART combinations (Velichkovska, Surnar, Nair, Dhar, & Toborek, 2019). In adult mice, DTG was shown to upregulate markers of oxidative stress in the cerebellum and frontal cortex, which was attenuated when DTG was delivered as a nanoformulation, illustrating the need for additional research into drug delivery alternatives (Montenegro-Burke et al., 2018).
Clinical data have also been reported that are consistent with a potential impact of various ART drugs on these pathways. An early observational study revealed mitochondrial respiratory chain dysfunction in CHEU infants exposed to AZT in utero that were associated with neurological deficits ranging from mild to severe (Blanche et al., 1999). More recent case study research of perinatal exposure to ART suggests that mitochondrial dysfunction may be transient. In one case, an AZT-exposed infant displayed neonatal encephalomyopathy, hyperlactemia, and decreased levels of mitochondria (Tovo, Chiapello, Gabiano, Zeviani, & Spada, 2005). By 30 months of age, the child had severe psychomotor delays and visual problems, which improved by 5 years of age, and the mitochondrial depletion was less severe by 6 years of age (Tovo et al., 2005). In another case report, two of three CHEU exposed to ART who had hyperlactemia and concurrent neurological symptoms showed complete resolution of clinical symptoms, and the other child showed improvement by 1 year of age (Noguera et al., 2004). In adult patients with HIV and taking ART, mitochondrial injury has been observed, which was associated with a significant decrease in concentrations of frontal white matter N-acetylaspartate that is sensitive to alterations in mitochondrial integrity (Schweinsburg et al., 2005). It is unclear from the literature whether potential mitochondrial deficits in adult patients resolve after ending or switching treatment regimens, which would be consistent with the transient disruption observed in some children with a limited exposure to ART. Children with HIV who are continuously exposed to ART show sustained mitochondrial damage, which is partially ameliorated by more recent drug formulations including second generation NRTIs (Moren et al., 2011). Because some measures of mitochondrial function can be evaluated through peripheral biomarkers, it is feasible to conduct longitudinal monitoring in populations with long-term exposure to ART to determine the extent and duration of mitochondrial changes to drug exposure.
Structural and synaptic deficits
The regulation of morphological and synaptic development is crucial in the formation of neural circuits. These processes occur on a global scale during early development, but also occur in a more restricted manner throughout life to allow for the continuous refinement of these circuits to support learning and memory. Synapse development, as measured by the expression of the synaptic marker synaptophysin, was impacted by RTV or SQV, but not AZT, in primary rat cortical neuroglial cultures (Akay et al., 2014). In pigtail macaque monkeys infected with the primate form of HIV, simian immunodeficiency virus (SIV), that were administered combination ART regimens consisting of TDF, ATV, SQV, and an integrase inhibitor, there were decreased levels of synaptophysin, compared to SIV-infected, non-ART treated control groups (Akay et al., 2014). In the same study, adult rats treated with AZT, SQV, and RTV also showed decreased expression of synaptophysin and MAP2 compared to vehicle-treated rats, suggesting both synaptic deficits and neuronal injury (Akay et al., 2014).
Studies have also reported alterations in gross morphology of brain structure. In mice perinatally exposed to a combination of abcavir, 3TC, ATC, and RTV, micro-CT imaging showed significantly reduced volumes of various brain regions such as the neocortex, amygdala, and hypothalamus, compared to unexposed controls (Serghides, 2018). These structural changes correlated with delays in motor skills and tactile and olfactory reflexes, as well as lower memory indices (Serghides, 2018). In humans, magnetic resonance imaging revealed hyperintensity in the white matter and pons in CHEU exposed perinatally to AZT, which also correlated with reports of mitochondrial dysfunction (Tardieu et al., 2005). Using diffusion tensor imaging, other studies have reported higher fractional anisotropy in several brain regions, including the cerebellum, in CHEU compared to unexposed children, which has generally been interpreted as indicating higher connectivity but could also reflect abnormalities in axon number or structure (Jankiewicz et al., 2017; Tran et al., 2016). Similar to the previously mentioned mice studies, one study reported behavioral correlates of these changes in neonates with lower scores on the Dubowitz scale, which is a neurological examination used to measure neurobehavioral status in newborn infants (Tran et al., 2016). Another study observed aberrant brainstem auditory evoked potentials in children exposed in utero to AZT alone or in combination with 3TC, suggesting ART exposure may have induced toxicity in lower regions of the brainstem (Poblano, Figueroa, Figueroa-Damian, & Schnaas, 2004). However, other studies have reported no differences in neuroanatomical or brain integrity measures in CHEU versus unexposed children (Jahanshad et al., 2015; McHenry et al., 2019). Although the reports of correlations among mitochondrial deficits, structural changes to white or gray matter, and/or behavioral outcomes in CHEU are provocative, additional longitudinal studies are needed to demonstrate a reliable association and to determine the duration of any potential effects following exposure to ART drugs.
Using human induced pluripotent stem cells to study ART toxicity
Despite the mechanistic convergence suggested by some cell culture studies, animal models, and human patient data, there is still a need to conduct controlled investigations of toxicity in human cells that are representative of the developing CNS. With the rapid advancement of stem cell technologies, pluripotent stem cell-derived neural and glial cells enable the generation of human cells that are the most relevant to investigate the impact of ART on the CNS. Although human embryonic stem cells were long considered the gold standard to differentiate into specific cell types for disease modeling, there are only a few approved lines and it is unclear how well these lines may capture genetic diversity relevant to individual variability in disease susceptibility, virus-host interactions, and drug responses. Induced pluripotent stem cells (iPSCs) hold a considerable advantage in being isogenic to the donor individual, with the differentiated cells retaining at least a permissive genetic context for the condition under investigation. iPSCs can be differentiated to nearly any cell type in the body, including constitutive populations of the CNS (Chambers, Mica, Lee, Studer, & Tomishima, 2016; Engle, Blaha, & Kleiman, 2018) (Figure 3). The differentiation and fate specification of iPSCs and NSCs rely on the same patterning factors expressed endogenously during neuro- and gliogenesis (Mertens, Marchetto, Bardy, & Gage, 2016), thus providing an advantageous model system for studying the developmental effects of exposure to HIV and ART. In particular, pluripotent stem cell-derived neural and glial cells may serve as a much needed bridge between studies in model organisms and clinical observations in humans (Dolmetsch & Geschwind, 2011).
Fig. 3. In vitro models to study the effects of antiretroviral therapy on neurodevelopment.
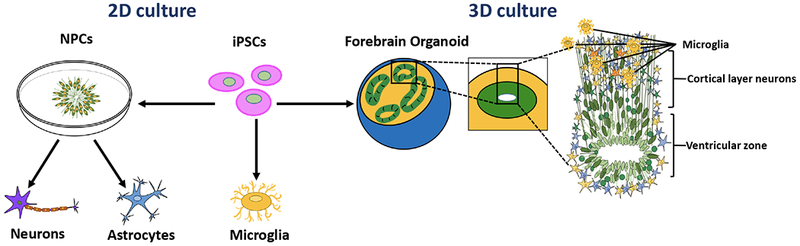
Induced pluripotent stem cells (iPSCs) offer new opportunities to study the effects of environmental factors and drugs on neurodevelopment. Targeted differentiated protocols can be used to generate neural progenitor cells (NPCs) and further patterning can lead to specific populations of neurons or glial cells. 2D cultures allow for highly enriched populations to study the effects of drugs in specific cell types. 3D culture approaches allow for the generation of organoids, which can recapitulate the heterogeneity of multiple cell populations and self-organization that is characteristic of fetal brain development. Forebrain cortical organoids exhibit proliferative ventricular zones and laminar stratification of neuronal layers reminiscent of the cerebral cortex. Microglia are generated from a distinct lineage and can be co-cultured with either 2D monolayer populations or 3D organoids to model virus-host interactions.
Cell-type specificity and 2D cultures
One of the most critical outstanding questions with respect to the potential for ART to directly impact the developing or adult nervous system is to determine whether there are specific brain regions and/or cell types that are vulnerable to dysregulation. iPSC-based models offer an opportunity to perform controlled experiments in highly enriched populations of specific cell types. Among the cell types that are relevant for modeling HIV-ART interactions in the CNS are NPCs, neurons, astrocytes, oligodendrocytes, and microglia (Jensen et al., 2015; Latronico et al., 2018). Because the embryonic origin and patterning factors that determine cell fate vary across these cell types, it can be useful to employ targeted differentiation protocols to generate largely homogenous populations of these cell types individually. These cells can then be examined in isolation or in co-culture systems to understand cell-cell interactions in the context of ART and/or HIV exposure.
Notably, 2D iPSC-based cultures are compatible and adaptable to a number of high throughput screening platforms, enabling rapid large-scale studies for mechanistic insight or preclinical therapeutic development (Kondo et al., 2017). Importantly, these monolayer cultures are also amenable to synchronization via the introduction of anti-mitotic agents to stop proliferation and induce differentiation and maturation of the cells at the same time, which further reduces heterogeneity in the culture dish and allows for more reproducible experiments. Phenotypic screening can be performed across iPSC lines in conjunction with DNA sequencing to identify donor or patient-specific differences. Introducing perturbagens, such as drugs or viruses, at various stages from iPSCs to progenitors can reveal changes in proliferation, cell death, fate specification, and cell viability. At later stages with postmitotic cells, cultures can be screened for markers of cellular function such as synapse development and electrophysiological activity. Future advancements in stem cell-derived neuron and glia methodology will likely come from a more thorough characterization of cell maturity, optimized differentiation protocols to generate specific cellular subtypes, and improved physiological fidelity to the desired cell type.
3D cell cultures
Although 2D cultures are extremely useful for analysis of homogenous or highly enriched populations of specific cell types, they do not recapitulate cellular organization and features of cytoarchitecture of in vivo organs. iPSC-derived 3D culture models have become prominent over the past decade as they provide opportunities to study additional phenotypes that are not evident in 2D models. Many 3D organoid models recapitulate salient features of cellular diversity and structural organization of the parent tissue. A critical issue in determining the diversity of cell types present in organoids is the differentiation protocol used and the extent to which patterning factors are used to generate specific brain regions. One of the initial reports describing 3D culturing of iPSCs to generate organoids used a very limited differentiation protocol, resulting in vast heterogeneity (Lancaster et al., 2013). To perform controlled experiments to evaluate the effect of drugs or exogenous factors, it is better to generate organoids with more consistency to facilitate the reliable observation of phenotypes and to quantify differences between samples. To date, cerebral organoids have been generated that model several different brain regions including the cerebellum, midbrain, and hippocampus (Di Lullo & Kriegstein, 2017; Lancaster et al., 2013; Qian, Song, & Ming, 2019). Although all organoids rely to some extent on cell-intrinsic programs for fate specification and organization, more directed differentiation protocols allow for more reproducible organoids and greater specificity of region identity. Less restrictive differentiation protocols typically result in more heterogeneous organoids, both in terms of intra-organoid cell types as well as variability across cultures. More recently, techniques have been developed to generate models of neural systems by fusing together different region-specific organoids (Marton et al., 2019; Sloan, Andersen, Pasca, Birey, & Pasca, 2018; Sloan et al., 2017).
One example of a region-specific model is a forebrain cortical organoid in which 3D culturing and a specific complement of patterning factors leads to the self-organized formation of ventricular zones and neurons expressing markers of all cortical layers and the basic laminar structure of the human cortex (Qian et al., 2018) (Figure 3). In this model, RNA sequencing at different time points of organoid development revealed a dynamic transcriptional profile that resembled the gene expression changes observed in the developing fetal cortex through the first two trimesters of gestation. These results suggest that organoids can recapitulate both structural and molecular features of early brain development to allow for mechanistic investigations of both acute and chronic drug and virus exposure. There is an increasing appreciation for the role of epigenetic and epitranscriptomic regulation of brain development under basal conditions (Yoon, Ringeling, et al., 2017), as well as its role in mediating the effects of maternal challenges such as stress or infection on fetal development (Bale, 2015). Organoids provide an opportunity to model these environmental factors and identify changes in the epigenome and epitranscriptome that may encode long-lasting signatures of these adverse events that could affect neural function in later development.
Organoids have been used effectively to study exposure to toxins as well as neurotropic virus infection to yield important insights into mechanisms of disease (Qian et al., 2018; Yoon, Song, et al., 2017). Both 2D and 3D models have illustrated the tropism of various viruses for specific cell types, including the preferential targeting of neural progenitor cells by the Zika virus (Qian et al., 2016; Tang et al., 2016). Importantly, iPSC-derived organoids can be differentiated to organ systems outside of the CNS as well, to evaluate the efficacy of therapeutic drugs, as well as off-target effects, on multiple systems.
Remaining challenges and future directions
Despite the promise of iPSCs to provide a new preclinical model for drug development and a diagnostic tool for personalized medicine approaches, there are still many challenges that remain to establish a more physiological platform that recapitulates all of the relevant factors for a given condition. Currently, iPSC models are more representative of early stages of development and it is unclear the extent to which we can model mature pathology using this system. 3D models can give rise to self-organized neural architecture that resembles the developing brain, but there are still critical components that have yet to be modeled reliably in a single integrated system, which include placental and blood brain barriers, vasculature, and the full complement of relevant cell types, including microglia (Figure 3).
Ultimately, the goal is to identify safe, effective ART regimens for the entire population of HIV+ or at-risk individuals, including women of reproductive age. The variability among patients in terms of individual responses to particular drugs or drug combinations remains a challenge, along with the pharmacokinetics during pregnancy and its impact on vertical transmission. To identify biomarkers that are predictive of an individual’s response, we will need to integrate data from observational and clinical studies with animal models and iPSC-based cell culture studies. Drug-drug interactions and the chronological history of drug exposure for a given patient may all play a role in determining the acute and chronic response to a particular drug combination. The advent of single cell biology approaches through RNA sequencing and epigenomics could allow for the stratification of patient groups in terms of treatment response and the identification of potential biomarkers.
Summary
Emerging and convergent evidence suggests that antiretroviral drugs may have a direct impact on the human CNS. The extent and manner in which these drugs may also affect the developing CNS is not well understood, in part due to the lack of physiologically relevant models of human brain formation. Although animal models are important to investigate acute and chronic effects of in utero ART exposure in an intact physiological system, there are species-specific differences in the placental barrier and neuroimmune processes that ultimately limit the utility of these studies to model human fetal brain development. Traditional in vitro models facilitate mechanistic studies on the effects of infectious agents and therapeutics in specific cell types, but historically we have not had access to many of the human neural cell types that are most relevant to investigating the effects of ART on development. Observational and imaging data in humans can provide valuable information after the emergence of pathology, but we need more longitudinal studies of CHEU infants and children who are also exposed to ART perinatally to determine the extent and duration of any consequences on cognitive development. But we also need a better predictive preclinical model to study the potential for deleterious effects of existing and newly developed drugs. iPSC-based studies offer a new approach to provide complementary information needed to bridge this gap between preclinical studies and observation of patient outcomes.
ACKNOWLEDGMENTS
This work was supported grants from National Institutes of Health (R35NS097370 and U19AI131130 to G-l.M.) and (R21MH118037 to K.M.C.).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- Akay C, Cooper M, Odeleye A, Jensen BK, White MG, Vassoler F, … Jordan-Sciutto KL (2014). Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. J Neurovirol, 20(1), 39–53. doi: 10.1007/s13365-013-0227-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alimenti A, Forbes JC, Oberlander TF, Money DM, Grunau RE, Papsdorf MP, … Burdge DR (2006). A prospective controlled study of neurodevelopment in HIV-uninfected children exposed to combination antiretroviral drugs in pregnancy. Pediatrics, 118(4), e1139–1145. doi: 10.1542/peds.2006-0525 [DOI] [PubMed] [Google Scholar]
- Allavena C, Le Moal G, Michau C, Chiffoleau A, & Raffi F (2006). Neuropsychiatric adverse events after switching from an antiretroviral regimen containing efavirenz without tenofovir to an efavirenz regimen containing tenofovir: a report of nine cases. Antivir Ther, 11(2), 263–265. [PubMed] [Google Scholar]
- Apostolova N, Funes HA, Blas-Garcia A, Alegre F, Polo M, & Esplugues JV (2015). Involvement of nitric oxide in the mitochondrial action of efavirenz: a differential effect on neurons and glial cells. J Infect Dis, 211(12), 1953–1958. doi: 10.1093/infdis/jiu825 [DOI] [PubMed] [Google Scholar]
- Arts EJ, & Hazuda DJ (2012). HIV-1 antiretroviral drug therapy. Cold Spring Harb Perspect Med, 2(4), a007161. doi: 10.1101/cshperspect.a007161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL (2015). Epigenetic and transgenerational reprogramming of brain development. Nat Rev Neurosci, 16(6), 332–344. doi: 10.1038/nrn3818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barre-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, … Montagnier L (1983). Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science, 220(4599), 868–871. [DOI] [PubMed] [Google Scholar]
- Becquet R, Bland R, Leroy V, Rollins NC, Ekouevi DK, Coutsoudis A, … Newell ML, (2009). Duration, pattern of breastfeeding and postnatal transmission of HIV: pooled analysis of individual data from West and South African cohorts. PLoS One, 4(10), e7397. doi: 10.1371/journal.pone.0007397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanche S, Tardieu M, Rustin P, Slama A, Barret B, Firtion G, … Delfraissy JF (1999). Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet, 354(9184), 1084–1089. doi: 10.1016/S0140-6736(99)07219-0 [DOI] [PubMed] [Google Scholar]
- Boyd SD, Sampson MR, Viswanathan P, Struble KA, Arya V, & Sherwat AI (2019). Cobicistat-containing antiretroviral regimens are not recommended during pregnancy: viewpoint. AIDS, 33(6), 1089–1093. doi: 10.1097/QAD.0000000000002163 [DOI] [PubMed] [Google Scholar]
- Brown LA, Jin J, Ferrell D, Sadic E, Obregon D, Smith AJ, … Giunta B (2014). Efavirenz promotes beta-secretase expression and increased Abeta1–40,42 via oxidative stress and reduced microglial phagocytosis: implications for HIV associated neurocognitive disorders (HAND). PLoS One, 9(4), e95500. doi: 10.1371/journal.pone.0095500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamandrei G, Valanzano A, Puopolo M, & Aloe L (2002). Developmental exposure to the antiretroviral drug zidovudine increases brain levels of brain-derived neurotrophic factor in mice. Neurosci Lett, 333(2), 111–114. [DOI] [PubMed] [Google Scholar]
- Caniglia EC, Cain LE, Justice A, Tate J, Logan R, Sabin C, … Collaboration H-C (2014). Antiretroviral penetration into the CNS and incidence of AIDS-defining neurologic conditions. Neurology, 83(2), 134–141. doi: 10.1212/WNL.0000000000000564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caniglia EC, Phillips A, Porter K, Sabin CA, Winston A, Logan R, … Hernan MA (2018). Commonly Prescribed Antiretroviral Therapy Regimens and Incidence of AIDS-Defining Neurological Conditions. J Acquir Immune Defic Syndr, 77(1), 102–109. doi: 10.1097/QAI.0000000000001562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalhal A, Gill MJ, Letendre SL, Rachlis A, Bekele T, Raboud J, … Centre for Brain Health in, H. A. (2016). Central nervous system penetration effectiveness of antiretroviral drugs and neuropsychological impairment in the Ontario HIV Treatment Network Cohort Study. J Neurovirol, 22(3), 349–357. doi: 10.1007/s13365-015-0404-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers SM, Mica Y, Lee G, Studer L, & Tomishima MJ (2016). Dual-SMAD Inhibition/WNT Activation-Based Methods to Induce Neural Crest and Derivatives from Human Pluripotent Stem Cells. Methods Mol Biol, 1307, 329–343. doi: 10.1007/7651_2013_59 [DOI] [PubMed] [Google Scholar]
- Chen Y, Balasubramaniyan V, Peng J, Hurlock EC, Tallquist M, Li J, & Lu QR (2007). Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nat Protoc, 2(5), 1044–1051. doi: 10.1038/nprot.2007.149 [DOI] [PubMed] [Google Scholar]
- Ciavatta VT, Bichler EK, Speigel IA, Elder CC, Teng SL, Tyor WR, & Garcia PS (2017). In vitro and Ex vivo Neurotoxic Effects of Efavirenz are Greater than Those of Other Common Antiretrovirals. Neurochem Res, 42(11), 3220–3232. doi: 10.1007/s11064-017-2358-x [DOI] [PubMed] [Google Scholar]
- Ciccarelli N, Fabbiani M, Di Giambenedetto S, Fanti I, Baldonero E, Bracciale L, … Silveri MC (2011). Efavirenz associated with cognitive disorders in otherwise asymptomatic HIV-infected patients. Neurology, 76(16), 1403–1409. doi: 10.1212/WNL.0b013e31821670fb [DOI] [PubMed] [Google Scholar]
- Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O’Sullivan MJ, … et al. (1994). Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med, 331(18), 1173–1180. doi: 10.1056/NEJM199411033311801 [DOI] [PubMed] [Google Scholar]
- Culnane M, Fowler M, Lee SS, McSherry G, Brady M, O’Donnell K, … Oleske J (1999). Lack of long-term effects of in utero exposure to zidovudine among uninfected children born to HIV-infected women. Pediatric AIDS Clinical Trials Group Protocol 219/076 Teams. JAMA, 281(2), 151–157. [DOI] [PubMed] [Google Scholar]
- De Schacht C, Mabunda N, Ferreira OC, Ismael N, Calu N, Santos I, … Jani IV (2014). High HIV incidence in the postpartum period sustains vertical transmission in settings with generalized epidemics: a cohort study in Southern Mozambique. J Int AIDS Soc, 17, 18808. doi: 10.7448/IAS.17.1.18808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decloedt EH, Rosenkranz B, Maartens G, & Joska J (2015). Central nervous system penetration of antiretroviral drugs: pharmacokinetic, pharmacodynamic and pharmacogenomic considerations. Clin Pharmacokinet, 54(6), 581–598. doi: 10.1007/s40262-015-0257-3 [DOI] [PubMed] [Google Scholar]
- Demir M, & Laywell ED (2015). Neurotoxic effects of AZT on developing and adult neurogenesis. Front Neurosci, 9, 93. doi: 10.3389/fnins.2015.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lullo E, & Kriegstein AR (2017). The use of brain organoids to investigate neural development and disease. Nat Rev Neurosci, 18(10), 573–584. doi: 10.1038/nrn.2017.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch R, & Geschwind DH (2011). The human brain in a dish: the promise of iPSC-derived neurons. Cell, 145(6), 831–834. doi: 10.1016/j.cell.2011.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden C, Peyriere H, Peytavin G, & Reynes J (2011). Severe insomnia related to high concentrations of raltegravir. AIDS, 25(5), 725–727. doi: 10.1097/QAD.0b013e32834465c8 [DOI] [PubMed] [Google Scholar]
- Engle SJ, Blaha L, & Kleiman RJ (2018). Best Practices for Translational Disease Modeling Using Human iPSC-Derived Neurons. Neuron, 100(4), 783–797. doi: 10.1016/j.neuron.2018.10.033 [DOI] [PubMed] [Google Scholar]
- Etherton MR, Lyons JL, & Ard KL (2015). HIV-associated Neurocognitive Disorders and Antiretroviral Therapy: Current Concepts and Controversies. Curr Infect Dis Rep, 17(6), 485. doi: 10.1007/s11908-015-0485-6 [DOI] [PubMed] [Google Scholar]
- Fischl MA, Richman DD, Grieco MH, Gottlieb MS, Volberding PA, Laskin OL, … et al. (1987). The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med, 317(4), 185–191. doi: 10.1056/NEJM198707233170401 [DOI] [PubMed] [Google Scholar]
- Ford N, Mofenson L, Shubber Z, Calmy A, Andrieux-Meyer I, Vitoria M, … Renaud F (2014). Safety of efavirenz in the first trimester of pregnancy: an updated systematic review and meta-analysis. AIDS, 28 Suppl 2, S123–131. doi: 10.1097/QAD.0000000000000231 [DOI] [PubMed] [Google Scholar]
- Fowler MG, Flynn P, & Aizire J (2018). What is new in perinatal HIV prevention? Curr Opin Pediatr, 30(1), 144–151. doi: 10.1097/MOP.0000000000000579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler MG, Qin M, Fiscus SA, Currier JS, Flynn PM, Chipato T, … Team, I. B. F. P. S. (2016). Benefits and Risks of Antiretroviral Therapy for Perinatal HIV Prevention. N Engl J Med, 375(18), 1726–1737. doi: 10.1056/NEJMoa1511691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funes HA, Apostolova N, Alegre F, Blas-Garcia A, Alvarez A, Marti-Cabrera M, & Esplugues JV (2014). Neuronal bioenergetics and acute mitochondrial dysfunction: a clue to understanding the central nervous system side effects of efavirenz. J Infect Dis, 210(9), 1385–1395. doi: 10.1093/infdis/jiu273 [DOI] [PubMed] [Google Scholar]
- Garcia-Otero L, Lopez M, Gomez O, Gonce A, Bennasar M, Martinez JM, … Gratacos E (2016). Zidovudine treatment in HIV-infected pregnant women is associated with fetal cardiac remodelling. AIDS, 30(9), 1393–1401. doi: 10.1097/QAD.0000000000001066 [DOI] [PubMed] [Google Scholar]
- Gatch MB, Kozlenkov A, Huang RQ, Yang W, Nguyen JD, Gonzalez-Maeso J, … Schetz JA (2013). The HIV antiretroviral drug efavirenz has LSD-like properties. Neuropsychopharmacology, 38(12), 2373–2384. doi: 10.1038/npp.2013.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert EM, Darin KM, Scarsi KK, & McLaughlin MM (2015). Antiretroviral Pharmacokinetics in Pregnant Women. Pharmacotherapy, 35(9), 838–855. doi: 10.1002/phar.1626 [DOI] [PubMed] [Google Scholar]
- Goodsell DS (2015). Illustrations of the HIV life cycle. Curr Top Microbiol Immunol, 389, 243–252. doi: 10.1007/82_2015_437 [DOI] [PubMed] [Google Scholar]
- Gordon J, Amini S, & White MK (2013). General overview of neuronal cell culture. Methods Mol Biol, 1078, 1–8. doi: 10.1007/978-1-62703-640-5_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J, & Young B (2009). Acute onset insomnia associated with the initiation of raltegravir: a report of two cases and literature review. AIDS Patient Care STDS, 23(9), 689–690. doi: 10.1089/apc.2009.0012 [DOI] [PubMed] [Google Scholar]
- Greene ND, & Copp AJ (2014). Neural tube defects. Annu Rev Neurosci, 37, 221–242. doi: 10.1146/annurev-neuro-062012-170354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin TZ, Kang W, Ma Y, & Zhang M (2015). The HAND Database: a gateway to understanding the role of HIV in HIV-associated neurocognitive disorders. BMC Med Genomics, 8, 70. doi: 10.1186/s12920-015-0143-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha JC, Nosbisch C, Abkowitz JL, Conrad SH, Mottet NK, Ruppenthal GC, … Unadkat JD (1998). Fetal, infant, and maternal toxicity of zidovudine (azidothymidine) administered throughout pregnancy in Macaca nemestrina. J Acquir Immune Defic Syndr Hum Retrovirol, 18(1), 27–38. [DOI] [PubMed] [Google Scholar]
- Harris M, Larsen G, & Montaner JS (2008). Exacerbation of depression associated with starting raltegravir: a report of four cases. AIDS, 22(14), 1890–1892. doi: 10.1097/QAD.0b013e32830e0169 [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR Jr., Woods SP, Ake C, Vaida F, … Group C, (2010). HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology, 75(23), 2087–2096. doi: 10.1212/WNL.0b013e318200d727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshad N, Couture MC, Prasitsuebsai W, Nir TM, Aurpibul L, Thompson PM, … Groups, P. S. (2015). Brain Imaging and Neurodevelopment in HIV-uninfected Thai Children Born to HIV-infected Mothers. Pediatr Infect Dis J, 34(9), e211–216. doi: 10.1097/INF.0000000000000774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankiewicz M, Holmes MJ, Taylor PA, Cotton MF, Laughton B, van der Kouwe AJW, & Meintjes EM (2017). White Matter Abnormalities in Children with HIV Infection and Exposure. Front Neuroanat, 11, 88. doi: 10.3389/fnana.2017.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen BK, Monnerie H, Mannell MV, Gannon PJ, Espinoza CA, Erickson MA, … Grinspan JB (2015). Altered Oligodendrocyte Maturation and Myelin Maintenance: The Role of Antiretrovirals in HIV-Associated Neurocognitive Disorders. J Neuropathol Exp Neurol, 74(11), 1093–1118. doi: 10.1097/NEN.0000000000000255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Grimmig B, Izzo J, Brown LAM, Hudson C, Smith AJ, … Giunta B (2016). HIV Non-Nucleoside Reverse Transcriptase Inhibitor Efavirenz Reduces Neural Stem Cell Proliferation in Vitro and in Vivo. Cell Transplant, 25(11), 1967–1977. doi: 10.3727/096368916X691457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL, Rodriguez VJ, Alcaide ML, Weiss SM, & Peltzer K (2019). The Use of Efavirenz During Pregnancy is Associated with Suicidal Ideation in Postpartum Women in Rural South Africa. AIDS Behav, 23(1), 126–131. doi: 10.1007/s10461-018-2213-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilewo C, Karlsson K, Ngarina M, Massawe A, Lyamuya E, Swai A, … Mitra Plus Study, T. (2009). Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: the Mitra Plus study. J Acquir Immune Defic Syndr, 52(3), 406–416. doi: 10.1097/QAI.0b013e3181b323ff [DOI] [PubMed] [Google Scholar]
- Knapp KM, Brogly SB, Muenz DG, Spiegel HM, Conway DH, Scott GB, … Read JS (2012). Prevalence of congenital anomalies in infants with in utero exposure to antiretrovirals. Pediatr Infect Dis J, 31(2), 164–170. doi: 10.1097/INF.0b013e318235c7aa [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Imamura K, Funayama M, Tsukita K, Miyake M, Ohta A, … Inoue H (2017). iPSC-Based Compound Screening and In Vitro Trials Identify a Synergistic Anti-amyloid beta Combination for Alzheimer’s Disease. Cell Rep, 21(8), 2304–2312. doi: 10.1016/j.celrep.2017.10.109 [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, … Knoblich JA (2013). Cerebral organoids model human brain development and microcephaly. Nature, 501(7467), 373–379. doi: 10.1038/nature12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange SC, Bak LK, Waagepetersen HS, Schousboe A, & Norenberg MD (2012). Primary cultures of astrocytes: their value in understanding astrocytes in health and disease. Neurochem Res, 37(11), 2569–2588. doi: 10.1007/s11064-012-0868-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latronico T, Pati I, Ciavarella R, Fasano A, Mengoni F, Lichtner M, … Liuzzi GM (2018). In vitro effect of antiretroviral drugs on cultured primary astrocytes: analysis of neurotoxicity and matrix metalloproteinase inhibition. J Neurochem, 144(3), 271–284. doi: 10.1111/jnc.14269 [DOI] [PubMed] [Google Scholar]
- Lian H, Roy E, & Zheng H (2016). Protocol for Primary Microglial Culture Preparation. Bio Protoc, 6(21). doi: 10.21769/BioProtoc.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Vaida F, Wong J, Sanders CA, Kao YT, Croteau D, … Group, C. (2016). Long-term efavirenz use is associated with worse neurocognitive functioning in HIV-infected patients. J Neurovirol, 22(2), 170–178. doi: 10.1007/s13365-015-0382-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra CM, Zhao Y, Clifford DB, Letendre S, Evans S, Henry K, … Team, A. C. T. G. S. (2009). Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS, 23(11), 1359–1366. doi: 10.1097/QAD.0b013e32832c4152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton RM, Miura Y, Sloan SA, Li Q, Revah O, Levy RJ, … Pasca SP (2019). Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat Neurosci, 22(3), 484–491. doi: 10.1038/s41593-018-0316-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack SA, & Best BM (2014). Protecting the fetus against HIV infection: a systematic review of placental transfer of antiretrovirals. Clin Pharmacokinet, 53(11), 989–1004. doi: 10.1007/s40262-014-0185-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry MS, Balogun KA, McDonald BC, Vreeman RC, Whipple EC, & Serghides L (2019). In utero exposure to HIV and/or antiretroviral therapy: a systematic review of preclinical and clinical evidence of cognitive outcomes. J Int AIDS Soc, 22(4), e25275. doi: 10.1002/jia2.25275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens J, Marchetto MC, Bardy C, & Gage FH (2016). Evaluating cell reprogramming, differentiation and conversion technologies in neuroscience. Nat Rev Neurosci, 17(7), 424–437. doi: 10.1038/nrn.2016.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet LJ, & Gillette MU (2012). Over a century of neuron culture: from the hanging drop to microfluidic devices. Yale J Biol Med, 85(4), 501–521. [PMC free article] [PubMed] [Google Scholar]
- Mitsuya H, Weinhold KJ, Furman PA, St Clair MH, Lehrman SN, Gallo RC, … Broder S (1985). 3’-Azido-3’-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type NI/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci U S A, 82(20), 7096–7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenegro-Burke JR, Woldstad CJ, Fang M, Bade AN, McMillan J, Edagwa B, … Siuzdak G (2018). Nanoformulated Antiretroviral Therapy Attenuates Brain Metabolic Oxidative Stress. Mol Neurobiol. doi: 10.1007/s12035-018-1273-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moren C, Noguera-Julian A, Rovira N, Garrabou G, Nicolas M, Cardellach F, … Fortuny C (2011). Mitochondrial assessment in asymptomatic HIV-infected paediatric patients on HAART. Antivir Ther, 16(5), 719–724. doi: 10.3851/IMP1806 [DOI] [PubMed] [Google Scholar]
- Nikolopoulou E, Galea GL, Rolo A, Greene ND, & Copp AJ (2017). Neural tube closure: cellular, molecular and biomechanical mechanisms. Development, 144(4), 552–566. doi: 10.1242/dev.145904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguera A, Fortuny C, Munoz-Almagro C, Sanchez E, Vilaseca MA, Artuch R, … Jimenez R (2004). Hyperlactatemia in human immunodeficiency virus-uninfected infants who are exposed to antiretrovirals. Pediatrics, 114(5), e598–603. doi: 10.1542/peds.2004-0955 [DOI] [PubMed] [Google Scholar]
- Nozyce ML, Huo Y, Williams PL, Kapetanovic S, Hazra R, Nichols S, … Pediatric, H. C. S. (2014). Safety of in utero and neonatal antiretroviral exposure: cognitive and academic outcomes in HIV-exposed, uninfected children 5–13 years of age. Pediatr Infect Dis J, 33(11), 1128–1133. doi: 10.1097/INF.0000000000000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyandiko WM, Otieno-Nyunya B, Musick B, Bucher-Yiannoutsos S, Akhaabi P, Lane K, … Wools-Kaloustian K (2010). Outcomes of HIV-exposed children in western Kenya: efficacy of prevention of mother to child transmission in a resource-constrained setting. J Acquir Immune Defic Syndr, 54(1), 42–50. doi: 10.1097/QAI.0b013e3181d8ad51 [DOI] [PubMed] [Google Scholar]
- Pariente G, Leibson T, Carls A, Adams-Webber T, Ito S, & Koren G (2016). Pregnancy-Associated Changes in Pharmacokinetics: A Systematic Review. PLoS Med, 13(11), e1002160. doi: 10.1371/journal.pmed.1002160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poblano A, Figueroa L, Figueroa-Damian R, & Schnaas L (2004). Effects of prenatal exposure to Zidovudine and Lamivudine on brainstem auditory evoked potentials in infants from HIV-infected women. Proc West Pharmacol Soc, 47, 46–49. [PubMed] [Google Scholar]
- Prieto LM, Gonzalez-Tome MI, Munoz E, Fernandez-Ibieta M, Soto B, Alvarez A, … Madrid Cohort of, H. I. V. I. M.-I. P. (2014). Birth defects in a cohort of infants born to HIV-infected women in Spain, 2000–2009. BMC Infect Dis, 14, 700. doi: 10.1186/s12879-014-0700-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Jacob F, Song MM, Nguyen HN, Song H, & Ming GL (2018). Generation of human brain region-specific organoids using a miniaturized spinning bioreactor. Nat Protoc, 13(3), 565–580. doi: 10.1038/nprot.2017.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, … Ming GL (2016). Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell, 165(5), 1238–1254. doi: 10.1016/j.cell.2016.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Song H, & Ming GL (2019). Brain organoids: advances, applications and challenges. Development, 146(8). doi: 10.1242/dev.166074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ML, Zeldow B, Siberry GK, Purswani M, Malee K, Hoffman HJ, … Pediatric, H. C. S. (2013). Evaluation of risk for late language emergence after in utero antiretroviral drug exposure in HIV-exposed uninfected infants. Pediatr Infect Dis J, 32(10), e406–413. doi: 10.1097/INF.0b013e31829b80ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson K, Liner J, & Meeker RB (2012). Antiretroviral neurotoxicity. J Neurovirol, 18(5), 388–399. doi: 10.1007/s13365-012-0120-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KR, Su Z, Margolis DM, Krambrink A, Havlir DV, Evans S, … Team, A. S. (2010). Neurocognitive effects of treatment interruption in stable HIV-positive patients in an observational cohort. Neurology, 74(16), 1260–1266. doi: 10.1212/WNL.0b013e3181d9ed09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondinini C, Venerosi A, Branchi I, Calamandrei G, & Alleva E (1999). Long-term effects of prenatal 3’-azido-3’-deoxythymidine (AZT) exposure on intermale aggressive behaviour of mice. Psychopharmacology (Berl), 145(3), 317–323. [DOI] [PubMed] [Google Scholar]
- Rubin LH, Maki PM, Springer G, Benning L, Anastos K, Gustafson D, … Women’s Interagency, H. I. V. S. (2017). Cognitive trajectories over 4 years among HIV-infected women with optimal viral suppression. Neurology, 89(15), 1594–1603. doi: 10.1212/WNL.0000000000004491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh A, Hull AD, Franklin P, & Spector SA (2005). Myelomeningocele in an infant with intrauterine exposure to efavirenz. J Perinatol, 25(8), 555–556. doi: 10.1038/sj.jp.7211343 [DOI] [PubMed] [Google Scholar]
- Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, … McArthur JC (2016). HIV-associated neurocognitive disorder--pathogenesis and prospects for treatment. Nat Rev Neurol, 12(4), 234–248. doi: 10.1038/nrneurol.2016.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg BC, Taylor MJ, Alhassoon OM, Gonzalez R, Brown GG, Ellis RJ, … Group, H. (2005). Brain mitochondrial injury in human immunodeficiency virus-seropositive (HIV+) individuals taking nucleoside reverse transcriptase inhibitors. J Neurovirol, 11(4), 356–364. doi: 10.1080/13550280591002342 [DOI] [PubMed] [Google Scholar]
- Serghides L (2018, March 4-7, 2018). Changes in Brain Volume and Cognition in Mice Exposed In Utero to ABC/3TC-ATV/RTV. Paper presented at the Conference on Retroviruses and Opportunistic Infections (CROI), Boston, Massachusetts. [Google Scholar]
- Shallie PD, & Naicker T (2019). The placenta as a window to the brain: A review on the role of placental markers in prenatal programming of neurodevelopment. Int J Dev Neurosci, 73, 41–49. doi: 10.1016/j.ijdevneu.2019.01.003 [DOI] [PubMed] [Google Scholar]
- Shamsuddin H, Raudenbush CL, Sciba BL, Zhou YP, Mast TC, Greaves WL, … Straus W (2019). Evaluation of Neural Tube Defects (NTD) After Exposure to Raltegravir During Pregnancy. J Acquir Immune Defic Syndr. doi: 10.1097/QAI.0000000000002031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibiude J, Le Chenadec J, Bonnet D, Tubiana R, Faye A, Dollfus C, … Viral Hepatitis French Perinatal Cohort/Protease Inhibitor Monotherapy Evaluation, T. (2015). In utero exposure to zidovudine and heart anomalies in the ANRS French perinatal cohort and the nested PRIMEVA randomized trial. Clin Infect Dis, 61(2), 270–280. doi: 10.1093/cid/civ260 [DOI] [PubMed] [Google Scholar]
- Sibiude J, Mandelbrot L, Blanche S, Le Chenadec J, Boullag-Bonnet N, Faye A, … Warszawski J (2014). Association between prenatal exposure to antiretroviral therapy and birth defects: an analysis of the French perinatal cohort study (ANRS CO1/CO11). PLoS Med, 11(4), e1001635. doi: 10.1371/journal.pmed.1001635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirois PA, Huo Y, Williams PL, Malee K, Garvie PA, Kammerer B, … Pediatric, H. C. S. (2013). Safety of perinatal exposure to antiretroviral medications: developmental outcomes in infants. Pediatr Infect Dis J, 32(6), 648–655. doi: 10.1097/INF.0b013e318284129a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan SA, Andersen J, Pasca AM, Birey F, & Pasca SP (2018). Generation and assembly of human brain region-specific three-dimensional cultures. Nat Protoc, 13(9), 2062–2085. doi: 10.1038/s41596-018-0032-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan SA, Darmanis S, Huber N, Khan TA, Birey F, Caneda C, … Pasca SP (2017). Human Astrocyte Maturation Captured in 3D Cerebral Cortical Spheroids Derived from Pluripotent Stem Cells. Neuron, 95(4), 779–790 e776. doi: 10.1016/j.neuron.2017.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slogrove AL, Becquet R, Chadwick EG, Cote HCF, Essajee S, Hazra R, … Powis KM (2018). Surviving and Thriving-Shifting the Public Health Response to HIV-Exposed Uninfected Children: Report of the 3rd HIV-Exposed Uninfected Child Workshop. Front Pediatr, 6, 157. doi: 10.3389/fped.2018.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Puka K, Sehra R, Read SE, & Bitnun A (2017). Longitudinal development of cognitive, visuomotor and adaptive behavior skills in HIV uninfected children, aged 3–5 years of age, exposed pre- and perinatally to anti-retroviral medications. AIDS Care, 29(10), 1302–1308. doi: 10.1080/09540121.2017.1325436 [DOI] [PubMed] [Google Scholar]
- Smurzynski M, Wu K, Letendre S, Robertson K, Bosch RJ, Clifford DB, … Ellis R (2011). Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS, 25(3), 357–365. doi: 10.1097/QAD.0b013e32834171f8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son G, & Han J (2018). Roles of mitochondria in neuronal development. BMB Rep, 51(11), 549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RS, Shapiro DE, McSherry GD, Britto P, Cunningham BE, Culnane M, … Delfraissy JF, (1998). Safety of the maternal-infant zidovudine regimen utilized in the Pediatric AIDS Clinical Trial Group 076 Study. AIDS, 12(14), 1805–1813. [DOI] [PubMed] [Google Scholar]
- Stern AL, Lee RN, Panvelker N, Li J, Harowitz J, Jordan-Sciutto KL, & Akay-Espinoza C (2018). Differential Effects of Antiretroviral Drugs on Neurons In Vitro: Roles for Oxidative Stress and Integrated Stress Response. J Neuroimmune Pharmacol, 13(1), 64–76. doi: 10.1007/s11481-017-9761-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, … Ming GL (2016). Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell, 18(5), 587–590. doi: 10.1016/j.stem.2016.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, & Zhang SC (2016). Neural Subtype Specification from Human Pluripotent Stem Cells. Cell Stem Cell, 19(5), 573–586. doi: 10.1016/j.stem.2016.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu M, Brunelle F, Raybaud C, Ball W, Barret B, Pautard B, … Blanche S (2005). Cerebral MR imaging in uninfected children born to HIV-seropositive mothers and perinatally exposed to zidovudine. AJNR Am J Neuroradiol, 26(4), 695–701. [PMC free article] [PubMed] [Google Scholar]
- Tau GZ, & Peterson BS (2010). Normal development of brain circuits. Neuropsychopharmacology, 35(1), 147–168. doi: 10.1038/npp.2009.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeraananchai S, Kerr SJ, Amin J, Ruxrungtham K, & Law MG (2017). Life expectancy of HIV-positive people after starting combination antiretroviral therapy: a meta-analysis. HIV Med, 18(4), 256–266. doi: 10.1111/hiv.12421 [DOI] [PubMed] [Google Scholar]
- Tierney AL, & Nelson CA 3rd. (2009). Brain Development and the Role of Experience in the Early Years. Zero Three, 30(2), 9–13. [PMC free article] [PubMed] [Google Scholar]
- Tovar-y-Romo LB, Bumpus NN, Pomerantz D, Avery LB, Sacktor N, McArthur JC, & Haughey NJ (2012). Dendritic spine injury induced by the 8-hydroxy metabolite of efavirenz. J Pharmacol Exp Ther, 343(3), 696–703. doi: 10.1124/jpet.112.195701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovo PA, Chiapello N, Gabiano C, Zeviani M, & Spada M (2005). Zidovudine administration during pregnancy and mitochondrial disease in the offspring. Antivir Ther, 10(6), 697–699. [PubMed] [Google Scholar]
- Tran LT, Roos A, Fouche JP, Koen N, Woods RP, Zar HJ, … Donald KA (2016). White Matter Microstructural Integrity and Neurobehavioral Outcome of HIV-Exposed Uninfected Neonates. Medicine (Baltimore), 95(4), e2577. doi: 10.1097/MD.0000000000002577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS. (2018). Global HIV & AIDS Statistics - 2018 Fact Sheet. Retrieved from http://www.unaids.org/en/resources/fact-sheet
- Underwood J, Robertson KR, & Winston A (2015). Could antiretroviral neurotoxicity play a role in the pathogenesis of cognitive impairment in treated HIV disease? AIDS, 29(3), 253–261. doi: 10.1097/QAD.0000000000000538 [DOI] [PubMed] [Google Scholar]
- van de Wijer L, Garcia LP, Hanswijk SI, Rando J, Middelman A, Ter Heine R, … Homberg JR (2019). Neurodevelopmental and behavioral consequences of perinatal exposure to the HIV drug efavirenz in a rodent model. Transl Psychiatry, 9(1), 84. doi: 10.1038/s41398-019-0420-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke RB, Chadwick EG, Hazra R, Williams PL, & Seage GR 3rd. (2016). The PHACS SMARTT Study: Assessment of the Safety of In Utero Exposure to Antiretroviral Drugs. Front Immunol, 7, 199. doi: 10.3389/fimmu.2016.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannappagari V, Koram N, Albano J, Tilson H, & Gee C (2016). Association between in utero zidovudine exposure and nondefect adverse birth outcomes: analysis of prospectively collected data from the Antiretroviral Pregnancy Registry. BJOG, 123(6), 910–916. doi: 10.1111/1471-0528.13542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velichkovska M, Surnar B, Nair M, Dhar S, & Toborek M (2019). Targeted Mitochondrial C0Q10 Delivery Attenuates Antiretroviral-Drug-Induced Senescence of Neural Progenitor Cells. Mol Pharm, 16(2), 724–736. doi: 10.1021/acs.molpharmaceut.8b01014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venerosi A, Cirulli F, Capone F, & Alleva E (2003). Prolonged perinatal AZT administration and early maternal separation: effects on social and emotional behaviour of periadolescent mice. Pharmacol Biochem Behav, 74(3), 671–681. [DOI] [PubMed] [Google Scholar]
- Venerosi A, Cirulli F, Lil’p IG, Fiore M, Calamandrei G, & Alleva E (2000). Prolonged perinatal exposure to AZT affects aggressive behaviour of adult CD-1 mice. Psychopharmacology (Berl), 150(4), 404–411. [DOI] [PubMed] [Google Scholar]
- Venerosi A, Valanzano A, Alleva E, & Calamandrei G (2001). Prenatal exposure to anti-HIV drugs: neurobehavioral effects of zidovudine (AZT) + lamivudine (3TC) treatment in mice. Teratology, 63(1), 26–37. doi: [DOI] [PubMed] [Google Scholar]
- Volberding PA, Lagakos SW, Koch MA, Pettinelli C, Myers MW, Booth DK, … et al. (1990). Zidovudine in asymptomatic human immunodeficiency virus infection. A controlled trial in persons with fewer than 500 CD4-positive cells per cubic millimeter. The AIDS Clinical Trials Group of the National Institute of Allergy and Infectious Diseases. N Engl J Med, 322(14), 941–949. doi: 10.1056/NEJM199004053221401 [DOI] [PubMed] [Google Scholar]
- W.H.O. (2018). Mother-to-child transmission of HIV. Retrieved from https://www.who.int/hiv/topics/mtct/about/en/
- White AJ (2001). Mitochondrial toxicity and HIV therapy. Sex Transm Infect, 77(3), 158–173. doi: 10.1136/sti.77.3.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead N, Potterton J, & Coovadia A (2014). The neurodevelopment of HIV-infected infants on HAART compared to HIV-exposed but uninfected infants. AIDS Care, 26(4), 497–504. doi: 10.1080/09540121.2013.841828 [DOI] [PubMed] [Google Scholar]
- Williams PL, Crain MJ, Yildirim C, Hazra R, Van Dyke RB, Rich K, … Pediatric, H. I. V. A. C. S. (2015). Congenital anomalies and in utero antiretroviral exposure in human immunodeficiency virus-exposed uninfected infants. JAMA Pediatr, 169(1), 48–55. doi: 10.1001/jamapediatrics.2014.1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PL, Marino M, Malee K, Brogly S, Hughes MD, Mofenson LM, & Team PC (2010). Neurodevelopment and in utero antiretroviral exposure of HIV-exposed uninfected infants. Pediatrics, 125(2), e250–260. doi: 10.1542/peds.2009-1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise ME, Mistry K, & Reid S (2002). Drug points: Neuropsychiatric complications of nevirapine treatment. BMJ, 324(7342), 879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Wang Y, Qin Z, Qiu L, Zhang M, Huang Y, & Zheng JC (2017). Combined Medication of Antiretroviral Drugs Tenofovir Disoproxil Fumarate, Emtricitabine, and Raltegravir Reduces Neural Progenitor Cell Proliferation In Vivo and In Vitro. J Neuroimmune Pharmacol, 12(4), 682–692. doi: 10.1007/s11481-017-9755-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KJ, Ringeling FR, Vissers C, Jacob F, Pokrass M, Jimenez-Cyrus D, … Song H, (2017). Temporal Control of Mammalian Cortical Neurogenesis by m(6)A Methylation. Cell, 171(4), 877–889 e817. doi: 10.1016/j.cell.2017.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KJ, Song G, Qian X, Pan J, Xu D, Rho HS, … Ming GL, (2017). Zika-Virus-Encoded NS2A Disrupts Mammalian Cortical Neurogenesis by Degrading Adherens Junction Proteins. Cell Stem Cell, 21(3), 349–358 e346. doi: 10.1016/j.stem.2017.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zash R, Makhema J, & Shapiro RL (2018). Neural-Tube Defects with Dolutegravir Treatment from the Time of Conception. N Engl J Med, 379(10), 979–981. doi: 10.1056/NEJMc1807653 [DOI] [PMC free article] [PubMed] [Google Scholar]


