Abstract
Background:
Existing data on altered membrane phospholipid (MPL) metabolism in schizophrenia are diverse. We conducted a meta-analysis of phosphorus magnetic spectroscopy (31P MRS) studies -a non-invasive imaging approach that can assess molecular biochemistry of cortex by measuring phosphomonoester and phosphodiester levels in schizophrenia that can provide evidence of altered biochemical processes involved in neuropil membrane expansion/contraction.
Methods:
We analyzed phosphomonoester and phosphodiester data on the frontal and temporal lobes in schizophrenia from 24 peer-reviewed publications using the MAVIS package in R by building random and fixed effects models. Heterogeneity of effect sizes, effects of publication bias and file drawer analysis were also assessed.
Results:
Schizophrenia subjects showed lower phosphomonoester levels in the frontal (p=0.008) and elevated phosphodiester levels in the temporal (p<0.001) regions with significant heterogeneity. We noted significant publication bias and file drawer effect for the frontal PME and PDE, and temporal PDE but not for the temporal PME levels. Fail-safe analysis estimated that a high number of negative studies were required to provide non-significant results.
Conclusions:
Despite methodological differences, these 31P MRS studies demonstrate regionally specific imbalance in MPL metabolism related to neuropil in schizophrenia compared to controls reflecting neuropil contraction. Specifically, decreased phosphomonoester levels in the frontal and elevated phosphodiester levels in the temporal regions provide evidence of decreased synthesis and increased degradation of neuropil membrane, respectively. Notwithstanding significant heterogeneity and publication bias, a large number of negative studies are required to render the results of this meta-analysis non-significant. These findings warrant further postmortem and animal studies.
Keywords: Magnetic resonance spectroscopy, Phosphorus magnetic resonance spectroscopy, schizophrenia, neurodevelopment, membrane phospholipids, phosphomonoester, phosphodiester, neuropil, synaptic pruning
Introduction
Extant human, postmortem and animal data supports association of altered prenatal and/or postnatal brain development as a core basis of schizophrenia(1). Cumulative evidence suggests neurodevelopmental aberrations of the neuropil evidenced by decreased formation or increased pruning of synapses, altered dendritic architecture, dendritic spine loss, decreased soma volume(2), increased neuronal packing density(3, 4), and alterations in interneurons(5). Gray matter metrics of structural magnetic resonance imaging-based approaches represent composite physical measures of axons, dendrites, synapses, glia, neuronal soma, interneurons, interneuronal space and microvasculature, and hence, considered relatively coarse(6).
Phosphorus magnetic resonance spectroscopy (31P MRS) is a non-invasive neuroimaging approach that reliably assesses differences in cellular membrane expansion/contraction by quantifying precursors (phosphomonoesters, PMEs; phosphocholine, PC, and phosphoethanolamine, PE) and catabolites (phosphodiesters, PDEs; glycerophosphocholine, GPC, and glycerophosphoethanolamine, GPE) of membrane phospholipids (MPL; phosphatidylcholine, phosphatidylethanolamine and phosphatidylserine)(7). MPLs naturally form lipid bilayers that physically separate the intracellular and extracellular environments. MPLs are not “visible” to 31P MRS on typical human MR scanners, hence, cannot be directly measured(8). However, PMEs and PDEs that are smaller and more mobile (i.e. not part of the less mobile MPLs) can be quantified based on their degree of mobility by time domain processing of the free induction decay(9). Continual brain remodeling during development and disease involves membrane expansion/contraction***. Membrane expansion associated with synapse formation, dendritic arborization, increased neuronal soma size, increased glia and myelin requires active MPL synthesis. Neuromaturation involves synapse elimination, decreased dendritic arborization, neuronal soma shrinking, neuronal or glial loss requires increased MPL breakdown. During such MPL turnover, periods of greater imbalance of MPL precursors over catabolites with membrane expansion, and periods of greater imbalance of MPL catabolites over MPL precursors with membrane contraction are reported(10, 11). Thus, three major patterns are important: membrane expansion observed as higher PME with lower PDE or higher PME with no PDE change; membrane contraction represented by lower PME with either no change or increased PDE; and neuropil elimination by higher PDE with no change in PME.
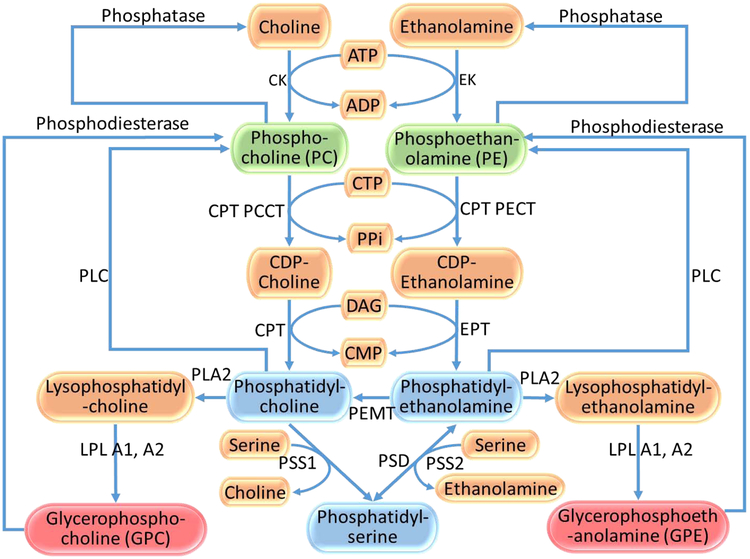
Animal lesion(10), human postmortem(12) and cellular(13) studies suggest that membrane expansion/contraction were primarily observed during increased/decreased axonal branching, neurite sprouting/retraction and synapse formation/elimination. Significantly greater membrane expansion occurs along dendrites, axons and synapses compared to soma, glia and myelin(14, 15). Although neuronal proliferation contributes to elevated PME levels, active postnatal neuronal proliferation is not reported in humans. Elevated MPL precursor and phosphatidylcholine levels was observed at the time and site of neurite sprouting in the hippocampus following unilateral entorhinal cortex lesion in rats(10). Elevated PDE levels were observed at the site and time of the entorhinal lesion in the same study. In vitro rat brain 31P MRS study revealed that during early neurodevelopment, elevated MPL precursor levels and reduced MPL catabolite levels reflects active MPL synthesis for membrane expansion required for increased dendrite and synapse formation(11). This was followed by decreased MPL precursor and increased MPL catabolite levels coinciding with maturation (i.e., pruning/neuropil elimination)(11, 16). These convergent data support that MPL metabolite levels may be sensitive to changes in neuropil density, myelin and other cellular components. Since well-defined biochemical steps underlie synthesis and degradation of MPLs (Fig 1), assessing MPL metabolites can provide important clues to biochemical pathway alterations underlying membrane expansion/contraction.
Fig 1:
Selected steps in the metabolism of membrane phospholipids (Kennedy pathway). Blue boxes: Membrane phospholipids (MPLs); Green boxes: MPL precursors (Phosphomonoesters, PME); Pink boxes: MPL breakdown products (Phosphodiesters, PDE)
The application of in vivo 31P MRS as a promising strategy started in the early 90’s with a seminal 31P MRS study(17) that provided the first direct in vivo evidence of exaggerated neuropil pruning (or contraction) in the prefrontal cortex (PFC) of first-episode neuroleptic-naïve schizophrenia patients reflected by decreased PMEs and increased PDEs, which was later supported by postmortem neuropil morphology data(18, 19). Several studies since then reported inconsistent results on metabolite levels and involved brain regions (reviewed in(20)) that may be due to heterogeneity in illness, progression, regional pathology, medication exposure, comorbidity, acquisition methodology, spectral quantification and localization scheme of the 31P MRS(9). Recently, association of MPL metabolite levels with peripheral blood inflammatory mediator levels(21) and genetic variations(22) are reported. There is renewed interest in examining neuropil after a persuasive study that reported association of Complement 4 (C4) gene copy numbers with schizophrenia and association of decreasing number of C4A gene repeats with decreased synaptic pruning(23). We conducted a meta-analysis of published data on PME and PDE levels among schizophrenia patients and healthy controls, and examined the effect sizes of case-control PME and PDE differences in the frontal and temporal lobes. We selected these two regions because more 31P MRS studies investigated these two regions, and prior postmortem and animal studies, functional imaging studies, electrophysiological approaches reported abnormalities in these regions in schizophrenia.
Methods
Data Sources:
In vivo human 31P MRS studies examining PME and PDE levels among schizophrenia patients and healthy controls were obtained through PubMed, PsychInfo and Web of Science search, using keywords, schizophrenia, psychosis, phosphorus magnetic spectroscopy, phosphodiester, phosphomonoester, phosphoethanolamine, phosphocholine, glycerophosphoethanolamine and glycerophosphocholine using different Boolean combinations (Supplemental Table 1). Initial broad search yielded 2047 studies. Many of these studies were not in vivo investigations and, thus, not relevant to this meta-analysis. Restricting the search to “(schizophrenia) AND (phosphorus magnetic resonance spectroscopy)” generated 87 studies. The advanced search structure was altered to generate a more reliable output, e.g. “(schizophrenia) AND (phosphorus magnetic resonance spectroscopy)) AND phosphodiester) OR phosphomonoester) OR glycerophosphoethanolamine) AND glycerophosphocholine)” of 89 studies. The search was conducted in October-November, 2017. A trained researcher (KMP) determined the eligibility of each publication for inclusion in the meta-analysis. Another author (CSH) read the full texts of all studies selected in the first step and excluded the studies that did not meet study criteria given below. After the list was finalized, full text manuscripts were reviewed again by KMP. We excluded 5 manuscripts that were not full publications, 5 studies that were not in vivo studies and 2 studies that did not include control subjects. Using the cited bibliography in these manuscripts, we manually searched other published studies not included in the above search but found no additional studies that met the criteria.
Study Selection:
We included manuscripts that reported: (1) in vivo 31P MRS studies on frontal and/or temporal region(s) on schizophrenia patients and healthy controls, (2) mean and standard deviations of PME and PDE levels, and (3) relevant clinical and demographic data. The studies were, then, organized by the brain regions examined. PME levels were reported by 16 studies for the frontal lobe(8, 17, 21, 24-36) and 8 for the temporal lobe(21, 25, 37-42). Seventeen studies reported frontal lobe PDE(8, 17, 21, 24-36, 43), and 9 studies reported temporal lobe PDE(21, 25, 37-42, 44). A study that tested a different hypothesis on most subjects already reported in a previous report(45) was not included in the meta-analysis (Supplemental figure; Table 1).
Data Analysis:
Data were analyzed using the MAVIS: Meta-Analysis Via Shiny v1.0.4 written on R 3.2.0 for both fixed effects (FE) and random effects (RE) models for each region separately. A forest and a funnel plot were generated for each region and metabolite along with significance levels and effect sizes for each model.
Heterogeneity was tested to investigate whether the results were influenced by unique study variables using the Cochran’s heterogeneity (Q) statistic. The Q statistic was calculated as the weighted sum of squared differences between individual study effects and the pooled effect sizes across studies. Next, we combined the effect sizes using Hedges’s g. We calculated the percentage of total variation in effect sizes due to heterogeneity rather than by chance (I2= 100%*((Q-df/Q))) to determine whether sensitivity analyses were needed. Sensitivity analyses for subgroup or moderators were conducted where the I2 was high. An absolute measure of heterogeneity of effect sizes across the studies that measures the dispersion of true effect sizes among the studies was also calculated (τ2 score).
To test for publication bias, a regression test for the funnel plot asymmetry was conducted. A file drawer analysis was conducted to calculate the fail-safe N using the Rosenthal’s approach that provides an estimate of the number of non-significant studies required to yield non-significant results on the meta-analysis: the larger the fail-safe N, the more robust the findings of the meta-analysis.
Sensitivity and Subgroup Analyses
We conducted sensitivity analysis by repeating the primary analysis to test the influence of publications with large effects by removing such publications. Similarly, we examined hemispheric differences, field strength of the magnet and certain clinical characteristics to assess their influence on the results.
Results
Study characteristics
The magnet strength varied between 1.5 and 4.0 Tesla: 23 studies used 1.5 T, 13 used 2 T, 1 used 3 T, and 4 used 4 T magnets. Four studies used surface coils and others used birdcage head coil; one study used proton (1H)-decoupled 31P MRS data. We noted decreasing voxel sizes with improving resolution over the years (Table 1). Sample sizes of studies varied between 10-60 for patients and 10-38 for controls. Studies with medication-naïve patients, those on medications and chronic patients were selected for the analysis. There was variability in reporting on the duration of illness, dose and duration of each antipsychotic administered, and the clinical status of patients at MRS data acquisition, all of which can affect the results.
Table 1:
STROBE Checklist of studies included in the meta-analysis
| Study | Region(s) examined | Objective and descriptive information |
Study Design | Statistical methods |
a) Summary of key results b) Limitations, sources of potential bias |
Source of Funding |
|---|---|---|---|---|---|---|
| FRONTAL LOBE | ||||||
| Pettegrew et al, Arch Gen Psychiatry, 48(6):563, 1991 (17) | Dorsal prefrontal cortex | To examine exaggerated synaptic pruning in the dorsal prefrontal cortex in SZ | Data collected on a 1.5 T Signa system with the spectroscopy research accessory 11 neuroleptic-naïve SZ patients (7 males and 4 females; mean age, 24.4 ±1.8 years) and 10 healthy controls (6 males and 4 females; mean age, 24.1±1.8 years). Duration of illness = 19.1 ±5.9 months |
Pooled variance t-test. Shapiro-Wilks test | a) SZ patients showed lower PME levels and higher PDE and β-ATP levels compared to controls. b) The findings were interpreted to be due to abnormal brain development c) First study to show MPL metabolite differences in first episode SZ |
NIMH grants AG05657 and AG08371 |
| Williamson et al, Arch Gen Psychiatry, 48(6):578, 1991 (24) | Dorsal PFC | Preliminary results of localized 31P MRS in chronic SZ patients and healthy controls | Data acquired on a 2 Tesla Siemens whole body imager using 5-cm transmit/receive surface dual tuned coil. Spectra were exponentially filtered, 0-fitted to 4096 data points, baseline corrected, and fitted with Lorentzian line shapes 10 SZ patients from outpatient (9M/1F; age: 33- 54 years). Mean CPZ equivalent dose=660±436 mg. Length of illness=15.9±5.2 years. 8 patients were on anticholinergic drug and two on a benzodiazepine. 7 controls matched for age and sex also examined. |
ANOVA | a) Lower PME levels in SZ as a U"trait" marker. No differences in PDEs. b) Lower PME may be observed early in the illness b) Small sample size; medicated patients |
Not Reported |
| Fujimoto et al, Acta Psychiatr Scand, 86(6): 455, 1992 (25)* | 10 regions that mainly consisted of frontal, temporal and parietal regions | To examine 31P MRS metabolites in chronic SZ | 16 medicated chronic male SZ patients (age=39±5 years, Mean illness duration=12 yrs), 20 controls (age=34±2 years, 15M/5F). Age of onset=22±4 years (range 18-30 years) Siemens-Asahi scanner at 2.0 Tesla. CSI with a voxel size of 36 cc |
Mann-Whitney U test | a) Elevated PDE levels on the left temporal lobe b) ATP and PCr levels were decreased |
None reported |
| Stanley et al, Schiz Res, 13(3):209, 1994 (8) | Left dorsal prefrontal cortex | Hypothesized that the increase in breakdown of membrane phospholipid metabolites will only be present early in the illness. | 5 cm diameter transmit/receive surface coil switchable between 1H and 31P frequencies on a 2 Tesla whole-body MRI using short pulse sequence repetition time 19 medicated SZ patients (age: 16-54 years; 16 were on anticholinergic medication for side effects) and 18 male controls (age: 18-53 years). The length of illness =1 to 24 years. |
ANOVA | a) Prefrontal PME levels in SZ lower than in controls, which was independent of the length of illness and medication status. PDE levels were increased in the newly diagnosed SZ b. Area of concern is the partial volume effect. CSF contamination could be different between the two groups. |
The Ontario Mental Health Foundation, London Upjohn Neurosciences Program, and Department of Psychiatry Research Trust |
| Deicken et al, Biol Psychiatry, 36(8):503, 1994 (35) | Frontal and parietal lobes | To examine MPL metabolite and high energy phosphate levels in the frontal and parietal lobes of SZ patients |
31P MRSI over single volume MRS technique used in previous studies. 20 male SZ patients and 16 male controls (mean age = 40.3 ± 11.0 years). 6 patients were neuroleptic free. 7 patients on neuroleptics only. 3 on neuroleptic + diphenhydramine and 4 on neuroleptic + benztropine. BPRS, inter correlation coefficient was 0.91 |
Multivariate repeated measures analysis of variance and regression analysis | a) PDE and PCr levels were elevated in SZ patients in bilateral frontal lobes but not parietal lobes compared to controls. b) Higher PDE and PCr levels correlated with hostility-suspiciousness and anxiety-depression subscales of BPRS |
NARSAD YI Award; BRSG grant S07-RR05755 NIH; VA Career Scientist award; UCSF Individual Investigator award; Philips Medical Systems |
| Shioiri et al., Psychiatry Res, 55(4): 223, 1994 (26) | Frontal lobe regions | To examine frontal lobe regions for PME and PDE differences in SZ and controls, and to examine association of neuroleptics with metabolite levels | Data acquired on a 1.5-T SIGNA MR system using a surface coil and depth resolved surface coil spectroscopy (DRESS). VOI was 30 mm slice between the frontal pole and tip of the corpus callosum. 26 SZ inpatients (16 M/10 F; age=31.8±12.4 years; range: 15-63 years; duration of illness 8.7 ± 9.5 years). 6 SZ patients were neuroleptic free for ≥2 weeks. Mean CPZ equivalent dose: 747 ± 622 mg/day (range: 200 mg – 2150 mg). 10 patients were on benzodiazepines. 26 controls (16 M/10 F) matched with patients for age and sex (mean age=31.9 ± 12.4 years) participated in the study. | ANOVA, Pearson correlation coefficient, regression analysis and Studen’s t test | a) PME and PDE levels did not show differences between SZ and controls. b) Lower PME levels seen in high negative symptom-SZ patients compared to low negative symptom-SZ. Elevated β-ATP levels in high negative symptom SZ patients compared to low negative symptom SZ. Lower PDE levels in SZ with low negative symptoms compared to controls. c) PME levels and CPZ-equivalent antipsychotic dose were not correlated |
Grant-in-Aid from the Japanese Ministry of Education. |
| Deicken et al, Schiz Res 14(2): 177:1995 (43) | Frontal lobe | Examined whether decreased PME and elevated PDE levels in the frontal lobe would be associated with impaired performance on the WCST | Philips Gyroscan at 2 Tesla with multi-slice images. PME and PDE levels calculated for the left and right frontal lobe voxels (25 cc each) on 16 SZ patients and 13 male controls who were part of a larger cohort of 20 SZ and 16 controls. Duration of illness = 14.2 ±7.5 (range 1-27 years). 5 neuroleptic-free for ≥7 days; 9 patients were on neuroleptics only; and 2 patients were on neuroleptic + benztropine mesylate. Within 48 h of the MRSI study, all subjects were administered the WCST | ANOVA and Pearson correlation | a) Both left and right frontal PDE levels were elevated in SZ than in controls b) Left frontal PME levels positively correlated with number of categories completed and concept formation, and negatively correlated with total but not perseverative errors |
NARSAD YI Award; BRSG Grant (Divn of Research Resources, UCSF Academic Senate Individual Investigator Grant; VA Research Associate Career Development Award |
| Kato et al, Psychiatry Res, 61(3): 151, 1995 (27) | Left and right frontal lobes | Examined both the left and right frontal lobes simultaneously by 31P MRS in SZ patients using a onedimensional chemical shift imaging technique to evaluate for left-right differences | MRS data acquired on a Signa 1.5-Tesla MR system. 27 inpatients (mean age=30.1±10.4 years; 15 F/12 M; duration of illness = 6.8± 7.9 years). 10 patients were off neuroleptics for ≥1 week and never given depot neuroleptics. 15 on butyrophenones and remaining 2 required additional phenothiazines for agitation before MRS study. 26 healthy subjects (mean age = 34.2 ±12.9 years; 15 F/ 11 M) | Paired t-test for left-right, and t test for case-control differences. Spearman’s correlation test | a) Reduced PME levels in the frontal lobes of SZ patients. b) Metabolite ratio measures were used |
Grant 5B-2-13 for Nervous and Mental Disorders from the Japanese Ministry of Health and Welfare. |
| Volz et al, Psychiatry Res: Neuroimaging, 76(2-3): 123, 1997 (28) | Dorsolateral Prefrontal Cortex | Examined changes in PDE and PCr levels linked to neuroleptic treatment and whether the reduced PDE levels are observed in neuroleptic-free patients | Image selected in vivo spectroscopy (ISIS)-sequence with double volume selection. Compared 50 medicated SZ patients (age=37.64±11.18 years; 31M/19F) with 10 SZ patients off neuroleptics for 3 days to 10 years (2 neuroleptic-naïve; age=33.60±13.29 years; 6M/4/F) and36 age-matched controls. | Kruskal Wallace H-test and Spearman correlation test | a) Decreased PDE levels in neuroleptic-free patients compared to controls. b) Resonance offset effects might have resulted in measuring signals from slightly different but overlapping VOIs |
BMBF |
| Hinsberger et al, Journal of psychiatry & neuroscience: JPN 22(2):111-117, 1997 (29) | Prefrontal lobe | Examined the relationship between 31P MRS data and left prefrontal gray and white matter volumes in SZ patients and controls predicting that previous findings of decreased PME levels in SZ patients compared to healthy subjects after controlling for gray and white volumes | Whole-body magnetic resonance imager at 2.0 T. The surface coil placed over the left dorsolateral prefrontal cortex. 10 patients and 10 controls were studied. 4 subjects were in their 1st episode and had not received antipsychotics prior to the study. One had received 1 mg of lorazepam in the 24 h before the study. The other SZ subjects received an average chlorpromazine-equivalent dose of 917±499 mg/d. 4 patients were taking an anticholinergic medication regularly. Mean duration of illness from the onset of positive symptoms was 12.2 ±8.6 years. | ANOVA | a) PME levels not correlated with left prefrontal grey matter volumes in either group or groups combines. b) Reduced PME levels might precede grey matter losses since patients showed reduced PME but not yet any volume loss. |
Canadian Psychiatric Research Foundation |
| Volz et al, Biol Psychiatry 44(6): 399-404, 1998 (30) | Dorsolateral prefrontal cortex | Examined MPL metabolites in a larger sample of SZ patients and controls since initial results were observed in smaller samples in the dorsolateral prefrontal region of SZ patients | Data acquired on a Philips Gyroscan ACS II system at 1.5 T. Each spectrum measured simultaneously in both VOIs was averaged over 768 measurements. The VOIs, located in both frontal brain areas, mainly in the DLPFC, had a mean volume of 28 × 28 × 50 mm3. 47 of the 50 patients were on stable neuroleptic medication of 10–15 mg haloperidol or equivalent. In this sample, the 13 SZ patients and 14 controls whose results were already reported as preliminary findings were also included. | Mann–Whitney U test with Bonferroni correction. Spearman correlation test and t test | a) Lower PDE and higher PME/PDE. b) PDE peak composed of glycerol-3-PC, glycerol-3-PE, and mobile phospholipids c) Spectra strongly T1-weighted; T1-weighted spin lattice relaxation times of PDE at 1.2–3.6 sec suggest that altered chemical/physical environment leading to T1 changes may have influenced measured peaks |
BMBF |
| Volz et al, Biol Psychiatry, 41(6):644, 1997 (36) | Dorsolateral prefrontal region | Pilot study, a volume selective 31P MRS technique was used allowing localization of the voxel in the prefrontal cortex | Image selected in vivo spectroscopy (ISIS)-sequence with double volume selection. Each spectrum was averaged over 768 measurements. 13 SZ inpatients and 14 age-matched controls were investigated. 10 SZ were on a neuroleptic, 3 of them on clozapine, and 3 received lithium | Mann-Whitney U-test | a) Decreased PDE levels in SZ compared to controls. PME/PDE ratio increased. b) No correlation between neuroleptic dose, duration of illness, BPRS, CGI, SANS or SAPS and PME and PDE levels |
BMBF |
| Potwarka et al, Biol Psychiatry 45(6): 687-693, 1999 (31) | Prefrontal, motor, and parieto-occipital regions. | Examined in vivo individual components of PME and PDE from prefrontal, motor and parieto-occipital cortex on both sides using 1H-decoupled 31P MRS 2-D chemical shift imaging to resolve elevated PDE in SZ to broad MPL peak. | Measurements performed on a Siemens Helicon SP4000 whole body imager at 1.5 T. Data acquired using a 2-dimensional chemical shift imaging sequence from a voxel of 50 cm3. This study examined 11 chronic medicated SZ patients on 461 ± 232 mg chlorpromazine equivalent and 11 healthy controls. 7 had taken an anticholinergic and 2 a benzodiazepine in the 24 hours before imaging. The average length of illness from the onset of positive symptoms was 20.6 ± 7.6 years. | 2-tailed t test, 2-factor MANCOVA, ANCOVA for the individual metabolite parameters. Pearson correlations | a) ↓ PC, and ↑ PDE and mobile phospholipid in SZ compared to controls in bilateral prefrontal cortex. b) No significant correlation with CPZ-equivalent medication dose. c) Metabolites were reported as a ratio to total phosphate signal. d) Not fully relaxed signal might have introduced partial saturation effects depending on T1 times; thus, measured metabolite level changes may be from T1 changes |
EJLB Foundation, The Medical Research Council of Canada (grant MT-12078), and NIMH |
| Riehemann et al., NMR Biomed 12(8):483-489. 1999 (32) | Frontal lobe | Investigated the influence of gender on the concentrations of MPL metabolites | 32 controls and 51 SZ inpatients (19 on stable meds) (50 men [19 controls, age=35.4± 12.5 years; 31 inpatients, age=37.5±12.4 years] and 33 women [13 controls, age=34.0±11.7 years; 20 inpatients, age=36.4±10.3 years]). ISIS on a 1.5 T scanner. 1024 data points from voxel of 28×28×50 mm3 | Mann–Whitney U-test with Bonferroni correction | a) The main results were increased Pi levels and decreased PCr levels in the brain of healthy females compared to healthy males b) Different spectrum analysis methodology used in studies |
German Ministry of Education, Science, Research, and Technology. BMBF |
| Shiryama et al, Eur J Neurosci 20(3): 749-756. 2004 (33) | Prefrontal cortex | Aim was to detect differences in MPL metabolite levels among medicated SZ patients | 11 male SZ outpatients (age: 28.6 ± 8.6 years) and 15 controls (28.4 ± 8.1 years). NMR measurements performed on a Philips Gyroscan S20MRI / MRS system at 2 Tesla. VOI 35 × 45 × 70 mm. Patients clinically stable for ≥1 year, receiving neuroleptics (CPZ equivalent dose, 317 ± 139 mg / day for 6.6±7.6 years) and anticholinergic medication. | Two-tailed Student’s t-tests for case-control differences | a) SZ patients showed an increased concentration of GPC but no other MPL metabolites such as GPE, PE and PC. b) Spectral quality was high with an SD of each MPL metabolite levels of < 15%. |
Ministry of Health and Welfare of Japan |
| Du et al, JAMA Psychiatry 71(1): 19-27, 2014 (34) | Frontal Lobe | To investigate cerebral bioenergetics in SZ via measurement of creatine kinase activity using in vivo 31P magnetization transfer spectroscopy. | Cross-sectional case-control study. most acquired signal comes from a 6 × 6 × 4 cc region in the frontal lobes. 26 participants with chronic SZ and 26 age-matched and sex-matched healthy controls (14 males and 12 females, 31.9± 8.9 years old) and 26 SZ patients (13 males and 13 females, 34.5 ± 8.4 years old). |
SPSS version 17; SPSS, Inc | a) A substantial (22%) and statistically significant reduction in creatine kinase kf in SZ. PCr/ATP, Pi /ATP, and PME/ATP ratios were not altered in SZ, but PDE/ATP ratio was reduced. SZ and schizoaffective disorder did not differ. b) To minimize low SNR, collected data from a large brain region. Most SZ patients were on medication. Results may be secondary to medications. |
NIH and the Shervert Frazier Research Institute at McLean Hospital |
| Prasad et al, Biological Psychiatry: Cognitive Neuroscience & Neuroimaging 1(6): 528-538, 2016 (21)* | a. Cross Sectional b. Multiple brain regions |
Examined MPL metabolite levels using whole-brain multi-voxel 3D CSI 31P MRS in early-course SZ and controls, and their relationship with IL-6 and CRP levels, performance on sustained attention, executive function and verbal memory tasks, and psychopathology scores | Data acquired on Siemens Tim Trio at 3 Tesla from 12 unique brain regions. Examined MPL metabolites and their cognitive, clinical and immunologic correlates among 28 early-course SZ patients (M:F=18/10; age=25.15±8.03 years; illness duration 1.99±1.33 years; antipsychotic-naïve=18; mean antipsychotic CPZ equivalent dose=317.53±270.20 mg) and 21 controls (M:F=5/16; age=25.24±6.25 years) | Generalized linear mixed models including bilateral VOI controlling for age, sex and GM fraction, and partial correlation with multiple test corrections | a) Higher PDE levels in thalamus and hippocampus but lower levels in the DLPFC and inferior frontal cortex in SZ. b) Higher PME levels in SZ compared to controls in the hippocampus and anterior cingulate but lower in inferior frontal and parietal lobule c) Antipsychotic dose did not correlate with PDE or PME levels. d) The left thalamus PDE correlated negatively with sustained attention. e) PDE levels positively correlated with IL-6 levels in the thalamus in SZ |
NIMH MH72995 and MH93540 |
| TEMPORAL LOBE | ||||||
| O’Callaghan et al, Biol Psychiatry, 29(11):1149, 1991 (37) | Temporo-parietal cortex | To examine the 31P MRS metabolites in SZ patients and controls | 18 medicated SZ patients (age=31 years, ll M/7 F, Illness duration=10 years), 10 controls (age=36 years, 6 M/4 F) Used surface coil on 1.5 T scanner |
Multiple regression | a) PME and PDE were not elevated b) pH was elevated in SZ compared to controls |
St John of God Order |
| Calabrese et al, Biol Psychiatry, 32(1):26, 1992 (38) | Temporal lobes | To examine PME metabolites, ATP and PCr in the temporal lobes of SZ patients | 11 SZ patients (2 neuroleptic-free, 9 medicated, age=39 years, lO M/l F), 9 male controls (age=35 years) Used ISIS, voxel size=87 cc on a 2 Tesla scanner |
MANOVA | a) SZ patients showed elevated PDE in the left temporal lobe b) β-ATP and PCr elevations inversely correlated with thought disorder severity c) PCr/β-ATP and PCr/Pi effects reflected higher ratios on the right side in SZ; percentage of β-ATP reflected higher relative concentrations in the left temporal lobe SZ |
R01DK33293, Philips Medical Systems, VA Medical Research Service, VA Psychiatrist Research Training Program, BRSG Grant S07-RR05755 |
| Fujimoto et al, Acta Psychiatr Scand, 86(6): 455, 1992 (25)* | 10 regions that mainly consisted of frontal, temporal and parietal regions | To examine 31P MRS metabolites in chronic SZ | 16 medicated chronic male SZ patients (age=39±5 years, Mean illness duration=12 yrs), 20 controls (age=34±2 years, 15M/5F). Age of onset=22±4 years (range 18-30 years) Siemens-Asahi scanner at 2.0 Tesla. CSI with a voxel size of 36 cc |
Mann-Whitney U test | a) Elevated PDE levels on the left temporal lobe b) ATP and PCr levels were decreased |
None reported |
| Fukuzako et al, Eur Arch Psychiatr Clin Neurosci, 244(5): 236, 1994 (39) | Temporal lobes; Also examined frontal and parietal lobes | Selected a relatively homogeneous group of neuroleptic-resistant SZ patients. | Data acquired on a 2.0 Tesla MR system. Voxel size=36 cc (3 ×3× 4 cm). Peak areas were automatically calculated by means of a Lorentzian curve fitting after baseline correction. SZ patients with persistent positive symptoms such as auditory hallucinations and delusions for > 2 years despite neuroleptic treatment were enrolled (n=16; 8 men and 8 women; mean age 39.6±8.1 years | Wilks-Shapiro test, MANOVA Pearson and Kendall’s rank-order correlation test | a) Observed elevated PDE in the bilateral medial temporal lobes of SZ patients compared to controls. b) No significant correlation between the PDE levels and daily dose of neuroleptics or anticholinergics. |
National Center of Psychiatry and Neurology of the Ministry of Health and Welfare; Japan Ministry of Education, Science and Culture |
| Deicken et al, Biol Psychiatry, 38(5):279, 1995 (40) | Temporal Lobes | To replicate and extend initial findings using new samples of patients and controls. Aim to test the hypothesis of metabolic asymmetry in the temporal lobes of SZ patients compared to healthy controls. | MRSI studies performed on a Philips Gyroscan system at 2 Tesla. MRSI raw data volume consisted of 1728 phase-encoded half-echo time signals, each with 256 samples and a bandwidth of 3 kHz. 18 male SZ patients (age =39.4 ± 6.0 years) and 14 male control subjects (age = 39.0 ± 5.2 years). 5 patients were neuroleptic-free; 8 on neuroleptics only; 3 were on neuroleptic plus diphenhydramine and 2 patients on a neuroleptic plus benztropine | MANOVA and Regression analysis | a) PME and PDE did not differ between SZ and controls b) PCr/Pi and PCr/ β-ATP showed significant group × side interaction c) Negative correlation between left temporal lobe PCr and the thinking disturbance on the BPRS. Degree of PCr asymmetry positively correlated with thinking disturbance on the BPRS. |
NARSAD YI Award: BRSG Grant of the Division of Research Resources, Academic Senate Individual Investigator Grant, A Research Associable Career Development Award |
| Fukuzako et al, Prog Neuropsychop harmacol & Biol Psychiatr, 20(4):629, 1996 (41) | Temporal lobes | Prior study did not investigate the relationship between MRS abnormalities and clinical characteristics. | Spectroscopy was performed on a Siemens-Asahi Meditec MR system at 2.0 tesla. Voxel size: 36 cc (3×3×4 cm3). 31 medicated patients (22 men, 9 women; mean age: 38.7±7.8 years; mean daily CPZ-equivalent dose of neuroleptics=481.8±374.6 mg) and 31 controls (22 men and 9 women; age=40.3±9.4 years) | MANOVA; Kendall’s correlation coefficient test | a) Increased PDE levels and decreased β-ATP in bilateral temporal lobe of SZ patients, especially on the left side. b) PDE levels positively correlated with BPRS positive symptom severity c) Daily neuroleptic dose did not correlate with PME or PDE. |
Ministry of Science, Culture, and Education; National Center of Psychiatry and Neurology of Ministry of Health and Welfare, Japan |
| Fukuzako et al, Neuropsychop harmacology 21(4): 542-549, 1999 (42) | Temporal Lobes | Longitudinal 31P MRS to investigate metabolite changes in the temporal lobes of SZ patients after 12-week haloperidol treatment | Data acquired on a Siemens-Asahi Meditec MR system at 2 Tesla. Voxel size 36 cc containing mainly the temporal lobes and small part of the frontal and parietal lobes. 25 first-episode, drug-naive Japanese SZ outpatients (14/11 men/women aged 16-32 years; mean 23.1) and 13 controls (15-31 years, mean 22.2 years). 16 SZ patients completed MRS scans before and after haloperidol; data on 13 patients and 13 controls reported | 2-way ANOVA; RMANOVA followed by Scheffé multiple-comparison method. | a) Significant effect of scan and side on PME and PDE in SZ but not in controls b) Significant diagnosis effect on PME and PDE c) PDE elevated on both sides in drug-naïve patients d) Haloperidol reduced the excess PDE after 12 weeks e) Large voxel size |
Ministry of Science, Culture, and Education; National Center of Psychiatry and Neurology of the Ministry of Health and Welfare of Japan |
| Fukuzako et al, Am J Psychiatry 156(8): 1205-1208, 1999 (44) | Temporal lobes | To examine 31P MRS metabolite changes in the temporal lobes of medication-naive SZ patients. | MRS data acquired on Siemens-Asahi Meditec MR system at 2.0 T. FOV=24 cm with an 8×8 data matrix and a 4-cm section thickness. 17 first-episode, drug-naive Japanese SZ patients (10/7 men/7 women; mean age=23.1 years) from outpatient clinics. Illness Duration: 6.6±6.2 months. 17 age and sex-matched controls (age=22.5 years) |
ANOVA | a) Elevated PDE levels and decreased PME levels in bilateral temporal lobes. b) Results were not corrected for multiple tests because of small n and exploratory nature of the study. c) Metabolites were modeled as single spectral peaks |
Ministry of Science, Culture & Education; National Center of Psychiatry and Neurology, Ministry of Health and Welfare, Japan |
| Prasad et al, Biological Psychiatry: Cognitive Neuroscience & Neuroimaging 1(6): 528-538, 2016 (21)* | a. Cross Sectional b. Multiple brain regions |
Examined MPL metabolite levels using whole-brain multi-voxel 3D CSI 31P MRS in early-course SZ and controls, and their relationship with IL-6 and CRP levels, performance on sustained attention, executive function and verbal memory tasks, and psychopathology scores | Data acquired on Siemens Tim Trio at 3 Tesla from 12 unique brain regions. Examined MPL metabolites and their cognitive, clinical and immunologic correlates among 28 early-course SZ patients (M:F=18/10; age=25.15±8.03 years; illness duration 1.99±1.33 years; antipsychotic-naïve=18; mean antipsychotic CPZ equivalent dose=317.53±270.20 mg) and 21 controls (M:F=5/16; age=25.24±6.25 years) | Generalized linear mixed models including bilateral VOI as repeated measures controlling for age, sex and GM fraction, and partial correlation with multiple test corrections | a) PDE levels elevated in the thalamus and hippocampus but reduced in the DLPFC and inferior frontal cortex in SZ compared to controls. b) PME levels elevated in SZ compared to controls in the hippo-campus and anterior cingulate but reduced in inferior frontal and parietal lobule c) Antipsychotic dose did not correlate with PDE or PME levels. d) The left thalamus PDE levels correlated negatively with sustained attention. e) PDE levels positively correlated with IL-6 levels in the thalamus in SZ |
NIMH MH72995 and MH93540 |
These studies examined both frontal and temporal lobes and hence are included in both groups. Total number of studies are the same as in the CONSORT diagram
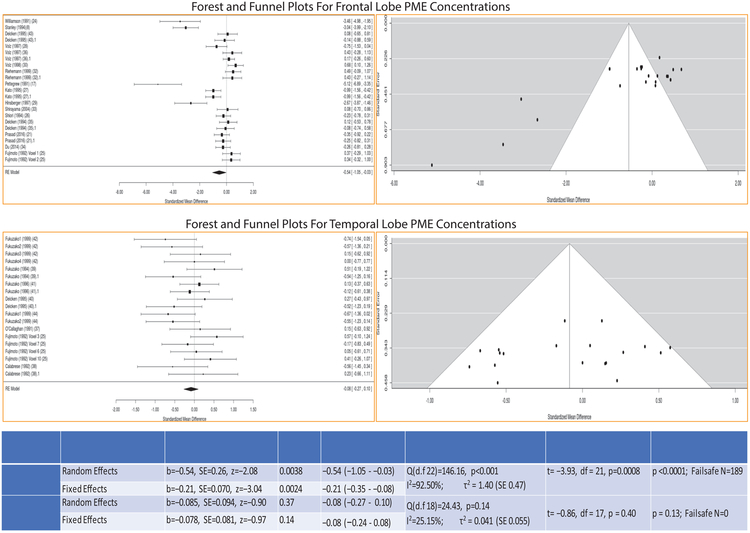
Frontal lobe PME levels (PC+PE)
This analysis included 404 schizophrenia patients (age: 32.02±5.15 years) and 340 controls (age: 30.75±4.90 years) from 18 studies. Decreased frontal PME levels in schizophrenia compared to control subjects were noted in both RE (b=−0.54; 95% CI:−1.05 to −0.03; SE 0.26; z=−2.08, p=0.038) and FE (b=−0.21; 95% CI=−0.35 to −0.08; SE 0.07, z=−3.04, p=0.002) models. The effect size was medium in the RE (Hedges’ g=−0.54; 95% CI:−1.05 to −0.03) but small in the FE (Hedges’ g=0.21; 95% CI:−0.35 to −0.08) model, and were considerably heterogeneous (Q=146.16, df=22, p<0.001; I2=92.50%; τ2=1.40). Publication bias (t=−3.93, df=21, p=0.0008) and fail-safe analysis (p<0.0001) were also significant with a high fail-safe number (N=189) (Fig 2).
Fig 2:
Forest and funnel plots for differences in PME concentrations in the frontal lobe and the temporal lobe among the studies. Table below shows the results of meta-analysis for both the random and fixed effects models along with effect sizes (Hedges’s g), heterogeneity of effect sizes, publication bias and fail-safe analysis
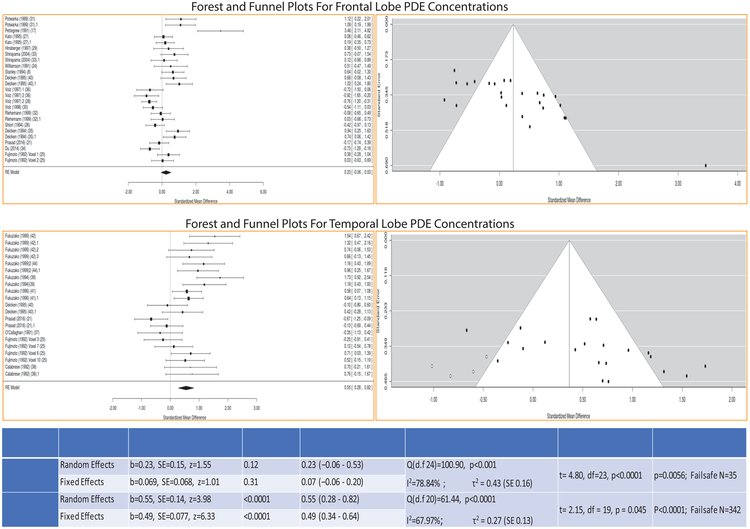
Frontal lobe PDE levels (GPC+GPE)
This analysis included 415 schizophrenia patients (age: 35.88±4.65 years) and 377 controls (age: 33.60±3.75 years) from 17 studies. PDE levels did not differ in both RE (b=0.23, 95% CI:−0.06 to −0.53, SE=0.15, z=1.55, p=0.12) and FE (b=0.069, 95% CI:−0.06 to −0.20, SE=0.07, z=0.07, p=0.31) models with significant heterogeneity (Q=100.90, df=24, p<0.001; I2=78.84%; τ2=0.43). Effect sizes were small in both models (RE: Hedges’s g=0.23; 95% CI:−0.06 to 0.53; FE: Hedges’s g=0.07; 95% CI:−0.06 to 0.20). Publication bias (t=4.80, df=23, p<0.0001) and fail-safe analysis were significant (p=0.0056) with a fail-safe N=35 (Fig 3).
Fig 3:
Forest and funnel plots for differences in PDE concentrations in the frontal lobe and the temporal lobe among the studies. Table below shows the results of meta-analysis for both the random and fixed effects models along with effect sizes (Hedges’s g), heterogeneity of effect sizes, publication bias and fail-safe analysis
Temporal lobe PME levels (PC+PE)
This analysis included 140 schizophrenia patients (age: 34.06±6.98 years) and 130 controls (age: 33.70±6.88 years) (n=8 studies). PME of patients did not differ from controls (RE: b=−0.085, 95% CI:−0.27 to 0.10, SE=0.09, z=−0.90, p=0.37; FE: b=−0.078, 95% CI:−0.24 to 0.08, SE=0.081, z=−0.97, p=0.33) with similar effect sizes in both models (RE: Hedges’s g=−0.08; 95% CI: −0.27 to 0.10; FE: Hedges’s g=−0.08; 95% CI:−0.24 to 0.08). The test for heterogeneity (Q=24.43, df=18, p=0.14; I2=25.15%, τ2=0.04), publication bias (t=−0.86, df=17, p=0.40, NS) and fail-safe analysis (p=0.40) were not significant with a fail-safe N=0 (Fig 2).
Temporal region PDE levels (GPC+GPE)
This analysis included 168 schizophrenia patients (age: 33.07±7.15 years) and 151 controls (age: 32.76±7.01 years) from 9 studies. Both RE (b=0.55, 95% CI:0.28 to 0.82, SE=0.14, z=3.98, p<0.0001) and FE (b=0.49, 95% CI: 0.34 – 0.64, SE=0.08, z=6.33, p<0.0001) models showed increased temporal PDE levels among patients compared to controls with similar effect sizes (RE: Hedges’s g=0.55; 95% CI: 0.28 to 0.82; FE: Hedges’s g=0.49; 95% CI: 0.34 – 0.64). Test of heterogeneity (Q(df=20)=61.44, p<0.0001; I2=67.97%, τ2=0.27), publication bias (t=2.15, df=19, p=0.045) and fail-safe analysis were significant (p<0.0001) with a fail-safe N=342 (Fig 3).
Sensitivity Analysis: PME Frontal Lobe
Two studies(8, 17) reported large effect sizes. Sensitivity analysis after excluding each of these studies, and then both showed that the frontal lobe PME remained significant in FE model after excluding either one of these studies but not when both were excluded. RE model remained significant after excluding Pettegrew et al(17) but not after Stanley et al(8) study. Effect sizes marginally decreased when either one of these studies was excluded. However, frontal lobe PME did not show significant changes after excluding both studies in both models (Supplemental Table 2).
Sensitivity Analysis PDE Frontal Lobe
Similarly, frontal lobe PDE was no longer significant after excluding either Pettegrew et al(17) or Stanley et al(8) studies alone or together in both models (Supplemental Table 2).
Sensitivity analyses for the temporal region were not conducted because no study/studies had substantially large/small effect sizes.
Sensitivity Analysis for Subgroup
Separate subgroup analysis for left-vs-right temporal lobes, patient age, and magnet strength entered showed significantly elevated PDE but not PME levels in RE and FE models in both left and right temporal regions. Age and magnet strength did not contribute to elevated temporal lobe PDE and lower frontal PME levels (Supplemental Table 3).
DISCUSSION
Our meta-analysis reveals elevated temporal region PDE levels and reduced frontal PME levels among schizophrenia patients compared to controls suggesting distinctly different biochemical processes related to imbalances in the MPL metabolism that is consistent with reduced MPL content. This assumes reduced precursor levels reflects reduced synthesis of MPLs on cellular processes in the frontal region and higher catabolites reflect increased degradation of temporal region MPLs. Sensitivity analyses did not show that these findings may be contributed by age, magnet field strength and hemisphere. A large number of negative studies needed to negate these results supports the robustness. Publication bias was noted for all except the temporal lobe PME levels. Although existing evidence (see Introduction) supports membrane formation/degradation primarily in the dendrites, synapses and neurites, other possibilities are discussed below.
Convergent evidence from animal lesion(10, 46), postmortem(12) and cellular(13) studies suggests reduced PME is associated with decreased neurite formation but may not be with larger soma since the ratio of soma volume/surface area of neurons is estimated to be about 1:40(14, 15) with dendrites, axons and synapses mainly contributing to the surface area. This is further supported by three-fold elevation in PME levels in the developing brain during neurite and myelin formation(46). Additionally, decreased choline kinase, phosphodiesterase and/or phospholipase activity, and increased phosphatase activity can lead to reduced PME since PME can be synthesized from phosphorylation of choline and ethanolamine by their respective kinases, phospholipase cleavage of PC and PE, and conversion from their respective PDEs by phosphodiesterase (see (17) (Fig 1). Phosphatase cleaves PME to choline/ethanolamine. Besides, low MR “visibility” of PME due to chelation with divalent cations and increased correlation times (τ), which are more prominent in lower strength magnets, can also contribute to reduced PME. Our sensitivity analysis showed that field strength did not contribute significantly to these results.
Similar mechanisms may underlie higher temporal region PDE levels in schizophrenia. Increased phospholipase A1 and A2 breaks down phosphatidylcholine and phosphatidylethanolamine to GPC and GPE, respectively. Decreased phosphodiesterase activity could reduce conversion of GPC/GPE to PC/PE. Increased MR “visibility” of PDE due to decreased chelation with divalent cations and decreased τ may contribute to elevated PDE levels. As discussed, increased PDE is associated with decreased neurites suggested by animal(10, 46), postmortem(12) and cellular(13) studies.
Evidence for altered activity/expression of enzymes involved in phospholipid metabolism in schizophrenia is inconsistent. Decreased phospholipase C-β1 expression is demonstrated in the prefrontal cortex of schizophrenia patients(47). Elevated intracellular phospholipase A2 in the temporal region by 45% but not in the prefrontal and occipital cortices was reported in both medication-naïve and medicated patients(48, 49). Similar alterations in the peripheral blood are also reported(49). Increased phosphodiesterase 10A activity is reported in striatal medium spiny neurons but a PET study did not reveal differences in any examined region between patients and controls(50). There are no published data on other enzymes in the system. However, the relationship of altered levels/expression of these enzymes can be more complex because several isoforms exist that warrants careful examination in schizophrenia.
Postmortem studies show age-related synaptic elimination in healthy individuals continuing until age 30 years(51, 52). Exaggerated synaptic pruning during adolescence may underlie schizophrenia onset(17). Our findings may reflect a summation of putative exaggerated pruning, other neurite and myelin changes before and during the early phase of the illness, progression, medication effects and comorbidities in patients and controls in their early 30s. However, synaptic pruning alone may not account for the entire variance in MPL metabolite alterations because replicated human postmortem studies show dendritic spine loss as a proxy to synaptic loss in the basilar dendrites of layer 3 of the cortex(53) along with decreased pyramidal cell volume(2) and increased packing density(18). Thus, postmortem data reflects the end-result of different processes that are challenging to distinguish unless individuals at different ages are examined. In contrast, in vivo 31P MRS can directly assess shifts in MPL metabolism related to neuropil membrane expansion/contraction by measuring differences in MPL precursors and catabolites. Similar observations were made in Alzheimer’s disease (AD) that show elevated PME in mild AD and elevated PDE levels in severe AD suggesting that when neurons are able to synthesize phospholipids in mild AD, elevated PME are observed as a mechanism to repair but in severe disease the ability of the brain to synthesize phospholipids may be compromised leading to lower PMEs and elevated PDE(54, 55). Further, white matter changes associated with these factors can also contribute to MPL metabolites through changes in myelin. An early study reported similar or higher PMEs and PDEs in the white matter(56) although not replicated. This meta-analysis provides data on a large sample that is hard to prospectively enroll. Although age, field-strength and hemisphere did not contribute to heterogeneity of the findings, other untested variables such as illness duration, sample size, illness stage, clinical severity, comorbidities, voxel size, and the acquisition, processing and quantification approach of the 31P MRS might have contributed. Despite publication bias, a very high fail-safe study numbers on both frontal and temporal regions suggests that these findings are persuasive for future post-mortem and animal studies. Elucidation of molecular basis of such distinct mechanisms can provide useful insights on novel targets for new treatment.
Animal and postmortem studies show regionally distinct molecular mechanisms underlying neurite elimination(57, 58), e.g., the Bergman glia with C1QL1 involvement in the cerebellum, astrocytes and microglia in the retinal ganglion to geniculate nucleus synapse elimination, microglial chemokine (CX3CL1) in the hippocampal Schaffer collateral to CA1 pyramidal neuron connections, microglial P2Y12 receptor system in the primary visual cortex(57), caspase-based digestion in the peripheral nervous system, and semaphorin-based synapse elimination in the dentate gyrus(58). Similarly, brain derived neurotrophic factor (BDNF) is associated with regulation of hippocampal neurogenesis(59) and inhibition of dendritic arborization(60). These regionally distinct mechanisms may vary during the development and illness course. Longitudinal studies on children and adolescents show increased PME until late childhood that plateaus off followed by increased PDE suggesting slowing down of neuropil formation and possibly followed by pruning(16, 61). Thus, cross-sectional and longitudinal 31P MRS studies at different stages of illness, such as high-risk individuals, first-episode medication-naïve, and chronic schizophrenia may highlight illness-stage specific membrane changes.
Detailed antipsychotic dose and duration of administration were unavailable in all studies precluding us from including these as covariates. A longitudinal study showed no significant change in MPL metabolites in patients on antipsychotics for up to a year(62). Two-week olanzapine treatment of antipsychotic-naïve patients showed decreased temporal cortex high energy phosphates but not of MPL metabolites(63). Chronic patients who were off-treatment for 4-weeks and then treated for 6-weeks with haloperidol/risperidone showed lower high energy phosphates and higher GPC+GPE in the prefrontal cortex(64). No changes in GPC+GPE were noted after 4-week treatment with haloperidol(65). The dose of neuroleptic correlated with change in ATP but not MPL metabolites(66). This review of direct investigation of medication effects suggests that antipsychotics may affect PME and PDE with short-term but not long-term administration.
Although size of the MRS voxels have continually decreased with increasing field strength, voxels include varying proportions of gray and white matter. Prior studies examining larger voxels might have included larger proportions of white matter that might have affected the results. Later studies using smaller voxels and more sophisticated techniques to parcellate gray and white matter have reported similar findings compared to the older studies. Studies that directly examine this question need to be conducted.
Some limitations of the study involve patient variables, differences in spectroscopy technique, and lack of consensus on the effects of antipsychotics on PME and PDE levels. These inconsistencies may be resolved by increasing the statistical power of future studies and standardizing acquisition and processing. Contribution of white matter changes to MPL metabolites need greater attention since white matter pathology is extensively reported in schizophrenia.
These results suggest distinctly different MPL metabolism in the frontal and temporal regions in schizophrenia compared to controls. These findings support postmortem and primate studies that reported neuropil contraction and raise the possibility of different underlying mechanisms. Despite significant heterogeneity, medium effect sizes and a high number of fail-safe studies required to negate these findings suggest that these results require further investigation. At high-field of 3 Tesla or greater, or with protondecoupling, 31P MRS can resolve and quantify the individual PMEs and PDEs(67) to better understand biochemical pathways.
Supplementary Material
Acknowledgments:
This work was funded through grants from the National Institute of Mental Health MH112584 and MH115026 (KMP).
Legend:
- ATP
Adenosine 1,2,3 Triphosphate
- ADP
Adenosine 2,3 diphosphate
- CTP
Cytidine 1,2,3 triphosphate
- PPi
Inorganic phosphate
- DAG
Diacylglycerol
- CMP
Cytidine monophosphate
- CK
Choline kinase
- EK
Ethanolamine kinase
- CPT PCCT
CPT-phosphocholine cytidyltransferase
- CPT PECT
CPT-phosphoethanolamine cytidyltransferase
- CPT
CDP-choline:1,2-diacylglycerol cholinephosphotransferase
- EPT
CDP-choline:1,2-diacylglycerol ethanolaminephosphotransferase
- PLC
Phospholipase C
- PLA2
Phospholipase A2
- PEMT
Phosphatidylethanolamine N-methyltransferase
- PSS1
Phosphatidylserine synthase 1
- PSS2
Phosphatidylserine synthase 2
- LPL A1, A2
Lysophospholipase A1 & A2
- PSD
Phosphatidylserine decarboxylase
Footnotes
Disclosures: All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lewis DA, Levitt P (2002): Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 25:409–432. [DOI] [PubMed] [Google Scholar]
- 2.Pierri JN, Volk CL, Auh S, Sampson A, Lewis DA (2001): Decreased somal size of deep layer 3 pyramidal neurons in the prefrontal cortex of subjects with schizophrenia. Arch Gen Psychiatry. 58:466–473. [DOI] [PubMed] [Google Scholar]
- 3.Selemon LD, Rajkowska G, Goldman-Rakic PS (1995): Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry. 52:805–818; discussion 819-820. [DOI] [PubMed] [Google Scholar]
- 4.Selemon LD, Rajkowska G, Goldman-Rakic PS (1998): Elevated neuronal density in prefrontal area 46 in brains from schizophrenic patients: application of a three-dimensional, stereologic counting method. J Comp Neurol. 392:402–412. [PubMed] [Google Scholar]
- 5.Lewis DA, Gonzalez-Burgos G (2008): Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 33:141–165. [DOI] [PubMed] [Google Scholar]
- 6.Weinberger DR, Radulescu E (2016): Finding the Elusive Psychiatric "Lesion" With 21st-Century Neuroanatomy: A Note of Caution. Am J Psychiatry. 173:27–33. [DOI] [PubMed] [Google Scholar]
- 7.Stanley JA (2002): In vivo magnetic resonance spectroscopy and its application to neuropsychiatric disorders. Can J Psychiatry. 47:315–326. [DOI] [PubMed] [Google Scholar]
- 8.Stanley JA, Williamson PC, Drost DJ, Carr TJ, Rylett RJ, Morrison-Stewart S, et al. (1994): Membrane phospholipid metabolism and schizophrenia: an in vivo 31P-MR spectroscopy study. Schizophr Res. 13:209–215. [DOI] [PubMed] [Google Scholar]
- 9.Stanley JA, Pettegrew JW (2001): Postprocessing method to segregate and quantify the broad components underlying the phosphodiester spectral region of in vivo (31)P brain spectra. Magn Reson Med. 45:390–396. [DOI] [PubMed] [Google Scholar]
- 10.Geddes JW, Panchalingam K, Keller JN, Pettegrew JW (1997): Elevated phosphocholine and phosphatidylcholine following rat entorhinal cortex lesions. Neurobiol Aging. 18:305–308. [DOI] [PubMed] [Google Scholar]
- 11.Pettegrew JW, Klunk WE, Panchalingam K, McClure RJ, Stanley JA (2000): Molecular insights into neurodevelopmental and neurodegenerative diseases. Brain Res Bull. 53:455–469. [DOI] [PubMed] [Google Scholar]
- 12.DeVries GH, Zetusky WJ, Zmachinski C, Calabrese VP (1981): Lipid composition of axolemma-enriched fractions from human brains. Journal of lipid research. 22:208–216. [PubMed] [Google Scholar]
- 13.Marcucci H, Paoletti L, Jackowski S, Banchio C (2010): Phosphatidylcholine biosynthesis during neuronal differentiation and its role in cell fate determination. J Biol Chem. 285:25382–25393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfenninger KH (2009): Plasma membrane expansion: a neuron's Herculean task. Nat Rev Neurosci. 10:251–261. [DOI] [PubMed] [Google Scholar]
- 15.Pfenninger KH, Johnson MP (1983): Membrane biogenesis in the sprouting neuron. I. Selective transfer of newly synthesized phospholipid into the growing neurite. The Journal of cell biology. 97:1038–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanley JA, Kipp H, Greisenegger E, MacMaster FP, Panchalingam K, Keshavan MS, et al. (2008): Evidence of developmental alterations in cortical and subcortical regions of children with attention-deficit/hyperactivity disorder: a multivoxel in vivo phosphorus 31 spectroscopy study. Arch Gen Psychiatry. 65:1419–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pettegrew JW, Keshavan MS, Panchalingam K, Strychor S, Kaplan DB, Tretta MG, et al. (1991): Alterations in brain high-energy phosphate and membrane phospholipid metabolism in first-episode, drug-naive schizophrenics. A pilot study of the dorsal prefrontal cortex by in vivo phosphorus 31 nuclear magnetic resonance spectroscopy. Arch Gen Psychiatry. 48:563–568. [DOI] [PubMed] [Google Scholar]
- 18.Selemon LD, Goldman-Rakic PS (1999): The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 45:17–25. [DOI] [PubMed] [Google Scholar]
- 19.Glantz LA, Lewis DA (2000): Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 57:65–73. [DOI] [PubMed] [Google Scholar]
- 20.Yuksel C, Tegin C, O'Connor L, Du F, Ahat E, Cohen BM, et al. (2015): Phosphorus magnetic resonance spectroscopy studies in schizophrenia. J Psychiatr Res. 68:157–166. [DOI] [PubMed] [Google Scholar]
- 21.Prasad KM, Burgess A, Nimgaonkar VL, Keshavan MS, Stanley JA (2016): Neuropil pruning in Early-Course Schizophrenia: Immunological, Clinical and Neurocognitive Correlates. Biological Psychiatry: Cognitive Neuroscience & Neuroimaging. 1:528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prasad KM, Chowdari KV, D'Aiuto LA, Iyengar S, Stanley JA, Nimgaonkar VL (2018): Neuropil contraction in relation to Complement C4 gene copy numbers in independent cohorts of adolescent-onset and young adult-onset schizophrenia patients-a pilot study. Translational psychiatry. 8:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, et al. (2016): Schizophrenia risk from complex variation of complement component 4. Nature. 530:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williamson P, Drost D, Stanley J, Carr T, Morrison S, Merskey H (1991): Localized phosphorus 31 magnetic resonance spectroscopy in chronic schizophrenic patients and normal controls. Arch Gen Psychiatry. 48:578. [DOI] [PubMed] [Google Scholar]
- 25.Fujimoto T, Nakano T, Takano T, Hokazono Y, Asakura T, Tsuji T (1992): Study of chronic schizophrenics using 31P magnetic resonance chemical shift imaging. Acta Psychiatr Scand. 86:455–462. [DOI] [PubMed] [Google Scholar]
- 26.Shioiri T, Kato T, Inubushi T, Murashita J, Takahashi S (1994): Correlations of phosphomonoesters measured by phosphorus-31 magnetic resonance spectroscopy in the frontal lobes and negative symptoms in schizophrenia. Psychiatry Res. 55:223–235. [DOI] [PubMed] [Google Scholar]
- 27.Kato T, Shioiri T, Murashita J, Hamakawa H, Inubushi T, Takahashi S (1995): Lateralized abnormality of high-energy phosphate and bilateral reduction of phosphomonoester measured by phosphorus-31 magnetic resonance spectroscopy of the frontal lobes in schizophrenia. Psychiatry Res. 61:151–160. [DOI] [PubMed] [Google Scholar]
- 28.Volz HP, Rzanny R, Rossger G, Hubner G, Kreitschmann-Andermahr I, Kaiser WA, et al. (1997): Decreased energy demanding processes in the frontal lobes of schizophrenics due to neuroleptics? A 31P-magneto-resonance spectroscopic study. Psychiatry Res: Neuroimaging. 76:123–129. [DOI] [PubMed] [Google Scholar]
- 29.Hinsberger AD, Williamson PC, Carr TJ, Stanley JA, Drost DJ, Densmore M, et al. (1997): Magnetic resonance imaging volumetric and phosphorus 31 magnetic resonance spectroscopy measurements in schizophrenia. J Psychiatry Neurosci. 22:111–117. [PMC free article] [PubMed] [Google Scholar]
- 30.Volz HP, Rzanny R, Rossger G, Hubner G, Kreitschmann-Andermahr I, Kaiser WA, et al. (1998): 31Phosphorus magnetic resonance spectroscopy of the dorsolateral prefrontal region in schizophrenics--a study including 50 patients and 36 controls. Biol Psychiatry. 44:399–404. [DOI] [PubMed] [Google Scholar]
- 31.Potwarka JJ, Drost DJ, Williamson PC, Carr T, Canaran G, Rylett WJ, et al. (1999): A 1H-decoupled 31P chemical shift imaging study of medicated schizophrenic patients and healthy controls. Biol Psychiatry. 45:687–693. [DOI] [PubMed] [Google Scholar]
- 32.Riehemann S, Volz HP, Wenda B, Hubner G, Rossger G, Rzanny R, et al. (1999): Frontal lobe in vivo (31)P-MRS reveals gender differences in healthy controls, not in schizophrenics. NMR Biomed. 12:483–489. [DOI] [PubMed] [Google Scholar]
- 33.Shirayama Y, Yano T, Takahashi K, Takahashi S, Ogino T (2004): In vivo 31P NMR spectroscopy shows an increase in glycerophosphorylcholine concentration without alterations in mitochondrial function in the prefrontal cortex of medicated schizophrenic patients at rest. Eur J Neurosci. 20:749–756. [DOI] [PubMed] [Google Scholar]
- 34.Du F, Cooper AJ, Thida T, Sehovic S, Lukas SE, Cohen BM, et al. (2014): In vivo evidence for cerebral bioenergetic abnormalities in schizophrenia measured using 31P magnetization transfer spectroscopy. JAMA Psychiatry. 71:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deicken RF, Calabrese G, Merrin EL, Meyerhoff DJ, Dillon WP, Weiner MW, et al. (1994): 31phosphorus magnetic resonance spectroscopy of the frontal and parietal lobes in chronic schizophrenia. Biol Psychiatry. 36:503–510. [DOI] [PubMed] [Google Scholar]
- 36.Volz HP, Rzanny R, May S, Hegewald H, Preussler B, Hajek M, et al. (1997): 31P magnetic resonance spectroscopy in the dorsolateral prefrontal cortex of schizophrenics with a volume selective technique--preliminary findings. Biol Psychiatry. 41:644–648. [DOI] [PubMed] [Google Scholar]
- 37.O'Callaghan E, Redmond O, Ennis R, Stack J, Kinsella A, Ennis JT, et al. (1991): Initial investigation of the left temporoparietal region in schizophrenia by 31P magnetic resonance spectroscopy. Biol Psychiatry. 29:1149–1152. [DOI] [PubMed] [Google Scholar]
- 38.Calabrese G, Deicken RF, Fein G, Merrin EL, Schoenfeld F, Wiener MW (1992): 31Phosphorus magnetic resonance spectroscopy of the temporal lobes in schizophrenia. Biological Psychiatry. 32:26–32. [DOI] [PubMed] [Google Scholar]
- 39.Fukuzako H, Takeuchi K, Ueyama K, Fukuzako T, Hokazono Y, Hirakawa K, et al. (1994): 31P magnetic resonance spectroscopy of the medial temporal lobe of schizophrenic patients with neuroleptic-resistant marked positive symptoms. Eur Arch Psychiatry Clin Neurosci. 244:236–240. [DOI] [PubMed] [Google Scholar]
- 40.Deicken RF, Calabrese G, Merrin EL, Vinogradov S, Fein G, Weiner MW (1995): Asymmetry of temporal lobe phosphorous metabolism in schizophrenia: a 31phosphorous magnetic resonance spectroscopic imaging study. Biol Psychiatry. 38:279–286. [DOI] [PubMed] [Google Scholar]
- 41.Fukuzako H, Fukuzako T, Takeuchi K, Ohbo Y, Ueyama K, Takigawa M, et al. (1996): Phosphorus magnetic resonance spectroscopy in schizophrenia: correlation between membrane phospholipid metabolism in the temporal lobe and positive symptoms. Prog Neuropsychopharmacol Biol Psychiatry. 20:629–640. [DOI] [PubMed] [Google Scholar]
- 42.Fukuzako H, Fukuzako T, Kodama S, Hashiguchi T, Takigawa M, Fujimoto T (1999): Haloperidol improves membrane phospholipid abnormalities in temporal lobes of schizophrenic patients. Neuropsychopharmacology. 21:542–549. [DOI] [PubMed] [Google Scholar]
- 43.Deicken RF, Merrin EL, Floyd TC, Weiner MW (1995): Correlation between left frontal phospholipids and Wisconsin Card Sort Test performance in schizophrenia. Schizophr Res. 14:177–181. [DOI] [PubMed] [Google Scholar]
- 44.Fukuzako H, Fukuzako T, Hashiguchi T, Kodama S, Takigawa M, Fujimoto T (1999): Changes in levels of phosphorus metabolites in temporal lobes of drug-naive schizophrenic patients. Am J Psychiatry. 156:1205–1208. [DOI] [PubMed] [Google Scholar]
- 45.Keshavan MS, Sanders RD, Pettegrew JW, Dombrowsky SM, Panchalingam KS (1993): Frontal lobe metabolism and cerebral morphology in schizophrenia: 31P MRS and MRI studies. Schizophrenia Research. 10:241–246. [DOI] [PubMed] [Google Scholar]
- 46.Pettegrew JW, Panchalingam K, Withers G, McKeag D, Strychor S (1990): Changes in brain energy and phospholipid metabolism during development and aging in the Fischer 344 rat. J Neuropathol Exp Neurol. 49:237–249. [DOI] [PubMed] [Google Scholar]
- 47.Kim SW, Cho T, Lee S (2015): Phospholipase C-beta1 Hypofunction in the Pathogenesis of Schizophrenia. Frontiers in psychiatry. 6:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ross BM, Turenne S, Moszczynska A, Warsh JJ, Kish SJ (1999): Differential alteration of phospholipase A2 activities in brain of patients with schizophrenia. Brain Res. 821:407–413. [DOI] [PubMed] [Google Scholar]
- 49.Ross BM, Hudson C, Erlich J, Warsh JJ, Kish SJ (1997): Increased phospholipid breakdown in schizophrenia. Evidence for the involvement of a calcium-independent phospholipase A2. Arch Gen Psychiatry.54:487–494. [DOI] [PubMed] [Google Scholar]
- 50.Marques TR, Natesan S, Niccolini F, Politis M, Gunn RN, Searle GE, et al. (2016): Phosphodiesterase 10A in Schizophrenia: A PET Study Using [(11)C]IMA107. Am J Psychiatry. 173:714–721. [DOI] [PubMed] [Google Scholar]
- 51.Nimgaonkar VL, Prasad KM, Chowdari KV, Severance EG, Yolken RH (2017): The complement system: a gateway to gene-environment interactions in schizophrenia pathogenesis. Mol Psychiatry. 22:1554–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, et al. (2011): Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A. 108:13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glausier JR, Lewis DA (2013): Dendritic spine pathology in schizophrenia. Neuroscience. 251:90–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pettegrew JW, Panchalingam K, Moossy J, Martinez J, Rao G, Boller F (1988): Correlation of phosphorus-31 magnetic resonance spectroscopy and morphologic findings in Alzheimer's disease. Arch Neurol. 45:1093–1096. [DOI] [PubMed] [Google Scholar]
- 55.Pettegrew JW, Moossy J, Withers G, McKeag D, Panchalingam K (1988): 31P nuclear magnetic resonance study of the brain in Alzheimer's disease. J Neuropathol Exp Neurol. 47:235–248. [DOI] [PubMed] [Google Scholar]
- 56.Buchli R, Duc CO, Martin E, Boesiger P (1994): Assessment of absolute metabolite concentrations in human tissue by 31P MRS in vivo. Part I: Cerebrum, cerebellum, cerebral gray and white matter. Magn Reson Med. 32:447–452. [DOI] [PubMed] [Google Scholar]
- 57.Neniskyte U, Gross CT (2017): Errant gardeners: glial-cell-dependent synaptic pruning and neurodevelopmental disorders. Nat Rev Neurosci. 18:658–670. [DOI] [PubMed] [Google Scholar]
- 58.Riccomagno MM, Kolodkin AL (2015): Sculpting neural circuits by axon and dendrite pruning. Annu Rev Cell Dev Biol. 31:779–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katoh-Semba R, Asano T, Ueda H, Morishita R, Takeuchi IK, Inaguma Y, et al. (2002): Riluzole enhances expression of brain-derived neurotrophic factor with consequent proliferation of granule precursor cells in the rat hippocampus. FASEB J. 16:1328–1330. [DOI] [PubMed] [Google Scholar]
- 60.Gao X, Smith GM, Chen J (2009): Impaired dendritic development and synaptic formation of postnatal-born dentate gyrus granular neurons in the absence of brain-derived neurotrophic factor signaling. Exp Neurol. 215:178–190. [DOI] [PubMed] [Google Scholar]
- 61.Pettegrew JW, Kopp SJ, Minshew NJ, Glonek T, Feliksik JM, Tow JP, et al. (1987): 31P nuclear magnetic resonance studies of phosphoglyceride metabolism in developing and degenerating brain: preliminary observations. J Neuropathol Exp Neurol. 46:419–430. [DOI] [PubMed] [Google Scholar]
- 62.Jayakumar PN, Gangadhar BN, Venkatasubramanian G, Desai S, Velayudhan L, Subbakrishna D, et al. (2010): High energy phosphate abnormalities normalize after antipsychotic treatment in schizophrenia: a longitudinal 31P MRS study of basal ganglia. Psychiatry Res. 181:237–240. [DOI] [PubMed] [Google Scholar]
- 63.Nenadic I, Dietzek M, Langbein K, Rzanny R, Gussew A, Reichenbach JR, et al. (2013): Effects of olanzapine on 31P MRS metabolic markers in schizophrenia. Hum Psychopharmacol. 28:91–93. [DOI] [PubMed] [Google Scholar]
- 64.Smesny S, Langbein K, Rzanny R, Gussew A, Burmeister HP, Reichenbach JR, et al. (2012): Antipsychotic drug effects on left prefrontal phospholipid metabolism: a follow-up 31P-2D-CSI study of haloperidol and risperidone in acutely ill chronic schizophrenia patients. Schizophr Res. 138:164–170. [DOI] [PubMed] [Google Scholar]
- 65.Keshavan MS, Pettegrew JW, Panchalingam K (1995): MRS in the study of psychoses: psychopharmacological studies. In: Nasrallah HA, Pettegrew JW, editors. NMR Spectroscopy in Psychiatric Brain Disorders. Washington DC, : American Psychiatric Press, pp 131–146. [Google Scholar]
- 66.Volz HP, Hubner G, Rzanny R, Rossger G, Preussler B, Eichhorn M, et al. (1998): High-energy phosphates in the frontal lobe correlate with Wisconsin Card Sort Test performance in controls, not in schizophrenics: a 31phosphorus magnetic resonance spectroscopic and neuropsychological investigation. Schizophr Res. 31:37–47. [DOI] [PubMed] [Google Scholar]
- 67.Jensen JE, Drost DJ, Menon RS, Williamson PC (2002): In vivo brain (31)P-MRS: measuring the phospholipid resonances at 4 Tesla from small voxels. NMR Biomed. 15:338–347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.