Abstract
Background:
Atopic dermatitis is a common childhood disease, potentially influenced by prenatal nutritional exposures such as polyunsaturated fatty acids (PUFAs).
Objective:
In a racially diverse cohort, we hypothesized that childhood atopic dermatitis would be associated with higher prenatal omega-6 (n-6) and lower omega-3 (n-3) PUFAs.
Methods:
We included mother-child dyads, births 2006–2011, enrolled in the UTHSC Conditions Affecting Neurocognitive Development in Early Childhood (CANDLE) cohort. Primary exposures included 2nd trimester plasma n-3 and n-6 PUFA status and the ratio of the two (n-6:n-3). We assessed child current atopic dermatitis symptoms in the previous 12 months at approximately age 4–6 years. We investigated the association between PUFA exposures and atopic dermatitis using multivariable logistic regression, adjusting for potential confounders. We assessed for effect modification by maternal prenatal smoking, atopic disease history, and child sex.
Results:
Among 1131 women, 67% were African-American and 42% had an atopic disease history; 17% of children had atopic dermatitis. Higher prenatal n-6 PUFAs were associated with increased relative odds of child atopic dermatitis (aOR: 1.25; CI: 1.01–1.54 per interquartile range difference) and interaction models demonstrated that this association was seen in dyads in which the women had a history of atopic disease. Neither prenatal n-3 PUFAs nor n-6:n-3 were associated with child atopic dermatitis.
Conclusion:
In this racially diverse cohort, higher 2nd trimester n-6 PUFAs were associated with atopic dermatitis in children of women with atopy. PUFAs may represent a modifiable risk factor for atopic dermatitis, particularly in individuals with a familial predisposition.
Keywords: Prenatal, Polyunsaturated Fatty Acids, Atopic Dermatitis, Child
INTRODUCTION
Atopic dermatitis is a common chronic inflammatory skin disease linked to healthcare costs of more than $5.2 billion per year in the United States (U.S.).(1) Atopic dermatitis affects more than 10% of U.S. children, but African-Americans are disproportionately affected, with approximately 19% reporting active atopic dermatitis within the past year.(2),(3) Because atopic dermatitis begins early in life and is associated with acute and chronic morbidity, it is important to understand how prenatal factors may influence disease development.(1) Current hypotheses regarding the pathophysiology of atopic dermatitis include epidermal barrier defects and deregulation of the innate or adaptive immune systems in response to environmental triggers in susceptible individuals.(4) Prenatal dietary and environmental exposures have been linked to development of several chronic diseases(5) and prenatal polyunsaturated fatty acids (PUFAs) status is a potential modifiable exposure in the development of atopic dermatitis.(6, 7)
Concurrent with increasing global prevalence of atopic diseases in recent decades,(3) there have been changes in dietary trends related to PUFA consumption. Consumption of omega-3 (n-3) PUFAs, most abundant in oily fish, has declined while intake of plant-based omega-6 (n-6) PUFAs has increased.(8, 9) PUFAs are known to influence the body’s inflammatory response, and in general, n-6 PUFAs promote inflammation while n-3 PUFAs, particularly eicosapentaenoic acid (EPA) and docasahexaenoic acid (DHA), have anti-inflammatory properties.(9–11) Because of these opposing actions, it has been hypothesized that the higher ratio of n-6 to n-3 PUFAs in American diets may contribute to growing rates of atopic dermatitis.(12)
Prior investigations of prenatal PUFA status and child eczema/atopic dermatitis have included assessment of maternal fish intake or characterization of PUFA intake during pregnancy,(13–20) prenatal or cord blood PUFA concentrations,(21–26) and supplementation trials.(27–32) These studies have had conflicting results, with several finding protective associations of n-3 PUFAs or fish intake or adverse associations of n-6 PUFAs,(14, 17–20, 22, 24, 28, 29) while others have reported opposite associations,(15, 23) or inconclusive or null findings.(13, 16, 21, 25–27, 30–32) The relationship between PUFAs and atopic dermatitis has not been well characterized in African Americans, despite the high burden of disease in this population.(21–24, 26, 33) In a large, racially diverse US prenatal cohort, we investigated the association between maternal n-3 and n-6 PUFA status during pregnancy and child atopic dermatitis at age 4–6 years.
METHODS
Study cohort and population
Study participants were dyads enrolled in the University of Tennessee Health Sciences Center (UTHSC) Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) cohort in Shelby County (Memphis), Tennessee.(34, 35) This prospective prenatal cohort includes 1503 women who were recruited between 16 and 40 years of age during their 2nd trimester of a singleton pregnancy from local community-based obstetric practices, referrals from community members, and media advertisements from December 2006 to July 2011.(34, 35) Maternal demographic and psychosocial characteristics, health history and biospecimens were obtained at 2nd trimester, 3rd trimester, and delivery visits. Dyads were followed annually at child age 1–3 years and once at approximately 4–6 years. Additional details regarding the study design, recruitment, and follow up are described elsewhere.(34–38)
A total of 1131 dyads were included in this study. We restricted our cohort analysis to White and African American dyads, as women who identified as other races were too few to study (n=31). Dyads missing 2nd trimester biospecimens for PUFA analysis (n=7) or child follow-up at 4–6 years for atopic dermatitis assessment (n=334) were not included. The study protocol was approved by UTHSC and Vanderbilt University. Informed consent was obtained from the women for herself and her child at enrollment and at the 4–6 year follow up visit.
Assessment and characterization of prenatal PUFA status
Maternal biospecimens collected during the 2nd trimester of pregnancy were analyzed for fatty acid content. The fasting status of the women at the time of blood draw was not known. Whole blood samples were collected, processed, and centrifuged at 300 rpm for 10 minutes to separate plasma and subsequently stored long term in the UTHSC Department of Pathology at −80 degrees Celsius. Specimens were sent in batches to the Vanderbilt University Medical Center Lipid Core where phospholipid PUFA concentrations were determined by standard techniques.(39, 40) We utilized the plasma phospholipid pool in this analysis to take advantage of the stability of this sampling method and to minimize the variability related to the postprandial triacylglycerol pool.(11) Phospholipid fatty acid concentrations were expressed as a percentage of total fatty acids (%s). In quantifying lipid classes, the inter-assay coefficient of variation was less than 1.5%. The fatty acid percentage variation was less than 1%.
To determine n-3 PUFA status, we summed the percentages for alpha-linolenic acid (C18:3n-3), eicosapentaenoic acid (EPA, C20:5n-3), omega-3 docosapentaenoic acid (C22:5n-3), and docosahexaenoic acid (DHA, C22:6n-3) (Table I).(41) We determined n-6 PUFA status by summing the percentages for linoleic acid (C18:2n-6), gamma-linolenic acid (C18:3n-6), homo-gamma-linolenic acid (C20:3n-6), arachidonic acid (C20:4n-6), docosatetraenoic acid (C22:4n-6), and omega-6 docosapentaenoic acid (C22:5n-6) (Table I).(41) We then assessed the ratio of n-6 to n-3 PUFA percentages of total (n-6:n-3). Additional n-3 PUFA sub analyses were conducted using the sum of EPA and DHA.
Table I:
Characteristics of dyads enrolled in the Conditions Affecting Neurocognitive Development and Learning in Early childhood (CANDLE) cohort with follow up visit at 4–6 years
| Characteristic | N | Median (IQR) or % (N) |
|---|---|---|
| Maternal Characteristics | ||
| Age, median (IQR), years | 1131 | 26 (22–30) |
| Race, % (n) | 1131 | |
| African-American | 67 (759) | |
| White | 33 (372) | |
| Education, % (n) | 1131 | |
| ≤ High school | 59 (662) | |
| > High school | 41 (469) | |
| Medicaid insurance, % (n) | 1131 | 56 (627) |
| Married/living with a partner, % (n) | 1131 | 56 (636) |
| Pre-pregnancy BMI, median (IQR), (kg/m2)2 | 1127 | 26 (22–32) |
| Atopy (atopic dermatitis/rhinitis/asthma), % (n) | 1118 | 43 (474) |
| Primiparous, % (n) | 1131 | 40 (453) |
| Smoked during current pregnancy, % (n) | 1130 | 9 (102) |
| Cesarean delivery, % (n) | 1125 | 38 (427) |
| Plasma PUFA status, median (IQR), % of total fatty acid content | ||
| n-3† | 1131 | 5.3 (4.8–6.1) |
| n-6‡ | 1129¶ | 41.1 (40.0–42.2) |
| Plasma n-6:n-3 PUFA ratio, median (IQR) | 1129¶ | 7.7 (6.7–8.7) |
| Plasma EPA + DHA,§ median (IQR), % of total fatty acid content | 1131 | 4.2 (3.7–5.0) |
| Child characteristics | ||
| Sex, male, % (n) | 1131 | 49 (559) |
| Gestational age, median (IQR), weeks | 1125 | 39 (38–40) |
| Birthweight, median (IQR), grams | 1124 | 3250 (2964–3569) |
| Ever breastfed, yes, % (n) | 1131 | 64 (728) |
| Atopic dermatitis outcomes | ||
| Current atopic dermatitis at 4 to 6 years, yes, % (n) | 1121 | 17 (189) |
| Location-specific atopic dermatitis at 4 to 6 years, yes, % (n) | 1121 | 15 (169) |
| Ever atopic dermatitis, yes, % (n) | 1120 | 33 (365) |
n-3 = alpha-linolenic acid (C18:3n-3) + eicosapentaenoic acid (EPA, C20:5n-3) + omega-3 docosapentaenoic acid (C22:5n-3) + docosahexaenoic acid (DHA, C22:6n-3)
n-6 = linoleic acid (C18:2n-6) + gamma-linolenic acid (C18:3n-6) + homo-gamma-linolenic acid (C20:3n-6) + arachidonic acid (C20:4n-6) + docosatetraenoic acid (C22:4n-6) + omega-6 docosapentaenoic acid (C22:5n-6)
EPA + DHA = eicosapentaenoic acid (C20:5n-3) + docosahexaenoic acid (C22:5n-3)
Two women were excluded due to incomplete n-6 PUFA measures, but their complete n-3 PUFA measures were used in analyses.
Outcome assessments
Child current atopic dermatitis was our primary study outcome and was ascertained via questionnaire completed by parents/caregivers at the 4 to 6 year follow up. The questions were based on the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire.(42) Current atopic dermatitis was defined as affirmative responses to the questions “has your child ever had an itchy rash which came and went for at least 6 months” and “has your child ever had this itchy rash at any time in the last 12 months?”(42) Location-specific atopic dermatitis, a secondary outcome designed to increase specificity of characterization, was defined as current atopic dermatitis with additional criterion related to distribution on the body typical in this age group (affirmative response to “has this itchy rash at any time affected any of the following places: the folds of the elbows, behind the knees, in front of the ankles, under the buttocks, or around the neck, ears, or eyes?”).(42) Finally, we considered a broad outcome, ever eczema, defined as an affirmative response to the question, “has your child ever had eczema?”(42)
Covariates
Sociodemographic characteristics and clinical history, including child and maternal medical history and anthropometrics, were collected at enrollment and at subsequent study visits. Current literature and other plausible associations with maternal PUFA status and child atopic dermatitis were considered a priori in selecting maternal and child characteristics to control for potential confounding in multivariable models.(43) We included the following maternal covariates that were collected at enrollment: age (years), self-reported race (White or African American), education level (high school or less/beyond high school), smoking during pregnancy (yes/no), pre-pregnancy body mass index (BMI) (kg/m2), and number of previous live births (none or ≥ one). We obtained route of delivery (vaginal or cesarean section) and child sex (male or female) at delivery. We assessed maternal history of atopic disease during the follow-up visit at approximately child age 4–6 years (yes/no, maternal report of ever having at least one of the following: asthma, hay fever/allergic rhinitis, or eczema/atopic dermatitis).
Statistical analysis
Maternal and child characteristics were summarized by reporting median and interquartile ranges (IQRs) for continuous variables and frequencies and percentages for categorical variables. We used multivariable logistic regression to assess the relationship between 2nd trimester PUFA exposures (n-3 percentage, n-6 percentage, n-6:n-3 ratio, and summed EPA and DHA percentage) and child atopic disease outcomes. We modeled PUFA exposures as continuous linear terms as appropriate after considering potential non-linear associations. We calculated and report all odds ratios (ORs) with 95% confidence intervals (CI) per IQR increase in PUFA percentage. For non-linear associations, we modeled the continuous predictor using restricted cubic splines (4 knots).(44) Multivariable models included covariates as previously described. In secondary analyses, we assessed effect modification of the relationship between prenatal PUFAs and our primary outcome, current atopic dermatitis, by maternal atopic history, smoking during pregnancy, and infant sex using interaction terms for each PUFA exposure and the specific covariate of interest in separate models.
Data management was performed using SAS version 9.4 (Cary, North Carolina) and analyses were conducted with R version 3.4.0 (The R Foundation, Vienna, Austria) using a two-sided type 1 error rate of 0.05 for statistical significance.
RESULTS
Maternal and child characteristics
Our study included 1131 dyads with maternal 2nd trimester PUFA determinations and child follow-up at the 4 to 6-year visit. Sixty-seven percent of women were African-American and 33% were White with a median age of 26 years at enrollment. The median pre-pregnancy BMI was 26 kg/m2. Additionally, 59% of women had a high school education or less, 56% had Medicaid insurance, 40% were primiparous, 9% reported smoking during pregnancy, and 43% had a history of atopic disease. Median gestational age was 39 weeks and median birth weight was 3250 grams. Overall, 49% of children were male and 38% were born by cesarean section (Table I).
Median (IQR) maternal plasma n-3 and n-6 PUFA percentage were 5.3% (4.8–6.1%) and 41.1% (40.0–42.2%) of the total fatty acid content, respectively (Table I). The median (IQR) n-6:n-3 PUFA ratio was 7.7 (6.7–8.7) (Table I). Compared to their White counterparts, African American women had lower n-3 PUFA data spread as summarized by the 25th and 75th interquartile ranges (IQR 4.7–5.8% for African Americans vs. 4.8–6.7% for Whites, p<0.001), higher n-6 PUFA as per the 25th and 75th quartiles (IQR 40–42% for African Americans vs. 39–42% for Whites, p<0.001), and higher n-6:n-3 PUFA ratios (IQR 7.0–8.8 for African Americans vs. 6.0–8.4 for Whites, p<0.001). The overall median sum (IQR) of EPA and DHA was 4.2% (3.7–5.0%), with higher interquartile ranges in Whites (IQR 3.8–5.6%) than African Americans (IQR 3.7–4.7%) (p<0.001).
The median child age at the follow-up visit was 4.2 years with an IQR of 4.1–4.6 years. Overall, 17% of children met criteria for current atopic dermatitis and 15% met the more stringent, location-specific criteria (Table I). Current atopic dermatitis was found in 18% of African American children and 14% of White children. Additionally, one-third of children reported ever having eczema (Table I), with a higher proportion among African Americans (37%) compared to Whites (24%).
Prenatal PUFA status and child atopic dermatitis
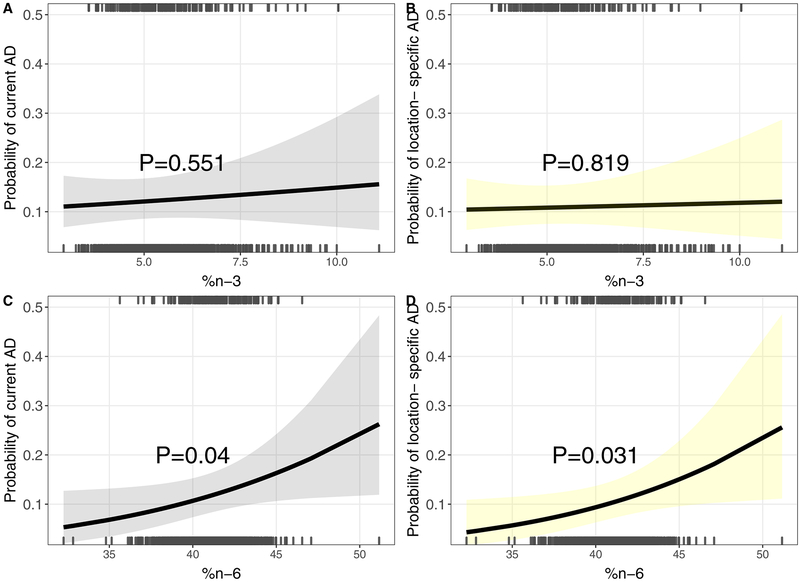
We did not detect a statistically significant association between 2nd trimester plasma n-3 PUFA status and our primary current atopic dermatitis outcome (adjusted odds ratio per IQR increase [aOR] [95% confidence interval (CI)]: 1.07 [0.86–1.32]) or the secondary outcomes of location-specific atopic dermatitis (1.03 [0.82–1.28]) or ever eczema (1.09 [0.92–1.30]) (Table II and Figure 1). However, we found a positive association between prenatal n-6 PUFAs and relative odds of current atopic dermatitis (1.25 [1.01–1.54]) (Table II and Figure 1). Prenatal n-6 PUFAs were also positively associated with relative odds of location-specific atopic dermatitis (1.27 [1.02–1.59]) (Table II and Figure 1) and ever eczema, although the latter did not reach statistical significance (1.16 [0.98–1.37]) (Table II).
Table II:
Association between prenatal plasma PUFA and child atopic dermatitis (AD) outcomes at 4–6 years in dyads enrolled in CANDLE
| Maternal plasma PUFA percentage (continuous) | Current AD | Location-specific AD | Ever AD | |||
|---|---|---|---|---|---|---|
| Unadjusted OR [CI]† | Adjusted OR [CI]†,‡ | Unadjusted OR [CI]† | Adjusted OR [CI]†,‡ | Unadjusted OR [CI]† | Adjusted OR [CI]†,‡ | |
| Omega-3 (n-3)§ | 0.99 (0.82–1.20) | 1.07 (0.86–1.32) | 0.95 (0.78–1.16) | 1.03 (0.82–1.28) | 0.98 (0.84–1.14) | 1.09 (0.92–1.30) |
| Omega-6 (n-6)§ | 1.24 (1.01–1.51)* | 1.25 (1.01–1.54)* | 1.27 (1.03–1.56)* | 1.27 (1.02–1.59)* | 1.19 (1.02–1.40)* | 1.16 (0.98–1.37) |
| n-6:n-3 | 1.05 (0.86–1.27) | 1.00 (0.81–1.24) | 1.09 (0.89–1.33) | 1.04 (0.83–1.30) | 1.04 (0.89–1.21) | 0.96 (0.80–1.14) |
OR: Odds Ratio; CI: 95% Confidence Interval; ORs are estimated per interquartile range increase in respective PUFA value
Adjusted for maternal age at enrollment, self-reported race, education level, smoking during pregnancy, pre-pregnancy BMI, atopic disease history, parity, route of delivery, and child sex
As percentage of total plasma fatty acids
Figure 1: Association between 2nd trimester PUFAs and child atopic dermatitis (AD) at 4–6 years using multivariable logistic regression.
Prenatal n3% and A) current and B) location-specific AD. Prenatal n6% and C) current and D) location-specific AD. Location-specific AD incorporates rash occurring in commonly affected areas. Adjustment for maternal age, race, education, smoking, pre-pregnancy BMI, atopic disease, parity, delivery route, child sex. Rug plots at top and bottom indicate PUFA levels among children with (top) and without (bottom) AD.
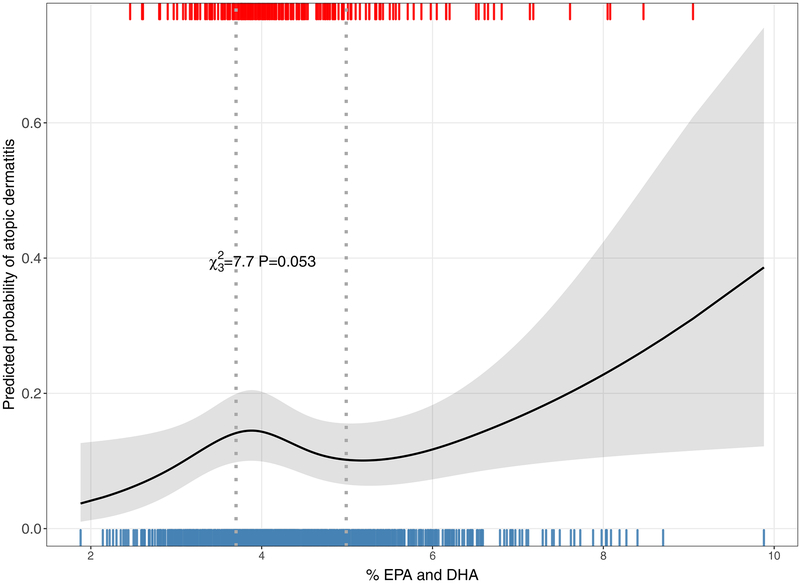
Assessing the ratio of prenatal n-6:n-3 PUFAs and odds of child atopic dermatitis outcomes, we did not observe an association between current atopic dermatitis (1.00 [0.81–1.24]), location-specific atopic dermatitis (1.04 [0.83–1.30]) or ever eczema (0.96 [0.80–1.14]) (Table II). In secondary analyses assessing the relationship between combined EPA and DHA and current atopic dermatitis, we detected a nonlinear relationship, indicating a non-monotonic dose-response in which odds of current atopic dermatitis appeared to be decreased across the IQR difference from 3.70–4.99% (0.69 [0.44–1.07] (Figure 2).
Figure 2: Association between prenatal EPA and DHA and child atopic dermatitis (AD).
A nonlinear relationship was found between prenatal EPA and DHA and AD suggesting a dose response in which AD odds increase at low and high concentrations but decrease with mid-range values. Rug plots at the top and bottom of the figure indicate prenatal PUFA levels among children with AD (top) and children without AD (bottom), respectively.
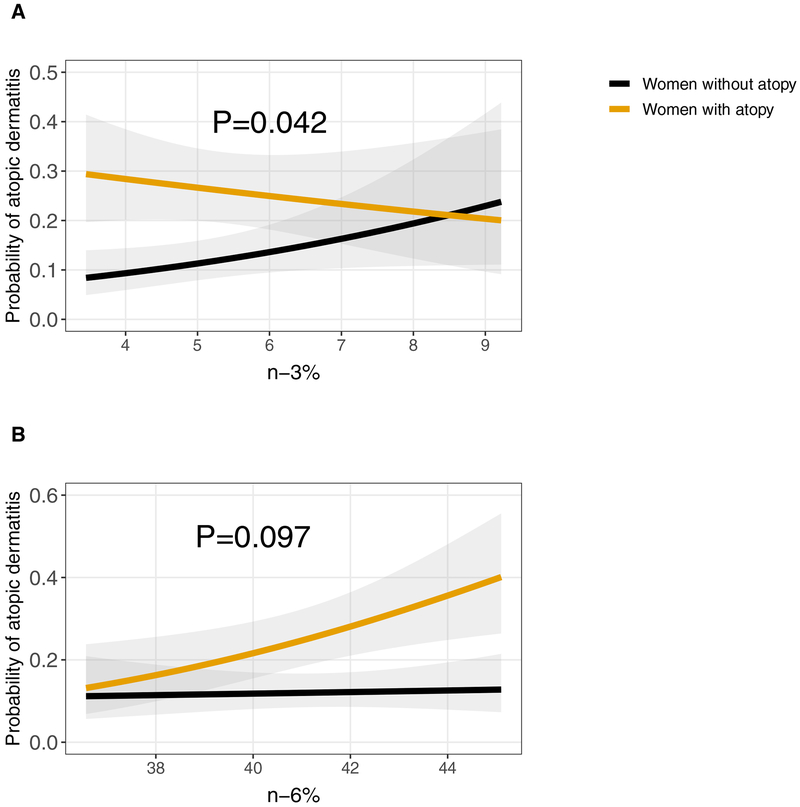
Lastly, we assessed for potential effect modification by maternal atopic disease history, smoking during pregnancy, and child sex in the association between prenatal PUFAs and current atopic dermatitis. For maternal atopic history, higher n-6 PUFAs were associated with increased relative odds of atopic dermatitis among children of women with a history of atopic disease (1.48 [1.10–1.99]) but not among children whose mothers did not have an atopic history (1.04 [0.77–1.40]) (p for interaction = 0.10) (Figure 3). In contrast, we found that higher n-3 PUFAs were associated with increased odds of child atopic dermatitis among non-atopic women (1.33 [0.99–1.77]) but not in atopic women (0.89 [0.67–1.18]) (p for interaction = 0.04) (Figure 3). We did not detect interaction by prenatal smoking with either n-3 PUFAs (p for interaction=0.31) or n-6 PUFAs (p for interaction=0.40) or interaction by child sex with n-3 PUFAs (p for interaction= 0.44) or n-6 PUFAs (p for interaction=0.30).
Figure 3: Association between prenatal PUFAs and child atopic dermatitis (AD) among those with and without a history of maternal atopy.
A. An increase in prenatal n-3 PUFAs was associated with higher probability of AD among children of non-atopic women but not among children of atopic women. B. An increase in prenatal n-6 PUFAs was associated with higher probability of AD among children of atopic women but not among children whose mothers were not atopic.
DISCUSSION
In our diverse study population, which includes a large proportion of African-American women, current atopic dermatitis was reported in 17% of children. Because of the potential influence of PUFAs on the development of the fetal immune system through inflammatory pathways,(9, 10) we investigated the association of prenatal PUFAs and child atopic dermatitis. We found that higher prenatal n-6 PUFAs were associated with increased relative odds of child atopic dermatitis at age 4–6 years, particularly in children whose mothers had history of atopic disease.
Polyunsaturated fatty acids are incorporated into plasma membranes of cells and are involved in the biosynthesis of allergic mediators.(9, 10) Their inflammatory influences include changes in membrane fluidity and cellular responsiveness, production of eicosanoids, production of anti-inflammatory resolvins and protectins, and regulation of the pro-inflammatory transcription factor NF-κB.(10) Arachidonic acid, an n-6 PUFA, serves as a substrate for the synthesis of eicosanoids, such as prostaglandins and leukotrienes, that drive allergic inflammation.(9, 10) Some n-3 PUFAs, such as EPA and DHA, oppose the inflammatory action of arachidonic acid.(9, 10) Because of these actions, the balance between n-3 and n-6 PUFAs may influence an individual’s propensity to develop allergic inflammation. Therefore, PUFAs are a potentially modifiable risk factor for the development of atopic dermatitis.
Several prior studies have assessed the relationship between prenatal and cord blood PUFAs and childhood atopic dermatitis with inconsistent findings. Some studies of prenatal PUFAs and childhood atopic dermatitis have reported null associations,(25, 26) while others have reported protective and adverse associations depending on the characterization of PUFAs. For example, in contrast to expected associations, Notenboom et al. found a protective association between plasma n-6:n-3 PUFA in the 3rd trimester and atopic dermatitis at 6–7 years in a Dutch cohort.(23) In a Spanish study of non-atopic women and their children, Montes et al. found that higher total first trimester plasma PUFAs and higher cord blood n-3 PUFAs had a protective association with child atopic dermatitis at age 14 months.(22) Finally, in a Dutch cohort, Rucci et al. detected an adverse association between higher second trimester n-6 PUFAs and childhood atopic dermatitis at 6 years of age.(24) We also assessed the relationship between PUFAs and atopic dermatitis, but did so in a predominately African American, U.S.-based cohort, which is important as there is some support for potential racial differences in PUFA metabolism.(45) Similar to Rucci et al., we found that n-6 PUFAs were associated with increased odds of atopic dermatitis.
While our study utilized biomarkers, several other studies in the literature have assessed dietary report of PUFA or fish intake, focusing on EPA and DHA. Several cohorts have reported protective associations between maternal dietary n-3 PUFA or fish intake and child atopic dermatitis, (17–20, 46) however some studies have reported adverse associations between n-6 PUFA intake and atopic dermatitis (14, 20) or no association.(13, 47–51) Randomized controlled trials (RCTs) of EPA and DHA supplementation have also yielded conflicting results (27–30) although variability in supplementation dose and timing may have contributed to different findings.(27–30) We detected a non-linear association in the relationship of combined EPA and DHA percentage of total and child atopic dermatitis. While a nonlinear relationship between EPA and DHA and atopic dermatitis has not been well characterized in the literature, other nutritional exposures have been shown to have nonlinear relationships with various diseases.(52–54)
Few studies have assessed whether the association between prenatal PUFAs and atopic dermatitis in children is modified by maternal atopic disease. (15, 20, 25) We found that higher prenatal n-6 PUFAs were associated with increased odds of atopic dermatitis in children of women with a history of atopic disease. The reason for this effect modification is uncertain; it is possible that the pro-inflammatory effects of prenatal n-6 PUFAs are of greater importance in children with an underlying familial predisposition for atopy. Alternatively, it is possible that higher n-6 PUFAs are associated with active atopic disease although evidence is limited.(55, 56) Active asthma during pregnancy potentially confers an increased risk of asthma in the offspring;(57) while it has not yet been described, it is plausible that a similar mechanism may be seen regarding atopic dermatitis. Our study also found that higher n-3 PUFAs were associated with increased odds of child atopic dermatitis among children of non-atopic mothers but not atopic mothers. Although this is contrary to the expected relationship, there has been report of higher prenatal fatty fish intake and increased eczema in children, although associations were not modified by maternal atopy.(15)
Our study has both strengths and limitations. We used biomarkers for our exposures, which provide an objective measure of maternal PUFA status. Although the fasting status of the women at the time of blood draw was not known, we assessed PUFAs from the plasma phospholipid fatty acid pool, which is less susceptible to oxidative stress than PUFAs from erythrocyte membranes and is less affected by postprandial triacylglycerols than the plasma total lipid pool.(11) Additional limitations include that PUFAs were measured from a single time point in the 2nd trimester of pregnancy. We also lack PUFA measurement in children. While there is potential for misclassification of outcomes given our reliance on parental report, we used the well-validated ISAAC questionnaire and findings were consistent when we used a definition that incorporated the affected areas on the body, which was designed to increase specificity of the diagnosis. Unmeasured confounding variables may also be present.
In conclusion, in this racially-diverse prospective prenatal cohort, higher 2nd trimester n-6 PUFAs were associated with increased relative odds of child atopic dermatitis. Furthermore, in our study population, associations were detected among children of women with a history of atopic disease. Although, results were inconclusive on the association of n-3 PUFAs and atopic dermatitis, when examining the combined EPA and DHA percentage and atopic dermatitis, IQR difference was suggestive of a protective association. This work is supportive of future studies that investigate whether PUFA intake during pregnancy may influence the development atopic dermatitis in children, particularly children at higher risk of developing disease.
HIGHLIGHTS BOX:
What is already known about this topic?
Atopic dermatitis is a chronic inflammatory skin condition, which may be influenced by prenatal nutritional exposures. Prior evidence that prenatal polyunsaturated fatty acid (PUFA) exposure affects atopic dermatitis development in children is inconclusive.
What does this article add to our knowledge?
This study finds that higher 2nd trimester omega-6 PUFAs are associated with atopic dermatitis in children of women with atopy.
How does this study impact current management guidelines?
Higher prenatal omega-6 PUFA exposure may increase the likelihood that a child with a familial predisposition to atopy develops atopic dermatitis, supporting that dietary PUFAs represent a modifiable risk factor in this disease.
Funding
This work was supported by the Urban Child Institute and by National Institutes of Health grants NHLBI HL109977 and HL132338.
ABBREVIATIONS USED:
- AD
atopic dermatitis
- aOR
Adjusted odds ratio
- BMI
Body mass index
- CANDLE
Conditions Affecting Neurocognitive Development and Learning in Early Childhood
- CI
Confidence interval
- DHA
Docasahexaenoic acid
- EPA
Eicosapentaenoic acid
- IQR
Interquartile range
- ISAAC
International Study of Asthma and Allergies in Childhood
- n-3
Omega-3
- n-6
Omega-6
- n-6:n-3
Omega-6:omega-3 ratio
- OR
Odds ratio
- PUFA
Polyunsaturated fatty acid
- RTCs
Randomized controlled trials
- U.S.
United States
- UTHSC
University of Tennessee Health Sciences Center
Footnotes
Conflict of Interest: The authors have declared that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Drucker AM, Wang AR, Li WQ, Sevetson E, Block JK, Qureshi AA. The Burden of Atopic Dermatitis: Summary of a Report for the National Eczema Association. J Invest Dermatol 2017;137(1):26–30. [DOI] [PubMed] [Google Scholar]
- 2.Bloom B, Jones LI, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2012. Vital Health Stat 10 2013(258):1–81. [PubMed] [Google Scholar]
- 3.Thomsen SF. Epidemiology and natural history of atopic diseases. Eur Clin Respir J 2015;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider L, Tilles S, Lio P, Boguniewicz M, Beck L, LeBovidge J, et al. Atopic dermatitis: a practice parameter update 2012. J Allergy Clin Immunol 2013;131(2):295–9.e1–27. [DOI] [PubMed] [Google Scholar]
- 5.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of In Utero and Early-Life Conditions on Adult Health and Disease. N Engl J Med 2008;359(1):61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kremmyda LS, Vlachava M, Noakes PS, Diaper ND, Miles EA, Calder PC. Atopy risk in infants and children in relation to early exposure to fish, oily fish, or long-chain omega-3 fatty acids: a systematic review. Clin Rev Allergy Immunol 2011;41(1):36–66. [DOI] [PubMed] [Google Scholar]
- 7.Prescott SL, Dunstan JA. Prenatal fatty acid status and immune development: the pathways and the evidence. Lipids 2007;42(9):801–10. [DOI] [PubMed] [Google Scholar]
- 8.Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev 2015(7):Cd010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miles EA, Calder PC. Maternal diet and its influence on the development of allergic disease. Clin Exp Allergy 2015;45(1):63–74. [DOI] [PubMed] [Google Scholar]
- 10.Calder PC. n-3 fatty acids, inflammation and immunity: new mechanisms to explain old actions. Proc Nutr Soc 2013;72(3):326–36. [DOI] [PubMed] [Google Scholar]
- 11.Brenna JT, Plourde M, Stark KD, Jones PJ, Lin YH. Best practices for the design, laboratory analysis, and reporting of trials involving fatty acids. Am J Clin Nutr 1082018. p. 211–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miles EA, Calder PC. Can Early Omega-3 Fatty Acid Exposure Reduce Risk of Childhood Allergic Disease? Nutrients 2017;9(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyake Y, Tanaka K, Okubo H, Sasaki S, Arakawa M. Maternal fat intake during pregnancy and wheeze and eczema in Japanese infants: the Kyushu Okinawa Maternal and Child Health Study. Ann Epidemiol 2013;23(11):674–80. [DOI] [PubMed] [Google Scholar]
- 14.Miyake Y, Sasaki S, Tanaka K, Ohfuji S, Hirota Y. Maternal fat consumption during pregnancy and risk of wheeze and eczema in Japanese infants aged 16–24 months: the Osaka Maternal and Child Health Study. Thorax 2009;64(9):815–21. [DOI] [PubMed] [Google Scholar]
- 15.Leermakers ETM, der Voort S-vAMM, Heppe DHM, de Jongste JC, Moll HA, Franco OH, et al. Maternal fish consumption during pregnancy and risks of wheezing and eczema in childhood: The Generation R Study. European Journal of Clinical Nutrition 2013;67(4):353. [DOI] [PubMed] [Google Scholar]
- 16.Nwaru BI, Erkkola M, Lumia M, Kronberg-Kippilä C, Ahonen S, Kaila M, et al. Maternal intake of fatty acids during pregnancy and allergies in the offspring. British Journal of Nutrition 2011;108(4):720–32. [DOI] [PubMed] [Google Scholar]
- 17.Jedrychowski W, Perera F, Maugeri U, Mrozek-Budzyn D, Miller RL, Flak E, et al. Effects of prenatal and perinatal exposure to fine air pollutants and maternal fish consumption on the occurrence of infantile eczema. International archives of allergy and immunology 2011;155(3):275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romieu I, Torrent M, Garcia-Esteban R, Ferrer C, Ribas-Fito N, Anto JM, et al. Maternal fish intake during pregnancy and atopy and asthma in infancy. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology 2007;37(4):518–25. [DOI] [PubMed] [Google Scholar]
- 19.Willers SM, Devereux G, Craig LC, McNeill G, Wijga AH, Abou El-Magd W, et al. Maternal food consumption during pregnancy and asthma, respiratory and atopic symptoms in 5-year-old children. Thorax 2007;62(9):773–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sausenthaler S, Koletzko S, Schaaf B, Lehmann I, Borte M, Herbarth O, et al. Maternal diet during pregnancy in relation to eczema and allergic sensitization in the offspring at 2 y of age. Am J Clin Nutr 2007;85(2):530–7. [DOI] [PubMed] [Google Scholar]
- 21.Newson RB, Shaheen SO, Henderson AJ, Emmett PM, Sherriff A, Calder PC. Umbilical cord and maternal blood red cell fatty acids and early childhood wheezing and eczema. J Allergy Clin Immunol 2004;114(3):531–7. [DOI] [PubMed] [Google Scholar]
- 22.Montes R, Chisaguano AM, Castellote AI, Morales E, Sunyer J, Lopez-Sabater MC. Fatty-acid composition of maternal and umbilical cord plasma and early childhood atopic eczema in a Spanish cohort. European journal of clinical nutrition 2013;67(6):658–63. [DOI] [PubMed] [Google Scholar]
- 23.Notenboom ML, Mommers M, Jansen EH, Penders J, Thijs C. Maternal fatty acid status in pregnancy and childhood atopic manifestations: KOALA Birth Cohort Study. Clin Exp Allergy 2011;41(3):407–16. [DOI] [PubMed] [Google Scholar]
- 24.Rucci E, den Dekker HT, de Jongste JC, Steenweg-de-Graaff J, Gaillard R, Pasmans SG, et al. Maternal fatty acid levels during pregnancy, childhood lung function and atopic diseases. The Generation R Study. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology 2016;46(3):461–71. [DOI] [PubMed] [Google Scholar]
- 25.Yu YM, Chan YH, Calder PC, Hardjojo A, Soh SE, Lim AL, et al. Maternal PUFA status and offspring allergic diseases up to the age of 18 months. Br J Nutr 2015;113(6):975–83. [DOI] [PubMed] [Google Scholar]
- 26.Standl M, Demmelmair H, Koletzko B, Heinrich J. Cord blood LC-PUFA composition and allergic diseases during the first 10 yr. Results from the LISAplus study. Pediatr Allergy Immunol 2014;25(4):344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunstan JA, Mori TA, Barden A, Beilin LJ, Taylor AL, Holt PG, et al. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. J Allergy Clin Immunol 2003;112(6):1178–84. [DOI] [PubMed] [Google Scholar]
- 28.Furuhjelm C, Warstedt K, Larsson J, Fredriksson M, Bottcher MF, Falth-Magnusson K, et al. Fish oil supplementation in pregnancy and lactation may decrease the risk of infant allergy. Acta Paediatr 2009;98(9):1461–7. [DOI] [PubMed] [Google Scholar]
- 29.Furuhjelm C, Warstedt K, Fageras M, Falth-Magnusson K, Larsson J, Fredriksson M, et al. Allergic disease in infants up to 2 years of age in relation to plasma omega-3 fatty acids and maternal fish oil supplementation in pregnancy and lactation. Pediatr Allergy Immunol 2011;22(5):505–14. [DOI] [PubMed] [Google Scholar]
- 30.Palmer DJ, Sullivan T, Gold MS, Prescott SL, Heddle R, Gibson RA, et al. Effect of n-3 long chain polyunsaturated fatty acid supplementation in pregnancy on infants’ allergies in first year of life: randomised controlled trial. Bmj 2012;344:e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noakes PS, Vlachava M, Kremmyda LS, Diaper ND, Miles EA, Erlewyn-Lajeunesse M, et al. Increased intake of oily fish in pregnancy: effects on neonatal immune responses and on clinical outcomes in infants at 6 mo. Am J Clin Nutr 2012;95(2):395–404. [DOI] [PubMed] [Google Scholar]
- 32.Palmer DJ, Sullivan T, Gold MS, Prescott SL, Heddle R, Gibson RA, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy 2013;68(11):1370–6. [DOI] [PubMed] [Google Scholar]
- 33.Chisaguano AM, Montes R, Castellote AI, Morales E, Julvez J, Vioque J, et al. Elaidic, vaccenic, and rumenic acid status during pregnancy: association with maternal plasmatic LC-PUFAs and atopic manifestations in infants. Pediatr Res 2014;76(5):470–6. [DOI] [PubMed] [Google Scholar]
- 34.Sontag-Padilla L, Shih RA, Chandra A, Tylavsky FA, Martin LT, Burns RM, et al. The Urban Child Institute CANDLE study : methodological overview and baseline sample description. [Google Scholar]
- 35.Roy A, Kocak M, Hartman TJ, Vereen S, Adgent M, Piyathilake C, et al. Association of prenatal folate status with early childhood wheeze and atopic dermatitis. Pediatr Allergy Immunol 2018;29(2):144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Völgyi E, Carroll KN, Hare ME, Ringwald-Smith K, Piyathilake C, Yoo W, et al. Dietary patterns in pregnancy and effects on nutrient intake in the Mid-South: the Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study. Nutrients 2013;5(5):1511–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmer FB, Anand KJ, Graff JC, Murphy LE, Qu Y, Völgyi E, et al. Early adversity, socioemotional development, and stress in urban 1-year-old children. J Pediatr 2013;163(6):1733–9.e1. [DOI] [PubMed] [Google Scholar]
- 38.Tylavsky FA, Kocak M, Murphy LE, Graff JC, Palmer FB, Völgyi E, et al. Gestational Vitamin 25(OH)D Status as a Risk Factor for Receptive Language Development: A 24-Month, Longitudinal, Observational Study. Nutrients 72015 p. 9918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226(1):497–509. [PubMed] [Google Scholar]
- 40.Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J Lipid Res 1964;5:600–8. [PubMed] [Google Scholar]
- 41.Prevention USCfDCa. Second national report on biochemical indicators of diet and nutrition in the U.S. population 20132012. Available from: http://www.cdc.gov/nutritionreport. [DOI] [PMC free article] [PubMed]
- 42.Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J 1995;8(3):483–91. [DOI] [PubMed] [Google Scholar]
- 43.Nurmatov U, Nwaru BI, Devereux G, Sheikh A. Confounding and effect modification in studies of diet and childhood asthma and allergies. Allergy 2012;67(8):1041–59. [DOI] [PubMed] [Google Scholar]
- 44.Harrell FE. Regression modeling strategies : with applications to linear models, logistic and ordinal regression, and survival analysis Second edition ed 2015. xxv, 582 pages p. [Google Scholar]
- 45.Chilton FH, Murphy RC, Wilson BA, Sergeant S, Ainsworth H, Seeds MC, et al. Diet-gene interactions and PUFA metabolism: a potential contributor to health disparities and human diseases. Nutrients 2014;6(5):1993–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alm B, Aberg N, Erdes L, Mollborg P, Pettersson R, Norvenius SG, et al. Early introduction of fish decreases the risk of eczema in infants. Archives of disease in childhood 2009;94(1):11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willers SM, Wijga AH, Brunekreef B, Kerkhof M, Gerritsen J, Hoekstra MO, et al. Maternal food consumption during pregnancy and the longitudinal development of childhood asthma. Am J Respir Crit Care Med 2008;178(2):124–31. [DOI] [PubMed] [Google Scholar]
- 48.Saito K, Yokoyama T, Miyake Y, Sasaki S, Tanaka K, Ohya Y, et al. Maternal meat and fat consumption during pregnancy and suspected atopic eczema in Japanese infants aged 3–4 months: the Osaka Maternal and Child Health Study. Pediatr Allergy Immunol 2010;21(1 Pt 1):38–46. [DOI] [PubMed] [Google Scholar]
- 49.Oien T, Storro O, Johnsen R. Do early intake of fish and fish oil protect against eczema and doctor-diagnosed asthma at 2 years of age? A cohort study. J Epidemiol Community Health 2010;64(2):124–9. [DOI] [PubMed] [Google Scholar]
- 50.Suarez-Varela MM, Alvarez LG, Kogan MD, Ferreira JC, Martinez Gimeno A, Aguinaga Ontoso I, et al. Diet and prevalence of atopic eczema in 6 to 7-year-old schoolchildren in Spain: ISAAC phase III. J Investig Allergol Clin Immunol 2010;20(6):469–75. [PubMed] [Google Scholar]
- 51.Nwaru BI, Erkkola M, Lumia M, Kronberg-Kippila C, Ahonen S, Kaila M, et al. Maternal intake of fatty acids during pregnancy and allergies in the offspring. Br J Nutr 2012;108(4):720–32. [DOI] [PubMed] [Google Scholar]
- 52.Xu H, Akesson A, Orsini N, Hakansson N, Wolk A, Carrero JJ. Modest U-Shaped Association between Dietary Acid Load and Risk of All-Cause and Cardiovascular Mortality in Adults. J Nutr 2016;146(8):1580–5. [DOI] [PubMed] [Google Scholar]
- 53.Yoon CY, Noh J, Lee J, Kee YK, Seo C, Lee M, et al. High and low sodium intakes are associated with incident chronic kidney disease in patients with normal renal function and hypertension. Kidney Int 2018;93(4):921–31. [DOI] [PubMed] [Google Scholar]
- 54.Seidelmann SB, Claggett B, Cheng S, Henglin M, Shah A, Steffen LM, et al. Dietary carbohydrate intake and mortality: a prospective cohort study and meta-analysis. Lancet Public Health 2018;3(9):e419–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miyake Y, Tanaka K, Sasaki S, Arakawa M. Polyunsaturated fatty acid intake and prevalence of eczema and rhinoconjunctivitis in Japanese children: the Ryukyus Child Health Study. BMC Public Health 2011;11:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kompauer I, Demmelmair H, Koletzko B, Bolte G, Linseisen J, Heinrich J. Association of fatty acids in serum phospholipids with hay fever, specific and total immunoglobulin E. The British journal of nutrition 2005;93(4):529–35. [DOI] [PubMed] [Google Scholar]
- 57.Liu X, Agerbo E, Schlünssen V, Wright RJ, Li J, Munk-Olsen T. Maternal asthma severity and control during pregnancy and risk of offspring asthma. J Allergy Clin Immunol 2018;141(3):886–92.e3. [DOI] [PubMed] [Google Scholar]