TO THE EDITOR
Hypersensitivity reactions (HSRs) are a well-known and frequent complication of platin chemotherapy and range from itching to life-threatening anaphylaxis.1 After a HSR, providers often switch to second-line chemotherapeutic agents to avoid further HSRs. Risk stratification with skin testing (ST) is a well-studied guideline that allows patients to safely continue first line treatment despite a HSR.2–7 However, risk stratification protocols require multiple office visits for repeat multi-step ST. These additional appointments for ST are time-consuming and resource-intensive especially for patients with complex illnesses, which limits progression through the risk stratification protocol. Literature on venom ST may offer an alternative approach; 1-step intradermal (ID) venom testing and simultaneous ID venom testing at different concentrations have both been shown to be safe in evaluating high-risk patients with a history of systemic venom reaction.8,9 Extrapolating from these studies, we sought to simplify the platin ST process while maintaining safety and efficacy by studying a modified 1-step platin ID ST protocol in patients with a history of platin HSR who have tolerated an initial desensitization. We hypothesized that a 1-step ST protocol using only the highest concentration is safe in low-risk patients (who have tolerated prior desensitization without HSR), but requires less time and fewer resources. If true, the 1-step ST protocol could improve access to platin risk stratification protocols.
Oncologic risk stratification protocols utilize ST prior to each desensitization to identify patients that are not allergic (or at lower risk of a HSR) to enable them to return for treatment safely to the outpatient setting.2–4, 6 Our institution uses a standard 4-step platin ST protocol including one epicutaneous and three ID ST steps, without duplicate ST performed.3, 5 Positive skin tests require a wheal to be 3 mm greater than the negative control. The final ID ST concentrations for carboplatin, cisplatin, and oxaliplatin are 5 milligrams per milliliter (mg/mL), 1 mg/mL, and 5 mg/mL, respectively. Rapid desensitization has 8 steps and intermediate desensitization has 13 steps at our institution. False-negative results can occur if ST is performed less than six weeks or more than six months from the initial HSR.2,10
Our study was conducted in two phases: initial retrospective chart and literature review to evaluate the safety of 1-step ID testing, followed by prospective data collection applying this model. First we retrospectively assessed the safety of 1-step ID testing by performing a chart review of all platin desensitization patients from 2013 to 2017 at our institution who had a positive platin ST. We assessed whether these patients reacted during the first step of their first desensitization after ST because the first desensitization bag administers more milligrams than the final ID step. Thus, if patients tolerate the first step of desensitization without a HSR, this would imply that ST with only the highest ID concentration is safe. However, it is important to acknowledge that the volumes of distribution differ in these scenarios. Our chart review identified 60 ST positive patients, and none had objective HSRs during the first desensitization step. One patient noted subjective itching during the first step of desensitization but improved with diphenhydramine and corticosteroids and completed the desensitization without a HSR. Additionally, a literature review on platin ST between 2000 to 2018 found that systemic HSRs related to platin ST were extremely rare, occurring in only 0.1% of patients. Our patient experience combined with the literature review support the safety of 1-step platin ID ST.
The modified protocol, modeled after our previously published risk stratification protocols but simplified to 1-step ID ST using the highest concentration only, was piloted over six months from January to August 2018 (Figure 1).3 While 4-step ST takes 60 minutes, 1-step testing takes 15 minutes. Modified 1-step ST was completed on either the night prior to or the day of the patient’s next desensitization, and desensitization plans were adjusted based on ST results. We evaluated adults (age 18 years or older) with a history of HSR to carboplatin, cisplatin, or oxaliplatin, who had tolerated at least one intermediate desensitization (and were thus considered lower risk) without HSR, with no prior ST or negative ST. We obtained patient characteristics and assessed safety and outcomes of the modified risk stratification protocol using 1-step ID ST. To ensure safety, patients were closely monitored by highly trained staff who could respond appropriately should a HSR occur.
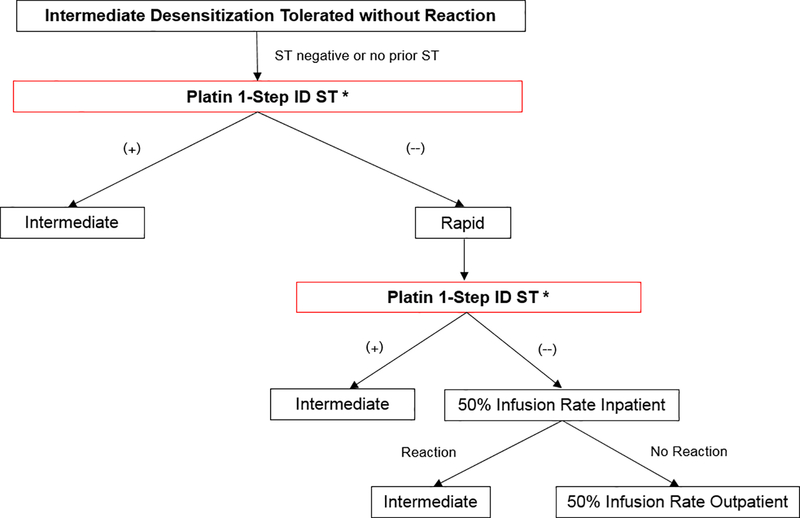
Figure 1.
Risk stratification algorithm with modified skin testing
ST, skin testing; ID, intradermal.
* Point at which patient can enter pathway.
1-step platin ST was performed at the highest ID concentration. ST was done on the night prior to, or the morning of, the patient’s next desensitization. Antihistamines were held for five days prior to ST.
We enrolled 10 patients with carboplatin (n=6) and oxaliplatin HSR (n=4) (Figure 2). The majority were female (70%) and white (90%). Nine patients (90%) completed ST the same day as their admissions for desensitization, while one patient (10%) completed ST one day prior to each desensitization admission. We performed 17 ST and decreased ST time by 765 minutes (or 12.75 hours).
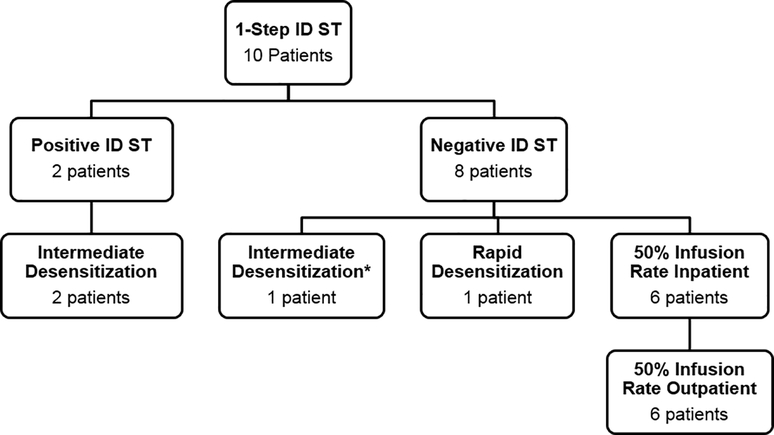
Figure 2.
Patient outcomes using risk stratification algorithm
ST, skin testing; ID, intradermal.
* ST occurred > 6 months after the hypersensitivity reaction which likely represents a false-negative result, so the algorithm supports intermediate desensitization.6
No HSRs occurred during ST. One oxaliplatin HSR patient who was ST negative twice developed a subcutaneous nodule at the ID site within one hour of testing. The first nodule resolved in two weeks, while the second nodule persisted for one month. No nodules developed with the third ST.
Two patients (20%) developed a positive ID ST with only a local wheal-and-flare reaction. The first was an ovarian cancer patient that developed hot flashes, diaphoresis, hypotension, and rigors with cycle 12 of carboplatin. She initially underwent four intermediate desensitizations (without HSR). Due to disease recurrence during our study, she had 1-step ID ST, which was positive. She continued carboplatin intermediate desensitization safely per protocol.
The second was a colon cancer patient who developed headache, itching, erythema, nausea, and diaphoresis with oxaliplatin. He empirically underwent two intermediate desensitizations without ST. He had mild diaphoresis with the first desensitization (resolved without treatment), but tolerated the second desensitization. At this point he was enrolled into the study, and 1-step ID ST was negative. Per protocol, he underwent a rapid desensitization which was complicated by diaphoresis but responded to diphenhydramine and fexofenadine. Repeat 1-step ID ST was positive. He subsequently completed five oxaliplatin intermediate desensitizations. All patients followed the risk stratification protocol similar to standard 4-step ST. Of the eight patients with negative ST, six tolerated 50%-slowed inpatient infusions and progressed back to outpatient infusions.
Modified 1-step ID ST is as safe as standard 4-step ST in low-risk platin HSR patients who have tolerated an intermediate desensitization when performed by experienced staff. There were no systemic reactions observed with 1-step platin ID ST using only the highest concentration, even in two ST positive patients. The 1-step protocol reduced ST time and number of visits to our institution enabling patients to safely but more easily progress through risk stratification. The main limitations of our study are the small sample size and that all patients were from one center. We do not recommend 1-step ID ST for initial evaluation of patients after initial HSR, as we evaluated this only in patients with known tolerance of desensitization and negative or no prior ST (who were thus lower risk). More safety data are needed; however, 1-step ID ST may offer a useful way to improve patient care in a select group of lower risk patients that are following a platin risk stratification pathway. Future steps include evaluating larger numbers of platin HSR patients and performing cost-effectiveness analyses. In conclusion, this study suggests the clinical safety and effectiveness of 1-step ID ST during risk stratification of platin HSRs for low-risk patients.
Clinical Implications.
Evaluation of platin hypersensitivity reactions requires multiple office visits and repeat skin testing. Modified 1-step skin testing is safe and effective but requires reduced time and resources compared to traditional 4-step skin testing.
Acknowledgments
Funding: A.S. Levin was supported by the National Institutes of Health award T32HL116275. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Polyzos A, Tsavaris N, Gogas H, Souglakos J, Vambakas L, Vardakas N, et al. Clinical features of hypersensitivity reactions to oxaliplatin: a 10-year experience. Oncology. 2009;76(1):36–41. [DOI] [PubMed] [Google Scholar]
- 2.Patil SU, Long AA, Ling M, Wilson MT, Hesterberg P, Wong JT, et al. A protocol for risk stratification of patients with carboplatin-induced hypersensitivity reactions. J Allergy Clin Immunol. 2012;129(2):443–7. [DOI] [PubMed] [Google Scholar]
- 3.Wang AL, Patil SU, Long AA, Banerji A. Risk-stratification protocol for carboplatin and oxaliplatin hypersensitivity: repeat skin testing to identify drug allergy. Ann Allergy Asthma Immunol. 2015;115(5):422–8. [DOI] [PubMed] [Google Scholar]
- 4.Wong JT, Ling M, Patil S, Banerji A, Long A. Oxaliplatin hypersensitivity: evaluation, implications of skin testing, and desensitization. J Allergy Clin Immunol Pract. 2014;2(1):40–5. [DOI] [PubMed] [Google Scholar]
- 5.Castells MC, Tennant NM, Sloane DE, Hsu FI, Barrett NA, Hong DI, et al. Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitization in 413 cases. J Allergy Clin Immunol. 2008;122(3):574–80. [DOI] [PubMed] [Google Scholar]
- 6.Lax T, Long A, Banerji A. Skin Testing in the Evaluation and Management of Carboplatin-Related Hypersensitivity Reactions. J Allergy Clin Immunol Pract. 2015;3(6):856–62. [DOI] [PubMed] [Google Scholar]
- 7.Levin A, Bhattacharya G, Blumenthal K, Camargo CA Jr., Banerji A. Platin chemotherapy hypersensitivity reactions: expanding the scope of practice and improving care. J Allergy Clin Immunol Pract. 2019;7(5):1691–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quirt JA, Wen X, Kim J, Herrero AJ, Kim HL. Venom allergy testing: is a graded approach necessary? Ann Allergy Asthma Immunol. 2016;116(1):49–51. [DOI] [PubMed] [Google Scholar]
- 9.Strohmeier B, Aberer W, Bokanovic D, Komericki P, Sturm GJ. Simultaneous intradermal testing with hymenoptera venoms is safe and more efficient than sequential testing. Allergy. 2013;68(4):542–4. [DOI] [PubMed] [Google Scholar]
- 10.Hesterberg PE, Banerji A, Oren E, Penson RT, Krasner CN, Seiden MV, et al. Risk stratification for desensitization of patients with carboplatin hypersensitivity: Clinical presentation and management. Journal of Allergy and Clinical Immunology. 2009;123(6):1262–7.e1. [DOI] [PubMed] [Google Scholar]