Abstract
Although contemporary chronic total occlusion (CTO) percutaneous coronary intervention (PCI) is performed with high success rates, 10-13% of patients presenting with CTOs have chronic kidney disease (CKD), and the comparative safety, efficacy and health status benefit of CTO PCI in these patients, has not been well defined. We examined the association of baseline renal function with periprocedural major adverse cardiovascular and cerebral events (MACCE) and health status outcomes in 957 consecutive patients (mean age 65.3 ± 10.3 years, 19.4% women, 90.3% white, 23.6 CKD [eGFR <60]) in the OPEN-CTO (Outcomes, Patients Health Status, and Efficiency in Chronic Total Occlusions Registry) study. Hierarchical multivariable regression models were used to examine the independent association of baseline eGFR with technical success, periprocedural complications and change in health status, using Seattle Angina Questionnaire (SAQ) over 1-year. Crude rates of acute kidney injury (AKI) were higher (13.5% vs 4.4% p <0.001) and technical success lower (81.8% vs 88.4% p=0.01) in patients with CKD. There were no significant differences in other periprocedural complications. After adjustment for confounding factors, there was no significant association of baseline eGFR with technical success or periprocedural MACCE (death, myocardial infarction, emergent bypass surgery, stroke, perforation), though patients with lower eGFR had higher rates of AKI. The difference in SAQ summary score, between patients on the 10th and 90th percentile for baseline eGFR distribution was not clinically significant (1-month: −0.91; 1-year: −3.06 points). In conclusion CTO PCI success, complication rates and the health status improvement after CTO PCI is similar in patients across a range of baseline eGFRs.
Keywords: Chronic Total Occlusion, Percutaneous Coronary Intervention, Chronic Kidney Disease, Health Status
INTRODUCTION
Chronic total occlusion (CTO) percutaneous coronary intervention (PCI) is associated with improved symptoms, improvement in left ventricular function and an increase in exercise tolerance. 1–4 About 10-13% of patients presenting with CTOs have chronic kidney disease (CKD), and the comparative safety and efficacy of treatment in these patients, as compared with patients without CKD, has not been well defined. 3,5,6 Since CTO PCI frequently involves longer procedures and greater iodinated contrast exposure, patients with CKD might experience lower technical success rates, higher periprocedural complication rates. 7–9 Importantly, although the primary indication for CTO PCI is symptom relief, no prior studies have assessed whether CTO PCI in patients with CKD is associated with similar symptom and quality of life (QoL) benefit as compared to those without renal dysfunction. To address these gaps in knowledge, we leveraged the 12-center Outcomes, Patient Health Status and Efficiency in Chronic Total Occlusions (OPEN CTO) registry 10 which included detailed baseline and follow-up health status assessments using Seattle Angina Questionnaire (SAQ) and Rose Dyspnea Scale, as well as corelab adjudicated technical success and detailed periprocedural complication data, among a consecutive series of patients undergoing CTO PCI using the hybrid approach.10
METHODS
The OPEN CTO registry is a prospective, single arm study that enrolled consecutive patients with CTOs who underwent attempted CTO PCI at 12 high-volume US centers between January 21,2014 to July 22, 2015. Details of the study have been described previously. 10 Briefly, eligible patients were >18 years of age, had symptoms consistent with ischemic heart disease and had a CTO defined as a lesion with Thrombolysis in Myocardial Infarction antegrade flow grade 0 that was thought to have been present for at least 3 months. Patients were treated according to the hybrid algorithm by all operators. 11 All operators were required to have at least 2 years of experience in performing CTO PCI using the hybrid approach and to have performed at least 100 CTO PCI procedures. 10 All consenting patients at the participating sites were included. Consecutive enrollment was confirmed via an audit of each site’s National Cardiovascular Data Registry Cath PCI data to prevent bias related to failure to include all patients undergoing CTO PCI at each institution. 10 The institutional review board at each site approved the study protocol. The OPEN CTO registry enrolled 1000 patients. After exclusion of patients who were missing baseline creatinine assessment (n=1), and those who did not have complete 1-year follow up health status assessment (n=42), the final analytic cohort for the present analysis included 957 patients. Supplemental Figure 1 describes the final analytical cohort derivation.
Estimated Glomerular Filtration Rate (eGFR) was calculated at baseline for each patient using the Modification in Diet in Renal Disease equation.12 Patients were categorized as having CKD if their eGFR was less than 60 ml/min/1.73m2, consistent with prior literature.13 Outcomes of interest included in-hospital major adverse cardiovascular and cerebral events (MACCE), acute kidney injury (AKI), technical success of the index CTO PCI and health status at 1-month and 12-month after the CTO PCI procedure. MACCE was defined as composite of in-hospital death, periprocedural myocardial infarction, emergency coronary bypass graft surgery, in-hospital stroke and clinically significant perforation. Periprocedural myocardial infarction was defined as type 4a and 5 myocardial infarction according to American College of Cardiology/American Heart Association/European Society of Cardiology universal definition of myocardial infarction.14 Perforations were classified according to the Ellis classification after review of procedural angiograms by the angiographic core lab (Saint Luke’s Mid America Heart Institute, Kansas City, Missouri) using QAngio XA 7.3 (Medis Medical Imaging Systems, Leiden, the Netherlands) software.15 Any perforation that required treatment was determined to be clinically significant. Technical success of CTO PCI procedure was defined as <50% residual stenosis and a Thrombolysis in Myocardial Infarction flow grade ≥2 without significant side branch occlusions as assessed by the OPEN CTO angiographic core lab.3 Post-procedure acute kidney injury was defined as increase in serum creatinine of 0.3mg/dl, in accordance with the Acute Kidney Injury Network definition.16
Disease-specific patient reported health status was assessed using the 19-item SAQ. Trained study personnel administered the SAQ during the baseline interview when enrolling each patient. Follow-up health status assessments were completed by a centralized telephone interview conducted by study coordinators at 1, 6 and 12 months after CTO PCI. The SAQ is a valid and reliable 19-item questionnaire with a 4-week recall period.17 It measures 5 domains of health in patients with coronary artery disease, which are angina frequency (SAQ AF), angina stability, QoL (SAQ QoL), physical limitation (SAQ PL) and treatment satisfaction.18 Domain scores range from 0-100, with higher scores indicating fewer symptoms and better QoL. Overall health status was summarized using the SAQ summary score (SAQ SS) which reflects the average of SAQ PL, AF and QoL domains.19 A mean change of ≥ 5 in the SAQ SS is considered clinically meaningful, although individual patients within a population are expected to have much larger changes. 20 Since dyspnea is a common angina equivalent in patients with CTOs 21, it was assessed using the Rose Dyspnea Scale. The Rose Dyspnea Scale is a 4-item questionnaire with a 1-month recall period that assesses the patient’s level of dyspnea with common activities.22 Each activity associated with dyspnea is assigned 1 point, hence scores range from 0-4, with score of 0 indicating no dyspnea and increasing scores indicating greater limitation from dyspnea. The Rose Dyspnea Scale has been used to assess symptoms in patients with coronary artery disease, and has shown to be associated with QoL, rehospitalization, procedure success and long-term outcomes.23 Complete revascularization was defined by operators as successful treatment of all physiologically significant stenoses.
For descriptive purposes we dichotomized our analytical cohort into patients with and without CKD. Baseline differences in patient characteristics, CTO lesion severity, comorbid conditions and procedural details were compared in patients with and without CKD using independent t tests for continuous variables and chi-square tests for categorical variables. We first conducted unadjusted analyses to compare the crude rates of MACCE, technical success and health status at baseline and follow up for patients with and without CKD. Then we fit hierarchical multivariable regression models, with a random effect for site to account for clustering at the site level, to assess the relationship of baseline eGFR as a continuous variable with outcomes. Using these models, for the outcome of technical success and MACCE, we plotted the odds ratio (with an eGFR of 90 as control) as a function of patient’s baseline eGFR. For longitudinal health status outcomes, we plotted the baseline adjusted 1-month and 12-month health status scores as a function of baseline eGFR. All models contained a nonlinear spline term for eGFR and were adjusted for potential confounders known to be associated with outcomes in patients undergoing CTO PCI identified a priori from previous studies 24 and clinical experience. These included age, sex, race, diabetes mellitus, congestive heart failure, peripheral arterial disease, use of diuretics, use of angiotensin converting enzyme inhibitors, any non-CTO lesion, complete revascularization, J-CTO score, prior bypass surgery and baseline health status. For the outcome of post-procedure AKI, we fit another hierarchical multivariable regression model adjusting for patients age, history of heart failure, diabetes and contrast volume, to assess the independent relationship of baseline eGFR with acute kidney injury. We plotted the odds ratio (in comparison to an eGFR of 90 as control) of acute kidney injury for each patient based on their baseline eGFR. To aid the clinical interpretability of our results we compared the proportions of patients having improvement in SAQ SS ≥ 5 at 12-months among patients across CKD stages; Stage 4 eGFR <30, Stage 3 eGFR 30-60, Stage 2 eGFR 61-90, and Stage 1 eGFR >90. We also fit a logistic regression model with CKD stage as an independent variable and clinically meaningful improvement in SAS SS (≥ 5) as the dependent variable. We compared the odds ratio of having a clinically meaningful improvement in SAQ SS at 1-year of patients with eGFR <30, 30-60 and 60-90 compared to patients with a baseline eGFR of ≥ 90. This type of responder analysis facilitates interpretation of the mean scores by showing the proportion of population experiencing varying degrees of clinical change in health status. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC). All statistical tests were 2-tailed, and significance was determined using α = 0.05. Patients with missing baseline creatinine, health status scores were excluded. Twenty-four% (n=225) of the patients had missing follow-up creatinine. These patients were not included in any analysis to examine the association of baseline eGFR with AKI. For covariates in the multivariable models, data was complete with <1% of the patients having missing data for all covariates.
RESULTS
At baseline, 225 patients (23.6%) had CKD and 732 (76.4%) did not. Overall, the mean age was 65.3 ± 10.3 years, 19.4% were females and 90.3% were Caucasian. Comorbidities were highly prevalent with 40.5% having diabetes, 22.4% having congestive heart failure and 13.2% reporting active smoking. Table 1 compares baseline characteristics in patients with and without CKD. Patients with CKD had a greater burden of comorbidities including, diabetes mellitus, congestive heart failure, peripheral artery disease and history of bypass surgery.
Table 1.
Baseline Characteristics of patients with and without CKD.
| Chronic Kidney Disease | |||
|---|---|---|---|
| Variable | Yes (n=225) | No (n=732) | P value |
| Age [years] (Mean ± SD) | 69.2 ± 10.1 | 64.1 ± 10.0 | < 0.001 |
| Women | 65 (28.9%) | 121 (16.5%) | < 0.001 |
| White | 202 (89.8%) | 662 (90.4%) | 0.30 |
| Diabetes Mellitus | 120 (53.3%) | 268 (36.6%) | < 0.001 |
| Congestive Heart Failure | 84 (37.3%) | 130 (17.8%) | < 0.001 |
| Ejection Fraction (Mean ± SD) | 48.7 ± 14.3 | 52.0 ± 13.2 | 0.002 |
| Current Smoker | 21 (9.5%) | 104 (14.4%) | 0.06 |
| Last HbgA1c (Mean ± SD) | 7.2 ± 1.6 | 6.9 ± 1.6 | 0.12 |
| History of Myocardial Infarction | 118 (52.4%) | 337 (46.0%) | 0.09 |
| Previous coronary bypass | 109 (48.4%) | 242 (33.1%) | < 0.001 |
| eGFR [[ml/min/1.73m2] (Mean ± SD) | 44.2 ± 14.5 | 87.3 ± 19.1 | < 0.001 |
| Medications on arrival | |||
| Diuretic | 139 (61.8%) | 224 (30.6%) | < 0.001 |
| Angiotensin Converting Enzyme Inhibitor | 86 (38.2%) | 346 (47.3%) | 0.02 |
| Procedural Variables | |||
| Initial Crossing Strategy | 0.006 | ||
| Antegrade wire escalation | 120 (48.9%) | 412 (56.3%) | |
| Antegrade dissection and reentry | 30 (13.3%) | 106 (14.5%) | |
| Retrograde wire escalation | 27 (12.0%) | 100 (13.7%) | |
| Retrograde dissection and reentry | 58 (25.8%) | 114 (15.6%) | |
| Successful Strategy | 0.43 | ||
| Antegrade wire escalation | 76 (37.8%) | 280 (41.1%) | |
| Antegrade dissection and reentry | 50 (24.9%) | 168 (24.7%) | |
| Retrograde wire escalation | 18 (9.0%) | 75 (11.0%) | |
| Retrograde dissection and reentry | 57 (28.4%) | 158 (23.2%) | |
| Target CTO Vessel | 0.61 | ||
| Left Main | 3 (1.3%) | 5 (0.7%) | |
| LAD/Diagonal | 42 (18.6%) | 154 (21.1%) | |
| LCX/Obtuse Marginal | 45 (20.0%) | 118 (16.2%) | |
| RCA/PDA/RPLV | 135 (59.9%) | 453 (62.2%) | |
| Non-CTO-PCI | 30 (13.3%) | 97 (13.3%) | 0.97 |
| Complete Revascularization | 162 (72.3%) | 566 (77.6%) | 0.10 |
| JCTO score (Mean ± SD) | 2.3 ± 1.2% | 2.3 ± 1.3 | 0.70 |
| Procedure Time (minutes) (Mean ± SD) | 124.6 ± 60.0 | 118.5 ± 65.0 | 0.21 |
| Contrast Used (cc) (Mean ± SD) | 229.9 ± 124.5 | 269.5 ± 141.7 | < 0.001 |
| Fluoroscopy Time (minutes) (Mean ± SD) | 53.0 ± 32.8 | 49.4 ± 34.7 | 0.18 |
| Radiation Dose (Air Kerma) (Mean ± SD) | 2481.8 ± 1856.1 | 2531.6 ± 1901.8 | 0.73 |
Abbreviations: estimated Glomerular Filtration Rate (eGFR), Chronic Total Occlusion (CTO), Left Anterior Descending coronary artery (LAD), Left Circumflex Coronary Artery (LCX), Right Coronary Artery (RCA), Posterior Descending Coronary Artery (PDA), Right Posterolateral Ventricular Coronary Artery (RPLV)
There was no significant difference between the crude rate of MACCE or any of the MACCE components in patients with and without CKD as depicted in Table 2 (7.1% vs 5.5 % p=0.36). Figure 1 describes the adjusted OR for MACCE as a function of patient’s baseline eGFR (with eGFR of 90 as the reference). There was no significant association of odds of MACCE with eGFR at baseline. The crude rate of technical success was lower in patients with CKD (81.8% vs 88.4%, p = 0.01). However, in adjusted models there was no significant association of eGFR with technical success of index CTO PCI (Figure 1). There was no difference in adjusted OR for having technical success of the index CTO PCI, in patients across a range of eGFR (with eGFR of 90 as reference). In patients with follow-up creatinine levels (n=732) AKI rate was 9%. Patients with CKD had higher rates of AKI compared to patients without CKD (13.5 vs 4.4% p<0.001). In the adjusted model, lower eGFR at baseline was associated in a non-linear fashion with higher odds of post-procedure acute kidney injury, with eGFR=90 as the reference (Figure 1). Patients with eGFR <40, had incrementally higher risk of AKI.
Table 2.
Periprocedural complications comparing patients with and without CKD
| Chronic Kidney Disease | |||
|---|---|---|---|
| Yes (n-225) | No (n=732) | P value | |
| Peri-procedural Complications | |||
| Clinical Perforation | 9 (4.0%) | 27 (3.7%) | 0.83 |
| Septal Hematoma | 5 (2.2%) | 8 (1.1%) | 0.20 |
| Pericardial Effusion | 7 (3.1%) | 12 (1.6%) | 0.18 |
| Access Site Hematoma | 14 (6.2%) | 26 (3.6%) | 0.08 |
| Retroperitoneal Bleed | 0 (0.0%) | 2 (0.3%) | 1.00 |
| Death during hospitalization | 0 (0.0%) | 0 (0.0%) | |
| Myocardial Infarction | 7 (3.1%) | 15 (2.0%) | 0.35 |
| Emergency surgery | 0 (0.0%) | 4 (0.5%) | 0.58 |
| Stroke | 0 (0.0%) | 0 (0.0%) | |
| Technical success | 184 (81.8%) | 647 (88.4%) | 0.01 |
| MACCE | 16 (7.1%) | 40 (5.5%) | 0.36 |
Abbreviations: Major Adverse Cardiac and Cerebral Events (MACCE)
Figure 1.
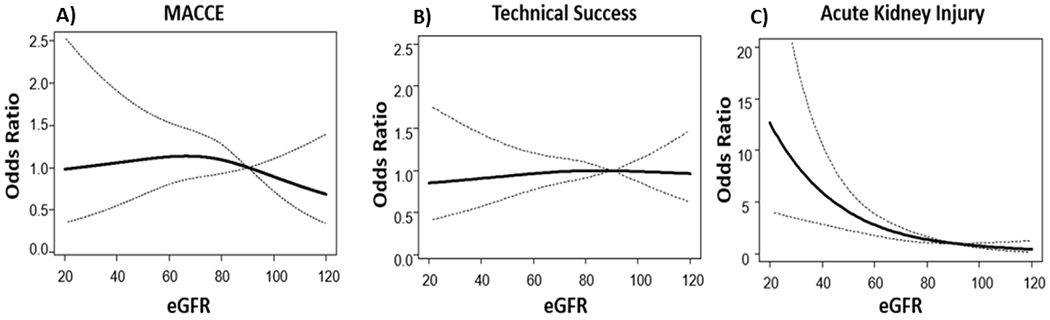
Multi-variable adjusted odds of having MACCE, technical success and AKI according to eGFR at baseline [eGFR of 90 as control].
(MACCE= Major Adverse Cardiac or Cerebral Event, AKI= Acute Kidney Injury, eGFR= estimated Glomerular Filtration Rate)
Overall, patients with and without CKD experienced large, early (1-month) and sustained (1-year) health status improvements after CTO PCI as measured by each SAQ domain and the Rose Dyspnea Scale (Table 3). Patients with CKD had a greater burden of physical limitation due to angina as reflected in lower baseline and follow up SAQ PL and more symptoms of dyspnea as reflected in higher scores on the Rose Dyspnea Scale (Table 3). Figure 2 and 3 describe the baseline health-status and multivariable adjusted SAQ scores at 1-month and 1-year respectively. There was no clinically meaningful difference in SAQ scores in patients across a spectrum of baseline eGFRs. The difference in SAQ SS, between patients on the 10th and 90th percentile for the eGFR distribution at 1-month and 1-year was −0.91 and −3.06 points. Supplemental Figure 2 describes the multivariable adjusted RDS at 1-month and 1-year after CTO PCI. Patients with lower eGFR had more symptoms of dyspnea at 1-year, with a difference in Rose Dyspnea Scale of 0.49 points between patients in the 10th and 90th percentile of the eGFR distribution. Overall 89% of the eligible patients (baseline SAQ SS <95) had a clinically meaningful improvement in health status (≥ 5-point improvement in SAQ SS) at 12-months. Figure 4 compares the proportion of patients by CKD stage who had a clinically meaningful improvement. There was no significant trend in achieving a clinically significant health status improvement across all stages of CKD. Supplemental Figure 3 describes the odds of patients having a clinically meaningful improvement in SAQ SS (≥ 5-points) at 1-year after CTO PCI, across different stages of CKD (compared to patients with eGFR >90). Patients with stage 4 CKD (eGFR <30) had a borderline significant lower odd of having a clinically meaningful change in SAQ SS. However, patients with stage 2 and stage 3 CKD had similar odds of having a clinically significant health status improvement after CTO PCI.
Table 3.
Unadjusted Health Status at baseline and in follow-up by CKD status.
| CKD (n=225) | No CKD (n=732) | P value | |
|---|---|---|---|
| SAQ Quality of Life (Mean ± SD) | |||
| Baseline | 49.7 ± 29.0 | 49.0 ± 26.7 | 0.72 |
| 1-month | 73.9 ± 23.6 | 75.4 ± 21.5 | 0.38 |
| 1 year | 76.5 ± 24.1 | 79.6 ± 21.1 | 0.08 |
| SAQ Angina Frequency (Mean ± SD) | |||
| Baseline | 70.1 ± 29.2 | 70.1 ± 26.4 | 0.99 |
| 1-month | 91.3 ± 18.3 | 90.7 ± 19.0 | 0.71 |
| 1 year | 92.0 ± 19.1 | 93.4 ± 16.7 | 0.29 |
| SAQ Physical Limitations (Mean ± SD) | |||
| Baseline | 58.0 ± 27.8 | 67.3 ± 25.3 | < 0.001 |
| 1-month | 91.1 ± 17.0 | 96.7 ± 11.2 | < 0.001 |
| 1 year | 91.8 ± 17.3 | 95.6 ± 12.3 | 0.003 |
| SAQ Summary Score (Mean ± SD) | |||
| Baseline | 59.4 ± 24.6 | 62.1 ± 21.8 | 0.12 |
| 1-month | 83.7 ± 16.8 | 86.1 ± 16.1 | 0.06 |
| 1 year | 85.2 ± 17.7 | 88.7 ± 14.6 | 0.004 |
| Rose Dyspnea Scale (Mean ± SD) | |||
| Baseline | 2.5 ± 1.5 | 2.2 ± 1.5 | 0.005 |
| 1-month | 1.5 ± 1.6 | 1.0 ± 1.3 | < 0.001 |
| 1 year | 1.7 ± 1.6 | 1.0 ± 1.3 | < 0.001 |
Abbreviations: Seattle Angina Questionnaire (SAQ)
Figure 2.
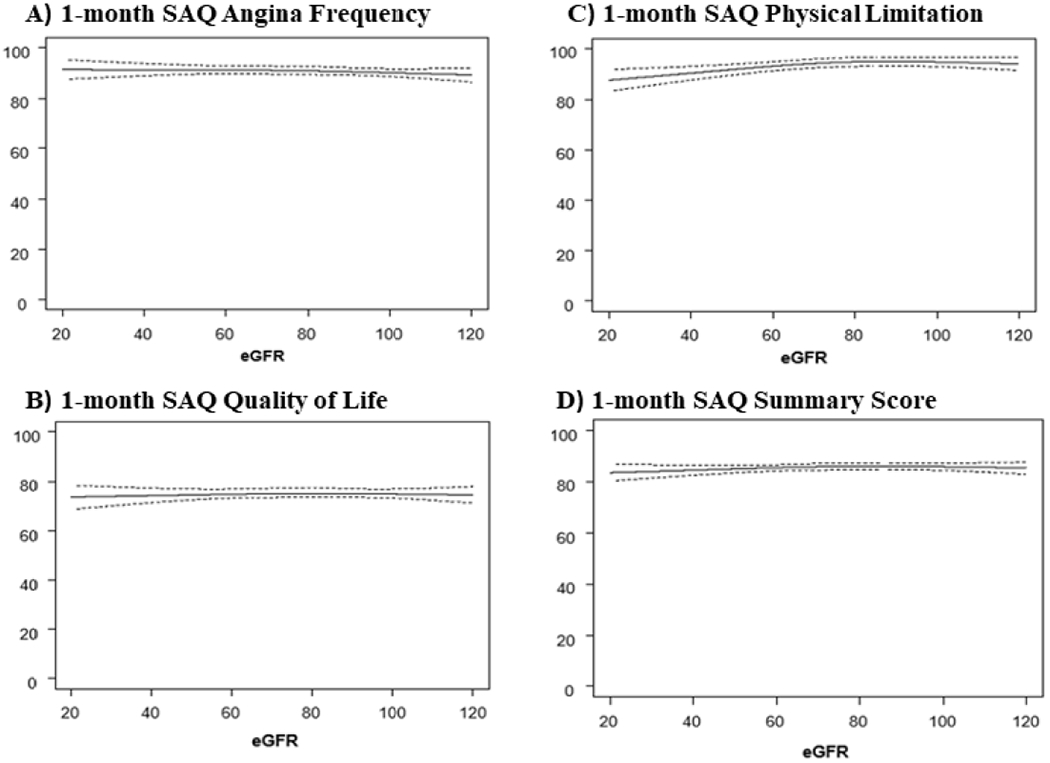
Baseline health status and multivariable adjusted SAQ scores 1-month after CTO PCI across range of baseline renal function.
(SAQ= Seattle Angina Questionnaire, CTO PCI=Chronic Total Occlusion Percutaneous Coronary Intervention)
Figure 3.
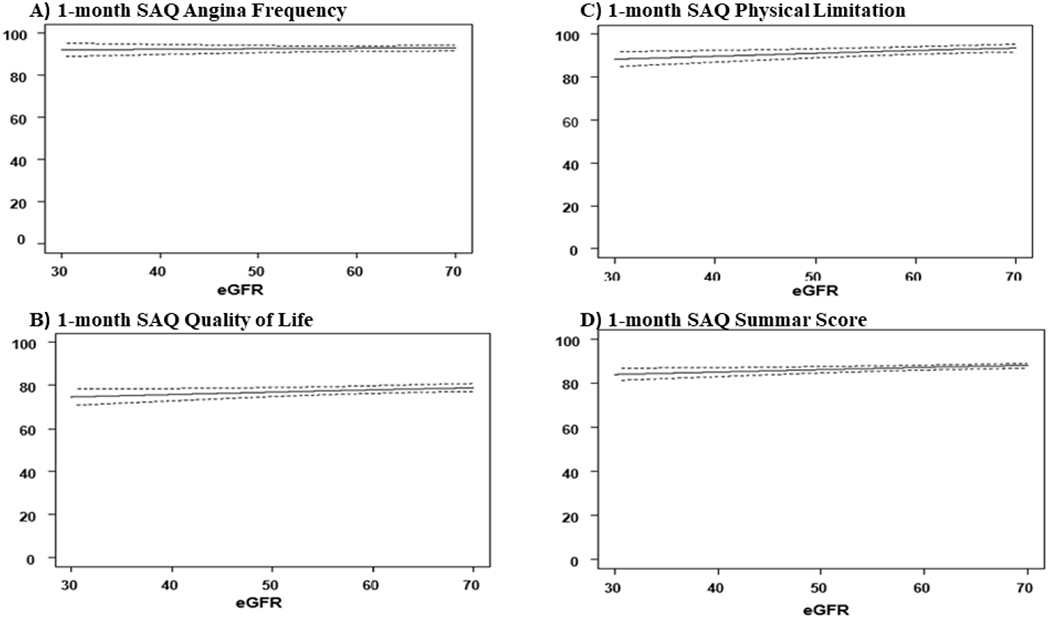
Baseline health status and multivariable adjusted SAQ scores 1-year after CTO PCI across range of eGFRs.
(SAQ= Seattle Angina Questionnaire, CTO PCI=Chronic Total Occlusion Percutaneous Coronary Intervention)
Figure 4.
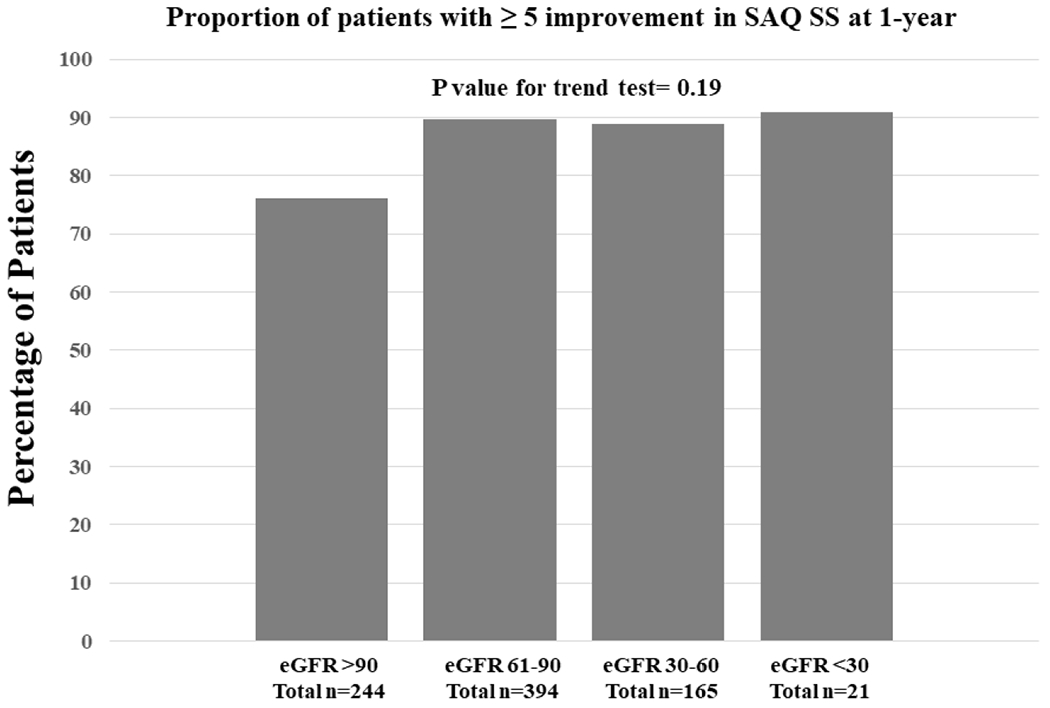
Responder analysis showing proportion of patients stratified by CKD stage having a clinically meaningful improvement in SAQ SS (≥ 5-points) at 1-year.
(CKD=Chronic Kidney Disease, SAQ SS= Seattle Angina Questionnaire Summary Score)
DISCUSSION
CKD in patients presenting with symptomatic CTOs is common, and comparative safety and effectiveness of PCI in these patients has not been thoroughly described in the prior literature. In the first analysis to examine the association between renal function and health status outcomes in a large, consecutive cohort of patients undergoing CTO PCI, we observed no clinically meaningful association of health status outcomes in patient with lower eGFR. Using coronary-disease specific health status assessments through 12-month follow-up, as well as assessment of dyspnea symptoms, we found that although patients with CKD had slightly lower SAQ SS and PL scores, these differences did not reach the threshold of clinical significance, and the health status improvement following the procedure was robust and sustained over 12-month follow-up in patients across the spectrum of renal function. Importantly, we found that patients with renal dysfunction had similar adjusted rates of technical success and periprocedural complications as patients without renal dysfunction even though patients with CKD had higher rates of AKI. In total, these findings suggest that CTO PCI can be performed safely in patients with CKD, and clinicians can expect similar improvement in health status, physical function and QoL following the procedure.
Rates of periprocedural complications including coronary perforations, in-hospital myocardial infarction and stroke in our experience were low and comparable to other studies.25 Prior studies of the outcomes of CTO PCI in patients with CKD have shown an association of CKD with worse outcomes including lower technical success, higher periprocedural complications, contrast nephropathy and death.5–9,26 However, most of those studies were retrospective analyses with the possibility of selective inclusion of patients that could bias the results. By leveraging a unique prospective registry that enrolled consecutive patients, confirmed by an audit of each enrolling center’s National Cardiovascular Data Registry CathPCI data 10, we were able to limit selection bias compared to these prior studies. Although the crude rate of technical success was lower in patients with CKD in OPEN CTO, this finding was primarily a reflection of a greater rate of prior bypass surgery in patients with CKD. Prior bypass surgery is a recognized correlate of lower success in patients undergoing CTO PCI 27 , and in multivariable adjusted analysis, technical success, MACCE as well as individual components of MACCE were similar across the range of baseline eGFRs. These data are reassuring, confirming that high rates of success similar to patients without CKD can be achieved in patients with renal dysfunction despite requirements that operators limit contrast exposure in the setting of CKD. As expected, there were higher rates of acute kidney injury following CTO PCI in patients with baseline CKD. Our rates of acute kidney injury were similar to some studies 28 but substantially higher than other reports.29 It remains critically important that operators focus on safe contrast limits and aggressive periprocedural hydration in these patients to limit the risk of periprocedural acute kidney injury.
No prior study, to our knowledge, has examined the relationship between renal function and health status after CTO PCI using validated instruments such as SAQ and Rose Dyspnea Scale. Since renal dysfunction has been shown to be associated with worse disease specific health status 30, and the principal indication for CTO PCI in most patients is symptomatic relief, our study addresses this critical knowledge gap regarding patient’s symptoms, function and QoL after CTO PCI over 1-year follow up. We found that patients with impaired renal function experienced large, sustained improvements in health status following CTO PCI, of similar magnitude to that experienced by patients without CKD. Additionally, an important clinical question is to understand the association of severity of CKD with outcomes after CTO PCI, since patients with severe CKD might portend poorer outcomes than those with mild or moderate CKD. Our analyses demonstrate that even though patients with markedly reduced eGFR had comparatively lower health status scores (SAQ and RDS) at baseline and follow-up, the recovery in health status and QoL after CTO PCI was substantial, and similar in magnitude to that experienced by patients without CKD.
These results should be considered in the context of the following potential limitations. OPEN-CTO was a prospective single arm registry and did not include patients who were managed with strategies other than PCI. Hence, our study cannot provide any insights into the outcomes experienced by patients with CKD and CTOs who were managed with medical therapy or surgical revascularization. Additionally, since post procedure collection of creatinine was not mandated the rates of post-procedure acute kidney injury may have been underestimated. Furthermore, the trajectories of renal function in follow-up were unknown, and the association between further decline in renal function and outcomes remains unclear. Additionally, operators in OPEN-CTO were highly experienced, and only those with at least 2-years of hybrid CTO experience were selected for participation. Thus, these results may not be generalizable to low-volume operators of CTO-PCI. Finally, our study included a modest number of patients with eGFR <30 (n=21), and whether technical success, complication rates and recovery of health status might be different in these patients with advanced CKD are important areas of further research.
In summary we found that technical success of the CTO PCI procedure, complication rates, and symptomatic benefit and recovery of health status after CTO PCI was consistent across the spectrum of severity of renal dysfunction, and similar to patients who did not have CKD, although patients with renal dysfunction had higher rates of AKI. These findings support of the safety and effectiveness of CTO PCI in appropriately selected patients with renal dysfunction. When performed by experienced operators, CKD should not be considered a deterrent to consideration of CTO PCI for patients with refractory symptoms.
Supplementary Material
Acknowledgments
Funding
The OPEN CTO study was supported by an unrestricted grant from Boston Scientific (Marlborough, MA). The sponsor was not involved with the design of the analysis or with writing, reviewing or approval of the manuscript.
Funding Disclosures
Dr. Malik, Dr. Peri-Okonny and Dr. Al Badarin are supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number T32HL110837. Dr. Grantham has received research grant support from and served on the advisory board for Boston Scientific; has received speaking fees and honoraria from Boston Scientific and Abbott Vascular; and is a part-time employee of and owns equity in Corindus. Dr. Lombardi owns equity in BridgePoint Medical. Dr. Spertus owns copyright for the Seattle Angina Questionnaire. He serves as a consultant to United Healthcare, Bayer and Novartis (modest). He has research grants from Abbott Vascular, Novarits and is the PI of an analytic center for the American College of Cardiology (significant). He has an equity interest in Health Outcomes Sciences (significant). Dr. Salisbury received institutional research grant support from Boston Scientific and Gilead, and consulting or speaking fees from Abiomed and Medtronic. Other authors do not report any disclosure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rossello X, Pujadas S, Serra A, Bajo E, Carreras F, Barros A, Cinca J, Pons-Llado G, Vaquerizo B. Assessment of Inducible Myocardial Ischemia, Quality of Life, and Functional Status After Successful Percutaneous Revascularization in Patients With Chronic Total Coronary Occlusion. Am J Cardiol 2016;117:720–726. [DOI] [PubMed] [Google Scholar]
- 2.Pujadas S, Martin V, Rossello X, Carreras F, Barros A, Leta R, Alomar X, Cinca J, Sabate M, Pons-Llado G. Improvement of myocardial function and perfusion after successful percutaneous revascularization in patients with chronic total coronary occlusion. Int J Cardiol 2013;169:147–152. [DOI] [PubMed] [Google Scholar]
- 3.Sapontis J, Salisbury AC, Yeh RW, Cohen DJ, Hirai T, Lombardi W, McCabe JM, Karmpaliotis D, Moses J, Nicholson WJ, Pershad A, Wyman RM, Spaedy A, Cook S, Doshi P, Federici R, Thompson CR, Marso SP, Nugent K, Gosch K, Spertus JA, Grantham JA. Early Procedural and Health Status Outcomes After Chronic Total Occlusion Angioplasty: A Report From the OPEN-CTO Registry (Outcomes, Patient Health Status, and Efficiency in Chronic Total Occlusion Hybrid Procedures). JACC Cardiovasc Interv 2017;10:1523–1534. [DOI] [PubMed] [Google Scholar]
- 4.Gao L, Wang Y, Liu Y, Cao F, Chen Y. Long-term clinical outcomes of successful revascularization with drug-eluting stents for chronic total occlusions: A systematic review and meta-analysis. Catheter Cardiovasc Interv 2017;89:574–581. [DOI] [PubMed] [Google Scholar]
- 5.George S, Cockburn J, Clayton TC, Ludman P, Cotton J, Spratt J, Redwood S, de Belder M, de Belder A, Hill J, Hoye A, Palmer N, Rathore S, Gershlick A, Di Mario C, Hildick-Smith D. Long-term follow-up of elective chronic total coronary occlusion angioplasty: analysis from the U.K. Central Cardiac Audit Database. J Am Coll Cardiol 2014;64:235–243. [DOI] [PubMed] [Google Scholar]
- 6.Tomasello SD, Boukhris M, Giubilato S, Marza F, Garbo R, Contegiacomo G, Marzocchi A, Niccoli G, Gagnor A, Varbella F, Desideri A, Rubartelli P, Cioppa A, Baralis G, Galassi AR. Management strategies in patients affected by chronic total occlusions: results from the Italian Registry of Chronic Total Occlusions. Eur Heart J 2015;36:3189–3198. [DOI] [PubMed] [Google Scholar]
- 7.Naganuma T, Tsujita K, Mitomo S, Ishiguro H, Basavarajaiah S, Sato K, Kobayashi T, Obata J, Nagamatsu S, Yamanaga K, Komura N, Sakamoto K, Yamamoto E, Izumiya Y, Kojima S, Kaikita K, Ogawa H, Nakamura S. Impact of Chronic Kidney Disease on Outcomes After Percutaneous Coronary Intervention for Chronic Total Occlusions (from the Japanese Multicenter Registry). Am J Cardiol 2018;121:1519–1523. [DOI] [PubMed] [Google Scholar]
- 8.Stahli BE, Gebhard C, Gick M, Ferenc M, Mashayekhi K, Buettner HJ, Neumann FJ, Toma A. Outcomes after percutaneous coronary intervention for chronic total occlusion according to baseline renal function. Clin Res Cardiol 2018;107:259–267. [DOI] [PubMed] [Google Scholar]
- 9.Zhang QB, Chen LM, Li M, Cui YQ, Zhao CY, Cui LQ. Influence of chronic kidney disease on the outcome of patients with chronic total occlusion. Am J Transl Res 2016;8:196–208. [PMC free article] [PubMed] [Google Scholar]
- 10.Sapontis J, Marso SP, Cohen DJ, Lombardi W, Karmpaliotis D, Moses J, Nicholson WJ, Pershad A, Wyman RM, Spaedy A, Cook S, Doshi P, Federici R, Thompson CR, Nugent K, Gosch K, Spertus JA, Grantham JA. The Outcomes, Patient Health Status, and Efficiency IN Chronic Total Occlusion Hybrid Procedures registry: rationale and design. Coron Artery Dis 2017;28:110–119. [DOI] [PubMed] [Google Scholar]
- 11.Brilakis ES, Grantham JA, Rinfret S, Wyman RM, Burke MN, Karmpaliotis D, Lembo N, Pershad A, Kandzari DE, Buller CE, DeMartini T, Lombardi WL, Thompson CA. A percutaneous treatment algorithm for crossing coronary chronic total occlusions. JACC Cardiovasc Interv 2012;5:367–379. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247–254. [DOI] [PubMed] [Google Scholar]
- 13.Webster AC, Nagler EV, Morton RL, Masson P. Chronic Kidney Disease. Lancet 2017;389:1238–1252. [DOI] [PubMed] [Google Scholar]
- 14.Fourth universal definition of myocardial infarction (2018). Rev Esp Cardiol (Engl Ed) 2019;72:72. [DOI] [PubMed] [Google Scholar]
- 15.Ellis SG, Ajluni S, Arnold AZ, Popma JJ, Bittl JA, Eigler NL, Cowley MJ, Raymond RE, Safian RD, Whitlow PL. Increased coronary perforation in the new device era. Incidence, classification, management, and outcome. Circulation 1994;90:2725–2730. [DOI] [PubMed] [Google Scholar]
- 16.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Fihn SD. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol 1994;74:1240–1244. [DOI] [PubMed] [Google Scholar]
- 18.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol 1995;25:333–341. [DOI] [PubMed] [Google Scholar]
- 19.Chan PS, Jones PG, Arnold SA, Spertus JA. Development and validation of a short version of the Seattle angina questionnaire. Circ Cardiovasc Qual Outcomes 2014;7:640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wyrwich KW, Spertus JA, Kroenke K, Tierney WM, Babu AN, Wolinsky FD. Clinically important differences in health status for patients with heart disease: an expert consensus panel report. Am Heart J 2004;147:615–622. [DOI] [PubMed] [Google Scholar]
- 21.Qintar M, Grantham JA, Sapontis J, Gosch KL, Lombardi W, Karmpaliotis D, Moses J, Salisbury AC, Cohen DJ, Spertus JA, Arnold SV. Dyspnea Among Patients With Chronic Total Occlusions Undergoing Percutaneous Coronary Intervention: Prevalence and Predictors of Improvement. Circ Cardiovasc Qual Outcomes 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rose GA, Blackburn H. Cardiovascular survey methods. Monogr Ser World Health Organ 1968;56:1–188. [PubMed] [Google Scholar]
- 23.Arnold SV, Spertus JA, Jones PG, Xiao L, Cohen DJ. The impact of dyspnea on health-related quality of life in patients with coronary artery disease: results from the PREMIER registry. Am Heart J 2009;157:1042–1049.e1041. [DOI] [PubMed] [Google Scholar]
- 24.Salisbury AC, Sapontis J, Grantham JA, Qintar M, Gosch KL, Lombardi W, Karmpaliotis D, Moses J, Cohen DJ, Spertus JA, Kosiborod M. Outcomes of Chronic Total Occlusion Percutaneous Coronary Intervention in Patients With Diabetes: Insights From the OPEN CTO Registry. JACC Cardiovasc Interv 2017;10:2174–2181. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto E, Natsuaki M, Morimoto T, Furukawa Y, Nakagawa Y, Ono K, Mitsudo K, Nobuyoshi M, Doi O, Tamura T, Tanaka M, Kimura T. Long-term outcomes after percutaneous coronary intervention for chronic total occlusion (from the CREDO-Kyoto registry cohort-2). Am J Cardiol 2013;112:767–774. [DOI] [PubMed] [Google Scholar]
- 26.Shimura T, Yamamoto M, Tsuchikane E, Teramoto T, Kimura M, Matsuo H, Kawase Y, Suzuki Y, Kano S, Habara M, Nasu K, Kinoshita Y, Terashima M, Matsubara T, Suzuki T. Rates of future hemodialysis risk and beneficial outcomes for patients with chronic kidney disease undergoing recanalization of chronic total occlusion. Int J Cardiol 2016;222:707–713. [DOI] [PubMed] [Google Scholar]
- 27.Azzalini L, Ojeda S, Karatasakis A, Maeremans J, Tanabe M, La Manna A, Dautov R, Ybarra LF, Benincasa S, Bellini B, Candilio L, Demir OM, Hidalgo F, Karacsonyi J, Gravina G, Micciche E, D’Agosta G, Venuti G, Tamburino C, Pan M, Carlino M, Dens J, Brilakis ES, Colombo A, Rinfret S. Long-Term Outcomes of Percutaneous Coronary Intervention for Chronic Total Occlusion in Patients Who Have Undergone Coronary Artery Bypass Grafting vs Those Who Have Not. Can J Cardiol 2018;34:310–318. [DOI] [PubMed] [Google Scholar]
- 28.Aguiar-Souto P, Ferrante G, Del Furia F, Barlis P, Khurana R, Di Mario C. Frequency and predictors of contrast-induced nephropathy after angioplasty for chronic total occlusions. Int J Cardiol 2010;139:68–74. [DOI] [PubMed] [Google Scholar]
- 29.Lin YS, Fang HY, Hussein H, Fang CY, Chen YL, Hsueh SK, Cheng CI, Yang CH, Chen CJ, Hang CL, Yip HK, Wu CJ. Predictors of contrast-induced nephropathy in chronic total occlusion percutaneous coronary intervention. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology 2014;9:1173–1180. [DOI] [PubMed] [Google Scholar]
- 30.Odden MC, Whooley MA, Shlipak MG. Association of chronic kidney disease and anemia with physical capacity: the heart and soul study. J Am Soc Nephrol 2004;15:2908–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


