Abstract
Background.
Bronchodilator reversibility measures are often associated with poor asthma outcomes in children. Whether bronchodilator dose responsiveness is similarly useful in children is unclear.
Objective.
We hypothesized that children and adolescents requiring higher doses of bronchodilator to achieve maximal bronchodilation would have unique risk factors and increased risk of future exacerbation.
Methods.
Children (6–11 years, N=299) and adolescents (12–21 years, N=331) with confirmed asthma underwent clinical phenotyping procedures and a test of maximal bronchodilation with escalating doses of albuterol sulfate up to 720 mcg. Outcome measures were assessed at 12 months and included exacerbations treated with systemic corticosteroids, emergency department (ED) visits and hospitalizations for asthma.
Results.
6.7% of children and 9.3% of adolescents had poor bronchodilator dose responsiveness, defined as attainment of maximal FEV1 with 720 mcg albuterol. Risk factors included Type-2 inflammation, prior exacerbations, and greater asthma severity; historical pneumonia and tobacco exposure were also risk factors in children. Children and adolescents with poor bronchodilator dose responsiveness did not have increased current symptoms or impaired quality of life, but had ~2-fold increased odds of exacerbation or ED visit and ~3-fold increased odds of hospitalization by 12 months, independent of airflow obstruction.
Conclusions.
Bronchodilator dose responsiveness may be useful for phenotyping and may be of utility in practice and future studies focused on asthma outcomes or quantification of treatment responses. In children and adolescents, this phenotype of poor bronchodilator responsiveness may be associated with periods of relatively stable disease yet marked airway constriction in response to triggers, including tobacco smoke, respiratory infections/pneumonia, and aeroallergens.
Keywords: Asthma in children, Exacerbation, Airway physiology, Phenotype, Lung function, Bronchodilator reversibility, Type-2 inflammation, Sensitization
Introduction
Airway lability is a characteristic feature of children with asthma. As such, bronchodilator reversibility testing is routinely performed in the clinical setting to confirm the diagnosis of asthma and quantify asthma control.1 However, the measure is not always repeatable2 and specific cut-points for the purpose of asthma diagnosis and evaluation in children are debatable.3 Furthermore, given the variable nature of asthma control, not all children with asthma display a bronchodilator response.4
Nonetheless, bronchodilator reversibility has been identified in phenotypic subgroups of children with asthma5–11 and may contribute to differing clinical outcomes and disease trajectories.12–14 For example, in the Childhood Asthma Management Program, a small subset of children with consistent improvement in forced expiratory volume in one second (FEV1) of 12% or greater after bronchodilator administration at each study visit had more nocturnal symptoms, prednisone bursts, missed days of school and hospitalizations.13 Other studies have likewise identified greater bronchodilator reversibility in children with difficult-to-control or severe asthma, who also have greater symptom burden despite inhaled corticosteroid (ICS) treatment.5–8, 15 Therefore, the Global Strategy for Asthma Management and Prevention recently updated their asthma treatment guidelines to include markedly increased bronchodilator reversibility as a potentially modifiable risk factor for future exacerbations, even in patients with few symptoms.1
Although the clinical importance of bronchodilator reversibility has been demonstrated, whether bronchodilator dose responsiveness is also useful for asthma phenotype and and outcome assessment is not clear. We therefore characterized bronchodilator dose responsiveness and its associations with asthma exacerbations over 12 months in a sample of children and adolescents 6–21 years of age enrolled in observational asthma research studies at Children’s Healthcare of Atlanta and Emory University in Atlanta, Georgia. Using a test of maximal bronchodilation with up to 720 mcg of albuterol sulfate, we hypothesized that participants achieving maximal FEV1 with 720 mcg albuterol, compared to participants achieving maximal FEV1 with 360 mcg or 540 mcg of albuterol, would be distinguished by unique phenotypic risk factors and would have increased odds of an exacerbation, emergency department (ED) visit and hospitalization for asthma by 12 months, independent of airflow obstruction.
Methods
Children and adolescents 6 to 21 years of age within the Children’s Healthcare of Atlanta medical system were eligible for the study if they had: 1) a physician diagnosis of asthma, 2) no self-reported acute illness or an asthma exacerbation treated with systemic corticosteroids within the preceding four weeks, and 3) historical evidence of either ≥ 12% reversibility in FEV1 or airway hyperresponsiveness evidenced by a provocative concentration of methacholine causing a 20% drop in FEV1 (PC20) ≤16 mg/mL. Exclusion criteria included premature birth before 35 weeks of gestation, current smoking <5 pack years, or other chronic airway disorders such as cystic fibrosis, pulmonary aspiration or vocal cord dysfunction. Permission to proceed with this study was granted by the Emory University Institutional Review Board. Informed written consent was obtained from legal guardians or participants (if ≥18 years of age). Verbal assent was obtained from children 6–10 years and written assent was obtained from children and adolescents 11 to 17 years.
Study design and procedures.
Participants completed an outpatient study visit where questionnaires pertaining to demographics, medical history and symptoms were administered. Composite Asthma Severity Index (CASI) scores were calculated according to the methods of Wildfire et al.16 with a 4-week modification for symptom recall and a 12-month modification for asthma exacerbations. Asthma-related quality of life over the preceding two weeks was assessed with the Asthma Quality of Life Questionnaire (AQLQ);17 participants 6–17 years of age completed the pediatric version of the AQLQ instrument that excludes assessment of the environmental domain.18 Neighborhood (i.e., ZIP code) characteristics were obtained from the 2010–2014 American Community Survey 5-years estimates, Tables S101, S1501, and DP03, available at www.factfinder.census.gov.19 Exhaled nitric oxide concentrations were measured with a commercial device (NIOX MINO®, Circrassia Pharmaceuticals, Chicago, IL). Aeroallergen sensitization was assessed by specific IgE testing (Children’s Healthcare of Atlanta, Atlanta, GA) or skin prick testing with 12 extracts: tree mix, grass mix, weed mix, Ambrosia artemisiifolia, Alternaria alternata, Aspergillus fumagatis, Cladosporium herbarum, dog dander, cat dander, Blatella germanica, Dermatophagoides farinae, and Dermatophagoides pteronyssinus (Greer® Laboratories, Lenoir, NC). Venipuncture was also performed for quantification of blood eosinophils (Children’s Healthcare of Atlanta).
Spirometry (KoKo® PDS, Ferraris, Louisville, CO) was performed following a bronchodilator withhold as recommended by guidelines from the European Respiratory Society (ERS)/American Thoracic Society (ATS) for bronchodilator reversibility testing.20 Participants withheld short-acting bronchodilators for a minimum of 4 hours, long-acting beta-agonists for a minimum of 12 hours, and leukotriene antagonists for a minimum of 24 hours. Spirometry was repeated after 360 mcg (4 inhalations) of albuterol sulfate administered through a valved holding chamber with a mouthpiece (Aerochamber,® Monaghan Medical Corporation, Plattsburg, NY). Fifteen minutes after the 360 mcg dose, 2 additional albuterol inhalations were administered (total albuterol dose, 540 mcg). If FEV1 differed by 5% or more between the 360 and 540 mcg albuterol dosages, then the final 2 inhalations of albuterol were given (720 mcg total dose). Percent difference in FEV1 between albuterol dosages was calculated as a relative change as follows: (FEV1post − FEV1previous)/FEV1previous × 100. Best FEV1, forced vital capacity (FVC), and forced expiratory flow at 25–75% of vital capacity (FEF25–75) values from 3 reproducible maneuvers were recorded fifteen minutes after each bronchodilation and were interpreted according to Global Lung Function Initiative prediction equations.21 A subset of participants, on separate days, also underwent lung volume measurement with a body plethysmograph (MedGraphics Elite Series; Medical Graphics Corporation, St Paul, Minn) for quantification of residual volume (RV) and total lung capacity (TLC) and bronchoprovocation testing with methacholine concentrations of 0 to 16 mg/mL (Provocholine; Methapharm Inc, Coral Springs, Fla) delivered by a Rosenthal dosimeter (Pulmonary Data Service Instrumentation, Louisville, Colo). Bronchoprovocation was limited to participants with baseline FEV1 > 70% predicted.
Outcomes.
Participants were telephoned at 6 and 12 months after the study visit and questioned about asthma exacerbations treated with systemic corticosteroids22 and exacerbations resulting in ED visits and hospitalizations. Hospitalizations were verified by a review of medical records.
Statistical analyses.
Data were analyzed with SPSS® Statistics (Version 24, IBM, Armonk, NY) with stratification by age group (6–11 vs. 12–21 years). Clinical features and of children and adolescents achieving maximal bronchodilation with 360 mcg (4 inhalations), 540 mcg (6 inhalations), or 720 mcg (8 inhalations) of albuterol sulfate were compared with chi-square tests and analysis of variance. Post-hoc testing was performed using Tukey’s Least Significant Difference tests. The predictive capacity of poor bronchodilator response, defined as needing 720 mcg albuterol to achieve maximal bronchodilation, was assessed with logistic regression. Models were adjusted for age group, sex, ethnicity, race, and baseline airflow obstruction, defined as FEV1/FVC below the lower limit of normal.21 A p value <0.05 was used as the threshold for statistical significance without adjustment for multiple comparisons.
Results
Six hundred thirty children (N = 299) and adolescents (N = 331) were enrolled. Demographic features of the participants, stratified by age group, are shown in Table 1. Age groups differed with regard to race, sex, other self-reported medical conditions including eczema, chronic sinusitis and gastroesophageal reflux, asthma controller medication use, indoor exposures and asthma-related healthcare utilization in the previous year. Household educational attainment and features of the neighborhoods (i.e., ZIP codes) in which the participants resided did not differ between age groups (Table 1).
Table 1.
Features of the participants. Data represent the mean ± standard deviation or the number of participants (%).
| All participants N = 630 | Children 5–11 years N = 299 | Adolescents 12–21 years N = 331 | |
|---|---|---|---|
| Age (years) | 12.8 ± 4.5 | 9.1 ± 1.7 | 16.2 ± 3.3 |
| Asthma duration (years) | 9.4 ± 4.7 | 6.4 ± 2.8 | 12.1 ± 4.5 |
| Males | 351 (55.7) | 180 (60.2) | 171 (51.7) |
| Race | |||
| White | 200 (31.7) | 79 (26.4) | 121 (36.6) |
| Black | 368 (58.4) | 193 (64.5) | 175 (52.9) |
| More than one race | 52 (8.3) | 25 (8.4) | 27 (8.2) |
| Other | 10 (1.6) | 2 (0.7) | 8 (2.4) |
| Hispanic ethnicity | 44 (7.0) | 24 (8.0) | 20 (6.0) |
| Household education level | |||
| Refused | 130 (20.6) | 49 (16.4) | 81 (24.5) |
| Did not complete high school | 26 (4.1) | 9 (3.0) | 17 (5.1) |
| High school diploma | 82 (13.0) | 41 (13.7) | 41 (12.4) |
| Some college/technical training | 174 (27.6) | 86 (28.8) | 88 (26.6) |
| College degree | 218 (34.6) | 114 (38.1) | 104 (31.4) |
| ZIP code features | 34.6 ± 16.9 | 35.0 ± 17.3 | 34.2 ± 16.2 |
| Population (in thousands) | |||
| Unemployment (%) | 12.7 ± 5.8 | 12.6 ± 5.6 | 12.8 ± 6.1 |
| Bachelor’s degree (%) | 31.2 ± 16.2 | 30.5 ± 15.4 | 32.1 ± 17.1 |
| Families below poverty threshold (%) | 19.4 ± 11.0 | 18.9 ± 10.3 | 19.9 ± 11.9 |
| Family history | |||
| Father with asthma | 149 (23.7) | 74 (24.7) | 75 (22.7) |
| Mother with asthma | 234 (37.1) | 115 (38.5) | 119 (36.0) |
| Other medical conditions | |||
| Allergic rhinitis | 558 (88.6) | 262 (87.6) | 296 (89.4) |
| Eczema | 343 (54.4) | 188 (62.9) | 155 (46.8) |
| Obesity | 172 (27.3) | 82 (27.4) | 90 (27.2) |
| Recurrent pneumonia | 289 (45.9) | 128 (42.8) | 161 (48.6) |
| Chronic sinusitis | 178 (28.3) | 64 (21.4) | 114 (34.4) |
| Gastroesophageal reflux | 149 (23.7) | 49 (16.4) | 100 (30.2) |
| Asthma controller medications | |||
| Inhaled corticosteroid | 492 (78.1) | 261 (87.3) | 231 (69.8) |
| Long-acting beta agonist | 360 (57.1) | 160 (53.5) | 200 (60.4) |
| Montelukast | 356 (56.5) | 185 (61.9) | 171 (51.7) |
| Omalizumab | 20 (3.2) | 6 (2.0) | 14 (4.2) |
| Indoor exposures | |||
| Cat | 98 (15.6) | 35 (11.7) | 63 (19.0) |
| Dog | 234 (37.1) | 103 (34.4) | 131 (39.6) |
| Tobacco smoke | 113 (18.0) | 33 (11.0) | 80 (24.2) |
| Asthma-related healthcare utilization | |||
| Unscheduled physician visit (past year) | 424 (67.3) | 222 (74.2) | 202 (61.0) |
| Emergency department (past year) | 300 (47.7) | 169 (56.5) | 131 (39.7) |
| Hospitalization (past year) | 148 (23.5) | 84 (28.1) | 64 (19.3) |
| Hospitalization (ever) | 348 (55.2) | 154 (51.5) | 194 (58.6) |
| Intensive care admission (ever) | 175 (27.8) | 75 (25.1) | 100 (30.2) |
| Intubation for asthma (ever) | 80 (12.7) | 35 (11.7) | 45 (13.6) |
Lung function and bronchodilation patterns.
In children, maximal bronchodilation was achieved with 360 mcg albuterol in 136 participants (45.5%), 540 mcg in 143 participants (47.8%), and 720 mcg in 20 participants (6.7%). In adolescents, maximal bronchodilation was achieved with 360 mcg albuterol in 121 participants (36.6%), 540 mcg in 179 participants (54.1%), and 720 mcg in 31 participants (9.4%).
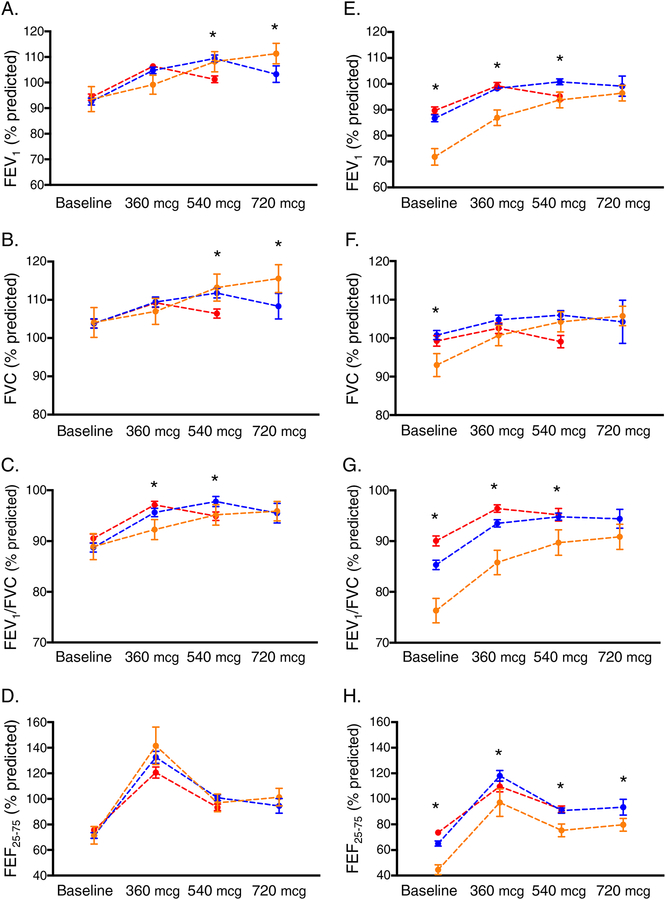
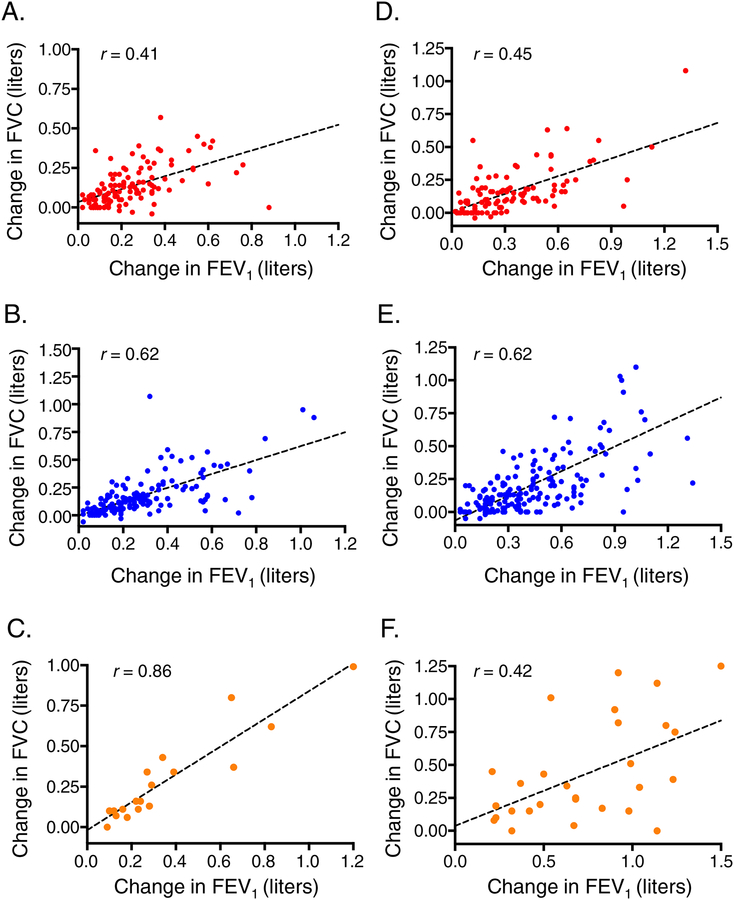
Patterns of bronchodilation with progressive albuterol dosages, stratified by age group, are shown in Figure 1. In children, there were no differences in baseline FEV1, FVC, FEV1/FVC or FEF25–75 percent predicted values between the albuterol dose response groups. However, by definition, children who achieved maximal bronchodilation with 720 mcg albuterol had progressive improvement in FEV1.; this was mirrored by progressive improvements in FVC and FEV1/FVC but not FEF25–75 (Figure 1A–D). In adolescents, baseline FEV1, FVC, FEV1/FVC and FEF25–75 percent predicted values differed between albuterol dose response groups and were significantly lower in participants who achieved maximal bronchodilation with 720 mg albuterol. After maximal bronchodilation, FEF25–75, but not FEV1 or FVC, remained lower in this group (Figure 1E–H). Associations between the maximal change in FEV1 and FVC for each albuterol dose response group are shown in Figure 2. Baseline RV/TLC values, methacholine PC20 values, and lung function reversibility measures are also shown in Table 2.
Figure 1.
FEV1, FVC, FEV1/FVC, and FEF25–75 percent predicted values in children (panels A–D, respectively) and adolescents (panels E–H, respectively) at baseline and after 360 mcg, 540 mcg, and 720 mcg of albuterol sulfate. Data are stratified by the dosage of albuterol at which maximal bronchodilation was obtained (red = 360 mcg, blue = 540 mcg, orange = 720 mcg). *p < 0.05
Figure 2.
Associations between the maximal change in FEV1 and FVC for each albuterol dose response group (red = 360 mcg, blue = 540 mcg, orange = 720 mcg).
Table 2.
Baseline RV/TLC and methacholine PC20 values and absolute and relative change in FEV1, FVC, FEV1/FVC, stratified by the dose of albuterol at which maximum bronchodilation was obtained. Data represent the mean ± standard deviation.
| Lung function measure | Children 5–11 years (N = 299) | Adolescents 12–21 years (N = 331) | ||||
|---|---|---|---|---|---|---|
| 360 mcg N = 136 | 540 mcg N = 143 | 720 mcg N = 20 | 360 mcg N = 121 | 540 mcg N = 179 | 720 mcg N = 31 | |
| Baseline RV/TLC (ratio)1 | 0.28 ± 0.07 | 0.28 ± 0.09 | 0.37 ± 0.11*,** | 0.26 ± 0.08 | 0.26 ± 0.10 | 0.36 ± 0.13*,** |
| Baseline Methacholine PC20 (mg/mL)2 | 3.60 ± 3.87 | 3.16 ± 4.30 | 1.22 ± 1.53*,** | 6.35 ± 8.73 | 4.43 ± 7.40 | 0.43 ± 0.29*,** |
| Absolute change1 | ||||||
| FEV1 (% predicted) | 11.2 ± 9.7 | 16.8 ± 12.7* | 17.8 ± 13.4* | 8.9 ± 7.6 | 14.1 ± 9.3* | 24.6 ± 13.9*,** |
| FVC (% predicted) | 6.1 ± 6.6 | 9.2 ± 10.1* | 12.3 ± 11.0* | 3.9 ± 5.7 | 6.1 ± 7.6* | 13.0 ± 13.6*,** |
| FEV1/FVC (% predicted) | 6.0 ± 7.3 | 8.0 ± 8.9* | 6.3 ± 6.6 | 5.8 ± 6.0 | 8.8 ± 6.2* | 14.3 ± 12.4*,** |
| FEF25–75 (% predicted) | 45.0 ± 51.1 | 61.0 ± 57.7* | 70.0 ± 63.9* | 36.0 ± 41.7 | 52.9 ± 52.8* | 56.2 ± 46.7* |
| Relative change5 | ||||||
| FEV1 (% predicted) | 14.0 ± 13.4 | 20.1 ± 19.7* | 22.7 ± 23.4* | 12.0 ± 13.3 | 18.9 ± 17.4* | 39.6 ± 28.7*,** |
| FVC (% predicted) | 6.3 ± 8.1 | 9.4 ± 11.3* | 13.2 ± 14.7* | 4.4 ± 6.6 | 6.6 ± 9.6 | 16.8 ± 23.0*,** |
| FEV1/FVC (% predicted) | 7.2 ± 9.3 | 9.7 ± 10.9* | 7.9 ± 9.3 | 7.1 ± 7.9 | 11.3 ± 9.4* | 20.3 ± 18.5*,** |
| FEF25–75 (% predicted) | 80.2 ± 117.4 | 113.6 ± 136.1* | 118.1 ± 107.5 | 59.2 ± 80.1 | 108.3 ± 146.1* | 140.8 ± 132.4* |
p< 0.05 vs. 360 mcg,
p<0.05 vs. 540 mcg
Children, 360 mcg, n = 40; 540 mcg, n = 36; 720 mcg, n = 8. Adolescents, 360 mcg, n = 40; 540 mcg, n = 53; 720 mcg, n = 8.
Children, 360 mcg, n = 55; 540 mcg, n = 47; 720 mcg, n = 9. Adolescents, 360 mcg, n = 65; 540 mcg, n = 104; 720 mcg, n = 10.
Data were logarithmically transformed prior to analyses.
Relative change = ((post-bronchodilator value in liters – pre-bronchodilator value in liters)/pre-bronchodilator value in liters)*100
Absolute change = post-bronchodilator percent predicted value – pre-bronchodilator percent predicted value
Clinical features associated with bronchodilation patterns.
Other clinical features of children and adolescents, stratified by the dose of albuterol at which maximum bronchodilation was obtained, are shown in Table 3. Children who achieved maximal bronchodilation with 720 mcg albuterol were more likely to report a history of recurrent pneumonia treated with antibiotics, indoor tobacco smoke exposure, a greater intensity of asthma treatment with higher dosages of ICS, more prior hospitalizations, intensive care unit admissions and intubations for asthma exacerbations, and greater asthma severity reflected by higher baseline CASI scores. Adolescents who achieved maximal bronchodilation with 720 mcg albuterol had a slightly longer duration of asthma, more hospitalizations for asthma in the previous year, increased lifetime hospitalizations and intensive care unit admissions for asthma exacerbations, and a higher baseline CASI score (Table 3).
Table 3.
Clinical features that distinguish participants who achieved maximal bronchodilation with 720 mcg albuterol. Data represent the mean ± standard deviation or the number of participants (%).
| Children 5–11 years (N = 299) | Adolescents 12–21 years (N = 331) | |||||
|---|---|---|---|---|---|---|
| 360 mcg N = 136 | 540 mcg N = 143 | 720 mcg N = 20 | 360 mcg N = 121 | 540 mcg N = 179 | 720 mcg N = 31 | |
| Asthma duration (years) | 6.2 ± 3.0 | 6.7 ± 2.5 | 6.1 ± 3.3 | 11.1 ± 4.6 | 12.6 ± 4.3 | 12.7 ± 4.4* |
| Recurrent pneumonia | 50 (36.8) | 65 (45.5) | 13 (65.0)* | 51 (42.1) | 93 (52.0) | 17 (54.8) |
| Inhaled corticosteroid | ||||||
| Any dose | 112 (82.4) | 130 (90.9)* | 19 (95.0)* | 79 (65.3) | 127 (70.9) | 25 (80.6) |
| High dose1 | 67 (49.3) | 91 (63.6)* | 13 (65.0)* | 51 (42.1) | 75 (41.9) | 16 (51.6) |
| Long-acting beta agonist | 61 (44.9) | 86 (60.1)* | 13 (65.0)* | 70 (57.9) | 109 (60.9) | 21 (67.7) |
| Montelukast | 77 (56.6) | 92 (64.3) | 16 (80.0)* | 69 (57.0) | 87 (48.6) | 15 (48.4) |
| Tobacco smoke exposure | 10 (7.4) | 18 (12.6) | 5 (25.0)* | 26 (21.7) | 43 (24.0) | 11 (35.5) |
| Hospitalization (ever) | 61 (44.9) | 79 (55.2) | 14 (70.0)* | 67 (55.4) | 100 (55.9) | 27 (87.1)*,** |
| Hospitalization (past year) | 34 (25.0) | 43 (30.1) | 7 (35.0) | 21 (17.4) | 32 (17.9) | 11 (35.5)*,** |
| Intensive care admission (ever) | 25 (18.4) | 40 (28.0) | 10 (50.0)*,** | 36 (29.8) | 47 (26.3) | 17 (54.8)*,** |
| Intubation for asthma (ever) | 17 (12.5) | 14 (9.8) | 6 (30.0)*,** | 16 (13.2) | 23 (12.8) | 6 (19.4) |
| Composite Asthma Severity Index (CASI) score | 7.9 ± 4.0 | 8.6 ± 3.6 | 10.2 ± 4.1* | 7.6 ± 4.2 | 7.8 ± 4.2 | 10.1 ± 3.6*,** |
p < 0.05 vs. 360 mcg,
p < 0.05 vs. 540 mcg
As defined by Global Initiative for Asthma 2018 guidelines.1
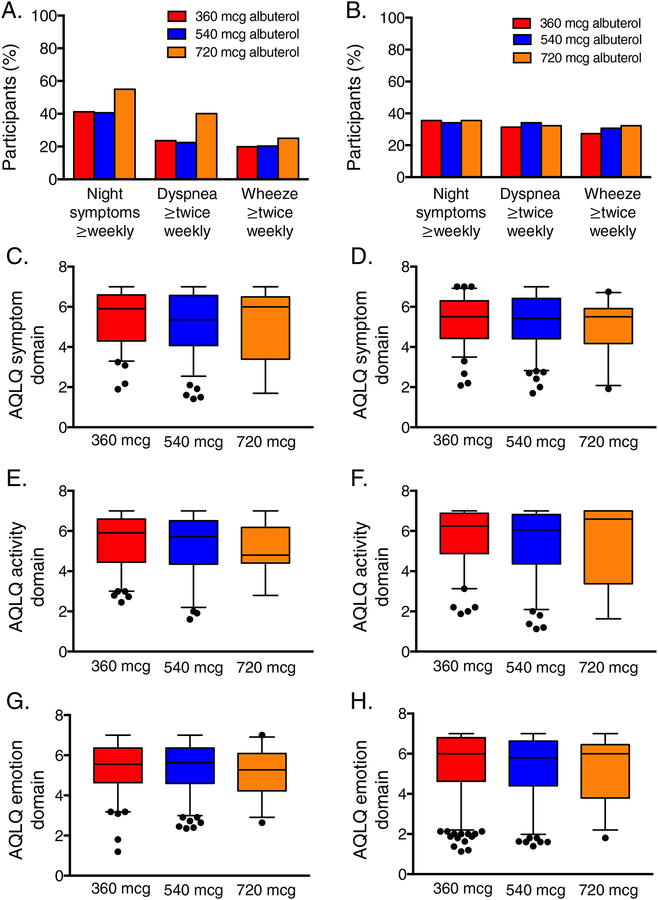
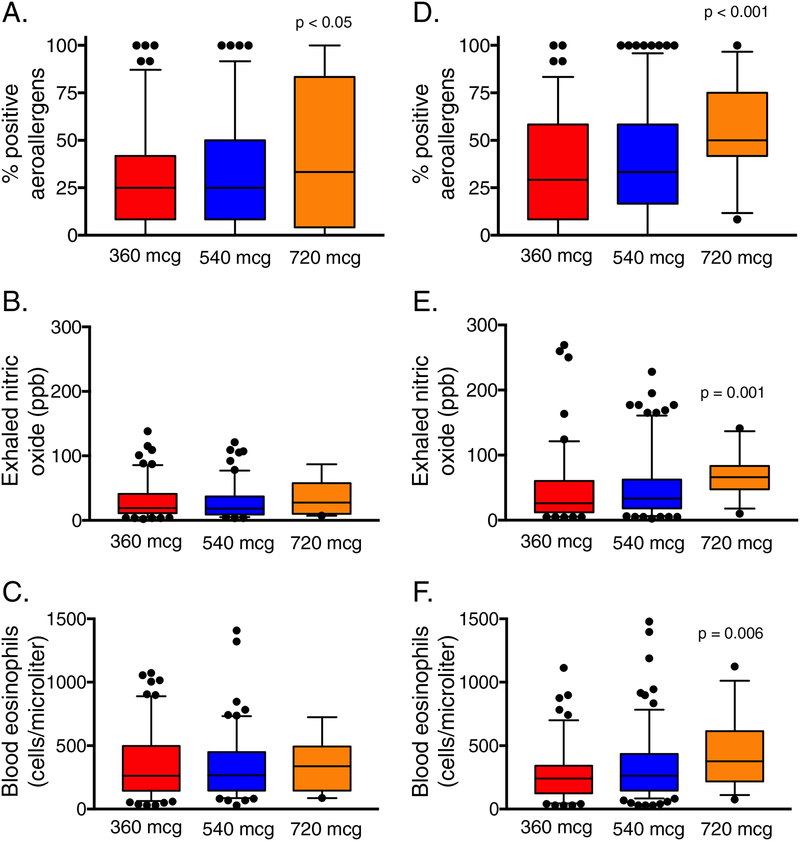
Other asthma-related and inflammatory features of children and adolescents are shown in Figures 3–4. There were no differences in current asthma symptoms (Figure 3A,B) or asthma-related quality of life domain scores (Figure 3C–H) in children or adolescents who achieved maximal bronchodilation with 720 mcg of albuterol compared to the other groups. However, markers of Type-2 inflammation differed; children and adolescents who achieved maximal bronchodilation with 720 mcg of albuterol were both distinguished by a higher percentage of positive aeroallergens (Figure 4) Adolescents (but not children) who achieved maximal bronchodilation with 720 mcg of albuterol also had higher exhaled nitric oxide concentrations and higher blood eosinophil counts (Figure 4).
Figure 3.
Self-reported symptoms over the previous two weeks (panels A, B) and Asthma Quality of Life Questionnaire (AQLQ) symptom (panels C, D), activity (panels E, F) and emotion (panels G, H) domain scores in children (left) and adolescents (right). Higher AQLQ scores reflect greater quality of life. Whiskers and dots represent 5th–95th percentiles and outliers, respectively. Groups are not statistically different.
Figure 4.
Percentage of positive aeroallergens, exhaled nitric oxide concentrations, and blood eosinophil counts in children (panels A–C, respectively) and adolescents (panels D–F, respectively) who achieved maximal bronchodilation with 360 mcg (red), 540 mcg (blue), and 720 mcg (orange) of albuterol sulfate. Whiskers and dots represent 5th–95th percentiles and outliers, respectively.
Predictive capacity of poor bronchodilator dose responsiveness.
The clinical relevance of poor bronchodilator dose responsiveness was assessed with multivariate logistic regression, with the albuterol dose at which maximal bronchodilation was obtained as the predictor and exacerbations treated with systemic corticosteroids, ED visits, and hospitalizations within 12 months of the study visit as outcomes. 12-month outcomes were available from 293 children (98.0%) and 243 adolescents (73.4%). Features of this subset did not differ from those of the larger sample (data not shown). Exacerbations treated with systemic corticosteroids occurred in 104 children (36%) and 75 adolescents (30.7%). ED visits occurred in 79 children (29%) and 52 adolescents (21%) and hospitalizations occurred in 24 children (8%) and 20 adolescents (8%). To improve power, age groups were merged for outcome analyses and adjusted for covariates including age group.
In analyses adjusted only for age group, maximal bronchodilation with 720 mcg albuterol (versus 360 mcg albuterol) was associated with more than 2-fold increased odds of exacerbation by 12 months (Table 3). This association persisted after adjustment for demographic variables, ICS use, and baseline airflow obstruction. Similarly, children and adolescents who achieved maximal bronchodilation with 720 mcg albuterol also had ~2-fold increased odds of an ED visit and ~3.5-fold increased odds of hospitalization by 12 months in adjusted analyses (Table 3).
Discussion
Our findings highlight the features and clinical importance of a low-prevalent (<10%) phenotype of children and adolescents with poor bronchodilator dose responsiveness, defined as achievement of maximal bronchodilation with 720 mcg of albuterol sulfate, who are at greater risk for life-threatening future exacerbations independent of baseline airflow obstruction. Although poor bronchodilator dose responsiveness not associated with increased self-reported symptoms or decreased quality of life (perhaps due to poor symptom perception), children and adolescents in this group tended to have more Type-2 inflammatory features, greater airway hyperresponsiveness to methacholine, and greater asthma burden/severity reflected by higher CASI scores. These same participants also had ~2-fold increased odds of a subsequent exacerbation or ED visit and ~3-fold increased odds of a subsequent hospitalization for status asthmaticus. Although additional studies are needed, this phenotype may be associated with periods of relatively stable disease yet marked airway constriction in response to triggers, including tobacco smoke, lower respiratory infections/pneumonia, and aeroallergens.
Although the clinical importance of bronchodilator reversibility measurements in children with asthma have been well established,5–15 the majority of asthma phenotyping work in children has focused on baseline measures of lung function (namely FEV1) without consideration of bronchodilation as a variable.23 In other studies where FEV1 bronchodilator reversibility was assessed, it did not consistently distinguish phenotypic groups of children.6, 10, 24–26 These disparate findings may be due to poor specificity of FEV1 in children. Indeed, others have shown that FEV1 values are often normal in children with asthma27, 28 and may not discriminate subtle differences in clinical presentation.29 Bronchodilator dose responsiveness studies in children are quite limited but may ultimately better phenotypic heterogeneity. For example, previous reports from the National Heart, Lung and Blood Institute’s Severe Asthma Research Program (SARP) examined maximal post-bronchodilator pulmonary function indices in children and adolescents 6–17 years with asthma and noted greater baseline air trapping and airflow limitation that persisted in a subset of participants after maximal bronchodilation.5–7, 30 These studies suggested that: 1) at least some children and adolescents have patterns of airway physiology similar to those of adults with severe persistent asthma,31 and 2) these patients with air trapping and airflow limitation may be at increased risk for exacerbation.5 However, those studies did not examine the dose of albuterol at which maximal bronchodilation was obtained and focused on comparisons between severe and non-severe asthma defined by ERS/ATS criteria.32
Our observations of increased exacerbation occurrence and severity in children and adolescents with poor bronchodilator dose responsiveness, defined as achievement of maximal bronchodilation with 720 mcg of albuterol sulfate, do have biologic plausibility. These patients with poor bronchodilator responsiveness also had more historical exacerbations and hospitalizations. Other studies have likewise shown that prior exacerbations are a significant predictor of future exacerbations regardless of disease severity or use of controller medications.33–37 Moreover, previous intensive care unit admissions are also strongly predictive of future intensive care unit admissions in children with asthma.38 One study also noted increased bronchodilator reversibility in children with self-reported asthma exacerbations in the previous year irrespective of asthma severity, although that report found no differences in the absolute FEV1 percent change between children stratified by exacerbation rate (i.e., 1–2 versus >2 exacerbations).39 However, a recent study of 560 inner-city children from Sorkness et al.40 identified three obstruction phenotypes in children (none, airflow limitation and air-trapping) and found that children with air trapping had the highest RV/TLC, greatest airway hyperresponsiveness to methacholine, greatest variability in FEV1 across multiple visits, and greatest exacerbation frequency. Similarly, a cluster of children in the Childhood Asthma Management Research Program with the greatest bronchodilator reversibility also had severe airway hyperresponsiveness, the most reports of prior hospitalizations and the highest rate of emergency department visits, and the highest risk of future exacerbation.26 Similar to our study, the prevalence of this phenotypic cluster was 9.3%.26 A separate analysis of this same population of children also noted associations between exacerbation frequency and the percent increase in post-bronchodilator FEV1 after methacholine challenge.41
Sorkness40 and others26, 42–44 have also noted more features of type-2 inflammation in children and adolescents with air trapping and bronchodilator reversibility, including greater aeroallergen sensitization, higher serum IgE concentrations, higher exhaled nitric oxide concentrations, and higher blood eosinophils. Furthermore, in the cluster of children in the Childhood Asthma Management Research Program with the greatest bronchodilator reversibility, the prevalence of skin test reactivity approached 100%.26 However, those studies did not stratify by age groups. In the present study, although greater aeroallergen sensitization was present in both children and adolescents, exhaled nitric oxide concentrations and blood eosinophils were only elevated in adolescents, perhaps due to greater ICS use and higher ICS dosages in children which may suppress Type-2 inflammatory pathways.45 It is also possible that markers of Type-2 inflammation vary by age, as optimal cut-points have not yet been defined for pre-adolescent children. Our observation of greater tobacco smoke exposure in children with marked bronchodilator reversibility is also aligned with other reports of increased bronchodilator responsiveness in tobacco-exposed infants46 and children.47
Nonetheless, this study does have a number of limitations. Particularly in children, the albuterol dose response curves did not plateau at the highest albuterol dose. Therefore, we cannot rule out fatigue or issues with albuterol delivery given that delivery can be impacted by multiple variables such as airway closures, airflow heterogeneity, tachyphylaxis, and proximal mucous plugging. It is therefore possible that these physiological factors limited the effects of the initial albuterol dose, and therefore resulted in greater distribution of albuterol with subsequent dosages. Second, inclusion was limited to children with historical evidence of at least 12% FEV1 bronchodilator reversibility or airway hyperresponsiveness to methacholine. This inclusion was consistent with ERS/ATS guidelines20 which define a change in FEV1 >12% of baseline (and >200 mL) as “significant bronchodilation” irrespective of age. Therefore, we did not detect phenotypes of fixed airflow obstruction with bronchodilator unresponsiveness that have been previously described in children.48 Third, bronchodilator reversibility may not adequately reflect the complex nature of airway smooth muscle tone. Indeed, the degree of bronchodilator reversibility also depends on excitation-contraction signaling pathways and activation of mechanisms that drive smooth muscle shortening, which are also thought to be abnormal in patients with asthma.49 There is also no consensus on the dose of bronchodilator to be used for bronchodilator reversibility testing. While most pulmonary function laboratories deliver four separate doses of short-acting beta-agonist (i.e., 400 total mcg salbutamol or 360 mcg albuterol) in accordance with guideline recommendations,20 dose-response studies of salbutamol50 and albuterol51 in adults with asthma demonstrate a log-linear cumulative dose response for FEV1 evident up to the final dose of 16 cumulative inhalations. Identical dose response studies in children are lacking, but limited evidence suggests a possible plateau of the dose response curve in children,52–56 similar our observation of maximal FEV1 attainment with 360–540 mcg albuterol in the majority of participants in the present study.
It is also difficult to ascertain the role of bronchodilator reversibility independent of airflow obstruction in the assessment of exacerbation risk. Other studies of similar populations of children have clearly shown that the level of airflow obstruction and reversal with bronchodilation contribute to poor symptom control and exacerbation.15, 44 For this reason, we adjusted for baseline airflow obstruction in our outcome analyses and found that poor bronchodilator dose responsiveness, defined as attainment of maximal bronchodilation with 720 mcg albuterol, remained a significant independent predictor of exacerbations, ED visits and hospitalizations. We also expressed bronchodilator reversibility measures as both a relative and absolute change in the percentage of predicted values, consistent with recommendations from others who have argued that relative measures are overly dependent on participant morphology57 and tend to advantage low initial values,58 rendering those values more susceptible to regression toward the mean.2
Finally, because the participants in our study were grouped according to abuterol dose responsiveness on a single day, we also acknowledge that the temporal stability and reproducibility of the dose response groups may be poor and may reflect adherence or access to controller medication and environmental controls and current asthma burden/severity. Indeed, in a large retrospective analysis of over 30,000 patients with confirmed asthma and historical FEV1 reversibility of at least 12%, the proportion of bronchodilator reversible patients decreased by 30% and 50% in the placebo and inhaled fluticasone treatment arms at study completion.59 However, among the patients who remained reversible, the degree of bronchodilator reversibility was essentially unchanged (26% change for placebo and 15% change for inhaled fluticasone).59 A separate report of adults stratified by asthma severity similarly demonstrated low concordance of bronchodilator reversibility measures over 12 months of follow-up, although concordance was somewhat improved in severe patients with greater obstruction and higher baseline bronchodilator reversibility.60 Our study population included a convenience sample of children and adolescents presenting to an academic medical center for evaluation and care, irrespective of asthma severity. We did not have enough information to define participants as having “severe” or “non-severe” asthma in the present study since consensus treatment guidelines emphasize the need for 12 months of asthma specialty follow-up with assessment of ICS adherence and management of co-morbid conditions.32 However, the overall prevalence of airflow obstruction (defined as an FEV1/FVC less than the lower limit of normal) was 43.1% in children and 48.6% in adolescents in our study; this is similar to that observed in non-severe asthma participants enrolled in SARP7 and a recent study of inner-city children with persistent asthma irrespective of asthma severity.40
In conclusion, poor bronchodilator dose responsiveness necessitating 720 mcg albuterol sulfate for attainment of maximal FEV1 is present in a small proportion of children and adolescents with asthma but is associated with unique risk factors and increased risk for life-threatening future exacerbations independent of airflow obstruction. Although future studies are needed in diverse populations to confirm the reproducibility of the phenotype and outcome associations, we contend that bronchodilator dose responsiveness may be a useful measure for the purpose of asthma phenotyping and may be of utility in clinical practice and future studies focused on asthma outcomes or quantification of asthma treatment responses, such as biologic studies.
Table 4.
Association between albuterol dose response and outcome occurrence by 12 months.
| Adjusted odds ratio (95% confidence interval) | |||
|---|---|---|---|
| Exacerbation | ED visit | Hospitalization | |
| Model 11 | |||
| 540 mcg vs. 360 mcg | 1.28 (0.87, 1.88) | 1.13 (0.74, 1.73) | 2.28 (1.07, 4.85)* |
| 720 mcg vs. 360 mcg | 2.65 (1.36, 5.17)* | 2.70 (1.35, 5.38)* | 4.93 (1.82, 13.41)* |
| Model 22 | |||
| 540 mcg vs. 360 mcg | 1.29 (0.87, 1.90) | 1.13 (0.73, 1.74) | 2.57 (1.20, 5.52)* |
| 720 mcg vs. 360 mcg | 2.59 (1.32, 5.08)* | 2.59 (1.29, 5.20)* | 4.78 (1.73, 13.21)* |
| Model 33 | |||
| 540 mcg vs. 360 mcg | 1.15 (0.77, 1.71) | 0.97 (0.62, 1.52) | 2.26 (1.04, 4.90)* |
| 720 mcg vs. 360 mcg | 2.19 (1.10, 4.34)* | 2.12 (1.03, 4.32)* | 3.87 (1.39, 10.80)* |
| Model 44 | |||
| 540 mcg vs. 360 mcg | 1.07 (0.71, 1.60) | 0.93 (0.59, 1.45) | 1.98 (0.91, 4.34) |
| 720 mcg vs. 360 mcg | 2.10 (1.05, 4.20)* | 2.05 (1.01, 4.20)* | 3.55 (1.25, 10.10)* |
p < 0.05
Adjusted for age group
Adjusted for age group and demographic features (sex, race, ethnicity)
Adjusted for age group, demographic features and ICS use
Adjusted for age group, demographic features, ICS use, and baseline airflow obstruction
Highlights Box.
What is already known about this topic?
Bronchodilator reversibility has been identified in phenotypic subgroups of children with asthma and may contribute to differing clinical outcomes and disease trajectories. Whether bronchodilator dose responsiveness is also useful for phenotype definition and outcome assessment is not clear.
What does this add to our knowledge?
Poor bronchodilator dose responsiveness was identified in <10% of participants but was associated with unique features (i.e., Type-2 inflammation, indoor exposures, prior severe exacerbations) and increased odds of future exacerbation and hospitalization, independent of airflow obstruction.
How does this study impact current management guidelines?
In children and adolescents, poor bronchodilator dose responsiveness may be an independent predictor of future risk and may identify a group of patients at highest risk for life-threatening exacerbations.
Support:
This study was supported in part by R01 NR013700, R01 NR012021, R01 HL69170, UL1 TR000454, the Children’s Healthcare of Atlanta Pediatric Research Alliance Center for Clinical Outcomes Research and Public Health, and the Atlanta Pediatric Scholars Program K12HD072245.
Abbreviations
- ATS
American Thoracic Society
- AQLQ
Asthma Quality of Life Questionnaire
- CASI
Composite Asthma Severity Index
- ED
Emergency department
- ERS
European Respiratory Society
- FEF25–75
Forced expiratory flow at 25–75% of vital capacity
- FEV1
Forced expiratory volume in one second
- FVC
Forced vital capacity
- ICS
Inhaled corticosteroid
- PC20
Provocative concentration of methacholine causing a 20% drop in FEV1
- RV
Residual volume
- SARP
Severe Asthma Research Program
- TLC
Total lung capacity
Footnotes
- Jocelyn R. Grunwell: Nothing to disclose.
- Khristopher M. Nguyen: Nothing to disclose.
- Alice C. Bruce: Nothing to disclose.
- Anne M. Fitzpatrick: Dr. Fitzpatrick has received grants from the National Institutes of Health and the Children’s Healthcare of Atlanta Pediatric Research Alliance Center for Clinical Outcomes Research and Public Health for aspects of the submitted work. All albuterol used in this study was purchased directly with grant funds.
References
- 1.Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2018. Available from: www.ginasthma.org.
- 2.Dompeling E, van Schayck CP, Molema J, Akkermans R, Folgering H, van Grunsven PM, et al. A comparison of six different ways of expressing the bronchodilating response in asthma and COPD; reproducibility and dependence of prebronchodilator FEV1. Eur Respir J 1992; 5:975–81. [PubMed] [Google Scholar]
- 3.Tse SM, Gold DR, Sordillo JE, Hoffman EB, Gillman MW, Rifas-Shiman SL, et al. Diagnostic accuracy of the bronchodilator response in children. J Allergy Clin Immunol 2013; 132:554–9 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray C, Foden P, Lowe L, Durrington H, Custovic A, Simpson A. Diagnosis of asthma in symptomatic children based on measures of lung function: an analysis of data from a population-based birth cohort study. Lancet Child Adolesc Health 2017; 1:114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzpatrick AM, Gaston BM, Erzurum SC, Teague WG, National Institutes of Health/National Heart L, Blood Institute Severe Asthma Research P. Features of severe asthma in school-age children: Atopy and increased exhaled nitric oxide. J Allergy Clin Immunol 2006; 118:1218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H, et al. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol 2011; 127:382–9 e1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teague WG, Phillips BR, Fahy JV, Wenzel SE, Fitzpatrick AM, Moore WC, et al. Baseline Features of the Severe Asthma Research Program (SARP III) Cohort: Differences with Age. J Allergy Clin Immunol Pract 2018; 6:545–54 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming L, Murray C, Bansal AT, Hashimoto S, Bisgaard H, Bush A, et al. The burden of severe asthma in childhood and adolescence: results from the paediatric U-BIOPRED cohorts. Eur Respir J 2015; 46:1322–33. [DOI] [PubMed] [Google Scholar]
- 9.Duijts L, Granell R, Sterne JA, Henderson AJ. Childhood wheezing phenotypes influence asthma, lung function and exhaled nitric oxide fraction in adolescence. Eur Respir J 2016; 47:510–9. [DOI] [PubMed] [Google Scholar]
- 10.Bacharier LB, Beigelman A, Calatroni A, Jackson DJ, Gergen PJ, O’Connor GT, et al. Longitudinal Phenotypes of Respiratory Health in a High-Risk Urban Birth Cohort. Am J Respir Crit Care Med 2019; 199:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granell R, Henderson AJ, Sterne JA. Associations of wheezing phenotypes with late asthma outcomes in the Avon Longitudinal Study of Parents and Children: A population-based birth cohort. J Allergy Clin Immunol 2016; 138:1060–70 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tantisira KG, Fuhlbrigge AL, Tonascia J, Van Natta M, Zeiger RS, Strunk RC, et al. Bronchodilation and bronchoconstriction: predictors of future lung function in childhood asthma. J Allergy Clin Immunol 2006; 117:1264–71. [DOI] [PubMed] [Google Scholar]
- 13.Sharma S, Litonjua AA, Tantisira KG, Fuhlbrigge AL, Szefler SJ, Strunk RC, et al. Clinical predictors and outcomes of consistent bronchodilator response in the childhood asthma management program. J Allergy Clin Immunol 2008; 122:921–8 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGeachie MJ, Yates KP, Zhou X, Guo F, Sternberg AL, Van Natta ML, et al. Patterns of Growth and Decline in Lung Function in Persistent Childhood Asthma. N Engl J Med 2016; 374:1842–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pongracic JA, Krouse RZ, Babineau DC, Zoratti EM, Cohen RT, Wood RA, et al. Distinguishing characteristics of difficult-to-control asthma in inner-city children and adolescents. J Allergy Clin Immunol 2016; 138:1030–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wildfire JJ, Gergen PJ, Sorkness CA, Mitchell HE, Calatroni A, Kattan M, et al. Development and validation of the Composite Asthma Severity Index--an outcome measure for use in children and adolescents. J Allergy Clin Immunol 2012; 129:694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juniper EF, Guyatt GH, Ferrie PJ, Griffith LE. Measuring quality of life in asthma. Am Rev Respir Dis 1993; 147:832–8. [DOI] [PubMed] [Google Scholar]
- 18.Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in children with asthma. Qual Life Res 1996; 5:35–46. [DOI] [PubMed] [Google Scholar]
- 19.Unites States Census Bureau. 2010–2014 American Community Survey 5-Year Estimates. Available from: http://www.factfinder.census.gov. Last accessed January 26.
- 20.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J 2005; 26:948–68. [DOI] [PubMed] [Google Scholar]
- 21.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40:1324–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuhlbrigge A, Peden D, Apter AJ, Boushey HA, Camargo CA Jr., Gern J, et al. Asthma outcomes: exacerbations. J Allergy Clin Immunol 2012; 129:S34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fitzpatrick AM, Moore WC. Severe Asthma Phenotypes - How Should They Guide Evaluation and Treatment? J Allergy Clin Immunol Pract 2017; 5:901–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deliu M, Yavuz TS, Sperrin M, Belgrave D, Sahiner UM, Sackesen C, et al. Features of asthma which provide meaningful insights for understanding the disease heterogeneity. Clin Exp Allergy 2018; 48:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schatz M, Hsu JW, Zeiger RS, Chen W, Dorenbaum A, Chipps BE, et al. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. J Allergy Clin Immunol 2014; 133:1549–56. [DOI] [PubMed] [Google Scholar]
- 26.Howrylak JA, Fuhlbrigge AL, Strunk RC, Zeiger RS, Weiss ST, Raby BA, et al. Classification of childhood asthma phenotypes and long-term clinical responses to inhaled anti-inflammatory medications. J Allergy Clin Immunol 2014; 133:1289–300, 300 e1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quanjer PH, Weiner DJ. Interpretative consequences of adopting the Global Lungs 2012 reference equations for spirometry for children and adolescents. Pediatr Pulmonol 2014; 49:118–25. [DOI] [PubMed] [Google Scholar]
- 28.Mahut B, Bokov P, Delclaux C. Abnormalities of plethysmographic lung volumes in asthmatic children. Respir Med 2010; 104:966–71. [DOI] [PubMed] [Google Scholar]
- 29.Bacharier LB, Strunk RC, Mauger D, White D, Lemanske RF Jr., Sorkness CA. Classifying asthma severity in children: mismatch between symptoms, medication use, and lung function. Am J Respir Crit Care Med 2004; 170:426–32. [DOI] [PubMed] [Google Scholar]
- 30.Sorkness RL, Teague WG, Penugonda M, Fitzpatrick AM, National Institutes of Health NHL, Blood Institute’s Severe Asthma Research P. Sex dependence of airflow limitation and air trapping in children with severe asthma. J Allergy Clin Immunol 2011; 127:1073–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorkness RL, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Chung KF, et al. Lung function in adults with stable but severe asthma: air trapping and incomplete reversal of obstruction with bronchodilation. J Appl Physiol (1985) 2008; 104:394–403. [DOI] [PubMed] [Google Scholar]
- 32.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43:343–73. [DOI] [PubMed] [Google Scholar]
- 33.Puranik S, Forno E, Bush A, Celedon JC. Predicting Severe Asthma Exacerbations in Children. Am J Respir Crit Care Med 2017; 195:854–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forno E, Celedon JC. Predicting asthma exacerbations in children. Curr Opin Pulm Med 2012; 18:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu AC, Tantisira K, Li L, Schuemann B, Weiss ST, Fuhlbrigge AL, et al. Predictors of symptoms are different from predictors of severe exacerbations from asthma in children. Chest 2011; 140:100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Covar RA, Szefler SJ, Zeiger RS, Sorkness CA, Moss M, Mauger DT, et al. Factors associated with asthma exacerbations during a long-term clinical trial of controller medications in children. J Allergy Clin Immunol 2008; 122:741–7 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haselkorn T, Zeiger RS, Chipps BE, Mink DR, Szefler SJ, Simons FE, et al. Recent asthma exacerbations predict future exacerbations in children with severe or difficult-to-treat asthma. J Allergy Clin Immunol 2009; 124:921–7. [DOI] [PubMed] [Google Scholar]
- 38.Tse SM, Samson C. Time to Asthma-Related Readmission in Children Admitted to the ICU for Asthma. Pediatr Crit Care Med 2017; 18:1099–105. [DOI] [PubMed] [Google Scholar]
- 39.Denlinger LC, Phillips BR, Ramratnam S, Ross K, Bhakta NR, Cardet JC, et al. Inflammatory and Comorbid Features of Patients with Severe Asthma and Frequent Exacerbations. Am J Respir Crit Care Med 2017; 195:302–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sorkness RL, Zoratti EM, Kattan M, Gergen PJ, Evans MD, Visness CM, et al. Obstruction phenotype as a predictor of asthma severity and instability in children. J Allergy Clin Immunol 2018; 142:1090–9 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park HW, Song WJ, Chang YS, Cho SH, Datta S, Weiss ST, et al. Bronchodilator response following methacholine-induced bronchoconstriction predicts acute asthma exacerbations. Eur Respir J 2016; 48:104–14. [DOI] [PubMed] [Google Scholar]
- 42.Colon-Semidey AJ, Marshik P, Crowley M, Katz R, Kelly HW. Correlation between reversibility of airway obstruction and exhaled nitric oxide levels in children with stable bronchial asthma. Pediatr Pulmonol 2000; 30:385–92. [DOI] [PubMed] [Google Scholar]
- 43.Silvestri M, Sabatini F, Sale R, Defilippi AC, Fregonese L, Battistini E, et al. Correlations between exhaled nitric oxide levels, blood eosinophilia, and airway obstruction reversibility in childhood asthma are detectable only in atopic individuals. Pediatr Pulmonol 2003; 35:358–63. [DOI] [PubMed] [Google Scholar]
- 44.Zoratti EM, Krouse RZ, Babineau DC, Pongracic JA, O’Connor GT, Wood RA, et al. Asthma phenotypes in inner-city children. J Allergy Clin Immunol 2016; 138:1016–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fitzpatrick AM, Jackson DJ, Mauger DT, Boehmer SJ, Phipatanakul W, Sheehan WJ, et al. Individualized therapy for persistent asthma in young children. J Allergy Clin Immunol 2016; 138:1608–18 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldstein AB, Castile RG, Davis SD, Filbrun DA, Flucke RL, McCoy KS, et al. Bronchodilator responsiveness in normal infants and young children. Am J Respir Crit Care Med 2001; 164:447–54. [DOI] [PubMed] [Google Scholar]
- 47.Comhair SA, Gaston BM, Ricci KS, Hammel J, Dweik RA, Teague WG, et al. Detrimental effects of environmental tobacco smoke in relation to asthma severity. PLoS One 2011; 6:e18574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGarry ME, Castellanos E, Thakur N, Oh SS, Eng C, Davis A, et al. Obesity and bronchodilator response in black and Hispanic children and adolescents with asthma. Chest 2015; 147:1591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koziol-White CJ, Panettieri RA Jr.. Modulation of Bronchomotor Tone Pathways in Airway Smooth Muscle Function and Bronchomotor Tone in Asthma. Clin Chest Med 2019; 40:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kleerup EC, Tashkin DP, Cline AC, Ekholm BP. Cumulative dose-response study of non-CFC propellant HFA 134a salbutamol sulfate metered-dose inhaler in patients with asthma. Chest 1996; 109:702–7. [DOI] [PubMed] [Google Scholar]
- 51.Ramsdell JW, Colice GL, Ekholm BP, Klinger NM. Cumulative dose response study comparing HFA-134a albuterol sulfate and conventional CFC albuterol in patients with asthma. Ann Allergy Asthma Immunol 1998; 81:593–9. [DOI] [PubMed] [Google Scholar]
- 52.Turner DJ, Landau LI, LeSouef PN. The effect of age on bronchodilator responsiveness. Pediatr Pulmonol 1993; 15:98–104. [DOI] [PubMed] [Google Scholar]
- 53.Kemp JP, Furukawa CT, Bronsky EA, Grossman J, Lemanske RF, Mansfield LE, et al. Albuterol treatment for children with asthma: a comparison of inhaled powder and aerosol. J Allergy Clin Immunol 1989; 83:697–702. [DOI] [PubMed] [Google Scholar]
- 54.Weinstein S, Chervinsky P, Pollard SJ, Bronsky EA, Nathan RA, Prenner B, et al. A one-week dose-ranging study of inhaled salmeterol in children with asthma. J Asthma 1997; 34:43–52. [DOI] [PubMed] [Google Scholar]
- 55.de Benedictis FM, Tuteri G, Pazzelli P, Niccoli A, Mezzetti D, Vaccaro R. Salmeterol in exercise-induced bronchoconstriction in asthmatic children: comparison of two doses. Eur Respir J 1996; 9:2099–103. [DOI] [PubMed] [Google Scholar]
- 56.Lenney W, Pedersen S, Boner AL, Ebbutt A, Jenkins MM. Efficacy and safety of salmeterol in childhood asthma. Eur J Pediatr 1995; 154:983–90. [DOI] [PubMed] [Google Scholar]
- 57.Ouksel H, Meslier N, Badatcheff-Coat A, Racineux JL. Influence of predicted FEV1 on bronchodilator response in asthmatic patients. Respiration 2003; 70:54–9. [DOI] [PubMed] [Google Scholar]
- 58.Quanjer PH, Ruppel GL, Langhammer A, Krishna A, Mertens F, Johannessen A, et al. Bronchodilator Response in FVC Is Larger and More Relevant Than in FEV1 in Severe Airflow Obstruction. Chest 2017; 151:1088–98. [DOI] [PubMed] [Google Scholar]
- 59.Yancey SW, Ortega HG. Retrospective characterization of airway reversibility in patients with asthma responsive to bronchodilators. Curr Med Res Opin 2007; 23:3205–7. [DOI] [PubMed] [Google Scholar]
- 60.Silkoff PE, Laviolette M, Singh D, FitzGerald JM, Kelsen S, Backer V, et al. Longitudinal stability of asthma characteristics and biomarkers from the Airways Disease Endotyping for Personalized Therapeutics (ADEPT) study. Respir Res 2016; 17:43. [DOI] [PMC free article] [PubMed] [Google Scholar]