Abstract
Background
Defining the neurobiological underpinnings of suicidal ideation (SI) is crucial to improving our understanding of suicide. This study used magnetoencephalographic gamma power as a surrogate marker for population-level excitation-inhibition balance to explore the underlying neurobiology of SI and depression. In addition, effects of pharmacological intervention with ketamine—which has been shown to rapidly reduce SI and depression—were assessed.
Methods
Data were obtained from 29 drug-free patients with major depressive disorder who participated in an experiment comparing subanesthetic ketamine to a placebo-saline infusion. Magnetoencephalographic recordings were collected at baseline and following ketamine and placebo infusions. During scanning, patients rested with their eyes closed. SI and depression were assessed, and a linear mixed-effects model was used to identify brain regions where gamma power and both SI and depression were associated. Two regions of the salience network (anterior insula, anterior cingulate) were then probed using dynamic causal modeling to test for ketamine effects.
Results
Clinically, patients showed significantly reduced SI and depression following ketamine. In addition, distinct regions in anterior insula were found to be associated with SI compared with depression. When modeling insula-anterior cingulate connectivity, ketamine lowered the membrane capacitance for superficial pyramidal cells. Finally, connectivity between insula and anterior cingulate was associated with improvements in depression symptoms.
Conclusions
These findings suggest the anterior insula plays a key role in SI, perhaps via its role in salience detection. In addition, transient changes in superficial pyramidal cell membrane capacitance and subsequent increases in cortical excitability might be one mechanism via which ketamine improves SI.
Clinical Trial Name
Rapid Antidepressant Effects of Ketamine in Major Depression; URL: https://clinicaltrials.gov/ct2/show/NCT00088699; Registration Number: 00088699
Keywords: suicidal ideation, ketamine, insula, magnetoencephalography, gamma, depression
1. Introduction
Suicide is a leading cause of death worldwide. In the United States, suicide rates are increasing, particularly among adolescents (1) and middle-aged adults (2). There is a relative paucity of treatments for acute suicide risk, which is at least partly due to the sparse neurobiological literature on suicidal thoughts and behavior. In this context, the need to define the neurobiological underpinnings of suicidal thoughts is critical to advancing the science on risk, with the ultimate goal of improving treatment for suicidal individuals. Nevertheless, a number of logistical obstacles exist to conducting research into suicide, particularly with actively suicidal individuals. Current methods for defining risk rely on patient self-report, which can be influenced by biases such as the desire to avoid stigma, as well as rapid, hour-to-hour changes in suicidal thoughts themselves (3). Consequently, a need exists to connect objective biomarkers to current indicators of risk—such as suicidal thoughts—that have been linked to later suicide attempts and death. Here we aimed to measure the neurobiology underlying suicidal ideation (SI) “in real-time” using magnetoencephalography (MEG), as this measure provides a direct, population-level assessment of neural activity.
A limited number of electrophysiological studies have explored the neural correlates of SI. Most of the literature focuses on history of suicide attempts, given that lifetime attempt is the best predictor of future behavior (4). Differences in scalp electroencephalographic recordings have been identified between suicide attempters and non-attempters with major depressive disorder (MDD) (5, 6) as well as healthy participants (7). These findings included scalp electrode changes in wide-band ERP amplitude and theta and alpha frequency changes associated with suicidal behavior, but they tell us little about the underlying brain regions that mediate suicidal thoughts. In addition, a preliminary analysis of resting-state MEG data from a group of depressed subjects classified according to suicide attempt risk found caudothalamic network coupling abnormality in a small group at high risk for suicide attempt (8). Given these limited findings, a more comprehensive understanding of the neural underpinnings of SI would help define targeted regions involved in suicide risk that may also respond to treatment.
A growing body of non-electrophysiological neuroimaging work has explored suicide risk in the context of psychiatric disorders including MDD (9–16), bipolar disorder (17), schizophrenia (18, 19), borderline personality disorder (20, 21), and post-traumatic stress disorder (22). Many of these studies have looked at changes in cortical thickness (14, 15, 18), grey matter volume (10, 12, 13, 16, 17, 19–21), and resting-state functional connectivity (22) associated with suicidal thoughts and symptoms. In addition, recent positron emissions tomography (PET) work has looked at neurotransmitter binding, in paticular serotonin, and its relation to SI and lethality of suicide attempt (11). Finally, work with healthy subjects has also explored the neural correlates of implicit, death-related associations using tasks (23, 24). These studies have suggested that changes in insular cortex (10, 11, 14, 18, 20, 23, 24) and anterior cingulate (11, 12, 15, 16, 18, 20, 21) may be important for mediating suicidal thoughts. Both regions are key nodes in the salience network, whose dysregulation is known to be implicated in several psychiatric disorders and associated psychopathology (25). The insula in particular is thought to play a key role in salience detection, interoception, and emotion regulation (26) and regulating subjective feelings associated with cognition and motivation (27). Thus, signaling deficits both within anterior insula and between anterior insula and other salience network nodes could have important implications for suicide risk.
In addition to defining neurobiological correlates of SI, few studies have explored changes in suicidal thoughts following pharmacological intervention. Of potential treatments, the glutamatergic modulator ketamine has found the most promise as a rapid-acting agent, with potential effects on SI occurring within hours and lasting up to one week (28). These effects have found to be at least partially independent of effects on mood (28). With regards to the neurobiology of suicidal thoughts and treatment, a PET study found that changes in glucose metabolism within the infralimbic cortex correlated with reductions in SI following ketamine administration (29). More generally, ketamine has been found to directly induce spontaneous gamma synchrony within cortical networks (30–33). This increased gamma synchrony is thought to result from NMDA receptor blockade by ketamine on GABA-ergic interneurons and disinhibition of excitatory pyramidal neurons (34), coupled with a glutamate “burst” and subsequent increased activation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors and strong synaptic excitation (35). Thus, gamma can be considered a putative surrogate marker of neural, population-level excitation-inhibition (E/I) balance (36) and synaptic potentiation (31), whose disruption can lead to synaptic loss, neuronal atrophy, and altered network-level connections (37). While these changes have been implicated in conditions such as depression (38), little research has explored the association between gamma power and SI directly.
This secondary analysis of a double-blind, crossover, placebo-controlled study used MEG to measure associations between resting-state gamma power and SI and depression scores in subjects with treatment-resistant MDD. All participants were scanned at baseline and following ketamine and placebo-saline infusions. In addition to measuring associations between gamma power and clinical variables, ketamine’s effect on connectivity between the anterior insula and anterior cingulate was modeled using dynamic causal modeling (DCM), as these regions have been implicated in both depression and risk for suicide (10, 11, 14, 23). DCM fits a biophysically plausible model of neural dynamics to measured electrophysiological signals, providing intrinsic and extrinsic estimates of synaptic response in specified neuronal ensembles (39). We tested for effects of ketamine on modeled parameter estimates, in addition to explicitly testing for associations between AMPA- and NMDA-mediated connectivity and changes in SI and depression scores. We hypothesized that SI would be associated with reduced gamma power in distinct brain regions important for mediating suicidal thoughts compared with depression.
2. Methods and Materials
2.1. Participants
All participants were studied at the National Institute of Mental Health (NIMH) Mood Disorders Research Unit in Bethesda, Maryland between September 2011 and August 2016. The present study used data drawn from a larger clinical trial (NCT#00088699) conducted to assess ketamine’s antidepressant effects (n=60) (33). The present sample comprised 29 subjects with a DSM-IV-TR diagnosis of MDD (40) without psychotic features (18F/11M, mean age=35.8±10.0 years) who were assessed for SI. MDD subjects were 18–65 years old, were experiencing a depressive episode lasting at least four weeks, had not responded to at least one adequate antidepressant trial during the current depressive episode (as assessed using the Antidepressant Treatment History Form), and had a Montgomery-Asberg Depression Rating Scale (MADRS) (41) score of > 20 at screening. Diagnosis was determined via the Structured Clinical Interview for Axis I DSM-IV-TR Disorders (SCID)-Patient Edition (42). All MDD subjects were hospitalized for the duration of the study and drug-free from psychotropic medications for at least two weeks prior to randomization to ketamine or saline infusion and throughout the study.
Participants were in good health as evaluated by a medical and psychiatric history screening that included a SCID as well as an extensive panel of laboratory testing (acute care panel, hepatic panel, mineral panel, LDH, uric acid, Creatinine Kinase, total protein), toxicology screens, urinalysis, clinical magnetic resonance imaging (MRI) without contrast, chest x-ray, and electrocardiogram. As stated in the original write-up of the clinical trial, patients had a mean number of 6.4 (SD=3.4) failed trials of antidepressant medication and had been depressed for an average of 41.4 months (SD=6.8) in the current episode. The Combined Neuroscience Institutional Review Board at the NIH approved the study. All participants provided written informed consent and were matched with an NIMH advocate from the Human Subjects Protection Unit to monitor consent and participation.
2.2. Clinical Measures
Due to concerns of the overlap between SI and other symptoms of depression, SI was assessed using the results of an exploratory factor analysis (EFA) across multiple assessments of depression and anhedonia, specifically the MADRS, HAMD, SHAPS and BDI (43). The resulting EFA identified several unidimensional constructs representing the core symptoms of depression, allowing us to separate SI from other depressive symptoms such as depressed mood, negative cognitions and anhedonia. The scaled SI score from the EFA is continuous (rather than dichotomizing suicide ideators versus non-ideators) and included the hopelessness and suicidal thoughts items from the Beck Depression Inventory (44) in addition to the suicidal thoughts item from the MADRS (41). Depressive symptomatology was assessed using the MADRS, without the corresponding suicidal thoughts item used to calculate SI (41).
Clinical measures were administered 60 minutes prior to infusion and at multiple timepoints (40, 80, 120, and 230 minutes post-infusion, as well as Days 1, 2, 3, 10, and 11) following both ketamine and placebo-saline infusions. Infusions occurred 14 days apart. For our analyses, we focused on the 60 minutes pre- and 230 minutes post-infusion timepoints. For the MEG data analysis, we classified the first pre-infusion rating as a baseline measurement and the 230-minute post-infusion rating as either the ketamine or placebo rating, depending on condition. These timepoints were selected because they were the closest ratings to the corresponding MEG scans. To measure the corresponding clinical changes in SI and depression scores, we compared the baseline (infusion 1, 60 minutes pre-infusion), placebo (230 minutes post-infusion), and ketamine (230 minutes post-infusion) session scores using linear mixed-effects models with restricted maximum likelihood estimation using IBM SPSS 24.0.0.0.
2.3. MEG Acquisition and Preprocessing
MEG recordings were collected two to four days prior to the first infusion (baseline) and six to nine hours after both the ketamine and placebo experimenter-blinded infusions (Figure 1A). The order of ketamine and placebo infusions was randomized across participants. During each session, participants were instructed to relax with their eyes closed and remain still. Resting state neuromagnetic data were acquired using a 275-channel CTF system with SQUID-based axial gradiometers (VSM MedTech Ltd., Couquitlam, BC, Canada) housed in a magnetically-shielded room (Vacuumschmelze, Germany). Data were collected at 1200 Hz with a bandwidth of 0–300 Hz. Synthetic third order balancing was used for active noise cancellation. During scanning, one or two 250-second resting state scans were acquired per subject. The first resting scan was acquired at the beginning of the scan session, while the second occurred approximately 45 minutes later after a series of tasks. In addition to MEG resting scans, T1 weighted MRI scans were acquired on a 3 Tesla GE scanner. MEG data were coregistered to each subject’s MRI image using MRI-visible fiducial markers that were placed on the head prior to MRI scanning.
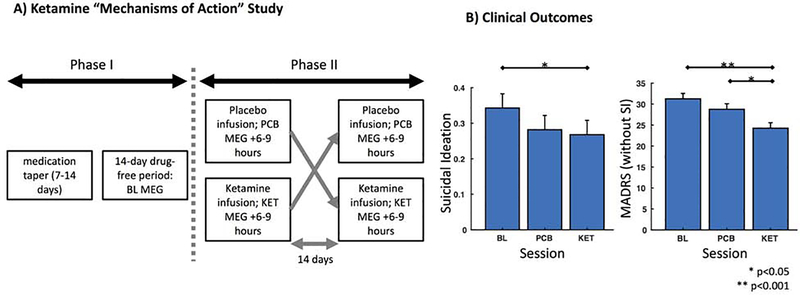
Figure 1. Study Design and Clinical Outcomes.
A) The study included a double-blind, placebo-controlled, crossover design. During Phase I, patients tapered off their medications and completed a 14-day drug-free period prior to entering Phase II. A baseline (BL) MEG recording was collected during this time-period (specifically, 2 to 4 days prior to the first infusion). During Phase II, patients received both ketamine (KET) and placebo saline (PCB) infusions, with a 14-day period between crossover. MEG recordings were collected 6–9 hours following each infusion. B) Clinically, patients showed significant reductions in suicidal ideation and depression scores following ketamine administration compared to baseline. In addition, patients showed significant reductions in depression scores between the ketamine and placebo sessions.
2.4. MEG Data Analysis
This work used the computational resources of the NIH HPC Biowulf cluster (http://hpc.nih.gov). MEG data preprocessing procedures have been previously described (33). Briefly, data were high-pass filtered at 2 Hz, then visually inspected for muscular, ocular, and movement artifacts. These artifacts were marked as bad time segments. Up to 10 segments of 15-second time periods in which no artifacts were present were then identified within each dataset. Subsequent analyses were performed using these clean datasets.
For source-level analysis, synthetic aperture magnetometry (SAM) was used to localize MEG data to source space using a 5 mm grid (45) and a multisphere head model. Beamformer weights were calculated using a bandpass frequency of 2–100 Hz, and power was normalized by the projected noise floor of the virtual sensor. Gamma band (30–50 Hz) power was then projected at each voxel, normalized by the square root of the sum of squared images for the six canonical bands between 2–100 Hz (delta, theta, alpha, beta, gamma, and high gamma). All normalized RMS gamma source images were warped to Talairach space using Analysis of Functional Neuroimages (AFNI) for group-level comparisons (46).
A linear mixed-effects model implemented in AFNI was used to assess group-level effects (47). For the analysis, either one or two resting scans per participant were included, coded as to whether the recording occurred before (pre) or after (post) task-based scans not reported here. Model factors included session (baseline, placebo, ketamine), recording (pre, post), SI factor score, and depression score. The model tested for the main effects of SI and depression on gamma power.
Two regions within the salience network were subsequently probed by extracting source activity and modeling the influence of ketamine on parameter estimates. These so-called ‘virtual electrodes’ were constructed in left anterior insula (Talairach coordinates: −32, 13, −8) and anterior cingulate (Talairach coordinates: −2, 43, 2). Source estimates from these locations were calculated using the previous wide-band (2–100 Hz) beamformer weights over the entire duration of artifact-free segments of data. These timeseries estimates were subsequently imported into SPM12 (http://www.fil.ion.ucl.ac.uk/spm/), then epoched into 2 s trial lengths. Subsequent modeling analyses used these epoched virtual timeseries signals.
2.5. Dynamic Causal Modeling
DCM uses a biophysical model of neural responses based on neural mass models to predict recorded electrophysiological data (48). The present study specifically used a conductance-based neural mass model for electrophysiology, the ‘CMM_NMDA’ model, to model responses between anterior insula and anterior cingulate. The model has been extensively described in the literature, and detailed equations can be found elsewhere (49–51). This model was selected because it includes extrinsic connection parameters for both fast (AMPA-mediated) and slow (NMDA-mediated) glutamatergic signaling. Within the model, superficial pyramidal cells (SPCs) encode and carry feedforward signaling to stellate cells, while deep pyramidal cells carry feedback signaling to SPCs and inhibitory interneurons (Figure 3A). This canonical microcircuit architecture makes it suitable for estimating connectivity in regions of cortex which largely conform to a similar layer-resolved architecture. While anterior insula includes several cortical layer types organized radially from agranular to granular, the orientation of the cortex within the region and the summed activity necessary to produce a measurable signal suggest that the measured MEG signal in insula should reflect contributions from agranular, dysgranular, and granular layers. Driving current input is included in the model, entering spiny stellate cells.
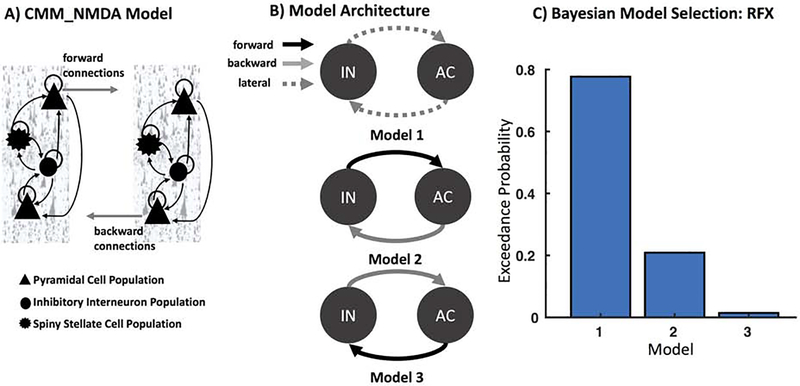
Figure 3. Models of Connectivity: Insula to Anterior Cingulate.
A). The CMM_NMDA model includes four distinct cell layers: superficial pyramidal cells, spiny stellates, inhibitory interneurons, and deep pyramidal cells. Forward connections originate from superficial pyramidal cells to excitatory spiny stellate cells, while backward connections originate from deep pyramidal cells to both superficial pyramidal cells and inhibitory interneurons. B) We constructed three plausible models of message-passing between insula (IN) and anterior cingulate (AC). Model 1 included lateral connections between IN and AC, while Model 2 included feedforward connections from IN to AC and feedback connections from AC to IN, and Model 3 included feedforward connections from AC to IN and feedback connections from IN to AC. C) Bayesian Model Selection (BMS) was used to adjudicate between models, demonstrating that Model 1 with fully interconnected feedforward and feedback connections between each region had the largest exceedance probability.
For the DCM analyses, MEG activity for the extracted time series were fitted over all 2 s rest periods using a wide frequency band (1–58 Hz) and an LFP model to capture cross-spectral density estimates from anterior insula and anterior cingulate. For computational efficiency, DCM optimizes a posterior density over free parameters (parameterized by its mean and covariance) via a standard variational Bayesian inversion procedure (52). In the present analysis, three plausible models of message-passing between anterior insula and anterior cingulate were considered. In Model 1, anterior insula and anterior cingulate were connected via lateral connections, which includes fully reciprocated feedforward and feedback connections between each region. In Model 2, anterior insula carried feedforward signals to anterior cingulate, with recurrent feedback connections carrying signals from anterior cingulate to anterior insula. Model 3 was the reciprocal of Model 2, with feedforward connections originating from anterior cingulate to anterior insula, with recurrent feedback connections from anterior insula to anterior cingulate. To adjudicate between these models, we used the negative free energy bound on the log-model evidence, selecting the model with the highest log-model evidence for subsequent analyses. Parameter estimates were harvested from optimized DCMs for the winning model for each subject and session separately to compare ketamine-mediated effects.
To determine the mixture of parameters which mediated ketamine effect, we applied a second-level modeling extension of DCM called parametric empirical Bayesian analysis (53). This analysis refits a full model (where all parameters can covary according to grouping - here, session) and provides reduced models where smaller combinations of parameters are considered and informed by differences between sessions. Here, we specifically tested for ketamine’s effect in our second-level design matrix, where the first column represented the average effect over all sessions (i.e., ones for all subjects/sessions) while the second column tested for the ketamine effect (i.e., zeros for baseline and placebo, ones for ketamine).
Finally, as an additional exploratory analysis, we specifically harvested extrinsic AMPA and NMDA connectivity estimates between anterior insula and anterior cingulate and correlated these with change in SI and depression scores from the ketamine session (i.e., 60 minutes prior to ketamine infusion minus 230 minutes post-ketamine infusion). As these were exploratory analyses, we used a liberal criterion of p<0.05 uncorrected to determine significant associations between changes in connectivity and clinical symptom improvement.
3. Results
We assessed clinical changes in SI and depression by comparing the corresponding clinical SI factor scores and depression scores by session (baseline, ketamine, placebo). For SI, the fixed effects model demonstrated a significant session effect on SI factor score (F3,29=25.3, p<0.001) (Figure 1B). Post-hoc tests with Bonferroni correction indicated a significant (p<0.05) reduction in SI factor scores following ketamine (Mean=0.268, SE=0.04) compared to baseline (Mean=0.343, SE=0.04), but no differences between placebo (Mean=0.282, SE=0.04) and either ketamine or baseline sessions. For depression, the fixed effects model demonstrated a significant session effect on depression score (F3,29=290.0, p<0.001) (Figure 1B). Post-hoc tests with Bonferroni correction indicated significant (p<0.05) reductions in depression scores following ketamine (Mean=24.3, SE=1.3) compared to baseline (Mean=31.3, SE=1.3) and ketamine compared to placebo (Mean=28.8, SE=1.3), but not between baseline and placebo sessions.
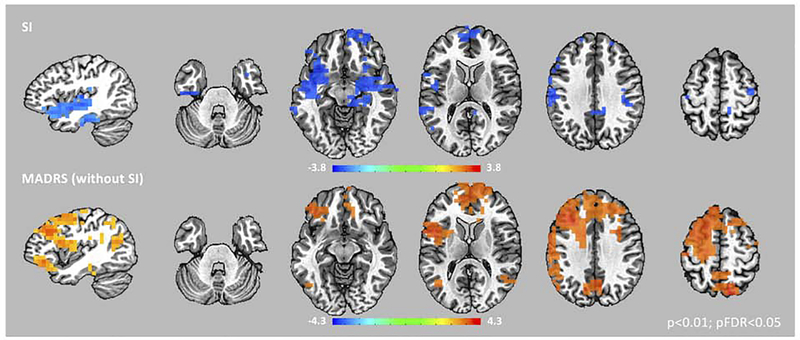
We computed whole-brain, source-level estimates of gamma power and used a linear mixed-effects model to measure the corresponding association with SI and depression scores. Figure 2 shows regions where SI and depression scores were significantly associated with gamma power from the whole-brain model (pFDR<0.05). Regions associated with SI included bilateral medial temporal regions, bilateral insula, and medial prefrontal cortex. These regions showed a negative association between gamma power and SI, suggesting higher SI scores were associated with lower gamma power. Regions associated with depression were localized to more dorsal regions, including posterior insula, bilateral frontal and parietal regions, and anterior and posterior cingulate cortices. These regions showed a positive association between gamma power and depression, suggesting higher depression scores were associated with higher gamma power. Critically, in evaluating the role of anterior insula in both SI and depression, we found that gamma power in more anterior regions of bilateral insula were associated with SI, while more dorsal, posterior (and left-lateralized) regions were associated with depression.
Figure 2. Gamma Power and Suicidal Ideation and Depression.
Images of normalized, root mean square gamma power (30–50 Hz) estimates associated with suicidal ideation (SI) and depression (MADRS – without SI) from the linear mixed-effects model are superimposed on a high-resolution structural scan. Images are thresholded at pFDR<0.05. A network of regions centered in the insular cortex showed associations with SI scores, while a more dorsal, distributed network of regions showed associations with MADRS (without SI) scores in our patients. In addition, within insular cortex, bilateral anterior regions showed significant associations with SI, while more dorsal, posterior (and left-lateralized) regions were associated with MADRS (without SI) scores.
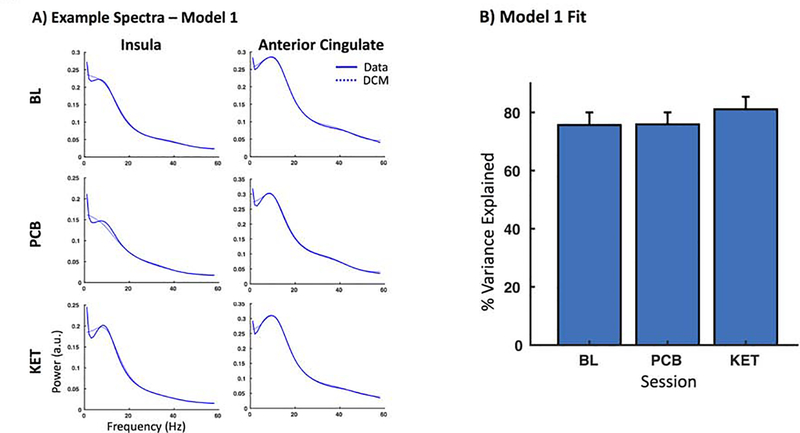
We focused on characterizing the influence of ketamine on effective connectivity between anterior insula and anterior cingulate, motivated by literature on the role of these regions in both suicidal thoughts and depression. We used DCM to model connectivity between these two regions, constructing three models of proposed connectivity (Figure 3B). Using Bayesian model selection to adjudicate between these models, we found that Model 1, with fully reciprocated feedforward and feedback connections between anterior insula and anterior cingulate, had the strongest model evidence (Figure 3C). Model fits across subjects/sessions were then computed for the winning model by correlating the estimated model spectra to the extracted virtual electrode spectra. Example spectral data are shown in Figure 4A. To confirm that there was no biasing in model fits by session, we compared the variance explained by the model across sessions. We found that our model provided good fit in terms of the average variance explained across sessions (75.7% for baseline, 75.9% for placebo, and 81.1% for ketamine), and a one-way analysis of variance confirmed no biasing in model fits based on session (F=0.54, p=0.58) (Figure 4).
Figure 4. Model Fits.
A) Spectral densities from Model 1 for the insula and anterior cingulate from the baseline (BL), placebo (PCB), and ketamine (KET) sessions for three example subjects. Solid lines indicate the data, while dashed lines represent the model fit. B) Model fits were compared across sessions in terms of the percent of the variance explained. No biasing in model fits was found between sessions.
We used parametric empirical Bayes, an analysis approach which allows for testing of random effects of model parameters at the group level, in order to test for meaningful parameters that contributed to ketamine’s effect. We focused on parameters that exhibited significant effects by focusing on those identified parameters having a probability of P>0.95. This analysis yielded a single parameter that significantly contributed to ketamine’s effect: the membrane capacitance of SPCs (given by Ep.CV(2) in the model) (Table 1). We extracted mean values for this parameter across sessions, and found that ketamine lowered the estimated membrane capacitance of SPCs compared to baseline and placebo sessions (baseline Mean=0.0406, placebo Mean=0.0416, ketamine Mean=0.0311). As membrane capacitance is inversely related to voltage change in the model, these findings suggest ketamine leads to faster voltage change across SPCs.
Table 1.
Ketamine Effect.
| Parameter | Parameter Estimate (Ep) | Posterior Probability (Pp) | |
|---|---|---|---|
| Rate Constants: | |||
| 1 | AMPA rate constant - IN* | −0.0981 | 0.6344 |
| 2 | AMPA rate constant - AC* | −0.0647 | 0.5202 |
| 3 | GABA rate constant - IN | 0 | |
| 4 | GABA rate constant - AC | 0 | |
| 5 | NMDA rate constant -IN | 0 | |
| 6 | NMDA rate constant - AC | 0 | |
| Membrane Capacitance | |||
| 7 | Membrane Capacitance - SS | 0 | |
| 8 | Membrane Capacitance - SPY** | −0.5777 | 1 |
| 9 | Membrane Capacitance - II | 0 | |
| 10 | Membrance Capacitance - DPY | 0 | |
| Extrinsic Connectivity | |||
| 11 | AMPA Forward Connectivity: AI to AC | 0 | |
| 12 | AMPA Forward Connectivity: AC to AI | 0 | |
| 13 | AMPA Backward Connectivity: AI to AC | 0 | |
| 14 | AMPA Backward Connectivity: AC to AI* | −0.2598 | 0.605 |
| 15 | NMDA Forward Connectivity: AI to AC | 0 | |
| 16 | NMDA Forward Connectivity: AC to AI | 0 | |
| 17 | NMDA Backward Connectivity: AI to AC | 0 | |
| 18 | NMDA Backward Connectivity: AC to AI | 0 | |
| Intrinsic Connectivity (one each for AI and AC) | |||
| 19 | Excitatory - SS to SPY | 0 | |
| 20 | Excitatory - SS to II | 0 | |
| 21 | Excitatory - SPY to DPY | 0 | |
| 22 | Excitatory - DPY to II | 0 | |
| 23 | Inhibitory - II to SS | 0 | |
| 24 | Inhibitory - II to SPY | 0 | |
| 25 | Inhibitory - II to DPY | 0 | |
| 26 | Inhibitory Self-Connection - SS | 0 | |
| 27 | Inhibitory Self-Connection - SPY | 0 | |
| 28 | Inhibitory Self-Connection - II | 0 | |
| 29 | Inhibitory Self-Connection -DPY | 0 |
Parametric empirical Bayes was used to identify the mixing of parameters that contributed to ketamine’s effect. Note that the timing of data collection (6–9 hours following ketamine administration) is past the half-life of ketamine. We defined meaningful parameters as those with a probability (Pp) of greater than 0.95. A single parameter, the membrane capacitance of superficial pyramidal cells (which are thought to be the primary contributor to the MEG signal), was found to significantly contribute to ketamine’s effect. In addition, other parameters modeling AMPA effects, including the AMPA rate constants in insula (IN) and anterior cingulate (AC) and AMPA backward connectivity between AC and AI were found to contribute to ketamine’s effect, though not at the significance level we defined. SPY=superficial pyramidal cells, SS=spiny stellate cells, II=inhibitory interneurons, DPY=deep pyramidal cells.
Pp>0.5
Pp>0.95
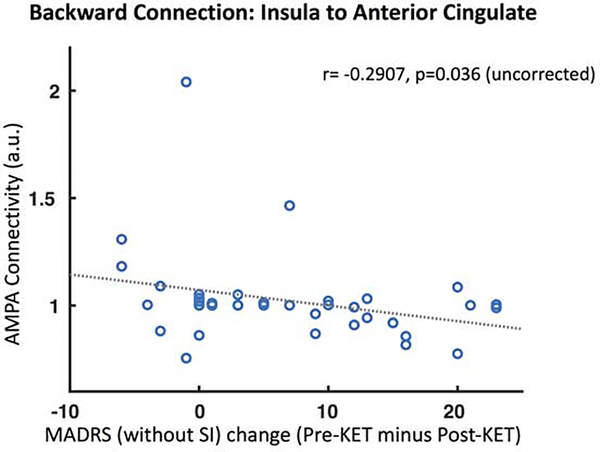
As a further exploratory analysis, we compared extrinsic AMPA and NMDA connectivity estimates for the four connections in our model with change in SI and depression scores during the ketamine infusion. Using a liberal criterion (p<0.05 uncorrected), we found a correlation between the backward AMPA-mediated extrinsic connection from insula to anterior cingulate and change in depression scores (r=−0.29, p=0.04). These findings suggest that lower estimates of AMPA connectivity were associated with reduced depression scores in our group. There were no associations between SI and AMPA and NMDA connectivity estimates.
4. Discussion
This study used MEG recordings acquired during rest to investigate the neurobiological underpinnings of SI in a group of MDD patients and to measure how connectivity was altered by ketamine administration. Clinically, ketamine administration significantly reduced both SI and depression scores in our sample, in line with previous findings (28, 54, 55). In addition, for SI a significant, negative association was observed with gamma power in a network of regions that included key nodes within the salience network, in line with our hypothesis. For depression scores, a significant, positive association was observed with gamma power in more dorsal regions including bilateral frontal and parietal cortices and anterior and posterior cingulate cortices. In the insular cortex in particular, we found an interesting distinction between SI and depression scores, such that gamma power in bilateral anterior insula was associated with SI while more dorsal and posterior (and left-lateralized) regions were associated with depression scores.
Gamma power is proposed to be a proxy measure for network-level E/I balance (36). Thus our findings for SI suggest that aberrant E/I balance and network slowing in anterior insular cortex might contribute significantly to suicide risk. This is in line with other findings noting an association between insula and both suicidal thoughts (23, 24) and risk for suicide (11, 14, 18, 20, 21). Broadly, the anterior insula has been implicated in regulating emotional states, self-awareness, and decision-making in MDD (56). The insular cortex is considered to be part of the salience network (57), which is involved in emotional salience processing and mediates between the central executive and default mode networks (26). Dysfunction within the salience network, particularly bottom-up salience detection, control switching between other large-scale brain networks, and aberrant reactivity to salient events within the anterior insula (in addition to aberrant coupling between the anterior insula and anterior cingulate), have been suggested to underlie a host of psychiatric conditions (25). Our findings suggest that SI could represent a salience network deficit that manifests via reduced gamma power, reflecting an imbalance in E/I within bilateral anterior insula.
To explore how ketamine modulates connectivity within the salience network, we modelled ketamine’s effect using DCM. Motivated by the extant literature on the role of anterior insula and anterior cingulate in suicidal thoughts and depression (11, 14, 18, 20, 21, 23, 24), we constructed several models of connectivity to explore message-passing between these regions,. We found that a model including fully interconnected feedforward and feedback connections provided the highest model evidence. We then used a Bayesian analysis to determine ketamine’s effect on parameter estimates within our model. This analysis showed that ketamine lowered SPC membrane capacitance, suggesting faster voltage change across SPCs following ketamine compared with baseline or placebo. While this effect has not previously been reported with SPCs, several studies have suggested that ketamine influences astrocytic membrane capacitance (58, 59), leading to increased bursting which is thought to be caused by stabilization of the fusion pore (59). In this context, SPCs are important because they are the thought to be the primary generators of the MEG signal (60, 61). Thus, if a similar mechanism exists for SPCs, stabilization of the fusion pore and increased bursting could be one mechanism accounting for increased cortical excitation following ketamine. In addition, parametric empirical Bayes showed that ketamine slowed AMPA rate constants (the rate of AMPA opening and closing) in both anterior insula and anterior cingulate, while lowering AMPA-mediated connectivity between cingulate and insula, though these effects did not reach our significance threshold. However, these findings add to a growing literature that suggest that ketamine alters AMPA receptor synaptic potentiation (31).
Finally, we examined associations between extrinsic AMPA- and NMDA-mediated connections in our model and changes in both SI and depression scores following ketamine administration. Here, we found a single connection which demonstrated an association with clinical change: lower AMPA connectivity in the backward connection from anterior insula to anterior cingulate was associated with reduced depression symptomatology, but not SI. Similar findings have been reported elsewhere (62), with AMPA connectivity correlating with sustained changes in depression symptoms following ketamine administration, even out to 14-days following ketamine administration.
One important limitation of our study design is that participants had a primary diagnosis of MDD. That is, subjects were not selected because they were acutely suicidal, though many of them did report SI as a feature of their larger symptomatology. In addition, our data was drawn from a larger clinical trial exploring ketamine’s antidepressant effects, so we could not differentiate SI from depression explicitly. Thus, it remains to be seen whether our findings can generalize to patients who are acutely suicidal without a corresponding MDD diagnosis. In addition, there was not a direct assessment of SI on the day of the baseline scan, thus future studies are needed with daily assessment of SI to capture potential variability in symptomatology. Lastly, our model of brain dynamics is based on a canonical microcircuit that includes four subpopulations of cell types: superficial pyramidal cells, spiny stellate cells, inhibitory interneurons, and deep pyramidal cells. As mention previously, anterior insula includes several cortical layer types organized radially. Thus, further work should consider applying different models to measure connectivity within insula which do not rely on a fully granular cortical architecture.
Nevertheless, this study has broad implications for future research into suicide risk. First, our findings demonstrate that network-level deficits in E/I within anterior insula are associated with suicide risk distinct from depression symptoms. Second, they suggest that ketamine leads to transient changes in pyramidal cell membrance capacitance within the salience network. As this network is important for both salience detection and switching between other large-scale networks, this could have important implications for how ketamine influences clinical outcomes related to risk for suicide more broadly. Third, our findings add to the literature on the importance of AMPA for improvements in depression symptoms (62). Finally, our findings add to a growing literature illustrating the usefulness of gamma power as a biomarker for measuring synaptic homeostasis and its dysregulation in psychiatric conditions.
Figure 5. AMPA Connectivity and Depression.
We compared NMDA and AMPA extrinsic connectivity estimates following ketamine (KET) administration with changes in both suicidal ideation (SI) and depression (MADRS – without SI) scores. Only MADRS (without SI) scores showed an association with connectivity – lower AMPA connectivity for the backward connection from insula to anterior cingulate was associated with lower depression symptomatology.
Acknowledgements
Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIA MH002857), by a NARSAD Independent Investigator Award to Dr. Zarate, and by a Brain and Behavior Mood Disorders Research Award to Dr. Zarate. The authors thank the 7SE research unit and staff for their support. Ioline Henter (NIMH) provided invaluable editorial assistance.
Footnotes
Disclosures
Dr. Zarate is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain; and as a co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. All other authors report no biomedical financial interests or potential conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Curtin SC, Heron M, Minino AM, Warner M (2018): Recent Increases in Injury Mortality Among Children and Adolescents Aged 10–19 Years in the United States: 1999–2016. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 67:1–16. [PubMed] [Google Scholar]
- 2.Curtin SC, Warner M, Hedegaard H (2016): Increase in Suicide in the United States, 1999–2014. NCHS data brief.1–8. [PubMed] [Google Scholar]
- 3.Kleiman EM, Turner BJ, Fedor S, Beale EE, Huffman JC, Nock MK (2017): Examination of real-time fluctuations in suicidal ideation and its risk factors: Results from two ecological momentary assessment studies. Journal of abnormal psychology. 126:726–738. [DOI] [PubMed] [Google Scholar]
- 4.Suominen K, Isometsa E, Suokas J, Haukka J, Achte K, Lonnqvist J (2004): Completed Suicide After a Suicide Attempt: A 37-Year Follow-Up Study. The American journal of psychiatry. 161:562–563. [DOI] [PubMed] [Google Scholar]
- 5.Jandl M, Steyer J, Kaschka WP (2010): Suicide risk markers in major depressive disorder: a study of electrodermal activity and event-related potentials. J Affect Disord. 123:138–149. [DOI] [PubMed] [Google Scholar]
- 6.Graae F, Tenke C, Bruder G, Rotheram MJ, Piacentini J, Castro-Blanco D, et al. (1996): Abnormality of EEG alpha asymmetry in female adolescent suicide attempters. Biological psychiatry. 40:706–713. [DOI] [PubMed] [Google Scholar]
- 7.Lee SM, Jang KI, Chae JH (2017): Electroencephalographic Correlates of Suicidal Ideation in the Theta Band. Clinical EEG and neuroscience. 48:316–321. [DOI] [PubMed] [Google Scholar]
- 8.Chattun MR, Zhang S, Chen Y, Wang Q, Amdanee N, Tian S, et al. (2018): Caudothalamic dysfunction in drug-free suicidally depressed patients: an MEG study. Eur Arch Psychiatry Clin Neurosci. [DOI] [PubMed] [Google Scholar]
- 9.Dombrovski AY, Siegle GJ, Szanto K, Clark L, Reynolds CF, Aizenstein H (2012): The temptation of suicide: striatal gray matter, discounting of delayed rewards, and suicide attempts in late-life depression. Psychological medicine. 42:1203–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang JP, Lee TW, Tsai SJ, Chen TJ, Yang CH, Lirng JF, et al. (2010): Cortical and subcortical abnormalities in late-onset depression with history of suicide attempts investigated with MRI and voxel-based morphometry. Journal of geriatric psychiatry and neurology. 23:171–184. [DOI] [PubMed] [Google Scholar]
- 11.Oquendo MA, Galfalvy H, Sullivan GM, Miller JM, Milak MM, Sublette ME, et al. (2016): Positron Emission Tomographic Imaging of the Serotonergic System and Prediction of Risk and Lethality of Future Suicidal BehaviorPositron Emission Tomographic Imaging of the Serotonergic SystemPositron Emission Tomographic Imaging of the Serotonergic System. JAMA psychiatry. 73:1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng H, Wu K, Li J, Qi H, Guo S, Chi M, et al. (2014): Increased suicide attempts in young depressed patients with abnormal temporal-parietal-limbic gray matter volume. J Affect Disord. 165:69–73. [DOI] [PubMed] [Google Scholar]
- 13.Rizk MM, Rubin-Falcone H, Lin X, Keilp JG, Miller JM, Milak MS, et al. (2019): Gray matter volumetric study of major depression and suicidal behavior. Psychiatry research Neuroimaging. 283:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor WD, Boyd B, McQuoid DR, Kudra K, Saleh A, MacFall JR (2015): Widespread white matter but focal gray matter alterations in depressed individuals with thoughts of death. Progress in neuro-psychopharmacology & biological psychiatry. 62:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner G, Schultz CC, Koch K, Schachtzabel C, Sauer H, Schlosser RG (2012): Prefrontal cortical thickness in depressed patients with high-risk for suicidal behavior. Journal of psychiatric research. 46:1449–1455. [DOI] [PubMed] [Google Scholar]
- 16.Wagner G, Koch K, Schachtzabel C, Schultz CC, Sauer H, Schlosser RG (2011): Structural brain alterations in patients with major depressive disorder and high risk for suicide: evidence for a distinct neurobiological entity? Neuroimage. 54:1607–1614. [DOI] [PubMed] [Google Scholar]
- 17.Duarte DGG, Neves MCL, Albuquerque MR, Turecki G, Ding Y, de Souza-Duran FL, et al. (2017): Structural brain abnormalities in patients with type I bipolar disorder and suicidal behavior. Psychiatry research Neuroimaging. 265:9–17. [DOI] [PubMed] [Google Scholar]
- 18.Besteher B, Wagner G, Koch K, Schachtzabel C, Reichenbach JR, Schlosser R, et al. (2016): Pronounced prefronto-temporal cortical thinning in schizophrenia: Neuroanatomical correlate of suicidal behavior? Schizophrenia research. 176:151–157. [DOI] [PubMed] [Google Scholar]
- 19.Giakoumatos CI, Tandon N, Shah J, Mathew IT, Brady RO, Clementz BA, et al. (2013): Are structural brain abnormalities associated with suicidal behavior in patients with psychotic disorders? Journal of psychiatric research. 47:1389–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soloff P, White R, Diwadkar VA (2014): Impulsivity, aggression and brain structure in high and low lethality suicide attempters with borderline personality disorder. Psychiatry research. 222:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soloff PH, Pruitt P, Sharma M, Radwan J, White R, Diwadkar VA (2012): Structural brain abnormalities and suicidal behavior in borderline personality disorder. Journal of psychiatric research. 46:516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barredo J, Aiken E, van ‘t Wout-Frank M, Greenberg BD, Carpenter LL, Philip NS (2019): Neuroimaging Correlates of Suicidality in Decision-Making Circuits in Posttraumatic Stress Disorder. Frontiers in psychiatry. 10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballard ED, Reed JL, Szczepanik J, Evans JW, Yarrington JS, Dickstein DP, et al. (2019): Functional Imaging of the Implicit Association of the Self With Life and Death. Suicide & life-threatening behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi Z, Han S (2013): Transient and sustained neural responses to death-related linguistic cues. Social cognitive and affective neuroscience. 8:573–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menon V (2011): Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 15:483–506. [DOI] [PubMed] [Google Scholar]
- 26.Menon V, Uddin LQ (2010): Saliency, switching, attention and control: a network model of insula function. Brain structure & function. 214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Namkung H, Kim S-H, Sawa A (2017): The Insula: An Underestimated Brain Area in Clinical Neuroscience, Psychiatry, and Neurology. Trends Neurosci. 40:200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, et al. (2018): The Effect of a Single Dose of Intravenous Ketamine on Suicidal Ideation: A Systematic Review and Individual Participant Data Meta-Analysis. The American journal of psychiatry. 175:150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ballard ED, Lally N, Nugent AC, Furey ML, Luckenbaugh DA, Zarate JCA (2015): Neural Correlates of Suicidal Ideation and Its Reduction in Depression. The international journal of neuropsychopharmacology. 18:pyu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong LE, Summerfelt A, Buchanan RW, O’Donnell P, Thaker GK, Weiler MA, et al. (2010): Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 35:632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cornwell BR, Salvadore G, Furey M, Marquardt CA, Brutsche NE, Grillon C, et al. (2012): Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biological psychiatry. 72:555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw AD, Saxena N, L EJ, Hall JE, Singh KD, Muthukumaraswamy SD (2015): Ketamine amplifies induced gamma frequency oscillations in the human cerebral cortex. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 25:1136–1146. [DOI] [PubMed] [Google Scholar]
- 33.Nugent AC, Ballard ED, Gould TD, Park LT, Moaddel R, Brutsche NE, et al. (2018): Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Homayoun H, Moghaddam B (2007): NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 27:11496–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren Z, Pribiag H, Jefferson SJ, Shorey M, Fuchs T, Stellwagen D, et al. (2016): Bidirectional Homeostatic Regulation of a Depression-Related Brain State by Gamma-Aminobutyric Acidergic Deficits and Ketamine Treatment. Biological psychiatry. 80:457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buzsaki G, Wang X-J (2012): Mechanisms of Gamma Oscillations. Annual Review of Neuroscience. 35:203–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duman RS, Aghajanian GK (2012): Synaptic Dysfunction in Depression: Potential Therapeutic Targets. Science. 338:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drevets WC (2004): Neuroplasticity in mood disorders. Dialogues in clinical neuroscience. 6:199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moran Rosalyn J, Symmonds M, Stephan Klaas E, Friston Karl J, Dolan Raymond J (2011): An In Vivo Assay of Synaptic Function Mediating Human Cognition. Current Biology. 21:1320–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.American Psychiatric Association (1994): Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psyciatric Association. [Google Scholar]
- 41.Montgomery SA, Asberg M (1979): A new depression scale designed to be sensitive to change. The British journal of psychiatry : the journal of mental science. 134:382–389. [DOI] [PubMed] [Google Scholar]
- 42.First MB, Spitzer RL, Gibbon M, Williams JB (2002): Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. SCID-I/P.
- 43.Ballard ED, Yarrington JS, Farmer CA, Lener MS, Kadriu B, Lally N, et al. (2018): Parsing the heterogeneity of depression: An exploratory factor analysis across commonly used depression rating scales. J Affect Disord. 231:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beck AT, Ward CH, Mendelson MM, Mock JJ, Erbaugh JJ (1961): An inventory for measuring depression. Arch Gen Psychiatry. 4:561–571. [DOI] [PubMed] [Google Scholar]
- 45.Vrba J, Robinson SE (2001): Signal Processing in Magnetoencephalography. Methods. 25:249–271. [DOI] [PubMed] [Google Scholar]
- 46.Cox RW (1996): AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Computers and Biomedical Research. 29:162–173. [DOI] [PubMed] [Google Scholar]
- 47.Chen G, Saad ZS, Britton JC, Pine DS, Cox RW (2013): Linear mixed-effects modeling approach to FMRI group analysis. Neuroimage. 73:176–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.David O, Kiebel SJ, Harrison LM, Mattout J, Kilner JM, Friston KJ (2006): Dynamic causal modeling of evoked responses in EEG and MEG. NeuroImage. 30:1255–1272. [DOI] [PubMed] [Google Scholar]
- 49.Moran RJ, Stephan KE, Dolan RJ, Friston KJ (2011): Consistent spectral predictors for dynamic causal models of steady-state responses. NeuroImage. 55:1694–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muthukumaraswamy SD, Shaw AD, Jackson LE, Hall J, Moran R, Saxena N (2015): Evidence that Subanesthetic Doses of Ketamine Cause Sustained Disruptions of NMDA and AMPA-Mediated Frontoparietal Connectivity in Humans. The Journal of Neuroscience. 35:11694–11706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Symmonds M, Moran CH, Leite MI, Buckley C, Irani SR, Stephan KE, et al. (2018): Ion channels in EEG: isolating channel dysfunction in NMDA receptor antibody encephalitis. Brain. 141:1691–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friston K, Mattout J, Trujillo-Barreto N, Ashburner J, Penny W (2007): Variational free energy and the Laplace approximation. Neuroimage. 34:220–234. [DOI] [PubMed] [Google Scholar]
- 53.Friston KJ, Litvak V, Oswal A, Razi A, Stephan KE, van Wijk BCM, et al. (2016): Bayesian model reduction and empirical Bayes for group (DCM) studies. Neuroimage. 128:413–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ballard ED, lonescu DF, Vande Voort JL, Niciu MJ, Richards EM, Luckenbaugh DA, et al. (2014): Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. Journal of psychiatric research. 58:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ballard ED, Luckenbaugh DA, Richards EM, Walls TL, Brutsche NE, Ameli R, et al. (2015): Assessing measures of suicidal ideation in clinical trials with a rapid-acting antidepressant. Journal of psychiatric research. 68:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGrath CL, Kelley ME, Holtzheimer PE, Dunlop BW, Craighead WE, Franco AR, et al. (2013): Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA psychiatry. 70:821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. (2007): Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. The Journal of neuroscience : the official journal of the Society for Neuroscience. 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stenovec M, Lasic E, Bozic M, Bobnar ST, Stout RF, Grubisic V, et al. (2016): Ketamine Inhibits ATP-Evoked Exocytotic Release of Brain-Derived Neurotrophic Factor from Vesicles in Cultured Rat Astrocytes. Mol Neurobiol. 53:6882–6896. [DOI] [PubMed] [Google Scholar]
- 59.Lasic E, Rituper B, Jorgacevski J, Kreft M, Stenovec M, Zorec R (2016): Subanesthetic doses of ketamine stabilize the fusion pore in a narrow flickering state in astrocytes. J Neurochem. 138:909–917. [DOI] [PubMed] [Google Scholar]
- 60.Llinas R (1974): Analysis of field potentials in the central nervous system. Anonymous Handbook of Electroencephlography and Clinical Neurophysiology.61–83. [Google Scholar]
- 61.Murakami S, Okada Y (2006): Contributions of principal neocortical neurons to magnetoencephalography and electroencephalography signals. J Physiol. 575:925–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gilbert JR, Yarrington JS, Wills KE, Nugent AC, Zarate CA Jr. (2018): Glutamatergic Signaling Drives Ketamine-Mediated Response in Depression: Evidence from Dynamic Causal Modeling. The international journal of neuropsychopharmacology. 21:740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]