Abstract
Protein-protein interactions can be modulated by phosphorylation of either binding partner, thereby altering subcellular localization and/or physiological function. Shank3, a master postsynaptic scaffolding protein that controls the developmental maturation of excitatory synapses, was recently shown to be phosphorylated by Protein Kinase A (PKA) at Ser685 in vivo. Mutation of Shank3 Ser685 was shown to modulate the binding of Abelson interactor 1 (ABI1), a component of the WAVE regulatory complex for actin remodeling, but a direct effect of Ser685 phosphorylation on ABI1 binding was not investigated. Here, we demonstrate that Ca2+/calmodulin-dependent protein kinase II alpha (CaMKIIα) also phosphorylates Shank3 at Ser685. Mutation of Ser685 to phospho-null alanine (S685A) prevented both CaMKIIα and PKA phosphorylation of a GST-Shank3 fusion protein. The co-immunoprecipitation of ABI1 with Shank3 from HEK293 cell extracts is reduced by mutation of Ser685 to either Ala or Asp. However, pre-phosphorylation of GST-Shank3 by purified CaMKIIα significantly increased binding of ABI1, and this effect was abrogated by Ser685 to Ala mutation in GST-Shank3. Taken together, our data suggest that neuronal ABI1-Shank3 interactions may be convergently regulated by Shank3 Ser685 phosphorylation in response to both Ca2+ and cAMP signaling, potentially modulating dendritic spine morphology.
Keywords: Protein phosphorylation, Shank3, CaMKIIα, PKA
Introduction
The scaffolding protein Shank3 interacts directly or indirectly with receptors, ion channels, cytoskeletal proteins, and signaling molecules to regulate their targeting and anchoring to postsynaptic densities and dendritic spines in excitatory neurons [1, 2]. These interactions are mediated by well-defined binding domains in Shank3, including multiple ankyrin repeats, SH3 and PDZ domains, a novel CaMKII binding motif, distinct proline-rich regions for binding to ABI1, homer, and cortactin, and a C-terminal SAM domain [3–7]. Mutations in the SHANK3 gene are often linked to neurodevelopmental and neuropsychiatric disorders, such as autism spectrum disorder (ASD) [8]. Moreover, disruptions of Shank3 expression in multiple mouse models result in deficits in synaptic transmission, abnormal neuronal morphology, and diverse behavioral phenotypes [9, 10]. Since phenotypes of these mouse models vary with the specific Shank3 mutation, which often target different exons, understanding physiological signaling mechanisms coupled to different regions of Shank3 will provide better targeted therapy for Shank3related neuropsychiatric disorders.
Phosphorylation of scaffolding proteins can regulate their binding interactions, subcellular distribution, and ultimately their physiological function [11]. Several phospho-proteomics studies have reported that Shank3 is phosphorylated at multiple sites when isolated from brain tissues, and in some instances the kinase and residue have been identified. For example, ribosomal S6 kinase 2 (RSK2) phosphorylates Shank3 at Ser1648 in vitro and in primary neurons [12]. Recently, Shank3 was shown to be phosphorylated in vivo at Ser685, immediately adjacent to the ABI1 binding motif [13]. A phospho-null S685A mutation or a missense S685I mutation, identified in a patient with ASD, reduced the ABI1-Shank3 interaction and impaired dendritic spine development and synaptic transmission. In vitro studies indicated that PKA, but not ERK2, GSK3β, or casein kinase 2, phosphorylated Ser685 [13]. However, a direct effect of Shank3 Ser685 phosphorylation, or of a phospho-mimetic mutation of Ser685 to an acidic residue, on ABI1 binding was not reported.
Synaptic plasticity involves dynamic changes in the actin cytoskeleton and spine morphology that are modulated by both Ca2+ and cAMP signaling [14–18]. While CaMKII and PKA can phosphorylate the same residues in some shared substrates, such as connexin43 [19, 20] or the Rpt6 subunit of the 26 S proteasome [21, 22], they also target distinct sites in other shared substrates, such as the GluA1 subunit of the AMPAtype glutamate receptor [23, 24]. Notably, phospho-proteomics analyses revealed that phosphorylation of several sites in Shank3 is increased following CaMKII activation in isolated synaptic fractions [25, 26], Thus, it seems likely that both PKA and CaMKII can target Shank3, but the potential role of CaMKII in Shank3 Ser685 phosphorylation has not been investigated.
In this study, we tested the hypothesis that Shank3 Ser685 is also targeted by CaMKII. We found that both PKA and CaMKII can phosphorylate a GST-Shank3 fusion protein containing residues 572–691, and that this phosphorylation is prevented by mutation of Ser685 to a phospho-null alanine residue. Consistent with prior studies, any mutation of Ser685 disrupts Shank3 interactions with ABI1. However, we show for the first time that Ser685 phosphorylation by CaMKII significantly enhances ABI1 interaction with Shank3.
Materials and Methods
Antibodies
The following antibodies and dilutions were used for immunoblotting: mouse anti-GFP (Vanderbilt Antibody and Protein Resource catalog 1C9A5, 1:3000), mouse anti-HA (Biolegend catalog 901503, 1:3000), mouse anti-CaMKIIα 6G9 (Thermo Fisher Scientific catalog MA1–048, 1:3000), goat anti-GST (GE Healthcare Life Sciences catalog 27–4577-01, 1:5000), HRP-conjugated anti-mouse (Promega catalog W4021, 1:3000), and HRP-conjugated anti-goat (Abcam catalog ab6741, 1:5000).
Cloning and Protein Expression
Constructs to express GFP-Shank3 (rat) and HA-tagged CaV1.3-CTD (rat) in HEK293T cells, and to express GST-Shank3 fusion proteins in BL21 (DE3) pLysS E. coli, were described previously [7]. The GST-GluA1 was previously described [23]. The eGFP-ABI1 vector was purchased from Addgene (#74905), and DNA encoding full-length ABI1 was PCR amplified and inserted into the pCGNh vector, a gift from Dr. Winship Herr (Université de Lausanne), between Xba1 and BamHI restriction sites to generate N-terminal HA-tagged ABI1. Point mutations were generated using Q5 Site-Directed Mutagenesis (New England Biolabs); the numbering of Shank3 residues is from the canonical rat sequence (UniProtKB: Q9JLU4 Isoform 2). All constructs were confirmed by DNA sequencing.
Protein Purification
Purification of recombinant mouse CaMKIIα and GST fusion proteins was previously described [27]. Purified bovine heart PKA was a generous gift from Dr. Jackie Corbin (Vanderbilt University).
Radiolabeled Phosphorylation Assays
GST fusion proteins were incubated at 30°C for the indicated time in 50 mM HEPES, pH 7.5, 10 mM magnesium acetate, 1 μM dithiothreitol, 400 μM [γ−32P] ATP (700–1,000 c.p.m./pmol) containing either purified CaMKIIα (10 nM), 2 mM CaCl2, and 2 μM calmodulin or purified PKA (10 or 50 nM). After addition of LDS sample buffer (Invitrogen), samples were heated (70°C, 10 min) an d resolved by SDS-PAGE. Gels were stained (InstantBlue, VWR) and exposed to X-ray film (Phenix) for 2–4 hours at 80°C.
Cell Culture and Co-Immunoprecipitation
HEK293T cells (ATCC catalog CRL-3216) were cultured and maintained in DMEM containing 10% FBS and 1% penicillin/streptomycin at 37°C with 5% CO 2. Cells (70% confluency; passages 3–18) plated on 10 cm cell culture dishes were transfected using Lipofectamine 2000 (Thermo Fisher Scientific) and plasmid DNA (3:1 Lipo/DNA). Next day, cells were lysed in 1 ml of lysis buffer (1% Triton X-100, 150 mM NaCl, 25 mM HEPES, pH 7.5, 0.2 mM PMSF, 1 mM benzamidine, 10 μg/ml leupeptin, 10 μM pepstatin, and 1 μM microcystin) and centrifuged (12,000 × g, 10 min). Where indicated, the soluble fraction was immunoprecipitated using rabbit anti-GFP (Thermo Fisher Scientific A-11222) (1 μl per 500 μl soluble fraction) and Protein A magnetic beads (Invitrogen) at 4°C for 1 hr. Beads were washed 3 t imes with lysis buffer, suspended in 30 μl of 2X SDS sample buffer and heated (70°C, 10 min). Inputs and immune complexes were immunoblotted as indicated.
GST Fusion Protein Phosphorylation for Co-Sedimentation Assays
GST fusion proteins or GST alone (2.5 μM) were phosphorylated (30°C, 20 minutes) using purified CaMKIIα (10 nM) as described above. After addition of EDTA (12 mM final) samples were diluted ten-fold in HEK293T cells soluble fractions containing HAAbi1. GST proteins and associated proteins were isolated using glutathione agarose, and analyzed by immunoblotting as previously described [27].
Results
CaMKIIα and PKA phosphorylate Shank3 in vitro
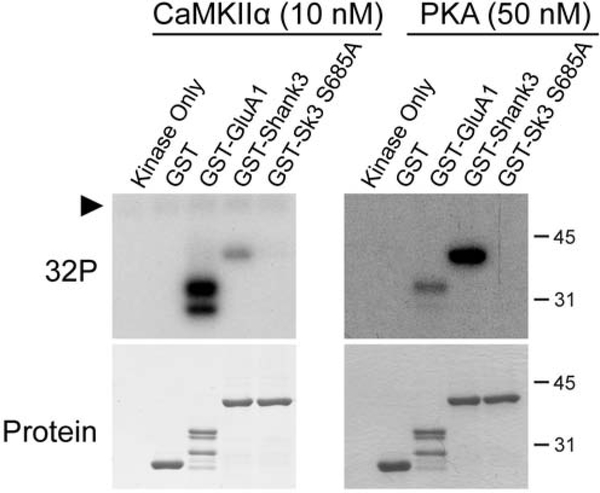
As an initial test of our hypothesis that CaMKII can phosphorylate Shank3 at Ser685, a site previously shown to be phosphorylated by PKA, we generated a GST fusion protein containing Shank3 amino acids 572–691, which includes the PDZ domain and the adjacent ABI1-binding motif. GST was used as a negative control and a GST fusion protein containing the AMPA receptor subunit GluA1 C-terminal residues 827901 was used as a positive control because CaMKII and PKA specifically phosphorylate Ser831 [23] and Ser845 [24], respectively. These three GST proteins were separately incubated with equimolar catalytic domain concentrations of purified CaMKIIα or PKA for 1–5 minutes and then analyzed by SDS-PAGE followed by autoradiography (Fig. 1). As expected, GST alone was not phosphorylated, but GST-GluA1 was phosphorylated by both CaMKIIα and PKA. GST-Shank3 was similarly phosphorylated by both CaMKIIα and PKA over a 5-min time course. While PKA reproducibly phosphorylated Shank3 more efficiently than GluA1, CaMKII displayed the reverse substrate preference. Nevertheless, these in vitro data show that CaMKII can phosphorylate Shank3 in a region of the protein that is also phosphorylated by PKA.
Figure 1: CaMKIIα and PKA phosphorylate Shank3 in vitro.
The indicated GST fusion proteins were incubated with CaMKIIα (10 nM) or PKA (10 nM) and [γ−32P]ATP (see Methods), and then analyzed by SDS-PAGE followed by Coomassie blue staining (bottom) and autoradiography to detect 32P-incorporation (top). Reactions ran for 1–5 minutes as indicated, before stopping with 4X sample buffer. Black arrowhead indicates CaMKIIα autophosphorylation. Representative of at least 3 biological replicates.
CaMKIIα and PKA target Shank3 Ser685
Ser685 had been previously identified as the main site of PKA phosphorylation within this region of Shank3 [13]. To confirm this finding and also test whether CaMKIIα also phosphorylates Ser685 within GST-Shank3 (572–691), we mutated Ser685 in the GST-Shank3 fusion protein to a phospho-null Ala residue (S685A). GST-Shank3-WT or -S685A were incubated with CaMKIIα or PKA (Fig. 2), with GST-GluA1 as a positive control, as in Fig. 1. While GST-Shank3-WT was strongly phosphorylated by both CaMKIIα and PKA, 32P-incorporation into GST-Shank3-S685A was barely detectable following incubation with either kinase. These data demonstrate that Ser685 in Shank3 is the major site within this domain that can be phosphorylated in vitro by both CaMKII and PKA.
Figure 2: CaMKIIα and PKA phosphorylate Shank3 at Ser685.
The indicated GST fusion proteins were phosphorylated by CaMKIIα (10 nM) or PKA (50 nM) for 5 min and then analyzed as in Figure 1. Black arrowhead indicates CaMKIIα autophosphorylation. Representative of at least 3 biological replicates.
Mutation of Ser685 interferes with ABI1 binding
Ser685 lies immediately C-terminal to the ABI1 binding motif in Shank3 [5]. WT Shank3 may be partially phosphorylated at Ser685 when expressed in HEK293 cells because a phospho-null S685A mutation reduces ABI1 co-immunoprecipitation with Shank3 [13]. Therefore, we hypothesized that a phospho-mimetic Ser685 to aspartate (S685D) mutation may enhance the interaction with ABI1 relative to the S685A mutant. To test this hypothesis, we co-expressed HA-tagged ABI1 and GFP-tagged full length Shank3 with either the wild type (WT) sequence or with mutations of Ser685 to alanine (S685A), or a phospho-mimetic aspartate (S685D) in HEK293T cells. Cells were lysed and Shank3 was immunoprecipitated using a GFP antibody (Fig. 3A). We detected a robust and specific co-immunoprecipitation of ABI1 with GFP-Shank3-WT, as previously demonstrated. Moreover, S685A mutation in GFP-Shank3 significantly reduced the levels of co-immunoprecipitated HA-ABI1, consistent with a prior report. However, there was a quantitatively similar reduction in the co-immunoprecipitation of HA-ABI1 with the GFP-Shank3-S685D mutant. As a control, we used a similar approach to test for effects of the Shank3 S685A and S685D mutations on co-immunoprecipitation of the HA-tagged C-terminal domain of the L-type Ca2+ channel CaV1.3 (HA-CaV1.3-CTD), which binds to the canonical PDZ domain (residues 572–663) in Shank3 [28] in close proximity to the ABI1 binding site and Ser685. However, neither Ser685 mutation had an effect on the levels of co-immunoprecipitated HA-CaV1.3-CTD. Therefore, the Ser685 mutations specifically interfere with ABI1-Shank3 binding, presumably by disrupting interactions involving amino acids adjacent to the core ABI1 binding motif. However, since introduction of negative charge by S685D mutation has the same effect as the uncharged S685A mutation, it is unclear whether Shank3 Ser685 phosphorylation affects the interaction with ABI1.
Figure 3: Mutation of GFP-Shank3 Ser685 disrupts ABI1 binding.
A, B. Lysates of HEK293T cells expressing HA-ABI1 (A) or HA-CaV1.3-CTD (B) and either empty vector, GFP-Shank3-WT, GFP-Shank3-S685A, or GFP-Shank3-S685D were immunoprecipitated (IP) using a GFP antibody. Input samples and IP complexes were resolved by SDS-PAGE and immunoblotted for GFP-Shank3 and HA-ABI1 (A) or HA-CaV1.3-CTD (B). HA/GFP signals for each IP lane were calculated and normalized to GFP-Shank3-WT. Both GFP-Shank3–685A and GFP-Shank3–685D significantly reduce HA-ABI1 co-immunoprecipitation (GFP-Shank3-S685A: 37±11% reduced compared to WT, ** p < 0.01, GFP-Shank3-S685D: 32±8% reduced compared to WT, *** p < 0.001, one sample Student’s t-test with equal variance compared to theoretical mean of 100). Mutation of S685 had no effect on HA-CaV1.3-CTD co-immunoprecipitation (one sample Student’s t-test with equal variance compared to theoretical mean of 100). The immunoblots shown are representative of 4–5 biological replicates that were quantified. Error bars, mean ± SEM.
Phosphorylation of Ser685 by CaMKIIα enhances ABI1-Shank3 binding
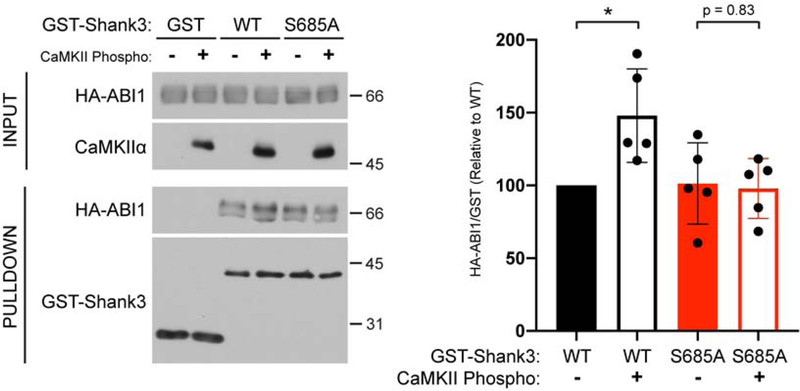
We directly tested for an effect of Shank3 Ser685 phosphorylation on ABI1 binding in vitro. The GST-Shank3-WT or GST-Shank3-S685A proteins were phosphorylated for an extended time (20 min) using purified CaMKIIα (see Methods) or incubated under control conditions (lacking CaMKIIα) and then mixed with lysates of HEK293T cells expressing HA-ABI1. Immunoblot analysis of GST protein complexes revealed that specific binding of HA-ABI1 to non-phosphorylated control GST-Shank3WT and GST-Shank3-S685A was indistinguishable (Fig. 4). However, pre-phosphorylation of GST-Shank3-WT by CaMKIIα increased ABI1 binding by about 50%, and this effect was abrogated by S685A mutation (Fig. 4). These data directly demonstrate that Shank3 Ser685 phosphorylation can enhance the association with ABI1.
Figure 4: CaMKIIα phosphorylation of Shank3 Ser685 enhances ABI1 binding.
Left, GST, GST-Shank3 WT, or GST-Shank3-S685A were pre-phosphorylated with CaMKIIα and incubated with HEK293T cell lysate expressing HA-ABI1. Control protein samples were pre-incubated in the absence of CaMKIIα. Isolated GST complexes were then analyzed by immunoblotting as indicated (see Methods). Immunoblots are representative of 5 independent experiments. Right, HA/GST signals from each pulldown lane were normalized to GST-Shank3-WT/no CaMKII condition. Incubation with CaMKIIα significantly increases HA-ABI1 binding to GST-Shank3-WT (148±14% increased relative to no CaMKII condition, * p < 0.05, one sample Student’s t-test with equal variance compared to theoretical mean of 100), which is not observed with GST-Shank3-S685A (unpaired Student’s t-test with equal variance). Error bars, mean ± SEM.
Discussion
Dynamic changes in the size and morphology of dendritic spines and postsynaptic densities during synaptic development and synaptic plasticity are mediated in part by the re-organization of multiprotein complexes that are assembled by numerous scaffolding proteins [29]. Phosphorylation of some synaptic proteins has been shown to modulate their interaction with scaffolding proteins [30, 31]. Here, we provide direct evidence that phosphorylation of a major synaptic scaffolding protein, Shank3, specifically modulates the binding of ABI1, a key synaptic modulator of F-actin cytoskeleton.
Shank3 is a member of a larger family of proteins that also includes Shank1 and Shank2. All three Shanks have been linked to ASD, as well as intellectual disability and schizophrenia [2, 32, 33]. They also share a similar overall domain organization, with high amino acid sequence similarity in SH3, PDZ, and SAM domains. Although there is low overall sequence similarity outside of the canonical protein interaction domains, the proline-rich ABI1 binding motif is conserved between Shank2 and Shank3, but not Shank1 [5, 34]. Our data significantly extend prior studies by showing that Ser685 in Shank3, immediately adjacent to the ABI1 binding motif (residues 677–684), can be phosphorylated by CaMKII, and confirm a prior report that PKA phosphorylates this residue. Although several proteomics studies have shown that Shank3 phosphorylation in isolated postsynaptic densities is increased under conditions that favor CaMKII activation [25, 26], this is the first study to show that CaMKII phosphorylates Shank3 using purified proteins. Moreover, we show for the first time that Ser685 phosphorylation directly enhances binding of ABI1 in vitro. Interestingly, comparison of the amino acid sequences surrounding the ABI1-binding sites in Shank3 and Shank2 reveals that Shank3 Ser685 is conserved as Shank2 Thr153. Further studies will be required to determine whether phosphorylation of Thr153 in Shank2 by CaMKII, PKA or other kinases can also modulate ABI1 binding.
As noted above, a prior study reported Shank3 Ser685 phosphorylation by PKA, and confirmed that Ser685 is phosphorylated in vivo by proteomics analysis and using phospho-site specific antibodies [13]. Ser685 mutation to alanine was also shown to decrease ABI1 binding to Shank3, consistent with the hypothesis that Shank3 Ser685 phosphorylation by PKA enhances the interaction with ABI1 in situ. Moreover, co-immunoprecipitation of the two proteins from lysates of cortical neurons was decreased following pre-treatment with H89, a PKA inhibitor [13]. However, neither the putative effect of H89 to decrease Ser685 phosphorylation in neurons nor a direct effect of Ser685 phosphorylation, or a phospho-mimetic mutation of Ser685 to aspartate or glutamate, to enhance the interaction with ABI1 was reported in this prior study. Nevertheless, given these prior findings, it was surprising that our current studies revealed that phospho-mimetic S685D and phospho-null S685A mutations had quantitatively similar effects to decrease the association of ABI1. However, neither mutation affected binding of the C-terminal domain of CaV1.3 to the nearby Shank3 PDZ domain.
Since our findings using the GFP-Shank3-S685D protein called into question the role of Ser685 phosphorylation in modulating the interaction with ABI1, it was important to directly investigate the putative effect of phosphorylation. We found that CaMKII phosphorylation of the GST-Shank3-WT protein in vitro enhanced the binding of ABI1, while the pre-incubation of GST-Shank3-S685A with CaMKII and ATP had no effect on ABI1 binding. Thus, our findings directly demonstrate for the first time that Shank3 Ser685 phosphorylation indeed enhances the binding of ABI1. Notably, while S685A mutation had no effect on in vitro binding of ABI1 to GST-Shank3, the same mutation significantly reduced co-immunoprecipitation of HA-ABI1 with GFP-Shank3 when the proteins were co-expressed in mammalian (HEK293) cells. This discrepancy may be explained by the partial phosphorylation of GFP-Shank3 at Ser685 by endogenous PKA, CaMKII or another kinase in mammalian cells, enhancing the interaction with HA-ABI1, whereas GST-Shank3 purified from bacteria is not phosphorylated. It is also noteworthy that indistinguishable amounts of HA-ABI1 co-immunoprecipitate with GFPShank3-S685A and -S685D; thus, the S685D mutation is not a phospho-mimetic mutation in this context. The failure of phospho-mimetic mutations is not unprecedented and is presumably under-reported in the literature because it is a negative result. This may be explained by the fact that aspartate (or glutamate) residues carry only a single negative charge as compared to the nominal two negative charges on much larger phosphorylated serine or threonine residues at physiological pH.
In summary, the present findings directly demonstrate that phosphorylation of Shank3 at Ser685 can enhance ABI1 binding. The potential importance of this mechanism is reinforced by studies of an ASD-linked de novo S685I mutation in Shank3, which has the same effect as the S685A mutation to decrease ABI1 binding by preventing phosphorylation [13]. Expression of Shank3-S685I in cultured neurons results in complex synaptic changes and knock-in Shank3-S685I mutant mice display a subset of ASD-related behavioral phenotypes [13]. Since Ser685 can be phosphorylated by either PKA or CaMKII, it will be important to investigate the relative importance of cAMP and Ca2+ signaling in physiological regulation of Ser685 phosphorylation and ABI1 interaction with Shank3, and the role of these mechanisms in synaptic plasticity. Further studies should determine whether Shank3 Ser685 phosphorylation is altered in mouse models of neuropsychiatric disorders, including mice carrying CaMKII mutations that have been linked to ASD and intellectual disability [35–37].
Article Highlights:
The synaptic scaffolding protein Shank3 binds CaMKII, ABI1, and many other proteins
We show that Ser685 in Shank3 can be phosphorylated by CaMKII or PKA
We show that Ser685 phosphorylation of Shank3 enhances ABI1 binding
Thus, Ca2+ and cAMP signaling may modulate Shank3 scaffolding functions
Acknowledgements
We thank Drs. Craig Garner, Winship Herr, and Jackie Corbin for generously providing various plasmids and reagents, as detailed above.
Funding: This work was supported in part by the National Institute of Health [T32DK007563 to TLP, R01-MH063232 and R01-NS078291 to RJC] and the American Heart Association [18PRE33960034 to TLP].
Abbreviations
- ABI1
Abelson interactor 1
- CaMKIIα
Ca2+/calmodulin-dependent protein kinase II alpha
- GFP
green fluorescent protein
- GST
Glutathione S-transferase
- PDZ
PSD95/Dlg1/zo-1
- PKA
protein kinase A
- Shank3
SH3 and multiple ankyrin repeat domains 3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interests
☒The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Sala C, Piech V, Wilson NR, Passafaro M, Liu G, Sheng M, Regulation of dendritic spine morphology and synaptic function by Shank and Homer, Neuron, 31 (2001) 115–130. [DOI] [PubMed] [Google Scholar]
- [2].Monteiro P, Feng G, SHANK proteins: roles at the synapse and in autism spectrum disorder, Nat Rev Neurosci, (2017). [DOI] [PubMed] [Google Scholar]
- [3].Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, Weinberg RJ, Worley PF, Sheng M, Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin, Neuron, 23 (1999) 569–582. [DOI] [PubMed] [Google Scholar]
- [4].Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF, Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins, Neuron, 23 (1999) 583592. [DOI] [PubMed] [Google Scholar]
- [5].Proepper C, Johannsen S, Liebau S, Dahl J, Vaida B, Bockmann J, Kreutz MR, Gundelfinger ED, Boeckers TM, Abelson interacting protein 1 (Abi-1) is essential for dendrite morphogenesis and synapse formation, The EMBO journal, 26 (2007) 13971409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Du Y, Weed SA, Xiong WC, Marshall TD, Parsons JT, Identification of a novel cortactin SH3 domain-binding protein and its localization to growth cones of cultured neurons, Mol Cell Biol, 18 (1998) 5838–5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Perfitt TL, Wang X, Stephenson JR, Nakagawa T, Colbran RJ, Neuronal L-Type Calcium Channel Signaling to the Nucleus Requires a Novel CaMKIIα-Shank3 Interaction, bioRxiv, (2019) 551648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, Nygren G, Rastam M, Gillberg IC, Anckarsater H, Sponheim E, Goubran-Botros H, Delorme R, Chabane N, Mouren-Simeoni MC, de Mas P, Bieth E, Roge B, Heron D, Burglen L, Gillberg C, Leboyer M, Bourgeron T, Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders, Nat Genet, 39 (2006) 25–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang X, Xu Q, Bey AL, Lee Y, Jiang YH, Transcriptional and functional complexity of Shank3 provides a molecular framework to understand the phenotypic heterogeneity of SHANK3 causing autism and Shank3 mutant mice, Molecular autism, 5 (2014) 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhou Y, Kaiser T, Monteiro P, Zhang X, Van der Goes MS, Wang D, Barak B, Zeng M, Li C, Lu C, Wells M, Amaya A, Nguyen S, Lewis M, Sanjana N, Zhang M, Zhang F, Fu Z, Feng G, Mice with Shank3 Mutations Associated with ASD and Schizophrenia Display Both Shared and Distinct Defects, Neuron, 89 (2015) 147–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Iasevoli F, Tomasetti C, de Bartolomeis A, Scaffolding proteins of the postsynaptic density contribute to synaptic plasticity by regulating receptor localization and distribution: relevance for neuropsychiatric diseases, Neurochemical research, 38 (2012) 1–22. [DOI] [PubMed] [Google Scholar]
- [12].Thomas GM, Rumbaugh GR, Harrar DB, Huganir RL, Ribosomal S6 kinase 2 interacts with and phosphorylates PDZ domain-containing proteins and regulates AMPA receptor transmission, Proc Natl Acad Sci U S A, 102 (2005) 15006–15011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang L, Pang K, Han K, Adamski CJ, Wang W, He L, Lai JK, Bondar VV, Duman JG, Richman R, Tolias KF, Barth P, Palzkill T, Liu Z, Holder JL Jr., Zoghbi HY, An autism-linked missense mutation in SHANK3 reveals the modularity of Shank3 function, Mol Psychiatry, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Strack S, Translocation of Autophosphorylated Calcium/Calmodulin-dependent Protein Kinase II to the Postsynaptic Density, Journal of Biological Chemistry, 272 (1997) 13467–13470. [DOI] [PubMed] [Google Scholar]
- [15].Lee SJ, Escobedo-Lozoya Y, Szatmari EM, Yasuda R, Activation of CaMKII in single dendritic spines during long-term potentiation, Nature, 458 (2009) 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Borovac J, Bosch M, Okamoto K, Regulation of actin dynamics during structural plasticity of dendritic spines: Signaling messengers and actin-binding proteins, Mol Cell Neurosci, 91 (2018) 122–130. [DOI] [PubMed] [Google Scholar]
- [17].Dell’Acqua ML, Smith KE, Gorski JA, Horne EA, Gibson ES, Gomez LL, Regulation of neuronal PKA signaling through AKAP targeting dynamics, Eur J Cell Biol, 85 (2006) 627–633. [DOI] [PubMed] [Google Scholar]
- [18].Zhong H, Sia GM, Sato TR, Gray NW, Mao T, Khuchua Z, Huganir RL, Svoboda K, Subcellular dynamics of type II PKA in neurons, Neuron, 62 (2009) 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Huang RYC, Laing JG, Kanter EM, Berthoud VM, Bao M, Rohrs HW, Townsend RR, Yamada KA, Identification of CaMKII Phosphorylation Sites in Connexin43 by High-Resolution Mass Spectrometry, Journal of proteome research, 10 (2011) 10981109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lampe PD, Lau AF, The effects of connexin phosphorylation on gap junctional communication, Int J Biochem Cell Biol, 36 (2004) 1171–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang F, Hu Y, Huang P, Toleman CA, Paterson AJ, Kudlow JE, Proteasome function is regulated by cyclic AMP-dependent protein kinase through phosphorylation of Rpt6, J Biol Chem, 282 (2007) 22460–22471. [DOI] [PubMed] [Google Scholar]
- [22].Djakovic SN, Schwarz LA, Barylko B, DeMartino GN, Patrick GN, Regulation of the proteasome by neuronal activity and calcium/calmodulin-dependent protein kinase II, J Biol Chem, 284 (2009) 26655–26665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Barria A, Derkach V, Soderling T, Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the alpha-amino-3hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor, J Biol Chem, 272 (1998) 32727–32730. [DOI] [PubMed] [Google Scholar]
- [24].Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL, Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit, Neuron, 16 (1996) 1179–1188. [DOI] [PubMed] [Google Scholar]
- [25].Jaffe H, Vinade L, Dosemeci A, Identification of novel phosphorylation sites on postsynaptic density proteins, Biochem Biophys Res Commun, 321 (2004) 210–218. [DOI] [PubMed] [Google Scholar]
- [26].Dosemeci A, Jaffe H, Regulation of phosphorylation at the postsynaptic density during different activity states of Ca2+/calmodulin-dependent protein kinase II, Biochem Biophys Res Commun, 391 (2010) 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Robison AJ, Bartlett RK, Bass MA, Colbran RJ, Differential modulation of Ca2+/calmodulin-dependent protein kinase II activity by regulated interactions with Nmethyl-D-aspartate receptor NR2B subunits and alpha-actinin, J Biol Chem, 280 (2005) 39316–39323. [DOI] [PubMed] [Google Scholar]
- [28].Zhang H, Maximov A, Fu Y, Xu F, Tang TS, Tkatch T, Surmeier DJ, Bezprozvanny I, Association of CaV1.3 L-type calcium channels with Shank, J Neurosci, 25 (2005) 1037–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sheng M, Hoogenraad CC, The postsynaptic architecture of excitatory synapses: a more quantitative view, Annual review of biochemistry, 76 (2007) 823–847. [DOI] [PubMed] [Google Scholar]
- [30].Zacchi P, Antonelli R, Cherubini E, Gephyrin phosphorylation in the functional organization and plasticity of GABAergic synapses, Front Cell Neurosci, 8 (2014) 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chung HJ, Huang YH, Lau LF, Huganir RL, Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand, J Neurosci, 24 (2004) 10248–10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Eltokhi A, Rappold G, Sprengel R, Distinct Phenotypes of Shank2 Mouse Models Reflect Neuropsychiatric Spectrum Disorders of Human Patients With SHANK2 Variants, Frontiers in molecular neuroscience, 11 (2018) 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sala C, Vicidomini C, Bigi I, Mossa A, Verpelli C, Shank synaptic scaffold proteins: keys to understanding the pathogenesis of autism and other synaptic disorders, J Neurochem, 135 (2015) 849–858. [DOI] [PubMed] [Google Scholar]
- [34].Lim S, Naisbitt S, Yoon J, Hwang JI, Suh PG, Sheng M, Kim E, Characterization of the Shank family of synaptic proteins. Multiple genes, alternative splicing, and differential expression in brain and development, J Biol Chem, 274 (1999) 29510–29518. [DOI] [PubMed] [Google Scholar]
- [35].Doshi-Velez F, Ge Y, Kohane I, Comorbidity clusters in autism spectrum disorders: an electronic health record time-series analysis, Pediatrics, 133 (2013) e5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Stephenson JR, Wang X, Perfitt TL, Parrish WP, Shonesy BC, Marks CR, Mortlock DP, Nakagawa T, Sutcliffe JS, Colbran RJ, A Novel Human CAMK2A Mutation Disrupts Dendritic Morphology and Synaptic Transmission, and Causes ASDRelated Behaviors, J Neurosci, 37 (2017) 2216–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cohen SM, Suutari B, He X, Wang Y, Sanchez S, Tirko NN, Mandelberg NJ, Mullins C, Zhou G, Wang S, Kats I, Salah A, Tsien RW, Ma H, Calmodulin shuttling mediates cytonuclear signaling to trigger experience-dependent transcription and memory, Nature communications, 9 (2018) 2451. [DOI] [PMC free article] [PubMed] [Google Scholar]